Abstract
As multi-cellular organisms evolved from small clusters of cells to complex metazoans, biological tubes became essential for life. Tubes are typically thought of as mainly playing a role in transport, with the hollow space (lumen) acting as a conduit to distribute nutrients and waste, or for gas exchange. However, biological tubes also provide a platform for physiological, mechanical, and structural functions. Indeed, tubulogenesis is often a critical aspect of morphogenesis and organogenesis. C. elegans is made up of tubes that provide structural support and protection (the epidermis), perform the mechanical and enzymatic processes of digestion (the buccal cavity, pharynx, intestine, and rectum), transport fluids for osmoregulation (the excretory system), and execute the functions necessary for reproduction (the germline, spermatheca, uterus and vulva). Here we review our current understanding of the genetic regulation, molecular processes, and physical forces involved in tubulogenesis and morphogenesis of the epidermal, digestive and excretory systems in C. elegans.
Keywords: aECM, actin, compaction, cytoskeleton, epithelia, fusion, intercalation, microtubules, morphogenesis, myosin, outgrowth, tubulogenesis
INTRODUCTION
The body plan of C. elegans can be described as a set of nested biological tubes (Figure 1). Internally there are alimentary, reproductive and excretory systems, consisting of unicellular and multicellular tubes. These are packed inside the tube-like epidermis, which protects the animal from the external environment, anchors the muscles and nervous system that allow for movement, and gives C. elegans its characteristic roundworm shape.
Figure 1. Tubular systems that make up the C. elegans body plan.
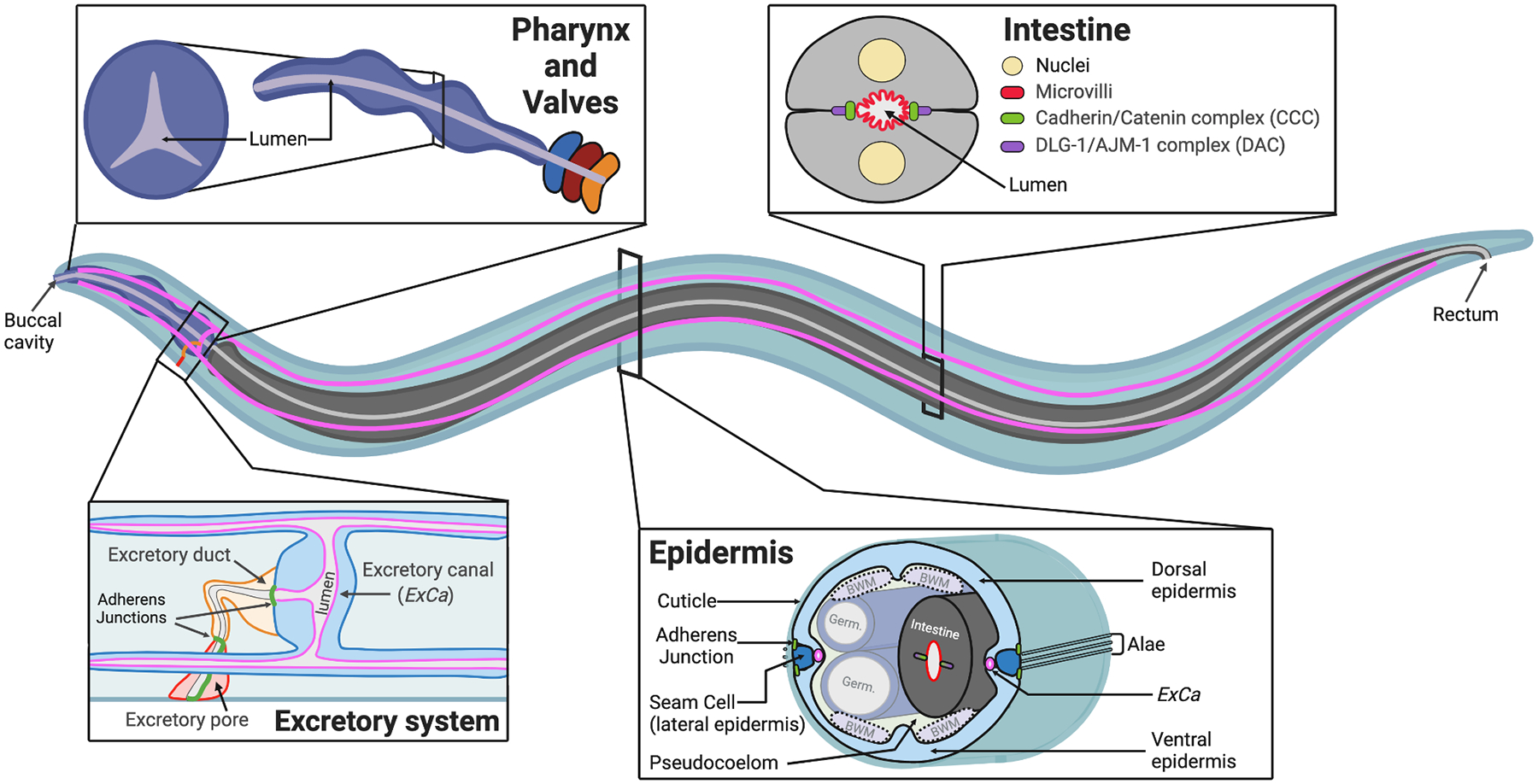
In this review we focus on four tissues in C. elegans: the pharynx, the intestine, the excretory system, and the epidermis. Each forms one, or more, tubes that perform specialized physiological functions. The pharynx lumen grinds food and transports it to the intestine, where it is digested and distributed systemically by secretion into the pseudocoelomic space. The excretory system is made up of three unicellular tubes that collectively function in osmoregulation. The epidermis forms a multi-cellular tube during embryogenesis and many epidermal cells fuse into a syncytial hypodermis as development proceeds. The epidermis secretes a protective collagen-rich cuticle; the dorsal and ventral regions display circumferential ridges termed annuli (not shown), while the lateral seam cells generate cuticle with specialized longitudinal ridges (alae) during the L1, dauer, and adult stages. The epidermis encloses the pseudocoelomic cavity, germline (germ.) and intestine. Four quadrants of body wall muscles (BWM) are attached to the dorsal and ventral epidermis, and these attachments provide force for morphogenesis and movement (see main text). Embedded in the epidermis are mechano-sensory and motor neurons (not shown), and the hollow excretory canal (ExCa) projections that stretch along the entire length of the worm (magenta). Diagrams inspired by WormAtlas (www.wormatlas.org), Sundaram and Buechner (2016), and Pasti and Labouesse (2014).
The powerful imaging, transgenic, and genetic approaches available to C. elegans researchers have allowed us to define, often to the single-cell level, how morphogenesis and tubulogenesis are regulated to form the structures that make the worm. Work from many groups has revealed the signal-transduction pathways and transcription factors that establish cell fates in these tissues, and the downstream effectors (regulators of cell polarity, the cytoskeleton, intracellular trafficking, cell adhesion, and the extracellular matrix) that control the cell behaviors (growth, adhesion, intercalation, fusion, and regulation of shape) required for morphogenesis and tubulogenesis.
Here we summarize how the epidermal, alimentary and excretory system tubes are formed, with a focus on what is known, and what remains to be discovered, about the forces that regulate their morphogenesis. We will not discuss morphogenesis of the reproductive system, as several excellent reviews, including a chapter elsewhere in this issue by Iosilevskii and Podbilewicz, describe these (Kelley and Cram, 2019; Schindler and Sherwood, 2013; Sherwood and Plastino, 2018). We hope this review stimulates new discussions of old findings, puts recent findings into broader context, and motivates future work, using new technologies for precise genome editing and in vivo manipulation of protein activities, to further define the mechanisms by which forces regulate tubulogenesis and morphogenesis.
THE EPIDERMIS
We begin with the epidermis, which includes at least three distinct sub-tissues, dorsal, lateral and ventral cells, that remain attached during their movements (Figure 2). Each epidermal region undergoes uniquely programmed movements, always connected to the rest of the epidermis, and in contact with other developing tissues, to form the tube that defines the shape of the worm.
Figure 2. Epidermal movements and forces.
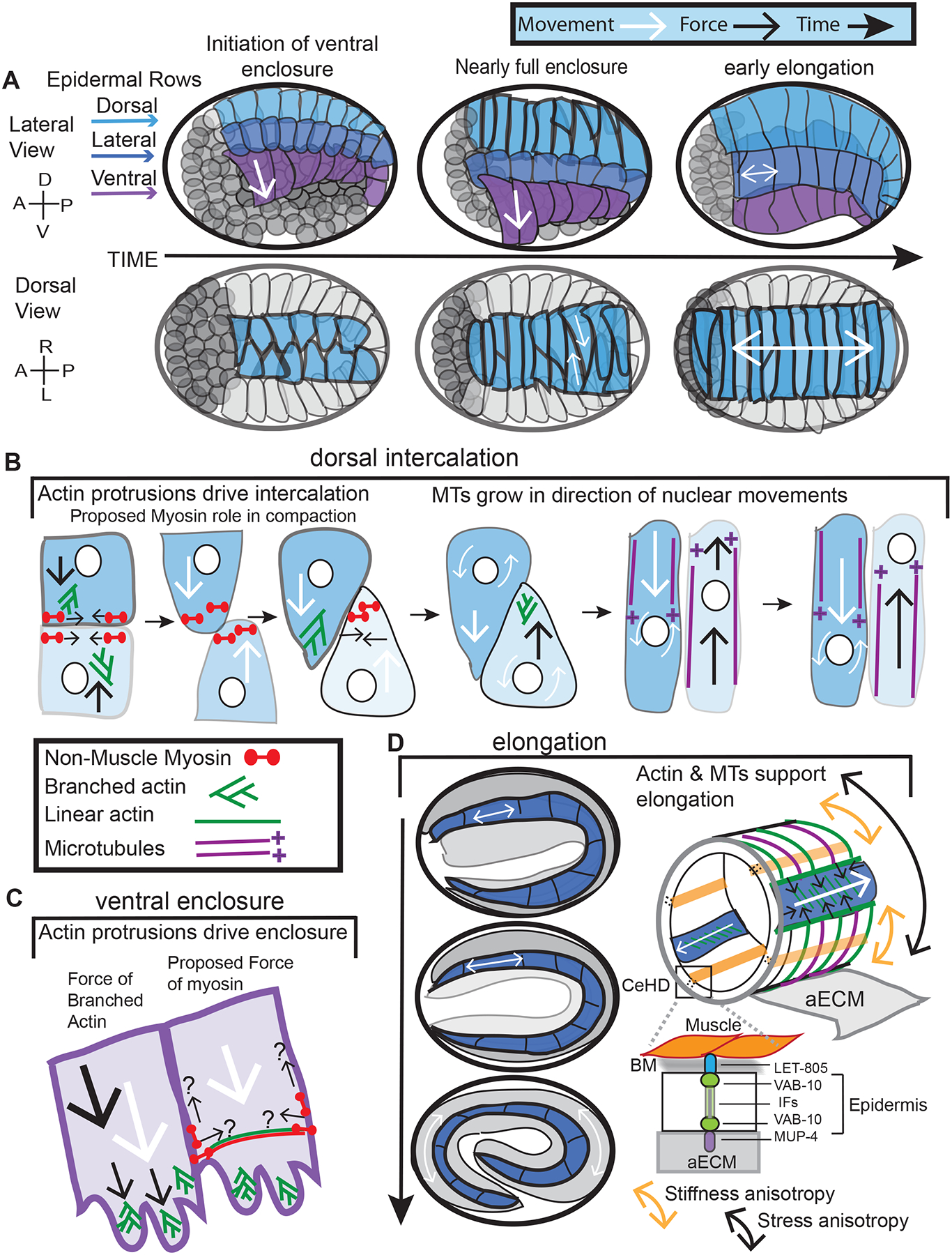
A) The epidermis begins as a sheet of cells that organize into 6 anterior/posteriorly oriented rows: 2 dorsal, 2 lateral and 2 ventral. The forces (black arrows) known to drive the morphogenetic movements of these rows: (B) dorsal intercalation, (C) ventral enclosure and (D) elongation, are described in the text. White arrows: direction of movement. The actin and microtubule cytoskeleton and their regulators drive these movements. MT and nuclear movements during dorsal intercalation based on Fridolfsson and Starr (2010). Proposed myosin role during dorsal intercalation based on Wernike et al. (2016) and during ventral enclosure based on Wernike et al. (2016) and Wallace et al. (2018). MTs working with actomyosin during elongation from 2-fold to 4-fold based on Vuong-Brender et al. (2017a). Elongation details from (Vuong-Brender et al., 2016a) and other References cited. CeHD: Hemidesmosome, BM: Basement Membrane, IFs: Intermediate Filaments.
Overview of Epidermal Morphogenesis
The epidermis forms from cells derived from the AB and C founder cell lineages that assemble by the 400-cell stage into a dorsal cap (Sulston et al., 1983). The cells arrange into six adherent lateral rows that then begin to move. The two dorsal-most rows begin dorsal intercalation, a process analogous to vertebrate convergent extension, which leads the two rows to interdigitate thereby becoming one longer row that lengthens the embryo along the anterior/posterior axis (Figure 2A, 2B). Meanwhile, the two outer, ventral rows begin to migrate towards the ventral midline in a process called ventral enclosure (Figure 2A. Priess and Hirsh, 1986; Sulston et al., 1983; Williams-Masson et al., 1998). After the ventral rows meet at the midline, the anterior head region is covered by epidermis, in a poorly understood process that is only now being analyzed (Fan et al., 2019), though specific transcription factors, like REF-1, are known to affect head epidermal fate (Neves and Priess, 2005; Sulston et al., 1983; Williams-Masson et al., 1998). Once the embryo is enclosed by epidermis, elongation, the lengthening process that transforms the oval embryo into a tubular worm, begins with dramatic cell shape changes in another two rows, the lateral seam cells, coupled with increased stiffness in the dorsal and ventral epidermal cells (Priess and Hirsh, 1986; Vuong-Brender et al., 2016b. See Fig. 2A, D)
Collective cell behaviors form the epidermal tube
Dorsal Intercalation
During dorsal intercalation cells in the two dorsal rows of epidermis become wedge shaped, with narrowed regions protruding toward the dorsal midline, and then alternating cells interdigitate across the midline (Sulston et al., 1983. Fig. 2A, B). Williams-Masson and colleagues used transmission and scanning electron microscopy (TEM and SEM) to show that during the midline-directed movements of dorsal intercalation the cells form basolateral protrusions at midline regions, that coincide with apparent decreased adhesion at apical junctions (Williams-Masson et al., 1998). This suggested these basolateral protrusions forced cells apart and overcame adhesion to permit the cellular rearrangements. These findings raised the question of what factors were needed to rearrange the cytoskeleton to promote these basolateral protrusions.
Priess and Hirsh (1986) showed that laterally aligned microtubules (MTs) and filamentous actin fibers (F-actin) assemble in the embryonic epidermis, and F-actin disruption using Cytochalasin D interrupted all cell shape changes. Genetic screens for regulators of morphogenesis identified specific actin nucleation and elongation factors required for the formation of medial protrusions during dorsal intercalation. In particular, some regulators of branched actin were shown to be essential for dorsal intercalation including the components of the WAVE complex of C. elegans, GEX-2/CYFIP/Sra-1/p140, GEX-3/KETTE/NAP1 (Soto et al., 2002), WVE-1/WAVE (originally GEX-1) and ABI-1/ABI (Patel et al., 2008). The Arp2/3 complex assembles networks of branched actin filaments that drive cell motility (Machesky et al., 1994; Mullins et al., 1998; Pollard and Borisy, 2003). Arp2/3 activity is stimulated by nucleation promoting factors (NPFs) like WAVE and WASP, which are themselves activated by the GTPases CED-10/Rac1 and CDC-42 respectively (Reviewed in Pollard, 2007). While loss of WAVE components completely blocked both ventral enclosure and dorsal intercalation, null mutations in CED-10/Rac1 did not, which suggested other GTPases may help regulate the WAVE complex (Soto et al., 2002). Loss of WSP-1/WASP and UNC-34, the sole ortholog of Enabled/VASP proteins that promote elongation of bundled F-actin (Bachmann et al., 1999), also blocks most morphogenic movements (Withee et al., 2004), although dorsal intercalation was not examined. These studies suggested that Arp2/3-dependent branched actin was essential for protrusions.
Examination of dorsal intercalation using dominant negative or constitutively active GTPases in embryos showed that the GTPases CED-10/Rac1, MIG-2 (a second Rac-like GTPase) and CDC-42 contribute to dorsal intercalation through WAVE and WASP (Walck-Shannon et al., 2016; Walck-Shannon et al., 2015). These studies also identified a role for the guanine exchange factor (GEF) UNC-73/TRIO in dorsal intercalation, likely through CED-10 and MIG-2. Additionally, CRML-1/CARMIL (Capping Arp2/3 myosin I linker) protein, first identified as an UNC-73 regulator (Vanderzalm et al., 2009), limits UNC-73 activity during dorsal intercalation (Walck-Shannon et al., 2015). These studies support the model that dorsal intercalation requires the forces provided by Arp2/3-dependent branched actin. The WAVE complex appears to make the largest contribution since loss of function mutations completely block intercalation (Patel et al., 2008; Soto et al., 2002), while ced-10, wsp-1 and cdc-42 mutations have partial penetrance, and require loss of other actin regulators to become significant (Soto et al., 2002; Walck-Shannon et al., 2016; Walck-Shannon et al., 2015). These studies do not rule out a role for other actin nucleators like formins, which mediate formation of linear F-actin (Reviewed in Goode and Eck, 2007; Pruyne, 2016), in dorsal intercalation.
Dorsal intercalation occurs independently of lateral epidermal cells, or the neighboring muscle or intestinal cells (Williams-Masson et al., 1998). Therefore the cell fate determinants of the dorsal hypodermis may hold clues as to how a tissue, or in this case, a sub-set of a tissue, organizes force for intercalations. Overall epidermal fate is specified by the transcription factors ELT-1/GATA (Page et al., 1997), which activates the zinc finger protein LIN-26 (Labouesse et al., 1996; Labouesse et al., 1994), and ELT-3/GATA (Gilleard et al., 1999). LIN-26 and ELT-1 are expressed in all epidermal cells (Quintin et al., 2001) while ELT-3 is expressed only in dorsal and ventral cells (Gilleard and McGhee, 2001). The die-1 gene, encoding a zinc finger C2H2 transcription factor, was one of the first identified to affect dorsal intercalation, although its loss affects other cells including muscle, seam cells, pharynx, and intestine (Heid et al., 2001).
Unanswered questions in dorsal intercalation
Nuclear migrations accompany dorsal intercalation, with nuclei migrating in the direction of the midline oriented protrusions, and MTs transition from centrosome-based organization to a non-centrosomal microtubule organizing center (ncMTOC. Reviewed in Sanchez and Feldman, 2017) just before becoming polarized in the direction of nuclear movements (Fig. 2B. Fridolfsson and Starr, 2010). While nuclear migrations are not required for dorsal intercalation or for viability (Starr et al., 2001), chemical disruption of MTs partially disrupted intercalation (Williams-Masson et al., 1998). Therefore, there may be additional roles for MTs in providing or reinforcing force during the intercalations.
Dorsal intercalation is distinct from anterior to posterior, with anterior, AB blastomere-derived cells intercalating first (Williams-Masson et al., 1998). Some mutations that block epidermal dorsal intercalation, like loss of transcription factor die-1 (Heid et al., 2001), appear to mainly affect the posterior C blastomere-derived cells. Therefore, our current understanding of how transcription factors, like die-1, regulate intercalation only applies to a subset of the dorsal epidermis. Perhaps genes required specifically for anterior intercalations are redundant, or they have earlier essential roles, so forward genetic screens have failed to identify them. Moreover, the targets of transcription factors that promote intercalations remain unknown, so we are as of yet unable to connect fate specification to cell behaviors.
In addition to anterior/posterior differences in cell behaviors during dorsal intercalation, there is evidence of lateral asymmetries. For example, a die-1::gfp transcriptional reporter was expressed equally in left and right cells as intercalation begins, but later appeared higher in the left cells (Heid et al., 2001). Other transgenic reporters expressed in the epidermis, like the fatty acid binding protein LBP-1 (Plenefisch et al., 2000) and LAT-1/latrophilin, which is structurally related to the planar cell polarity protein Flamingo/CELSR (Langenhan et al., 2009), become enriched asymmetrically during dorsal intercalation. This raises the intriguing question of whether left/right force asymmetry promotes medial movements during dorsal intercalation.
Given that the anterior/posterior and left/right asymmetries of cell behaviors and protein expression appear to be transient, and that dorsal intercalation is relatively rapid, lasting under 100 min., new tools for rapid and tissue-specific depletion of proteins using degrons (Armenti et al., 2014b; Nance and Frokjaer-Jensen, 2019; Wang et al., 2017; Zhang et al., 2015) with carefully selected sub-tissue enriched promoters, may help clarify the role and regulation of the asymmetries seen in dorsal intercalation.
Ventral enclosure
Ventral enclosure begins when two anterior cells of the two ventral rows of the epidermal sheet, known as the leading cells, begin to pull the entire epidermis ventrally (Fig. 2A). This migration continues until the leading cells meet at the ventral midline, and ends when the more posterior cells, called pocket cells, complete an actomyosin-based constriction that allows them to zip up the ventral surface (Figure 2A). Genetic screens identified the WAVE complex as a major force in initiating the ventral enclosure, as 100% of embryos mutant for any component of the WAVE complex fail to initiate ventral enclosure, despite the correct specification of epidermis (Patel et al., 2008). In wild-type embryos the migrating ventral epidermal cells enrich F-actin at the leading edge, and display dynamic protrusions and retractions (Figure 2C). Loss of WAVE-dependent branched actin eliminates the ventral F-actin enrichment and dynamic protrusion, although less dynamic and more filopodial-like protrusions remain (Bernadskaya et al., 2012; Soto et al., 2002). Other branched F-actin NPFs contribute to this process, including WASP/WSP-1 (Sawa et al., 2003; Withee et al., 2004). The GTPases activating these NPFs, CED-10/Rac1 and CDC-42 are also thought to contribute (Ouellette et al., 2016; Patel et al., 2008; Soto et al., 2002; Zilberman et al., 2017).
Unanswered questions in ventral enclosure
Myosin-based contractility must contribute to ventral enclosure, but exactly how remains to be determined. Studies showed that the GTPase RHO-1/RhoA and its effectors, two C. elegans non-muscle myosin homologs, nmy-1 and nmy-2, were required for migration during ventral closure. Unexpectedly, genetic experiments supported that the non-muscle myosin activity was required in the neuroblasts underlying the epidermis (Fotopoulos et al., 2013; Piekny et al., 2003; Piekny and Mains, 2003; Wernike et al., 2016). However, NMY-2 was also shown to be present in the ventral epidermis and likely contributing to ventral enclosure (See Fig. 2C. Wallace et al., 2018; Wernike et al., 2016). Additionally, we discovered that HUM-7, a myosin-like protein that contains a Rho GTPase-activating protein domain, negatively regulates RHO-1, as well as the enrichment of F-actin and myosin in ventral epidermal cells during enclosure, and we found that elevated myosin and F-actin in epidermal cells leads many hum-7 mutant embryos to enclose faster (Wallace et al., 2018). Finally, although the specific role of myosin in migrating ventral epidermal cells remains unclear, transient purse-string-like myosin structures form dorsal to the F-actin-rich protrusions (See Fig. 2C. Wallace et al., 2018; Wernike et al., 2016), suggesting a role for myosin in these protrusions.
Several explanations for myosin’s apparent non-autonomous role in neuroblasts during ventral enclosure have been proposed. Perhaps myosin-based mechanical forces in neuroblasts are coupled to constriction of the overlying epidermal cells, or neuroblasts promote a chemical cue that guides myosin activation in the epidermis during ventral enclosure (Wernike et al., 2016). Several candidate cues exist. Guidance proteins that guide axonal migrations later in development have been shown to guide ventral enclosure in the early embryo including SAX-3/ROBO, VAB-1/Ephrin Receptor, UNC-40/DCC and PLX-2/plexin (Bernadskaya et al., 2012; George et al., 1998; Ghenea et al., 2005; Ikegami et al., 2012). These guidance cues organize ventrally polarized F-actin in the leading edge cells, and regulate membrane localization of CED-10/Rac1 and WVE-1 in the epidermis during this time (Bernadskaya et al., 2012). How these guidance cues organize myosin contractility in support of ventral enclosure is not known.
Epidermal Elongation
Elongation is the best studied of the epidermal movements (reviewed recently in Carvalho and Broday, 2020; Vuong-Brender et al., 2016a). The pioneering studies of Priess and Hirsh (1986) introduced the idea that circumferentially organized F-actin and MTs create the forces that establish the tube-like shape of the worm. Laser ablation of pharynx, intestine, neurons and body wall muscle (BWM) precursors did not interfere with elongation. In contrast, any ablation of hypodermal cells affected elongation, suggesting the epidermis is under tension. Inhibition of actin polymerization and laser ablation studies showed that the force for elongation likely came from the actin cytoskeleton, was reinforced by the microtubule cytoskeleton, and was then maintained by connections between the epidermal cells and extracellular structures secreted by the epidermis (Priess and Hirsh, 1986).
Elongation has been separated into two phases (Figure 2D). The first, which elongates the embryo to the 2-fold stage, involves changes in the shape of the seam cells. The molecular regulation of myosin in all three sub-tissues of the epidermis contributes to force regulation in this phase of elongation. Myosin activity in the lateral seam cells appears to generate a high level of tension required for elongation; for example, myosin regulatory light chain, MLC-4, and activators of myosin, like RHO-1 and the Rho Kinase ortholog LET-502, help generate high levels of tension required for elongation (Gally et al., 2009; Piekny and Mains, 2003; Shelton et al., 1999; Wissmann et al., 1997). Conversely, myosin tension is kept at lower levels in dorsal and ventral epidermal rows by Rho-family GTPase-activating proteins (RhoGAPs) including RGA-2 (Diogon et al., 2007), and the myosin phosphatase, MEL-11, and this region of lower tension is thought to contribute to elongation by distributing tension throughout the circumference of the epidermis (Gally et al., 2009; Piekny et al., 2003; Wissmann et al., 1997). Thus passive anisotropic (direction-dependent) force in the dorsal/ventral cells was proposed to promote elongation, and circumferential MTs in dorsal/ventral cells were proposed to contribute passive force (Ciarletta et al., 2009; Priess and Hirsh, 1986). However, MTs in dorsal/ventral cells may instead play an active role, by supporting transport of junctional proteins (Quintin et al., 2016).
While the force for the first phase of elongation is connected to non-muscle myosin contractility, the myosin in the elongating epidermal cells is not polarized, and contractile pulses have yet to be detected there, raising the issue of how actomyosin force is translated into cell shape changes. Results from laser nano-ablations to measure the stress anisotropy (direction-dependent stress) in the lateral seam cells and the stiffness anisotropy in the dorsal and ventral cells were used to generate a model for force generation during elongation that incorporated the measured force anisotropies and related them to the organization of actin and spectrin. Actin thus provides a passive resistance force by inducing stiffness anisotropy in dorsal and ventral cells (Vuong-Brender et al., 2017a. Fig. 2D). Additionally, laser ablation data were used to develop a nonlinear elasticity model that proposes that ventral enclosure (Figure 2C) contributes pre-strains and pre-stresses, which are distributed by compression imposed by the apical extracellular matrix (aECM), to promote elongation (Ben Amar et al., 2018). These results demonstrate that coordinated interplay of forces in the epidermis (actomyosin contractility in lateral cells, F-actin-mediated stiffness in dorsal-ventral cells) drives the first phase of embryonic elongation.
The second phase of elongation, which takes the embryo from the 2-fold to the final, 4-fold stage, involves contractions of BWM cells connected to the dorsal and ventral epidermis through complex structures called fibrous organelles (FOs). FOs span from the aECM outside the epidermis, through the epidermal cells, to their basal ECM, which is linked to muscle (Francis and Waterston, 1991; Gieseler et al., 2017). Each fibrous organelle is composed of two hemidesmosome-like junctions (CeHDs), one apical and one basal, that each contain the plectin VAB-10 bound to intermediate filaments (the formation, structure and function of FO’s and CeHDs are reviewed in Vuong-Brender et al., 2016a; Zhang and Labouesse, 2010). The CeHDs were proposed to transmit mechanical tension from the contracting muscles to the epidermis (Zhang et al., 2011b). Therefore mechanotransduction, the process that allows cells to sense and respond to mechanical stimuli and convert them to biochemical signals, was proposed to support elongation. VAB-10 plays a central role in this mechanotransduction process, as it likely brings together the hemidesmosome transmembrane receptor LET-805 and GIT-1 (G-protein-coupled receptor kinase interacting ArfGAP1) at the CeHDs, through the VAB-10 central plakin domain, which is also appears to act as a mechanosensor (Suman et al., 2019).
A model for how contractile forces from the muscles drive the second phase of elongation came from an RNAi screen for enhancers of the p21-activated kinase, PAK-1 (Gally et al., 2009), which regulates myosin during elongation. Mutations in α-spectrin, spc-1, enhanced mild pak-1 mutations so that embryos elongated and then retracted, in a process that required muscle contraction, and which altered bundles of actin filaments present in the dorsoventral epidermis (Costa et al., 1997; Priess and Hirsh, 1986). This suggested PAK-1 and SPC-1 normally stabilize elongation promoted by the muscles. Spinning disc imaging demonstrated that muscle contractions induced actin-filament bending. Modeling the embryo as a Kelvin-Voight material (spring in parallel with a dashpot, or damper, which resists motion via viscous friction) was able to predict the elongation patterns of wildtype embryos and the retraction of the spc-1 pak-1 embryos (Lardennois et al., 2019). Muscle contractions were also proposed to orient F-actin fibers in the seam cells by regulating adherens junctions (AJs), and PAR-dependent planar polarity (Gillard et al., 2019). Finally, the formin FHOD-1, which has been shown to regulate epidermal morphogenesis (Vanneste et al., 2013), was proposed to contribute to this process using an actin bundling function (Lardennois et al., 2019).
Recent work has also identified a role for the apical CeHDs and overlying embryonic sheath, which is the aECM of the embryonic epidermis, during this second phase of elongation. An RNAi screen identified a role for apical ECM proteins in elongation, including the zona pellucida (ZP) domain proteins NOAH-1, NOAH-2 and FBN-1/Fibrillin, and the extracellular leucine-rich repeat (eLRR) proteins SYM-1 and LET-4 (Mancuso et al., 2012; Vuong-Brender et al., 2017b). These proteins provide embryonic integrity and relay mechanical stress required for elongation, from the epidermis to the muscle and vice-versa. aECM proteins appear to play important roles in the morphogenesis of other biological tubes in C. elegans, as we discuss below.
THE ALIMENTARY SYSTEM
The C. elegans digestive tract is composed of several tubes. From anterior to posterior they are the buccal cavity, pharynx, or foregut, three valve cells that connect the pharynx to the intestine, which is then followed by the rectal valve and rectal epithelium (Figs. 1, 3 and not shown). Here we mainly focus on the pharynx and the intestine, because the genetic control and cell biological processes regulating morphogenesis of these have been better studied.
Figure 3. Forces forming the Intestine and Pharynx.
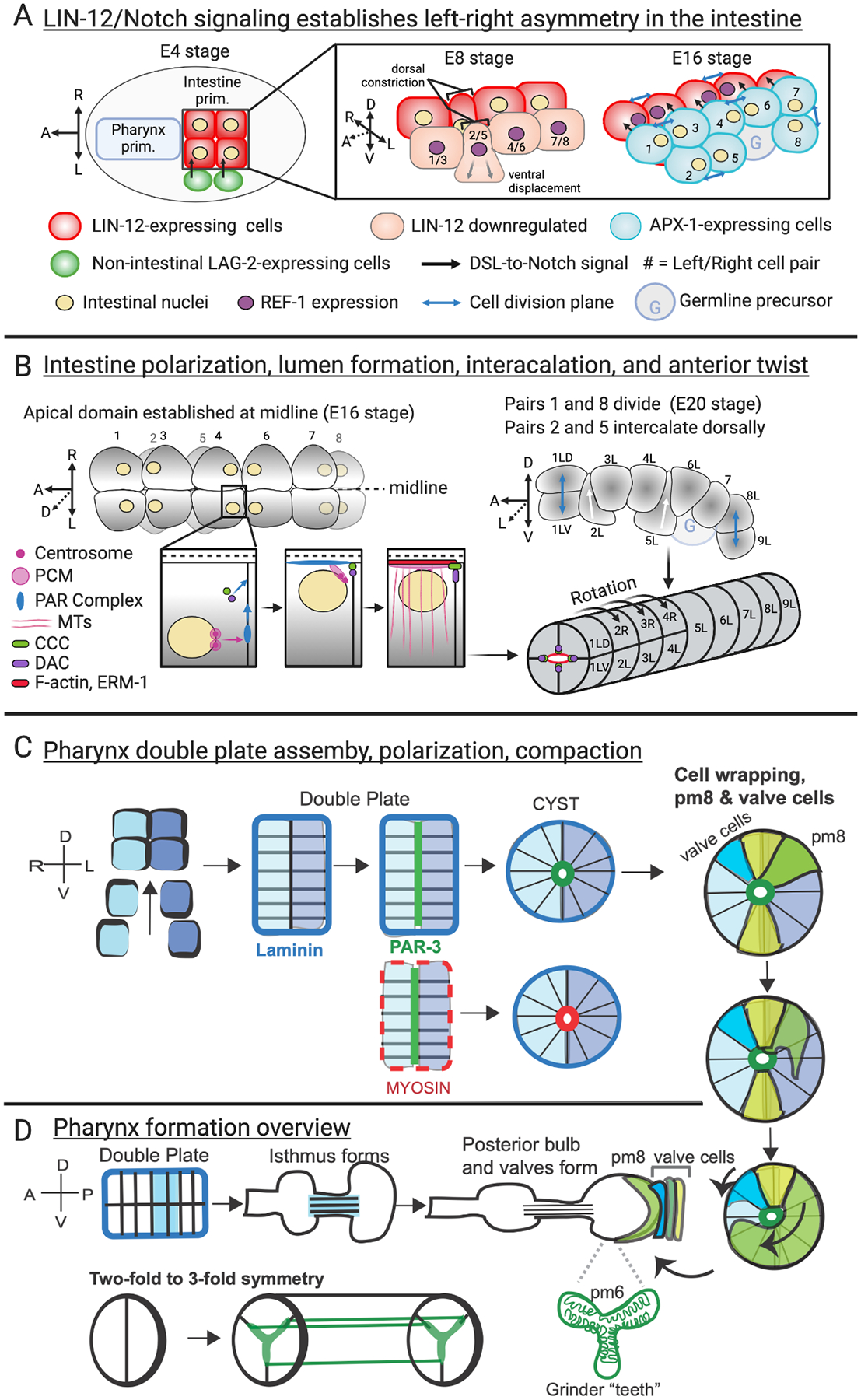
The transcription factors required for pharynx and intestine formation are known, and some of the movements have also been described. The roles of some of the signaling cascades, extracellular matrix, cell polarity regulators, and cytoskeletal remodeling are highlighted. A, B) The intestine is a simple tube made up of only one type of cell and it displays 2-fold symmetry for most of its length. It is composed of 9 segments, or rings, derived from 20 cells: four in the first ring, and pairs of cells in the remaining eight. Nomenclature is based on the cell pairs present at the E16 stage and the rings that they form in the final structure, as described in Asan et al. (2016). (C, D) The more complex pharynx is made up of 5 types of cells and develops 3-fold symmetry. A) Asymmetric LIN-12/Notch signaling during early intestinal development (E4 to early E16) establishes left-right asymmetry within the primordium that translates into a directional “twist” later (shown in B). At the E4 stage cells on the left are exposed to the Notch ligand, LAG-2, which induces expression of the LIN-12/Notch target REF-1, a bHLH transcription factor that transcriptionally downregulates LIN-12. By the E16 stage only right intestinal cells express LIN-12, so that APX-1, a second LIN-12/Notch signal, triggers REF-1 expression specifically in these cells. At E8 the pair 2/5 precursors are ventrally displaced, and during the E8-to-E16 division the pair 7/8 precursors divide along the dorso-ventral axis. This creates a two-layered primordium at the E16 stage that wraps around the germline precursor cells. B) After the E8-to-E16 divisions the intestinal nuclei lie next to the lateral membrane created by cytokinesis. Around 30 minutes after division, intestinal polarization begins, as nuclei, PAR complex components, and a ncMTOC assemble at the apical membrane. Polarization requires formation of Cadherin-catenin complex (CCC) and Discs-Large/AJM-1 complex (DAC) junctions, and accumulation of apical cytoskeletal components such as F-actin and ERM-1. The last two intestinal divisions (pairs 1 and 8) occur as the ventral cell pairs (2 and 5) intercalate dorsally, always maintaining apical/basal polarity, creating a continuous tube. Finally, three anterior rings (2, 3, and 4) undergo a left-handed twist, which is regulated via unknown mechanisms established by asymmetric LIN-12/Notch signaling (panel A). This twist may help align the developing lumen between consecutive intestinal rings (Asan et al., 2016). Wnt signaling limits the twist to the anterior intestine (Hermann et al., 2000; Neves et al., 2007; Neves and Priess, 2005). Diagrams are based on Asan et al. (2016), Bernadskaya et al. (2011), Feldman and Priess (2012), Gobel et al. (2004), Leung et al. (1999), and Segbert et al. (2004). C) Pharynx double plate assembly, polarization, compaction and cell wrapping adapted from Rasmussen et al. (2012) and (2013). The pharyngeal double plate undergoes compaction into the cyst stage (Rasmussen et al., 2012). The posterior cells of the cyst, pm8 and the valve cells, undergo wrapping and fusions to form the doughnut-shaped pm8 and the three valve cells (Rasmussen et al., 2013). D) Pharynx overview based on Mango et al. (1994) and Pilon (2014). Grinder “teeth” adapted from (Altun, 2009b).
The intestine: a 20-cell tube derived from a single lineage
Formation of the intestine
The C. elegans intestine is a simple tube made up of 20 cells arranged in nine adherent rings. The anterior ring, composed of 4 cells, attaches to the pharynx, a muscular tube worms use for feeding, through rings formed by the valve cells. The posterior 16 cells of the intestine are arranged in rings of only 2 cells. The cells are attached at AJs, to form an apical, central lumen covered in microvilli. The basal region faces out and is in contact with germ cells, BWMs, epidermal cells and ventral neurons (Figures 1, 3A, B).
The entire C. elegans intestinal tube is derived from the E blastomere, a cell that forms at the 8-cell stage (Deppe et al., 1978; Sulston et al., 1983). The E blastomere is different from its sister MS due to Wnt and Src signaling (Bei et al., 2002; Rocheleau et al., 1997). Mutations in pop-1, encoding the Wnt-regulated TCF/LEF transcription factor, cause both EMS daughters to acquire E fate, and all the cells derived from EMS daughters in this mutant organize in what appears to be a longer single intestinal tube (Lin et al., 1995), suggesting that E fate specification is sufficient to initiate the morphogenetic program that forms intestine.
Studies to identify genes that promote E-fate specification have identified a pathway of transcription factors that establish intestinal fate. Therefore the transcriptional control of intestinal cell fate is well-characterized (Reviewed in Maduro and Rothman, 2002; McGhee, 2013). When the E blastomere is cultured in isolation, its descendants form a cyst of cells expressing intestinal markers. However, these cells do not form a tube. Therefore external signals, or physical interactions with neighboring tissues, likely contribute to tube morphogenesis (Leung et al., 1999; reviewed in McGhee, 2007).
How forces shape the intestine
Formation of the intestinal tube requires first that the cells form and maintain their positions within a planar primordium, even as they divide from 4 to 8 and then 8 to 16 cells (named E4 to E16). The tube undergoes a twist at the E16 stage, and as the embryo elongates, the intestine also elongates, maintaining a narrow apical lumen. The forces driving these events are in response to signals, some of which are known.
Forces creating the planar primordium of the intestine through cellular intercalations
The simple shape of the intestinal tube involves surprisingly complex cell rearrangements. For example, the E4 cells form a plane, then divide and split the plane into two layers (Figure 3A) before cell intercalation returns the E8 cells to a single plane (Figure 3B). The planar cell polarity protein, VANG-1/Strabismus/Van Gogh maintains the E4 to E8 cells in a planar primordium, because vang-1 loss led E4 to E8 cells to display variable orientations, suggesting that planar cell polarity cues help position the E8 primordium (Asan et al., 2016). As the cells go from E8 to E16, specific cells undergo intercalations, which involve extending thin lateral protrusions while basal regions become rounded and flattened. The forces required for these protrusions have not been examined, though mutants in axonal guidance cues, including Netrin (unc-6, unc-40), Robo (sax-3), Semaphorins (mab-20), and Ephrins (efn-4) are required for correct position of intestinal cells, in particular for anterior movements that result in the first intestinal ring having 4 cells and 2 cells in the second ring (Asan et al., 2016).
Early asymmetric LIN-12/Notch signaling sets up a later intestinal twist
After cells in the intestinal tube “pack” with a reproducible asymmetry or handedness, such that the intestinal cells complete the intercalations to form the final tube, a subset of cells rotate to create a “twist” in the intestine (Figure 3B). This twist depends on Notch signaling and left/right asymmetry (Figure 3A). Intestinal cells in the E4 to E8 primordia express the receptor LIN-12/Notch, but only cells on the left side contact ligand-expressing cells outside the intestine. This LIN-12/Notch interaction is required for the uniform, asymmetric packing of intestinal cells. In addition, this interaction down regulates LIN-12/Notch in the left cells, so that a later LIN-12/Notch signaling event affects only the right cells. The result of this second Notch interaction is that a subset of anterior cells will later revolve around the long axis of the intestinal tube, thus “twisting” the intestine. Only the anterior cells participate in the twist due to anterior/posterior patterning that requires Wnt signaling including LIT-1/Nemo-like Kinase and POP-1 (Asan et al., 2016; Hermann et al., 2000; Neves et al., 2007; Neves and Priess, 2005).
Polarization and Lumen formation
At the E16 stage intestinal cells develop apical/basal polarity (Figure 3B). One of the earliest indicators of cell polarity in the intestine is the MT-dependent movement of nuclei towards the midline between intestinal cell pairs, the site of the future apical membrane and lumen (Leung et al., 1999). At the time of nuclear polarization these cells also exhibited asymmetric MTs, which were found to emerge “in a fountain-like array” from the apical membrane, suggesting that this membrane might function as an MTOC (Leung et al., 1999). Indeed, later work showed that MTOC function is transferred from nuclei-associated centrosomes to the apical membrane during polarization, that MTs promote apical accumulation of PAR-3, while centrosomes are required for the apical accumulation of gamma tubulin (Feldman and Priess, 2012).
The lumen forms at 270 minutes after first cleavage, as a tube sets up after the establishment of apical junctions containing at least two distinct junctional complexes, the Cadherin/Catenin complex (CCC) and the DLG-1/AJM-1 complex (DAC) (See Figure 3B. Reviewed in Pasti and Labouesse, 2014; Segbert et al., 2004). Conserved polarity proteins are required for this event, including apical PAR complex components PAR-3 and PAR-6, which help position and condense apical junction proteins, like HMR-1/E-Cadherin and DLG-1/Discs Large, to set up a continuous lumen (Achilleos et al., 2010; Totong et al., 2007). Surprisingly, loss of the apical PAR complex does not block polarity establishment, which may reflect the robust redundant polarity pathways active in embryos (Liro et al., 2018). However, recent work using transient depletion of the apical PAR complex suggests that this complex may be needed to establish a robust apical domain, because loss of PAR-6 during mid-embryogenesis, as the cells divide from E16 to E20 (Figure 3B), caused lumen defects in larvae (see bioRxiv preprint by Sallee et al., 2020).
Once polarity and apical junctions are established, lumen formation requires ERM-1, an Ezrin, Radixin, Moesin homolog, as ERM-1 loss results in cystic lumens (Gobel et al., 2004; van Fürden et al., 2004). ERM-1 has membrane and F-actin-binding domains, and loss of ACT-5, an actin enriched in the intestine and in the excretory canal (see below), or SMA-1/βH-spectrin, genetically interacted with erm-1 mutants (Gobel et al., 2004), and RNAi against erm-1 reduced apical F-actin (Van Furden et al., 2004), indicating that ERM-1 plays a role in actin regulation. Loss of ERM-1 reduced apical WAVE complex accumulation, while loss of WAVE components led to elevated ERM-1 levels, but the basis for this reciprocal effect has not been investigated further (Bernadskaya et al., 2011). The apical lumen membranes are further supported by fatty acid biosynthesis through sphingolipid synthesis (Zhang et al., 2011) and clathrin/AP-1 apically-directed transport (Shafaq-Zadah et al., 2012; Zhang et al., 2011a). The formation and width of the intestinal lumen also requires intermediate filaments (IFs), because loss of IFB-2/LMN2 or IFO-1 (intestinal filament organizer) resulted in reduced apical F-actin and defective lumens, and EMs showed that ifo-1 mutations alter the terminal web, and the apical junctions (Carberry et al., 2012). A recent study of the function of individual IF proteins, using CRISPR knockouts, showed that loss of ifc-2 resulted in a thinner endotube and abnormalities in the microvilli, while depletion of ifb-2 altered the actin cytoskeleton and other structures differently, suggesting that distinct IF proteins play specific roles in lumen formation (Geisler et al., 2020).
Regulating the diameter of the intestinal lumen is likely important because it defines the membrane surface area where microvilli can form (Fig. 1). Genetic and RNAi studies suggest that myosin contractility helps determine lumen diameter. Mutations in UNC-94/tropomodulin, an F-actin pointed-end capping protein predicted to stabilize filaments, results in an abnormally large lumen, despite apparently normal apical junctions, and this defect can be partially rescued by RNAi-mediated depletion of MEL-11/myosin phosphatase (Cox-Paulson et al., 2014). Loss of branched actin regulators does not interfere with apical/basal polarity (Patel and Soto, 2013), yet mutants display a wider intestinal lumen that may be caused by changes in the establishment and maintenance of apical AJs, as shown by changes in Cadherin and DLG-1/AJM-1 junctions over time (Bernadskaya et al., 2011; Patel and Soto, 2013; Sasidharan et al., 2018).
Unanswered questions in intestinal morphogenesis
The forces downstream of the left/right asymmetry leading to the intestinal twist, or rotation, are not described. Perhaps higher resolution microscopy will reveal asymmetries in adhesive molecules that could explain how initial cell fate asymmetry promotes the twist in just a section of the anterior intestine. The source for the signals that promote the twist includes the dorsal epidermis, but apparently not adjacent BWMs (Asan et al., 2016). This suggests that cues that promote dorsal/ventral polarity, like UNC-6/Netrin and SAX-3/Robo, might contribute. To address this will require tissue-specific transient depletion of these guidance cues. Since actin is downstream of these cues, there may also be dorsal/ventral differences in F-actin enrichment, and the effect of linear or branched F-actin on intestinal cell rearrangements has not been examined.
Several peri-centriolar material (PCM)-associated proteins that are non-redundantly required for MT nucleation from, and anchoring to, centrosomes are re-distributed to the apical ncMTOC during polarization (See Figure 3B, and Feldman and Priess, 2012; Sallee et al., 2018). Surprisingly, intestine-specific depletion of these proteins did not abrogate ncMTOC function (Sallee et al., 2018), suggesting that there may be redundant mechanisms, and/or new players, that promote MT growth from the apical membrane. Recent work using biotin-based proximity labeling and mass-spectroscopy (see bioRxiv preprint by Sanchez et al., 2020) should allow for identification of proteins whose loss disrupts ncMTOC function, which will help us further understand the interplay of polarity factors, MTs, and actin in the intestine, which will enrich our understanding of how MTs and ncMTOCs contribute to morphogenesis.
The pharynx: cells from multiple lineages combine to form a tube
The pharynx is derived from the ABa and MS lineages of the 4-cell and 8-cell embryo, respectively, which generate five types of cells: muscles, neurons, marginal cells, duct cells, and epithelia. The signals and transcription factors that promote pharyngeal cell fate are well understood. The forkhead transcription factor PHA-4/FoxA is thought to be the master regulator for pharynx identity (Kalb et al., 1998; Mango et al., 1994). PHA-4 is activated in ABa descendants by Notch signaling (Christensen et al., 1996; Good et al., 2004; Moskowitz and Rothman, 1996), and in MS descendants by SKN-1 (bZIP transcription factor) and the GATA factors MED-1 and MED-2 (Broitman-Maduro et al., 2006). A PHA-4 consensus binding site has been defined, and shown to regulate expression by PHA-4 (Gaudet and Mango, 2002), and its targets, including the T-box transcription factors TBX-37/TBX-38 and TBX-35 (Broitman-Maduro et al., 2006; Good et al., 2004). The cellular events that promote formation of the tubular pharynx are well described (Leung et al., 1999; Portereiko and Mango, 2001; Rasmussen et al., 2013), but many questions remain about the formation of this complex tube.
How forces shape the pharynx
Gastrulation
Gastrulation in C. elegans is tissue-specific, and happens over a long period, with the precursors of the pharynx and intestine moving from the ventral surface internally to form tubes at different times. Pharyngeal cells ingress later than intestine, MS progeny first, then ABa progeny, and the ventral cleft closes by 290 min (Sulston et al., 1983). While pharyngeal cell fate specification is required for normal pharyngeal gastrulation (Harrell and Goldstein, 2011), and properly polarized cell divisions contribute (Rasmussen et al., 2012), the mechanisms that promote pharyngeal cell ingression are not well understood. Presumably similar processes as those that promote intestine ingression, such as myosin-driven apical cell constrictions, are involved (Reviewed in Goldstein and Nance, 2020).
Compaction into a cyst
As pharyngeal cells ingress from the ventral surface, they form a dorsal/ventral-oriented structure called the double plate that is only two cells wide. By 290 minutes, cell in the double plate rearrange into a cyst by apical constriction. Cyst formation involves multiple cytoskeletal changes, including a shift of non-muscle myosin from basolateral to apical regions, forming a rosette. Apical myosin enrichment seems to follow apical/basal polarization, as it follows apical enrichment of PAR-3 (See Figure 3C, and Rasmussen et al., 2012). The cyst extends anterior/posteriorly into a tube; later events shape the tube along the anterior/posterior axis, such that the final pharynx has two bulbs, separated by the narrow isthmus, and in cross section the tube has 3-fold symmetry (See Figure 3D, and Albertson and Thomson, 1976).
Lumen formation requires the basement membrane protein LAM-1/laminin
Unlike the intestine, which appears to polarize without basement membrane (BM), signals from the BM are crucial for multiple events of pharyngeal polarization and movements (Rasmussen et al., 2012). LAM-1 is the single C. elegans laminin β subunit, so it is a component of all C. elegans BMs. Pharyngeal cells form in lam-1 mutants, but can have reversed apical/basal polarity. Wild-type embryos accumulate PAR-3 internally, where the apical region forms. In lam-1 mutants, some cells accumulate PAR-3 on the external surface. Despite this major defect in cell polarity, neighboring cells can rearrange themselves to position the PAR-3 surface to the interior of the primordium and these movements can create a pharynx with two lumens. This polarity defect of lam-1 mutants can be rescued by LAM-1 expressed from external, non-pharyngeal cells, such as the intestinal cells or BWMs, suggesting that laminin can move about. Finally, pha-4 mutants do not deposit laminin between pharynx and BWM, suggesting that pharyngeal fate specification somehow promotes correct laminin accumulation.
Extension of the pharyngeal primordium
Portereiko and Mango (2001) described the anterior movements of the ABa derived cells, which result in reorientation of these cells towards the central, apical region, and extension of the anterior pharynx, which connects the pharynx to the epidermis through the arcade cells, specialized epithelial cells that join the pharyngeal epithelium to form the buccal cavity (Altun and Hall, 2009a; White, 1988; Wright and Thomson, 1981). These events happen after establishment of a BM, visible at 400 min., and anterior movements are independent of the MS-derived pharyngeal cells, suggesting that these movements depended on tension between the pharynx, buccal cavity and epidermis, to pull the entire pharynx forward (Portereiko and Mango, 2001). The aECM protein FBN-1/fibrillin, expressed in epidermis, was proposed to transmit this tension, supporting ingression of the anterior epidermis to form the buccal cavity by transducing inward pulling forces from the elongating pharynx (Kelley et al., 2015). PAR-6 is required for reorientation of the arcade cells, and deposition of HMR-1/Cadherin in the apical regions, suggesting an important role for PAR-6 in the anterior pharynx, (Von Stetina and Mango, 2015), as appears to be the case in the anterior intestine (see bioRxiv preprint by Sallee et al., 2020).
Posterior bulb and valve formation
Individual cells of the pharynx acquire distinct shapes and roles, and we have a few clues about how some of those specific events are regulated. In the posterior pharynx, pm8 (pharyngeal muscle 8) cells and valve cells form by undergoing rearrangements, while maintaining apical/basal polarity (Figure 3C, D). The AFF-1 fusogen regulates autofusion of pm8 while the EFF-1 fusogen regulates autofusion of valve1/vp1 (Rasmussen et al., 2008). Signals that coordinate this process include Notch signaling (which regulates pm8), targets of Notch (Rasmussen et al., 2008), and PHA-4 independent targets including EGL-43 (Rasmussen et al., 2013). In the absence of signals, cellular movements fail, suggesting they are somehow translated into F-actin protrusions that drive specific cell intercalations and rotational movements. The extracellular matrix is highly regulated during this process, and, for example, laminin is required to polarize pharynx but not intestine (Rasmussen et al., 2012).
During pm8 and valve cell morphogenesis, LAM-1/β−laminin must be correctly deposited in specific regions to promote specific cellular migrations. If LAM-1, or its proposed receptor INA-1/α-integrin, is missing, inappropriate movements occur (Rasmussen et al., 2008; Rasmussen et al., 2013; Rasmussen et al., 2012). Cells throughout the pharynx normally have radial apical/basal polarity, and cells at the posterior end of the pharynx, which contact the intestine, inappropriately develop anterior/posterior polarity and inappropriate laminin accumulation, when intestinal contacts are removed. Since laminin normally acts as a polarizing cue for pharyngeal cells, the intestinal cells might promote pharyngeal development by blocking laminin from reaching the posterior surface (Rasmussen et al., 2013). Mutations in two other BM proteins, TEN-1/teneurin and NID-1/nidogen, also disrupt the BM surrounding the pharynx and their loss causes a Pun (Pharynx unattached) phenotype (Trzebiatowska et al., 2008). ECM mutants also cause the Twp (twisted pharynx) phenotype, so ECM is required to maintain correct shape, perhaps by connecting to adhesion molecules (Avery, 1993; Axang et al., 2007).
As we saw for the epidermis, mutations in the transcription factor, DIE-1 affect intestinal, pharyngeal and valve intercalations. These incorrect movements can detach the pharynx and valve cells from the intestine, and lead to ectopic laminin accumulation (Rasmussen et al., 2013). Loss of DIE-1, or the transcription factors AST-1/ETS, UNC-39/Six5, CED-43/distal-less or ELT-5/GATA result in Pun phenotypes, suggesting they regulate anterior pharynx movements or adhesion (Aspock et al., 2003; Heid et al., 2001; Koh et al., 2002; Schmid et al., 2006).
Unusual neuronal migrations contribute to isthmus formation
The bulbs of the pharynx become separated by a thinner region called the isthmus (Figure 3D). This process is supported by the atypical migrations of pharyngeal neurons, like M2. This neuron does not extend a growth cone; instead, in the “fishing line” model, M2’s axon is pulled from the cell body by its connection to other cells, and a growth cone does not form until later to finalize connections (Morck et al., 2006; Morck et al., 2004; and reviewed in Pilon, 2014). This is reminiscent of the movements of amphid sensory neurons and glia which form a rosette, attached to anteriorly migrating epidermis, and the cell bodies move posteriorly in a process that requires specialized matrix proteins, DYF-7 and DEX-1 (Heiman and Shaham, 2009).
Unanswered questions in pharynx tube formation
The constrictions that transform the double plate, with two-fold symmetry, into a cylinder with three-fold symmetry have been partially described, but the cues and forces that transforms two-fold symmetry to three-fold symmetry, are not known (Figure 3D). This seems like an opportunity for creative mathematical modeling, using known parameters (Hollo, 2017), that could be tested with genetic techniques.
Many of the events of pharynx formation require regulated tissue constriction, and how these constrictions are regulated is not clear. The pharynx expresses two pharynx-specific myosin heavy chains, MYO-1 and MYO-2, which are regulated by homeobox transcription factor CEH-22 (Okkema and Fire, 1994). However, ceh-22 loss has only a mild pharyngeal phenotype (Okkema et al., 1997), and NMY-2 is enriched apically during cyst formation (Rasmussen et al., 2012. See Figure 3C).
While we have long known that the pharyngeal lumen is covered by a specialized cuticle (Albertson and Thomson, 1976; Wright and Thomson, 1981), and recent work shows specific roles for gland cells in secreting digestive enzymes (Rochester et al., 2017), exactly how the apical cuticle is formed during development is open to investigation. A detailed characterization of changes in the cuticle of the pharynx, as worms transition from larval stage 4 to adulthood, showed that the pm6 and pm7 cells of the posterior bulb transiently become secretory, and contribute to the adult grinder “teeth” (Figure 3D), composed of a 5-layered aECM (Sparacio et al., 2020). The exact composition of this aECM is not known, but intriguing components include Prion-like molecules of the ABU/PQN Paralog Group (APPG), which are expressed in pharyngeal cells. Some of these were shown to localize to the cuticle and to promote cuticular function (George-Raizen et al., 2014).
THE EXCRETORY SYSTEM
The structure and function of the excretory system was described nearly 40 years ago (Nelson and Riddle, 1984; Nelson et al., 1983), when it was proposed that it mediated osmoregulation and fluid excretion, functions that have led to comparisons to renal and lymphatic systems (Buechner et al., 1999; Kolotuev et al., 2013). For an in-depth review of the development and function of the excretory system, we suggest an excellent recent article by Sundaram and Buechner (2016). Here we will focus on what is known about the forces that regulate morphogenesis of the three tubes that make up the excretory system: the excretory canal (ExCa), duct and pore cells (Fig. 1).
Overview of the excretory system and unicellular tubes
The excretory system provides a model to study the smallest of tubes: those made by single cells. The ExCa cell body lies in the ventral midline, below the posterior pharynx bulb, and it extends long hollow processes, or canals, embedded in the left and right basolateral surfaces of the epidermis along the entire length of the animal. Two smaller tubes: the duct (which abuts the ExCa) and the pore (which connects the duct to the epidermis) link the ExCa to the outside (Fig. 1).
Animals that lack a functional excretory system (due to mutations that affect cell fate specification, or after laser ablation of the ExCa cell) die as turgid, fluid-filled larvae; a phenotype described as a “rod-like” lethality. This led to the proposal that the ExCa collects excess fluids from the epidermis and/or pseudocoelomic space, and transports this liquid, via its lumen, for excretion through the duct and pore. To date, fluid flow within the excretory system has not been directly observed. However, early observations showed that duct pulsations are inversely correlated to environmental osmolarity (Nelson and Riddle, 1984), and careful analysis of mutants that affect duct and pore tubulogenesis shows that defects in these tubes cause fluid accumulation (edema) along the entire system (Cohen et al., 2019; Forman-Rubinsky et al., 2017; Gill et al., 2016; Mancuso et al., 2012; Stone et al., 2009), consistent with the proposed physiological function.
Unicellular tubes are classified into two broad categories: “seamed” and “seamless”. (Hogan and Kolodziej, 2002; Lubarsky and Krasnow, 2003). Seamed tubes have “auto-cellular” junctions along the length of their apical, lumen-lining, surface. They were postulated to arise by wrapping of the cell upon itself, enclosing extracellular material into a lumen, followed by the formation of a self-sealing auto-cellular junction that maintains tube integrity. The excretory pore and duct are both, at least transiently, seamed tubes. In contrast, seamless unicellular tubes do not display auto-cellular junctions, and they were postulated to arise mainly by “hollowing”; a mechanism where the lumen forms via polarized trafficking and fusion of intracellular vesicles (Hogan and Kolodziej, 2002; Lubarsky and Krasnow, 2003). The ExCa appears to forms a tube by hollowing. Additionally, studies of unicellular tubes in the alimentary system (see above) and of the excretory duct revealed that seamless tubes also arise by “auto-fusion” of seamed tubes; defining a new mechanism for the formation of seamless tubes.
Tubulogenesis of the excretory duct and pore
Cell migration
The duct and pore precursors are born on the ventral side of the embryo and migrate towards the midline during ventral enclosure (Fig. 4A). The cues and forces that regulate migration remain unknown, and to date the only gene implicated is mls-2, encoding an HMX/Nkx5 transcription factor (Walton et al., 2015). MLS-2 expression during ventral enclosure is restricted to a discreet subset of cells, including the duct and pore; consistent with MLS-2 functioning cell-autonomously (Abdus-Saboor et al., 2011; Abdus-Saboor et al., 2012). Identification of MLS-2 targets may help identify proteins that regulate migration. Intriguingly, mls-2 mutants also exhibit defects in duct and pore cell shape later in development (Abdus-Saboor et al., 2012), suggesting that MLS-2 targets might include cytoskeletal components that may also regulate migration.
Fig. 4: Excretory Duct and Pore tubulogenesis.
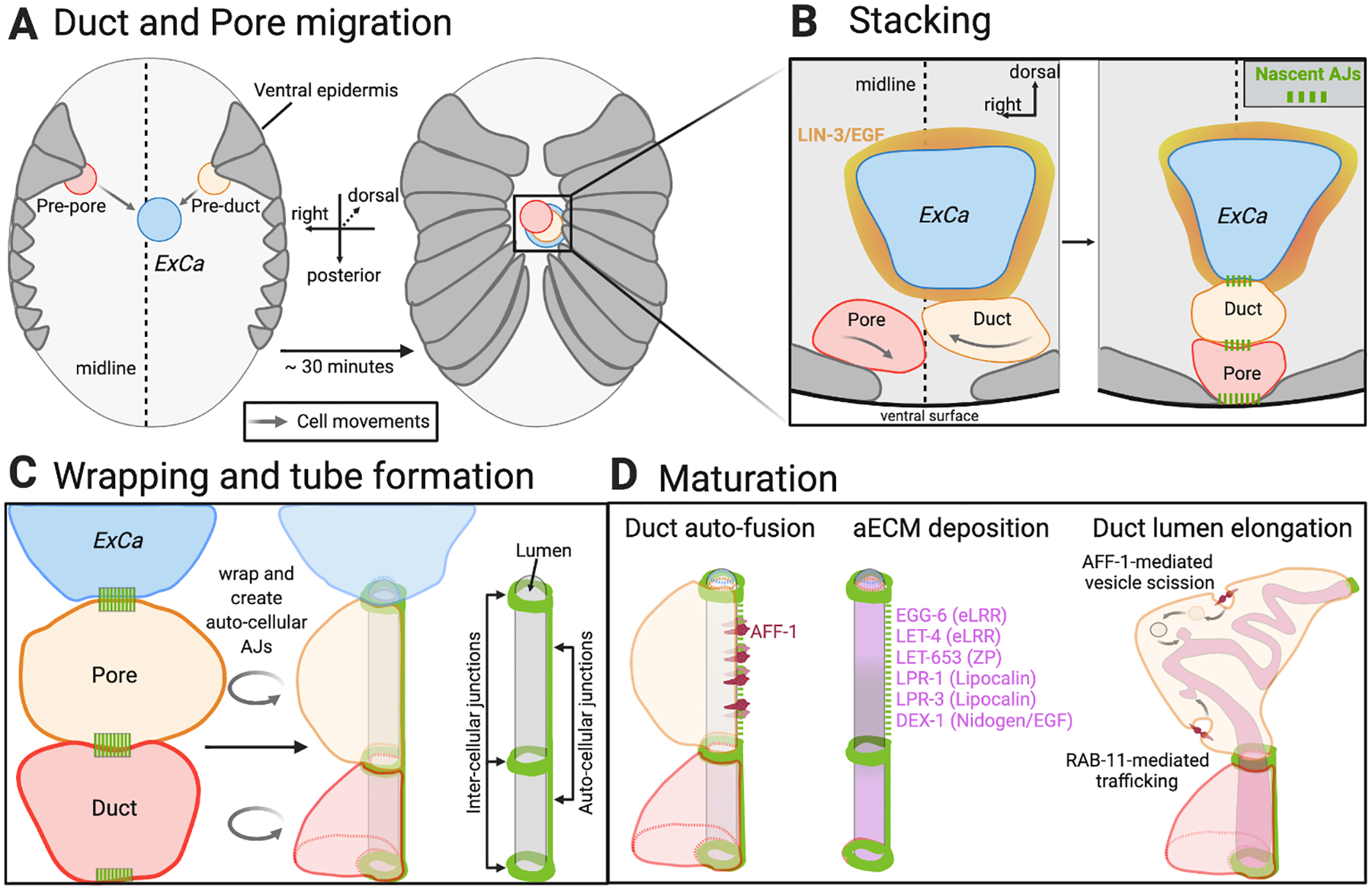
A) Before their migration the presumptive (pre) duct (yellow) and pore (red) are in contralateral positions over the ventral epidermis, and then proceed to migrate towards the midline during ventral enclosure. The ExCa (blue) is born near the midline, slightly shifted to the left side. B) Even though the pre-duct and pre-pore both have the potential to adopt duct fate, the pre-duct invariably reaches the ExCa first and is induced to adopt duct fate by ExCa-produced LIN-3/EGF (orange), which activates EGFR signaling in the duct. The specified duct produces an unknown inhibitory signal, which prevents the pre-pore from adopting duct fate, and physically impedes the pore from contacting the ExCa. Soon after the three cells stack they form inter-cellular adherens junctions (AJ’s, green) that keeps them adhered to each other. The pore also forms AJs with neighboring epidermal cells. C) The duct and pore form tubes by wrapping and creating auto-cellular AJs. D) After the duct forms a tube, it matures by undergoing auto-fusion (becoming a “seamless” tube), increasing its lumen length, and secreting a specialized aECM important for structural stability (orange). Auto-fusion and lumen growth both require the fusogen AFF-1 (burgundy). Diagrams inspired by WormAtlas (www.wormatlas.org), Sundaram and Buechner (2016), and Soulavie et al. (2018).
Fate specification, stacking, wrapping, and auto-cellular junction formation
The presumptive duct and pore express the receptor LET-23/EGFR, and the ExCa cell expresses the ligand LIN-3/EGF (Abdus-Saboor et al., 2011). During embryogenesis the ExCa cell lies slightly to the left of the midline, thus the duct precursor, born on the left side of the embryo, invariably reaches the LIN-3 source first (Fig. 4A). This presumably leads to stronger and/or earlier activation of EGFR-Ras-ERK signaling, which specifies duct fate via the LIN-1/ETS and EOR-1/BTB transcription factors (Abdus-Saboor et al., 2011; Howard and Sundaram, 2002). Once specified, the duct prevents the presumptive pore from adopting the duct fate via an unknown inhibitory signal (Yochem et al., 1997).
After duct and pore fates are specified, the three excretory system cells “stack”: the duct moves dorsally, into the embryo, to contact the ExCa cell, while the pore adopts a position ventral to the duct and contacts the duct and the ventral surface of the embryo (Fig. 4B). The forces that drive stacking remain unknown, but factors that regulate this behavior are likely targets of LET-60/Ras signaling, because loss-of-function let-60 alleles that do not affect duct fate specification disrupt stacking (Abdus-Saboor et al., 2011). The ExCa not only provides the signal (EGF) that regulates duct fate-specification. This cell appears to also provide mechanical support for stacking, because laser ablation of the ExCa after EGF-promoted duct specification also leads to stacking defects (Abdus-Saboor et al., 2011).
After, or during, stacking, the pore, duct, and ExCa generate inter-cellular AJs (Abdus-Saboor et al., 2011; Stone et al., 2009) that promote adhesion between these cells (Fig. 4C). The duct and pore then wrap upon themselves and generate auto-cellular junctions, or “seams”, along the length of the cell body, creating “doughnut-like” unicellular tubes (reviewed in Sundaram and Cohen, 2016). The mechanisms that regulate this change in cell shape and formation of auto-cellular junctions remain poorly understood.
Duct maturation: auto-fusion, lumen remodeling and aECM deposition
Around an hour after the duct wraps and forms an auto-cellular junction, this cell fuses to itself and the auto-cellular junction disappears (Soulavie et al., 2018; Stone et al., 2009. See Fig. 4D). Duct auto-fusion is promoted by AFF-1, which is transcriptionally upregulated by LET-60/Ras signaling, LIN-1/Ets, and EOR-1/BTB (Soulavie et al., 2018). AFF-1 is a “fusogen” that induces homotypic fusion between cells that express this protein (Sapir et al., 2007), or, as is the case for the duct (and for the pharyngeal valve pm8, discussed above) fusion between apposed membrane regions of the same cell (for a review of cell fusions, see chapter by Iosilevskii and Podbilewicz in this collection). Why does the duct undergo auto-fusion? Unlike the pore, which exhibits developmental plasticity and trans-differentiates during L1 (reviewed in Rothman and Jarriault, 2019; Sundaram and Buechner, 2016), the duct remains a tube throughout the life of the animal. In addition the duct undergoes remodeling of cell shape and increased lumenogenesis (see below). Removal of the auto-cellular junction may make the duct more resistant to the stresses associated with remodeling and throughout the worms’ lifespan.
Soon after auto-fusion the duct remodels (Fig. 4D): it elongates and the lumen grows, becomes meandering, until it is ~1.5x the length of the cell (Abdus-Saboor et al., 2011; Soulavie et al., 2018; Stone et al., 2009). LET-60/Ras signaling is also required for remodeling, at least in part via its regulation of AFF-1 expression. Studies using precisely-timed degron-dependent AFF-1 depletion showed that this protein plays a role in lumen elongation, independent of its role in duct auto-fusion. Using FM4–64, a dye that enters cells via endocytosis, it was also shown that AFF-1 regulates a post-internalization endocytic step at the basolateral plasma membrane, and analysis of endocytic mutants showed that the GTPase RAB-11, involved in endocytic recycling, also plays a role in duct lumen elongation (Soulavie et al., 2018). These observations led to a model where AFF-1 regulates scission (another type of a membrane-merging event) of basolateral endocytic vesicles, and that these vesicles are transcytosed to that apical membrane to increase its length (Fig. 4D).
Screens for mutants that cause “rod-like lethality” have uncovered numerous aECM proteins required for duct integrity, including some that play a role in epidermal elongation (see above), such as the eLRR proteins LET-4 and EGG-6 (Mancuso et al., 2012), the PAN-Apple-ZP-family protein LET-653 (Gill et al., 2016), and the Nidogen-EGF protein DEX-1 (Cohen et al., 2019). The secreted lipocalins LPR-1 and LPR-3, which function non-autonomously and are thought to regulate extracellular transport, or accumulation, of lipids and/or signaling molecules, are also required for aECM function (Forman-Rubinsky et al., 2017; Pu et al., 2017; Stone et al., 2009). Confocal microscopy and TEM analyses show that mutations in these genes alter the structure of the duct lumen aECM, leading to defects in lumen length, width and patency (i.e., openness), and secondary consequences like disruption of the inter-cellular junction between the duct and pore. Importantly, the excretory phenotypes associated with disruption of the aECM only become apparent at, or after, the time of excretory duct remodeling and/or when fluid begins to flow through the excretory system. This is consistent with a model where the aECM plays a critical role in maintaining tube integrity in response to mechanical stresses, like morphogenic changes (as is the case during embryonic elongation) or fluid flow.
Remaining questions in excretory duct and pore tubulogenesis
We still do not know how the transcription factors (MLS-2/Hmx5, LIN-1/Ets, EOR-1/BTB) and signal-transduction pathways (LET-23/EGFR) that regulate pore and duct tubulogenesis are translated to forces that promote migration, stacking, wrapping, and elongation. F-actin accumulation has been visualized in the duct and pore after their morphogenesis, but a detailed analysis of the cytoskeleton in earlier steps is lacking; most likely because it is difficult to image these small cells during morphogenesis, and/or because overexpression of fluorescently-tagged cytoskeletal proteins in these cells may affect their behavior. Perhaps endogenous tagging of cytoskeletal components, or low-level expression from single-copy transgenes (Nance and Frokjaer-Jensen, 2019), combined with brighter fluorescent proteins (El Mouridi et al., 2017; Heppert et al., 2016), and new sensitive, and fast, live-imaging techniques (Fadero et al., 2018) will allow characterization of the duct and pore cytoskeletons to gain understanding into how these cells achieve the complex behaviors they undergo during morphogenesis.
Tubulogenesis of the excretory canal
The ExCa is the largest cell in C. elegans and it has a distinct “H” shape, with the cell body forming a “bridge” between two long hollow canals that extend along the entire length of the animal (Figure 1). Tubulogenesis begins in the embryo (Fig. 5A) and occurs via “hollowing”; a mechanism also observed in the Drosophila trachea (Gervais and Casanova, 2010), during angiogenesis (blood vessel formation) in zebrafish (Gebala et al., 2016; Yu et al., 2015), and in cell culture models of angiogenesis using human endothelial (blood vessel) cells (Kamei et al., 2006), consistent with the idea that mechanisms and players involved in ExCa tubulogenesis are conserved.
Fig. 5: ExCa tubulogenesis.
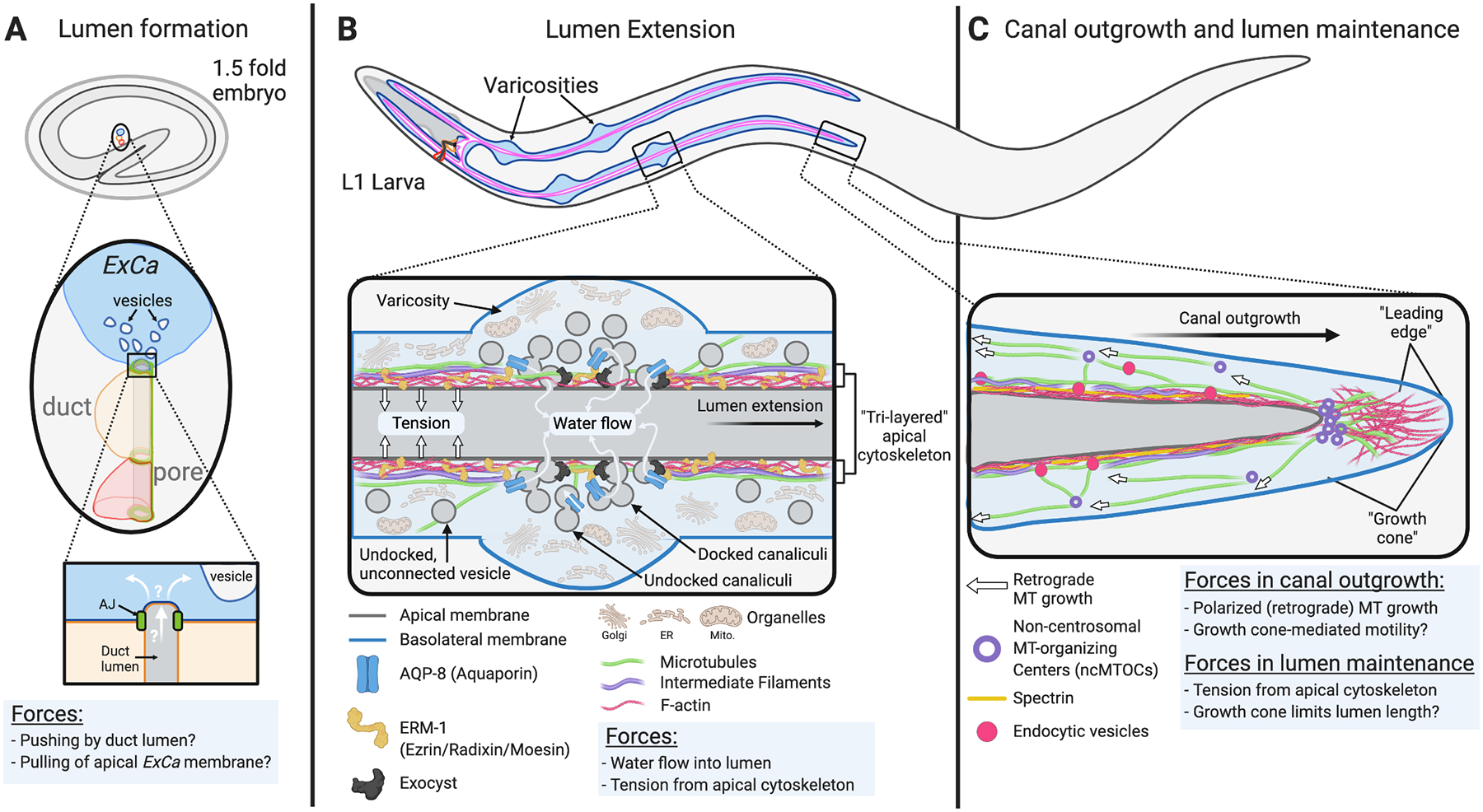
In this figure forces are denoted by white arrows and described in each panel. A) The earliest indication of ExCa lumenogenesis is an incursion of the duct apical membrane into the ExCa apical region. Intracellular vesicles that seem to coalesce at the site of the nascent lumen are seen by EM at this stage. B, C) In newly-hatched L1s the ExCa has extended ~1/2 of its final length. Cellular and molecular components (i.e., organelles, vesicles, proteins) shown separately in these panels are found at both regions of lumen extension, and where lumen maintenance and basolateral outgrowth occur. However, for simplicity they are only shown in the panel that highlights their role. B) The ERM-1 C-terminus interacts with F-actin or with aquaporin (AQP-8) on canalicular vesicles (grey). Areas of intense lumen growth form varicosities associated with increased frequency of canaliculi fusing to the apical membrane, and basolateral displacement of organelles (ER, Golgi, Mitochondria). A “tri-layered” apical cytoskeleton, made of F-actin, IFs and MTs, promotes directional lumen extension by applying tension and preventing lumen dilation. C) Polarized MT growth, nucleated by ncMTOCs along the basolateral domain, promotes ExCa outgrowth. A growth cone-like structure at ExCa tips may also promote outgrowth and regulate lumen extension. Lumen width is maintained by regulators of the apical cytoskeleton and endocytic trafficking. Diagrams inspired by Kolotuev et al. (2013), Khan et al. (2013), Khan et al. (2019), and Sundaram and Buechner (2016).
As the ExCa lumen grows during embryogenesis it bifurcates into left/right spurs, and the ExCa basolateral membrane extends in concert with the growing lumen. Left and right canals migrate dorsally along the ventral BWMs until they reach the lateral epidermis, where they once again bifurcate into anterior/posterior spurs, giving rise to the ExCa’s distinctive shape (reviewed in Sundaram and Buechner, 2016). At hatching (Fig. 5B, C) the anterior canals are fully grown, reaching the buccal cavity, while posterior canals have only grown ~1/2 of their final length, and they will continue to grow through the L1 until they reach the rectum (Buechner et al., 1999; Fujita et al., 2003; Hedgecock et al., 1987; Sundaram and Buechner, 2016). The embryonic stages of ExCa tubulogenesis are challenging to study because embryos are continuously moving at this time, making live imaging difficult. Instead, ExCa tubulogenesis from hatching onwards has been intensively analyzed and forms the basis of much of our knowledge about how it is regulated. ExCa outgrowth through the L1 stage has been described as “active” to contrast with growth from L2 through adulthood, which does not result in further canal extension and appears linked to growth of the underlying epidermis (Buechner et al., 1999; Fujita et al., 2003). However, it is not clear whether the mechanisms that regulate “active” vs. “passive” ExCa growth are distinct.
For simplicity we present ExCa tubulogenesis as occurring in discreet steps: lumen formation, extension, maintenance, and basolateral ExCa cell outgrowth. However it is important to keep in mind that these processes, and the players that regulate them, are interconnected, and disrupting one step can affect others. This makes assigning gene function to specific steps difficult, but it also makes the ExCa an excellent model to study how the forces that regulate tubulogenesis are balanced during development. Here we focus on the cell biological and force-generating aspects of ExCa tubulogenesis. We also suggest another recently-published review (Buechner et al., 2020) that focuses on genetic advances that have guided our understanding of the cell biology underlying ExCa development.
ExCa lumen formation
The earliest stages of ExCa tubulogenesis have, to date, only been visualized by electron microscopy (Fig. 5A). The first sign of tubulogenesis appears to be an invagination of the ExCa apical plasma membrane, which is surrounded by the AJ connecting the ExCa to the duct, into the cytoplasm (Abdus-Saboor et al., 2011; Stone et al., 2009; Sundaram and Buechner, 2016). How this invagination occurs, and whether it is required for tubulogenesis, remains unknown. It is possible that forces from the duct lumen push against the ExCa membrane creating a deformation. An alternative, and not mutually exclusive, possibility is that the duct-ExCa junction nucleates an endocytic process that promotes apical membrane invagination. Whether this early invagination is required for tubulogenesis, and how it is controlled, will require high-resolution imaging and precise manipulation of candidate gene activity in a duct and ExCa-specific manner.
At early stages of lumen formation many intracellular vesicles are seen within the ExCa and they appear to coalesce near the duct-ExCa junction (Berry et al., 2003. Fig. 5A). This finding led to a model where fusion of intracellular vesicles with the apical domain initiates lumen growth. Whether these vesicles are formed by transcytosis from the basolateral membrane, originate from cellular organelles, and/or via endocytosis remains unknown. Notably, RNAi against the endocytic regulators rab-5, rab-7 and rab-11 caused an early arrest in ExCa tubulogenesis (Khan et al., 2019), consistent with a model where these early vesicles are endocytic. However, whether the ExCa defects seen in rab-depleted embryos is due to a cell-autonomous function for these RAB proteins remains to be determined.
A role for intracellular vesicle fusion as a mechanism of lumen formation is consistent with findings that the PAR complex (PAR-3, PAR-6, and PKC-1), the GTPase RAL-1, and the Exocyst complex (which is activated by RAL-1 to promote vesicle tethering and fusion) accumulate at the lumen-lining apical membrane and are required for recruitment, docking, and fusion of “canalicular vesicles” (Armenti et al., 2014a), which differ from other vesicles in that they are larger (>50nm) and coated with ATPases (Buechner et al., 1999; Kolotuev et al., 2013; Nelson et al., 1983; Sundaram and Buechner, 2016). A recent report also implicates exc-5, which encodes an ortholog of the FGD-family of Cdc42-specific GEFs (Gao et al., 2001; Suzuki et al., 2001; Zheng et al., 1996), and the GTPase CDC-42 in recruiting the PAR complex to the lumen (see bioRxiv preprint by Abrams and Nance, 2020). EXC-5 and CDC-42 had been previously implicated in lumen maintenance (see below). This new finding reveals a molecular pathway that connects Rho GTPase signaling, cell polarity, and vesicle-tethering proteins to lumen extension, and suggests an earlier role for EXC-5 and CDC-42 than previously described. However, due to difficulties visualizing the earliest phases of lumen formation it is still not known whether EXC-5, CDC-42, PAR complex, and RAL-1/Exocyst-mediated vesicle docking plays a role at the earliest stages of lumen formation.
ExCa lumen growth: flow, vesicle fusion, and tension promote lumen extension
ERM-1 is expressed in the ExCa at the time of lumen formation (~1.5 fold embryo stage) and it accumulates at the apical (lumen-lining) membrane (Khan et al., 2013), co-localizing with the ExCa and intestine specific actin ACT-5 (Gobel et al., 2004; MacQueen et al., 2005. Fig. 5B). Genetic manipulation of ERM-1 levels suggested that it was involved in lumen growth: ERM-1 depletion led to formation of many unconnected vesicles instead of a continuous lumen, while overexpression led to widened and cystic lumens (Khan et al., 2013). An RNAi screen for modifiers of ERM-1 overexpression phenotypes identified the aquaporin water channel AQP-8 as a suppressor, and yeast two-hybrid studies showed that AQP-8 and ERM-1 physically interact (Khan et al., 2013). AQP-8 localizes to apical membrane-adjacent (peri-lumenal) regions, overlapping in confocal images with the ATPase VHA-5, which marks canaliculi (Kolotuev et al., 2013), and EM analyses showed that ERM-1 overexpression increased the number of canaliculi docking at, and fusing with, the lumenal membrane in an AQP-8-dependent manner (Khan et al., 2013). These results led to a model where ERM-1 recruits AQP-8-containing canaliculi to the apical membrane to promote lumen growth (Fig 5B).
A connection between water flow and lumen extension was hinted at by observations that the osmoregulatory kinases GCK-3 and WNK-1 are expressed in the ExCa and promote its growth (Choe and Strange, 2007; Denton et al., 2005; Hisamoto et al., 2008). However, a possible role for AQP-8 in lumen elongation may be that it interacts with ERM-1 only to recruit canaliculi to the lumen to provide a source of membrane. To directly assess the channel function of AQP-8, Khan et al. (2013) treated animals with sub-lethal concentrations of mercury, which inhibits water-channel activity, and found that this caused ExCa defects. Moreover, transgenes expressing AQP-8 in which residues required for channel activity were mutated failed to rescue aqp-8 mutants, demonstrating that water-channel activity is required for AQP-8 function.
By manipulating the environment of newly hatched L1’s, in which the ExCa is still growing and actively making lumen, Kolotuev et al. (2013) showed that lumen extension is osmoregulated. Placing L1’s in hypertonic media (reducing internal water concentration) followed by recovery on isotonic media (allowing water to re-enter) caused the ExCa to rapidly develop a “pearls-on-a-string” morphology (Fig. 5B). EM analysis of these pearls, previously called “varicosities” (Hahn-Windgassen and Van Gilst, 2009), showed that they are areas where canaliculi re-localize from the cytoplasm to the lumen and fuse to the lumen (Fig. 5B); suggesting that varicosities are regions of localized and active lumen growth. Consistent with this idea, hypertonic stress followed by recovery increased lumen extension rate by ~2 fold (Kolotuev et al., 2013).
Varicosities are seen in L1’s grown under normal conditions (Hahn-Windgassen and Van Gilst, 2009; Kolotuev et al., 2013), indicating that they are not artifacts of osmotic shock. Varicosities are transient, dissipating rapidly, presumably after the canaliculi fuse with the lumen. Consistent with this, mutations in genes that regulate canalicular function, like nhr-31 (which encodes a nuclear hormone receptor that drives VHA-5 expression), ral-1, and the Exocyst complex, cause varicosities to persist longer and fail to resolve (Armenti et al., 2014a; Hahn-Windgassen and Van Gilst, 2009).
How is force generated by water flowing into discrete lumenal regions focused so that extension proceeds in one direction? It appears that tension applied by the apical cytoskeleton, which has now been shown to consist of all three major players (F-actin, IFs, and MTs), plays an important role in this aspect of lumen extension. The intermediate filament proteins IFA-4, IFB-1 and IFC-2 abut apical F-actin (Fig. 5B), and their loss causes large cysts to form (Al-Hashimi et al., 2018; Khan et al., 2019; Kolotuev et al., 2013). Recent work by Khan et al. (2019) shows that the apical cytoskeleton also contains MTs that, as visualized by confocal imaging of TBB-2/β-tubulin, border the IF cytoskeleton (Fig. 5B). Loss of any of the components of this “Tri-layered” apical cytoskeleton causes dilation of the lumen, previously referred to as a cystic phenotype (Buechner et al., 1999), consistent with the cytoskeleton playing a role in restricting lumen growth. Classically, the cystic phenotype had been interpreted as a defect in lumen “maintenance” (discussed below), whereas these new studies suggest that the apical cytoskeleton is not just required for maintenance, but rather that it plays a critical role during lumen formation to promote extension.
ExCa outgrowth
As the lumen extends, the ExCa basolateral membrane grows with it in a coordinated manner. ExCa outgrowth has been compared to neuronal outgrowth, because both require shared players, including UNC-6/Netrin, and signaling via the FGF, and Wnt pathways (Hedgecock et al., 1987; Oosterveen et al., 2007; Polanska et al., 2011). ExCa and neuronal outgrowth also share many effectors, like the cytoskeletal regulators ABI-1, ARX-2, UNC-73, and WVE-1 (described in previous sections), the F-actin/MT cross-linker UNC-53/NAV2, the kinase MIG-15/NIK, MIG-10/Lamellipodin, and the cell adhesion molecule RIG-6/Contactin (Katidou et al., 2013; Marcus-Gueret et al., 2012; McShea et al., 2013; Schmidt et al., 2009; Stringham et al., 2002).
Live imaging of ExCa tips showed that, like neuronal growth cones (Lowery and Van Vactor, 2009), they are MT and F-actin rich (Shaye and Greenwald, 2015). We also found that canal tips may function as ncMTOCs, because they accumulate TBG-1/γ-tubulin (Shaye and Greenwald, 2015). Recent work shows that this is another similarity between neuronal growth cones and ExCa tips, because some dendritic growth cones in C. elegans and Drosophila also have ncMTOCs (Liang et al., 2020). We previously described the canal tips as “leading edge structures”. However, the neuronal leading edge is specifically the distal-most region within the growth cones, and it is defined by having F-actin-rich exploratory filopodia, lamellipodia-like veils, and containing few MTs (Lowery and Van Vactor, 2009). Confocal imaging of canal tips shows that their distal-most region are indeed F-actin rich and lack MT labeling (See Fig. 4A in Shaye and Greenwald, 2015), consistent with the idea that this distal-most region is similar to the neuronal leading edge, and that it may be more appropriate to refer to the entire tip structure as the ExCa’s “growth cone” (Fig 5C).
Where do the forces that promote ExCa outgrowth come from? Here we discuss three, not mutually-exclusive, models. The first model is that the extending lumen “pushes” on the ExCa tips to promote outgrowth. Lumen and basolateral outgrowth are coordinated, but several lines of evidence suggest that these processes are at least partially independent. Live imaging and TEM reconstructions of posterior canals in newly-hatched L1’s showed that basolateral outgrowth precedes lumen formation, and that the distal-most region of the ExCa lacks lumen (Kolotuev et al., 2013). Moreover, in mutants with compromised lumen extension (e.g., aqp-8 and gck-3) canals are shorter, suggesting that some outgrowth depends lumen extension, but they often exhibit long “lumen-less” cytoplasmic regions (Hisamoto et al., 2008; Khan et al., 2013), indicating that basolateral outgrowth can continue even when lumen extension is compromised.
Work on exc-6 suggested a second model for promoting ExCa outgrowth, dependent on the function of polarized dynamic MTs. A pioneering screen for mutants with cystic excretory canals, described in more detail in the next section, isolated the first exc-6 allele (Buechner et al., 1999). In exc-6 mutants the ExCa had only slightly widened regions, not large cysts, but their canals were short and the enlarged regions had multiple lumens of normal diameter, suggesting that basolateral outgrowth was compromised while lumen growth continued in these mutants. We found that exc-6 encodes an ortholog of the formin INF2 (Shaye and Greenwald, 2015), and even though formins are canonical actin-polymerizing factors (Breitsprecher and Goode, 2013), there was no obvious reduction in apical or growth cone-associated F-actin in exc-6 mutants. Moreover, a functional Venus::EXC-6 translational reporter mostly accumulated along basolateral MTs, only co-localizing with F-actin at the growth cone. In exc-6 mutants the growth cone disintegrates, suggesting that this structure plays a role in ExCa outgrowth (see below) and/or in limiting lumen extension (Shaye and Greenwald, 2015).
Live imaging of MT cytoskeleton components (the plus-end protein EBP-2, which marks growing MT ends, TBG-1/γ-tubulin, and the centrosome marker SAS-4) showed that the ExCa has ncMTOCs along the length of the canal, and that MTs grow in a polarized fashion; with the majority exhibiting retrograde (towards the cell body) growth (Shaye and Greenwald, 2015. Fig. 5C). Retrograde MTs, also known as “minus-end-out” MTs, are also enriched in growing dendrites in Drosophila and C. elegans (Liang et al., 2020; Maniar et al., 2012; Stone et al., 2008); providing yet another similarity between ExCa and neuronal outgrowth.
In exc-6 mutants the ncMTOCs along the canal disappear and MT polarity is lost. Genetic interactions between exc-6 and MT subunits, or regulators, like tbb-2/β-tubulin, the MT-severing Katanin complex (encoded by mei-1 and mei-2), and the kinesin light and heavy chains, klc-2 and unc-116, showed that mutations that stabilize MTs suppressed, while mutations that de-stabilize MTs enhanced, exc-6 mutants. These results suggested that EXC-6-promoted ExCa outgrowth is driven by its regulation of dynamic MTs (Shaye and Greenwald, 2015), consistent with reports identifying an emerging role for formins in MT regulation (Andres-Delgado et al., 2012; Chesarone et al., 2010; Daou et al., 2014; Gaillard et al., 2011; Roth-Johnson et al., 2014; Young et al., 2008)
A third model for how force may regulate ExCa outgrowth comes from the fact that neuronal growth cones drive outgrowth via dynamic cytoskeletal processes like F-actin protrusions and actomyosin-mediated contractions (Lowery and Van Vactor, 2009). Whether these mechanisms play a role in ExCa outgrowth has not been determined. Using RNAi, and mutant alleles, we did not find a role for nmy-1 or nmy-2 in ExCa outgrowth, and attempts to disrupt the F-actin cytoskeleton, with loss-of-function mutations in, or ExCa-specific overexpression of, the actin de-polymerizing factors UNC-60/Cofilin or GSNL-1/Gelsolin did not cause ExCa defects (D. Shaye, unpublished). However, these observations are tempered by the fact that genetic and/or functional redundancy between myosins (see “Summary and Open Questions” section) and actin regulators might mask a role for actomyosin forces in ExCa outgrowth.
Lumen maintenance
The screen by Buechner et al. (1999) identified ten genes (exc-1 through exc-9, and sma-1) whose loss led to cystic ExCa’s, suggesting a role for these genes in establishing and/or maintaining lumen diameter. The gene sma-1 was known to encode βH-spectrin (McKeown et al., 1998), and α/β-spectrin dimers bind F-actin and form a cytoskeletal network at the plasma membrane (Liem, 2016); therefore, finding sma-1 in this screen suggested that the F-actin cytoskeleton was important for lumen maintenance. Cloning exc-7 revealed that it encodes an RNA-binding protein similar to Drosophila ELAV and vertebrate ELAVL, involved in mRNA processing, and that EXC-7 binds to sma-1 mRNA and regulates SMA-1 expression (Fujita et al., 2003).
All the exc genes except exc-3 have been cloned and found to encode proteins that make up, or regulate, the F-actin, MTs, and/or IF cytoskeletons. Here we will briefly review the exc genes, with an emphasis on exc-5; as this gene and its function have been the most extensively studied. As discussed in the “Lumen extension” section, genes that regulate lumen diameter also play an important role in lumen growth; therefore, as mentioned in the introduction to this section, different aspects of ExCa tubulogenesis are intertwined and it can be difficult to assign discreet functions to players involved. The genes discussed here may thus be involved not just in lumen maintenance but also in earlier steps of lumen growth.
Mutations underlying exc-2 were recently found to affect the ifc-2 locus (Al-Hashimi et al., 2018), which, as discussed in the “Lumen Extension” section, encodes an intermediate filament protein. Further analysis of the exc-2 locus suggested the existence of a second gene encoding a novel protein, which has been called ecp-1, that could be affected by exc-2 mutations (Karabinos et al., 2019). However, there is uncertainty as to whether ecp-1 encodes a separate protein, or is an isoform of IFC-2 (termed ifc-2c) that lacks IF motifs. Moreover expression of ecp-1/ifc-2c has not been corroborated by Northern or Western blots (Al-Hashimi et al., 2018; Dodemont et al., 1994; Geisler et al., 2020), and although ecp-1 RNAi caused cystic ExCa (Karabinos et al., 2019), it is possible that this phenotype may be due to co-depletion of IFC-2. Therefore, more studies will be required to decipher ecp-1 and ifc-2 contributions to lumen maintenance. Besides its function in lumen extension, recent work demonstrates that the EXC-2/IFC-2 C-terminus promotes apical localization of EXC-9 (Yang et al., 2020), a protein that regulates endocytic trafficking in the ExCa (discussed further below). Of note, of the three IFs expressed in the ExCa (IFA-4, IFB-1 and EXC-2/IFC-2), only EXC-2 is required for EXC-9 apical localization (Al-Hashimi et al., 2018; Yang et al., 2020), suggesting specialization of IF function that remains to be explored.
Cloning exc-4 revealed that it encodes a C. elegans ortholog of Chloride Intracellular Channel (CLIC) proteins (Berry et al., 2003). Functional analyses showed that EXC-4 accumulates at the apical membrane, dependent on an N-terminal putative transmembrane domain (PTD). When human CLIC1 was expressed in the ExCa it accumulated in the cytoplasm and was unable to rescue exc-4 mutants. However when the CLIC1 N-terminus was replaced with the EXC-4 PTD the resulting chimera was localized to the apical membrane and rescued exc-4 (Berry and Hobert, 2006), demonstrating that EXC-4/CLIC function is conserved and that apical membrane localization is critical for function. The first CLIC protein was identified by its ability to bind a promiscuous chloride channel inhibitor. However, channel function in vivo, or under physiological conditions in vitro, has not been demonstrated, and structurally EXC-4/CLIC proteins resemble the glutathione-S-transferase (GST)-Ω family of proteins, and several CLICs have GST activity in vitro, although a cysteine residue required for GST function is absent in C. elegans and Drosophila CLICs (Argenzio and Moolenaar, 2016). Therefore EXC-4/CLIC function remains mysterious, although we have recently found that EXC-4 appears to regulate Gα and Rac signaling in the ExCa (A. Arena and D. Shaye, manuscript in preparation).
As mentioned in the “ExCa lumen formation” section, EXC-5 is an ortholog of the FGD-family of Cdc42-specific GEFs (Gao et al., 2001; Suzuki et al., 2001; Zheng et al., 1996), and CDC-42 is a well-known actin regulator, but it can play other roles including in endocytic trafficking and vesicle docking (see bioRxiv preprint by Abrams and Nance, 2020; Balklava et al., 2007). An analysis of endocytic trafficking markers in exc-5 mutants showed that early endosomal markers (RAB-5 and EEA-1) were upregulated and mis-localized, and the recycling endosome marker RME-1 was depleted (Mattingly and Buechner, 2011). This suggests that EXC-5 regulates endocytosis and recycling of cargo required to maintain lumen diameter. Two other genes, exc-1 (encoding the sole Immunity-related GTPase, or IRG, gene in C. elegans) and exc-9 (a homolog of vertebrate cysteine-rich intestinal proteins, or CRIP) function with exc-5 to regulate endocytic trafficking (Grussendorf et al., 2016; Tong and Buechner, 2008). Overexpression of the endocytic regulator RAB-8 suppresses exc-1 and exc-9 phenotypes, further supporting their putative role in trafficking (Yang et al., 2020). In contrast, RAB-8 overexpression did not suppress exc-5, consistent with the idea that EXC-5/FGD plays other roles in the ExCa, for example, regulation of formin-mediated actin polymerization (Shaye and Greenwald, 2016), which will be discussed further below.
CDC-42 and endocytic recycling are also regulated by the striatin-interacting phosphatase and kinase (STRIPAK) complex. Loss of STRIPAK components, like CCM-3 and its downstream effector, the kinase GCK-1, led to shortened and cystic canals, reduced cdc-42 activity (assessed with an mCherry-tagged GTPase-binding domain (GBD) of WSP-1), and loss of RAB-11-marked recycling endosomes (Lant et al., 2015). CCM-3 is the worm ortholog of PDCD10, whose loss causes cerebral cavernous malformations (CCMs); capillary swellings that can rupture and cause intra-cranial hemorrhage (Draheim et al., 2014). The role of PDCD10 in vascular disease draws an intriguing parallel between ExCa tubulogenesis and vertebrate angiogenesis.
Despite the fact that exc-5 and ccm-3 are both regulators of cdc-42, and their mutants display some similar phenotypes, there are notable differences (Lant et al., 2015; Mattingly and Buechner, 2011). For example, ccm-3 mutants have fragmented Golgi, which are not seen in exc-5 mutants. Conversely, exc-5 appears to regulate early endosomes, while ccm-3 does not. Loss of exc-5 and ccm-3 also affect distinct recycling compartments, those marked by RME-1 and RAB-11 respectively, suggesting that these genes regulate distinct endocytic recycling routes, as RME-1 preferentially promotes basolateral recycling (Chen et al., 2006). Finally, cdc-42 activity, reported by mCherry::(GBD)WSP-1, was reduced in ccm-3 but not in exc-5 mutants, which raised the question of whether exc-5 actually functions through cdc-42 (see below). Based on these observations, it is tempting to speculate that EXC-5 and CCM-3 not only activate CDC-42, but they may also modulate how this GTPase interacts with downstream effectors, thus leading to distinct outcomes downstream of cdc-42.
What proteins function downstream of cdc-42 in the ExCa? Lant et al. (2015) investigated the kinase MRCK-1, the ortholog of three vertebrate MRCK/CDC42BP kinases that bind to, and are activated by, Cdc42 (Leung et al., 1998). Loss of mrck-1 caused phenotypes similar to ccm-3 and gck-1, like decreased RAB-11 and Golgi accumulation. Surprisingly, mrck-1 loss also reduced CDC-42 and mCherry::(GBD)WSP-1 levels, suggesting that mrck-1 may function in a feedback loop to promote cdc-42 activity. Proteomic analysis of STRIPAK-interacting proteins found that MRCK-1 physically interacts with CCM-3 (Lant et al., 2015), consistent with the model proposed above where CCM-3 not only functions upstream of CDC-42, it may also regulate interactions with its downstream effector MRCK-1.
We undertook a candidate-gene approach to define proteins that function downstream of exc-5 to regulate apical F-actin accumulation. First, to ask whether exc-5 functions through cdc-42, we took advantage of the fact that increasing EXC-5 dosage caused canal shortening (Mattingly and Buechner, 2011), and found that cdc-42 loss suppressed exc-5-overxpression, demonstrating that cdc-42 indeed acts downstream of exc-5 (Shaye and Greenwald, 2016). Functional GFP::EXC-5 accumulates at the apical membrane (Mattingly and Buechner, 2011), consistent with a model where EXC-5, via CDC-42, promotes apical F-actin accumulation. We used genetic interaction studies and live imaging to show that the formins INFT-2/INF2 and CYK-1/DIAPH, both expressed in the ExCa, are regulated by EXC-5 and CDC-42, and also showed that cyk-1, exc-5 and inft-2 regulate apical F-actin levels (Shaye and Greenwald, 2016); revealing a pathway by which the actin cytoskeleton is built at the apical membrane. Intriguingly, Venus::CYK-1 accumulated in F-actin-rich motile punctae (Fig. 5C), which were lost in cdc-42 mutants (Shaye and Greenwald, 2016); raising the possibility that these punctae may be EXC-5 and CDC-42-regulated endocytic vesicles that promote lumen maintenance.
Unanswered questions in ExCa tubulogenesis
Although much progress has been made in understanding the regulation and mechanisms of ExCa tubulogenesis, many questions remain. For example, how do the signaling pathways that control guidance regulate the effectors that promote outgrowth? And how does the ExCa basolateral membrane interact with the substrate(s) over which it extend? During early outgrowth the canals migrate over the BWM basement membrane (BM). Once the canals contact the lateral hypodermis they appear to breach an existing BM and insinuate themselves between the lateral epidermis and its BM (Fig. 1, “Epidermis” panel), at which point the canals bifurcate and undergo anterior/posterior outgrowth. The BM-component EPI-1/Laminin, and its receptors, the integrins INA-1, PAT-2, and PAT-3, are required for ExCa outgrowth (Hedgecock et al., 1987; Huang et al., 2003), but little else is known about their function in this process. The recent development of tools for live imaging of BM components, and their receptors, in vivo (Keeley et al., 2020) will allow us to define how interactions between the canals and the BM regulate ExCa tubulogenesis.
Recent RNAi screens have identified >10 new genes involved in ExCa development (Al-Hashimi et al., 2019; A. Socovich and D. Shaye, manuscript in preparation). Perhaps among these novel players there will be genes that will help us address some of these remaining questions in ExCa tubulogenesis.
SUMMARY AND OPEN QUESTIONS
Our current understanding of tubulogenesis provides single cell precision in the understanding of transcription factors that must be active to promote morphogenetic events, and in some cases single cell understanding to explain how tissues form specific structures. The open questions remaining are in the interpretation and integration of the signals. For example, cell fate signals must be translated into cues to activate complex transcriptional programs so that individual cells follow the correct morphogenesis program. In addition, once a morphogenetic program launches, cells must combine polarity cues and the changing environment they encounter to reorganize their cytoskeleton, adhesive complexes, and polarized apical and basal extracellular matrices to establish and maintain correctly formed tubes.
While some cells and tissues mentioned above are fairly well understood, the movements of some tissues have yet to be fully described. For example, we still lack a proper description of how the epidermis encloses the head; the final section of the epidermal sheet to form a tube. Since migration of the anterior epidermis is required for it to join the developing alimentary canal, and form the buccal cavity, it is of great importance for development of the worm. Recent studies also show that this anterior epidermal migration is required to pattern many of the sensory structures, like the amphid neurons, that allow worms to detect their environment (Fan et al., 2019).
Further analysis of cellular force will require that we fully investigate the cellular force generators, including actin, MTs, the motors that work with them, and how these are constrained by adhesive forces. For example, a better understanding of how myosin is organized and controlled during distinct tissue movements will help clarify if novel myosin activities, or novel myosins, support the morphogenetic movements described here. C. elegans has 17 myosins (Berg et al., 2001), and most are poorly understood. For example, do cells use specific actomyosin or adhesive structures when they undergo a radial migration around a central axis, as happens when the valve cells form between the pharynx and intestine, or when the duct and pore wrap upon themselves? Another open mystery is how tissues are able to become distinct and cohesive, while still moving past each other during embryogenesis, at a time when the extracellular matrix is just being formed. In other words, how is intra-tissue adhesiveness maintained, even as junctions are remodeled by a tissue undergoing dynamic changes, like the rapidly migrating epidermis during ventral enclosure? Long-standing questions in tubular morphogenesis await new approaches and techniques that build on the strong foundation we have introduced here.
ACKNOWLEDGEMENTS
We apologize for any important work that may have been omitted due to length restrictions and our own limitations. We thank Jim Priess, Michel Labouesse, and members of the Soto and Shaye labs for comments on this review. Figures 1, 4 and 5 were created using BioRender.com. Our work is supported by grants from the National Institutes for Health: GM081670 and K12GM093854 to M.S., and GM134032 to D. S.
REFERENCES
- Abdus-Saboor I, Mancuso VP, Murray JI, Palozola K, Norris C, Hall DH, Howell K, Huang K, Sundaram MV, 2011. Notch and Ras promote sequential steps of excretory tube development in C. elegans. Development 138, 3545–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdus-Saboor I, Stone CE, Murray JI, Sundaram MV, 2012. The Nkx5/HMX homeodomain protein MLS-2 is required for proper tube cell shape in the C. elegans excretory system. Developmental Biology 366, 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams J, Nance J, 2020. A polarity pathway for exocyst-dependent intracellular tube extension. bioRxiv, 2020.2010.2005.327247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achilleos A, Wehman AM, Nance J, 2010. PAR-3 mediates the initial clustering and apical localization of junction and polarity proteins during C. elegans intestinal epithelial cell polarization. Development 137, 1833–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hashimi H, Chiarelli T, Lundquist EA, Buechner M, 2019. Novel exc Genes Involved in Formation of the Tubular Excretory Canals of Caenorhabditis elegans. G3 (Bethesda). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hashimi H, Hall DH, Ackley BD, Lundquist EA, Buechner M, 2018. Tubular Excretory Canal Structure Depends on Intermediate Filaments EXC-2 and IFA-4 in Caenorhabditis elegans. Genetics 210, 637–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson DG, Thomson JN, 1976. The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 275, 299–325. [DOI] [PubMed] [Google Scholar]
- Altun ZF, Hall DH, 2009a. Epithelial system, interfacial cells. In WormAtlas. doi: 10.3908/wormatlas.1.15 [DOI] [Google Scholar]
- Altun ZF, Hall DH, 2009b. Alimentary System, Pharynx. In WormAtlas. doi: 10.3908/wormatlas.1.3 [DOI] [Google Scholar]
- Andres-Delgado L, Anton OM, Bartolini F, Ruiz-Saenz A, Correas I, Gundersen GG, Alonso MA, 2012. INF2 promotes the formation of detyrosinated microtubules necessary for centrosome reorientation in T cells. J Cell Biol 198, 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argenzio E, Moolenaar WH, 2016. Emerging biological roles of Cl- intracellular channel proteins. J Cell Sci 129, 4165–4174. [DOI] [PubMed] [Google Scholar]
- Armenti ST, Chan E, Nance J, 2014a. Polarized exocyst-mediated vesicle fusion directs intracellular lumenogenesis within the C. elegans excretory cell. Developmental Biology 394, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenti ST, Lohmer LL, Sherwood DR, Nance J, 2014b. Repurposing an endogenous degradation system for rapid and targeted depletion of C. elegans proteins. Development 141, 4640–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asan A, Raiders SA, Priess JR, 2016. Morphogenesis of the C. elegans Intestine Involves Axon Guidance Genes. Plos Genetics 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspock G, Ruvkun G, Burglin TR, 2003. The Caenorhabditis elegans ems class homeobox gene ceh-2 is required for M3 pharynx motoneuron function. Development 130, 3369–3378. [DOI] [PubMed] [Google Scholar]
- Avery L, 1993. The genetics of feeding in Caenorhabditis elegans. Genetics 133, 897–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axang C, Rauthan M, Hall DH, Pilon M, 2007. The twisted pharynx phenotype in C. elegans. Bmc Dev Biol 7, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann C, Fischer L, Walter U, Reinhard M, 1999. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J Biol Chem 274, 23549–23557. [DOI] [PubMed] [Google Scholar]
- Balklava Z, Pant S, Fares H, Grant BD, 2007. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol 9, 1066–1073. [DOI] [PubMed] [Google Scholar]
- Bei Y, Hogan J, Berkowitz LA, Soto M, Rocheleau CE, Pang KM, Collins J, Mello CC, 2002. SRC-1 and Wnt signaling act together to specify endoderm and to control cleavage orientation in early C. elegans embryos. Dev Cell 3, 113–125. [DOI] [PubMed] [Google Scholar]
- Ben Amar M, Qiuyang-Qu P, Vuong-Brender TTK, Labouesse M, 2018. Assessing the Contribution of Active and Passive Stresses in C. elegans Elongation. Phys Rev Lett 121, 268102. [DOI] [PubMed] [Google Scholar]
- Berg JS, Powell BC, Cheney RE, 2001. A millennial myosin census. Mol Biol Cell 12, 780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernadskaya YY, Patel FB, Hsu HT, Soto MC, 2011. Arp2/3 promotes junction formation and maintenance in the Caenorhabditis elegans intestine by regulating membrane association of apical proteins. Mol Biol Cell 22, 2886–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernadskaya YY, Wallace A, Nguyen J, Mohler WA, Soto MC, 2012. UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph polarize F-actin during embryonic morphogenesis by regulating the WAVE/SCAR actin nucleation complex. PLoS Genet 8, e1002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry KL, Bulow HE, Hall DH, Hobert O, 2003. A C. elegans CLIC-like protein required for intracellular tube formation and maintenance. Science 302, 2134–2137. [DOI] [PubMed] [Google Scholar]
- Berry KL, Hobert O, 2006. Mapping functional domains of chloride intracellular channel (CLIC) proteins in vivo. J Mol Biol 359, 1316–1333. [DOI] [PubMed] [Google Scholar]
- Breitsprecher D, Goode BL, 2013. Formins at a Glance. J. of Cell Sci 126, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broitman-Maduro G, Lin KT, Hung WW, Maduro MF, 2006. Specification of the C. elegans MS blastomere by the T-box factor TBX-35. Development 133, 3097–3106. [DOI] [PubMed] [Google Scholar]
- Buechner M, Hall DH, Bhatt H, Hedgecock EM, 1999. Cystic canal mutants in Caenorhabditis elegans are defective in the apical membrane domain of the renal (excretory) cell. Dev Biol 214, 227–241. [DOI] [PubMed] [Google Scholar]
- Buechner M, Yang Z, Al-Hashimi H, 2020. A Series of Tubes: The C. elegans Excretory Canal Cell as a Model for Tubule Development. J Dev Biol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carberry K, Wiesenfahrt T, Geisler F, Stocker S, Gerhardus H, Uberbach D, Davis W, Jorgensen E, Leube RE, Bossinger O, 2012. The novel intestinal filament organizer IFO-1 contributes to epithelial integrity in concert with ERM-1 and DLG-1. Development 139, 1851–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho CA, Broday L, 2020. Game of Tissues: How the Epidermis Thrones C. elegans Shape. J Dev Biol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Schweinsberg PJ, Vashist S, Mareiniss DP, Lambie EJ, Grant BD, 2006. RAB-10 is required for endocytic recycling in the Caenorhabditis elegans intestine. Mol Biol Cell 17, 1286–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesarone MA, DuPage AG, Goode BL, 2010. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol 11, 62–74. [DOI] [PubMed] [Google Scholar]
- Choe KP, Strange K, 2007. Evolutionarily conserved WNK and Ste20 kinases are essential for acute volume recovery and survival after hypertonic shrinkage in Caenorhabditis elegans. Am J Physiol, Cell Physiol 293, C915–927. [DOI] [PubMed] [Google Scholar]
- Christensen S, Kodoyianni V, Bosenberg M, Friedman L, Kimble J, 1996. lag-1, a gene required for lin-12 and glp-1 signaling in Caenorhabditis elegans, is homologous to human CBF1 and Drosophila Su(H). Development 122, 1373–1383. [DOI] [PubMed] [Google Scholar]
- Ciarletta P, Ben Amar M, Labouesse M, 2009. Continuum model of epithelial morphogenesis during Caenorhabditis elegans embryonic elongation. Philos Trans A Math Phys Eng Sci 367, 3379–3400. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Flatt KM, Schroeder NE, Sundaram MV, 2019. Epithelial Shaping by Diverse Apical Extracellular Matrices Requires the Nidogen Domain Protein DEX-1 in Caenorhabditis elegans. Genetics 211, 185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Draper BW, Priess JR, 1997. The role of actin filaments in patterning the Caenorhabditis elegans cuticle. Dev Biol 184, 373–384. [DOI] [PubMed] [Google Scholar]
- Cox-Paulson E, Cannataro V, Gallagher T, Hoffman C, Mantione G, McIntosh M, Silva M, Vissichelli N, Walker R, Simske J, Ono S, Hoops H, 2014. The minus-end actin capping protein, UNC-94/tropomodulin, regulates development of the Caenorhabditis elegans intestine. Dev Dyn 243, 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daou P, Hasan S, Breitsprecher D, Baudelet E, Camoin L, Audebert S, Goode BL, Badache A, 2014. Essential and nonredundant roles for Diaphanous formins in cortical microtubule capture and directed cell migration. Mol Biol Cell 25, 658–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton J, Nehrke K, Yin X, Morrison R, Strange K, 2005. GCK-3, a newly identified Ste20 kinase, binds to and regulates the activity of a cell cycle-dependent ClC anion channel. J. Gen. Physiol 125, 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppe U, Schierenberg E, Cole T, Krieg C, Schmitt D, Yoder B, von Ehrenstein G, 1978. Cell lineages of the embryo of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A 75, 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogon M, Wissler F, Quintin S, Nagamatsu Y, Sookhareea S, Landmann F, Hutter H, Vitale N, Labouesse M, 2007. The RhoGAP RGA-2 and LET-502/ROCK achieve a balance of actomyosin-dependent forces in C. elegans epidermis to control morphogenesis. Development 134, 2469–2479. [DOI] [PubMed] [Google Scholar]
- Dodemont H, Riemer D, Ledger N, Weber K, 1994. Eight genes and alternative RNA processing pathways generate an unexpectedly large diversity of cytoplasmic intermediate filament proteins in the nematode Caenorhabditis elegans. EMBO J 13, 2625–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draheim KM, Fisher OS, Boggon TJ, Calderwood DA, 2014. Cerebral cavernous malformation proteins at a glance. J Cell Sci 127, 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mouridi S, Lecroisey C, Tardy P, Mercier M, Leclercq-Blondel A, Zariohi N, Boulin T, 2017. Reliable CRISPR/Cas9 Genome Engineering in Caenorhabditis elegans Using a Single Efficient sgRNA and an Easily Recognizable Phenotype. G3 (Bethesda) 7, 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadero TC, Gerbich TM, Rana K, Suzuki A, DiSalvo M, Schaefer KN, Heppert JK, Boothby TC, Goldstein B, Peifer M, Allbritton NL, Gladfelter AS, Maddox AS, Maddox PS, 2018. LITE microscopy: Tilted light-sheet excitation of model organisms offers high resolution and low photobleaching. J Cell Biol 217, 1869–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Kovacevic I, Heiman MG, Bao Z, 2019. A multicellular rosette-mediated collective dendrite extension. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Priess JR, 2012. A role for the centrosome and PAR-3 in the hand-off of MTOC function during epithelial polarization. Curr Biol 22, 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman-Rubinsky R, Cohen JD, Sundaram MV, 2017. Lipocalins Are Required for Apical Extracellular Matrix Organization and Remodeling in Caenorhabditis elegans. Genetics 207, 625–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotopoulos N, Wernike D, Chen Y, Makil N, Marte A, Piekny A, 2013. Caenorhabditis elegans anillin (ani-1) regulates neuroblast cytokinesis and epidermal morphogenesis during embryonic development. Dev Biol 383, 61–74. [DOI] [PubMed] [Google Scholar]
- Francis R, Waterston RH, 1991. Muscle cell attachment in Caenorhabditis elegans. J Cell Biol 114, 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridolfsson HN, Starr DA, 2010. Kinesin-1 and dynein at the nuclear envelope mediate the bidirectional migrations of nuclei. J Cell Biol 191, 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Hawkinson D, King KV, Hall DH, Sakamoto H, Buechner M, 2003. The role of the ELAV homologue EXC-7 in the development of the Caenorhabditis elegans excretory canals. Dev Biol 256, 290–301. [DOI] [PubMed] [Google Scholar]
- Gaillard J, Ramabhadran V, Neumanne E, Gurel P, Blanchoin L, Vantard M, Higgs HN, 2011. Differential interactions of the formins INF2, mDia1, and mDia2 with microtubules. Mol Biol Cell 22, 4575–4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gally C, Wissler F, Zahreddine H, Quintin S, Landmann F, Labouesse M, 2009. Myosin II regulation during C. elegans embryonic elongation: LET-502/ROCK, MRCK-1 and PAK-1, three kinases with different roles. Development 136, 3109–3119. [DOI] [PubMed] [Google Scholar]
- Gao JT, Estrada L, Cho SC, Ellis RE, Gorski JL, 2001. The Caenorhabditis elegans homolog of FGD1, the human Cdc42 GEF gene responsible for faciogenital dysplasia, is critical for excretory cell morphogenesis. Hum Mol Genet 10, 3049–3062. [DOI] [PubMed] [Google Scholar]
- Gaudet J, Mango SE, 2002. Regulation of organogenesis by the Caenorhabditis elegans FoxA protein PHA-4. Science 295, 821–825. [DOI] [PubMed] [Google Scholar]
- Gebala V, Collins R, Geudens I, Phng LK, Gerhardt H, 2016. Blood flow drives lumen formation by inverse membrane blebbing during angiogenesis in vivo. Nat Cell Biol 18, 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler F, Coch RA, Richardson C, Goldberg M, Bevilacqua C, Prevedel R, Leube RE, 2020. Intestinal intermediate filament polypeptides in C. elegans: Common and isotype-specific contributions to intestinal ultrastructure and function. Sci Rep 10, 3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SE, Simokat K, Hardin J, Chisholm AD, 1998. The VAB-1 Eph receptor tyrosine kinase functions in neural and epithelial morphogenesis in C. elegans. Cell 92, 633–643. [DOI] [PubMed] [Google Scholar]
- George-Raizen JB, Shockley KR, Trojanowski NF, Lamb AL, Raizen DM, 2014. Dynamically-expressed prion-like proteins form a cuticle in the pharynx of Caenorhabditis elegans. Biol Open 3, 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais L, Casanova J, 2010. In vivo coupling of cell elongation and lumen formation in a single cell. Curr Biol 20, 359–366. [DOI] [PubMed] [Google Scholar]
- Ghenea S, Boudreau JR, Lague NP, Chin-Sang ID, 2005. The VAB-1 Eph receptor tyrosine kinase and SAX-3/Robo neuronal receptors function together during C. elegans embryonic morphogenesis. Development 132, 3679–3690. [DOI] [PubMed] [Google Scholar]
- Gieseler K, Qadota H, Benian GM, 2017. Development, structure, and maintenance of C. elegans body wall muscle. WormBook 2017, 1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill HK, Cohen JD, Ayala-Figueroa J, Forman-Rubinsky R, Poggioli C, Bickard K, Parry JM, Pu P, Hall DH, Sundaram MV, 2016. Integrity of Narrow Epithelial Tubes in the C. elegans Excretory System Requires a Transient Luminal Matrix. PLoS Genet 12, e1006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard G, Nicolle O, Brugiere T, Prigent S, Pinot M, Michaux G, 2019. Force Transmission between Three Tissues Controls Bipolar Planar Polarity Establishment and Morphogenesis. Curr Biol 29, 1360–1368 e1364. [DOI] [PubMed] [Google Scholar]
- Gilleard JS, McGhee JD, 2001. Activation of hypodermal differentiation in the Caenorhabditis elegans embryo by GATA transcription factors ELT-1 and ELT-3. Mol Cell Biol 21, 2533–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleard JS, Shafi Y, Barry JD, McGhee JD, 1999. ELT-3: A Caenorhabditis elegans GATA factor expressed in the embryonic epidermis during morphogenesis. Dev Biol 208, 265–280. [DOI] [PubMed] [Google Scholar]
- Gobel V, Barrett PL, Hall DH, Fleming JT, 2004. Lumen morphogenesis in C. elegans requires the membrane-cytoskeleton linker erm-1. Dev Cell 6, 865–873. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Nance J, 2020. Caenorhabditis elegans Gastrulation: A Model for Understanding How Cells Polarize, Change Shape, and Journey Toward the Center of an Embryo. Genetics 214, 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good K, Ciosk R, Nance J, Neves A, Hill RJ, Priess JR, 2004. The T-box transcription factors TBX-37 and TBX-38 link GLP-1/Notch signaling to mesoderm induction in C. elegans embryos. Development 131, 1967–1978. [DOI] [PubMed] [Google Scholar]
- Goode BL, Eck MJ, 2007. Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem 76, 593–627. [DOI] [PubMed] [Google Scholar]
- Grussendorf KA, Trezza CJ, Salem AT, Al-Hashimi H, Mattingly BC, Kampmeyer DE, Khan LA, Hall DH, Gobel V, Ackley BD, Buechner M, 2016. Facilitation of Endosomal Recycling by an IRG Protein Homolog Maintains Apical Tubule Structure in Caenorhabditis elegans. Genetics 203, 1789–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn-Windgassen A, Van Gilst MR, 2009. The Caenorhabditis elegans HNF4α Homolog, NHR-31, Mediates Excretory Tube Growth and Function through Coordinate Regulation of the Vacuolar ATPase. PLoS Genet 5, e1000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell JR, Goldstein B, 2011. Internalization of multiple cells during C. elegans gastrulation depends on common cytoskeletal mechanisms but different cell polarity and cell fate regulators. Dev Biol 350, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock EM, Culotti JG, Hall DH, Stern BD, 1987. Genetics of cell and axon migrations in Caenorhabditis elegans. Development 100, 365–382. [DOI] [PubMed] [Google Scholar]
- Heid PJ, Raich WB, Smith R, Mohler WA, Simokat K, Gendreau SB, Rothman JH, Hardin J, 2001. The zinc finger protein DIE-1 is required for late events during epithelial cell rearrangement in C. elegans. Dev Biol 236, 165–180. [DOI] [PubMed] [Google Scholar]
- Heiman MG, Shaham S, 2009. DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell 137, 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppert JK, Dickinson DJ, Pani AM, Higgins CD, Steward A, Ahringer J, Kuhn JR, Goldstein B, 2016. Comparative assessment of fluorescent proteins for in vivo imaging in an animal model system. Mol Biol Cell 27, 3385–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann GJ, Leung B, Priess JR, 2000. Left-right asymmetry in C. elegans intestine organogenesis involves a LIN-12/Notch signaling pathway. Development 127, 3429–3440. [DOI] [PubMed] [Google Scholar]
- Hisamoto N, Moriguchi T, Urushiyama S, Mitani S, Shibuya H, Matsumoto K, 2008. Caenorhabditis elegans WNK-STE20 pathway regulates tube formation by modulating ClC channel activity. EMBO Rep 9, 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL, Kolodziej PA, 2002. Organogenesis: molecular mechanisms of tubulogenesis. Nat Rev Genet 3, 513–523. [DOI] [PubMed] [Google Scholar]
- Hollo G, 2017. Demystification of animal symmetry: symmetry is a response to mechanical forces. Biol Direct 12, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RM, Sundaram MV, 2002. C. elegans EOR-1/PLZF and EOR-2 positively regulate Ras and Wnt signaling and function redundantly with LIN-25 and the SUR-2 Mediator component. Genes Dev 16, 1815–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-C, Hall DH, Hedgecock EM, Kao G, Karantza V, Vogel BE, Hutter H, Chisholm AD, Yurchenco PD, Wadsworth WG, 2003. Laminin alpha subunits and their role in C. elegans development. Development 130, 3343–3358. [DOI] [PubMed] [Google Scholar]
- Ikegami R, Simokat K, Zheng H, Brown L, Garriga G, Hardin J, Culotti J, 2012. Semaphorin and Eph receptor signaling guide a series of cell movements for ventral enclosure in C. elegans. Curr Biol 22, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb JM, Lau KK, Goszczynski B, Fukushige T, Moons D, Okkema PG, McGhee JD, 1998. pha-4 is Ce-fkh-1, a fork head/HNF-3alpha,beta,gamma homolog that functions in organogenesis of the C. elegans pharynx. Development 125, 2171–2180. [DOI] [PubMed] [Google Scholar]
- Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM, 2006. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 442, 453–456. [DOI] [PubMed] [Google Scholar]
- Karabinos A, Schulze E, Baumeister R, 2019. Analysis of the novel excretory cell expressed ECP-1 protein and its proposed ECP-1/IFC-2 fusion protein EXC-2 in the nematode Caenorhabditis elegans. Gene Expr Patterns 34, 119061. [DOI] [PubMed] [Google Scholar]
- Katidou M, Tavernarakis N, Karagogeos D, 2013. The contactin RIG-6 mediates neuronal and non-neuronal cell migration in Caenorhabditis elegans. Dev Biol 373, 184–195. [DOI] [PubMed] [Google Scholar]
- Keeley DP, Hastie E, Jayadev R, Kelley LC, Chi Q, Payne SG, Jeger JL, Hoffman BD, Sherwood DR, 2020. Comprehensive Endogenous Tagging of Basement Membrane Components Reveals Dynamic Movement within the Matrix Scaffolding. Dev Cell 54, 60–74 e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley CA, Cram EJ, 2019. Regulation of Actin Dynamics in the C. elegans Somatic Gonad. J Dev Biol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley M, Yochem J, Krieg M, Calixto A, Heiman MG, Kuzmanov A, Meli V, Chalfie M, Goodman MB, Shaham S, Frand A, Fay DS, 2015. FBN-1, a fibrillin-related protein, is required for resistance of the epidermis to mechanical deformation during C. elegans embryogenesis. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan LA, Jafari G, Zhang N, Membreno E, Yan SY, Zhang HJ, Gobel V, 2019. A tensile trilayered cytoskeletal endotube drives capillary-like lumenogenesis. Journal of Cell Biology 218, 2403–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan LA, Zhang H, Abraham N, Sun L, Fleming JT, Buechner M, Hall DH, Gobel V, 2013. Intracellular lumen extension requires ERM-1-dependent apical membrane expansion and AQP-8-mediated flux. Nat Cell Biol 15, 143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K, Peyrot SM, Wood CG, Wagmaister JA, Maduro MF, Eisenmann DM, Rothman JH, 2002. Cell fates and fusion in the C. elegans vulval primordium are regulated by the EGL-18 and ELT-6 GATA factors -- apparent direct targets of the LIN-39 Hox protein. Development 129, 5171–5180. [DOI] [PubMed] [Google Scholar]
- Kolotuev I, Hyenne V, Schwab Y, Rodriguez D, Labouesse M, 2013. A pathway for unicellular tube extension depending on the lymphatic vessel determinant Prox1 and on osmoregulation. Nat Cell Biol 15, 157–168. [DOI] [PubMed] [Google Scholar]
- Labouesse M, Hartwieg E, Horvitz HR, 1996. The Caenorhabditis elegans LIN-26 protein is required to specify and/or maintain all non-neuronal ectodermal cell fates. Development 122, 2579–2588. [DOI] [PubMed] [Google Scholar]
- Labouesse M, Sookhareea S, Horvitz HR, 1994. The Caenorhabditis elegans gene lin-26 is required to specify the fates of hypodermal cells and encodes a presumptive zinc-finger transcription factor. Development 120, 2359–2368. [DOI] [PubMed] [Google Scholar]
- Langenhan T, Promel S, Mestek L, Esmaeili B, Waller-Evans H, Hennig C, Kohara Y, Avery L, Vakonakis I, Schnabel R, Russ AP, 2009. Latrophilin signaling links anterior-posterior tissue polarity and oriented cell divisions in the C. elegans embryo. Dev Cell 17, 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lant B, Yu B, Goudreault M, Holmyard D, Knight JD, Xu P, Zhao L, Chin K, Wallace E, Zhen M, Gingras AC, Derry WB, 2015. CCM-3/STRIPAK promotes seamless tube extension through endocytic recycling. Nat Commun 6, 6449. [DOI] [PubMed] [Google Scholar]
- Lardennois A, Pasti G, Ferraro T, Llense F, Mahou P, Pontabry J, Rodriguez D, Kim S, Ono S, Beaurepaire E, Gally C, Labouesse M, 2019. An actin-based viscoplastic lock ensures progressive body-axis elongation. Nature 573, 266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung B, Hermann GJ, Priess JR, 1999. Organogenesis of the Caenorhabditis elegans intestine. Dev Biol 216, 114–134. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Tan I, Manser E, Lim L, 1998. Myotonic dystrophy kinase-related Cdc42-binding kinase acts as a Cdc42 effector in promoting cytoskeletal reorganization. Mol Cell Biol 18, 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Kokes M, Fetter RD, Sallee MD, Moore AW, Feldman JL, Shen K, 2020. Growth cone-localized microtubule organizing center establishes microtubule orientation in dendrites. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem RK, 2016. Cytoskeletal Integrators: The Spectrin Superfamily. Cold Spring Harb Perspect Biol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Thompson S, Priess JR, 1995. pop-1 encodes an HMG box protein required for the specification of a mesoderm precursor in early C. elegans embryos. Cell 83, 599–609. [DOI] [PubMed] [Google Scholar]
- Liro MJ, Morton DG, Rose LS, 2018. The kinases PIG-1 and PAR-1 act in redundant pathways to regulate asymmetric division in the EMS blastomere of C. elegans. Dev Biol 444, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery LA, Van Vactor D, 2009. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol 10, 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubarsky B, Krasnow MA, 2003. Tube morphogenesis: making and shaping biological tubes. Cell 112, 19–28. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Atkinson SJ, Ampe C, Vandekerckhove J, Pollard TD, 1994. Purification of a cortical complex containing two unconventional actins from Acanthamoeba by affinity chromatography on profilin-agarose. J Cell Biol 127, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen AJ, Baggett JJ, Perumov N, Bauer RA, Januszewski T, Schriefer L, Waddle JA, 2005. ACT-5 is an essential Caenorhabditis elegans actin required for intestinal microvilli formation. Mol Biol Cell 16, 3247–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduro MF, Rothman JH, 2002. Making worm guts: the gene regulatory network of the Caenorhabditis elegans endoderm. Dev Biol 246, 68–85. [DOI] [PubMed] [Google Scholar]
- Mancuso VP, Parry JM, Storer L, Poggioli C, Nguyen KC, Hall DH, Sundaram MV, 2012. Extracellular leucine-rich repeat proteins are required to organize the apical extracellular matrix and maintain epithelial junction integrity in C. elegans. Development 139, 979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mango SE, Lambie EJ, Kimble J, 1994. The pha-4 gene is required to generate the pharyngeal primordium of Caenorhabditis elegans. Development 120, 3019–3031. [DOI] [PubMed] [Google Scholar]
- Maniar TA, Kaplan M, Wang GJ, Shen K, Wei L, Shaw JE, Koushika SP, Bargmann CI, 2012. UNC-33 (CRMP) and ankyrin organize microtubules and localize kinesin to polarize axon-dendrite sorting. Nat Neurosci 15, 48–U66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus-Gueret N, Schmidt KL, Stringham EG, 2012. Distinct cell guidance pathways controlled by the Rac and Rho GEF domains of UNC-73/TRIO in Caenorhabditis elegans. Genetics 190, 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly BC, Buechner M, 2011. The FGD homologue EXC-5 regulates apical trafficking in C. elegans tubules. Dev Biol 359, 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee JD, 2007. The C. elegans intestine. WormBook, 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee JD, 2013. The Caenorhabditis elegans intestine. Wiley Interdiscip Rev Dev Biol 2, 347–367. [DOI] [PubMed] [Google Scholar]
- McKeown C, Praitis V, Austin J, 1998. sma-1 encodes a ßH-spectrin homolog required for Caenorhabditis elegans morphogenesis. Development 125, 2087–2098. [DOI] [PubMed] [Google Scholar]
- McShea MA, Schmidt KL, Dubuke ML, Baldiga CE, Sullender ME, Reis AL, Zhang SBO, O’Toole SM, Jeffers MC, Warden RM, Kenney AH, Gosselin J, Kuhlwein M, Hashmi SK, Stringham EG, Ryder EF, 2013. Abelson interactor-1 (ABI-1) interacts with MRL adaptor protein MIG-10 and is required in guided cell migrations and process outgrowth in C. elegans. Developmental Biology 373, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morck C, Axang C, Goksor M, Pilon M, 2006. Misexpression of acetylcholinesterases in the C. elegans pha-2 mutant accompanies ultrastructural defects in pharyngeal muscle cells. Dev Biol 297, 446–460. [DOI] [PubMed] [Google Scholar]
- Morck C, Rauthan M, Wagberg F, Pilon M, 2004. pha-2 encodes the C. elegans ortholog of the homeodomain protein HEX and is required for the formation of the pharyngeal isthmus. Dev Biol 272, 403–418. [DOI] [PubMed] [Google Scholar]
- Moskowitz IP, Rothman JH, 1996. lin-12 and glp-1 are required zygotically for early embryonic cellular interactions and are regulated by maternal GLP-1 signaling in Caenorhabditis elegans. Development 122, 4105–4117. [DOI] [PubMed] [Google Scholar]
- Mullins RD, Heuser JA, Pollard TD, 1998. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci U S A 95, 6181–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance J, Frokjaer-Jensen C, 2019. The Caenorhabditis elegans Transgenic Toolbox. Genetics 212, 959–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson F, Riddle D, 1984. Functional Study of the Caenorhabditis elegans Secretory- Excretory System Using Laser Microsurgery. The Journal of Experimental Zoology 231, 45–56. [DOI] [PubMed] [Google Scholar]
- Nelson FK, Albert PS, Riddle DL, 1983. Fine structure of the Caenorhabditis elegans secretory-excretory system. J Ultrastruct Res 82, 156–171. [DOI] [PubMed] [Google Scholar]
- Neves A, English K, Priess JR, 2007. Notch-GATA synergy promotes endoderm-specific expression of ref-1 in C. elegans. Development 134, 4459–4468. [DOI] [PubMed] [Google Scholar]
- Neves A, Priess JR, 2005. The REF-1 family of bHLH transcription factors pattern C. elegans embryos through Notch-dependent and Notch-independent pathways. Dev Cell 8, 867–879. [DOI] [PubMed] [Google Scholar]
- Okkema PG, Fire A, 1994. The Caenorhabditis elegans NK-2 class homeoprotein CEH-22 is involved in combinatorial activation of gene expression in pharyngeal muscle. Development 120, 2175–2186. [DOI] [PubMed] [Google Scholar]
- Okkema PG, Ha E, Haun C, Chen W, Fire A, 1997. The Caenorhabditis elegans NK-2 homeobox gene ceh-22 activates pharyngeal muscle gene expression in combination with pha-1 and is required for normal pharyngeal development. Development 124, 3965–3973. [DOI] [PubMed] [Google Scholar]
- Oosterveen T, Coudreuse DY, Yang PT, Fraser E, Bergsma J, Dale TC, Korswagen HC, 2007. Two functionally distinct Axin-like proteins regulate canonical Wnt signaling in C. elegans. Dev Biol 308, 438–448. [DOI] [PubMed] [Google Scholar]
- Ouellette MH, Martin E, Lacoste-Caron G, Hamiche K, Jenna S, 2016. Spatial control of active CDC-42 during collective migration of hypodermal cells in Caenorhabditis elegans. J Mol Cell Biol 8, 313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page BD, Zhang W, Steward K, Blumenthal T, Priess JR, 1997. ELT-1, a GATA-like transcription factor, is required for epidermal cell fates in Caenorhabditis elegans embryos. Genes Dev 11, 1651–1661. [DOI] [PubMed] [Google Scholar]
- Pasti G, Labouesse M, 2014. Epithelial junctions, cytoskeleton, and polarity. WormBook, 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel FB, Bernadskaya YY, Chen E, Jobanputra A, Pooladi Z, Freeman KL, Gally C, Mohler WA, Soto MC, 2008. The WAVE/SCAR complex promotes polarized cell movements and actin enrichment in epithelia during C. elegans embryogenesis. Dev Biol 324, 297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel FB, Soto MC, 2013. WAVE/SCAR promotes endocytosis and early endosome morphology in polarized C. elegans epithelia. Dev Biol 377, 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekny AJ, Johnson J-LF, Cham GD, Mains PE, 2003. The Caenorhabditis elegans nonmuscle myosin genes nmy-1 and nmy-2 function as redundant components of the let-502/Rho-binding kinase and mel-11/myosin phosphatase pathway during embryonic morphogenesis. Development 130, 5695–5704. [DOI] [PubMed] [Google Scholar]
- Piekny AJ, Mains PE, 2003. Squeezing an egg into a worm: C. elegans embryonic morphogenesis. ScientificWorldJournal 3, 1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon M, 2014. Developmental genetics of the Caenorhabditis elegans pharynx. Wiley Interdiscip Rev Dev Biol 3, 263–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenefisch J, Xiao H, Mei B, Geng J, Komuniecki PR, Komuniecki R, 2000. Secretion of a novel class of iFABPs in nematodes: coordinate use of the Ascaris/Caenorhabditis model systems. Mol Biochem Parasitol 105, 223–236. [DOI] [PubMed] [Google Scholar]
- Polanska UM, Edwards E, Fernig DG, Kinnunen TK, 2011. The cooperation of FGF receptor and Klotho is involved in excretory canal development and regulation of metabolic homeostasis in Caenorhabditis elegans. J Biol Chem 286, 5657–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, 2007. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct 36, 451–477. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG, 2003. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465. [DOI] [PubMed] [Google Scholar]
- Portereiko MF, Mango SE, 2001. Early morphogenesis of the Caenorhabditis elegans pharynx. Dev Biol 233, 482–494. [DOI] [PubMed] [Google Scholar]
- Priess JR, Hirsh DI, 1986. Caenorhabditis elegans morphogenesis: the role of the cytoskeleton in elongation of the embryo. Dev Biol 117, 156–173. [DOI] [PubMed] [Google Scholar]
- Pruyne D, 2016. Revisiting the Phylogeny of the Animal Formins: Two New Subtypes, Relationships with Multiple Wing Hairs Proteins, and a Lost Human Formin. PLoS One 11, e0164067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu P, Stone CE, Burdick JT, Murray JI, Sundaram MV, 2017. The Lipocalin LPR-1 Cooperates with LIN-3/EGF Signaling To Maintain Narrow Tube Integrity in Caenorhabditis elegans. Genetics 205, 1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin S, Michaux G, McMahon L, Gansmuller A, Labouesse M, 2001. The Caenorhabditis elegans gene lin-26 can trigger epithelial differentiation without conferring tissue specificity. Dev Biol 235, 410–421. [DOI] [PubMed] [Google Scholar]
- Quintin S, Wang S, Pontabry J, Bender A, Robin F, Hyenne V, Landmann F, Gally C, Oegema K, Labouesse M, 2016. Non-centrosomal epidermal microtubules act in parallel to LET-502/ROCK to promote C. elegans elongation. Development 143, 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JP, English K, Tenlen JR, Priess JR, 2008. Notch signaling and morphogenesis of single-cell tubes in the C. elegans digestive tract. Dev Cell 14, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JP, Feldman JL, Reddy SS, Priess JR, 2013. Cell interactions and patterned intercalations shape and link epithelial tubes in C. elegans. PLoS Genet 9, e1003772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JP, Reddy SS, Priess JR, 2012. Laminin is required to orient epithelial polarity in the C. elegans pharynx. Development 139, 2050–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, Cha YH, Ali M, Priess JR, Mello CC, 1997. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell 90, 707–716. [DOI] [PubMed] [Google Scholar]
- Rochester JD, Tanner PC, Sharp CS, Andralojc KM, Updike DL, 2017. PQN-75 is expressed in the pharyngeal gland cells of Caenorhabditiselegans and is dispensable for germline development. Biol Open 6, 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth-Johnson EA, Vizcarra CL, Bois JS, Quinlan ME, 2014. Interaction between microtubules and the Drosophila formin Cappuccino and its effect on actin assembly. J Biol Chem 289, 4395–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J, Jarriault S, 2019. Developmental Plasticity and Cellular Reprogramming in Caenorhabditis elegans. Genetics 213, 723–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee MD, Pickett MA, Feldman JL, 2020. Apical PAR complex proteins protect against epithelial assaults to create a continuous and functional intestinal lumen. bioRxiv, 2020.2010.2028.359299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee MD, Zonka JC, Skokan TD, Raftrey BC, Feldman JL, 2018. Tissue-specific degradation of essential centrosome components reveals distinct microtubule populations at microtubule organizing centers. PLoS Biol 16, e2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AD, Branon TC, Cote LE, Papagiannakis A, Liang X, Pickett MA, Shen K, Jacobs-Wagner C, Ting AY, Feldman JL, 2020. Proximity labeling at non-centrosomal microtubule-organizing centers reveals VAB-10B and WDR-62 as distinct microtubule regulators. bioRxiv, 2020.2008.2029.272369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AD, Feldman JL, 2017. Microtubule-organizing centers: from the centrosome to non-centrosomal sites. Curr Opin Cell Biol 44, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir A, Choi J, Leikina E, Avinoam O, Valansi C, Chernomordik LV, Newman AP, Podbilewicz B, 2007. AFF-1, a FOS-1-regulated fusogen, mediates fusion of the anchor cell in C. elegans. Dev Cell 12, 683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasidharan S, Borinskaya S, Patel F, Bernadskaya Y, Mandalapu S, Agapito M, Soto MC, 2018. WAVE regulates Cadherin junction assembly and turnover during epithelial polarization. Dev Biol 434, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa M, Suetsugu S, Sugimoto A, Miki H, Yamamoto M, Takenawa T, 2003. Essential role of the C. elegans Arp2/3 complex in cell migration during ventral enclosure. J Cell Sci 116, 1505–1518. [DOI] [PubMed] [Google Scholar]
- Schindler AJ, Sherwood DR, 2013. Morphogenesis of the Caenorhabditis elegans vulva. Wires Dev Biol 2, 75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C, Schwarz V, Hutter H, 2006. AST-1, a novel ETS-box transcription factor, controls axon guidance and pharynx development in C. elegans. Dev Biol 293, 403–413. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Marcus-Gueret N, Adeleye A, Webber J, Baillie D, Stringham EG, 2009. The cell migration molecule UNC-53/NAV2 is linked to the ARP2/3 complex by ABI-1. Development 136, 563–574. [DOI] [PubMed] [Google Scholar]
- Segbert C, Johnson K, Theres C, van Fürden D, Bossinger O, 2004. Molecular and functional analysis of apical junction formation in the gut epithelium of Caenorhabditis elegans. Dev Biol 266, 17–26. [DOI] [PubMed] [Google Scholar]
- Shafaq-Zadah M, Brocard L, Solari F, Michaux G, 2012. AP-1 is required for the maintenance of apico-basal polarity in the C. elegans intestine. Development 139, 2061–2070. [DOI] [PubMed] [Google Scholar]
- Shaye DD, Greenwald I, 2015. The disease-associated formin INF2/EXC-6 organizes lumen and cell outgrowth during tubulogenesis by regulating F-actin and microtubule cytoskeletons. Dev Cell 32, 743–755. [DOI] [PubMed] [Google Scholar]
- Shaye DD, Greenwald I, 2016. A network of conserved formins, regulated by the guanine exchange factor EXC-5 and the GTPase CDC-42, modulates tubulogenesis in vivo. Development 143, 4173–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton CA, Carter JC, Ellis GC, Bowerman B, 1999. The nonmuscle myosin regulatory light chain gene mlc-4 is required for cytokinesis, anterior-posterior polarity, and body morphology during Caenorhabditis elegans embryogenesis. J Cell Biol 146, 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood DR, Plastino J, 2018. Invading, Leading and Navigating Cells in Caenorhabditis elegans: Insights into Cell Movement in Vivo. Genetics 208, 53–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto MC, Qadota H, Kasuya K, Inoue M, Tsuboi D, Mello CC, Kaibuchi K, 2002. The GEX-2 and GEX-3 proteins are required for tissue morphogenesis and cell migrations in C. elegans. Genes Dev 16, 620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulavie F, Hall DH, Sundaram MV, 2018. The AFF-1 exoplasmic fusogen is required for endocytic scission and seamless tube elongation. Nature Communications 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparacio AP, Trojanowski NF, Snetselaar K, Nelson MD, Raizen DM, 2020. Teething during sleep: Ultrastructural analysis of pharyngeal muscle and cuticular grinder during the molt in Caenorhabditis elegans. PLoS One 15, e0233059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr DA, Hermann GJ, Malone CJ, Fixsen W, Priess JR, Horvitz HR, Han M, 2001. unc-83 encodes a novel component of the nuclear envelope and is essential for proper nuclear migration. Development 128, 5039–5050. [DOI] [PubMed] [Google Scholar]
- Stone CE, Hall DH, Sundaram MV, 2009. Lipocalin signaling controls unicellular tube development in the Caenorhabditis elegans excretory system. Dev Biol 329, 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone MC, Roegiers F, Rolls MM, 2008. Microtubules have opposite orientation in axons and dendrites of Drosophila neurons. Mol Biol Cell 19, 4122–4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringham E, Pujol N, Vandekerckhove J, Bogaert T, 2002. unc-53 controls longitudinal migration in C. elegans. Development 129, 3367–3379. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN, 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 100, 64–119. [DOI] [PubMed] [Google Scholar]
- Suman SK, Daday C, Ferraro T, Vuong-Brender T, Tak S, Quintin S, Robin F, Grater F, Labouesse M, 2019. The plakin domain of C. elegans VAB-10/plectin acts as a hub in a mechanotransduction pathway to promote morphogenesis. Development 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram MV, Buechner M, 2016. The Caenorhabditis elegans Excretory System: A Model for Tubulogenesis, Cell Fate Specification, and Plasticity. Genetics 203, 35–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram MV, Cohen JD, 2016. Time to make the doughnuts: Building and shaping seamless tubes. Semin Cell Dev Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Buechner M, Nishiwaki K, Hall DH, Nakanishi H, Takai Y, Hisamoto N, Matsumoto K, 2001. A putative GDP-GTP exchange factor is required for development of the excretory cell in Caenorhabditis elegans. EMBO Reports 2, 530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Buechner M, 2008. CRIP homologues maintain apical cytoskeleton to regulate tubule size in C. elegans. Dev Biol 317, 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totong R, Achilleos A, Nance J, 2007. PAR-6 is required for junction formation but not apicobasal polarization in C. elegans embryonic epithelial cells. Development 134, 1259–1268. [DOI] [PubMed] [Google Scholar]
- Trzebiatowska A, Topf U, Sauder U, Drabikowski K, Chiquet-Ehrismann R, 2008. Caenorhabditis elegans teneurin, ten-1, is required for gonadal and pharyngeal basement membrane integrity and acts redundantly with integrin ina-1 and dystroglycan dgn-1. Mol Biol Cell 19, 3898–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Fürden D, Johnson K, Segbert C, Bossinger O, 2004. The C. elegans ezrin-radixin-moesin protein ERM-1 is necessary for apical junction remodelling and tubulogenesis in the intestine. Dev Biol 272, 262–276. [DOI] [PubMed] [Google Scholar]
- Vanderzalm PJ, Pandey A, Hurwitz ME, Bloom L, Horvitz HR, Garriga G, 2009. C. elegans CARMIL negatively regulates UNC-73/Trio function during neuronal development. Development 136, 1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste CA, Pruyne D, Mains PE, 2013. The role of the formin gene fhod-1 in C. elegans embryonic morphogenesis. Worm 2, e25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Stetina SE, Mango SE, 2015. PAR-6, but not E-cadherin and beta-integrin, is necessary for epithelial polarization in C. elegans. Dev Biol 403, 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong-Brender TT, Ben Amar M, Pontabry J, Labouesse M, 2017a. The interplay of stiffness and force anisotropies drives embryo elongation. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong-Brender TT, Yang X, Labouesse M, 2016a. C. elegans Embryonic Morphogenesis. Curr Top Dev Biol 116, 597–616. [DOI] [PubMed] [Google Scholar]
- Vuong-Brender TT, Yang X, Labouesse M, 2016b. C. elegans Embryonic Morphogenesis. Curr Top Dev Biol 116, 597–616. [DOI] [PubMed] [Google Scholar]
- Vuong-Brender TTK, Suman SK, Labouesse M, 2017b. The apical ECM preserves embryonic integrity and distributes mechanical stress during morphogenesis. Development 144, 4336–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walck-Shannon E, Lucas B, Chin-Sang I, Reiner D, Kumfer K, Cochran H, Bothfeld W, Hardin J, 2016. CDC-42 Orients Cell Migration during Epithelial Intercalation in the Caenorhabditis elegans Epidermis. PLoS Genet 12, e1006415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walck-Shannon E, Reiner D, Hardin J, 2015. Polarized Rac-dependent protrusions drive epithelial intercalation in the embryonic epidermis of C. elegans. Development 142, 3549–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace AG, Raduwan H, Carlet J, Soto MC, 2018. The RhoGAP HUM-7/Myo9 integrates signals to modulate RHO-1/RhoA during embryonic morphogenesis in Caenorhabditis elegans. Development 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton T, Preston E, Nair G, Zacharias AL, Raj A, Murray JI, 2015. The Bicoid class homeodomain factors ceh-36/OTX and unc-30/PITX cooperate in C. elegans embryonic progenitor cells to regulate robust development. PLoS Genet 11, e1005003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Tang NH, Lara-Gonzalez P, Zhao Z, Cheerambathur DK, Prevo B, Chisholm AD, Desai A, Oegema K, 2017. A toolkit for GFP-mediated tissue-specific protein degradation in C. elegans. Development 144, 2694–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernike D, Chen Y, Mastronardi K, Makil N, Piekny A, 2016. Mechanical forces drive neuroblast morphogenesis and are required for epidermal closure. Dev Biol 412, 261–277. [DOI] [PubMed] [Google Scholar]
- White JG, 1988. The Anatomy, in: Wood WB (Ed.), The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory, pp. Chapter 4. pp 81–122. [Google Scholar]
- Williams-Masson EM, Heid PJ, Lavin CA, Hardin J, 1998. The cellular mechanism of epithelial rearrangement during morphogenesis of the Caenorhabditis elegans dorsal hypodermis. Dev Biol 204, 263–276. [DOI] [PubMed] [Google Scholar]
- Wissmann A, Ingles J, McGhee JD, Mains PE, 1997. Caenorhabditis elegans LET-502 is related to Rho-binding kinases and human myotonic dystrophy kinase and interacts genetically with a homolog of the regulatory subunit of smooth muscle myosin phosphatase to affect cell shape. Genes Dev 11, 409–422. [DOI] [PubMed] [Google Scholar]
- Withee J, Galligan B, Hawkins N, Garriga G, 2004. Caenorhabditis elegans WASP and Ena/VASP proteins play compensatory roles in morphogenesis and neuronal cell migration. Genetics 167, 1165–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KA, Thomson JN, 1981. The buccal capsule of Caenorhabditis elegans (Nematoda: Rhabditoidea): an ultrastructural study. Canadian Journal of Zoology 59, 1952–1961. [Google Scholar]
- Yang Z, Mattingly BC, Hall DH, Ackley BD, Buechner M, 2020. Terminal web and vesicle trafficking proteins mediate nematode single-cell tubulogenesis. J Cell Biol 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochem J, Sundaram M, Han M, 1997. Ras is required for a limited number of cell fates and not for general proliferation in Caenorhabditis elegans. Mol Cell Biol 17, 2716–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KG, Thurston SF, Copeland S, Smallwood C, Copeland JW, 2008. INF1 is a novel microtubule-associated formin. Mol Biol Cell 19, 5168–5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JA, Castranova D, Pham VN, Weinstein BM, 2015. Single-cell analysis of endothelial morphogenesis in vivo. Development 142, 2951–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Abraham N, Khan LA, Hall DH, Fleming JT, Göbel V, 2011a. Apicobasal domain identities of expanding tubular membranes depend on glycosphingolipid biosynthesis. Nat Cell Biol 13, 1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Labouesse M, 2010. The making of hemidesmosome structures in vivo. Dev Dyn 239, 1465–1476. [DOI] [PubMed] [Google Scholar]
- Zhang H, Landmann F, Zahreddine H, Rodriguez D, Koch M, Labouesse M, 2011b. A tension-induced mechanotransduction pathway promotes epithelial morphogenesis. Nature 471, 99–103. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ward JD, Cheng Z, Dernburg AF, 2015. The auxin-inducible degradation (AID) system enables versatile conditional protein depletion in C. elegans. Development 142, 4374–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Fischer DJ, Santos MF, Tigyi G, Pasteris NG, Gorski JL, Xu Y, 1996. The faciogenital dysplasia gene product FGD1 functions as a Cdc42Hs-specific guanine-nucleotide exchange factor. J Biol Chem 271, 33169–33172. [DOI] [PubMed] [Google Scholar]
- Zilberman Y, Abrams J, Anderson DC, Nance J, 2017. Cdc42 regulates junctional actin but not cell polarization in the Caenorhabditis elegans epidermis. J Cell Biol 216, 3729–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]


