Abstract
Background:
Use of AlloDerm™ is highly suggested for the treatment of deep burns and burn sequela reconstruction. Scar formation and contracture are recognized as long-term consequences of split-thickness skin autografting, which is applied for full-thickness burn injuries. Mature fibroblasts, in the absence of dermis, seem to secrete collagen in the reformed scar pattern.
Objective:
To process AlloDerm™ from fresh allograft and use it as a dermal substitute for covering deep wounds in burn patients and evaluate its effectiveness.
Methods:
In this case-series, 7 patients with deep burn wounds involving different locations on the body surface were exposed to combined AlloDerm™ (processed from fresh human allograft) with thin split thickness skin autograft on it. On the 5th post-operative day, wound dressings were changed to evaluate the graft survival with the human acellular dermal matrix scaffold. To determine the skin profiles, follow-ups continued for at least 6 months.
Results:
The results showed excellent graft take, good elasticity, acceptable thickness, and little contracture and scarring according to fix surgeon assessment in 6 patients. Graft rejection happened only in one patient with chronic electrical injury.
Conclusion:
AlloDerm™ derived from cadaver skin and combination of it with thin split thickness skin auto grafting constitute a cost-effective and favorable option for the treatment of deep burn wounds in our center, considering the increased tendency of the population towards organ donation in the event of brain death.
Key Words: Skin, Graft, AlloDerm™, Burn, Wound
INTRODUCTION
Autologous full-thickness or split-thickness (meshed or non-meshed) skin graft provides the best burn wound coverage. However, performance of this method is limited due to the scarcity of available resources, especially for major burns [1, 2]. Donor site problems have always been a dilemma for the patient and surgeon. Thick donor sites do not heal without scar or contracture formation. Nearly all of these poor results are due to lack of dermis. Dermis, a cellular layer between the subcutaneous tissue and epidermis, protects the body against stress and human appearance is mostly related to it. The dermal layer is responsible for durability and elasticity power of the skin [3]. So donor site morbidities, such as scar formation, are the main autograft application concerns, especially when thick skin is harvested [4].
Thick allograft can compensate dermal loss but unfortunately the immunogenic characteristics of the skin allograft limit its potential use in the treatment of full-thickness deep burns. It should be noted that the skin allograft will ultimately be rejected [4, 5]. The non-cellular dermal components, including extracellular matrix collagens and proteins, seem to be non-immunogenic.
There are multiple skin substitutes in the market; almost all of them are expensive [6]. AlloDerm™ as a dermal substitute is produced as an acellular dermal matrix from fresh human allograft in a process, which removes the cells and epidermis from the dermis without changing the extracellular matrix structure.
Clinical studies have shown that AlloDerm™ is an effective dermal substitute; however, in a one-step procedure, the split-skin graft take rate on the AlloDerm™ surface is relatively lower than that of the split-skin graft alone [7].
Processing of AlloDerm™ from fresh allograft and combination of AlloDerm™ with thin split thickness skin auto-grafting can be a cost-effective and favorable option for covering deep wounds in our center considering the increased tendency of population towards organ donation in the event of brain death. This study aimed at evaluating the potential of split-thickness skin grafting with AlloDerm™ for covering deep burn wounds.
MATERIALS AND METHODS
Patient Selection and Surgical Procedure
The present case-series study was designed and included seven patients with deep burn wounds or deep donor sites involving different locations in the body who had referred to Shiraz Amir Almomenin Burn Center, Shiraz, southern Iran. The patients underwent one stage use of combined AlloDerm™ (processed from fresh allograft in our institution) with thin skin autograft only (composite graft) between February, 2016 and March, 2018.
In seven patients, composite graft was fixed and dressed with Vaseline gauze. Wound dressing was changed on the 5th post-operative day and examined by a fixed burn surgeon to evaluate the percentage of graft take. Follow-ups were performed to evaluate the skin profile, including thickness, elasticity, contracture, scarring and discoloration using Vancouver scar scale.
Informed written consents were obtained from the patients. The Ethics Committee of Shiraz University of Medical Sciences approved this study.
Harvesting, Processing, and Storage
In this study, skin allograft was harvested from dead-brain patients in the operation room of Namazi Hospital with power dermatome (0.3 thickness) under aseptic conditions. Upon harvesting, the skin was added to sterile 50% glycerol consisting of penicillin and gentamicin [8]. Serological screening tests for HIV I and II, syphilis, HBsAg, HCV, and HTLV I and II were conducted previously in the Organ Transplantation Center of Namazi Hospital, Iran.
The skin was kept at 4–8 °C; 1:1 meshing was performed for better nutrition and viability of the allograft; it was divided into 5×5 cm pieces. Another benefit of 1:1 meshing of allograft and subsequently formed AlloDerm™ is better thin autograft take placed on it due to better imbibition of fluid and nutrients via fine meshed AlloDerm™. For further processing, decellularizing solution (10 mM EDTA, 2% sodium deoxycholate, and 10 mM HEPES buffer), de-epidermizing solution (0.5% Triton X100, 1 M NaCl, and 10 mM EDTA), and washing solution (PBS containing 0.5% w/v Triton X100 and 10 mM EDTA) were used within 24–48 hours.
For this purpose, after removing the donor skin from the transportation container, it was placed on a piece of support with its reticular side down. Next, a piece of gauze (with an appropriate size) was added to the skin epidermal side. The skin was placed in a petri dish with its reticular side down, and de-epidermizing solution (50 mL) was added. The petri dish was then incubated at 37±2 °C for 24 hours. Following incubation, the petri dish was transferred to a laminar flow hood for de-epidermization.
After removing the gauze, the epidermis was held by forceps and pulled away from the dermis. Next, the excess de-epidermizing solution was aspirated, and dermis was washed with the tissue wash solution (50 mL) in the same petri dish. The petri dish was placed on a rotator for 5 min at 40±5 RPM at 20–26 °C. It was then transferred to the laminar flow hood for aspirating the tissue wash solution; this procedure was repeated two more times.
In the following step, the dermis was treated with the decellularizing solution (50 mL), and the petri dish was placed on a rotator for one hour at 40±5 RPM at 20–26 °C. After removing the decellularizing solution via aspiration, the dermis was rinsed in the tissue wash solution (50 mL). (Fig 1) The petri dish was then placed on a rotator at 40±5 RPM for 5 min (20–26 °C). Via aspiration, the tissue wash solution was removed. The washing procedure was repeated twice. After processing the AlloDerm™, it was added to a sterile bottle, containing gentamicin, 50% glycerol, and penicillin. Sterilization of solutions was done by vacuum filter (0.22 µm pore size) and all processing steps was done in a clean room at Shiraz Medical Faculty in aseptic condition.
Figure 1.
De-epidermization procedure for preparing fresh AlloDerm™. H&E staining approved the acellular dermal matrix with no cells
Hematoxylin and Eosin (H&E) Staining
H&E staining confirmed the acellularity of the prepared AlloDerm™ compared to normal skin (Fig 1).
Surgical Procedure
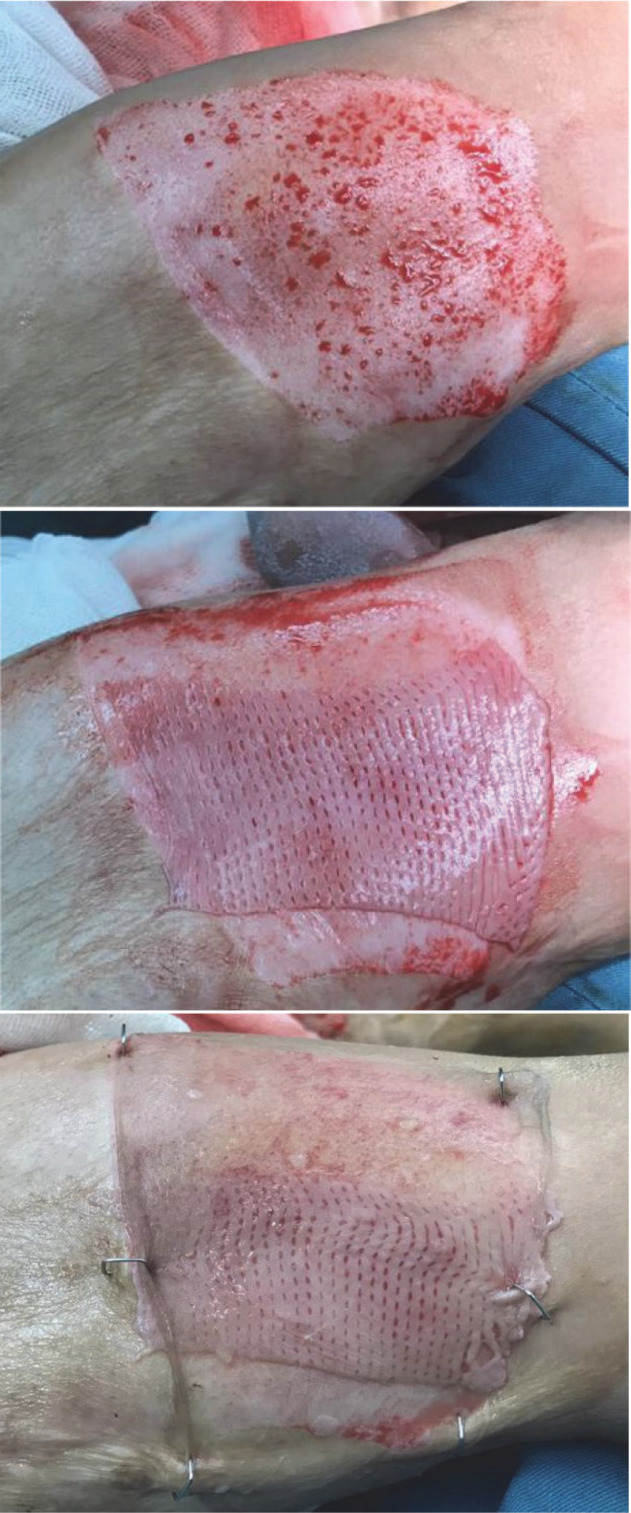
After preparation and drape of the operative field and under general or spinal anesthesia, and after debridement or excision of the wound to viable wound with pinpoint bleeding and after homeostasis a patch of previously prepared AlloDerm™ was inserted in the wound bed. Then, a thin split-thickness autograft (0.2 mm), which was harvested from the non-burnt area of the thigh, was grafted on the wound over the AlloDerm™ and fixed with skin staple. After fixing the graft, it was covered with Vaseline gauze and dressing applied. Wound dressings were removed after 5 days; wounds were examined by a fixed burn surgeon (Fig 2).
Figure 2.
Left panel: Deep wound of the right thigh; Middle panel: 1:1 meshed AlloDerm™ on deep wound of right thigh; Right panel: Thin autograft on AlloDerm™ fixed with skin staples
RESULTS
Seven (2 female and 5 male) patients were enrolled in this study. The mean age of patients was 31.3 years. The mean burn size was 2.3% TBSA (Table 1). Graft rejection was happened in only one patient.
Table 1.
Clinical characteristics of the patients
| Sex | Age | TBSA% | Etiology | Used AlloDerm™ area | Burn degree | Graft take after 1 week | Long-term follow-up | ||
|---|---|---|---|---|---|---|---|---|---|
| Thickness | Elasticity | Contracture | |||||||
| Male | 26 | 1 | Electrical injury | Left hand | Third degree | 100% | Acceptable | Good | Little |
| Male | 24 | 1 | Water burn injury | Right hand | Third degree | 100% | Acceptable | Good | Little |
| Male | 46 | 1 | Chronic electrical injury | Right foot | Third degree | 0% | — | — | — |
| Male | 7 | 2 | Burn injury of face | Right thigh donor site | Full thickness | 100% | Acceptable | Good | No |
| Male | 4 | 6 | Post burn contracture of toes | Right thigh donor site | Full thickness | 100% | Acceptable | Good | No |
| Female | 56 | 2.5 | Chronic burn of right hand | Right thigh donor site | Full thickness | 100% | Acceptable | Good | No |
| Female | 56 | 2.5 | Post burn contracture of right wrist | Right wrist | Full thickness cavity | 100% | Acceptable | Good | Little |
According to a fixed surgeon assessment using the Vancouver scar scale score in all patients, this case series showed excellent graft take, good elasticity, acceptable thickness and little contracture and scarring. The skin autografts appeared darker in color in short-time follow-up visit; no significant darkness was seen in the long-term follow-up (Fig 3).
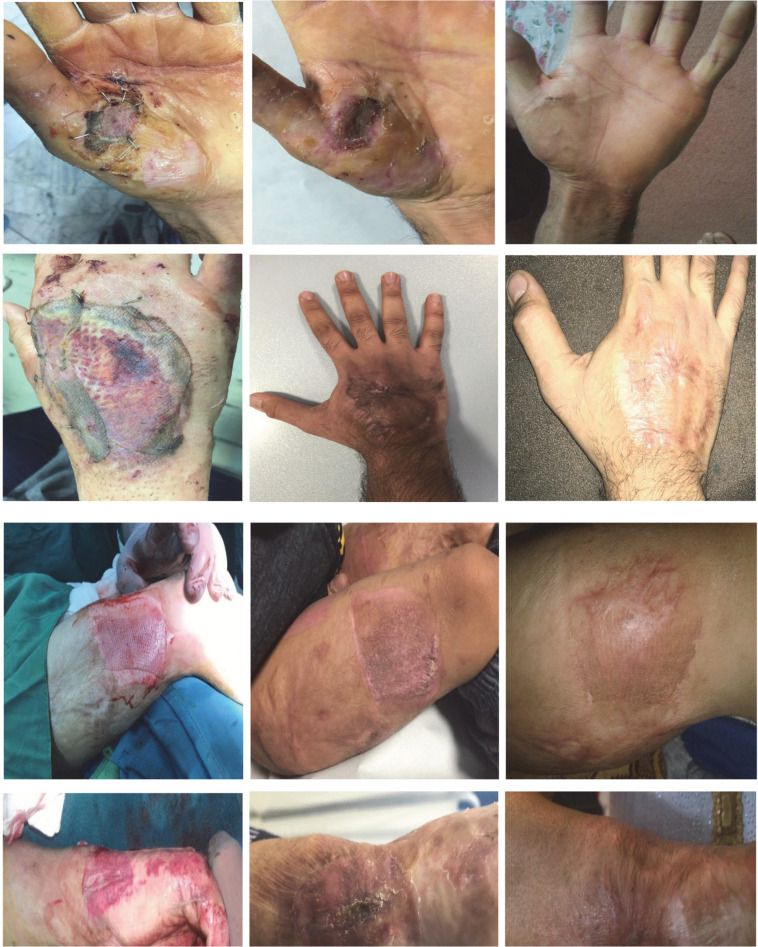
Figure 3.
First row panels) Post-operative follow-up (1, 3 and 12 weeks) photographs of case 1 with 2×2 cm AlloDerm™ graft plus split thickness skin graft on the left hand burn site cavity. Second row panels) Post-operative follow-up (1 week, 4 weeks, and 24 months) photographs of case 2 with 4×4 cm AlloDerm™ graft plus split thickness skin graft on the right hand 4th degree burn. Third row panels) Intra- and post-operative follow-up (3 weeks and 5 months) photographs of case 4 with 7×7 cm AlloDerm™ graft plus split thickness skin graft on deep donor site. Fourth row panels) Intra- and post-operative follow-up (1 and 6 months) photographs of case 7 with 3×3 cm AlloDerm™ graft plus split thickness skin graft on the right wrist deep burn site
DISCUSSION
Pliability and quality of skin is mostly related to the dermis. After dermal injury, regeneration is usually abnormal with scar formation. Full-thickness or thick split-thickness skin graft is the best way to compensate dermal defects, however, donor healing will be a new problem [9-11]. Dermal substitutes are the best alternatives for patient’s dermis. Resurfacing of dermal defect can be combined with the use of dermal regeneration templates that adds pliability to the grafted area and improves the skin quality [12,13]. Acellular matrices serve as a scaffold, which may assist in forming some of the structure, components, and signaling mechanism to assist in wound healing and regeneration [14-17].
AlloDerm™ is a de-cellularized allogenic dermal component without epidermis and dermal cells with no or little antigenicity. So, AlloDerm™ can be used in other individuals without fear of rejection with no need for immunosuppression.
The most popular dermal substitute in the market is Integra. Integra is placed on excised wound, and the wound is allowed to generate a new auto-dermis for approximately two weeks. Two weeks later, new dermis will be grated with a thin split-thickness skin after removing the superficial silicone layer of Integra. The disadvantages include the necessity of a two-staged procedure to complete the resurfacing and the need for meticulous wound care and also high cost of Integra.
Dermagraft and Transcyte are two kinds of cellular dermal products that due to their cellularity are antigenic and are used to increase wound healing when they degenerate. So they do not work as a permanent dermal substitute. Another dermal substitute is Matriderm, a product that consists of bovine collagen. Matriderm is a one-stage dermal substitute; however, it has animal source and is expensive.
There are small case series describing the use of AlloDerm™ for burn wound management [7, 12, 13]. Skin appearance is related to dermal layer and spontaneous dermal regeneration is concomitant with more scar formation and contraction due to low primary collagen. Scar formation is lesser when we use thicker skin graft. Damaged dermis cannot regenerate to native one and patients have limited donor sites for harvesting thicker skins. All these indicate the importance of dermis and the necessity for an ideal substitute such as AlloDerm™.
Due to unavailability of Integra and Matriderm in our country, Iran, and their high costs and need for two stages of operation for Integra, we tried to test preparing and using AlloDerm™ in our institution. Processing was performed; AlloDerm™ was derived from fresh allograft with thin autograft onlay as a full-thickness or thick split-thickness skin composite graft to cover deep burn wounds (3rd and 4th degrees) in one stage performed at our institute.
Shalom, et al, evaluated the application of acellular dermal matrix and thin autograft for grafting full-thickness burns over the knee and elbow joints in eight patients with good ROM and 75–100% graft take [18]. Also, SukJoon, et al, evaluated 27 patients with wide dyspigmented scar contracture in the upper extremity, undergoing combined thin skin and AlloDerm™ grafting. They reported complete graft take with mild contracture [19]. Moreover, a multi-center clinical study on 43 patients evaluated the functional capacity of an acellular allograft dermal matrix as a permanent dermal transplant in full-thickness and deep partial-thickness burns. According to wound assessments over time, thin split-thickness autografts plus AlloDerm™ were similar to thicker split-thickness autografts [7]. Another study by Bing Tang, et al, on 16 patients with deep facial burns during 2006–2009 evaluated one-stage, razor-thin skin autografting on the human acellular dermal matrix scaffold. Based on the results, the composite skin autograft take was 97.3% on the 12th post-operative day; the skin autografts had a normal color, good elasticity, and soft texture. Little scarring was observed on the skin junctures [20].
The result of our study is similar to other studies with respect to graft take, contracture formation and pliability of the graft. Only in one patient, the graft was rejection, probably due to poor blood supply of the wound bed. The graft site was dark in post-operative follow up but in long-term follow up visit there was no significant difference. Finally, processing of AlloDerm™ from allograft as a dermal substitute and using it with thin split thickness skin autograft on lay in one stage is an effective, cost-beneficial and favorable option for coverage of deep wounds, or when there is a need for thick skin graft. Processing and usage of AlloDerm™ in this manner makes it as an excellent substitute for more expensive and complex dermal products such as Integra with resolving the need for a two-stage operation.
Although processing and clinical usage of AlloDerm™ was performed for the first time in our institution, we think all the centers which treat burn wounds need some types of AlloDerm™ for burn reconstruction. AlloDerm™ preparation from fresh allograft is feasible in burn centers and comparing to other commercial products, it is less expensive.
This procedure is a good choice for burn reconstruction with less donor site problem. It also can be used for reconstruction of burn scars or donor sites. AlloDerm™ is applicable to other fields such as hernia repair, tendon coverage, and closure of fistula.
CONFLICTS OF INTEREST:
None declared.
ACKNOWLEDGMENTS
The present article was extracted from the thesis written by Abdolhamid Najafi.
References
- 1.Kearney L, Francis EC, Clover AJ. New technologies in global burn care-a review of recent advances. Int J Burns Trauma. 2018;8:77. [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammadi AA, Jafari SMS, Kiasat M, et al. Effect of fresh human amniotic membrane dressing on graft take in patients with chronic burn wounds compared with conventional methods. Burns. 2013;39:349–53. doi: 10.1016/j.burns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Gilaberte Y, Prieto-Torres L, Pastushenko I, Juarranz A. Nanoscience in Dermatology. Elsevier; 2016. Anatomy and Function of the Skin; pp. 1–14. [Google Scholar]
- 4.Agarwal P, Sharma D, Agarwal A, Patel L. Can removal of epidermis delay rejection of cadaveric dermis-only allograft? A proof of concept study. Indian Journal of Burns. 2018;26:66. [Google Scholar]
- 5.Sobhani Z, Mahdavi-Mazdeh M, Ayaz M, et al. The Use of Partial Thickness Skin Allograft as a Temporary Biologic Dressing of Burns: 1059. Transplantation. 2012;94:488. [Google Scholar]
- 6.Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21:243–8. doi: 10.1016/0305-4179(95)93866-i. [DOI] [PubMed] [Google Scholar]
- 7.Wainwright D, Madden M, Luterman A, et al. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. The Journal of burn care & rehabilitation. 1996;17:124–36. doi: 10.1097/00004630-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Livesey S, Del Campo A, Nag A, et al. Method for processing and preserving collagen-based tissues for transplantation: Google Patents. 2006. [Google Scholar]
- 9.Ayaz M, Karami MY, Deilami I, Moradzadeh Z. Effects of early versus delayed excision and grafting on restoring the functionality of deep burn injured hands: A double blind, randomized parallel clinical trial. J Burn Care Res. 2019;40:451–6. doi: 10.1093/jbcr/irz033. [DOI] [PubMed] [Google Scholar]
- 10.Ayaz M, Keshavarzi A, Bahadoran H, et al. Comparison of the Results of Early Excision and Grafting between Children and Adults; A Prospective Comparative Study. Bull Emerg Trauma. 2017;5:179–83. [PMC free article] [PubMed] [Google Scholar]
- 11.Ayaz M, Bahadoran H, Arasteh P, Keshavarzi A. Early Excision and Grafting versus Delayed Skin Grafting in Burns Covering Less than 15% of Total Body Surface Area; A Non- Randomized Clinical Trial. Bull Emerg Trauma. 2014;2:141–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Lattari V, Jones LM, Varcelotti JR, et al. The use of a permanent dermal allograft in full-thickness burns of the hand and foot: a report of three cases. J Burn Care Rehabil. 1997;18:147–55. doi: 10.1097/00004630-199703000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Callcut R, Schurr M, Sloan M, Faucher L. Clinical experience with Alloderm: a one-staged composite dermal/epidermal replacement utilizing processed cadaver dermis and thin autografts. Burns. 2006;32:583–8. doi: 10.1016/j.burns.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Cuono C, Langdon R, McGuire J. Use of cultured epidermal autografts and dermal allografts as skin replacement after burn injury. The Lancet. 1986;327:1123–4. doi: 10.1016/s0140-6736(86)91838-6. [DOI] [PubMed] [Google Scholar]
- 15.Heimbach D, Luterman A, Burke J, et al. Artificial dermis for major burns A multi-center randomized clinical trial. Ann Surg. 1988;208 doi: 10.1097/00000658-198809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purdue GF. Dermagraft-TC pivotal efficacy and safety study. J Burn Care Rehabil. 1997;18:S13–S4. doi: 10.1097/00004630-199701001-00007. [DOI] [PubMed] [Google Scholar]
- 17.Reyzelman A, Crews RT, Moore JC, et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. Int Wound J. 2009;6:196–208. doi: 10.1111/j.1742-481X.2009.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shalom A, Wong L, Munster A. Full-thickness burns across joints: results of coverage with acellular dermal matrix and thin autograft. Eur J Plast Surg. 2005;28:82–5. [Google Scholar]
- 19.Oh SJ, Kim Y. Combined AlloDerm® and thin skin grafting for the treatment of postburn dyspigmented scar contracture of the upper extremity. J Plast Reconstr Aesthet Surg. 2011;64:229–33. doi: 10.1016/j.bjps.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Tang B, Zhu B, Liang Y-Y, et al. Early escharectomy and concurrent composite skin grafting over human acellular dermal matrix scaffold for covering deep facial burns. Plast reconstr sur. 2011;127:1533–8. doi: 10.1097/PRS.0b013e31820a63e8. [DOI] [PubMed] [Google Scholar]