Abstract
Purpose
Patients’ chronic disease burden can influence the likelihood that providers will recommend cancer screening and that patients will participate in it. Using data from the STOP CRC pragmatic study, we examined associations between chronic disease burden and colorectal cancer screening recommendation and use.
Methods
Participating STOP CRC clinics (n = 26) received either usual care or training to implement a mailed fecal immunochemical test (FIT) outreach program. Selected clinic patients (n = 60,187 patients) were aged 50–74 and overdue for colorectal cancer screening. We used logistic regression to examine the associations between FIT recommendations and completion and patients’ chronic disease burden, calculated using the Charlson Comorbidity Index and the Chronic Illness and Disability Payment System.
Results
For each index, FIT recommendation odds were 8–9% higher among individuals with minimal chronic disease burden and 13–23% lower among individuals with high chronic disease burden (inverted U-shaped association). Among adults who were ordered a FIT, FIT completion odds were 20% lower for individuals with any, versus no, chronic condition, and diminished with increasing disease burden (inverse linear association).
Conclusions
Analysis showed an inverted U-shaped association between patients’ chronic disease burden and providers’ recommendation of a FIT, and an inverse linear association between patients’ chronic disease burden and FIT completion.
ClinicalTrials.gov registration:
Keywords: Multiple chronic conditions, colorectal cancer screening, Charlson comorbidity index, Chronic Illness and Disability Payment System, federally qualified health centers
Introduction
Colorectal cancer is a leading cause of cancer death in the United States and screening could reduce colorectal cancer mortality by more than 50 percent [1, 2]. Nevertheless, an estimated 28 million US adults aged 50–75 are not up-to-date with colorectal cancer screening recommendations in 2016 [3]. Many of these adults, an estimated 80%, experience one or more chronic health condition [4]. Little is known about how the presence and number of chronic conditions influences the use of screening services for colorectal cancer.
Previous investigations into patterns of colorectal cancer screening use among individuals with and without chronic health conditions have reported mixed results. Studies reporting higher colorectal cancer screening uptake generally attribute the pattern to individuals’ more frequent contact with the health care system, which creates multiple opportunities for providers to offer preventive care [5, 6]. Lower colorectal cancer screening uptake is often explained by the additional burden posed by chronic conditions in navigating the health system and in coordinating treatments for multiple conditions [7]. Moreover, providers who are focused on care for chronic conditions may overlook patients’ need for preventive services [8] or may, often appropriately, recommend the discontinuation of screening for individuals with chronic conditions who have a shortened lifespan and for whom the benefit of screening is less certain [9]. A limited number of recent studies have postulated an inverted U-shaped relationship, where preventive services use initially rises for individuals with a single chronic condition, then drops linearly with increasing disease burden [10].
While the use of preventive services is known to be influenced by both provider and patient behaviors, research is lacking on the influence of chronic conditions on recommendations of those services. Moreover, much of the research has relied on simple counts of co-morbid conditions or the Charlson comorbidity index (CCI), which is a commonly used weighted co-morbidity index [11]. Little is known about the relatively utility of other weighted indices, such as the Chronic Illness and Disability Payment System (CDPS) [12].
To address these literature gaps, we conducted a secondary analysis of data from the Strategies and Opportunities to Stop Colorectal Cancer (STOP CRC) study to examine the association between the presence and number of chronic conditions and completion of screening for colorectal cancer. We used electronic health record (EHR) data from participating STOP CRC federally qualified health center (FQHC) clinics (n = 26 clinics) on colorectal cancer screening test orders and completions to assess separately provider recommendations (i.e. fecal test orders) and patient use of colorectal cancer screening services (i.e. fecal test completion). We applied two comorbidity indices: the CCI and the CDPS. We chose these indices because CCI is the most used co-morbidity index for research,[13] and CDPS was developed using data from Medicaid enrollees (a large proportion of whom receive their care at FQHCs). Given the low completion rates of colorectal cancer screening generally, and specifically among adults who receive care at FQHCs [14], our findings may inform efforts to improve the delivery of preventive care services among the growing number of adults with one or more chronic conditions.
Materials and methods
STOP CRC was a large pragmatic study of mailed fecal immunochemical (FIT) outreach conducted in FQHC clinics (n = 8 health centers [26 clinics]) in Oregon and California. The study protocol and results have been reported previously [15]. Briefly, STOP CRC was a cluster-randomized study of an EHR-embedded mailed fecal immunochemical test (FIT) outreach program to improve rates of colorectal cancer screening [15, 16]. Participating clinics were randomized to either Usual Care (n = 13) or the EHR-embedded Intervention (n = 13) on February 4, 2014. Our primary outcome was the clinic-level proportion of eligible adults (accrued from February 4, 2014–February 3, 2015) who completed a FIT within 12 months or through August 3, 2015. The evaluation interval was February 4, 2014 – August 3, 2015, and data were gathered through August 3, 2017, for analysis of secondary endpoints (i.e., completion of follow-up colonoscopy). The findings showed significant but modest improvements in FIT completion in clinics assigned to the STOP CRC intervention [15]. The Institutional Review Board of Kaiser Permanente Northwest approved all study activities, and participating clinics ceded human subjects review authority to this IRB.
Patient Eligibility
Our analysis used patient data from the STOP CRC intervention. Eligible patients were 50–74 years old, had visited a clinic in the previous year, and were due for colorectal cancer screening. Due for screening was defined as having no EHR evidence of 1) a fecal test in the previous year, 2) a flexible sigmoidoscopy in the previous 4 years, 3) a colonoscopy in the previous 9 years, or 4) an order for a fecal testing in the previous 6 months or sigmoidoscopy/colonoscopy in the previous year. Patients were excluded if there was EHR evidence of a limited set of conditions that disqualified them for fecal testing (e.g., history of colorectal cancer, inflammatory bowel disease, or end-stage renal disease) [17].
Intervention
The intervention consisted of customized EHR tools and training to support the sequential mailing of outreach components—an introductory letter, a FIT kit, and a reminder letter—to those due for screening. Usual care differed among clinics but generally consisted of providers distributing FITs to patients attending clinic visits. The intervention was added to usual care. Intervention clinic staff placed lab orders for FITs that were mailed. Lab orders were also placed for FITs distributed in clinic. Because the FIT order did not distinguish between mailed and in-clinic distribution, we used the mailing of a FIT introductory letter as the proxy for a FIT distributed through the mailed outreach program.
Analytic Samples
The STOP CRC study developed real-time EHR tools that identified patients due for colorectal cancer screening. The full STOP CRC dataset included 60,187 unique study-eligible adults identified from February 4, 2014 – May 3, 2017. The study’s primary analyses used two patient samples: 1) patients due for colorectal cancer screening (eligible for a FIT) during the first year of STOP CRC (N=41,193; February 4, 2014 – February 3, 2015), and 2) patients who received a FIT lab order and were due for FIT completion during the 3 years of STOP CRC (N=35,655; February 4, 2014 – February 3, 2017). The STOP CRC intervention involved mailing an introductory letter, placing a FIT order and mailing a FIT, and sending a reminder letter to eligible patients. For the latter cohort, patients’ first FIT order was considered the index order and the evaluation interval was extended to May 3, 2017, to allow for at least 3 months’ follow-up. These samples were used for analysis of our primary outcomes—receipt of FIT order and completion of FIT— and secondary outcomes—receipt of any colorectal cancer screening order (i.e., FIT order or endoscopy referral) and completion of any CRC screening. Among the 41,193 patients due for a FIT, 21,134 (51%) were in intervention clinics; of these 7,163 (34%) were sent an introductory letter and ordered a FIT as part of the STOP CRC intervention. We stratify our analysis to assess difference in FIT ordering and completion for patients who received the mailed FIT outreach intervention and those who did not.
Multi-Morbidity Definitions
To understand the relationship between chronic disease burden and colorectal cancer screening recommendation and use, we calculated chronic disease burden separately using the CCI [18] and the CDPS [12]. For both indices, chronic conditions were ascertained by searching for ICD-9 or -10 codes up to 1 year before the FIT result date.
The CCI is a commonly used scale for assessing morbidity and predicts 1-year mortality for patients who may have a range of comorbid conditions [19]. Conditions included in the CCI are: AIDS; cerebrovascular disease; chronic pulmonary disease; congestive heart disease; dementia; diabetes with chronic complications; diabetes; hemiplegia or paraplegia; malignancy, including leukemia and lymphoma; metastatic solid tumor; mild liver disease; moderate or severe liver disease; myocardial infarction; peptic ulcer disease; peripheral vascular disorder; renal disease; and rheumatologic disease. Conditions are weighted (values 1–6) to calculate the score.
The CDPS was developed to predict medical expenditures in clinics serving Medicaid patients and to categorize diagnoses hierarchically; that is, a patient diagnosed with both a less severe condition and a more severe condition in the same disease category would be categorized as having the more severe condition. The indicators for the presence of a disease in each diagnostic and severity category are weighted to produce a single index of disease burden (CDPS version 6.3, University of San Diego, CA). The conditions included in CDPS are: AIDS/HIV, cancer, cardiovascular disease, cerebrovascular disease, central nervous system conditions, developmental disability, diabetes, eye conditions, genital disease, gastrointestinal diseases, hematological diseases, infectious disease, metabolic conditions, pregnancy, psychiatric conditions, pulmonary diseases, renal disease, skeletal conditions, skin conditions, and substance abuse (Table 3) [12] Each comorbid condition is weighted from 1 to 19 based on disease severity, producing a total score ranging from 0 to 37 [20]. The distribution of both indices was right-skewed, so we used discrete values from 0 to 4 and collapsed index values of 5 or greater into a single category.
Table 3.
Associations between Chronic Illness and Disability Payment System index category and FIT outcomes
| FIT Order N=41193 Adjusteda | FIT Result N=35655 Adjusteda | |||||
|---|---|---|---|---|---|---|
| N | OR | 95% CI | N | OR | 95% CI | |
| AIDS/HIV | 134 | 0.63 | (0.43–0.93) | 74 | 0.69 | (0.40–1.18) |
| Cancer | 2752 | 0.79 | (0.72–0.87) | 1831 | 0.97 | (0.88–1.07) |
| Cardiovascular | 21485 | 1.13 | (1.08–1.19) | 15889 | 0.87 | (0.83–0.91) |
| Cerebrovascular | 398 | 1.01 | (0.81–1.25) | 280 | 0.9 | (0.70–1.16) |
| Central Nervous System | 5053 | 0.97 | (0.91–1.04) | 4025 | 0.91 | (0.85–0.98) |
| Developmental Disability | 179 | 1.39 | (1.02–1.91) | 151 | 1.64 | (1.18–2.27) |
| Diabetes | 8980 | 1.13 | (1.07–1.20) | 7267 | 0.82 | (0.78–0.87) |
| Eye | 1098 | 1.11 | (0.97–1.26) | 903 | 0.89 | (0.78–1.03) |
| Genital | 2289 | 0.92 | (0.84–1.01) | 1654 | 0.96 | (0.86–1.06) |
| Gastrointestinal | 8865 | 0.84 | (0.80–0.89) | 6361 | 0.93 | (0.87–0.98) |
| Hematological | 716 | 1.02 | (0.86–1.20) | 577 | 0.85 | (0.71–1.02) |
| Infectious | 3627 | 0.91 | (0.84–0.98) | 2564 | 0.83 | (0.76–0.91) |
| Metabolic | 3002 | 0.96 | (0.88–1.04) | 2160 | 0.92 | (0.84–1.01) |
| Psychiatric | 13150 | 0.99 | (0.95–1.04) | 9968 | 0.84 | (0.80–0.89) |
| Pulmonary | 8627 | 0.97 | (0.92–1.03) | 6483 | 0.89 | (0.84–0.94) |
| Renal | 3132 | 0.99 | (0.91–1.07) | 2571 | 0.86 | (0.79–0.94) |
| Skeletal | 8377 | 0.92 | (0.87–0.98) | 6242 | 0.97 | (0.91–1.03) |
| Skin | 2937 | 1.04 | (0.96–1.14) | 2324 | 0.82 | (0.74–0.90) |
| Substance abuse | 4950 | 0.98 | (0.91–1.05) | 3985 | 0.78 | (0.72–0.84) |
Specific Chronic Illness and Disability Payment System categories significantly associated with FIT order receipt were: AIDS/HIV, cancer, gastrointestinal, infectious, and skeletal (lower FIT order receipt); Cardiovascular, developmental disability, and diabetes (higher FIT order receipt). Categories significantly associated with FIT completion were: Cardiovascular, central nervous system, diabetes, gastrointestinal, infectious, psychiatric, pulmonary, renal, skin, substance abuse (lower FIT completion); Developmental disability (higher FIT completion).
Statistical Methods
We report patient demographic characteristics for the two samples. We assessed associations between chronic disease index score (1 through 5) and each screening outcome (i.e., receipt of FIT order, completion of FIT screening) using logistic regression. We did not cluster by health center in the models, because there was a very small intraclass correlation coefficient (ICC=0.02 and 0.06) in the unconditional models. We report associations separately for the CCI and the CDPS. All models included age, sex, health center, and intervention arm assignment (a clinic-level variable, randomized within health center). We made additional adjustments for the number of clinic visits in the previous year. Models using CDPS were also adjusted for obesity, tobacco use, and preferred language. We adjusted the models using a consistent process, which resulted in different adjustment variables. This allowed for comparisons with published literature.
We hypothesized that the relationship between chronic disease burden and FIT outcomes might be modified according to whether the patient was sent a FIT invitation letter by mail (as a proxy for having been sent a FIT kit vs. receiving a FIT during an in-clinic visit). That is, a provider might make a personalized recommendation for a FIT based on a patient’s health status during a face-to-face visit but not in a mailed-FIT intervention. To evaluate this, we tested an interaction term for letter receipt by comorbidity index. An alpha level of 0.05 for the Wald test of this term was used to reject the null hypothesis of no interaction.
We further hypothesized that associations between each index and screening outcomes could be nonlinear. For example, odds of screening may increase from 0 to minimal morbidity but then decline with increasing morbidity burden (an inverted U shape). To evaluate this, we used dummy variables to test the associations between each category of co-morbid conditions (1–5), using no chronic disease conditions as the reference group. An alpha level of 0.05 for the logistic regression model was used to reject the null hypothesis of no association. We repeated these analyses for any colorectal cancer screening order and any colorectal cancer screening completion.
Results
The full STOP CRC dataset included 60,187 unique study-eligible adults identified from February 4, 2014 – May 3, 2017. Of these, 41,193 patients were due for a FIT during the first year of STOP CRC (sample 1) and 35.3% received a FIT order. Over all 3 years of STOP CRC data, 35,655 patients received a FIT order (sample 2) and approximately one-third (35.6%) completed a FIT within 3 months of the order.
For both analytic samples, the majority (>80%) of patients were aged 50–64, 12% were Hispanic, and 40% were obese (Table 1). More than one-third had Medicaid coverage, and approximately one-quarter had Medicare. Household income was low (~40% were under 100% of the federal poverty level). Half of the patients had one or two clinic visits in the previous year, and ~20% had six or more visits.
Table 1.
Baseline characteristics of patients in each analytic sample
| Eligible for FIT Order (n = 41,193) | Eligible for FIT completion (n = 35,655) | |||
|---|---|---|---|---|
| N | % | N | % | |
| Attending a clinic randomized to intervention | 21,134 | 51.3 | 19,898 | 55.8 |
| Age 65+ | 7,883 | 19.1 | 6,034 | 16.9 |
| Female | 22,994 | 55.8 | 19,614 | 55.0 |
| Hispanic | ||||
| Yes | 4,931 | 12.0 | 5,239 | 14.7 |
| No | 34,966 | 84.9 | 28,994 | 81.3 |
| Missing | 1,296 | 3.1 | 1,422 | 4.0 |
| Language | ||||
| English | 33,656 | 81.7 | 27,721 | 77.7 |
| Spanish | 3,804 | 9.2 | 4,251 | 11.9 |
| Other | 3,155 | 7.7 | 3,401 | 9.5 |
| Unknown | 578 | 1.4 | 282 | 0.8 |
| Race | ||||
| Asian | 2,034 | 4.9 | 2,161 | 6.1 |
| African American | 1,957 | 4.8 | 1,935 | 5.4 |
| White | 34,771 | 84.4 | 28,866 | 81.0 |
| Native American/Pacific Islander | 595 | 1.4 | 555 | 1.6 |
| Unknown | 1,836 | 4.5 | 2,138 | 6.0 |
| Obese (body mass index > 30) | 16,827 | 40.8 | 14,495 | 40.7 |
| Current tobacco use | ||||
| Yes | 10,679 | 25.9 | 9649 | 27.1 |
| No | 25,730 | 62.5 | 22,498 | 63.1 |
| Unknown | 4784 | 11.6 | 3508 | 9.8 |
| Insurance status | ||||
| Commercial | 6,087 | 14.8 | 4,764 | 13.4 |
| Medicaid | 15,326 | 37.2 | 15,997 | 44.9 |
| Medicare | 10,754 | 26.1 | 7,764 | 21.8 |
| Uninsured | 8,324 | 20.2 | 6,672 | 18.7 |
| Other | 234 | 0.6 | 177 | 0.5 |
| Unknown | 468 | 1.1 | 281 | 0.8 |
| Federal poverty level | ||||
| <100% | 17,531 | 42.6 | 14,007 | 39.3 |
| 100–200% | 10,293 | 25.0 | 8,192 | 23.0 |
| 200%+ | 4,733 | 11.5 | 4,696 | 13.2 |
| Unknown | 8,636 | 21.0 | 8,760 | 24.6 |
| Number of office visits in past year | ||||
| 1–2 | 20,698 | 50.2 | 19,344 | 54.3 |
| 3–5 | 11,501 | 27.9 | 9,290 | 26.1 |
| 6+ | 8,994 | 21.8 | 7,021 | 19.7 |
| Prior colorectal cancer screening, evera | 8,946 | 21.7 | 5,621 | 15.8 |
| Diabetes, ever | 8,756 | 21.3 | 7,348 | 20.6 |
| Flu shot in past year | 11,590 | 28.1 | 9,813 | 27.5 |
| Mammogram in past 2 yearsb | 9,237 | 40.2 | 7,194 | 36.7 |
| Pap test in past 3 yearsc | 7,927 | 43.2 | 6,849 | 42.6 |
Ever had fecal test, sigmoidoscopy, or colonoscopy
Among women (n=22,994 and 19,614)
Among women age 50–64 years (n=18,342 and 16,080
Distributions differed for the two indices. Most patients (53%) had a Charlson index of 0, about 27% had an index of 1, and about 8% had an index of 3 or more. For the CDPS index, 20% of patients had an index of 0, one-third had an index of 1, 19% had an index of 2, and 27% had an index of 3 or more.
Overall, the patterns of associations between chronic disease burden and FIT order differed by index. For the CCI, the associations followed an inverted U shape, demonstrating higher odds of FIT orders for individuals with a score of 1, with odds generally diminishing with increasing scores (a minor exception was the 5+ category). Compared to adults with a CCI score of 0, adults in the fourth highest CCI category were less likely to have received a FIT order (OR=0.77, 95% CI: 0.65–0.91). For CDPS, a different inverted U shape emerged, where higher odds of FIT order were observed for individuals in co-morbid categories 1–4, then odds diminished substantially for the 5+ category. Compared to adults with an CDPS score of 0, adults in the highest CDPS category were significantly less likely to receive a FIT order (OR=0.88, 95% CI: 0.79–0.98). In adjusted models, each index was statistically significantly associated with a FIT order (both p ≤ 0.03, Figure 1 and Figure 2). The two indices were moderately correlated (r for FIT order (sample 1) = 0.6; r for FIT completion (sample 2) = 0.5).
Fig. 1.

Probability of FIT order, FIT completion by morbidity score calculated using Charlson co-morbidity index and Chronic Illness and Disability Payment System index
Compared to individuals with a Charlson Comorbidity Index (CCI) of 0, receipt of a FIT order was slightly higher for individuals with a CCI of 1 and displayed a downward trend for CCI scores of 2–5. Odds of FIT return decreased with increasing CCI score.
Fig. 2.
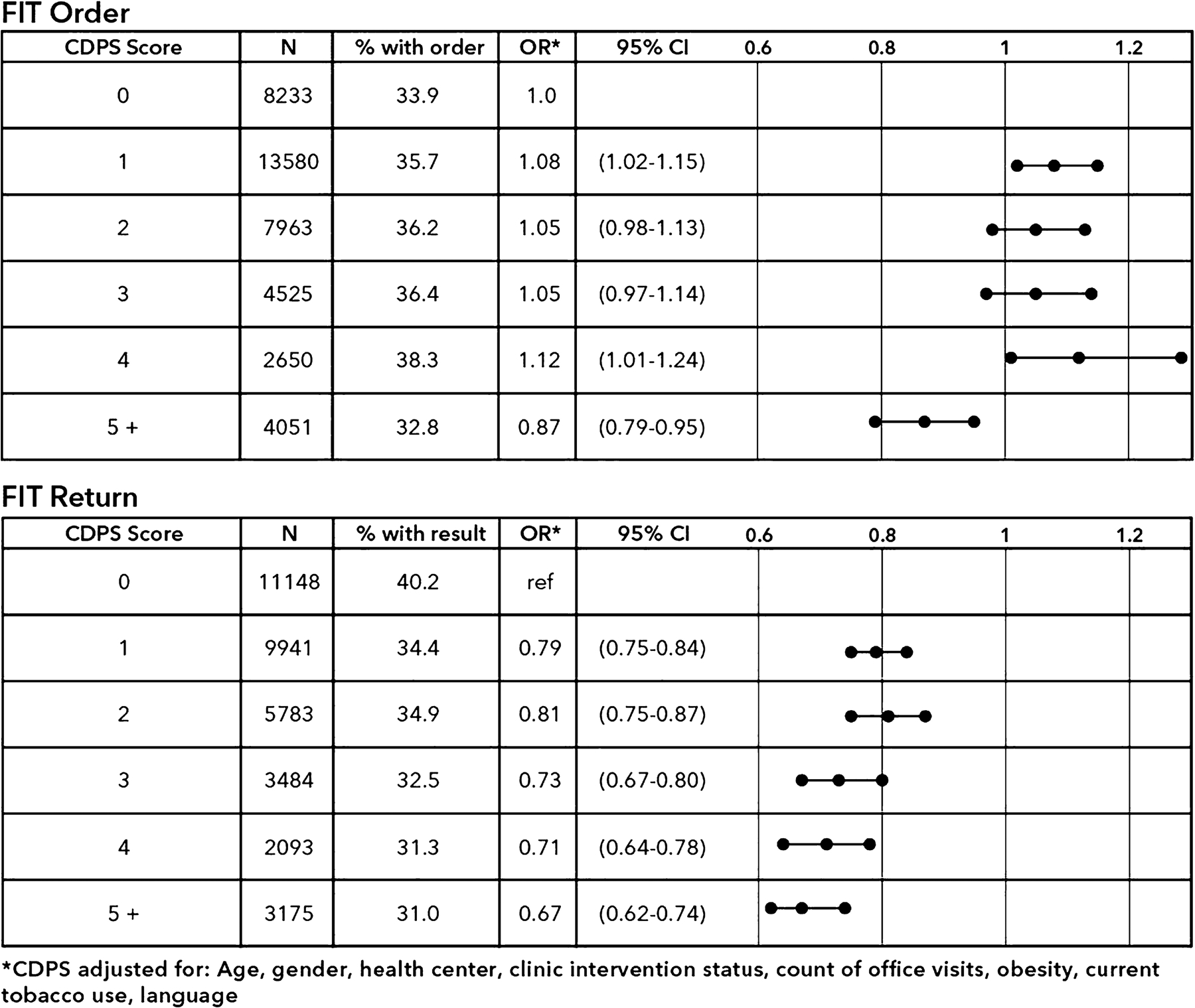
Probability of FIT order, FIT completion by morbidity score calculated using the Chronic Illness and Disability Payment System index
Compared to individuals with a Chronic Illness and Disability Payment System (CDPS) of 0, receipt of a FIT order was slightly higher for individuals with a CDPS of 1–4 and lower for individuals with a CDPS of 5. Odds of FIT return and completion of any CRC screening decreased with increasing CDPS score.
The association between the CCI and FIT order differed according to whether patients were mailed a FIT introductory letter (p for interaction = 0.002, Table 2), with no observed association among those mailed a letter and a non-linear association among those not mailed a letter. The CDPS association with FIT order did not differ by invitation letter stratum (p=0.12).
Table 2.
Associations between morbidity index score (Charlson Co-morbidity Index and Chronic Illness and Disability Payment System Illness index) and FIT order status by whether the participant was mailed a STOP CRC introduction letter, among eligible participants in STOP CRC intervention clinics (n = 21,134)
| Score | Eligible for FIT order (N = 21,134) N (%) | FIT order (%) | Adjusted OR (95% CI)a | FIT order N (%) | Adjusted OR (95% CI)a | P for interaction |
|---|---|---|---|---|---|---|
| Charlson Co-morbidity Index | ||||||
| Mailed a STOP CRC letter | Not mailed a STOP CRC letter | |||||
| 0 | 11,182 (53.1) | 3899 (87.9) | 1.0 --- | 7283 (23.2) | 1.0 --- | |
| 1 | 5,761 (27.4) | 1976 (88.5) | 0.95 (0.79–1.14) | 3785 (27.2) | 1.14 (1.04–1.26) | |
| 2 | 2,294 (10.9) | 742 (88.9) | 1.00 (0.76–1.31) | 1552 (21.9) | 0.83 (0.72–0.95) | |
| 3 | 935 (4.4) | 299 (92.0) | 1.25 (0.79–1.98) | 636 (22.8) | 0.85 (0.70–1.05) | |
| 4 | 363 (1.7) | 109 (83.5) | 0.57 (0.32–1.00) | 254 (21.3) | 0.76 (0.55–1.05) | |
| ≥ 5 | 503 (2.4) | 138 (84.8) | 0.71 (0.43–1.19) | 365 (20.0) | 0.82 (0.62–1.08) | 0.002 |
| Chronic Illness and Disability Payment System Index | ||||||
| Mailed a STOP CRC letter | Not mailed a STOP CRC letter | |||||
| 0 | 4,464 (21.1) | 1541 (86.6) | 1.0 --- | 2923 (22.0) | 1.0 --- | |
| 1 | 6,898 (32.6) | 2402 (87.9) | 1.03 (0.84–1.28) | 4496 (24.3) | 1.09 (0.97–1.23) | |
| 2 | 4,117 (19.5) | 1362 (89.7) | 1.17 (0.91–1.52) | 2755 (25.1) | 1.09 (0.95–1.25) | |
| 3 | 2,281 (10.8) | 779 (89.9) | 1.04 (0.77–1.42) | 1502 (26.1) | 1.14 (0.97–1.34) | |
| 4 | 1,367 (6.5) | 474 (89.2) | 1.04 (0.72–1.50) | 893 (26.5) | 1.13 (0.93–1.36) | |
| ≥ 5 | 2,007 (9.5) | 618 (87.7) | 0.85 (0.61–1.19) | 1389 (20.6) | 0.79 (0.66–0.94) | 0.12 |
Charlson co-morbidity index: adjusted for age, sex, health center, clinic visits; Chronic Illness and Disability Payment System: adjusted for age, sex, health center, clinic visits, obesity, tobacco use, and language
The relationship between Charlson Comorbidity Index score and FIT order receipt differed by whether an individual was mailed STOP CRC mailings or was offered a FIT during an in-clinic visit. This comorbidity-FIT order interaction was not significant when the Chronic Illness and Disability Payment System was used.
CCI and CDPS values were inversely associated with FIT return (Figures 1 and 2). The strongest associations were observed for CDPS and CCI values of 3 or more, which were associated with about 30% lower odds of FIT return, independent of age, number of clinic visits, and other adjustments. An inverse linear trend was also observed for completion of any CRC screening for each index (CCI and CDPS).
CDPS-specific chronic conditions associated with a substantial (≥20%) lower odds of FIT-order receipt were HIV/AIDS (OR=0.63; 95% CI: 0.43 – 0.93) and cancer (OR=0.79; 95% CI: 0.72 – 0.87); only substance abuse was associated with a substantially lower odds of FIT completion (OR=0.78; 95% CI: 0.72, 0.84). In contrast, individuals with a developmental disability were substantially more likely that those without to receive a FIT order (OR=1.39; 95% CI: 1.02 – 1.91) or complete a FIT (OR=1.64; 95% CI: 1.18 – 2.27; Table 3). Similar conditions were associated with any colorectal cancer screening outcomes: individuals with HIV/AIDS had a lower odds of receiving any colorectal cancer screening order (OR=0.63; 95% CI: 0.44 – 0.91) and individuals with a developmental disability had higher odds of order and completion (OR=1.33; 95% CI: 0.98 – 1.80 for any colorectal cancer screening order, and OR=1.59; 95% CI: 1.15 – 2.20 for any colorectal cancer screening completion). CCI-specific chronic conditions associated with FIT-order receipt and FIT-completion are provided in Supplemental Table 1.
We examined the odds of FIT order and FIT completion by disease severity for AIDS/HIV, cancer, substance abuse, developmental disabilities, and diabetes. When these conditions were examined by CDPS severity category, we observed an apparent trend for lower odds of FIT order with increasing disease severity for cancer and AIDS/HIV. A similar pattern was observed for FIT completion for cancer and substance abuse (Supplemental Table 2).
Discussion
In this study of colorectal cancer screening in eight community health centers in Oregon and California, we found that, compared to patients with no chronic disease diagnoses, patients with a chronic disease index score of 1 were 8–9% more likely to be offered a FIT; conversely, patients with a high chronic disease burden (a score of 4 or more) were 13–23% less likely to be offered a FIT. The pattern was most pronounced for individuals who received kits during in-clinic visits. For each morbidity index, we observed an inverse linear association between patients’ chronic disease burden and FIT completion. Patients having any chronic disease diagnosis were approximately 20% less likely to complete a FIT than patients having none (CCI: 1+ vs. 0; OR: 0.80; 95% CI: (0.76–0.84); CDPS: 1+ vs. 0: OR: 0.77; 95%CI: (0.73–0.80), and completion diminished with increasing chronic disease burden. Specific chronic conditions and greater disease severity were generally associated with lower odds of receiving a FIT order (e.g., HIV/AIDs, cancer) or completing a FIT (e.g., substance abuse). Developmental disability was associated with higher odds of FIT order and completion. Our findings may inform efforts to optimize rates of colorectal cancer screening among vulnerable patients with multiple chronic conditions.
Our observation that comorbidity burden is inversely associated with FIT return is consistent with previous reports. Haas and colleagues reported that integrated health system patients with higher CCI scores (≥3 vs 0) were less likely to complete a FIT (HR, 0.82; 95% CI, 0.79–0.87) [21]. In a population-based screening program in Barcelona, Spain, those with three or more dominant chronic diseases were less likely to participate, and those having multiple minor chronic diseases were more likely to participate, in FIT screening (8). In integrated health systems participating in the Population-Based Research Optimizing Screening through Personalized Regimens (PROSPR) consortium, timely follow-up of abnormal FIT (colonoscopy within 3 months) decreased linearly with increasing CCI among all age groups [22]. These patterns may reflect a competing focus on morbidities and the perception of a lack of screening benefit for individuals with comorbid conditions [23].
The appropriate discontinuation of screening among patients who would accrue relatively few benefits is supported by guidelines that recommend the consideration of overall health and life expectancy of at least 10 years in deciding whether to perform screening and/or continue it beyond age 75 [24–26]. Such discontinuation can ensure a screening benefit, reduce harms, [27] and enhance cost-effectiveness of screening [28]. Mailing FITs may eliminate the opportunity for providers to assess individual patients’ anticipated screening benefits and harms, even though such mailings can surmount provider forgetfulness and time constraints posed by in-clinic distribution. Moreover, many healthy patients who attend clinic visits infrequently may be offered FIT only sporadically through opportunistic in-clinic distribution. There is some evidence that providers inconsistently apply stopping-rules for screening— e.g., more than 10% would continue offering fecal testing to an 80-year-old with non-small cell lung cancer [29] —perhaps suggesting the need for ongoing provider training/education or automated provider notifications to prompt screening discontinuation in patients who would accrue little benefit. Our findings can inform provider education about how to support colorectal cancer screening among patients with co-morbid conditions. Among adults who meet the clinical criteria for follow-up colonoscopy, our findings might also support the use of FIT as a first-line, non-invasive screening strategy for individuals with multiple co-morbidities.
Individual disease categories associated with screening completion can be useful markers for patient groups that need tailored messaging or other assistance to detect and prevent colorectal cancer. Prior research on the associations among individual disease conditions and colorectal cancer screening have shown mixed results. Liu and colleagues reported lower colorectal cancer screening among men with cardiovascular disease, while Heflin and colleagues reported higher rates of fecal testing among adults with hypertension [6, 30]. In analysis using the CDPS, we found that adults with cardiovascular disease were 13% more likely to obtain an order for a FIT, but 23% less likely to complete it, a pattern that also was observed for any colorectal cancer screening (Supplement Table 2). Similarly, in analysis using the CCI, adults with congestive heart failure were 30% less likely to complete a FIT, than those without the condition (Supplement Table 1).
Our observation that patients with HIV/AIDS had substantially lower odds of FIT order and completion is notable given that the risk of colorectal cancer in those with HIV/AIDS is comparable to non-infected populations [31, 32]. Notably, the associations were mostly driven by lower odds of FIT orders among individuals with AIDS specifically. Antiretroviral treatments for HIV/AIDS are known to be rigorous (requiring over 90% adherence) [33], so completing a FIT may be a lower priority for these patients and the providers caring for them. While newer versions of FITs have no dietary or medication restrictions, providers may be uncertain about the accuracy of FIT results for patients on complex medication regimes. Our findings might underscore the need to communicate the importance of colorectal cancer screening participation among individuals with HIV and AIDS and their providers.
The only CDPS category associated with greater odds of FIT order and return was developmental disability. Diagnoses of mild and moderate intellectual disabilities predominated this condition category in our study. Although no previous studies have reported on colorectal cancer screening among developmentally disabled patients, breast and cervical cancer screening studies have reported inconsistent associations. For example, a study in Canada found that women with intellectual and developmental disabilities (IDD) were less likely to be screened than were women without IDD [34]. Another Canadian study found an inverted V-shaped association with disability (intellectual or other) severity, such that a higher proportion of women with moderate disability were up-to-date on breast cancer screening than those without disability or those with severe disability [35].
We explored associations of chronic condition burden and colorectal cancer screening using two distinct indices: the CCI and the CDPS. Our hypothesis was that providers and patients might place a lower priority on colorectal cancer screening if the burden of chronic conditions was high. We found that the CCI and the CDPS scores had similar relationships with screening completion outcomes, although their patterns differed somewhat for receipt of FIT order, with the CCI displaying a direct association in patients with a single co-morbid condition, then an inverse association with subsequent co-morbid conditions, and the CDPS suggestive of direct associations up through four co-morbid conditions.
With a few notable exceptions, our co-morbidity indices performed much as expected, suggesting that a higher number of co-morbid conditions and higher disease severity leads to lower rates of colorectal cancer screening. One exception was adults with diabetes. Analysis using both the CDPS and the CCI showed that compared to adults without diabetes, adults with diabetes had a higher odds of receiving a FIT order, but a lower odds of completing a FIT. This pattern was observed for diabetes with and without complications (Supplement Table 1). Given that diabetes is an established risk factor for colorectal cancer, our findings might suggest that efforts are needed to promote FIT completion in this subgroup.
The CCI and the CDPS each provided unique insight into the associations between co-morbid conditions and FIT testing; for several conditions, the two indices produced similar findings (e.g., HIV/AIDS, cancer, diabetes, renal disease) and for other conditions associations significant in one index were suggestive in the other (e.g., cardiovascular disease, pulmonary disease). Several other conditions were present in one index but not the other (e.g., liver disease and rheumatological disease were present in CCI and not in CDPS; central nervous system, developmental disabilities, gastroenterology, infectious conditions, psychiatric conditions, skeletal conditions, skin conditions, and substance abuse were present in CDPS and not CCI). The CDPS also offered a robust assessment of disease severity, which may be particularly important for colorectal cancer screening decisions.
This study has several limitations. Our study participants received care at one of eight FQHCs in Oregon and California, and our patient population was primarily urban. It is not known whether our findings generalize to patients in other geographic regions. We relied on EHR information for patient demographic characteristics, behaviors (e.g., tobacco and alcohol consumption), and diagnoses. These data are subject to common issues of incomplete capture and misclassification. We assessed patient diagnoses based on data obtained up to 2 years prior to patients’ identification as eligible for colorectal cancer screening and entry into the study cohort. This may have precluded capture of chronic conditions that were neither diagnosed nor treated during this timeframe. Finally, for obvious reasons, our STOP CRC eligibility criteria excluded patients who had end-stage renal disease or colorectal disease (i.e., colectomy, ulcerative colitis, or Crohn’s disease); this likely altered our associations with renal and gastrointestinal diseases.
Conclusion
Analysis showed a U-shaped association between patients’ chronic disease burden and providers’ recommendation of a FIT, and an inverse linear association between patients’ chronic disease burden and FIT completion. Our findings can inform efforts to promote colorectal cancer screening services use among patients with chronic conditions.
Supplementary Material
Table 4.
Associations between Chronic Illness and Disability Payment System index severity and each FIT outcome for categories significantly associated with FIT outcomes.
| FIT Order N=41193 Adjusteda |
FIT Result N= N=35655 Adjusteda |
|||||
|---|---|---|---|---|---|---|
| N | OR | 95% CI | N | OR | 95% CI | |
| AIDS/HIV | 134 | 0.63 | (0.43–0.93) | 74 | 0.69 | (0.40–1.18) |
| AIDS (high) | 110 | 0.58 | (0.38–0.89) | 54 | 0.81 | (0.45–1.49) |
| HIV (medium) | 24 | 0.93 | (0.38–2.27) | 20 | 0.39 | (0.11–1.36) |
| Cancer | 2752 | 0.79 | (0.72–0.87) | 1831 | 0.97 | (0.88–1.07) |
| Very high | 91 | 0.66 | (0.41–1.07) | 57 | 0.69 | (0.38–1.23) |
| High | 330 | 0.64 | (0.49–0.83) | 204 | 0.67 | (0.49–0.92) |
| Medium | 469 | 0.94 | (0.77–1.16) | 367 | 0.94 | (0.75–1.18) |
| Low | 1862 | 0.80 | (0.72–0.90) | 1203 | 1.06 | (0.94–1.20) |
| Substance abuse | 4950 | 0.98 | (0.91–1.05) | 3985 | 0.78 | (0.72–0.84) |
| Low | 2533 | 1.02 | (0.93–1.12) | 2221 | 0.69 | (0.62–0.77) |
| Very Low | 2417 | 0.94 | (0.86–1.04) | 1764 | 0.94 | (0.85–1.05) |
| Developmental Disability | 179 | 1.39 | (1.02–1.91) | 151 | 1.64 | (1.18–2.27) |
| Medium | 0 | - | - | 0 | - | - |
| Low | 179 | 1.39 | (1.02–1.91) | 151 | 1.64 | (1.18–2.27) |
| Diabetes | 8980 | 1.13 | (1.07–1.20) | 7267 | 0.82 | (0.78–0.87) |
| Type 1 High | 13 | - | - | 17 | - | - |
| Type 1 Medium | 252 | 1.15 | (0.88–1.51) | 219 | 0.83 | (0.62–1.11) |
| Type 2 Medium | 1567 | 1.11 | (1.00–1.24) | 1487 | 0.86 | (0.76–0.96) |
| Type 2 Low | 7148 | 1.11 | (1.05–1.18) | 5544 | 0.84 | (0.79–0.90) |
Adjusted for age, sex, health center, intervention arm, clinic visits, tobacco use, and language preference.
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute, Award Number UH3CA188640. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The study sponsor had no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Conflict of interest
Dr. Coronado served as a co-investigator on a study funded by Epigenomics and as a principal investigator on a study funded by Quidel Corporation. The studies had no influence on the design, conduct, or reporting of the present study. All other authors declare that they have no conflict of interest.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Research involving Human Participants and/or Animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (The Institutional Review Board of Kaiser Permanente Northwest; ClinicalTrials.gov registration: NCT01742065) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Institutional Review Board of Kaiser Permanente Northwest approved all study activities, and participating clinics ceded human subjects review authority to this IRB.
References
- 1.American Cancer Society (2019) Cancer Statistics Center.
- 2.Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD (2012) Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. The New England journal of medicine 366:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (2016) Behavioral Risk Factor Surveillance System Survey Data. Atlanta, Georgia: Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- 4.American Association for Retired Persons Chronic Conditions Among Older Americans; . In: Anderson G, ed. Analysis of Medical Expenditure Panel Survey data from 2005
- 5.Zhao G, Ford ES, Ahluwalia IB, Li C, Mokdad AH (2009) Prevalence and trends of receipt of cancer screenings among US women with diagnosed diabetes. Journal of general internal medicine 24:270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heflin MT, Pollak KI, Kuchibhatla MN, Branch LG, Oddone EZ (2006) The impact of health status on physicians’ intentions to offer cancer screening to older women. The journals of gerontology. Series A, Biological sciences and medical sciences 61:844–50. [DOI] [PubMed] [Google Scholar]
- 7.Fontana SA, Baumann LC, Helberg C, Love RR (1997) The delivery of preventive services in primary care practices according to chronic disease status. American journal of public health 87:1190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox J, Zikmund-Fisher BJ, Gross CP (2012) Older patient experiences in the mammography decision-making process. Arch Intern Med. 172:62–4; discussion 4. doi: 10.1001/archinternmed.2011.601. [DOI] [PubMed] [Google Scholar]
- 9.Feuer EJ, Lee M, Mariotto AB, Cronin KA, Scoppa S, Penson DF, Hachey M, Cynkin L, Carter GA, Campbell D, Percy-Laurry A, Zou Z, Schrag D, Hankey BF (2012) The Cancer Survival Query System: making survival estimates from the Surveillance, Epidemiology, and End Results program more timely and relevant for recently diagnosed patients. Cancer 118:5652–62. [DOI] [PubMed] [Google Scholar]
- 10.Guiriguet C, Pera G, Castells A, Toran P, Grau J, Rivero I, Buron A, Macia F, Vela-Vallespin C, Vilarrubi-Estrella M, Marzo-Castillejo M (2017) Impact of comorbid conditions on participation in an organised colorectal cancer screening programme: a cross-sectional study. BMC cancer 17:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz A, Kang J, Moore SP, Baade P, Langbecker D, Condon JR, Valery PC (2017) Association between comorbidity and participation in breast and cervical cancer screening: A systematic review and meta-analysis. Cancer Epidemiol 47:7–19. [DOI] [PubMed] [Google Scholar]
- 12.Kronick R, Gilmer T, Dreyfus T, Lee L (2000) Improving health-based payment for Medicaid beneficiaries: CDPS. Health care financing review 21:29–64. [PMC free article] [PubMed] [Google Scholar]
- 13.Oemrawsingh A, Swami N, Valderas JM, Hazelzet JA, Pusic AL, Gliklich RE, Bergmark RW (2020) Patient-Reported Morbidity Instruments: A Systematic Review. Value Health. 23:791–811. doi: 10.1016/j.jval.2020.02.006. Epub May 27. [DOI] [PubMed] [Google Scholar]
- 14.HRSA (2016) 2016 National Health Center Data.
- 15.Coronado GD, Petrik AF, Vollmer WM, Taplin SH, Keast EM, Fields S, Green BB (2018) Effectiveness of a Mailed Colorectal Cancer Screening Outreach Program in Community Health Clinics: The STOP CRC Cluster Randomized Clinical Trial. JAMA internal medicine 178:1174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coronado GD, Vollmer WM, Petrik A, Taplin SH, Burdick TE, Meenan RT, Green BB (2014) Strategies and Opportunities to STOP Colon Cancer in Priority Populations: Design of a cluster-randomized pragmatic trial. Contemp Clin Trials 38:344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrik AF, Green BB, Vollmer WM, Le T, Bachman B, Keast E, Rivelli J, Coronado GD (2016) The validation of electronic health records in accurately identifying patients eligible for colorectal cancer screening in safety net clinics. Family practice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology 45:613–9. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases 40:373–83. [DOI] [PubMed] [Google Scholar]
- 21.Haas CB, Phipps AI, Hajat A, Chubak J, Wernli KJ (2019) Time to fecal immunochemical test completion for colorectal cancer screening. Am J Manag Care. 25:174–80. [PMC free article] [PubMed] [Google Scholar]
- 22.Klabunde CN, Zheng Y, Quinn VP, Beaber EF, Rutter CM, Halm EA, Chubak J, Doubeni CA, Haas JS, Kamineni A, Schapira MM, Vacek PM, Garcia MP, Corley DA, consortium P (2016) Influence of Age and Comorbidity on Colorectal Cancer Screening in the Elderly. Am J Prev Med 51:e67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross CP, Fried TR, Tinetti ME, Ross JS, Genao I, Hossain S, Wolf E, Lewis CL (2015) Decision-making and cancer screening: a qualitative study of older adults with multiple chronic conditions. Journal of geriatric oncology 6:93–100. [DOI] [PubMed] [Google Scholar]
- 24.Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, Brawley OW, Wender RC (2018) Cancer screening in the United States, 2018: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 68:297–316. doi: 10.3322/caac.21446. Epub 2018 May 30. [DOI] [PubMed] [Google Scholar]
- 25.Cho H, Klabunde CN, Yabroff KR, Wang Z, Meekins A, Lansdorp-Vogelaar I, Mariotto AB (2013) Comorbidity-adjusted life expectancy: a new tool to inform recommendations for optimal screening strategies. Annals of internal medicine 159:667–76. [DOI] [PubMed] [Google Scholar]
- 26.Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson DJ (2017) Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 153:307–23. [DOI] [PubMed] [Google Scholar]
- 27.Braithwaite D, Walter LC, Izano M, Kerlikowske K (2016) Benefits and Harms of Screening Mammography by Comorbidity and Age: A Qualitative Synthesis of Observational Studies and Decision Analyses. Journal of general internal medicine 31:561–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Hees F, Saini SD, Lansdorp-Vogelaar I, Vijan S, Meester RG, de Koning HJ, Zauber AG, van Ballegooijen M (2015) Personalizing colonoscopy screening for elderly individuals based on screening history, cancer risk, and comorbidity status could increase cost effectiveness. Gastroenterology 149:1425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haggstrom DA, Klabunde CN, Smith JL, Yuan G (2013) Variation in primary care physicians’ colorectal cancer screening recommendations by patient age and comorbidity. Journal of general internal medicine 28:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu BY, O’Malley J, Mori M, Fagnan LJ, Lieberman D, Morris CD, Buckley DI, Heintzman JD, Carney PA (2014) The association of type and number of chronic diseases with breast, cervical, and colorectal cancer screening. Journal of the American Board of Family Medicine : JABFM 27:669–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Neill TJ, Nguemo JD, Tynan AM, Burchell AN, Antoniou T (2017) Risk of Colorectal Cancer and Associated Mortality in HIV: A Systematic Review and Meta-Analysis. Journal of acquired immune deficiency syndromes (1999) 75:439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mani D, Aboulafia DM (2013) Screening guidelines for non-AIDS defining cancers in HIV-infected individuals. Current opinion in oncology 25:518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mannheimer S, Friedland G, Matts J, Child C, Chesney M (2002) The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 34:1115–21. [DOI] [PubMed] [Google Scholar]
- 34.Cobigo V, Ouellette-Kuntz H, Balogh R, Leung F, Lin E, Lunsky Y (2013) Are cervical and breast cancer screening programmes equitable? The case of women with intellectual and developmental disabilities. Journal of intellectual disability research : JIDR 57:478–88. [DOI] [PubMed] [Google Scholar]
- 35.Guilcher SJ, Lofters A, Glazier RH, Jaglal SB, Voth J, Bayoumi AM (2014) Level of disability, multi-morbidity and breast cancer screening: does severity matter? Preventive medicine 67:193–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


