Abstract
Cooperative asymmetric catalysis with hydrogen chloride (HCl) and chiral dual-hydrogen-bond donors (HBDs) is applied successfully to highly enantioselective Prins cyclization reactions of a wide variety of simple alkenyl aldehydes. The optimal chiral catalysts were designed to withstand the strongly acidic reaction conditions and induce rate accelerations of 2 orders of magnitude over reactions catalyzed by HCl alone. We propose that the combination of strong mineral acids and chiral hydrogen-bond-donor catalysts may represent a general strategy for inducing enantioselectivity in reactions that require highly acidic conditions.
Graphical Abstract
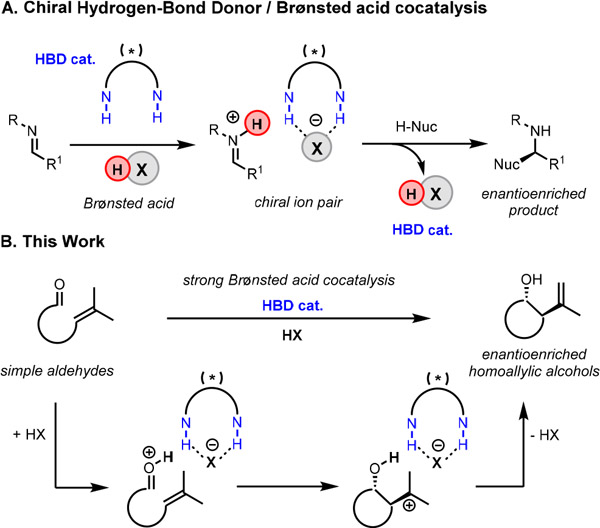
Reactions catalyzed by Brønsted acids are of central utility in organic chemistry, and extensive research efforts have been directed over the past two decades toward the development of enantioselective variants.1 Attainment of high levels of absolute stereocontrol requires the creation of well-defined and differentiated geometries about the protonated electrophile, and fundamentally different approaches have been taken to address this challenge. One involves the application of chiral Brønsted acids, and systems based on bis-aryloxide ligated phosphoric acids have been deployed with outstanding success, particularly in the promotion of reactions involving imine electrophiles.1,2 Extension to less basic functional groups such as simple carbonyl and olefin derivatives requires catalysts with enhanced Brønsted acidity,3 and effective catalyst platforms have been identified including N-triflyl phosphoramides by Yamamoto,4 imidodiphosphorimidates by List,5 pentacarboxycyclopentadienes by Lambert,6 and bis(sulfuryl)imides by Berkessel.7 Enhanced acidity of chiral Brønsted acid catalysts has also been achieved through the introduction of achiral cocatalysts including simple Lewis acids and hydrogen-bond donors.8
An alternative strategy for accessing enantioselective Brønsted acidic catalyst systems has relied on the anion binding abilities of chiral dual-hydrogen-bond donors (HBDs).9 Chiral urea, thiourea, and squaramide-based catalysts have been shown to impart enantioselectivity in reactions catalyzed by both weak10 and strong11 achiral Brønsted acids (Scheme 1A). While this cocatalytic strategy has found use in activation of several classes of nitrogenous electrophiles including aldimines10,11b and aziridines,11a we recognized the possibility that the innate acidity of a strong mineral acid could be leveraged to activate substantially less Lewis basic oxygen-centered electrophiles.12 In this regard, our group recently identified adventitious hydrogen bromide as an effective cocatalyst in highly enantioselective chiral squaramide catalyzed ring-opening reactions of oxetanes.11c We hypothesized that this mode of cooperative catalysis could be applied broadly to the activation of other challenging classes of electrophiles such as simple carbonyls.3b
Scheme 1.
(A) Cocatalytic strategy in activation of nitrogenous electrophiles (B) Application of Brønsted acid and HBD cocatalysis to enantioselective Prins cyclization reactions.
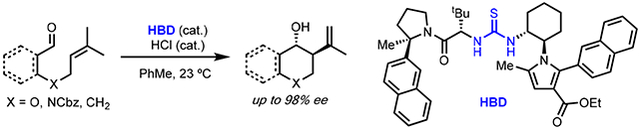
We chose to explore the concept of strong Brønsted acid/chiral HBD cocatalysis in the context of the addition of alkenes to carbonyl compounds (the Prins reaction), a venerable transformation that provides access to stereochemically defined homoallylic alcohol products (Scheme 1B).13 Although numerous advances in asymmetric Lewis14 and Brønsted15 acid catalysis of Prins and carbonyl-ene reactions have been made, protonation of simple aldehyde substrates toward reaction with simple alkenes remains a formidable challenge. Highly enantioselective methods operating on such substrates are rare and have been limited primarily to 5-membered ring-forming carbonyl-ene reactions.16,17 We recognized that enantioselective catalysis of Prins cyclizations could provide complementary strategies to access six-membered ring homoallylic alcohol products, for which general highly enantioselective methods have not yet been developed.18 Herein, we report highly enantioselective chiral HBD/HCl cocatalysis of Prins cyclizations of simple alkenyl aldehydes. The successful implementation of cooperativity between simple mineral acids and chiral hydrogen-bond donors points to a potentially general approach to enantioselective catalysis of organic reactions involving weakly Brønsted basic substrates.
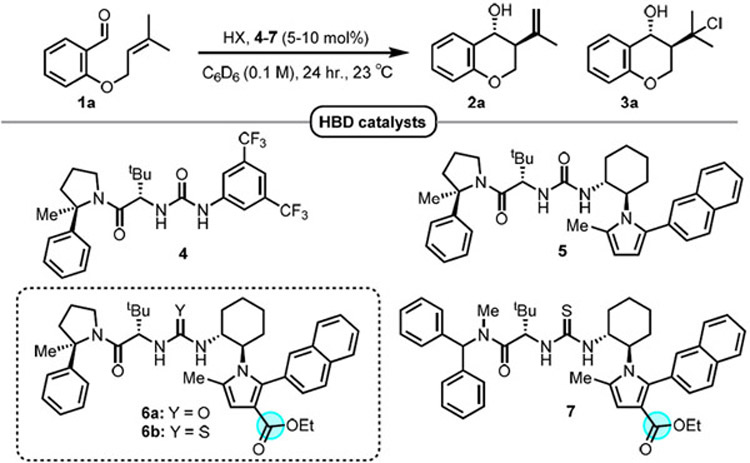
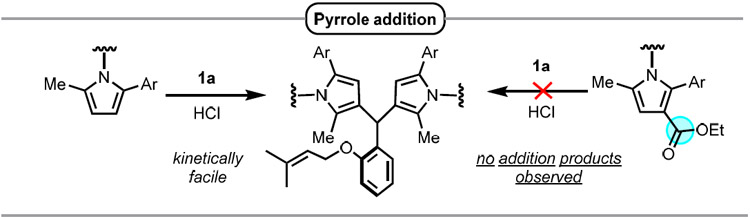
The cyclization of prenylated salicylaldehyde derivative 1a, which affords access to valuable 4-chromanol products, was selected as a model reaction.19 While the dual hydrogen-bond donor catalyst 4 alone did not catalyze the cyclization of 1a (Table 1, entry 1), the inclusion of catalytic hydrogen chloride (8 mol%, as a solution in Et2O) at room temperature promoted smooth conversion to homoallylic alcohol product 2a along with tertiary chloride product 3a arising from trapping of the presumed tertiary carbocation intermediate (Scheme 1B). Under these conditions, 2a and 3a were formed in a 3.5:1 ratio with similar enantiomeric excess (76% and 74% ee respectively) (Table 1, entry 2). A variety of other achiral Brønsted acids were surveyed with catalyst 4 (Table 1, entries 3-6), in all cases providing product 2a in diminished levels of both diastereo- and enantioselectivity relative to reactions carried out with HCl.20 Weaker Brønsted acids such as benzoic acid and diphenyl phosphoric acid failed to provide any measurable level of conversion after 24 hours (Table 1, entries 5 and 6). The use of urea catalyst 5 bearing a pyrrole moiety derived from trans-1,2 diaminocyclohexane led to improvements in both diastereo- and enantioselectivity as well as an increase in the 2a:3a ratio (Table 1, entry 7). However, HCl was found to promote undesired addition reactions21 between the electron-rich pyrrole of 5 and substrate 1a, resulting in catalyst decomposition (Table 1, bottom; see SI for details). Introduction of an electron-withdrawing ester substituent to attenuate the nucleophilicity of the pyrrole led to complete suppression of the undesired addition pathways, with catalyst 6a affording a significantly cleaner reaction while also providing products 2a and 3a with enhanced ee (Table 1, entry 8). A further improvement in enantioselectivity was achieved with the more reactive thiourea analog 6b, which delivered products 2a and 3a both in 98% ee using an 8 mol% loading of HCl (Table 1, entry 9).
Table 1.
Optimization of HBD catalyst structure and reaction conditionsa
| ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | HX (mol%) |
HBD (mol%) |
conversion (%)b |
yield (%)b |
d.r. 2ab | 2a : 3ab | ee 2a (%)c |
ee 3a (%)c |
| 1 | — | 4 (10) | n.r. | n.r. | — | — | — | — |
| 2 | HCl (8) | 4 (10) | 33 | 28 | 10 : 1 | 3.5 : 1 | 76 | 74 |
| 3 | MsOH (8) | 4 (10) | 100 | trace | — | — | — | — |
| 4 | TsOH H2O (8) | 4 (10) | 87 | 30 | 4 : 1 | — | 31 | — |
| 5 | BzOH (8) | 4 (10) | n.r. | n.r. | — | — | — | — |
| 6 | HOP(O)(OPh)2 (8) | 4 (10) | n.r. | n.r. | — | — | — | — |
| 7 | HCl (8) | 5 (10) | 41 | 32 | 22 : 1 | 6.5 : 1 | 89 | 77 |
| 8 | HCl (8) | 6a (10) | 45 | 44 | 23 : 1 | 4.4 : 1 | 96 | 95 |
| 9 | HCl (8) | 6b (10) | 34 | 32 | 49 : 1 | 3.0 : 1 | 98 | 98 |
| 10 | HCl (8) | 7 (10) | 35 | 25 | 12 : 1 | 2.4 : 1 | 87 | 89 |
| 11 | HCl (30) | 6b (10) | 96 | 94 | > 50 : 1 | 3.1 : 1 | 96 | 98 |
| 12 | HCl (30) | 6b (5) | 88 | 85 | 30 : 1 | 3.1 : 1 | 92 | 94 |
| 13 | AcCl + EtOH (30) | 6b (5) | 93 | 91 | 38 : 1 | 3.0 : 1 | 95 | 97 |
| 14d | AcCl + EtOH (30) | 6b (5) | — | 82 | 36 : 1 | — | 95 | — |
| ||||||||
Conducted using 0.08 mmol of 1a with Brønsted acid cocatalysts delivered as solutions in Et2O.
Determined from crude reaction mixtures using 1H NMR spectroscopy with mesitylene internal standard. Yield refers to the combined yield of products trans/cis 2a and 3a except for entry 14.
Determined by GC analysis using a chiral stationary phase. n.r. = no reaction.
Reaction was quenched by the addition of 60 mol% KHMDS.
The stoichiometric consumption of HCl in the generation of product 3a resulted in limited reaction conversions when 8 mol% loadings of HCl were employed. Increased conversion was achieved with higher (30 mol%) loadings of HCl, affording comparable enantioselectivity with 10 mol% 6b (Table 1, entry 11). However, lowering the loading of 6b to 5 mol% with the increased HCl loadings resulted in diminished enantioselectivity, presumably due to intervention of a competing racemic HCl-catalyzed background reaction (Table 1, entry 12). We surveyed methods for the controlled in situ generation of HCl, and found that the use of 30 mol% equimolar combinations of acetyl chloride (AcCl) and anhydrous ethanol (EtOH) restored nearly optimal levels of yield and ee with catalyst 6b (Table 1, entry 13).22 Finally, conversion of tertiary chloride 3a to 2a via potassium hexamethyldisilazide (KHMDS)-promoted elimination could be effected as a quench at the end of the reaction without the need for initial purification (Table 1, entry 14). Under these conditions, 2a was generated as the exclusive product in 82% NMR yield and 95% ee.23
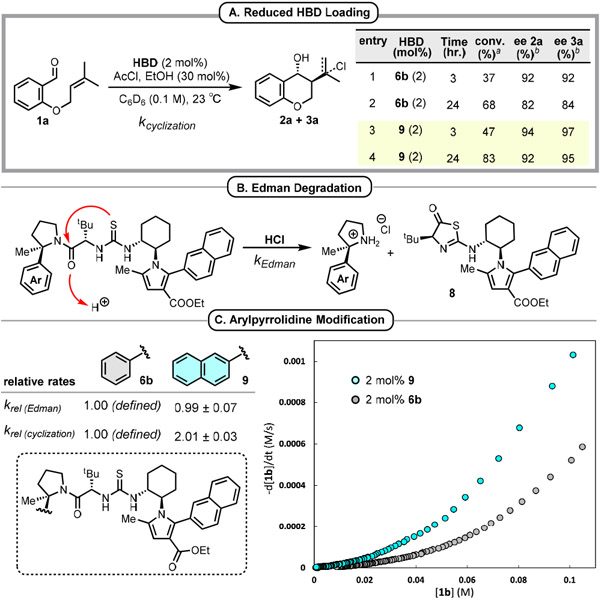
Efforts to further reduce the loading of 6b were met by the observation of decreasing enantioselectivity over the course of the reactions (Figure 1A, entries 1-2), suggesting the participation of a competitive catalyst deactivation pathway. Indeed, subjecting 6b to HCl in the absence of substrate led to the clean formation of heterocycle 8 and the corresponding aryl pyrrolidine arising from Edman degradation24,25 (Figure 1B, see SI for details). Variation of the arylpyrrolidine catalyst component led to the identification of 2-naphthyl derivative 9, which displayed minimal decreases in enantioselectivity over the same timeframe (Figure 1A, entries 3-4).26 In principle, the observed increased robustness of reactions catalyzed by 9 can be attributed to either increased catalyst stability to HCl or to increased reactivity in the Prins reactions.27 In fact, 6b and 9 were found to undergo Edman degradation at identical rates, while 9 catalyzes the Prins cyclization of 1b at twice the rate compared to 6b (Figure 1C). Hence, the higher rate of Prins cyclization allows 9 to more effectively outcompete Edman degradation, resulting in higher levels of enantioselectivity at lower catalyst loadings.28
Figure 1.
Optimization of HBD aryl pyrrolidine. aDetermined from crude reaction mixtures using 1H NMR spectroscopy with mesitylene internal standard. bDetermined by GC analysis using a chiral stationary phase.
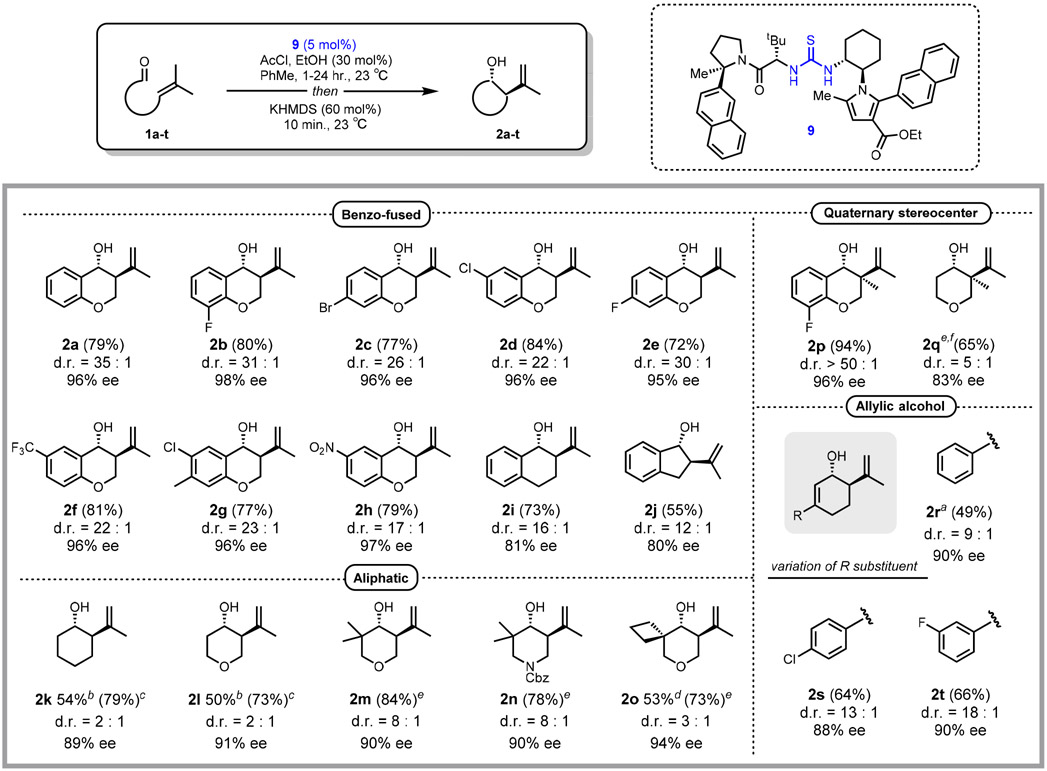
With optimal hydrogen-bond donor catalyst 9 and conditions for the cyclization of 1a in-hand, we evaluated the scope of the newly developed method (Figure 2). A variety of electronically and sterically differentiated analogs of 1a were found to undergo smooth cyclization and elimination at room temperature to provide chromanols 2b-2h in good yields with high diastereoselectivities (≥17:1) and enantioselectivities (95-98% ee). Lower but nonetheless significant levels of enantioselectivity were obtained in the cyclization of arylaldehyde derivatives lacking a ring-conjugated oxygen atom, with 1i reacting to form product in 81% ee and 1j affording the carbocylic five-membered ring product 2j in 80% ee.
Figure 2.
Catalytic enantioselective Prins cyclization reactions. Reactions were carried out using 0.3 mmol of substrates 1, except for substrates 1k and 1l which were carried out on 0.1 mmol scale. Isolated yields are reported, except where noted. d.r. determined using 1H NMR spectroscopy of crude reaction mixtures. Reported ee values correspond to the major trans products. The absolute stereochemical configuration of 2c was determined via X-ray crystallographic analysis. All stereochemistry of products 2 is inferred from this result. a10 mol% 9 used, bNMR yield of trans diastereomer, cNMR yield of diastereomeric mixture, disolated yield of trans diastereomer, eisolated yield of diastereomeric mixture, f15 mol% AcCl, EtOH used.
The new method was extended to simple olefinic aldehydes, with the cyclization of aliphatic substrates 1k-1o taking place in high ee and moderate-to-good yields. Although products 2k and 2l were formed with low diastereoselectivities, the trans cyclohexanol products were generated in high enantioselectivities (89% and 91% ee respectively), a noteworthy result considering that substrates without conformationally biasing disubstitution29 have rarely been documented in direct enantioselective Brønsted acid catalyzed additions of alkenes to carbonyls.30 In contrast to the benzo-fused products 2a and 2i, the presence or identity of a heteroatom linker had negligible effects on stereoselectivity, with oxygen-, nitrogen-, and carbon-linked alkenyl aldehydes 2k-2o all affording similar levels of ee and d.r. The cyclization of simple alkenyl aldehyde substrates represents a long-standing challenge in asymmetric Brønsted/Lewis acid catalysis, so the successful access provided by this method to trans-configured six-membered ring products in high enantioselectivities under mild reaction conditions is particularly significant.
Substrates 1p and 1q bearing tetrasubstituted alkenyl components also underwent highly enantioselective cyclizations, forging congested quaternary stereogenic centers in products 2p and 2q. Although compounds possessing quaternary centers of this type have been synthesized racemically,31 highly enantioselective methods for their preparation are rare.32 Finally, conjugated enal electrophiles provided access to new allylic alcohol products 2r-2t possessing natural product-like features in good enantioselectivity (88-90% ee) and d.r. (≥ 9:1).33
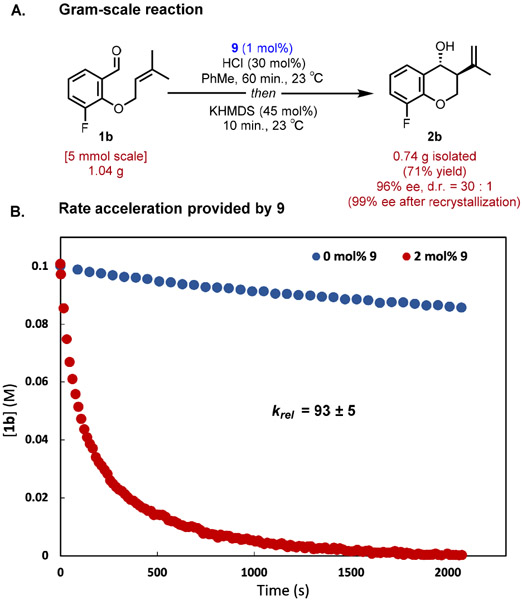
The chiral thiourea and HCl cocatalyzed Prins reaction was readily adapted to preparative synthesis, as demonstrated by the cyclization of 1b conducted on gram scale (Figure 3A). The loading of 9 could be reduced to 1 mol% while maintaining a highly enantioselective reaction outcome (96% ee), good yield (71%), and a short reaction time (1h). The enantiomeric purity of 2b was upgraded to 99% following a single recrystallization.
Figure 3.
(A) Gram-scale synthesis of 2b. (B) Rate acceleration using catalyst 9 in reaction of 1b.
Thiourea 9 was found to induce substantial rate acceleration in cyclization of substrates 1 relative to the HCl-catalyzed background reaction. Monitoring the HCl-catalyzed reaction of 1b in the presence and absence of 2 mol% 9 using in situ infrared spectroscopy revealed rate enhancement of approximately two orders of magnitude (93 times) (Figure 3B). The observation of such effects with a chiral cocatalyst is reminiscent of ligand-accelerated catalysis using transition metals.34 Our current efforts are directed towards elucidating the basis of this rate acceleration as well as identifying the mode of stereoinduction across multiple substrate classes.
In summary, we have developed a highly enantioselective Prins cyclization of alkenyl aldehydes catalyzed by the combination of hydrogen chloride and chiral hydrogen-bond donors. The catalytic method displays broad substrate scope, providing valuable chromanol derivatives and related natural product-like compounds in high levels of ee. Given the ability of dual hydrogen bond donors to recognize anions with widely varying steric and electronic properties,35 we anticipate general application of the cooperative action of strong Brønsted acids and chiral HBDs in asymmetric catalysis.
Supplementary Material
ACKNOWLEDGMENT
Financial support for this work was provided by the NIH through GM043214. We thank Dr. Shao-Liang Zheng for X-ray data collection and structure determination.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).For reviews, see: (a) Parmar D; Sugiono E; Raja S; Rueping M Complete Field Guide to Asymmetric BINOL-Phosphate Derived Brønsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Brønsted Acidity, Hydrogen Bonding, Ion Pairing and Metal Phosphates. Chem. Rev 2014, 114, 18, 9047–9153. [DOI] [PubMed] [Google Scholar]; (b) Parmar D; Sugiono E; Raja S; Rueping M Addition and Correction to Complete Field Guide to Asymmetric BINOL-Phosphate Derived Brønsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Brønsted Acidity, Hydrogen Bonding, Ion Pairing, and Metal Phosphates. Chem. Rev 2017, 117, 15, 10608–10620. [DOI] [PubMed] [Google Scholar]
- (2).For seminal reports, see: (a) Akiyama T; Itoh J; Yokota K; Fuchibe K. Enantioselective Mannich-Type Reaction Catalyzed by a Chiral Brønsted Acid. Angew. Chem., Int. Ed 2004, 43, 1566–1568. [DOI] [PubMed] [Google Scholar]; (b) Uraguchi D; Terada M Chiral Brønsted Acid-Catalyzed Direct Mannich Reactions via Electrophilic Activation J. Am. Chem. Soc 2004, 126, 17, 5356–5357. [DOI] [PubMed] [Google Scholar]
- (3).(a) Akiyama T; Mori K Stronger Brønsted Acids: Recent Progress. Chem. Rev 2015, 115, 17, 9277–9306. [DOI] [PubMed] [Google Scholar]; (b) Rueping M; Ieawsuwan W; Antonchick AP; Nachtsheim BJ Chiral Brønsted Acids in the Catalytic Asymmetric Nazarov Cyclization—The First Enantioselective Organocatalytic Electrocyclization Reaction. Angew. Chem. Int. Ed 2007, 46, 2097–2100. [DOI] [PubMed] [Google Scholar]; (c) Tsuji N; Kennemur JL; Buyck T; Lee S; Prevost S; Kaib PSJ; Bykov D; Fares C; List B Activation of olefins via asymmetric Brønsted acid catalysis. Science 2018, 359, 1501–1505. [DOI] [PubMed] [Google Scholar]
- (4).Yamamoto H; Nakashima D Design of Chiral N-Triflyl Phosphoramide as a Strong Chiral Brønsted Acid and Its Application to Asymmetric Diels-Alder Reaction. J. Am. Chem. Soc 2006, 128, 30, 9626–9627. [DOI] [PubMed] [Google Scholar]
- (5).Schreyer L; Properzi R; List B IDPi Catalysis. Angew. Chem. Int. Ed 2019, 58, 12761–12777. [DOI] [PubMed] [Google Scholar]
- (6).(a) Gheewala CD; Collins BE; Lambert TH An aromatic ion platform for enantioselective Brønsted acid catalysis. Science 2016, 351, 6276, 961–965. [DOI] [PubMed] [Google Scholar]; (b) Gheewala C; Hirschi JS; Lee W-H; Paley DW; Vetticatt MJ; Lambert TH Asymmetric Induction via a Helically Chiral Anion: Enantioselective PCCP Brønsted Acid-Catalyzed Inverse Electron-Demand Diels-Alder Cycloaddition of Oxocarbenium Ions. J. Am Chem. Soc 2018, 140, 3523–3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Berkessel A; Christ P; Leconte N; Neudörfl J-M; Schäfer M Synthesis and Structural Characterization of a New Class of Strong Chiral Brønsted Acids: 1,1’-Binaphthyl-2,2’-bis(sulfuryl)imides (JIN-GLEs). Eur. J. Org. Chem 2010, 2010, 5165–5170. [Google Scholar]
- (8).(a) Yamamoto H From designer Lewis acid to designer Brønsted acid towards more reactive and selective acid catalysis. Proc. Jpn. Acad., Ser. B Phys. Biol Sci 2008, 84, 134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ishihara K; Kaneeda M; Yamamoto H Lewis Acid Assisted Chiral Brønsted Acid for Enantioselective Protonation of Silyl Enol Ethers and Ketene Bis(trialkylsilyl) Acetals. J. Am. Chem. Soc 1994, 116, 11179–11180. [Google Scholar]; (c) Maskeri MA; O’Connor MJ; Jaworski AA; Bay AV; Scheidt KA A Cooperative Hydrogen Bond Donor-Based Bronsted Acid System for the Enantioselective Synthesis of Tetrahydropyrans. Angew. Chem. Int. Ed 2018, 57, 17225–17229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) Zhang Z; Schreiner PR (Thio)urea organocatalysis—What can be learnt from anion recognition? Chem. Soc. Rev 2009, 38, 1187–1198. [DOI] [PubMed] [Google Scholar]; (b) Brak K; Jacobsen EN Asymmetric Ion-Pairing Catalysis. Angew. Chem. Int. Ed 2013, 52, 534–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Klausen RS; Jacobsen EN Weak Brønsted Acid–Thiourea Co-catalysis: Enantioselective, Catalytic Protio-Pictet–Spengler Reactions. Org. Lett 2009, 11, 4, 887–890. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yoshida K; Inokuma T; Takasu K; Takemoto Y Brønsted Acid-Thiourea Co-catalysis: Asymmetric Synthesis of Functionalized 1,4-Dihydropyridines from β-Enamino Esters and α,β-Unsaturated Aldehydes. Synlett 2010, 12, 1865–1869. [Google Scholar]; (c) Yoshida K; Inokuma T; Takasu K; Takemoto Y Catalytic Asymmetric Synthesis of Both Enantiomers of 4-Substituted 1,4-Dihydropyridines with the Use of Bifunctional Thiourea-Ammonium Salts Bearing Different Counterions. Molecules 2010, 15, 8305–8326. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lee Y; Klausen RS; Jacobsen EN Thiourea-Catalyzed Enantioselective Iso-Pictet–Spengler Reactions. Org. Lett 2011, 13, 20, 5564–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zhao C; Chen SB, Seidel DS Direct Formation of Oxocarbenium Ions under Weakly Acidic Conditions: Catalytic Enantioselective Oxa-Pictet–Spengler Reactions. J. Am. Chem. Soc 2016, 138, 9053–9056. [DOI] [PubMed] [Google Scholar]; (f) Klausen RS; Kennedy CR; Hyde AM; Jacobsen EN Chiral Thioureas Promote Enantioselective Pictet–Spengler Cyclization by Stabilizing Every Intermediate and Transition State in the Carboxylic Acid-Catalyzed Reaction. J. Am. Chem. Soc 2017, 139, 35, 12299–12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).For select examples, see: (a) Mita T; Jacobsen EN Bifunctional Asymmetric Catalysis with Hydrogen Chloride: Enantioselective Ring Opening of Aziridines Catalyzed by a Phosphinothiourea. Synlett 2009, 10, 1680–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xu H; Zuend SJ; Woll MG; Tao Y; Jacobsen EN Asymmetric Cooperative Catalysis of Strong Brønsted Acid–Promoted Reactions Using Chiral Ureas. Science 2010, 327, 5968, 986–990. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Strassfeld DA; Algera RF; Wickens ZK; Jacobsen EN A Case Study in Catalyst Generality: Simultaneous, Highly Enantioselective Brønsted- and Lewis-Acid Mechanisms in Hydrogen-Bond-Donor Catalyzed Oxetane Openings. J. Am. Chem. Soc 2021, 143, 9585–9594. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Knowles RR; Lin S; Jacobsen EN Enantioselective Thiourea-Catalyzed Cationic Polycyclizations. J. Am. Chem. Soc 2010, 132, 5030–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).(a) Cooperative catalysis between achiral hydrogen-bond donors and achiral Brønsted acids has been applied to the activation of oxygen-based electrophiles. For examples, see Zhao C; Sojdak CA; Myint W; Seidel DA Reductive Etherification via Anion-Binding Catalysis. J. Am. Chem. Soc 2017, 139, 10224–10227. [DOI] [PubMed] [Google Scholar]; (b) Wel T; Kotke M; Kleiner CM; Schreiner PR Cooperative Bronsted Acid-Type Organocatalysis: Alcoholysis of Styrene Oxides. Org. Lett 2008, 10, 1513–1516. [DOI] [PubMed] [Google Scholar]
- (13).(a) Prins HJ On the Condensation of Formaldehyde with some Unsaturated Compounds. Chem. Weekbl 1919, 16, 51–56. [Google Scholar]; (b) Arundale E; Mikeska LA The Olefin-Aldehyde Condensation. The Prins Reaction. Chem. Rev 1952, 51, 505–555. [Google Scholar]
- (14).(a) For a review, see:Mikami K; Shimizu M Asymmetric Ene Reactions in Organic Synthesis. Chem Rev. 1992, 92, 1021–1050. For select examples of catalytic enantioselective carbonyl-ene reactions promoted by chiral Lewis acids, see: [Google Scholar]; (b) Sakane S; Maruoka K; Yamamoto H Asymmetric Cyclization of Unsaturated Aldehydes Catalyzed by a Chiral Lewis Acid. Tetrahedron 1986, 42, 8, 2203–2209. [Google Scholar]; (c) Mikami K; Terada M; Sawa E; Nakai T Asymmetric Catalysis by Chiral Titanium Perchlorate for Carbonyl-Ene Cyclization. Tetrahedron. Lett 1991, 32, 45, 6571–6574. [Google Scholar]; (d) Mikami K; Terada M; Sawa E; Nakai T Asymmetric Catalysis by Chiral Titanium Perchlorate for Carbonyl-Ene Cyclization. Tetrahedron Asymmetry 1991, 2, 12, 1403–1412. [Google Scholar]; (e) Mikami K Asymmetric catalysis of carbonyl-ene reactions and related carbon-carbon bond forming reactions. Pure & Appl. Chem 1996, 68, 3, 639–644. [Google Scholar]; (f) Mikami K; Terada M; Nakai T Catalytic Asymetric Glyoxylate-Ene Reaction: A Practical Access to a-Hydroxy Esters in High Enantiomeric Purities. J. Am. Chem. Soc 1990, 112, 10, 3949–3954. [Google Scholar]; (g) Evans DA; Tregay SW; Burgey CS; Paras NA; Vojkovsky T C2-Symmetric Copper (II) Complexes as Chiral Lewis Acids. Catalytic Enantioselective Carbonyl-Ene Reactions with Glyoxylate and Pyruvate Esters. J. Am. Chem. Soc 2000, 122, 7936–7943. [Google Scholar]; (h) Yang D; Yang M; Zhu N Chiral Lewis Acid-Catalyzed Enantioselective Intramolecular Carbonyl Ene Reactions of Unsaturated α-Keto Esters. Org. Lett 2003, 5, 3749–3752. [DOI] [PubMed] [Google Scholar]; (i) Zheng K; Yang Y; Zhao J; Yin C; Lin L; Liu X; Feng X The Magnesium(II)-Catalyzed Asymmetric Ketone–Ene Reaction under Solvent-Free Conditions: Stereocontrolled Access to Enantioenriched Trifluoromethyl-Substituted Compounds. Chem. Eur. J 2010, 16, 9969–9972. [DOI] [PubMed] [Google Scholar]; (j) Truong PM; Zavalij PY; Doyle MP Highly Enantioselective Carbonyl–Ene Reactions of 2,3-Diketoesters: Efficient and Atom-Economical Process to Functionalized Chiral α-Hydroxy-β-Ketoesters. Angew. Chem. Int. Ed 2014, 53, 6468–6472. [DOI] [PubMed] [Google Scholar]; (k) Xu X; Wang X; Liu Y; Doyle MP Enantioselective Carbonyl-Ene Reactions Catalyzed by Chiral Cationic Dirhodium(II,III) Carboxamidates. J. Org. Chem 2014, 79, 12185–12190. [DOI] [PubMed] [Google Scholar]; (j) Ruck RT; Jacobsen EN Asymmetric Catalysis of Hetero-Ene Reactions with Tridentate Schiff Base Chromium(III) Complexes. J. Am. Chem. Soc 2002, 124, 2882–2883. [DOI] [PubMed] [Google Scholar]; (l) Ruck RT; Jacobsen EN Asymmetric Hetero-Ene Reactions of Trimethylsilyl Enol Ethers Catalyzed by Tridentate Schiff Base Chromium(III) Complexes. Angew. Chem. Int. Ed 2003, 42, 4771–4774. [DOI] [PubMed] [Google Scholar]; (m) Rajapaska N; Jacobsen EN Enantioselective Catalytic Transannular Ketone-Ene Reactions. Org. Lett 2013, 15, 4238–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).(a) For select examples of catalytic enantioselective additions of alkenes to carbonyls promoted by chiral Brønsted acids, see Rueping M; Theissmann T; Kuenkel A; Koenigs RM Highly Enantioselective Organocatalytic Carbonyl-Ene Reaction with Strongly Acidic, Chiral Brønsted Acids as Efficient Catalysts. Angew. Chem. Int. Ed 2008, 47, 6798–6801. [DOI] [PubMed] [Google Scholar]; (b) Kikuchi J; Aramaki H; Okamoto H; Terada M F10 BINOL-derived chiral phosphoric acid-catalyzed enantioselective carbonyl-ene reaction: theoretical elucidation of stereochemical outcomes. Chem. Sci 2019, 10, 1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kikuchi J; Aizawa Y; Terada M Chiral strong Brønsted acid-catalyzed enantioselective addition reaction of simple olefins with ethyl glyoxylate. Org. Chem. Front 2020, 7, 1383–1387. [Google Scholar]; (d) Ishihara H; Huang J; Mochizuki T; Hatano M; Ishihara K Enantio- and Diastereoselective Carbonyl-Ene Cyclization–Acetalization Tandem Reaction Catalyzed by Tris(pentafluorophenyl)borane-Assisted Chiral Phosphoric Acids. ACS Catal. 2021, 11, 6121–6127. [Google Scholar]; (e) Zhao C; Sun Q; Hart-Cooper WM; DiPasquale AG; Toste FD; Bergman RG; Raymond KN Chiral Amide Directed Assembly of a Diastereo- and Enantiopure Supramolecular Host and its Application to Enantioselective Catalysis of Neutral Substrates. J. Am. Chem. Soc 2013, 135, 18802–18805. [DOI] [PubMed] [Google Scholar]
- (16).Grachan ML; Tudge MT; Jacobsen EN Enantioselective Catalytic Carbonyl-Ene Cyclization Reactions. Angew. Chem. Int. Ed 2008, 47, 1469–1472. [DOI] [PubMed] [Google Scholar]
- (17).Liu L; Leutzsch M; Zheng Y; Alachraf MW; Thiel W; List B Confined Acid-Catalyzed Asymmetric Carbonyl-Ene Cyclization. J. Am. Chem. Soc 2015, 137, 13268–13271. [DOI] [PubMed] [Google Scholar]
- (18).For select examples of enantioselective tetrahydropyran- and tetrahydrdofuranforming Prins reactions catalyzed by chiral Brønsted acids, see: (a) Liu L; Kaib PSJ; Tap A; List B A General Catalytic Asymmetric Prins Cyclization. J. Am. Chem. Soc 2016, 138, 10822–10825. [DOI] [PubMed] [Google Scholar]; (b) Xie Y; Cheng G-J; Lee S; Kaib PSJ; Thiel W; List B Catalytic Asymmetric Vinylogous Prins Cyclization: A Highly Diastereo- and Enantioselective Entry to Tetrahydrofurans. J. Am. Chem. Soc 2016, 138, 14538–14541. [DOI] [PubMed] [Google Scholar]; (c) Sun H-R; Zhao Q; Yang H; Yang S; Gou B-B; Chen J; Zhou L Org. Lett 2019, 21, 7143–7148. [DOI] [PubMed] [Google Scholar]
- (19).(a) 4-chromanols constitute the core structures of several natural products and biologically active molecules. For examples, see Ahmed SA; Bardshiri E; McIntyre CR; Simpson TJ Biosynthetic Studies on Tajixanthone and Shamixanthone, Polyketide Hemiterpenoid Metabolites of Aspergillus variecolor. Aust. J. Chem 1992, 45, 249–274. [Google Scholar]; (b) Wu Q; Wy C; Long H; Chen R, Liu D; Proksch P; Guo P; Lin W Varioxiranols A-G and 19-O-Methyl-22-methoxypre-shamixanthone, PKS and Hybrid PKS-Derived Metabolites from a Sponge-Associated Emercella variecolor Fungus. J. Nat. Prod 2015, 78, 2461–2470. [DOI] [PubMed] [Google Scholar]; (c) Koch K; Melvin LS Jr.; Reiter LA; Biggers MS; Showell HJ; Griffiths RJ; Pettipher ER; Cheng JB; Milici AJ; Breslow R; Conklyn MJ; Smith MA; Hackman BC; Doherty NS; Salter E; Farrell CA; Schulte G (+)-1-(3S,4R)-[3-(4-Phenylbenzyl)-4-hydroxychroman-7-yl] cyclopentane Carboxylic Acid, a Highly Potent, Selective Leukotriene B4 Antagonist with Oral Activity in the Murine Collagen-Induced Arthritis Model. J. Med. Chem 1994, 37, 3197–3199. [DOI] [PubMed] [Google Scholar]; (d) Nahar L; Das Talukdar A; Nath D; Nath S; Mehan A; Ismail FMD; Sarker SD Naturally Occurring Calanolides: Occurrence, Biosynthesis, and Pharmacological Properties Including Therapeutic Potential. Molecules 2020, 25, 4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).In these reactions, tertiary adducts analogous to 3a were not observed.
- (21).Sobral AJFN; Rebanda NGCL; Silva M; Lampreia SH; Silva MR; Beja AM; Paixano JA; Gonsalves A One-step synthesis of dipyrromethanes in water. Tetrahedron Lett. 2003, 44, 3971–3973. [Google Scholar]
- (22).Acetylated analogs of 2a and 3a were not detected using these reagents.
- (23).Although this derivatization is clean and rapid, approximately 40% of 3a is converted to 2a while the remaining material reverts to 1a, lowering the overall yield of the reaction. See SI for details.
- (24).Edman P Method for Determination of the Amino Acid Sequence in Peptides. Acta. Chem. Scand 1950, 283–293. [Google Scholar]
- (25).The products of Edman degradation were found to be catalytically inactive. See SI for details.
- (26).Other aromatic substituents were surveyed at this position with inferior results. See SI for details.
- (27).McCann SD; Reichert EC; Arrechea PL; Buchwald SL Development of an Aryl Amination Catalyst with Broad Scope Guided by Consideration of Catalyst Stability. J. Am. Chem. Soc 2020, 142, 15027–15037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).The observation of Edman degradation using these catalysts provides indirect evidence for complexation between HCl and the chiral thiourea amide. We hypothesize that the sequestration of HCl in such a manner is responsible for the high enantioselectivity observed in this report. This possibility is also supported by observations made in reference 11c.
- (29).Jung ME; Piizzi G gem-Disubstitution Effect: Theoretical Basis and Synthetic Applications. Chem. Rev 2005, 105, 1735–1766. [DOI] [PubMed] [Google Scholar]
- (30).For catalytic enantioselective homoallylic alcohol forming reactions in which simple substrates lacking Thorpe-Ingold-type substitution are used, see refs. 15d and 17.
- (31).Ren H; Dunet G; Mayer P; Knochel P Highly Diastereoselective Synthesis of Homoallylic Alcohols Bearing Adjacent Quaternary Centers Using Substituted Allylic Zinc Reagents J. Am. Chem. Soc 2007, 129, 5376–5377. [DOI] [PubMed] [Google Scholar]
- (32).(a) A moderately-enantioselective cyclization with a tetrasubstitued alkene nucleophile has been disclosed. See reference 16.Nguyen KD; Herkommer D; Krische MJ Enantioselective Formation of All-Carbon Quaternary Centers via C–H Functionalization of Methanol: Iridium-Catalyzed Diene Hydrohydroxymethylation. J. Am. Chem. Soc 2016, 138, 14210–14213. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dongbang S; Shen Z; Ellman JA Synthesis of Homoallylic Alcohols with Acyclic Quaternary Centers through CoIII-Catalyzed Three-Component C-H Bond Addition to Internally Substituted Dienes and Carbonyls. Angew. Chem. Int. Ed 2019, 58, 12590–12594. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chen X; Zhu L; Gui Y; Jing K; Jiang Y; Bo Z; Lan Y; Li J; Yu D Highly Selective and Catalytic Generation of Acyclic Quaternary Carbon Stereocenters via Functionalization of 1,3-Dienes with CO2. J. Am. Chem. Soc 2019, 141, 18825–18835. [DOI] [PubMed] [Google Scholar]
- (33).Moderate yields are observed in these reactions due to competing isomerization of 1 to the corresponding unreactive Z-enals. Presumably, this occurs through HCl conjugate addition followed by bond rotation and subsequent elimination.
- (34).Berrisford DJ; Bolm C; Sharpless KB Ligand-Accelerated Catalysis. Angew. Chem. Int. Ed 1995, 34, 1059–1070. [Google Scholar]
- (35).Schifferer L; Stinglhamer, Kaur, K.; Mancheño, O. G. Halides as versatile anions in asymmetric anion-binding organocatalysis. Beilstein J. Org. Chem 2021, 17, 2270–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.