Abstract
Mature B lymphocytes are unique in containing nuclear Rel proteins prior to cell stimulation. This activity consists largely of p50–c-Rel heterodimers, and its importance for B-cell function is exemplified by reduced B-cell viability in several genetically altered mouse strains. Here we suggest a mechanism for the cell specificity and the subunit composition of constitutive B-cell NF-κB based on the observed properties of Rel homo- and heterodimers and IκBα. We show that c-Rel lacks a nuclear export sequence, making the removal of c-Rel-containing complexes from the nucleus less efficient than removal of p65-containing complexes. Second, the nuclear import potential of p65 and c-Rel homodimers but not p50-associated heterodimers was attenuated when they were complexed to IκBα, leading to a greater propensity of heterodimers to be nuclear. We propose that subunit composition of B-cell NF-κB reflects the inefficient retrieval of p50–c-Rel heterodimers from the nucleus. Cell specificity may be a consequence of c-Rel–IκBα complexes being present only in mature B cells, which leads to nuclear c-Rel due to IκBα turnover and shuttling of the complex.
The Rel family of transcription factors regulate inducible gene transcription (11, 34). These proteins share an approximately 300 amino acid domain at the N terminus, the Rel homology domain (RHD), which is used for protein-protein interactions and DNA binding. Homo- or heterodimerization between Rel family members is essential for DNA binding and interactions with the regulatory IκB proteins which inhibit DNA binding and localize Rel proteins to the cell cytoplasm. In most cells, Rel proteins are located in the cell cytoplasm complexed to one of three IκB proteins—IκBα, IκBβ, or IκBɛ (15, 25, 28, 44). Upon cell stimulation, IκBs are phosphorylated at their N termini by IκB kinases, which targets them for proteasome-mediated degradation. This leaves the Rel proteins free to translocate to the nucleus and activate gene expression. Rel protein-dependent gene expression must be strictly controlled, as exemplified by the phenotype of IκBα-deficient mice (3, 24). These animals die soon after birth, presumably due to aberrant gene activation. Thus, regulation of DNA binding and subcellular distribution of Rel proteins by IκBs is a critical aspect of the biology of these transcription factors.
The three IκBs retain Rel proteins in the cytoplasm by different mechanisms. IκBα, but not IκBβ or IκBɛ, contains a strong nuclear export sequence (NES), and cytoplasmic location of p65-RelA by IκBα has been shown to require nuclear export via the exportin CRM1 (16, 19, 42). Specifically, inhibition of nuclear export by mutating the IκBα NES or affecting CRM1 function genetically or pharmacologically leads to nuclear accumulation of p65-IκBα complexes (16, 19, 42). Based on such studies, the current model is that cytoplasmic localization by IκBα is a dynamic process, with IκBα-containing complexes shuttling between the nucleus and the cytoplasm. Presumably, the net cytoplasmic appearance of these complexes occurs because the balance between nuclear entry and exit is tilted in favor of the latter.
In contrast, IκBβ and IκBɛ localize Rel proteins to the cytoplasm as effectively as IκBα but do not contain identifiable NESs. We have shown that nuclear export is not involved in this process and suggested that cytoplasmic localization by IκBβ and IκBɛ reflects sequestration of Rel proteins (43). That is, Rel-IκBβ (or -IκBɛ) complexes are cytoplasmic because they never make it into the nucleus. Furthermore, even when nuclear p65-IκBβ complexes were created by sequential expression of p65 followed by IκBβ, export of this complex to the cytoplasm was inefficient (43). Shuttling of IκBα-associated complexes but not IκBβ-associated complexes implies that there is residual nuclear localization sequence (NLS) activity in IκBα complexes. Our working model is that IκBβ association must hide the Rel NLSs more effectively.
Mature B lymphocytes are unique in containing nuclear Rel proteins prior to cell stimulation. The constitutive nuclear activity consists of p50–c-Rel heterodimers (13, 22, 27, 30), and its importance for B-cell function is exemplified by the lower viability of B cells from p50-deficient and xid mice, both of which have decreased constitutive NF-κB (2, 7, 20, 21, 23, 38, 39, 41). In c-Rel-deficient mice, p50-p65 (NF-κB) heterodimer levels are elevated in splenic B cells, suggesting that lack of p50–c-Rel heterodimers must be compensated for (10, 26). Moreover, B-cell viability is severely decreased in p65–c-Rel double-deficient mice (12). Several models have been proposed to explain the presence of nuclear NF-κB in B cells, such as increased turnover rate of IκBα in WEHI 231 cells (29) and protection of nuclear NF-κB by IκBβ (36). Miyamoto and colleagues have provided much of the support for the turnover model, in particular the recent demonstration that intracellular calcium controls IκBα degradation in mature B cells (9, 31, 40). However, the importance of IκBα half-life for constitutive nuclear NF-κB has not been directly demonstrated. Indeed, there is some evidence that IκBα is not turned over as fast in immunoglobulin G (IgG)-positive B cells, yet there is constitutive NF-κB in the nucleus (6). Finally, it is not clear why the constitutive DNA-binding activity is largely composed of p50–c-Rel heterodimers.
In this paper we show that p50–c-Rel heterodimers have a greater propensity to be nuclear than p50-p65 heterodimers or either homodimer alone. Two properties of Rel and IκB proteins dictate this preference. First, the NLSs of p65 and c-Rel homodimers are attenuated by association with IκBα, while p50 provides a strong NLS and thereby increases nuclear import of heterodimers. Second, c-Rel does not contain a nuclear export sequence like p65; therefore, c-Rel-containing complexes are exported out of the nucleus less efficiently. We propose that the subunit composition of B-cell NF-κB reflects the inefficient retrieval of p50–c-Rel heterodimers from the nucleus. The question of retrieval only arises in B cells because c-Rel is sequestered in the cytoplasm of pre-B cells and non-B cells by association with IκBβ. B-cell-specific association of c-Rel with IκBα may further increase nuclear c-Rel because of IκBα turnover. Thus, the presence of constitutive “NF-κB” in B-cell nuclei is the dynamic consequence of the properties of Rel and IκB proteins.
MATERIALS AND METHODS
Cell lines and strains.
D5h3 T hybridoma cells, WEHI-231 (immature B) cells, and DC27 (mature T) cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Gibco-BRL, New York, N.Y.) supplemented with 10% heat-inactivated fetal bovine serum, 50 μM β-mercaptoethanol, 50 U of penicillin per ml, and 50 μg of streptomycin per ml. A20 (mature B) cells, 70Z (pre-B) cells, M12 (mature B) cells, 22D6 (pre-B) cells, 38B9 (pre-B) cells, and 3A9 (mature T) cells were grown in RPMI 1640 medium (Gibco-BRL) with 10% heat-inactivated fetal bovine serum and the above supplements. COS cells were cultured in DMEM with 10% newborn calf serum and the above antibiotics. BOSC23 (human kidney epithelial cells) were maintained in DMEM with 10% heat-inactivated fetal bovine serum and antibiotics.
Splenic primary B cells were made by positively selecting mouse spleen cell suspensions using CD19 MicroBeads and MiniMACs with an MS column (Miltenyi Biotec). The sorted cells were then analyzed by flow cytometry, and over 95% of cells were CD19 positive. For splenic T cells, single-cell suspensions of mouse spleen cells were incubated with concanvalin A (4 μg/ml) (CalBiochem) for 3 days. Cells were then washed to remove concanavalin A and cultured in the presence of recombinant human interleukin-2 (IL-2) (50 U/ml) (BD Transduction Laboratories) for 7 days. Over 95% of T cells were T-cell receptor-beta positive by flow cytometry.
Plasmids.
EGFP-p65 and pCDNA3.HA-IκBα have been described previously (42). pCDNA3.HA-IκBαLIL3A49(NESn) and HA-IκBαNESnc were made by PCR using fragments from pGFP-IκBαNESc and pGFP-IκBαNESc-LIL3A49 (42) with a hemagglutinin (HA) tag containing 5′ primer and 3′ primer. The products were cloned into BamHI and EcoRI sites of pCDNA3 vector (Invitrogen Corporation). EGFP-p65(RHD) and EGFP-p65(364) contain mouse p65 (amino acids 1 to 306) and murine p65 (amino acids 1 to 364), respectively, in frame after the green fluorescent protein (GFP) gene of EGFP-C3 (Invitrogen Corporation) in the EcoRI and KpnI sites. EGFP-c-Rel, EGFP-c-Rel(RHD), and EGFP-c-Rel(344) contain full-length murine c-Rel, murine c-Rel (amino acids 1 to 297), mouse c-Rel (amino acids 1 to 344), respectively, in frame after GFP in the EcoRI and BamHI sites. The fragment in EGFP-p65(NES4A) was generated by PCR site-directed mutagenesis, with the four leucines at residues 434, 438, 441, and 443 changed to alanines and inserted in frame after GFP at EcoRI and XhoI sites. pCDNA3.hCRM1 was obtained by BamHI digestion of full-length human CRM1 cDNA clone in pBS-hCRM1 (a gift from G. Grosveld). pERVF2.p50 contained the full-length processed murine p50 (4), which was cloned after the cytomegalovirus promoter in plasmid pERVF2. The sequences of all plasmids used in this study were confirmed, and expression of proteins was verified by Western blot analysis.
Transfections.
COS cell and BOSC23 cell transfection was done by the calcium phosphate method as previously described (32) or with Fugene-6 transfection reagent according to the manufacturer's specifications (Roche Molecular Biochemicals). Leptomycin B (LMB) (10 ng/ml), a generous gift from M. Yoshida (University of Tokyo), was added 4 h prior to harvest.
Immunoprecipitation.
Cells were washed three times with phosphate-buffered saline (PBS). Whole-cell extracts were made by lysing the cells in TNT buffer (20 mM Tris-HCl [pH 7.5], 200 mM NaCl, 1% Triton X-100) with phosphatase and protease inhibitors. Then 100 μg of whole-cell extracts was incubated first with 30 μl of prewashed protein A-agarose beads in a 50% slurry (Roche Molecular Biochemicals) and then appropriate antibodies in TNT buffer with 0.1% Triton X-100 for 4 h. Anti-IκBα, anti-c-Rel, and anti-p65 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). The supernatants were subjected to secondary immunoprecipitation as indicated in the figure legends. The immunoprecipitates were washed three times with TNT buffer and eluted with 4 × sodium dodecyl sulfate (SDS) buffer by heating at 100°C for 5 min. The supernatants were separated by SDS–10% polyacrylamide gel electrophoresis (PAGE) for Western blot analysis.
Western blot analysis.
Polyacrylamide gels were transferred to enhanced chemiluminescence Hybond nitrocellulose membrane (Amersham). The membranes were blocked with 5% milk in TBST buffer and then incubated with anti-c-Rel, anti-p65, or anti-IκBα at a dilution of 1:500 for 1 h at room temperature. The membranes were washed and incubated with peroxidase-conjugated anti-rabbit Ig (Amersham) at a dilution of 1:2,000. The chemiluminescence signal was detected using SuperSignal substrate according to the manufacturer's specification (Pierce, Rockford, Ill.).
Immunostaining.
The procedures for immunostaining of adherent cells were the same as described previously (42). For staining suspension cells (T cells and B cells), the procedures were described previously (8). Cells were allowed to settle on UV-sterilized coverslips (Fisher) with or without poly-l-lysine treatment (Sigma) overnight. LMB (100 ng/ml) was added 45 min prior to the staining. Cells were fixed in 3% paraformaldehyde for 20 min at room temperature and then permeabilized with buffer WB (0.5% NP-40, 0.01% sodium azide in 1 × PBS). The blocking was done with 5% normal donkey serum (Jackson Laboratory) at room temperature for 30 min. After blocking, the cells were incubated with primary antibodies in buffer WB with 5% normal donkey serum for 30 min at room temperature and then washed three times with buffer WB. The secondary antibody, fluorescein isothiocyanate (FITC)-conjugated anti-rabbit Ig (Jackson Laboratory) was used at a 1:300 dilution in WB with 5% normal donkey serum for 30 min at room temperature. After several washes 4′,6′-diamidino-2-phenylindole (DAPI) (Molecular Probes, Eugene, Oreg.) was used at 1 μg/ml for counterstaining the nucleus. After further washing, the cells were mounted with ProLong Antifade (Molecular Probes) according to the manufacturer's specifications.
Fluorescence microscopy.
The subcellular localization of GFP and the immunofluorescence signals were observed by fluorescence microscopy (Axiophot II; Zeiss) with a GFP generic filter, FITC, rhodamine, and DAPI filter.
RESULTS
Multiple factors contribute to cytosolic localization by IκBα.
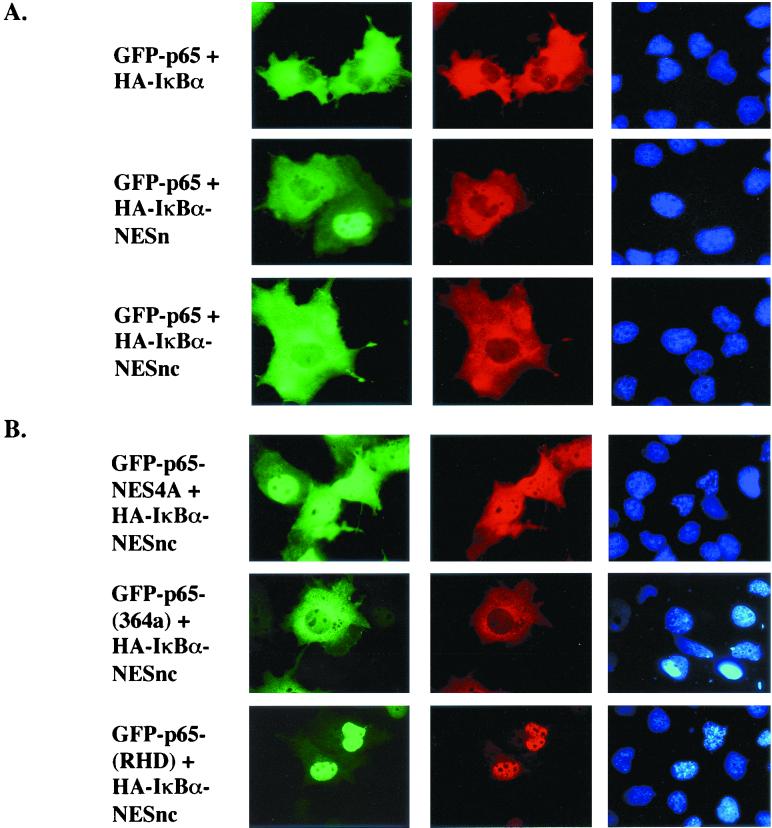
We have previously shown that IκBα contains an NES in its N-terminal domain, and cytosolic localization of p65 by IκBα requires nuclear export. IκBα also contains another NES in its C-terminal domain (1, 33, 37). To test whether these NESs were the only mediators of cytoplasmic localization, GFP-Rel proteins were coexpressed in COS cells with IκBα derivatives mutated in one or both NESs. GFP-p65 was entirely cytoplasmic in the presence of wild-type IκBα or derivatives mutated at the N-terminal NES (NESn) or both NESs (NESnc) (Fig. 1, top three panels). The observed cytoplasmic location was the result of nuclear export because treatment of the transfected cells with LMB led to nuclear accumulation of both p65 and IκBα proteins (data not shown). Therefore, neither known NES sequence in IκBα was essential for cytoplasmic retention of p65. We conclude that other CRM1-dependent export determinants were involved, which could lie within the ankyrin domains of IκBα or in p65. Mutation of an NES in p65 identified by Harhaj and Sun (14) resulted in mixed nuclear and cytoplasmic distribution of the protein in the presence of IκBαNESnc (Fig. 1B, top panel). Cytoplasmic expression of a complex that lacks all three NESs was more clearly evident in a C-terminal truncation mutant of p65, GFP-p65(364), which lacks the C-terminal NES. This protein was located in the cytoplasm in the presence of IκBαNESnc. We conclude that nuclear export is not the only mechanism by which IκBα regulates the subcellular distribution of p65.
FIG. 1.
Multiple determinants of cytosolic localization in p65-IκBα protein complex. GFP-p65 and derivatives thereof were expressed in COS cells with HA-IκBα (first row) or HA-IκBα derivatives containing mutations at the N-terminal NES (NESn), or both N- and C-terminal NESs (NESnc), as indicated. Thirty-six hours after transfection, half of the cells were treated with LMB and fixed for fluorescent visualization. Columns show GFP fluorescence, anti-HA antibodies detected by rhodamine-coupled secondary antibody, and DAPI staining, respectively. GFP-p65NES4A contains four alanine residues replacing leucines in the C-terminal NES of p65; GFP-p65(RHD) contains the first 306 amino acids of p65 that include the RHD and the NLS (residues 301 to 304); GFP-p65(364) contains the first 364 amino acids of p65. All p65 derivatives were coexpressed with the doubly mutated IκBαNESnc. Cells were fixed for fluorescent visualization as described in the text. Representative results are shown from one of three independent experiments.
A fusion protein containing only the RHD of p65, GFP-p65(RHD), was exclusively nuclear when coexpressed with IκBαNESnc (Fig. 1B), but cytoplasmic in the presence of wild-type IκBα (data not shown). Colocalization of the IκBα derivatives with GFP-p65(RHD) suggested that the proteins were complexed (compare GFP and anti-HA fluorescence in Fig. 1B, third line); this was directly confirmed by coimmunoprecipitation (data not shown). Location of GFP-p65(364) in the cytoplasm but GFP-p65(306) in the nucleus when complexed to IκBαNESnc suggested that IκBα interaction with residues beyond the NLS of p65 (which is within the RHD) attenuated NLS function. Thus, the mutated IκBα compensated for the attenuated NLS of GFP-p65(364) but not the exposed NLS of GFP-p65(RHD). These observations provide the first functional evidence for attenuation of the p65 NLS in complex with IκBα. Use of an export-deficient IκBα mutant was key in distinguishing the relative contributions of export and import in the net subcellular distribution of p65-IκBα complexes.
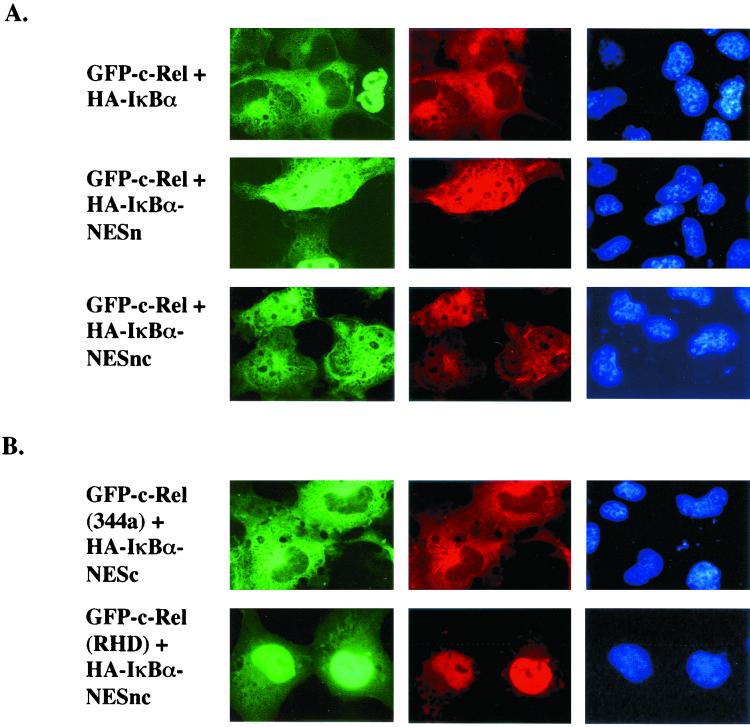
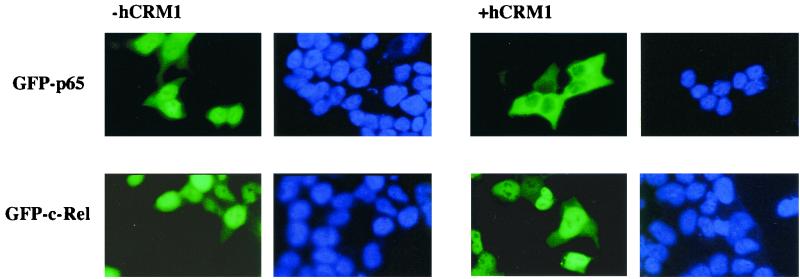
Unlike GFP-65, GFP-c-Rel showed a mixed distribution (nucleus plus cytoplasm) in the presence of IκBαNESnc (Fig. 2, third line). This pattern was similar to the NES-mutated p65 derivative, suggesting that c-Rel did not have an NES in its C-terminal domain. To directly compare CRM1 responsiveness of p65 and c-Rel, we coexpressed GFP derivatives of these proteins in BOSC23 cells together with human CRM1 (hCRM1) (Fig. 3). Both proteins were predominantly nuclear in the absence of CRM1; however, GFP-p65 but not GFP-c-Rel redistributed to the cytoplasm in the presence of hCRM1. This effect was mediated by the C-terminal NES of p65, because point mutations or deletion of this sequence made the protein unresponsive to hCRM1 (data not shown).
FIG. 2.
Multiple determinants of cytosolic localization in c-Rel–IκBα protein complex. GFP-c-Rel and derivatives thereof were transiently transfected with HA-IκBα (first row) or its derivative nuclear export mutant NESn or NESnc as described in the legend to Fig. 1. GFP-c-Rel(RHD) contains the first 297 residues of c-Rel that include the RHD and the NLS (residues 292 to 295). GFP-c-Rel(344) contains the first 344 residues of c-Rel. Thirty-six hours after transfection, cells were immunostained for fluorescent visualization. Results shown are representative of at least three independent experiments.
FIG. 3.
c-Rel does not contain a CRM1-dependent export sequence. BOSC23 cells were transiently transfected with expression plasmids encoding GFP-p65 or GFP-cRel proteins as indicated, with or without a plasmid encoding full-length hCRM1. The total amount of DNA used in transfections was normalized with empty vector. Thirty-six hours after transfection, cells were fixed for DAPI staining and fluorescence visualization. Results are from one of three independent experiments.
As observed with p65, the RHD of c-Rel, GFP-c-Rel(RHD), was exclusively nuclear in the presence of IκBαNESnc, but a longer derivative had considerable cytoplasmic expression (Fig. 2B). Our interpretation is that the NLS of c-Rel(344) is attenuated by interaction with IκBα, but that of the RHD is not. We conclude that p65 and c-Rel are similar to the extent that both NLSs are attenuated in association with IκBα; they are different in that p65 contains a C-terminal NES, but c-Rel does not. The biological implications of the differences are discussed below.
Effects of heterodimerization.
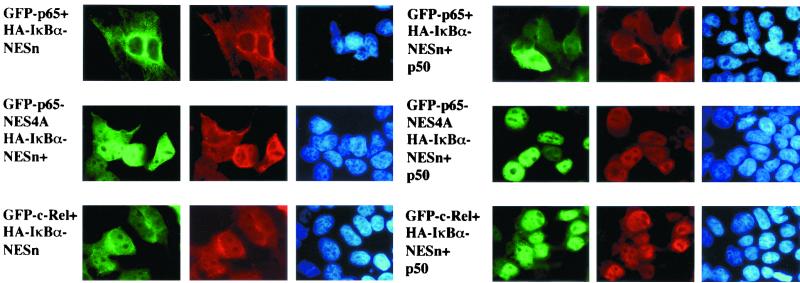
The major cellular Rel-DNA complexes detected by mobility shift assays contain heterodimers of p65 or c-Rel with p50 or p52 (27, 30). To explore how the subunit composition of the Rel dimer affected subcellular localization, we coexpressed GFP-p65 or GFP–c-Rel with p50 and IκBα. The GFP-p65–p50 heterodimer was located in the cytoplasm in the presence of either wild-type IκBα (data not shown) or IκBαNESn (Fig. 4). Mutation of the p65 NES did not affect the cytosolic location of the p65-p50 complex in the presence of wild-type IκBα (data not shown), but resulted in nuclear localization of the IκBαNESn-conaining heterotrimer (Fig. 4, fourth and fifth columns, second row).
FIG. 4.
Increased nuclear propensity of p50–c-Rel heterodimers. Left panels show the subcellular distribution of homodimers of GFP-p65 (first row), an NES-mutated GFP-p65 (second row), and GFP-c-Rel (third row) in the presence of HA-IκBαNESn (IκBα mutated in the N-terminal NES). Right panels show the distribution of heterodimers of these Rel proteins together with p50 in the presence of HA-IκBαNESn. In all cases, GFP fluorescence colocalized with anti-HA, indicating that IκBα was associated with the Rel homo- or heterodimers. All complexes were predominantly nuclear when the cells were treated with LMB for the last 3 h prior to harvest (data not shown). Results shown are from one of three independent experiments.
All cytosolic complexes moved to the nucleus when the cells were treated with LMB (data not shown), indicating that export was necessary to maintain NF-κB in the cytosol. Nuclear location of p50-p65NES heterodimers (Fig. 4, fourth column, second row) but not p65NES homodimers (Fig. 4, first column, second row) complexed to IκBαNESn suggested that the p50 NLS was more effective at directing nuclear entry than the attenuated p65 NLS in an IκBα-containing complex. We conclude that the p50 NLS is active in an NF-κB–IκBα complex and that cytosolic location of the complex requires ongoing nuclear export.
The data in Fig. 2 and 3 showed that c-Rel does not contain an NES. Consistent with this, GFP–c-Rel–p50 complexes were retained in the cytoplasm by wild-type IκBα (data not shown), but not IκBαNESn (Fig. 4, third row). Because c-Rel homodimers were brought to the cytoplasm by IκBαNESn, the nuclear location of p50–c-Rel heterodimers (Fig. 4, fourth column, third row) is most likely because the NLS of p50 is exposed in this complex. These observations suggest that various Rel homo- and heterodimers exhibit a hierarchy in their preference to be located in the cytoplasm when complexed to IκBα. At one extreme, the (p65)2-IκBα complex contains three NESs, which allows it to be removed most efficiently from the nucleus, while the p50–c-Rel–IκBα complex contains only one NES, making it more likely to be nuclear. As shown below, this may be, in part, the explanation for the preferential location of p50–c-Rel complexes in the nuclei of B cells. Conversely, the propensity for nuclear export may define the ordered removal of Rel complexes from the nucleus at the end of cell stimulation.
Rel complexes in lymphocytes.
From the studies described above, we drew two conclusions: first, that p65 and c-Rel differed in that p65 contained an NES while c-Rel did not, and second, that heterodimerization with p50 favored nuclear localization because its NLS was active when complexed to IκBα. We propose that the subunit specificity of p50–c-Rel complexes in B-cell nuclei may be a consequence of inefficient export of this complex from the nucleus compared to p65-containing complexes. B-cell specificity of the phenomenon is a consequence of c-Rel association with IκBα only in B cells (see below); this leads to increased c-Rel in the nucleus as it shuttles with IκBα and because IκBα is degraded more rapidly.
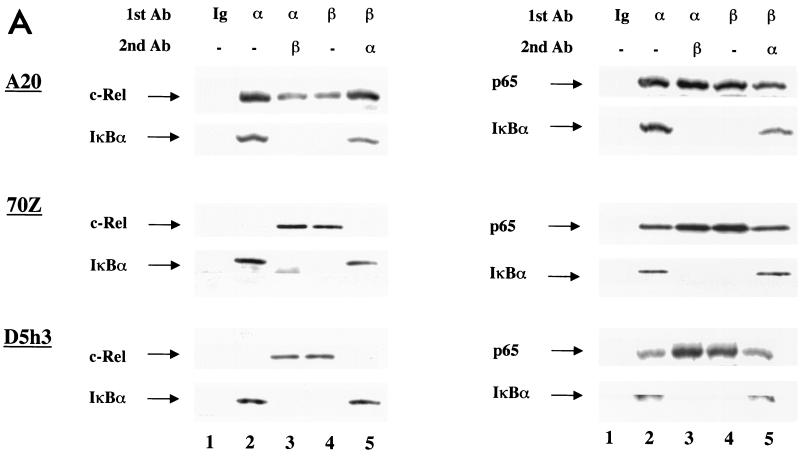
To identify c-Rel-associated IκBs, we carried out immunoprecipitations followed by immunoblotting. Whole-cell extracts from the indicated cells were immunoprecipitated with only one antibody or sequentially with two antibodies. The precipitates were fractionated by SDS-PAGE and immunoblotted with anti-c-Rel or anti-p65 antibodies. Anti-IκBα coprecipitated c-Rel only from A20 (mature) B cells, but not from 70Z (pre-B) cells or D5h3 (T hybridoma) cells (Fig. 5A, left panels, lane 2). In contrast, c-Rel–IκBβ association was detected in all these cell extracts (Fig. 5A, lane 4). Furthermore, preclearing the extracts with anti-IκBβ removed all the c-Rel from 70Z and D5h3 extracts, confirming that there was an insignificant amount of IκBα-associated c-Rel in these cells (Fig. 5A, lane 5). Conversely, preclearing with anti-IκBα reduced the residual IκBβ-associated c-Rel only in A20 extracts (Fig. 5A, lane 3). These observations indicate that c-Rel only associates with IκBα in mature B cells. Unlike c-Rel, endogenous p65 was associated with both IκBα and IκBβ in all three cell lines (Fig. 5A, right panels).
FIG. 5.
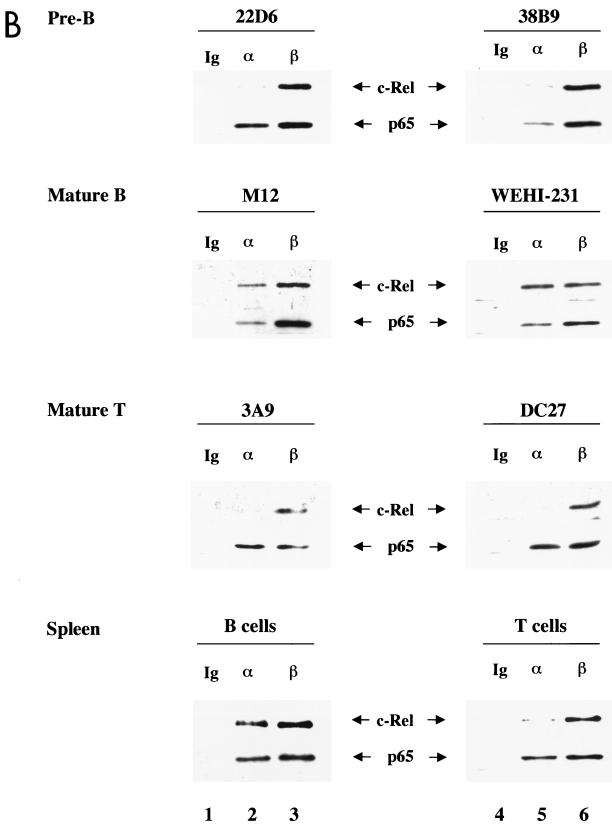
c-Rel associates with IκBα in mature B cells but not in pre-B cells and mature T cells. (A) Whole-cell extracts (100 μg) from mature B cells (A20), pre-B cells (70Z), or mature T cells (D5h3) were immunoprecipitated with nonspecific IgG (lane 1), anti-IκBα antibody (lane 2), or anti-IκBβ antibody (lane 4). For sequential immunoprecipitation, extracts were precleared with the first antibody and immunoprecipitated with the second antibody, as indicated above the lanes (lanes 3 and 5). Immunoprecipitates were separated by SDS-PAGE, and Rel proteins associated with IκBs were detected by immunoblotting. Left panel shows the results using anti-c-Rel and anti-IκBα antibodies (Ab) to probe the blots; right panel was probed with anti-p65 and anti-IκBα antibodies. IκBβ protein was not assayed for because it comigrated with the Ig heavy-chain protein used for immunoprecipitation. Results are representative of at least three independent experiments. (B) Whole-cell extracts from indicated cells were analyzed by immunoprecipitation using nonspecific IgG (lane 1), anti-IκBα (lane 2), and anti-IκBβ (lane 3), followed by immunoblotting as described above. For each cell type, the upper panel shows the results using anti-c-Rel antibodies to probe the blots, and the lower panel shows the results of probing with anti-p65 antibodies. Top three rows correspond to cell lines representing pre-B, mature B, and T cells. Bottom row shows the analysis of primary splenic B and T cells purified as described in the text. Results are representative of at least two independent experiments with each cell type.
We further confirmed this trend in additional cell lines. In these studies, anti-IκBα or anti-IκBβ was used for immunoprecipitation. IκB-associated proteins were fractionated by SDS-PAGE, and c-Rel or p65 was detected by immunoblotting. IκBα-associated p65 was detected in six additional cell lines tested representing pre-B, mature B, and mature T cells (Fig. 5B, top three panels, lanes 2 and 5), as well as primary splenic B and T cells (Fig. 5B, bottom panel). Anti-IκBβ also coprecipitated both c-Rel and p65 from all cells examined (Fig. 5B, lanes 3 and 6). However, IκBα-associated c-Rel was only detected in mature B-cell lines and in splenic B cells (Fig. 5B, second and fourth rows, lanes 2 and 5), but not in pre-B, mature T, or splenic T cells. Taken together with the results in Fig. 5A, these observations suggest that c-Rel–IκBα complexes are a unique feature of mature B lymphocytes.
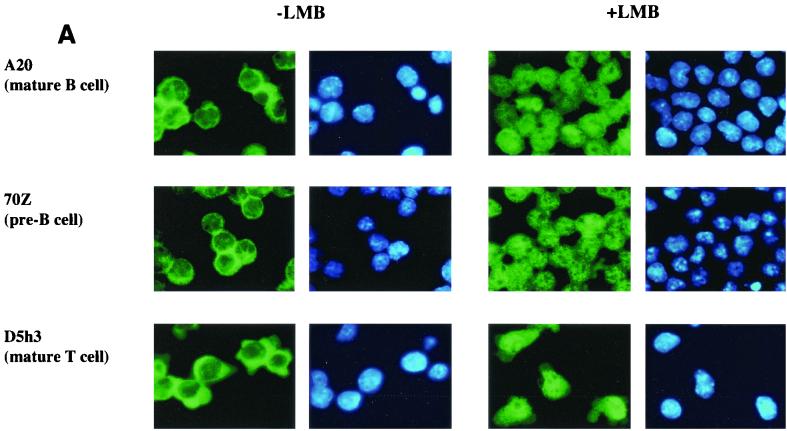
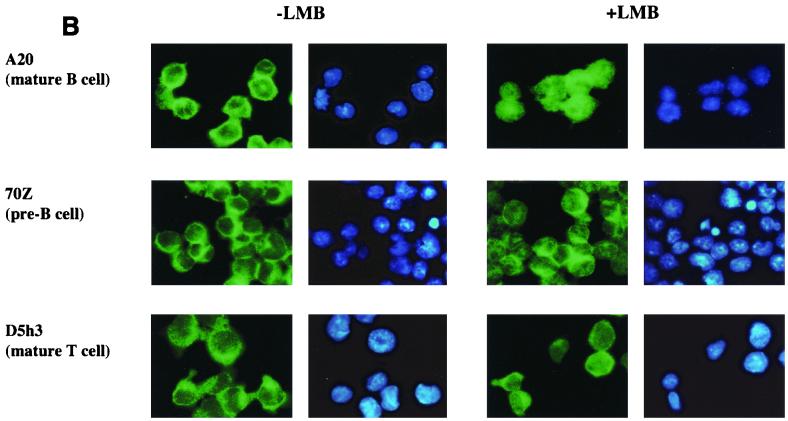
The proposed model makes the additional prediction that p65 shuttles between the nucleus and the cytoplasm in most cells because it is associated with IκBα, while c-Rel shuttles only in B cells. We tested this prediction using LMB to block CRM1-dependent export in the three cell lines used in Fig. 5A. Endogenous p65 was predominantly cytoplasmic in all three cell lines prior to treatment with LMB (Fig. 6A, left panels). In the presence of LMB, nuclear translocation of p65 was evident from its mixed nuclear and cytoplasmic distribution in all cells (Fig. 6A, right panels). The partial redistribution of p65 was probably because only a fraction of the total cytoplasmic pool of p65 was associated with IκBα; the rest that was associated with other IκBs did not shuttle, and therefore its subcellular location did not change with LMB. Immunostaining with anti-IκBα and anti-IκBβ antibodies confirmed that only IκBα redistributed to the nucleus in response to LMB treatment of these cells (data not shown). Unlike p65, the predominantly cytoplasmic distribution of c-Rel in untreated cells was only altered in A20 cells after LMB treatment (Fig. 6B). Note that the constitutively nuclear c-Rel in A20 cells is difficult to discern in these figures because the majority of the protein, and consequently the imunofluorescence, is located in the cytoplasm. We conclude that the subcellular location of c-Rel in B cells is regulated by competing nuclear import and export. In contrast, c-Rel in other cell types is sequestered in the cytoplasm by IκBβ or IκBɛ.
FIG. 6.
c-Rel shuttles only in mature B cells. A20, D5h3, and 70Z cells were settled on poly-l-lysine-coated coverslips overnight. Half of the coverslips with attached cells were treated with LMB (100 ng/ml) for 45 min. Cells with and without LMB treatment were fixed and permeabilized for immunostaining with anti-p65 antibodies (A) or anti-c-Rel antibodies (B). Green fluorescence in the first and the third columns shows endogenous p65 (A) and endogenous c-Rel (B) of A20 (top row), 70Z (second row), and D5h3 (third row) cells before and after LMB treatment as indicated. Blue fluorescence in the second and fourth columns shows DAPI staining of nuclei. Results shown are from one of three independent experiments.
DISCUSSION
We extended our earlier studies of nuclear-cytoplasmic dynamics of Rel-IκB proteins to (i) compare the contribution of Rel proteins to export-dependent cytoplasmic localization and (ii) study the effects of heterodimerization on subcellular distribution. Our observations lead to a mechanism for the constitutive presence of nuclear p50–c-Rel heterodimers in B lymphocytes. The crux of our model is that inefficient export of p50–c-Rel heterodimers from B-cell nuclei may lead to accumulation of this protein in the nucleus. Why does this only happen in B cells, and what determines subunit specificity? We found that c-Rel is mainly associated with IκBβ in non-B cells. The slow turnover rate of IκBβ and its lack of nucleocytoplasmic shuttling ensure that this c-Rel rarely enters the nucleus.
In contrast, IκBα-associated c-Rel in mature B cells can reach the nucleus in two ways. First, there is continuous generation of free p65 and c-Rel (homo- and p50-containing heterodimers) due to the higher turnover rate of IκBα. These proteins translocate to the nucleus, from which they must be exported to maintain the cytoplasmic pool. This must be mediated by IκBα, since the other IκBs do not have export potential. Newly synthesized IκBα that migrates to the nucleus finds a DNA-binding competent pool of Rel homo- and heterodimers with which to associate. We propose that the absence of an NES in c-Rel is manifest at this stage, and c-Rel is exported out of the nucleus less efficiently than p65. Association with p50 further enhances nuclear propensity of p50–c-Rel heterodimers because the p50 NLS is not attenuated by IκBα. The proposed discrimination based on efficiency of export suggests that export capacity is limiting under these conditions. At present we cannot distinguish whether the limitation is at the level of the export machinery or the export chaperone IκBα. Second, IκBα-associated c-Rel also enters the nucleus, because the complexes shuttle. Once in the nucleus, some of these complexes may dissociate if, for example, export is limiting and the intact complex is not rapidly removed. Again, the reduced export potential of c-Rel-containing complexes compared to p65-containing complexes would skew the subunit composition of the resulting DNA-binding activity towards c-Rel. Thus, B-cell-specific nuclear NF-κB is the dynamic consequence of the combined properties of Rel, IκB, and cellular export proteins.
Antibody accessibility studies had first led to the model that cytoplasmic retention of NF-κB by IκBs was the result of hiding the NLSs present on the Rel proteins (45). While this may be true for IκBβ and IκBɛ (43), it is clear that functional NLS activity is present in Rel-IκBα complexes, which undergo nucleocytoplasmic shuttling. Moreover, the finding that IκBα-dependent nuclear export is the major mechanism of cytoplasmic “retention” by IκBα circumvents the necessity to hide NLSs in Rel-IκBα complexes. This raises the question of whether association with IκBα affects the NLS of Rel proteins at all.
The crystal structure of the NF-κB–IκBα complex shows that IκBα is oriented such that the first two ankyrin domains contact the C terminus of the RHDs of p65 and p50 (17, 18). This interaction forces a 19-amino-acid stretch of p65 that contains the NLS into two α-helices (18). The NLS is located at the end of the first helix, and three of its side chains make salt bridges with IκBα. The close association of IκBα and the p65 NLS is consistent with inactivation or attenuation of NLS function. The NLS of p50 does not contact IκBα, leading to the speculation that it may be occluded indirectly to keep the p50-p65 complex in the cytoplasm (5).
We provide the first functional evidence that the NLSs of both p65 and c-Rel are attenuated by association with IκBα. This conclusion follows from the observation that the RHDs of p65 and c-Rel were nuclear in the presence of an NES-mutated IκBα derivative, whereas longer Rel proteins were located in the cytoplasm. Use of the NES-mutated IκBα in these studies was essential to distinguish export- from import-dependent cytoplasmic location. Because export did not play a role in the subcellular distribution of these complexes, our interpretation is that the nuclear import potential of longer Rel proteins is reduced when they are complexed to IκBα. This is most likely due to the secondary structure that is induced in the p65 NLS by IκBα when residues beyond the RHD are present (18). Recent biochemical studies also show that these additional residues contribute to IκBα binding to p65 (35).
We also present evidence that the p50 NLS is functionally available and mediates nuclear entry of p50-p65-IκBα and p50–c-Rel–IκBα complexes. These observations offer no evidence for “indirect” occlusion of the p50 NLS. Rather, cytoplasmic location of the heterodimers must involve compensation of the p50 NLS by the export potential of the IκBα NES. In other studies we have found that the p65 NES is weaker than the IκBα NES (W. Tam, unpublished observations). This makes good sense because a strong NES in p65 would make this protein constitutively cytoplasmic even in the absence of IκBα. Instead, the weaker NES accentuates nuclear exit in the presence of IκBα, but is dominated by the unattenuated NLS in the absence of IκBα. Modulating the strength of NESs and NLSs in Rel and IκB family members presumably allows the appropriate balance of nuclear and cytoplasmic expression of NF-κB proteins.
Our observations indicate a hierarchy of nuclear propensity among Rel homo- and heterodimers which is based on the presence or absence of NESs and association with p50. For example, p65 homodimers contain two NESs and two unattenuated NLSs; (p65)2 complexes are predominantly nuclear because the NLSs dominate. Association with IκBα attenuates the NLSs and adds an additional NES; the result is location in the cytoplasm. Compared to p65, c-Rel has a greater tendency to be nuclear because it lacks a well-defined NES. Association with p50 further accentuates this by providing an NLS that is active even when complexed to IκBα. As described above, one consequence of this hierarchy is to enrich c-Rel–p50 complexes in B-cell nuclei. We speculate that this hierarchy may also dictate the efficiency by which Rel proteins are removed from the nucleus at the end of cell stimulation. Accordingly, p65 homodimers would be removed most efficiently, followed by c-Rel homodimers, followed by p50-p65 and p50–c-Rel heterodimers. One reason may be that the most potent transcription activator must be downregulated effectively for the cell to reach its resting state.
ACKNOWLEDGMENTS
The human CRM1 gene and LMB were kindly provided by G. Grosveld and M. Yoshida (University of Tokyo), respectively. We thank Phil Gnatowski for help in preparation of the manuscript.
This work was supported by NIH grant AI-41035 to R.S.
REFERENCES
- 1.Arenzana-Seisdedos F, Turpin P, Rodriguez M, Thomas D, Hay R T, Virelizier J L, Dargemont C. Nuclear localization of IκBα promotes active transport of NF-κB from the nucleus to the cytoplasm. J Cell Sci. 1997;110:369–378. doi: 10.1242/jcs.110.3.369. [DOI] [PubMed] [Google Scholar]
- 2.Bajpai U D, Zhang K, Teutsch M, Sen R, Wortis H H. Bruton's tyrosine kinase links the B cell receptor to nuclear factor κB activation. J Exp Med. 2000;191:1735–1744. doi: 10.1084/jem.191.10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beg A A, Sha W C, Bronson R T, Baltimore D. Constitutive NF-κB activation, enhanced granulopoiesis, and neonatal lethality in Iκ Bα-deficient mice. Genes Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- 4.Blank V, Kourilsky P, Israel A. Cytoplasmic retention, DNA binding and processing of the NF-κB p50 precursor are controlled by a small region in its C-terminus. EMBO J. 1991;10:4159–4167. doi: 10.1002/j.1460-2075.1991.tb04994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cramer P, Muller C W. A firm hand on NFκB: structures of the IκBα-NFκB complex. Structure Fold Des. 1999;7:R1–R6. doi: 10.1016/s0969-2126(99)80002-1. [DOI] [PubMed] [Google Scholar]
- 6.Doerre S, Corley R B. Constitutive nuclear translocation of NF-κB in B cells in the absence of IκB degradation. J Immunol. 1999;163:269–277. [PubMed] [Google Scholar]
- 7.Ellmeier W, Jung S, Sunshine M J, Hatam F, Xu Y, Baltimore D, Mano H, Littman D R. Severe B cell deficiency in mice lacking the Tec kinase family members Tec and Btk. J Exp Med. 2000;192:1611–1624. doi: 10.1084/jem.192.11.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feske S, Draeger R, Peter H H, Eichmann K, Rao A. The duration of nuclear residence of NFAT determines the pattern of cytokine expression in human SCID T cells. J Immunol. 2000;165:297–305. doi: 10.4049/jimmunol.165.1.297. [DOI] [PubMed] [Google Scholar]
- 9.Fields E R, Seufzer B J, Oltz E M, Miyamoto S. A switch in distinct IκBα degradation mechanisms mediates constitutive NF-κB activation in mature B cells. J Immunol. 2000;164:4762–4767. doi: 10.4049/jimmunol.164.9.4762. [DOI] [PubMed] [Google Scholar]
- 10.Gerondakis S, Strasser A, Metcalf D, Grigoriadis G, Scheerlinck J Y, Grumont R J. Rel-deficient T cells exhibit defects in production of interleukin 3 and granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci USA. 1996;93:3405–3409. doi: 10.1073/pnas.93.8.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossmann M, Nakamura Y, Grumont R, Gerondakis S. New insights into the roles of Rel/NFκB transcription factors in immune function, hemopoiesis and human disease. Int J Biochem Cell Biol. 1999;31:1209–1219. doi: 10.1016/s1357-2725(99)00068-0. [DOI] [PubMed] [Google Scholar]
- 12.Grossmann M, O'Reilly L A, Gugasyan R, Strasser A, Adams J M, Gerondakis S. The anti-apoptotic activities of Rel and RelA required during B-cell maturation involve the regulation of Bcl-2 expression. EMBO J. 2000;19:6351–6360. doi: 10.1093/emboj/19.23.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grumont R J, Gerondakis S. The subunit composition of NF-κB complexes changes during B-cell development. Cell Growth Differ. 1994;5:1321–1331. [PubMed] [Google Scholar]
- 14.Harhaj E W, Sun S C. Regulation of RelA subcellular localization by a putative nuclear export signal and p50. Mol Cell Biol. 1999;19:7088–7095. doi: 10.1128/mcb.19.10.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hay R T, Vuillard L, Desterro J M, Rodriguez M S. Control of NF-κB transcriptional activation by signal induced proteolysis of IκBα. Phil Trans R Soc Lond B Biol Sci. 1999;354:1601–1609. doi: 10.1098/rstb.1999.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang T T, Kudo N, Yoshida M, Miyamoto S. A nuclear export signal in the N-terminal regulatory domain of IκBα controls cytoplasmic localization of inactive NF-κB/IκBα complexes. Proc Natl Acad Sci USA. 2000;97:1014–1019. doi: 10.1073/pnas.97.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huxford T, Huang D B, Malek S, Ghosh G. The crystal structure of the IκBα/NF-κB complex reveals mechanisms of NF-κB inactivation. Cell. 1998;95:759–770. doi: 10.1016/s0092-8674(00)81699-2. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs M D, Harrison S C. Structure of an IκBα/NF-κB complex. Cell. 1998;95:749–758. doi: 10.1016/s0092-8674(00)81698-0. [DOI] [PubMed] [Google Scholar]
- 19.Johnson C, Van Antwerp D, Hope T J. An N-terminal nuclear export signal is required for the nucleocytoplasmic shuttling of IκBα. EMBO J. 1999;18:6682–6693. doi: 10.1093/emboj/18.23.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerner J D, Appleby M W, Mohr R N, Chien S, Rawlings D J, Maliszewski C R, Witte O N, Perlmutter R M. Impaired expansion of mouse B cell progenitors lacking Btk. Immunity. 1995;3:301–312. doi: 10.1016/1074-7613(95)90115-9. [DOI] [PubMed] [Google Scholar]
- 21.Khan W N, Alt F W, Gerstein R M, Malynn B A, Larsson I, Rathbun G, Davidson L, Muller S, Kantor A B, Herzenberg L A, et al. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 22.Kistler B, Rolink A, Marienfeld R, Neumann M, Wirth T. Induction of nuclear factor-κB during primary B cell differentiation. J Immunol. 1998;160:2308–2317. [PubMed] [Google Scholar]
- 23.Klaus G G, Holman M, Johnson-Leger C, Elgueta-Karstegl C, Atkins C. A re-evaluation of the effects of X-linked immunodeficiency (xid) mutation on B cell differentiation and function in the mouse. Eur J Immunol. 1997;27:2749–2756. doi: 10.1002/eji.1830271102. [DOI] [PubMed] [Google Scholar]
- 24.Klement J F, Rice N R, Car B D, Abbondanzo S J, Powers G D, Bhatt P H, Chen C H, Rosen C A, Stewart C L. IκBα deficiency results in a sustained NF-κB response and severe widespread dermatitis in mice. Mol Cell Biol. 1996;16:2341–2349. doi: 10.1128/mcb.16.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krappmann D, Scheidereit C. Regulation of NF-κB activity by IκBα and IκBβ stability. Immunobiology. 1997;198:3–13. doi: 10.1016/s0171-2985(97)80022-8. [DOI] [PubMed] [Google Scholar]
- 26.Liou H C, Jin Z, Tumang J, Andjelic S, Smith K A, Liou M L. c-Rel is crucial for lymphocyte proliferation but dispensable for T cell effector function. Int Immunol. 1999;11:361–371. doi: 10.1093/intimm/11.3.361. [DOI] [PubMed] [Google Scholar]
- 27.Liou H C, Sha W C, Scott M L, Baltimore D. Sequential induction of NF-κB/Rel family proteins during B-cell terminal differentiation. Mol Cell Biol. 1994;14:5349–5359. doi: 10.1128/mcb.14.8.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.May M J, Ghosh S. Rel/NF-κB and IκB proteins: an overview. Semin Cancer Biol. 1997;8:63–73. doi: 10.1006/scbi.1997.0057. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto S, Chiao P J, Verma I M. Enhanced IκBα degradation is responsible for constitutive NF-κB activity in mature murine B-cell lines. Mol Cell Biol. 1994;14:3276–3282. doi: 10.1128/mcb.14.5.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyamoto S, Schmitt M J, Verma I M. Qualitative changes in the subunit composition of κB-binding complexes during murine B-cell differentiation. Proc Natl Acad Sci USA. 1994;91:5056–5060. doi: 10.1073/pnas.91.11.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyamoto S, Seufzer B J, Shumway S D. Novel IκBα proteolytic pathway in WEHI231 immature B cells. Mol Cell Biol. 1998;18:19–29. doi: 10.1128/mcb.18.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelsen B, Tian G, Erman B, Gregoire J, Maki R, Graves B, Sen R. Regulation of lymphoid-specific immunoglobulin μ heavy chain gene enhancer by ETS-domain proteins. Science. 1993;261:82–86. doi: 10.1126/science.8316859. [DOI] [PubMed] [Google Scholar]
- 33.Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 34.Pahl H L. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 35.Phelps C B, Sengchanthalangsy L L, Huxford T, Ghosh G. Mechanism of IκBα binding to NF-κB dimers. J Biol Chem. 2000;275:29840–29846. doi: 10.1074/jbc.M004899200. [DOI] [PubMed] [Google Scholar]
- 36.Phillips R J, Ghosh S. Regulation of IκBβ in WEHI 231 mature B cells. Mol Cell Biol. 1997;17:4390–4396. doi: 10.1128/mcb.17.8.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prigent M, Barlat I, Langen H, Dargemont C. IκBα and IκBα/NF-κB complexes are retained in the cytoplasm through interaction with a novel partner, RasGAP SH3-binding protein 2. J Biol Chem. 2000;275:36441–36449. doi: 10.1074/jbc.M004751200. [DOI] [PubMed] [Google Scholar]
- 38.Satterthwaite A B, Li Z, Witte O N. Btk function in B cell development and response. Semin Immunol. 1998;10:309–316. doi: 10.1006/smim.1998.0123. [DOI] [PubMed] [Google Scholar]
- 39.Sha W C, Liou H C, Tuomanen E I, Baltimore D. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 40.Shumway S D, Maki M, Miyamoto S. The PEST domain of IκBα is necessary and sufficient for in vitro degradation by μ-calpain. J Biol Chem. 1999;274:30874–30881. doi: 10.1074/jbc.274.43.30874. [DOI] [PubMed] [Google Scholar]
- 41.Snapper C M, Zelazowski P, Rosas F R, Kehry M R, Tian M, Baltimore D, Sha W C. B cells from p50/NF-κB knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switching. J Immunol. 1996;156:183–191. [PubMed] [Google Scholar]
- 42.Tam W F, Lee L H, Davis L, Sen R. Cytoplasmic sequestration of Rel proteins by IκBα requires CRM1-dependent nuclear export. Mol Cell Biol. 2000;20:2269–2284. doi: 10.1128/mcb.20.6.2269-2284.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tam W F, Sen R. IκB family member function by different mechanisms. J Biol Chem. 2001;276:7701–7704. doi: 10.1074/jbc.C000916200. [DOI] [PubMed] [Google Scholar]
- 44.Whiteside S T, Israel A. IκB proteins: structure, function and regulation. Semin Cancer Biol. 1997;8:75–82. doi: 10.1006/scbi.1997.0058. [DOI] [PubMed] [Google Scholar]
- 45.Zabel U, Henkel T, Silva M S, Baeuerle P A. Nuclear uptake control of NF-κB by MAD-3, an IκB protein present in the nucleus. EMBO J. 1993;12:201–211. doi: 10.1002/j.1460-2075.1993.tb05646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]