Abstract
Background:
Successful regeneration depends on four key elements of tissue engineering such as stem cells, growth factors, scaffold and sterile environment. Therefore, regenerative endodontic therapy requires higher degree of disinfection for successful outcomes.
Aims and Objective:
To evaluate the clinical and radiographic outcome using conventional and laser assisted disinfection in regenerative endodontics at 1, 3 and 6 months.
Materials and Methods:
18 children with necrotic young permanent teeth were selected for the study. In Group A, in the first appointment AAE (American Association of Endodontics, Clinical Consideration for Regenerative Endodontic Procedure - 2016) disinfection protocol was employed. In Group B laser assisted disinfection (810 nm diode laser, 1 W, 20 ms Pulse length and 20 ms interval duration) was performed. Microbial samples were obtained before and after disinfection to check the level of disinfection. The cases were followed up at 1 month, 3 months and 6 months interval and evaluated for clinical outcome, periapical healing and apical response. Data were statistically analyzed with level of significance set 1% or 5% accordingly (P<0.01 or P<0.05)
Results:
On intragroup comparison there was significant reduction in bacterial count before and after disinfection with respect to laser assisted disinfection group. (P value = 0.007) There was no significant change in the clinical outcome score in laser group at 1,3- and 6-months interval. Periapical healing score showed statistically significant results in laser group when followed from 3 months to 6 months (P value = 0.04).
Conclusion:
Along with AAE 2016 protocol, use of laser assisted disinfection resulted in improved quality of disinfection and an expeditious clinical outcome and periapical healing in necrotic young permanent teeth. Keywords: Disinfection; laser; regenerative endodontics; sodium hypochlorite; tooth apex
Keywords: Disinfection, laser, regenerative endodontics, sodium hypochlorite, tooth apex
INTRODUCTION
Regenerative endodontic technique stands as a preeminent solution for management of immature permanent teeth with pulpal necrosis when dental practitioners are challenged by the unique morphological features of these teeth showing incomplete root apex and thin dentinal walls.[1]
Even though apexification was the most practiced treatment modality for an immature necrotic tooth, several disadvantages such as long-time span of the treatment, multiple patient visits, and thin dentinal walls with increased fracture risk make this approach less popular.[2]
Tissue engineering field has shown an increase in the research attention in recent years and showed promising results in pulp regeneration which is a boon to dentistry. Regenerative endodontics can be practiced today by clinical translation of concepts such as tissue engineering, pulp biology, and pulp regeneration.[3] Hence, the American Association of Endodontists (AAE) recommends regenerative endodontic treatment for young permanent teeth with necrotic pulp.[4]
Successful regeneration depends on four key elements of tissue engineering, which are:[5]
Stem cells
Scaffolds
Signaling molecules
Sterile environment.
Therefore, root canal space must be completely disinfected, and it requires a higher degree of disinfection in regenerative endodontic therapy. Efficient removal of debris and microorganism can be achieved by various techniques such as irrigation, instrumentation, and intracanal medicaments.[6] Multiple researches have been published with significant differences in disinfection protocols using various disinfecting solutions and antibiotics in regenerative endodontics.[7] Disinfecting agents should not damage the survival and proliferative capacity of the stem cells. Disinfection using 1.5% sodium hypochlorite (NaOCl) followed by 17% ethylenediaminetetraacetic acid (EDTA) resulted in adequate disinfection without any detrimental effect on stem cells of apical papilla and the use of 17% EDTA resulted in increased expression of dentin sialophosphoprotein and survival of stem cells by reversing the adverse effects of NaOCl as well as the release of growth factors from dentin matrix.[8] Therefore, AAE 2016 protocol recommended the use of 1.5% NaOCl and 17% EDTA as irrigant and calcium hydroxide as intracanal medicament.[4]
The primary goal of laser in disinfection is to eradicate microorganisms, removal of debris and smear layer. These beneficial effects of laser will ensure successful endodontic treatment outcome.[9] Reduction in the bacterial count up to 74% was noted when an 810-nm diode laser was used. Efficacy of diode laser has been proved in maturogenesis of young permanent teeth.[10,11]
Recently, immature teeth treated with regenerative endodontics where photo-activated disinfection has been used as an adjunct with irrigating solutions showed root closure and increasing radicular thickness in necrotic immature teeth when followed for 10–12 months.[12,13]
Many aspects of regenerative endodontics are new and unanswered because of lack of research in this field. The AAE Foundation and Endodontic Community promotes researches on regenerative endodontic procedures even though the regenerative cases treated with AAE 2016 protocol were reported to be successful.[4]
The null hypothesis stated that there is no difference in clinical and radiographic outcome using conventional and laser-assisted disinfection in regenerative endodontics in necrotic young permanent teeth. The need for improvement in existing clinical protocol leads to the aim of this study to evaluate the clinical and radiographic outcome using conventional and laser-assisted disinfection in regenerative endodontics in necrotic young permanent teeth.
SUBJECTS AND METHODS
Trial design
The study is a randomized clinical trial between two parallel groups with an allocation ratio of 1:1.
Sample size determination
The calculated sample size was 8 (α: level of significance = 5%, 1−β: power of the study = 80%). Considering 20% dropout, 10 was the final sample size for each group. Calculation was done according to sampling method described by Chow et al.[14]
Study setting
The study was registered under Rajiv Gandhi University of Health Sciences, Karnataka. A total of 18 children aged 7–15 years whose parents provided consent to their participation in the study were selected. The present study was conducted in the Department of Pedodontics and Preventive Dentistry, Bapuji Dental College and Hospital, Davangere. The study was approved by the Ethical Committee (Reg.no. BDC/EXAM/467/2018-19), and informed consent was obtained from all the children's parents, who children were participating in this study.
Eligibility criteria[4]
Inclusion criteria
Following subjects are excluded from the study
Subjects who come under ASA I (American Society of Anesthesiologists) for children, both genders with age range from 7 to 15 years, tooth in question is restorable, permanent necrotic central incisors with incomplete root development defined by apical foramen size ≥1 mm, with or without periapical lesions, pulp involvement due to trauma, and selected teeth have never been subjected to any line of endodontic treatment.
Exclusion criteria
Following subjects are excluded from the study
Radiographic or clinical identification of ankylosis, root resorption, root fracture, uncooperative patients, legal guardians did not consent to participate in the study. Presence of draining sinus and presence of periodontal pockets, presence of periapical radiolucency more than 10 mm measured using DBSWIN software tool. (Durr dental Vista Scan).
Randomization and blinding
Sequence generation
Sequence generation was done for the patient's number from 1 to 20 using computer sequence generation (www.random.org).
Allocation concealment
The upper central incisors in each group were randomly assigned by a coin toss to be either the conventional disinfection on the head side or the laser-assisted disinfection on the tail side.
Dental examination and informed consent
A detailed case history was taken (demographic details, medical history, and dental history). General physical examination was carried out to rule out systemic illness. Extraoral and intraoral examination was carried out by visual, palpation, and percussion methods. Informed consent was obtained from each patient's parents before beginning any procedure explaining intended treatment, the possible outcomes, complications, follow-up period needed, and sequela of no treatment.
Radiographic diagnostic procedure and standardization
Periapical radiographic images were obtained by Dental RVG (DBSWIN software, Durr dental Vista Scan). Images were dimensionally standardized using a film holding device to obtain a constant tooth-film-cone relationship through the study. Obtained X-ray images were processed using automatic X-ray film processor and saved in a tiff format.
Treatment procedure
Regenerative endodontic treatment has been performed according to the AAE protocol.[4]
First appointment
A digital intraoral radiograph of concerned tooth was taken. Local anesthesia with vasoconstrictor was administered (Lignox 2%A – 1:80,000 adrenaline), and rubber dam application was carried out. Access cavity was prepared using round bur (ISO 012/25 mm). A sterile paper point was inserted into root canal and left for 60 s to soak up the contents of the root canal. The wet paper point was then dropped into 2 ml of BHI solution (Micropress). BHI broth was diluted in log 10 steps. For culture, 0.1 ml portion of the dilution (10-1–10-5) was applied and inoculated on blood agar (HiMedia, India) and placed in an incubator at 37°C for 24–48 h.[15] The total numbers of colony-forming units were determined for each sample using colony counter by a blinded assessor.
Group A (conventional disinfection group)
Minimal or no instrumentation of the dentinal walls is always advocated in regenerative endodontic procedures. The disinfection approach was chemical rather than chemo-mechanical.[4,16]
Copious, gentle irrigation was done with 20 ml of 1.5% NaOCl (Fisher Scientific) with side-vented closed needle (20 ml/canal, 5 min) followed by irrigation with 17% EDTA (PREVEST DenPro) (20 ml/canal, 5 min) by placing irrigating needle about 1 mm from root end as per AAE 2016 protocol.
Group B (laser-assisted disinfection group)
Copious, gentle irrigation was done with 20 ml of 1.5% NaOCl with side-vented closed needle (20 ml/canal, 5 min), followed by disinfection was performed with an 810-nm diode laser (AMD LASERS Picasso Dental Diode Laser) with power 1 W, 20-ms pulse length, and 20-ms interval duration. The optical fiber tip (200 micron) was introduced 1 mm short of the working length and laser irradiation was performed with the irrigant in the canal for 15 s[17] followed by 17% EDTA irrigation.
Microbial sample was collected with the help of sterile paper point and cultured from both the groups to assess total microbial count after disinfection in a similar manner as explained before. Ca(OH)2 medicament (prime dental) was placed as per AAE 2016 protocol.[4] Access cavity was sealed with restorative material (GC Fuji Glass Ionomer Cement), and the patient was recalled after 1–4 weeks.
Second appointment
Local anesthesia without vasoconstrictor (Lignox 2%) was administered. Copious, gentle irrigation was done with 20 ml of 17% EDTA. Blood clot scaffold was placed inside the root canal by over instrumenting with precurved 15 no. K-file (MANI Inc., Japan). Collagen plug (Colla Cote™) was placed over the blood clot. A 3–4-mm MTA (MTA Plus) coronal seal was placed.
Posttreatment evaluation
All patients were recalled at 1, 3, and 6 months to evaluate treated teeth. In this study, the cases were evaluated for clinical outcome, periapical healing, and apical response according to Miller's grading of tooth mobility, Ostravik Index, and Chen and Chen criteria, respectively.
Clinical outcome was assessed by Miller's grading of tooth mobility at 1-, 3-, and 6-month interval.[18]
Score 0 – Normal physiologic mobility
Score 1 – Mobility <1 mm
Score 2 – Mobility >1 mm
Score 3 – Mobility <1 mm in horizontal direction with vertical depressability.
Periapical healing was assessed through Ostravik index at 3- and 6-month interval[19] [Figures 1 and 2].
Figure 1.
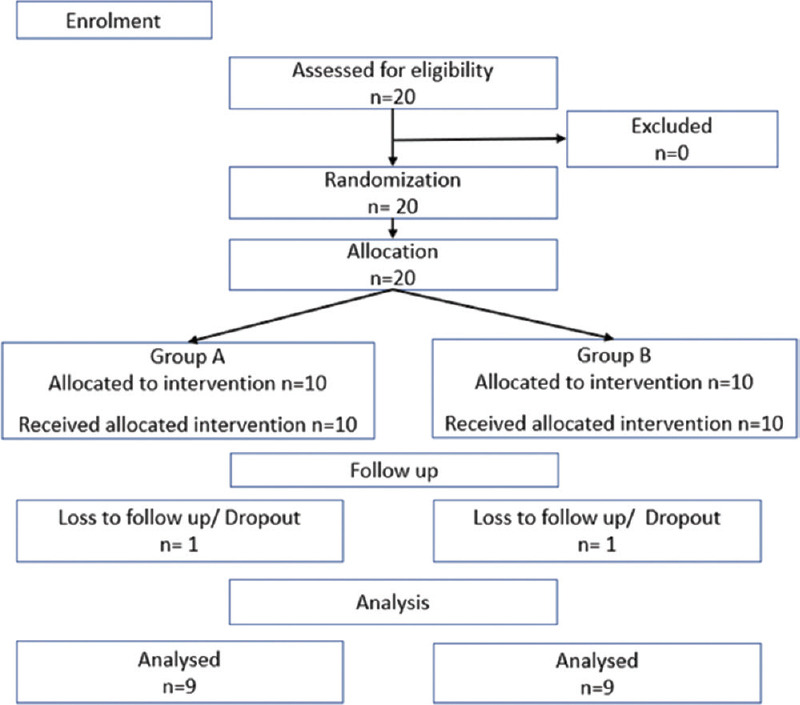
CONSORT 2010 flow diagram
Figure 2.
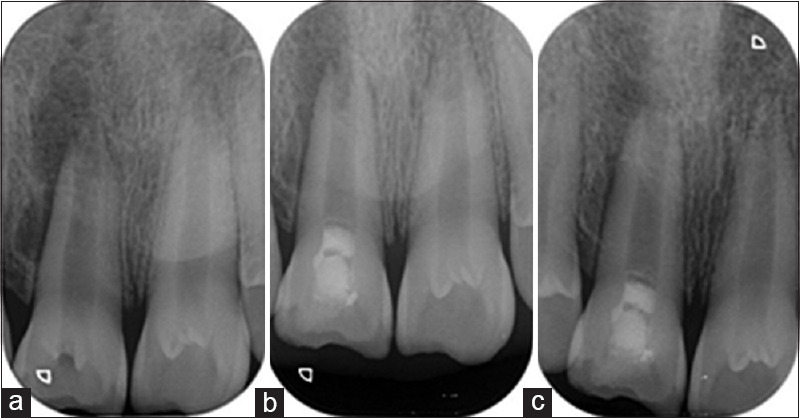
Representative radiograph of Group A (conventional disinfection) at (a) baseline, (b) 3 months, and (c) 6 months
Score 1 – Normal periapical structures
Score 2 – Small changes in bone structure
Score 3 – Changes in bone structure with some mineral loss
Score 4 – Periodontitis with well-defined radiolucent areas
Score 5 – Severe periodontitis with exacerbating features.
Five types of apical responses are observed in a necrotic immature tooth treated by revascularization procedure. In this study, a system proposed by Chen and Chen was used to evaluate the apical responses of teeth at 3 and 6 months[20] [Table 1 and Figures 2, 3].
Table 1.
Apical response types by Chen et al.
| Score | Criteria |
|---|---|
| Type 1 | Increased thickening of the canal walls and continued root maturation |
| Type 2 | No significant continuation of root development with root apex becoming blunt and closed |
| Type 3 | Continued root development with the apical foramen remaining open |
| Type 4 | Severe calcification (obliteration) of the canal space |
| Type 5 | A hard-tissue barrier formed in the canal between the coronal MTA plug and the root apex |
MTA: Mineral Trioxide Aggregate
Figure 3.
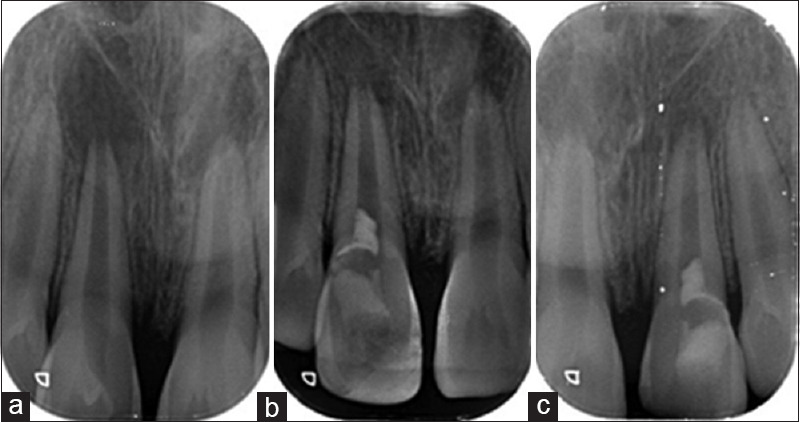
Representative radiograph of Group B (laser-assisted disinfection) at (a) baseline, (b) 3 months, and (c) 6 months
RESULTS
Out of 20 cases that were treated in the study, 18 cases reported complete follow-up of 6 months showing 2 cases dropout among study subjects due to COVID-19 pandemic.
Figure 1 shows the flow of patients through the flow.
Statistical analysis was accomplished using software program Statistical Package for Social Sciences version 22 (SPSS Inc).
Nonparametric tests are used. Intragroup comparison of microbial count before and after disinfection was done using Wilcoxon signed-rank test. Comparison of clinical outcome score between time intervals in each study group was done using Friedman test. Comparison of periapical healing score between the groups at 3 and 6 months was done using MannWhitney U-test. Data were statistically analyzed with level of significance set 1% or 5% accordingly (P < 0.01 or P < 0.05).
Microbial count
Table 2 shows on intragroup comparison, the median values for Group A before and after disinfection was 300 (50–800) and 0 (0–250) and for Group B before and after disinfection were 400 (100–1150) and 0 (0–50), respectively. P =0.02 had shown a statistically significant change in bacterial count before and after disinfection with respect to the conventional disinfection group. P =0.007 had shown a statistically highly significant change in bacterial count before and after disinfection with respect to the laser assisted disinfection group.
Table 2.
Intragroup comparison of microbial count before and after disinfection in Group A (disinfection using conventional irrigation) and Group B (laser-assisted disinfection) before and after disinfection
| Group | Microbial count | n | Mean (SD) | Median (Q1-Q3) | Wilcoxon signed-rank test |
|
|---|---|---|---|---|---|---|
| Z | P | |||||
| A | Before disinfection | 9 | 600.00 (884.59) | 300 (50-800) | -2.38 | 0.02* |
| After disinfection | 9 | 122.22 (156.35) | 0 (0-250) | |||
| B | Before disinfection | 9 | 666.67 (734.85) | 400 (100-1150) | -2.68 | 0.007* |
| After disinfection | 9 | 22.22 (44.10) | 0 (0-50) | |||
*P<0.05 statistically significant, P>0.05 NS. NS: Nonsignificant, SD: Standard deviation
Clinical outcome
Table 3 shows on statistical analysis, the median values for clinical outcome for Group A at 1, 3, and 6 months were 0 (0–0), 1 (0–1), and 0 (0–0.5) and for Group B were 0 (0–0), 0 (0–0), and 0 (0–0), respectively. P =0.02 had shown a statistically significant change in clinical outcome score in Group A at 1, 3, and 6 months. This indicates that laser assisted disinfection 3 showed no change in the clinical outcome scores indicating successful clinical outcome when followed for 6 months.
Table 3.
Comparison of clinical outcome score between time intervals in each study group
| Group | Clinical outcome score | n | Mean (SD) | Median (Q1-Q3) | Friedman test |
|
|---|---|---|---|---|---|---|
| χ 2 | P | |||||
| A | 1 month | 9 | 0 (0) | 0 (0-0) | 7.60 | 0.02* |
| 3 months | 9 | 0.56 (0.53) | 1 (0-1) | |||
| 6 months | 9 | 0.22 (0.44) | 0 (0-0.5) | |||
| B | 1 month | 9 | 0 (0) | 0 (0-0) | - | - |
| 3 months | 9 | 0 (0) | 0 (0-0) | |||
| 6 months | 9 | 0 (0) | 0 (0-0) | |||
*P<0.05 Statistically significant, P>0.05 NS. NS: Nonsignificant, SD: Standard deviation
Radiographic outcome
Periapical healing score
Table 4 shows on statistical analysis, the median values for apical closure score at 3 months for Group A and Group B were 2 (2–3) and 3 (2–3), respectively. The median values for apical closure score at 6 months for Group A and Group B were 2 (2–3) and 3 (2–3), respectively.
Table 4.
Comparison of periapical healing score between Group A (disinfection using conventional irrigation) and Group B (laser-assisted disinfection) at 3 months and 6 months
| Group Periapical healing score |
n | Mean (SD) | Median (Q1-Q3) | Mann-Whitney U-test |
|
|---|---|---|---|---|---|
| U statistic | P | ||||
| 3 months | |||||
| Group A | 9 | 2.00 (0.50) | 2 (2-2) | 32.5 | 0.33 (NS) |
| Group B | 9 | 1.78 (0.44) | 2 (1.5-2) | ||
| 6 months | |||||
| Group A | 9 | 1.67 (0.71) | 2 (1-2) | 22 | 0.04* |
| Group B | 9 | 1.11 (0.33) | 1 (1-1) | ||
*P<0.05 statistically significant, P>0.05 NS. NS: Nonsignificant, SD: Standard deviation
P = 0.04 had a shown statistically significant change in periapical healing score when Group A and Group B were compared at 6 months. This indicates that laser-assisted disinfection has shown good periapical healing when compared to the conventional disinfection group.
Apical closure score [Table 5]
Table 5.
Comparison of apical closure score between Group A (disinfection using conventional irrigation) and Group B (laser-assisted disinfection) at 3 months and 6 months
| Apical closure score | Group |
Total | |
|---|---|---|---|
| A | B | ||
| 3 months | |||
| 1 | 1 (11.1) | 2 (22.2) | 3 (16.7) |
| 2 | 4 (44.4) | 0 | 4 (22.2) |
| 3 | 3 (33.3) | 6 (66.7) | 9 (50.0) |
| 4 | 0 | 1 (11.1) | 1 (5.6) |
| 5 | 1 (11.1) | 0 | 1 (5.6) |
| 6 months | |||
| 1 | 1 (11.1) | 2 (22.2) | 3 (16.7) |
| 2 | 4 (44.4) | 0 | 4 (22.2) |
| 3 | 3 (33.3) | 6 (66.7) | 9 (50.0) |
| 4 | 0 | 1 (11.1) | 1 (5.6) |
| 5 | 1 (11.1) | 0 | 1 (5.6) |
Values within brackets are mentioned in percentages
The percentage values for apical closure scores are mentioned in Table 5.
DISCUSSION
Regenerative endodontics is one of the most exciting and developing fields in endodontics in the treatment of immature teeth with necrotic pulp. Several international guidelines are published regarding regenerative endodontic protocols.[19] AAE provides clinical recommendations for RET, known as AAE 2016 protocol, which are based on successful case reports and in vitro studies.[4]
The AAE guidelines fall under the category of T2 domain of translational research that was conducted under highly controlled experimental conditions (including microbial sampling), with well-defined outcome measures but a relatively small sample size. Translational research produces more meaningful, applicable results that directly benefit human health. The goal of translational research is to translate basic science discoveries more quickly and efficiently into practice by encouraging and promoting multidisciplinary collaboration among laboratory and clinical researchers.[21] Therefore, the concepts of basic stem cell researches are translated to in-vivo practice. Many procedural parameters are not well described in endodontics (e.g., diagnosis, indication, irrigation, disinfection, pretreatment, and root canal preparation). Therefore, it has to be revisited considering pulp regeneration.[3]
AAE 2016 protocol suggested the use of irrigation systems such as needle with closed end and side vents, or EndoVac.[4] The current study has utilized the use of needle with closed end and side vents. A study was conducted to assess the role of needle design on extrusion of irrigant during endodontic treatment. Open-ended beveled needle showed significantly high extrusion than that of closed-ended side-vented needle.[22]
As pulp regeneration is in its translational research stage, there is scope for improvement at each and every step of protocol that is followed. AAE also supports researchers to undertake more and more research in this field due to the evolving nature of regenerative endodontics. Therefore, the present study was conducted to check the clinical and radiographic outcome using conventional (AAE current regenerative endodontic protocol 2016) and laser-assisted disinfection in regenerative endodontics.[4]
The success of regenerative endodontic therapy depends on the selection of cases. Pulp regeneration is most predictable in teeth with an open apex in young individuals. The age being an influencing factor, the successful results are shown at younger age between 9 and 18 years. Teeth with apical diameter ≥1 mm were selected because of greater increase in root length, thickness, and apical narrowing in such teeth.[23] Teeth with periapical radiolucency more than 10 mm were excluded from the study as the major factor for failure of the regenerative endodontic cases is persistent infection.[24]
The success of regenerative endodontics depends on the disinfection of root canal. The presence of infection may influence the characteristics of tissues formed resulting in formation of ectopic tissues such as periodontal ligament, bone, and cementum. Whereas previously noninfected teeth showed regeneration of dentin and pulp.[25] Therefore, assessment of bacterial count in this study helps in the estimation and quantification of disinfection that is achieved by two different protocols.
The cases present in the study had shown that there was a statistically significant reduction in bacterial count before and after disinfection in both the groups. Even though bacterial reduction count was almost similar among both the groups, laser-assisted disinfection showed highly significant results (P = 0.007).
The degree of cleanliness of root canals is greater due to the evaporation of debris and sealing of dentinal tubules after using laser than after the conventional method. Laser has the ability to remove and disinfect the root canal by direct laser irradiation as well as an added advantage of activating chemical irrigants to increase their bactericidal effects by a mechanism known as cavitation suggesting the synergistic effect of diode laser combined with NaOCl.[10,26]
Miller's grading of tooth mobility was used in the study to assess clinical outcome as it is the most widely accepted method for routine clinical examinations of tooth mobility. It may provide valuable information for the diagnosis of moderate and severe periodontitis when clinical attachment level is not obtainable during routine practice.[27] Clinical outcome at 1, 3, and 6 months showed successful results in the laser group. Regenerative endodontic procedure using a conventional disinfectant approach appears to be capable of providing only satisfactory outcomes for a nonvital immature permanent tooth.[28]980-nm diode laser irradiation showed effective for disinfecting the root canals containing aerobic and anaerobic bacteria.[29] The laser activation of irrigating solutions such as EDTA or NaOCl will enhance the removal of the smear layer and bacterial biofilm. After absorption of laser energy by the solution, there will be formation of vapor bubbles, collapse of the bubbles, acoustic streaming, and finally cavitation.[30] Several case reports on regenerative endodontics used photoactivated disinfection method to achieve the goal of regenerative endodontics showed no pain on percussion or palpation tests when followed for 10 months to 12 months intervals.[12] Diode laser irradiation on stem cells from human exfoliated deciduous teeth did not cause a cytotoxic effect.[31] Therefore, laser-assisted disinfection showed an expeditious response with respect to clinical success.
Evaluation of periapical healing score in the current study was assessed by periapical index by Ostravik and apical response by Chen and Chen index. Periapical index processes a high degree of reliability validated for accuracy and reproducibility which is used to assess periapical healing in endodontically treated teeth.[32] Five types of apical responses were observed as an outcome of regenerative endodontic therapy as per Chen and Chen criteria. This is a new type of scoring that is quantifiable and easy for comparison to assess the apical closure types as an outcome of regenerative endodontic procedure.[33]
Periapical healing score showed statistically significant results in the laser group showing significant improvement in the healing when followed from 3–6 months. The type of apical response seen in Group A was Type 2 (44.4%) and Type 3 (33.3%) at both 3- and 6-month interval, respectively. The type of apical response seen in Group B was Type 3 (66.7%) and Type 1 (22.2%) at both 3 and 6 months, respectively. The effectiveness of photodynamic therapy on periapical healing was already documented in endodontic treatments when followed for 6–18 months. Clinical outcome of regenerative endodontics can be observed within 6 months of treatment such as no pain, soft-tissue swelling, or sinus tract. Radiographic outcomes such as resolution of apical radiolucency are often observed by 6 months (AAE).[4] Various studies on regenerative endodontics have shown resolution of periapical radiolucency at 6-month follow-up.[34,35]
A revascularization case study demonstrated resolution of apical periodontitis, whereas continued root development with apical closure occurred in 79.3% of cases. Revascularization-Associated Intracanal Calcification (RAIC) was a finding noted in 62.1% (calcific barrier and canal obliteration). A higher frequency of RAIC was noted in cases with bleeding induction and Ca(OH)2 medicament placement.[36] Therefore, laser-assisted disinfection demonstrated better periapical healing and increased root length and dentin thickness as radiographic outcome ascribable to its superior disinfection property.
In cell-homing-based approach, the patient's endogenous cells undertake tissue repair/regeneration. Currently, cell-homing approaches are followed in regenerative endodontics as they are simple and economical.[37] Blood clot scaffold is the gold standard for regenerative endodontic therapy in younger population because of its extremely favorable and clinically feasible properties by intentionally provoking periapical tissue intracanal bleeding can be obtained. Blood clot consists of fibrin matrix which traps cells necessary for tissue regeneration. It acts as a pathway for SCAP cells, macrophages, and fibroblasts to migrate into the root canal. Growth factors present in blood clot scaffold play an important role in cell differentiation. The growth factors such as platelet-derived growth factor, vascular endothelial growth factor, and platelet-derived epithelial growth factor from blood clot promote regeneration.[38]
The current study used blood clot as a scaffold for both the groups. An additional observation was made by comparing the scaffold and the type of apical response considering both the groups. The most prevalent apical closures observed in this study were Type 3 (50.0%) and Type 2 (22.2%) apical closure. Majority of the cases wrt blood clot scaffold system showed Type 2 apical closure.[39]
Calcium hydroxide has long been used as an intracanal medicament in endodontics. It can disinfect the canal, without any detrimental effect to stem cells, as well as helps in the release of growth factors such as TGF-beta.[40] Although antibiotics used in regenerative endodontic procedures are effective against bacteria related to endodontic infections, the main disadvantage of triple antibiotic paste is tooth discoloration caused by minocycline and detrimental effect on stem cells at higher concentration.[41] Triple antibiotic paste has a highly acidic pH (2.9) that affects the microhardness of dentin.[42]
Even though both the disinfection protocols showed successful clinical and radiographic outcomes in regenerative endodontics, laser-assisted disinfection demonstrated better outcomes when followed for 6 months. Thus, laser disinfection has a beneficial effect when used in regenerative endodontics.
The study has potential limitations such as smaller sample size and shorter duration of follow-up which gives a scope for more research with regard to same.
CONCLUSION
The current study suggests that along with AAE 2016 protocol, laser-assisted disinfection results in improved quality of disinfection, expeditious clinical outcome, and periapical healing in immature necrotic permanent teeth treated with regenerative endodontic procedure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lin J, Zeng Q, Wei X, Zhao W, Cui M, Gu J, et al. regenerative endodontics versus apexification in immature permanent teeth with apical periodontitis: A prospective randomized controlled study. J Endod. 2017;43:1821–7. doi: 10.1016/j.joen.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 2.Paulindraraj S, Venkatesan R, Suprasidh S, Christopher A. Apexification – Then and now: A review. Int J Dent Med Res. 2002;2:313–6. [Google Scholar]
- 3.Hargreaves KM, Diogenes A, Teixeira FB. Treatment options: Biological basis of regenerative endodontic procedures. Pediatr Dent. 2013;35:129–40. [PubMed] [Google Scholar]
- 4.American Association of Endodontists. AAE Clinical Consideration for a Regenerative Procedure. Revised 6-8-16. 2016. [Last accessed on 2017 Oct 12]. Available from: https://www.aae.org/specialty/wpcontent/uploads/sites/2/2019/02/19_TraumaGuidelines.pdf .
- 5.Nazzal H, Duggal MS. Regenerative endodontics: A true paradigm shift or a bandwagon about to be derailed? Eur Arch Paediatr Dent. 2017;18:3–15. doi: 10.1007/s40368-016-0265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiley J, Sons AS, Diogenes A, Mah Y, Teixeira FB, Hargreaves KM. An update on clinical regenerative endodontics. Br Dent J. 2013;215:289. [Google Scholar]
- 7.Lin LM, Kahler B. A review of regenerative endodontics: Current protocols and future directions. J Istanb Univ Fac Dent. 2017;51:S41–51. doi: 10.17096/jiufd.53911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin DE, De Almeida JF, Henry MA, Khaing ZZ, Schmidt CE, Teixeira FB, et al. Concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J Endod. 2014;40:51–5. doi: 10.1016/j.joen.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Kimura Y, Wilder-Smith P, Matsumoto K. Lasers in endodontics: A review. Int Endod J. 2000;33:173–85. doi: 10.1046/j.1365-2591.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- 10.Gutknecht N, van Gogswaardt D, Conrads G, Apel C, Schubert C, Lampert F. Diode laser radiation and its bactericidal effect in root canal wall dentin. J Clin Laser Med Surg. 2000;18:57–60. doi: 10.1089/clm.2000.18.57. [DOI] [PubMed] [Google Scholar]
- 11.El A, Ossama R, Mekkawi E, Abd-El M, Kataia R, Ali MM, et al. Evaluation of the Efficacy of diode laser in maturogenesis of immature teeth with necrotic pulps: An in vivo study “Part One”. Indian J Public Heal Res Dev. 2020;11:1387. [Google Scholar]
- 12.Abdel Hafiz Abdel Rahim AS, Abdelgawad F, Abd Alsamed AM, Moheb DM, Wahab El-Dokky NA. Case report: Single visit photo-activated disinfection in regenerative endodontics. F1000Res. 2019;8:1519. doi: 10.12688/f1000research.20118.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johns DA, Shivashankar VY, Krishnamma S, Johns M. Use of photoactivated disinfection and platelet-rich fibrin in regenerative Endodontics. J Conserv Dent. 2014;17:487–90. doi: 10.4103/0972-0707.139850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow S, Shao J, Wang H. Sample Size Calculation in Clinical Research. 2nd ed. Florida: Chapman and Hall; 2008. p. 62. [Google Scholar]
- 15.Moritz A, Gutknecht N, Schoop U, Goharkhay K, Doertbudak O, Sperr W. Irradiation of infected root canals with a diode laser in vivo: Results of microbiological examinations. Lasers Surg Med. 1997;21:221–6. doi: 10.1002/(sici)1096-9101(1997)21:3<221::aid-lsm1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 16.Geisler TM. Clinical considerations for regenerative endodontic procedures. Dent Clin North Am. 2012;56:603–26. doi: 10.1016/j.cden.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Naik R, Raviraj G, Yavagal C, Mandroli P. Diode lasers for pediatric endodontics: State-of-the-art! J Dent Lasers. 2017;11:7. [Google Scholar]
- 18.Prabhakar A, Rani NS, Yavagal C. Revascularization of immature necrotic teeth with platelet-rich fibrin and blood clot. Int J Oral Heal Sci. 2016;6:4. [Google Scholar]
- 19.Shivashankar VY, Johns DA, Maroli RK, Sekar M, Chandrasekaran R, Karthikeyan S, et al. Comparison of the effect of PRP, PRF and induced bleeding in the revascularization of teeth with necrotic pulp and open apex: A triple blind randomized clinical trial. J Clin Diagn Res. 2017;11:C34–9. doi: 10.7860/JCDR/2017/22352.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nazzal H, Tong H, Nixon P, Duggal M. Regenerative endodontic therapy for managing immature non-vital teeth: A national survey of UK paediatric dental specialists and trainees. Br Dent J. 2018;224:247–54. doi: 10.1038/sj.bdj.2018.122. [DOI] [PubMed] [Google Scholar]
- 21.Hargreaves KM, Diogenes A, Teixeira FB. Paradigm lost: A perspective on the design and interpretation of regenerative endodontic research. J Endod. 2014;40:S65–9. doi: 10.1016/j.joen.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Kalhoro FA, Anwar K, Shaikh MA, Rajput F. Rate of apical extrusion of sodium hypochlorite: Open ended versus closed ended needles. Pak Oral Dent J. 2012;34:159–63. [Google Scholar]
- 23.Estefan BS, El Batouty KM, Nagy MM, Diogenes A. Influence of age and apical diameter on the success of endodontic regeneration procedures. J Endod. 2016;42:1620–5. doi: 10.1016/j.joen.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Almutairi W, Yassen GH, Aminoshariae A, Williams KA, Mickel A. Regenerative endodontics: A systematic analysis of the failed cases. J Endod. 2019;45:567–77. doi: 10.1016/j.joen.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Becerra P, Ricucci D, Loghin S, Gibbs JL, Lin LM. Histologic study of a human immature permanent premolar with chronic apical abscess after revascularization/revitalization. J Endod. 2014;40:133–9. doi: 10.1016/j.joen.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Jurič IB, Anic I. The use of lasers in disinfection and cleaning of root canals: A review. Acta Stomatol Croat. 2014;48:6–15. doi: 10.15644/asc48/1/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu CP, Tu YK, Lu SL, Chang JH, Lu HK. Quantitative analysis of miller mobility index for the diagnosis of moderate to severe periodontitis – A cross-sectional study. J Dent Sci. 2018;13:43–7. doi: 10.1016/j.jds.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kharchi AS, Tagiyeva-Milne N, Kanagasingam S. Regenerative endodontic procedures, disinfectants and outcomes: A systematic review. Prim Dent J. 2020;9:65–84. doi: 10.1177/2050168420963302. [DOI] [PubMed] [Google Scholar]
- 29.Gutknecht N, Franzen R, Schippers M, Lampert F. Bactericidal effect of a 980-nm diode laser in the root canal wall dentin of bovine teeth. J Clin Laser Med Surg. 2004;22:9–13. doi: 10.1089/104454704773660912. [DOI] [PubMed] [Google Scholar]
- 30.Hmud R, Kahler WA, George R, Walsh LJ. Cavitational effects in aqueous endodontic irrigants generated by near-infrared lasers. J Endod. 2010;36:275–8. doi: 10.1016/j.joen.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Tunç H, Islam A, Kabadayı H, Vatansever HS, Çetiner S, Yilmaz HG. Evaluation of low-level diode laser irradiation and various irrigant solutions on the biological response of stem cells from exfoliated deciduous teeth. J Photochem Photobiol B. 2019;191:156–63. doi: 10.1016/j.jphotobiol.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Orstavik D. Reliability of the periapical index scoring system. Scand J Dent Res. 1988;96:108–11. [PubMed] [Google Scholar]
- 33.Chen MY, Chen KL, Chen CA, Tayebaty F, Rosenberg PA, Lin LM. Responses of immature permanent teeth with infected necrotic pulp tissue and apical periodontitis/abscess to revascularization procedures. Int Endod J. 2012;45:294–305. doi: 10.1111/j.1365-2591.2011.01978.x. [DOI] [PubMed] [Google Scholar]
- 34.Jha P, Virdi MS, Nain S. A Regenerative approach for root canal treatment of mature permanent teeth: Comparative evaluation with 18 months follow-up. Int J Clin Pediatr Dent. 2019;12:182–8. doi: 10.5005/jp-journals-10005-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pace R, Giuliani V, Di Nasso L, Pagavino G, Franceschi D, Franchi L. Regenerative endodontic therapy using a new antibacterial root canal cleanser in necrotic immature permanent teeth: Report of two cases treated in a single appointment. Clin Case Rep. 2021;9:1870–5. doi: 10.1002/ccr3.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song M, Cao Y, Shin SJ, Shon WJ, Chugal N, Kim RH, et al. Revascularization-associated intracanal calcification: Assessment of prevalence and contributing factors. J Endod. 2017;43:2025–33. doi: 10.1016/j.joen.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Huang GT, Al-Habib M, Gauthier P. Challenges of stem cell-based pulp and dentin regeneration: A clinical perspective. Endod Topics. 2013;28:51–60. doi: 10.1111/etp.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alshehadat SA, Thu HA, Hamid SS, Nurul AA, Rani SA, Ahmad A. Scaff olds for dental pulp tissue regeneration: A review. Int Dent Med J Adv Res. 2016;2:1–12. [Google Scholar]
- 39.Botero TM, Tang X, Gardner R, Hu JCC, Boynton JR, Holland GR. Clinical evidence for regenerative endodontic procedures: Immediate versus delayed induction? J Endod. 2017;43:S75–81. doi: 10.1016/j.joen.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng Q, Nguyen S, Zhang H, Chebrolu HP, Alzebdeh D, Badi MA, et al. Release of growth factors into root canal by irrigations in regenerative endodontics. J Endod. 2016;42:1760–6. doi: 10.1016/j.joen.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 41.Ruparel NB, Teixeira FB, Ferraz CC, Diogenes A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 2012;38:1372–5. doi: 10.1016/j.joen.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 42.Yilmaz S, Dumani A, Yoldas O. The effect of antibiotic pastes on microhardness of dentin. Dent Traumatol. 2016;32:27–31. doi: 10.1111/edt.12193. [DOI] [PubMed] [Google Scholar]


