The use of ultrasound (US) in conjunction with microbubbles (MB) to transiently open the blood-brain barrier (BBB) has been used to increase drug delivery to the brain. Recently, US-mediated BBB opening has been demonstrated to increase the release of biomarkers such as DNA, RNA, and proteins from the central nervous system (CNS) compartment into the blood.1–3 However, important variables such as the optimal time for blood collection following US-mediated BBB opening have not been characterized. This study characterizes the kinetics of cell-free DNA (cfDNA) release following US-mediated BBB opening in healthy and intracranial glioma mouse models.
Circulating tumor biomarkers offer the ability to glean important diagnostic information noninvasively.4 For example, tumor-derived DNA shed into the bloodstream of lung cancer patients have been used to identify drug targetable mutations as well as to predict tumor progression and relapse.5 However, the ability to detect circulating biomarkers from glioma patients within blood has remained difficult and unreliable.6 In part, this may be due to the BBB which limits exchange of biological materials between the CNS and blood. Indeed, a previous study of 42 patients with glioblastoma (GBM) identified the rate transfer content (ktrans), a direct marker for BBB permeability, as independently associated with increased cfDNA.7
US-mediated BBB opening is an emerging technology that safely and transiently opens the BBB. Many clinical trials are currently underway investigating the use of this technology to increase drug delivery into the brain for treatment of CNS disease such as GBM.8 Recently, studies in murine and porcine models as well as human GBM patients have shown that US-mediated BBB opening can increase the release of protein, RNA and DNA from the brain.1–3 Thus, there has been interest in repurposing this technology to increase the levels of circulating biomarkers from GBM. However, to date, there have been no studies investigating the optimal time for blood collection following sonication. In this study, we use a low-intensity US device to open the BBB in healthy mice and in an intracranial glioma mouse model as previously described with a few modifications.9 The distance between the US transducer and mouse head was increased by 10 mm and acoustic power level was increased from 0.3 MPa to 0.4 MPa in order to increase the area of BBB disruption. We then determined cfDNA concentration in plasma at various time points post sonication. BBB-opening was confirmed using sodium fluorescein as previously described9 (Figure 1A). All animal studies were performed and approved in compliance with Northwestern University’s Institutional Animal Care and Usage Committee.
Figure 1.
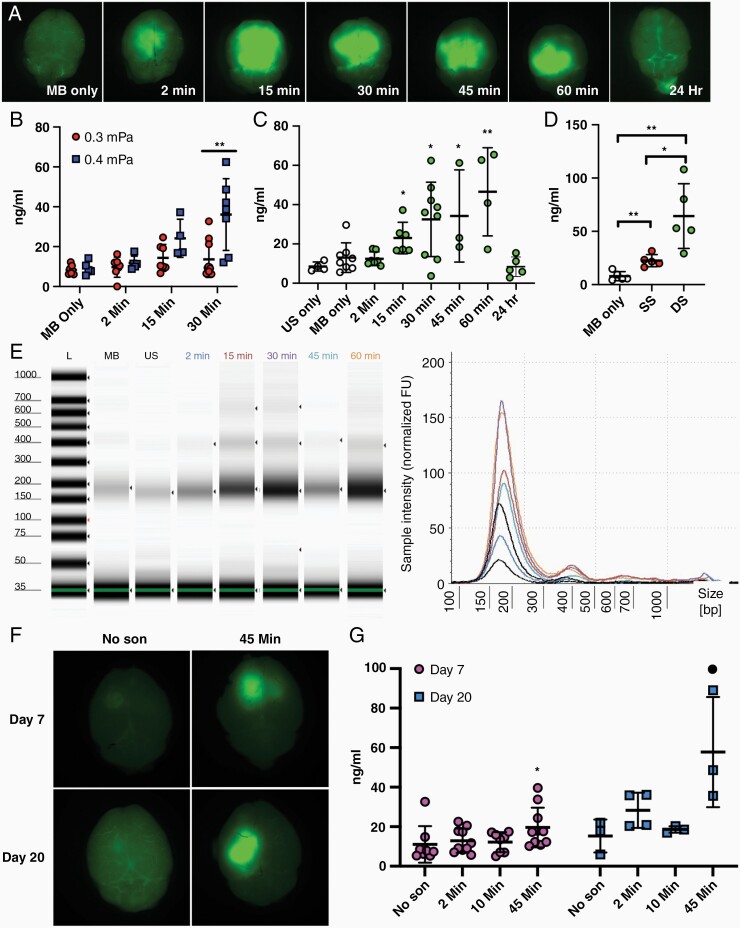
(A) Representative images of fluorescein visualization of BBB opening following US and MB treatment compared to mice injected with MB alone. Brains were harvested after blood collection at time shown and imaged with Nikon AZ100 microscope with FITC filter cube. Mice in 24-hour time group had fluorescein injected 30 minutes prior to blood collection the day after sonication. (B–D) Plasma cfDNA concentration following sonication. Blood samples were collected via terminal cardiac puncture (0.5–1.0 mL) which yielded 200–500 µL of plasma. Plasma was separated from blood using a double centrifugation protocol with soft deceleration (1600 g for 10 minutes followed by 16,000 g for 10 minutes). cfDNA was isolated using Thermofisher MagMax cfDNA isolation kit according to manufacturer instructions. (B,C) cfDNA concentrations in plasma was determined fluorometrically using Qubit High sensitivity dsDNA assay or by (D) digital droplet PCR determination of HBB1 copy number and converted to ng/mL. Data plotted are mean ± standard deviation, each circle represents 1 animal. Student’s 2-tailed T-test was used to determine significance. (B) Average cfDNA concentration increases with acoustic pressure for US-mediated BBB opening at 45 minutes (**P = .0038). (C) Average cfDNA concentration begins to increase at 15 minutes following US-mediated BBB opening at 0.4 mPa and remains elevated at 60 minutes post sonication before returning to baseline levels 24 hours following sonication. (*P = .0341; *P = .016; *P = .0379; **P = .0027). (D) Average cfDNA release induced by US-mediated BBB opening increases in dose-dependent manner. Blood samples were collected 30 minutes post-sonication treatment. SS, Single Sonication; DS, Double sonication (**P = .0017; *P = .016; **P = .0034). (E) Isolated cfDNA samples display characteristic cfDNA fragment ladder pattern with peaks between 150 and 200 bp. Two cfDNA samples from each experimental condition were analyzed using Agilent Cell-free Screen Tape assay run on Agilent 4150 Instrument. MB, Microbubble only; US, Ultrasound only. (F) Representative images of fluorescein visualization of BBB opening in PF8 intracranial tumor model at day 7 and day 20 post intracranial tumor implantation. (G) Average cfDNA levels following sonication in PF8 tumor model reveal cfDNA is increased 45 minutes following sonication compared to non-sonicated mice at day 7 and day 20 post intracranial injection (*P = .040;• P = .032).
First, we investigated whether changing acoustic power settings (0.3–0.4 MPa) while keeping MB dose (Lumason, Bracco, 10 mg/kg) and all other pulsing parameters constant (1 MHz, 25 ms pulse, 1 Hz pulse repetition frequency, 120 second sonication) impacted cfDNA release into the bloodstream (Figure 1B). At 0.4 MPa, cfDNA was elevated in the blood 30 minutes after sonication compared to the MB only control (36.09 vs 9.252 ng/mL, P = .0086, Student’s 2-tailed t-test) and lower acoustic power (0.3 MPa) group (36.09 vs 13.63 ng/mL, P = .0039, Student’s 2-tailed t-test), suggesting that higher acoustic powers were associated with increased BBB opening. CfDNA levels 30 minutes after sonication at 0.3MPa were increased but did not reach significance (13.63 vs 8.38 ng/mL, P = .159, Student’s 2-tailed t-test).
Next, in order to determine the optimal time for blood collection following US-mediated BBB opening, we sonicated mice at 0.4 MPa and collected peripheral blood samples at 2, 15, 30, 45, and 60 minutes following sonication (Figure 1C). At 15 minutes, cfDNA concentrations began to significantly increase compared to the non-sonicated control group. Average cfDNA concentrations were highest in the 60-minute post-sonication blood collection group compared to US only (46.54 vs 13.01 ng/mL, P = .0027, Student’s 2-tailed t-test; no MB used) and MB only controls (46.54 vs 8.48 ng/mL, P = .0149, Student’s 2-tailed t-test, no US used). To determine if the increase in cfDNA seen was transient, we sonicated mice and collected their blood 24 hours later. We found that average cfDNA levels in these mice had returned to baseline levels seen in US only (8.40 vs 8.5 ng/mL, P = .98, Student’s 2-tailed t-test) and MB only mice (8.40 vs 13.01 ng/mL, P = .253, Student’s 2-tailed t-test). Of note, no fluorescein leakage was seen in mice 24 hours post-sonication, suggesting cfDNA increase is correlated with BBB disruption (Figure 1A).
To further confirm that US-mediated BBB opening increases cfDNA concentrations, we compared cfDNA levels in C57/BL6 mice that received single (SS) or 2 sequential sonication (DS) treatments (Figure 1D). We found that mean cfDNA concentrations were increased in a dose-dependent manner. Mice receiving DS had significantly elevated cfDNA levels compared to MB control (64.32 vs 7.907 ng/mL, P = .0034, Student’s 2-tailed t-test) and SS group (64.32 vs 22.54 ng/mL, P = .0166, Student’s 2-tailed t-test).
We then evaluated if US-mediated BBB opening also increased cfDNA levels in tumor bearing mice (Figure 1F and G). 10,000 PF8 cells, a retrovirally generated murine glioma cell line,10 were intracranially injected into C57/BL6 mice. Blood samples were collected at 2-, 10-, and 45-minute post-sonication following US-mediated BBB opening at day 7 and day 20 post intracranial injection. At day 20 postinjection, cfDNA levels were found to be increased 45 minutes after sonication compared to non-sonicated controls (57.75 vs 15.31 ng/mL, P = .032, Student’s 1-tailed T-test), whereas cfDNA levels 2 minutes post sonication were not significantly increased (28.31 vs 15.31 ng/mL, P = .054, Student’s 1-tailed T-test). At day 7 postinjection, cfDNA levels 45 minutes following sonication also appeared increased compared to non-sonicated controls albeit to a lesser extent (19.60 vs 11.02 ng/mL, P = .040, Student’s 1-tailed T-test).
The repurposing of US-mediated BBB opening to increase circulating biomarker detection is an exciting new direction for the use of this technology. One barrier in the study of circulating glioma biomarkers is the inherent low amounts of biomaterial released by these tumors into the bloodstream.6 Meng et al. have recently shown that US can be used to increase circulating biomarkers from brain tumors.3 Our study demonstrates that it is critically important to optimize all variables in the collection, processing and downstream analysis of glioma-derived biomaterials within blood. One major limitation of our study is we did not perform experiments to analyze the source of the cfDNA released into the bloodstream following sonication. It will be important to see if the increase in cfDNA caused by US-mediated BBB opening will also release tumor derived DNA into the bloodstream as opposed to DNA from components of the brain vasculature; however given that only a small volume of plasma (<500 µL) can be obtained from each mouse, these studies may be better performed in human subjects where a larger volume of plasma may be collected.
In conclusion, our study demonstrates that cfDNA released by US-mediated BBB opening into the circulation is influenced by time. Specifically, we show that it takes 15 minutes for a noticeable increase in cfDNA following US-mediated BBB opening in both healthy and glioma bearing mice. These findings provide important considerations for future investigations involving US-mediated BBB opening induced release of glioma biomarkers.
Funding
Philanthropic support from Dan and Sharon Moceri, GLC is supported by National Institutes of Health, National Institute of Neurological Disorders and Stroke (DP2NS111506) and AMS is supported by National Cancer Institute (1R01CA245969-01A1).
Conflict of Interest: The authors declare no conflicts of interest
Authorship Statement. Experimental Design (D.Y.Z., A.G., H.C.H., G.L.C., A.M.S.), Data acquisition (D.Y.Z., A.G., S.J.K., M.C., A.M.S.), Data Analysis (D.Y.Z., A.G., H.C.H., M.W.Y., C.D., R.S., G.L.C., A.M.S.), Manuscript preparation (D.Y.Z., A.G., H.C.H., M.W.Y., C.D., R.S., G.L.C., A.M.S.), Study Supervision (A.M.S.).
References
- 1. Zhu L, Cheng G, Ye D, et al. . Focused ultrasound-enabled brain tumor liquid biopsy. Sci Rep. 2018;8(1):6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pacia CP, Zhu L, Yang Y, et al. . Feasibility and safety of focused ultrasound-enabled liquid biopsy in the brain of a porcine model. Sci Rep. 2020;10(1):7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meng Y, Pople CB, Suppiah S, et al. . MR-guided focused ultrasound liquid biopsy enriches circulating biomarkers in patients with brain tumors. Neuro Oncol. 2021;23(10):1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heitzer E, Haque IS, Roberts CES, Speicher MR. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet. 2019;20(2):71–88. [DOI] [PubMed] [Google Scholar]
- 5. Sabari JK, Offin M, Stephens D, et al. . A prospective study of circulating tumor DNA to guide matched targeted therapy in lung cancers. J Natl Cancer Inst. 2019;111(6):575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Westphal M, Lamszus K. Circulating biomarkers for gliomas. Nat Rev Neurol. 2015;11(10):556–566. [DOI] [PubMed] [Google Scholar]
- 7. Nabavizadeh SA, Ware JB, Guiry S, et al. . Imaging and histopathologic correlates of plasma cell-free DNA concentration and circulating tumor DNA in adult patients with newly diagnosed glioblastoma. Neurooncol Adv. 2020;2(1):vdaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sonabend AM, Stupp R. Overcoming the blood-brain barrier with an implantable ultrasound device. Clin Cancer Res. 2019;25(13):3750–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang DY, Dmello C, Chen L, et al. . Ultrasound-mediated delivery of paclitaxel for glioma: a comparative study of distribution, toxicity, and efficacy of albumin-bound versus cremophor formulations. Clin Cancer Res. 2020;26(2):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sonabend AM, Yun J, Lei L, et al. . Murine cell line model of proneural glioma for evaluation of anti-tumor therapies. J Neurooncol. 2013;112(3):375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]