Abstract
Background:
Sensorineural hearing loss (SNHL) is characteristic of Usher syndrome type 2 (USH2), but less is known about SNHL in nonsyndromic autosomal recessive retinitis pigmentosa (ARRP) and olfaction in USH2A-associated retinal degeneration.
Methods:
The Rate of Progression of USH2A-related Retinal Degeneration (RUSH2A) is a natural history study that enrolled 127 participants, 80 with USH2 and 47 with ARRP. Hearing was measured by pure-tone thresholds and word recognition scores, and olfaction by the University of Pennsylvania Smell Identification Test (UPSIT).
Results:
SNHL was moderate in 72% of USH2 participants and severe or profound in 25%, while 9% of ARRP participants had moderate adult-onset SNHL. Pure-tone thresholds worsened with age in ARRP but not in USH2 participants. The degree of SNHL was not associated with other participant characteristics in either USH2 or ARRP. Median pure-tone thresholds in ARRP participants were significantly higher than the normative population (P<0.001). Among 14 USH2 participants reporting newborn hearing screening results, 7 reported passing. Among RUSH2A participants, 7% had mild microsmia and 5% had moderate or severe microsmia. Their mean (±SD) UPSIT score was 35 (±3), similar to healthy controls [34 (±3); P=0.39]. Olfaction differed by country (P=0.02), but was not significantly associated with clinical diagnosis, age, gender, race/ethnicity, smoking status, visual measures, or hearing.
Conclusions:
Hearing loss in USH2A-related USH2 did not progress with age. ARRP patients had higher pure-tone thresholds than normal. Newborn hearing screening did not identify all USH2A-related hearing loss. Olfaction was not significantly worse than normal in participants with USH2A-related retinal degeneration.
Keywords: autosomal recessive retinitis pigmentosa, Usher Syndrome Type 2, Sensorineural hearing loss, Olfaction
Introduction
Dual sensory impairment associated with deaf-blindness is particularly challenging for patients (National Center on Deaf-Blindness, 2021). Many conditions can cause loss of hearing and vision, including prenatal or congenital complications such as congenital cytomegalovirus infection, hydrocephalus, asphyxia and injury, in addition to hereditary syndromes. Usher syndrome is a leading cause of autosomal recessive deaf-blindness, and is a ciliopathy characterized by retinitis pigmentosa (RP) with sensorineural hearing loss (SNHL). Clinically, Usher syndrome is divided into 3 types, based on the severity and onset of hearing loss (Friedman, Schultz, Ahmed, Tsilou, & Brewer, 2011; Mathur & Yang, 2015). Usher syndrome type 1 is associated with profound congenital hearing loss, absent vestibular responses, and retinitis pigmentosa (RP) (or rod greater than cone photoreceptor degeneration) beginning in the first decade of life and represents 33–44% of all Usher syndrome (Mathur & Yang, 2015). Usher syndrome type 2 (USH2) is associated with a moderate to severe congenital sensorineural hearing loss (SNHL) with a sloping configuration, normal vestibular function and RP beginning in the first or second decade, and represents 56–67% of all Usher syndrome (Eudy et al., 1998; Fishman, Kumar, Joseph, Torok, & Anderson, 1983). Usher syndrome type 3 is the least common form, accounting for <3% of Usher syndrome, and is associated with progressive hearing loss, variable vestibular responses, and variable onset of RP (Adato et al., 2002; Fields et al., 2002; Joensuu et al., 2001). Usher syndrome is genetically heterogeneous and at least 15 genes and 3 loci have been associated with autosomal recessive Usher syndrome (RetNet) (The Hereditary Hearing Loss Homepage, 2021). This report focuses on USH2.
Though multiple genes have been associated with USH2, the most commonly implicated is USH2A (Le Quesne Stabej et al., 2012; McGee, Seyedahmadi, Sweeney, Dryja, & Berson, 2010). Mutations in USH2A are among the most common causes of nonsyndromic autosomal recessive RP (ARRP) (Kaiserman, Obolensky, Banin, & Sharon, 2007; McGee et al., 2010; Oishi et al., 2014; Rivolta, Sweklo, Berson, & Dryja, 2000; Seyedahmadi, Rivolta, Keene, Berson, & Dryja, 2004). While congenital SNHL is a defining feature in USH2, less is known about hearing in persons with USH2A-related ARRP (Lenassi et al., 2015).
In addition to vision loss caused by photoreceptor degeneration and SNHL, other sensory deficits can be associated with the ciliary defect that underlies USH2A-related disease. Olfaction deficits in people with USH2 were first reported by Zrada et al (Zrada, Braat, Doty, & Laties, 1996). Studies in mice demonstrated expression of the Usher protein complex in the ciliary region of the olfactory epithelium, and abnormal electro-olfactograms in murine models of Usher syndrome (Jansen et al., 2016). However, subsequent reports of smell function in patients with USH2 have provided conflicting results (Ribeiro et al., 2016; Seeliger et al., 1999). Also, to the best of our knowledge, olfaction deficits have not been evaluated in USH2A-associated ARRP. Some reports suggest smell may be heightened in USH2 patients (Ribeiro et al., 2016), raising the possibility that sensory compensation to dual vision-hearing loss may be present in USH2 patients. Cross-sensory enhancements at the cortical level have been reported (Armel & Ramachandran, 1999; Gougoux, Zatorre, Lassonde, Voss, & Lepore, 2005; Myers, Iannaccone, & Bidelman, 2017). However, there is little evidence that olfaction is improved as a result of vision loss (Smith, Doty, Burlingame, & McKeown, 1993). Thus, the question whether olfaction is different from normal in USH2 or ARRP patients with USH2A mutations remains unresolved.
The Rate of Progression of USH2A-related Retinal Degeneration (RUSH2A) study is an ongoing multicenter, international, non-interventional investigation at multiple sites of individuals with USH2A-associated USH2 and ARRP (NCT03146078). Baseline ocular characteristics of the RUSH2A participants have been published (Birch et al., 2020; Duncan et al., 2020). Here, the RUSH2A study afforded the opportunity to investigate hearing and olfaction in patients with USH2A-related retinal degeneration.
Methods
Study Design
The RUSH2A study design has been described previously (Duncan et al., 2020). Briefly, 127 participants were enrolled between August 2017 and December 2018 at 16 sites in Canada, France, Germany, the Netherlands, the United Kingdom, and the United States. This investigation adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review boards (IRBs) or ethics boards associated with each participating site.
Participants had to be at least 8 years old with a rod-cone degeneration associated with at least 2 disease-causing USH2A sequence variants. ARRP participants for whom the phase of allele inheritance was not known underwent additional genetic testing of 1–2 first-degree relatives to confirm that inheritance was in trans. Subjects whose heterozygous variants in the USH2A gene were inherited in cis were not eligible to participate in the study. The segregation studies as well as pathogenicity or likely pathogenicity of all USH2A variants was reviewed by an expert genetics committee. Ultimately, participants with USH2 or with ARRP with homozygous or compound heterozygous USH2A variants inherited in trans were enrolled.
Best corrected visual acuity letter score was measured according to the Early Treatment of Diabetic Retinopathy Study (ETDRS) protocol (Ferris, Kassoff, Bresnick, & Bailey, 1982) to assign participants to a vision cohort. One hundred and five participants with 54 or greater (20/80 or better) letters in the better eye, central kinetic visual field III4e isopter extending at least 10 degrees diameter, and stable fixation were enrolled in the primary cohort. A secondary cohort of 22 patients had ETDRS letter score of 53 or worse (20/100 or worse), central kinetic visual field III4e isopter less than 10 degrees diameter, or unstable fixation. Both cohorts are combined in this baseline cross-sectional report.
Audiology
Audiologic evaluation including pure-tone and speech thresholds and word recognition tests was administered at study baseline by audiologists associated with study sites. Audiology data were reviewed by a single study expert audiologist [CCB] to confirm accuracy and completeness. Three participants with cochlear implants in both ears were excluded from the audiology analyses. Data from two other participants were not available since they missed the hearing examination. Participants’ self-reported history of hearing loss (e.g., newborn screening test results and age at onset of hearing loss) was collected at the baseline visit.
Hearing outcomes in this report include the pure-tone thresholds and word recognition scores (WRS). A four frequency (0.5, 1, 2 and 4 kHz) pure tone average (4F-PTA) was calculated for each ear and the mean value from two ears was used for analyses. Degree of hearing loss by air conduction was classified into 5 categories based on the 4F-PTA: normal (≤20 dB HL), mild (>20 to ≤40 dB HL), moderate (>40 to ≤70 dB HL), severe (>70 to ≤95 dB HL) and profound (>95 dB HL). If a patient had hearing loss by the 4F-PTA, the type of loss was determined by calculating the difference between the three frequency (0.5, 1 and 2 kHz) pure-tone averages (3F-PTA) for air and bone conduction (i.e., air-bone gap). When the average air-bone gap was less than 10 dB HL and there was hearing loss by the 3F-PTA (>20 dB HL), the hearing loss was classified as SNHL. When the air-bone gap was greater than 10 dB HL, the hearing loss was classified as conductive if the bone conduction 3F-PTA was normal (≤20 dB HL), or mixed when the bone conduction 3F-PTA indicated any degree of hearing loss (>20 dB HL). One ear of a single USH2 patient was excluded from analysis of hearing loss type due to insufficient data. A third PTA (WR-PTA) comprising the average of thresholds at 0.5, 1, 2 and 3 kHz was used for plotting the comparison between pure-tone thresholds with WRS (Gurgel, Jackler, Dobie, & Popelka, 2012).
Olfaction
The University of Pennsylvania Smell Identification Test (UPSIT, Sensonics International, Haddon Heights, NJ) was performed in all 127 participants (Doty, Shaman, & Dann, 1984). This reliable and sensitive test (Doty, Frye, & Agrawal, 1989) correlates well with more time-consuming tests, including olfactory threshold tests (Doty et al., 2019). In brief, the UPSIT consists of 40 “scratch-and-sniff” microencapsulated odorant patches that release an odor upon being scratched. Four forced-choice response alternatives, three foils and one correct answer, are associated with each odorant patch. The total number of correct responses out of the 40 odorants serves as the test score. Subjects with ongoing upper respiratory infections were excluded from testing. Testing was conducted in rooms with adequate air turnover and participants were requested not to wear lotions or perfumes on the test day to minimize confounding from such factors. Smoking status information was also collected. The smell function of each participant ≥15 years (N=126) was further classified into these categories: normosmia (UPSIT total score 32–40 for men and 31–40 for women), mild microsmia (UPSIT total score 28–31 for men and 29–30 for women) and moderate/severe microsmia (UPSIT total score 17–27 for men and 17–28 for women (Doty, 1995). All baseline testing occurred prior to February 2019.
Ocular Function and Structure Assessments
All measurements were performed by study certified technicians following standardized protocols. Following subjective refraction, best corrected visual acuity (BCVA) was measured as the ETDRS letter score obtained from the Electronic Visual Acuity (EVA) tester or standard ETDRS charts (Beck et al., 2003; Ferris et al., 1982). Only BCVA from study eyes was used for analyses. Full-field electroretinogram (ERG) tests were measured to obtain an objective estimate of the residual retinal function, and were performed following International Society for Clinical Electrophysiology of Vision (ISCEV) standards (McCulloch et al., 2015). The full-field ERG measures included amplitude of the dark adapted (DA) 0.01 ERG response b-wave (a rod-driven bipolar cell response), the amplitude of the DA 3.0 ERG response b-wave (the combined response of both the rod and the cone systems), and the trough-to-peak amplitude of the light-adapted (LA) 30 Hz flicker (a purely L- and M-cone-driven response).
The full-field stimulus test (FST) in response to white, blue and red stimuli was performed utilizing the Espion E3 system (Diagnosys LCC, Lowell, MA) (Klein & Birch, 2009) as described previously (Birch et al., 2020). This test provides non-localizing information on a participant’s ability to detect a light stimulus in the dark and indicates whether the perception is mediated by rod or cone photoreceptors.
Retinal structure was assessed using spectral-domain optical coherence tomography (SD-OCT) and the remaining ellipsoid zone (EZ) area was measured. Static perimetry (SP) was performed using the Octopus 900 (Haag-Streit, Mason, OH) and three measures were included in the analyses: full-field hill of vision, 30-degree hill of vision and mean sensitivity. For the evaluation of kinetic perimetry, the Octopus perimetry EyeSuite software calculated areas in squared degrees for each isopter automatically. Both SP and KP data were graded by the Casey Reading Center (Casey Eye Institute, Oregon Health Sciences University, Portland, OR). Mean macular sensitivity was measured by fundus-guided microperimetry, graded by the Duke Reading Center (Duke University, Durham, NC). Details on these measures have been reported elsewhere (Duncan et al., 2020).
Statistical Analysis
The distributions of 4F-PTA data and UPSIT scores were summarized using means and standard deviations (SDs). The 4F-PTA was compared with age- and gender-matched percentile bands of normative data from ISO standards (ISO 7029:2017 Acoustics — Statistical distribution of hearing thresholds related to age and gender, 2017) for USH2 and ARRP participants separately. For ARRP participants, the percentage below the age and sex–specific median was compared to 0.50 using one-proportion Z-test. The associations between participant characteristics and 4F-PTA thresholds within the USH2 and ARRP groups were also assessed separately by linear regression models. The UPSIT scores from RUSH2A participants were compared to healthy historical controls of similar age and gender from an UPSIT database at the University of Pennsylvania (N=77) using a linear regression model with adjustment for age and gender. Linear regression models were also used to assess the association between study participant characteristics and UPSIT scores. Correlations between UPSIT scores, 4F-PTA thresholds, and selected visual functional and structural measures were assessed with Spearman partial correlations with 95% confidence intervals adjusted for age. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and reported P values are two-sided.
Results
Study Population
A detailed description of the RUSH2A participant enrollment flow and baseline characteristics was previously published (Duncan et al., 2020). A total of 127 participants were enrolled into the study, with 63% (n= 80) classified as USH2 and 37% (n= 47) as ARRP based on self-reported hearing loss history (those with hearing loss before adulthood were classified as USH2, and ARRP otherwise, except one participant who reported childhood hearing loss due to Usher Syndrome was re-classified into ARRP based on normal 4F-PTA results at study baseline). The median age was 37 years (IQR, 27 to 44) for the USH2 group and 44 years (IQR, 36 to 50) for the ARRP group. Overall, 68 of the participants (54%) were female, 113 (89%) were white, and 83 (65%) were enrolled in the US or Canadian sites. The median age at onset of first noticed vision loss was 16 years (IQR, 13 to 22) for the USH2 group and 32 years (IQR, 20 to 41) in the ARRP group.
Auditory Findings
Among the 75 USH2 participants with audiology data, none had normal hearing; the degree of hearing loss was severe or profound in 19 (25%), moderate in 54 (72%), and mild in 2 (3%). Among the 47 ARRP participants, 4 (9%) had moderate adult-onset hearing loss, 8 (17%) had mild hearing loss and 35 (74%) had normal hearing (e-Figure 1). All 75 USH2 participants had SNHL except one with bilateral mixed hearing loss. Among the 12 ARRP participants with hearing loss, one had unilateral conductive hearing loss, and the remaining 11 had SNHL that was bilateral in 10 and unilateral in 1.
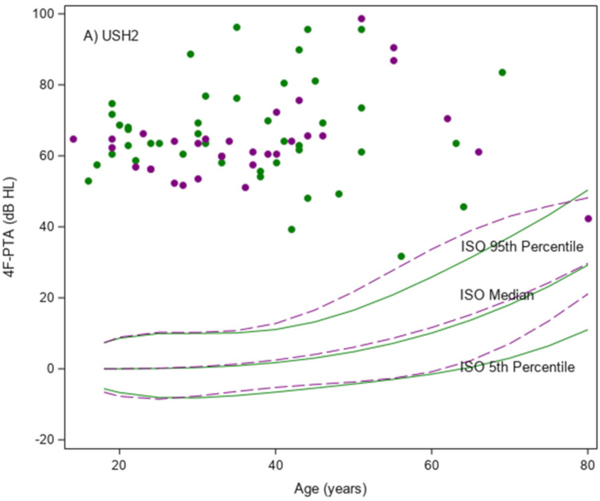
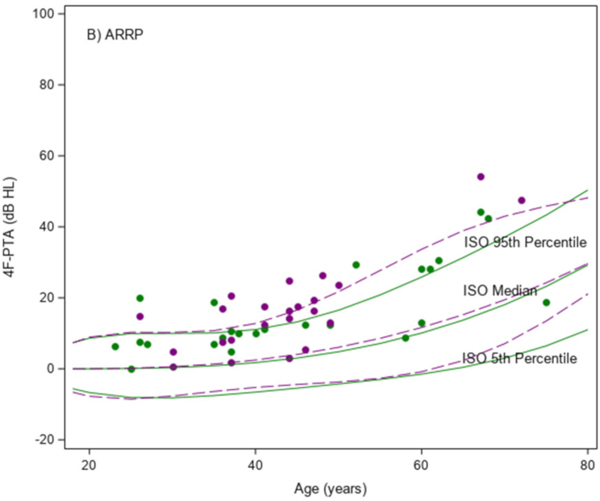
The mean 4F-PTA was 66 dB HL (SD, 13) in the USH2 group, and 17 dB HL (SD, 12) in the ARRP group. As shown in Figure 1A, 4F-PTAs did not increase (worsen) with age in the USH2 participants. The mean (95% C.I.) difference in the 4F-PTA was −0.01 (−0.34, 0.32) dB HL/year in females and 0.19 (−0.09, 0.46) dB HL/year in males in the USH2 group. All but one of the USH2 participants had 4F-PTAs above the International Organization for Standardization (ISO) 95th percentile curves for both males and females (ISO 7029:2017 Acoustics — Statistical distribution of hearing thresholds related to age and gender, 2017). As shown in Figure 1B, 4F-PTAs increased with age among the ARRP participants and showed a similar upward trend when compared to the ISO normative data. The mean (95% C.I.) difference in the 4F-PTA was 0.54 (0.31, 0.78) dB HL/year in females and 0.99 (0.65, 1.32) dB HL/year in males in the ARRP group. All but two ARRP participants (96%) had 4F-PTAs at or above the median ISO curves (P<0.001 for comparing to 50%).
Figure 1. Scatter plots of the mean four frequency (0.5, 1, 2 and 4kHz) pure-tone average (4F-PTA) from ears vs age at study baseline.
A) USH2 (N=75); B) ARRP (N=47) Green indicates female and purple indicates male. Solid and dashed lines are percentile bands of normative data from ISO standards for females and males, respectively.
Among the USH2 participants, the 4F-PTA was not significantly associated with age, gender, race/ethnicity or duration of disease (e-Table 1). Among the ARRP participants, higher 4F-PTAs were significantly associated with older age (P < 0.001) and longer disease duration (P = 0.03) from univariate models. Disease duration was confounded by age and was no longer significant once age was included in the multivariable model.
Self-reported newborn hearing screening data were collected; however, only 14 (22%) USH2 and 5 (19%) ARRP participants (mean age=31 years, range 15 to 50) reported that hearing screening tests were conducted during the newborn period. Interestingly, seven out of the 14 USH2 participants who recalled newborn hearing screening reported they passed this test (e-Figure 2).
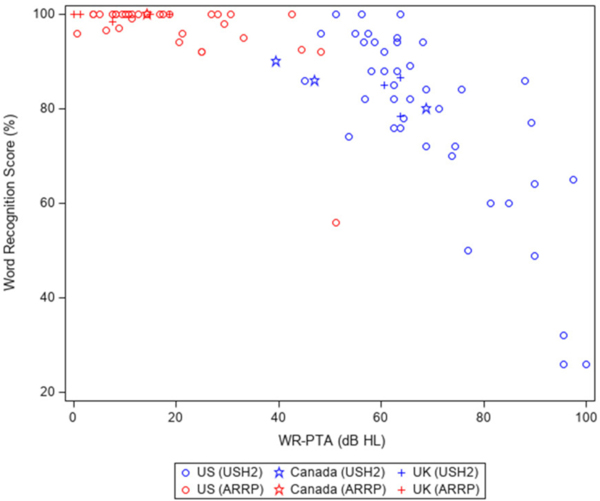
Scatter plots of WRS versus the WR-PTA are presented in Figure 2. WRS for the USH2A participants ranged from 26 to 100% and declined in a non-linear pattern as the WR-PTA increased; the decline of WRS was much faster when WR-PTA was greater than 60 dB HL compared to the decline when WR-PTA was below 60 dB HL. All but one of the ARRP participants had WRS of 90% or better despite the degree of hearing loss; the single outlier had moderate SNHL (age=67 years).
Figure 2.
Scatter plots of mean word recognition score vs pure-tone average of 0.5, 1, 2 and 3kHz (WR-PTA) averaged from both ears at study baseline (N=87)
Olfactory Findings
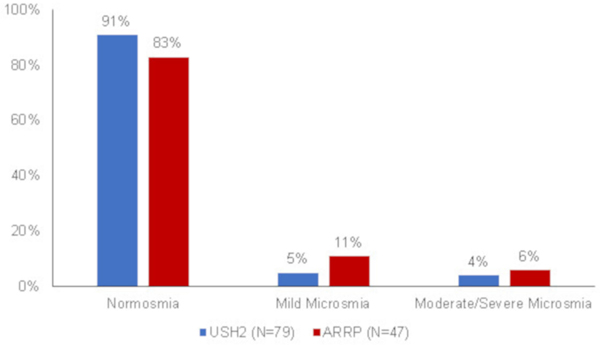
The majority of the RUSH2A participants had normal smell function at the study baseline visit. Among the 126 participants ≥15 years old, only 9 (7%) had mild microsmia and 6 (5%) had moderate or severe microsmia. The proportion of participants who had any smell function loss in the ARRP group (17%) was numerically higher than those in the USH2 group (9%) (Figure 3), but this was not statistically significant (P=0.17). The different age distributions among the two groups had no impact on this result.
Figure 3.
Smell function loss severity by clinical diagnosis.
The mean (±SD) UPSIT score was 35 (±3) in the RUSH2A participants and 34 (±3) in the age-similar healthy controls from the UPSIT database. After adjusting for age and gender, the mean adjusted difference (95% C.I.) between the two groups was 0.39 (−0.50, 1.28) (P = 0.39).
Within the RUSH2A cohort, there were significant differences in UPSIT scores among different countries (P = 0.02), with participants from Netherlands and UK having lower mean UPSIT total scores (32 and 33, respectively) and participants from Canada and Germany having higher mean UPSIT total scores (38 and 36, respectively) (Table 1). The UPSIT score was not significantly associated with clinical diagnosis, age, gender, race/ethnicity and smoking status at study baseline. No significant associations were found between UPSIT scores and visual functional and structural measures (Table 2), or with the 4F-PTA (Spearman correlation = 0.12, P = 0.18, adjusting for age). Sensitivity analyses show similar results for olfaction analyses by excluding participants who used carbonic anhydrase inhibitor at time of enrollment.
Table 1.
Association between UPSIT total score and participant characteristics
| Characteristic | N | UPSIT Score | Univariate P-value |
|---|---|---|---|
| Mean ± SD | |||
| All | 127 | 35 ± 3 | NA |
| Clinical Diagnosis | 0.55 | ||
| USH2 | 80 | 35 ± 3 | |
| ARRP | 47 | 34 ± 4 | |
| Age at Enrollment (yrs) | 0.90† | ||
| <30 | 30 | 34 ± 4 | |
| 30–39 | 34 | 34 ± 3 | |
| 40–49 | 37 | 35 ± 3 | |
| 50–59 | 11 | 34 ± 5 | |
| ≥60 | 15 | 35 ± 3 | |
| Gender | 0.34 | ||
| Female | 68 | 35 ± 4 | |
| Male | 59 | 34 ± 3 | |
| Race/Ethnicity | 0.63 | ||
| Non-Hispanic White | 113 | 34 ± 3 | |
| Other | 14 | 35 ± 3 | |
| Duration of Disease (yrs) ‡ | 0.38† | ||
| <10 | 37 | 34 ± 4 | |
| 10–19 | 46 | 34 ± 4 | |
| ≥20 | 43 | 35 ± 3 | |
| Daily Smoker Ever | 0.23 | ||
| Yes | 33 | 34 ± 3 | |
| No | 94 | 35 ± 3 | |
| Country | 0.02§ | ||
| Canada | 4 | 38 ± 1 | |
| France | 13 | 34 ± 5 | |
| Germany | 11 | 36 ± 2 | |
| Netherlands | 10 | 32 ± 3 | |
| UK | 10 | 33 ± 3 | |
| US | 79 | 35 ± 3 |
Variable was analyzed as continuous.
One participant was missing age of onset (a participant-reported field based on their awareness of visual symptoms) and duration of disease (computed based on age of onset and date of enrollment)
Four participants from Canada were excluded from analysis due to small sample size.
Table 2.
Spearman correlation between UPSIT total score and visual function and structure measures
| Visual Function Measures | Correlation with UPSIT Score (95% C.I.)† | P-value |
|---|---|---|
| Visual Acuity | −0.05 (−0.22, +0.13) | 0.57 |
| Full-field Stimulus Threshold | ||
| White Stimulus | +0.07 (−0.13, +0.27) | 0.49 |
| Blue Stimulus | +0.06 (−0.14, +0.27) | 0.55 |
| Red Stimulus | +0.11 (−0.09, +0.31) | 0.28 |
| Full-field Electroretinography | ||
| DA 0.01 ERG Response‡ | −0.11 (−0.28, +0.07) | 0.21 |
| DA 3.0 ERG Response§ | −0.14 (−0.30, +0.04) | 0.13 |
| LA 3.0 Flicker ERG Response¶ | −0.12 (−0.29, +0.06) | 0.19 |
| Kinetic Perimetry | ||
| I4e Seeing Area | −0.08 (−0.25, +0.10) | 0.37 |
| III4e Seeing Area | −0.09 (−0.26, +0.08) | 0.30 |
| V4e Seeing Area | −0.12 (−0.29, +0.06) | 0.20 |
| Ellipsoid Zone Area on Optical Coherent Tomography | −0.10 (−0.27, +0.08) | 0.26 |
| Static Perimetry | ||
| Full-field Hill of Vision | −0.11 (−0.28, +0.07) | 0.23 |
| 30 Degree Hill of Vision | −0.11 (−0.29, +0.07) | 0.21 |
| Mean Sensitivity | −0.10 (−0.27, +0.08) | 0.26 |
| Microperimetry Mean Retinal Sensitivity | −0.11 (−0.31, +0.10) | 0.32 |
Spearman partial correlation adjusted for age at enrollment.
The amplitude of the b-wave from the dark adapted 0.01 ERG response which reflects a rod-driven bipolar cell response.
The amplitude of the b-wave of the dark adapted 3.0 ERG response, which arises from a combined response of both rod and cone systems.
The trough-to-peak amplitude of the light-adapted 30 Hz flicker ERG response, which is cone-driven.
Discussion
The RUSH2A study provided the opportunity to assess olfactory and hearing deficits and to address some discrepancies in the literature, especially regarding smell dysfunction in participants with USH2A mutations. Strengths of the RUSH2A study include the largest (n=127) patient population to date of molecularly confirmed participants with USH2A-related retinal degeneration and the use of quantitative standard clinical testing methods.
The hearing loss observed previously in patients with USH2A-related USH2 ranged from mild to severe, and was sensorineural, bilaterally symmetrical, and gently down-sloping from low to high frequencies (Abadie et al., 2012; Pennings et al., 2003). The audiometric patterns observed in RUSH2A are largely consistent with these descriptions (e-Figure 3). Moderate SNHL was present in 72% of our cohort, comparable to previous reports showing moderate SNHL in 75–77% (Abadie et al., 2012; Blanco-Kelly et al., 2015).
Congenital hearing loss is a factor that differentiates USH2 from ARRP, and there is an expectation that auditory dysfunction, other than that attributable to normal aging, does not occur in the ARRP phenotype. Our data show clinically significant, mild to moderate SNHL in 26% (12/47) of participants with ARRP, in agreement with prior reports of hearing loss in 5 (26%) of 19 patients with ARRP (Lenassi et al., 2015). Hearing sensitivity declines with age, and faster in males than females, in otologically normal individuals (ISO 7029:2017 Acoustics — Statistical distribution of hearing thresholds related to age and gender, 2017). Cross-sectional analysis of PTAs across the ages in RUSH2A shows hearing progression in the ARRP group; however, the 4F-PTAs were above the age- and gender-based 95th percentile for 19 (40%) of 47. This suggests that there may be a subtle effect of USH2A mutations on the auditory system, where hearing thresholds are slightly elevated above age- and gender-based normative levels, although clinically normal, in persons with ARRP.
Hearing loss in USH2A-associated USH2 is generally considered stable without significant-age related decline (Reisser, Kimberling, & Otterstedde, 2002). However, cross-sectional data have shown hearing loss progression across test frequencies in an USH2 group that was attributable to aging for all but the low frequencies that declined at a rate independent of age (Pennings et al., 2003). Additional reports of longitudinal data have documented faster age-related hearing sensitivity decline in USH2A-associated USH2 than other types of USH2 (M. Sadeghi et al., 2004). Case studies show hearing loss progression in some (A. M. Sadeghi, Cohn, Kimberling, Halvarsson, & Möller, 2013) (van Aarem et al., 1996) and stability in others (A. M. Sadeghi et al., 2013) (Hmani-Aifa et al., 2002). In the current USH2 cohort, the cross-sectional data did not show hearing loss progression with age, not even the expected progression seen in a normal aging population or other USH2 groups (Pennings et al., 2003; M. Sadeghi et al., 2004). However, it is possible that better hearing was present at younger ages, especially since some participants reported that they passed a newborn screening test, and this cross-sectional study was not designed to assess progression, which is a limitation of the study.
Only 14 of the USH2 participants in this study reported having a newborn hearing screening test. This low number is largely attributable to the incorporation of newborn hearing screening as standard practice in only the past 20 years (“Year 2007 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs,” 2007). Seven of these USH2 participants reported that they passed the newborn hearing screening. While the hearing loss associated with USH2A is often described as congenital, age at hearing loss identification may be much later as evidenced by a mean age of diagnosis at 5.9 years reported by Abadie et al. (Abadie et al., 2012). In addition, newborn hearing screening tests are generally conducted at levels that may miss a mild hearing loss (Year 2019 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs, (2019)). Results of newborn hearing screenings in our small dataset suggest that hearing loss may be present at birth in some and that it may have a delayed onset in others with USH2A mutations. Further investigation of hearing screening results and age of hearing loss diagnosis in a larger cohort of persons with USH2A mutations may clarify the onset timeline.
The mean UPSIT score for all RUSH2A participants was not significantly different from normal and there was no significant difference between USH2 and ARRP participants. Only 12% of all participants exhibited olfaction deficits (7% had mild microsmia and 5% had moderate or severe microsmia). Thus, while cases of impaired smell function in conjunction with USH2A mutations are possible, they appear to be relatively uncommon and rarely severe.
Olfaction has been the object of investigation in Usher syndrome prior to RUSH2A. Zrada et al. first raised the possibility of olfaction deficits in a study of UPSIT results in USH1 and USH2 subjects with similar disease severity to our study participants (Zrada et al., 1996). A subsequent investigation by Seeliger et al. (Seeliger et al., 1999) did not confirm olfaction deficits in either USH1 or USH2 using the “Sniffin’ Sticks” test, a three-part approach that measures olfactory thresholds (T), odor discrimination (D), and the ability to identify (I) correctly 16 common odors. Ribeiro et al. (Ribeiro et al., 2016) used the same test and characterized olfaction in USH1 and USH2 patients with separate T, D and I scores plus a composite TDI olfaction score. These authors observed significantly lower T, I and TDI composite olfaction scores in USH2 than in USH1 patients, and observed faster olfaction aging in USH1 patients than in controls, but not in USH2 patients (Ribeiro et al., 2016). Importantly, however, the smell identification (I) scores in both USH1 and USH2 patients were not different from control subjects, in contrast to earlier findings (Zrada et al., 1996).
Olfaction deficits have also been reported in patients with other syndromic ciliopathies affecting vision associated with CEP290 variants (McEwen et al., 2007) or Bardet-Biedl syndrome (BBS). Utilizing either a 12-item version of the UPSIT (Kulaga et al., 2004) or the complete 40-item version used in the present study (Iannaccone et al., 2005), olfaction deficits have been documented in BBS. Some BBS-related protein expression in the murine olfactory epithelium has been documented, and both electro-olfactographic and behavioral evidence of olfaction deficits have been confirmed in BBS mouse models (Kulaga et al., 2004; Nishimura et al., 2004).
There are several potential reasons for the discrepancies among the previous Usher syndrome studies. The small studies by Zrada et al. (Zrada et al., 1996) (8 USH1 and 14 USH2 patients), Seeliger at al. (Seeliger et al., 1999) (8 USH1 and 31 USH2 subjects), and Ribeiro et al. (Ribeiro et al., 2016) (22 USH1 and 43 USH2) were genetically uncharacterized and it is likely the participants in these prior reports (Ribeiro et al., 2016; Seeliger et al., 1999; Zrada et al., 1996) were genetically heterogeneous. Thus, it is possible that subtypes of USH2 other than those carrying USH2A mutations may exhibit a greater proportion and/or severity of olfaction deficits than observed in RUSH2A.
Preclinical studies have shown Usher protein complex expression in the ciliary region of the murine olfactory epithelium (Jansen et al., 2016). Significantly abnormal electro-olfactograms have been observed in mice carrying mutant Usher genes: olfactory defects were especially severe in USH1C (harmonin) and USH1G (SANS) deficient mouse models but were not observed in a mouse model of MYO7A-related USH1B. Furthermore, only certain characteristics of the electro-olfactogram were abnormal in some of the mice, and in some cases, they were rather subtle (Jansen et al., 2016). Although mice rely on smell function far more than humans and there may be important inter-species differences in olfaction deficits resulting from Usher protein defects, a spectrum of olfaction abnormalities may exist among various forms of Usher syndrome. No model of USH2A-related disease was included in the investigation by Jansen et al. (Jansen et al., 2016) and it is not presently known whether a defect in USH2A is associated with an olfaction deficit in mice. Our findings predict either no deficit or a milder/subtler deficit in these mice than has been thus far observed in Usher syndrome models.
The UPSIT investigates odor identification (I) in a very reproducible and comprehensive fashion (40 separate odors vs 16 in the “Sniffin’ Sticks” test) and correlates well with olfactory thresholds and other measures of olfactory function. Prior to RUSH2A, an olfaction deficit was seen only with the UPSIT (Zrada et al., 1996), which has a higher reliability and sensitivity than the “Sniffin’ Sticks” test (Doty, 2007). While patients with USH2A-related retinal disease do not appear to have significant smell identification deficits, other subtypes of USH2 may, and may have been overrepresented in prior studies (Zrada et al., 1996). A recent larger study of genetically uncharacterized patients (Ribeiro et al., 2016) documented consistently abnormal D scores for both USH1 and USH2 patients, but not T scores. Thus, consistent with results suggested by the investigations conducted on mice (Jansen et al., 2016), it is possible that only certain olfaction characteristics may be abnormal in humans with Usher syndrome. In summary, the RUSH2A participants did not consistently exhibit smell identification deficits as measured by the UPSIT. However, a systematic investigation of genetically well-characterized USH1 and USH2 patients is warranted to determine which subtypes of Usher syndrome, if any, exhibit olfaction deficits (Jansen et al., 2016). If present, olfaction deficits could potentially add a third sensory dimension to the dual visual-auditory compromise that characterizes Usher syndrome.
Neither olfaction nor SNHL parameters were significantly associated with age, sex, race/ethnicity or smoking status at study baseline (with the exception that age was significantly associated with 4F-PTAs in ARRP participants). Olfaction was not associated with any visual functional or structural measures, and there was no correlation between UPSIT and 4F-PTA thresholds. All olfactory tests were completed by February 2019, prior to the discovery of the COVID-19 virus commonly associated with hyposmia and anosmia (Meng, Deng, Dai, & Meng, 2020).
In conclusion, USH2A-related USH2 participants did not show normal age-related increases in SNHL, and ARRP participants had higher pure-tone thresholds than a normative population. Amnestic accounts from this cohort suggest some patients with USH2A may pass a newborn screening for hearing loss. Olfaction was not significantly worse than normal in participants exhibiting either USH2A-related USH2 or ARRP by UPSIT score criteria. Pathogenic variants in USH2A variably affect the cochlea, and their impact on olfaction is modest as judged by UPSIT in the current study. Additional measures of olfactory function, such as olfactory thresholds, might be assessed in the future to determine if they are more sensitive to such pathogenic variants (e.g., Doty et al., 2019). However, in general, such tests are highly correlated with the UPSIT and the notion that threshold tests are primarily a measure peripheral olfactory neural function, i.e., olfactory epithelium, is questionable (Doty & Laing, 2015).
Supplementary Material
Acknowledgments:
Katarina Stingl and Isabelle Audo are members ot the ERN-EYE (European Reference Network for Rare Eye Diseases). This work was supported, in part, by the Intramural Research Programs of the NIH, NIDCD and NEI, NEI-P30 EY002162, Research to Prevent Blindness, Inc., New York, NY (unrestricted funds to UCSF and Duke Eye Center).
Funding/Support: Funded by Foundation Fighting Blindness
Conflict of Interest Statement: Alessandro Iannaccone receives financial support to his institution from Applied Genetic Technologies Corporation (AGTC), Allergan, 4D Molecular Therapeutics, ProQR Therapeutics, Stargazer Pharmaceuticals, MeiraGTx/Janssen and Acucela, is a consultant for IQVIA, Guidepoint Clinical, Frontera Therapeutics, Endogena, Evolution Medical, Kairos Ventures, Teladoc Health, Gyroscope, Ocugen, Rhythm Pharmaceuticals, ClearView Healthcare Partners, GLG, Janssen, M. Arkin Ltd., and serves as a board member for Alia Therapeutics. Richard L. Doty is president and major shareholder of Sensonics International, the manufacturer and distributor of smell and taste tests, including the smell test used in this study. He is a consultant to Eisai Co., Ltd., Merck Pharmaceuticals, the Michael J. Fox Foundation for Parkinson’s Research, and Johnson & Johnson. He receives royalties from Cambridge University Press, Elsevier, Johns Hopkins University Press, McGraw-Hill, and John Wiley & Sons, Inc. Maureen G. Maguire receives payment to her institution from the National Eye Institute for all support for the present manuscript, Jacque L. Duncan receives all support for the present manuscript from Foundation Fighting Blindness where she serves as chair, scientific advisory board; chair, Consortium executive committee; Study chair, RUSH2A Study, 4D Therapeutics, consultant, AGTC Therapeutics, data safety monitoring committee member, DTx Pharma, consultant, Editas, consultant, Astellas Institute for Regenerative Medicine, consultant, Eloxx, consultant, Eyevensys, consultant, Gyroscope Scientific advisory board member, ProQR Therapeutics, Data safety monitoring committee member
Spark Therapeutics, Data safety monitoring committee member, SparingVision, Scientific advisory board member, Vedere Bio Scientific advisory board member, Grants or contracts for clinical trial support are received from Acucela, Allergan/Abbvie, Biogen/Nightstarx, consulting fees are received from 4D Therapeutics, Editas, DTx Pharma, Astellas Institute for Regenerative Medicine. Eloxx, Eyevensys, participation on a data safety monitoring board from AGTC Therapeutics, ProQR Therapeutics, Spark Therapeutics. Gavin M. Bidelman receives grant support to institution where he serves as a P.I. from National Institutes of Health (NIH-NIDCD-R01DC016267). Katarina Stingl receives grants or contract payments to her institution from the Foundation Fighting Blindness, Kerstan Foundation, German Research Council, ProQR, ORA, ViGeneron, Novartis, she receives payment or honorarial for lectures, presentations, speaker bureaus, manuscript writing or educational events to her institution from Novartis, travel support is paid to her from ProQR, Novartis, ERN-EYE, French Retinal Clinical Research Network. Janet K. Cheetham receives payments to her personally from the Foundation Fighting Blindness for all support for the present manuscript, receives consulting payments to her personally from Foundation Fighting Blindness, and DTx Pharma, support for travel and attending meetings is received from Foundation Fighting Blindness.
Footnotes
Data Availability Statement: A de-identified database is available upon request through the public domain on the FFB/Jaeb public website.
References
- Abadie C, Blanchet C, Baux D, Larrieu L, Besnard T, Ravel P, . . . Roux AF (2012). Audiological findings in 100 USH2 patients. Clin Genet, 82(5), 433–438. doi: 10.1111/j.1399-0004.2011.01772.x [DOI] [PubMed] [Google Scholar]
- Adato A, Vreugde S, Joensuu T, Avidan N, Hamalainen R, Belenkiy O, . . . Lancet D. (2002). USH3A transcripts encode clarin-1, a four-transmembrane-domain protein with a possible role in sensory synapses. Eur J Hum Genet, 10(6), 339–350. doi: 10.1038/sj.ejhg.5200831 [DOI] [PubMed] [Google Scholar]
- Armel KC, & Ramachandran VS (1999). Acquired synesthesia in retinitis pigmentosa. Neurocase, 5(4), 293–296. doi: 10.1080/13554799908411982 [DOI] [Google Scholar]
- Beck RW, Moke PS, Turpin AH, Ferris FL 3rd, SanGiovanni JP, Johnson CA, . . . Kraker RT (2003). A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol, 135(2), 194–205. doi: 10.1016/s0002-9394(02)01825-1 [DOI] [PubMed] [Google Scholar]
- Birch DG, Cheng P, Duncan JL, Ayala AR, Maguire MG, Audo I, . . . Group f. t. F. F. B. C. I. (2020). The RUSH2A Study: Best-Corrected Visual Acuity, Full-Field Electroretinography Amplitudes, and Full-Field Stimulus Thresholds at Baseline. Transl Vis Sci Technol, 9(11), 9–9. doi: 10.1167/tvst.9.11.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Kelly F, Jaijo T, Aller E, Avila-Fernandez A, López-Molina MI, Giménez A, . . . Ayuso C. (2015). Clinical aspects of Usher syndrome and the USH2A gene in a cohort of 433 patients. JAMA Ophthalmol, 133(2), 157–164. doi: 10.1001/jamaophthalmol.2014.4498 [DOI] [PubMed] [Google Scholar]
- Doty RL (1995). The Smell Identification Test™ Administration Manual. Haddon Heights, NJ: Sensonics, Inc. [Google Scholar]
- Doty RL (2007). Office Procedures for Quantitative Assessment of Olfactory Function. American Journal of Rhinology, 21(4), 460–473. doi: 10.2500/ajr.2007.21.3043 [DOI] [PubMed] [Google Scholar]
- Doty RL, Frye RE, & Agrawal U. (1989). Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Perception & Psychophysics, 45(5), 381–384. doi: 10.3758/BF03210709 [DOI] [PubMed] [Google Scholar]
- Doty RL, & Laing DG (2015). Psychophysical measurement of human olfactory function. . In Handbook of Olfaction and Gustation (Doty RL ed., pp. 229–261). Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Doty RL, Shaman P, & Dann M. (1984). Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav, 32(3), 489–502. doi: 10.1016/0031-9384(84)90269-5 [DOI] [PubMed] [Google Scholar]
- Doty RL, Wylie C, Potter M, Beston R, Cope B, & Majam K. (2019). Clinical validation of the olfactory detection threshold module of the Snap & Sniff® olfactory test system. Int Forum Allergy Rhinol, 9(9), 986–992. doi: 10.1002/alr.22377 [DOI] [PubMed] [Google Scholar]
- Duncan JL, Liang W, Maguire MG, Audo I, Ayala AR, Birch DG, . . . Sahel J-A (2020). Baseline Visual Field Findings in the RUSH2A Study: Associated Factors and Correlation with Other Measures of Disease Severity. Am J Ophthalmol, 219, 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eudy JD, Weston MD, Yao S, Hoover DM, Rehm HL, Ma-Edmonds M, . . . Sumegi J. (1998). Mutation of a gene encoding a protein with extracellular matrix motifs in Usher syndrome type IIa. Science, 280(5370), 1753–1757. doi: 10.1126/science.280.5370.1753 [DOI] [PubMed] [Google Scholar]
- Ferris FL 3rd, Kassoff A, Bresnick GH, & Bailey I. (1982). New visual acuity charts for clinical research. Am J Ophthalmol, 94(1), 91–96. [PubMed] [Google Scholar]
- Fields RR, Zhou G, Huang D, Davis JR, Möller C, Jacobson SG, . . . Sumegi J. (2002). Usher syndrome type III: revised genomic structure of the USH3 gene and identification of novel mutations. Am J Hum Genet, 71(3), 607–617. doi: 10.1086/342098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman GA, Kumar A, Joseph ME, Torok N, & Anderson RJ (1983). Usher’s syndrome. Ophthalmic and neuro-otologic findings suggesting genetic heterogeneity. Arch Ophthalmol, 101(9), 1367–1374. doi: 10.1001/archopht.1983.01040020369005 [DOI] [PubMed] [Google Scholar]
- Friedman TB, Schultz JM, Ahmed ZM, Tsilou ET, & Brewer CC (2011). Usher syndrome: hearing loss with vision loss. Adv Otorhinolaryngol, 70, 56–65. doi: 10.1159/000322473 [DOI] [PubMed] [Google Scholar]
- Gougoux F, Zatorre RJ, Lassonde M, Voss P, & Lepore F. (2005). A Functional Neuroimaging Study of Sound Localization: Visual Cortex Activity Predicts Performance in Early-Blind Individuals. PLOS Biology, 3(2), e27. doi: 10.1371/journal.pbio.0030027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgel RK, Jackler RK, Dobie RA, & Popelka GR (2012). A new standardized format for reporting hearing outcome in clinical trials. Otolaryngol Head Neck Surg, 147(5), 803–807. doi: 10.1177/0194599812458401 [DOI] [PubMed] [Google Scholar]
- Hmani-Aifa M, Arab SB, Kharrat K, Orten DJ, Boulila-Elgaied A, Drira M, . . . Ayadi H. (2002). Distinctive audiometric features between USH2A and USH2B subtypes of Usher syndrome. Journal of Medical Genetics, 39(4), 281. doi: 10.1136/jmg.39.4.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannaccone A, Mykytyn K, Persico AM, Searby CC, Baldi A, Jablonski MM, & Sheffield VC (2005). Clinical evidence of decreased olfaction in Bardet–Biedl syndrome caused by a deletion in the BBS4 Gene. American Journal of Medical Genetics Part A, 132A(4), 343–346. doi: 10.1002/ajmg.a.30512 [DOI] [PubMed] [Google Scholar]
- ISO 7029:2017 Acoustics — Statistical distribution of hearing thresholds related to age and gender. (2017). (3 ed.). [Google Scholar]
- Jansen F, Kalbe B, Scholz P, Mikosz M, Wunderlich KA, Kurtenbach S, . . . Osterloh S. (2016). Impact of the Usher syndrome on olfaction. Hum Mol Genet, 25(3), 524–533. doi: 10.1093/hmg/ddv490 [DOI] [PubMed] [Google Scholar]
- Joensuu T, Hämäläinen R, Yuan B, Johnson C, Tegelberg S, Gasparini P, . . . Sankila E-M (2001). Mutations in a Novel Gene with Transmembrane Domains Underlie Usher Syndrome Type 3. The American Journal of Human Genetics, 69(4), 673–684. doi: 10.1086/323610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiserman N, Obolensky A, Banin E, & Sharon D. (2007). Novel USH2A mutations in Israeli patients with retinitis pigmentosa and Usher syndrome type 2. Arch Ophthalmol, 125(2), 219–224. doi: 10.1001/archopht.125.2.219 [DOI] [PubMed] [Google Scholar]
- Klein M, & Birch DG (2009). Psychophysical assessment of low visual function in patients with retinal degenerative diseases (RDDs) with the Diagnosys full-field stimulus threshold (D-FST). Doc Ophthalmol, 119(3), 217–224. doi: 10.1007/s10633-009-9204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulaga HM, Leitch CC, Eichers ER, Badano JL, Lesemann A, Hoskins BE, . . . Katsanis N. (2004). Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet, 36(9), 994–998. doi: 10.1038/ng1418 [DOI] [PubMed] [Google Scholar]
- Le Quesne Stabej P, Saihan Z, Rangesh N, Steele-Stallard HB, Ambrose J, Coffey A, . . . Bitner-Glindzicz M. (2012). Comprehensive sequence analysis of nine Usher syndrome genes in the UK National Collaborative Usher Study. J Med Genet, 49(1), 27–36. doi: 10.1136/jmedgenet-2011-100468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenassi E, Vincent A, Li Z, Saihan Z, Coffey AJ, Steele-Stallard HB, . . . Webster AR (2015). A detailed clinical and molecular survey of subjects with nonsyndromic USH2A retinopathy reveals an allelic hierarchy of disease-causing variants. Eur J Hum Genet, 23(10), 1318–1327. doi: 10.1038/ejhg.2014.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur P, & Yang J. (2015). Usher syndrome: Hearing loss, retinal degeneration and associated abnormalities. Biochim Biophys Acta, 1852(3), 406–420. doi: 10.1016/j.bbadis.2014.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, & Bach M. (2015). ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol, 130(1), 1–12. doi: 10.1007/s10633-014-9473-7 [DOI] [PubMed] [Google Scholar]
- McEwen DP, Koenekoop RK, Khanna H, Jenkins PM, Lopez I, Swaroop A, & Martens JR (2007). Hypomorphic CEP290/NPHP6 mutations result in anosmia caused by the selective loss of G proteins in cilia of olfactory sensory neurons. Proc Natl Acad Sci U S A, 104(40), 15917–15922. doi: 10.1073/pnas.0704140104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee TL, Seyedahmadi BJ, Sweeney MO, Dryja TP, & Berson EL (2010). Novel mutations in the long isoform of the USH2A gene in patients with Usher syndrome type II or non-syndromic retinitis pigmentosa. J Med Genet, 47(7), 499–506. doi: 10.1136/jmg.2009.075143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Deng Y, Dai Z, & Meng Z. (2020). COVID-19 and anosmia: A review based on up-to-date knowledge. Am J Otolaryngol, 41(5), 102581. doi: 10.1016/j.amjoto.2020.102581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MH, Iannaccone A, & Bidelman GM (2017). A pilot investigation of audiovisual processing and multisensory integration in patients with inherited retinal dystrophies. BMC Ophthalmology, 17(1), 240. doi: 10.1186/s12886-017-0640-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center on Deaf-Blindness. (2021). Causes of Deaf-Blindness. Retrieved from https://www.nationaldb.org/info-center/overview/causes/
- Nishimura DY, Fath M, Mullins RF, Searby C, Andrews M, Davis R, . . . Sheffield VC (2004). Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci U S A, 101(47), 16588. doi: 10.1073/pnas.0405496101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M, Oishi A, Gotoh N, Ogino K, Higasa K, Iida K, . . . Yoshimura N. (2014). Comprehensive molecular diagnosis of a large cohort of Japanese retinitis pigmentosa and Usher syndrome patients by next-generation sequencing. Invest Ophthalmol Vis Sci, 55(11), 7369–7375. doi: 10.1167/iovs.14-15458 [DOI] [PubMed] [Google Scholar]
- Pennings RJ, Huygen PL, Weston MD, van Aarem A, Wagenaar M, Kimberling WJ, & Cremers CW (2003). Pure tone hearing thresholds and speech recognition scores in Dutch patients carrying mutations in the USH2A gene. Otol Neurotol, 24(1), 58–63. doi: 10.1097/00129492-200301000-00013 [DOI] [PubMed] [Google Scholar]
- Reisser CF, Kimberling WJ, & Otterstedde CR (2002). Hearing loss in Usher syndrome type II is nonprogressive. Ann Otol Rhinol Laryngol, 111(12 Pt 1), 1108–1111. doi: 10.1177/000348940211101208 [DOI] [PubMed] [Google Scholar]
- RetNet. (January 21, 2021). RetNet: Summaries of Genes and Loci Causing Retinal Diseases. Retrieved from https://sph.uth.edu/RetNet/
- Ribeiro JC, Oliveiros B, Pereira P, António N, Hummel T, Paiva A, & Silva ED (2016). Accelerated age-related olfactory decline among type 1 Usher patients. Scientific reports, 6, 28309–28309. doi: 10.1038/srep28309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivolta C, Sweklo EA, Berson EL, & Dryja TP (2000). Missense mutation in the USH2A gene: association with recessive retinitis pigmentosa without hearing loss. Am J Hum Genet, 66(6), 1975–1978. doi: 10.1086/302926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi AM, Cohn ES, Kimberling WJ, Halvarsson G, & Möller C. (2013). Expressivity of hearing loss in cases with Usher syndrome type IIA. Int J Audiol, 52(12), 832–837. doi: 10.3109/14992027.2013.839885 [DOI] [PubMed] [Google Scholar]
- Sadeghi M, Cohn ES, Kelly WJ, Kimberling WJ, Tranebjoerg L, & Möller C. (2004). Audiological findings in Usher syndrome types IIa and II (non-IIa). Int J Audiol, 43(3), 136–143. doi: 10.1080/14992020400050019 [DOI] [PubMed] [Google Scholar]
- Seeliger M, Pfister M, Gendo K, Paasch S, Apfelstedt-Sylla E, Plinkert P, . . . Zrenner E. (1999). Comparative study of visual, auditory, and olfactory function in Usher syndrome. Graefes Arch Clin Exp Ophthalmol, 237(4), 301–307. doi: 10.1007/s004170050237 [DOI] [PubMed] [Google Scholar]
- Seyedahmadi BJ, Rivolta C, Keene JA, Berson EL, & Dryja TP (2004). Comprehensive screening of the USH2A gene in Usher syndrome type II and non-syndromic recessive retinitis pigmentosa. Exp Eye Res, 79(2), 167–173. doi: 10.1016/j.exer.2004.03.005 [DOI] [PubMed] [Google Scholar]
- Smith RS, Doty RL, Burlingame GK, & McKeown DA (1993). Smell and taste function in the visually impaired. Perception & Psychophysics, 54(5), 649–655. doi: 10.3758/BF03211788 [DOI] [PubMed] [Google Scholar]
- The Hereditary Hearing Loss Homepage. (2021). Retrieved from https://hereditaryhearingloss.org/usher
- van Aarem A, Pinckers AJLG, Kimberling WJ, Huygen PLM, Bleeker-Wagemakers EM, & Cremers WRJ (1996). Stable and Progressive Hearing Loss in Type 2A Usher’s Syndrome. Annals of Otology, Rhinology & Laryngology, 105(12), 962–967. doi: 10.1177/000348949610501206 [DOI] [PubMed] [Google Scholar]
- Year 2007 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs. (2007). Pediatrics, 120(4), 898. doi: 10.1542/peds.2007-2333 [DOI] [PubMed] [Google Scholar]
- Year 2019 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs. (2019). Journal of Early Hearing Detection and Intervention, 4(2), 1–44. doi: 10.15142/fptk-b748 [DOI] [Google Scholar]
- Zrada SE, Braat K, Doty RL, & Laties AM (1996). Olfactory loss in Usher syndrome: Another sensory deficit? Am. J. Med. Genet, 64(4), 602–603. doi: 10.1002/ajmg.1320640402 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.