Abstract
Background
The potential effects of aerobic and resistance training in patients with severe chronic kidney disease (CKD) are not fully elucidated. This study investigated the effects of a home‐based exercise programme on physical functioning and health‐related quality of life (HRQOL) in patients with Stage 4 CKD, equivalent to estimated glomerular filtration rate of 15–30 mL/min/1.73 m2.
Methods
Forty‐six patients with Stage 4 CKD (median age, 73 years; 33 men) were randomly assigned to exercise (n = 23) and control (n = 23) groups. Exercise group patients performed aerobic exercise at 40–60% peak heart rate thrice weekly and resistance training at 70% of one‐repetition maximum twice weekly at home for 6 months. Control patients received no specific intervention. Primary outcomes were distance in incremental shuttle walking test and HRQOL assessed using the Kidney Disease Quality of Life—Short Form questionnaire. Secondary outcomes included kidney function assessed with combined urea and creatinine clearance, urinary biomarkers, and anthropometric and biochemical parameters associated with CKD.
Results
Improvement in incremental shuttle walking test was significantly greater in the exercise group compared with controls (39.4 ± 54.6 vs. −21.3 ± 46.1; P < 0.001). Among Kidney Disease Quality of Life domains, significant mean differences were observed between the exercise group and the control group in work status, quality of social interaction, and kidney disease component summary outcomes (12.76 ± 5.76, P = 0.03; 5.97 ± 2.59, P = 0.03; and 4.81 ± 1.71, P = 0.007, respectively). There were greater reductions in natural log (ln)‐transformed urinary excretion of liver‐type fatty acid‐binding protein, ln serum C‐reactive protein, and acylcarnitine to free carnitine ratio in the exercise group compared with controls, with significant between‐group differences of −0.579 ± 0.217 (P = 0.008), −1.13 ± 0.35 (P = 0.003), and −0. 058 ± 0.024 (P = 0.01), respectively.
Conclusions
Our 6 month home‐based exercise programme improved aerobic capacity and HRQOL in patients with Stage 4 CKD, with possible beneficial effects on kidney function and CKD‐related parameters.
Keywords: Home‐based exercise, Chronic kidney disease, Randomized controlled trial, Incremental shuttle walking test, Quality of life
Introduction
Chronic kidney disease (CKD) is a worldwide health problem, and the number of patients rises with the increase in the ageing population and patients with hypertension or diabetes. It is complicated with various disease conditions. Muscle wasting is common and progressive in patients with CKD, because of multifactorial aetiologies associated with CKD including hormonal changes like insulin/insulin‐like growth factor resistance, systemic inflammation, metabolic acidosis, protein energy wasting, physical inactivity, overexpression of myostatin, and reduction in satellite cell function. 1 , 2 In addition, skeletal muscle mitochondrial dysfunction may be important in the pathogenesis of decreased endurance in uraemia. 3 These progressive and cumulative effects of CKD on skeletal muscles leading to reduced skeletal muscle mass and exercise capacity imply uraemic sarcopenia. Decreased exercise capacity has been shown to affect the quality of life and to be independently associated with high mortality in this population. 4
Aerobic and resistance training in patients with CKD has been increasingly demonstrated to be beneficial. Among patients with pre‐dialysis CKD, exercise may improve peak oxygen uptake (Vo2peak) measured using an incremental exercise protocol performed on treadmill or cycle ergometers, aerobic fitness assessed by the distance covered using a maximal graded test such as incremental shuttle walking test (ISWT) or using submaximal tests such as the 6 min walk test (6MWT), and muscle strength with muscle mass. 5 , 6 , 7 , 8 , 9 , 10 , 11 An exercise programme was also suggested to improve quality of life, arterial stiffness assessed with pulse wave velocity, indicators of nutritional status including serum total cholesterol, albumin, prealbumin, transferrin, inflammation, and estimated glomerular filtration rate (eGFR) assessed by creatinine or cystatin C. 5 , 6 , 7 , 8 , 9 , 10 , 11 These findings encourage nephrologists to recommend exercise for sedentary CKD patients. However, these trials were performed in patients with moderate (Stage 3) CKD or combined moderate and severe (Stage 3–4) CKD. Therefore, the benefit of exercise exclusively in patients with severe CKD has not been described.
We designed a randomized controlled trial (RCT) including only patients with Stage 4 CKD, equivalent to eGFR of 15–30 mL/min/1.73 m2, to clarify the effects of home‐based exercise programme in this group.
Methods
Study population
Outpatients with CKD at the nephrology department of Keio University Hospital in Tokyo, Japan, were evaluated for inclusion in this RCT. Recruitment commenced in November 2018, and the trial ended in June 2020. Patients with Stage 4 CKD (eGFR = 15–30 mL/min/1.73 m2), who were between 20 and 90 years old, and with the ability to understand and provide informed consent were assessed for eligibility to participate in this trial. Exclusion criteria included the following: uncontrolled hypertension (blood pressure >180/110 mmHg), severe anaemia (haemoglobin level <7 mg/dL), active and proliferative diabetic retinopathy, symptomatic coronary artery disease or cerebrovascular disease within 3 months before trial recruitment, current heart failure (New York Heart Association Classes III and IV), symptomatic or fatal arrhythmia, significant valvular heart disease, difficulty walking without aid due to orthopaedic problems, and a history of cerebrovascular or peripheral artery disease. Unstable patients with CKD, including those whose kidney function was acutely diminished and considered reversible, who would need renal replacement therapy within 6 months, and who underwent vascular access procedure or peritoneal dialysis catheter insertion, were also excluded.
Study design and randomization
This RCT evaluated the effects of a home‐based exercise programme involving aerobic exercise (AE) and resistance exercise (RE) in Stage 4 CKD outpatients.
After baseline assessment, block randomization with a block size of two was performed by an individual not associated with the trial, using computer‐generated random numbers. Additionally, randomization was stratified by age (65 years or older vs. less) and sex (male vs. female). The participants were divided into control and exercise groups. Blinding of participants and rehabilitation doctors to group assignment was impossible; however, nephrologists responsible for the CKD clinic and outcome assessors were blinded. The patients assigned to the exercise group were instructed by the same rehabilitation doctor to perform individualized AE and RE at home. The patients allocated to the control group received usual care of CKD and received advice on maintaining their lifestyles.
Outcome measures
Outcome measures were assessed at baseline and 24 weeks, regardless of adherence to the programme. The primary outcomes were changes in ISWT and health‐related quality of life (HRQOL), and secondary outcomes include changes in renal outcomes: biochemical and anthropometric data related to malnutrition, inflammation, and atherosclerosis syndrome, muscle mass and strength, and bone mineral density (BMD) and carnitine profile. Additionally, before intervention, correlation between ISWT and other outcomes, including HRQOL and secondary outcomes, was analysed using baseline parameters of all available patients.
Physical functioning
Aerobic capacity was measured with ISWT, in which heart rate (HR) was continuously monitored. Patients were required to walk between two cones 10 m apart, with the pace set by a beeper. The speed of the beeper increased gradually until the patient could not keep up with the pace or until they stopped because of fatigue. Total distance (m) was measured and used for analysis, while peak HR and rate of perceived exertion (RPE) at ISWT were used to prescribe AE. Moreover, handgrip and quadriceps strength were evaluated as parameters of muscle strength as previously described. 11 Briefly, handgrip strength was assessed using a dynamometer in standing position with both arms hanging down. Quadriceps strength was assessed using an isokinetic dynamometer (μTasF‐1, Anima, Tokyo, Japan), with the subject seated in a chair, hip joints at 90°, knees flexed at 90°, and ankles fixed at the chair leg by the dynameter, and the maximum isometric contraction of the knee extensors measured.
Health‐related quality of life
Health‐related quality of life was assessed using the Kidney Disease Quality of Life—Short Form (KDQOL‐SF) Japanese Version 1.3, including subscales on quality of life specific to kidney disease and dialysis (KDQOL) and general HRQOL [Medical Outcomes Study 36‐Item Short‐Form Health Survey (SF‐36)]. Kidney disease component summary (KDCS), physical component summary, mental component summary, and role/social component summary (RCS) were calculated from KDQOL‐SF, as previously described. 11
Anthropometric data
Body mass index (BMI; kg/m2), waist circumference (cm) at umbilicus, and average circumference of both legs (cm) at mid‐thigh were calculated. We simultaneously performed all body composition measurements in the succeeding text using multi‐frequency bioelectrical impedance analysis (MF‐BIA) instrument (InBody S‐10, InBody Japan Inc., Tokyo, Japan), according to manufacturer instructions. 12 , 13 Muscle mass was assessed and analysed as skeletal mass index (SMI): appendicular skeletal mass divided by height squared (kg/m2). MF‐BIA is a clinically validated method to assess SMI and to diagnose sarcopenia, as well as whole‐body dual‐energy X‐ray absorptiometry (DXA). 14 Additionally, considering the possible influence of lower extremity oedema on skeletal muscle mass in patients with CKD, upper extremity SMI and lower extremity SMI (LESMI) were calculated separately and used for analysis. Intracellular water (ICW) and extracellular water (ECW) were automatically calculated by MF‐BIA software, and ECW/ICW ratio was used for analysis as an indicator of CKD‐associated volume overload or decreased cell volume associated with ageing and muscle attenuation. 13 InBody S‐10 did not require any statistical correction to reflect the characteristics of the target population and could measure body composition with the same accuracy regardless of the subjects' characteristics (https://www.inbody.com/global/intro/Technology.aspx). 15 , 16 Basic research of the InBody's developer demonstrated the formula for the MF‐BIA principle underlying InBody, which could derive body composition accurately without having to perform any statistical correction. 17 The accuracy of ICW and ECW in patients with CKD using MF‐BIA was verified through this research using the heavy water dilution method, whereas the analysis method was not exactly the same as the current InBody. 17 On the other hand, MF‐BIA by InBody has been validated as a robust tool for measuring and monitoring body composition in haemodialysis patients, compared with DXA. 18 Additionally, the previous study using InBody indicated that ICW decreased with malnutrition in patients with CKD as well as that of healthy individuals, rather than increased because of fluid overload secondary to CKD. 12 Arterial stiffness was assessed with brachial‐ankle pulse wave velocity (baPWV) using a cardiovascular screening device (BP‐203RPEIII, Omron Healthcare, Kyoto, Japan) as previously described. 11 BMD (g/cm2) was assessed with whole‐body DXA (QDR 4500/A, Hologic, Waltham, MA, USA).
Biochemical data
Blood and 24 h urine samples were collected from participants for biochemical analyses. Combined urea and creatinine clearance, one of the most accurate ways to measure GFR independent of factors such as age, gender, or muscle mass, was corrected for body surface area and used to assess renal function (mL/min/1.73 m2). 19 Moreover, 24 h urine urinary albumin (mg/day) and liver‐type fatty acid‐binding protein (L‐FABP) (μg/day) were obtained as biomarkers of CKD progression. 20 , 21 Serum albumin (mg/dL) and inflammation markers including C‐reactive protein (CRP) and interleukin‐6 (IL‐6) were obtained from serum samples. Additionally, haemoglobin A1c, glycated albumin, and homeostasis model assessment of insulin resistance calculated from the fasting blood glucose and fasting insulin levels were measured as metabolic parameters. Serum carnitine profiles (total, free, and acylcarnitine) were determined using an enzyme cycling method, and decreased serum free carnitine level (μmol/L) and increased acylcarnitine to free carnitine ratio (AC/FC) were used to assess carnitine deficiency. 22
The nephrologists could adjust antihypertensive drugs, diuretics, erythropoiesis‐stimulating agents, phosphate‐lowering agents, anti‐diabetic drugs, and anti‐lipemic agents during the study period in both groups. However, carnitine supplementation was not used at baseline and was not started during the study period.
Exercise intervention
Patients in the exercise group were instructed to perform unsupervised home‐based individualized AE thrice weekly and RT twice weekly for 6 months, as previously described. 11 AE training target was 40–60% peak HR, as determined at baseline ISWT, with a rating of 11–13 on Borg RPE scale. Although both peak HR and RPE were used to prescribe AE in individuals, considering that the use of beta‐blockers affects their HR and the peak HR may be attenuated in CKD, 23 we gave priority to RPE rather than peak HR in setting the AE prescription (the target walking speed). The patients with appropriate HR response during ISWT were also set their target HR. RE target was 70% of one‐repetition maximum (1‐RM), which was targeted as the load that an individual could perform 10–15 times, because the measurement of 1‐RM strength was not recommended in patients with CKD. 23 We asked patients to perform one session per day for each muscle group including latissimus, erector spinae, deltoid, biceps, triceps, quadriceps, hamstrings, gluteus medius, adductor magnus, gastrocnemius, and tibialis anterior muscles using TheraBand (Hygenic Corp., Akron, OH, USA), with each session involving 10 repetitions. Additionally, we instructed that patients not decrease their daily activity while adding home‐based exercise. The exercise capacity of participants, including distance in ISWT and muscle strength, was reassessed bimonthly at CKD clinic visits, and the exercise prescription was adjusted accordingly.
Only patients in the exercise group were sent two postcards every other week to monitor their adherence to both AE and RE, and we collected one postcard each week (Supporting Information, Table S1). Specifically, whether the patients performed AE and/or RE or not was entered into the postcards every day. The adherence (%) was calculated as the number of weeks in which patients performed the target number of sessions (thrice weekly for AE and twice weekly for RE) divided by the total number of weeks during the intervention period. The weeks in which patients did not return the postcards were considered as weeks in which the patients did not do the exercise programme. Meanwhile, the exercise intensity, namely, HR and RPE in each session of AE and RE, was not monitored and was not required for the patients to enter. This was because we considered that it was rather difficult for patients to monitor their HR with %peak HR, to report their RPE using Borg scale, and to assess %1‐RM during every home‐based exercise session. Additionally, the number of steps per day, which was measured by the individual's smartphone, smartwatch, or pedometer, was entered into the postcards, although this number was not used to determine adherence to the exercise programme but used as an indicator that physical activity was not reduced as the exercise programme was initiated. These postcards were collected by post. If the patient did not have any device to assess steps, we gave the patient a pedometer (FB‐731‐BK, TANITA, Tokyo, Japan) and asked them to measure their steps. Although the difference in devices used to monitor the number of steps per day might lead to variability among the study participants, actually only a few patients requested to use their own smartphones/smartwatches, and the remaining patients were all given the same pedometer. We have calculated the average number of steps per day during the study period for each patient. Patients in the control group were not monitored regarding their physical performance, considering that monitoring itself might lead to increased physical activity: the Hawthorne effect. The same rehabilitation doctor oversaw these tasks.
Statistical analysis
The Kolmogorov–Smirnov test was used to test the normality of continuous data. Normally and non‐normally distributed data were presented as mean ± standard deviation and median (inter‐quartile range), respectively. Skewed data were log transformed before analyses. Categorical data were expressed as n (%).
The Pearson correlation was used to assess association between ISWT and HRQOL at baseline. Linear regression was performed with ISWT as dependent variable and each secondary outcome, grouped by age and sex, as independent variables. Unpaired Student's t‐test or Mann–Whitney U test for continuous variables and χ 2 test for categorical variables were used to compare baseline characteristics between groups and ISWT change (ΔISWT) during the study period between groups. Analysis of covariance (ANCOVA), with baseline values as covariates and 24 week values as dependent variables, was used to test for significant differences between HRQOL and secondary outcomes of the patient groups. Comparison of ISWT change between binary variables, as a priori‐specified subgroups [age (≥65 vs. <65 years), sex (male vs. female), diabetes (yes vs. no), history of cerebrovascular/cardiovascular disease (yes vs. no), and smoking (current or ex‐smoker vs. non‐smoker)], was also performed using ANCOVA. Additionally, as sub‐analyses among participants in the exercise group, comparison of exercise capacity between the patients with high adherence (HA) and low adherence (LA) to AE, RE, and AE + RE, or with high and low number of steps, divided by the median, was performed using ANCOVA.
Because few exercise intervention studies assessing ISWT among CKD patients are available, we based sample size calculation on previous meta‐analysis containing RCTs of non‐dialysis‐dependent CKD patients evaluating aerobic capacity by VO2peak. 24 Based on the forest plot that demonstrates the effects of exercise intervention on Vo2peak (ΔVo2peak) over time, we calculated that 23 patients per study group were required to detect 4.54 (mL/kg/min) improvement in Vo2peak with standard deviation of 4.3 and 4.9 (mL/kg/min) in the exercise and control groups, respectively (unpaired Student's t‐test; β = 0.20; α = 0.05). 6 , 25 , 26 With an estimated 30% attrition rate, an overall sample of 60 is required.
We used full analysis set for both primary and secondary outcomes, with those not assessed for baseline characteristics excluded. Missing data values were presumed to be data missing at random, and calculations were completed using multiple imputation by chained equations (100 imputations). SPSS software for Mac (Ver. 25; IBM Corp., Armonk, NY, USA) was used to perform all analyses. P‐value <0.05 was considered statistically significant.
Results
Patient flow and exercise programme adherence
Among 242 outpatients with Stage 4 CKD, who were between 20 and 90 years old, and with the ability to understand the study protocol, assessed for study eligibility, 176 fulfilled the criteria to participate, and 51 consented to participate (Figure 1). Of these, five patients withdrew before randomization, leaving 46 patients randomly assigned to the control group (n = 23) and the exercise group (n = 23).
Figure 1.
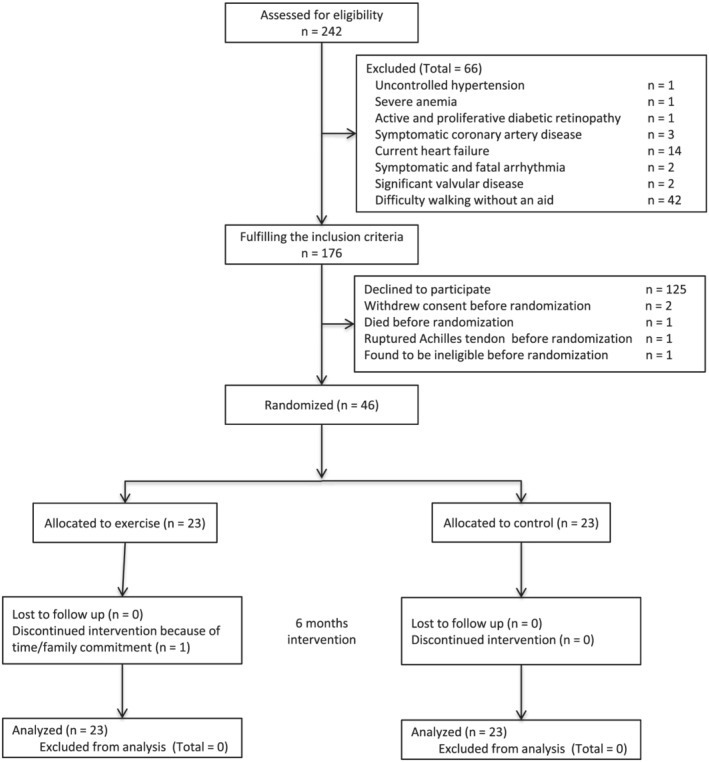
CONSORT diagram of the flow of patients through the various phases of the trial.
These 46 patients were predominantly male (72%) with median age of 73 (69–78) years, not significantly different from non‐consenting patients (n = 130), with male predominance (63%) and median age of 70 (63–78) years (P = 0.2 and 0.4, respectively). Among the exercise group, one patient discontinued the intervention because of time/family commitment, and another refused some assessments for fear of COVID‐19. In the first patient, all data, except MF‐BIA measurements, baPWV, and BMD, were obtained 24 weeks after baseline assessment. In the second patient, all parameters, except physical functioning, MF‐BIA measurements, and baPWV, were obtained at the final assessment. No patients were lost to follow‐up. Intervention‐related serious adverse events were not noted.
Among the 22 patients in the exercise group who underwent the assessment of their exercise capacity, collection rate of postcards was high [100% (96–100)], and median adherence to prescribed AE, RE, and AE + RE sessions were 92% (40–99), 96% (85–100), and 87% (34–96), respectively, according to data obtained from received postcards (Table 1). Median number of steps per day was 4629 (3481–6445). Postcard collection rate and adherence to the exercise sessions were not significantly different between genders, while adherence to AE + RE tended to be higher in men than women. Additionally, daily number of steps was significantly higher in patients <65 years than those ≥65 years and among men than women.
Table 1.
Postcard collection rate; adherence to aerobic exercise, resistance exercise, and both; and the number of steps per day in patients in the exercise group
| Groups | Overall | Age | Sex | ||||
|---|---|---|---|---|---|---|---|
| <65 years (n = 5) | ≥65 years (n = 17) | P‐value | Male (n = 16) | Female (n = 6) | P‐value | ||
| Collection rate (%) | 100 (96–100) | 100 (96–100) | 100 (96–100) | 0.96 | 100 (96–100) | 94 (87–100) | 0.23 |
| Adherence (%) | |||||||
| AE | 92 (40–99) | 92 (73–96) | 92 (29–100) | 0.75 | 96 (79–100) | 55 (19–90) | 0.16 |
| RE | 96 (85–100) | 88 (84–96) | 96 (85–100) | 0.35 | 94 (87–100) | 90 (29–99) | 0.36 |
| AE + RE | 87 (34–96) | 84 (73–88) | 92 (29–96) | 0.66 | 87 (67–97) | 52 (4–88) | 0.09 |
| The number of steps per day | 4629 (3481–6445) | 8936 (5732–12521) | 4144 (3414–5092) | 0.04 | 5059 (4001–6851) | 3268 (2493–3763) | 0.02 |
AE, aerobic exercise; RE, resistance exercise.
Baseline characteristics and association of parameters with aerobic capacity
The clinical characteristics of study patients at baseline are summarized in Table 2. The control and exercise groups showed no significant difference in demographic, clinical, and biochemical characteristics.
Table 2.
Demographic, clinical, and biochemical data of the study groups
| Variables | All (n = 46) | Control (n = 23) | Exercise (n = 23) | P‐value |
|---|---|---|---|---|
| Age (years) | 73 (69–78) | 76 (69–78) | 72 (69–79) | 0.7 |
| 65 years or older (%) | 36 (78%) | 18 (78%) | 18 (78%) | 1 |
| Male/female (%) | 33/13 (72/28%) | 16/7 (70/30%) | 17/6 (74/26%) | 1 |
| Diabetes (%) | 14 (30%) | 7 (30%) | 7 (30%) | 1 |
| CCVD (%) | 12 (26%) | 5 (22%) | 7 (30%) | 0.7 |
| Smoking (%) | 21 (46%) | 10 (44%) | 11 (48%) | 1 |
| Kidney function | ||||
| eGFR (mL/min/1.73 m2) | 23.2 ± 4.7 | 23.8 ± 4.5 | 22.4 ± 5.1 | 0.3 |
| Renal CrCl (mL/min/1.73 m2) | 32.6 ± 10.0 | 32.2 ± 8.1 | 34.8 ± 10.5 | 0.4 |
| Renal urea Cl (mL/min/1.73 m2) | 14.8 ± 4.7 | 14.7 ± 4.1 | 16.5 ± 5.3 | 0.2 |
| Average Cr and urea Cl (mL/min/1.73 m2) | 23.7 ± 7.1 | 23.5 ± 5.8 | 25.7 ± 7.5 | 0.3 |
| Urine protein (g/day) | 0.6 (0.2–2.3) | 0.6 (0.3–1.4) | 0.5 (0.2–3.4) | 0.8 |
| Urine albumin (mg/day) | 404.8 (77.9–1513.6) | 404.8 (82.6–948.5) | 390.0 (84.7–2151.0) | 0.6 |
| Urine L‐FABP (μg/day) | 16.9 (5.7–47.5) | 16.8 (7.9–31.2) | 17.1 (4.4–67.2) | 0.4 |
| Urinary Na excretion (mEq/day) | 143.8 ± 46.2 | 130.8 ± 44.4 | 156.8 ± 45.2 | 0.06 |
| BMI (kg/m2) | 23.9 ± 4.5 | 23.0 ± 4.3 | 24.7 ± 4.6 | 0.2 |
| GNRI | 100.7 ± 9.8 | 99.3 ± 9.3 | 102.2 ± 10.3 | 0.3 |
| nPCR (g/kg/day) | 0.93 ± 0.20 | 0.88 ± 0.17 | 0.99 ± 0.21 | 0.06 |
| Systolic blood pressure (mmHg) | 140.5 ± 15.7 | 140.8 ± 15.5 | 140.2 ± 16.3 | 0.9 |
| Diastolic blood pressure (mmHg) | 77.1 ± 13.3 | 75.7 ± 12.2 | 78.5 ± 14.4 | 0.5 |
| Mean blood pressure (mmHg) | 98.2 ± 12.8 | 97.4 ± 12.1 | 99.0 ± 13.8 | 0.7 |
| Haemoglobin (g/dL) | 12.0 ± 1.6 | 11.8 ± 1.2 | 12.3 ± 1.9 | 0.3 |
| Haemoglobin A1c (%) | 6.1 ± 0.7 | 6.1 ± 0.8 | 6.0 ± 0.6 | 0.8 |
| Glycated albumin (mmol/L) | 15.4 ± 2.7 | 16.0 ± 2.9 | 14.8 ± 2.4 | 0.1 |
| Fasting blood sugar (mg/dL) | 116.2 ± 21.6 | 115.1 ± 17.4 | 17.3 ± 25.5 | 0.7 |
| HOMA‐IR | 3.0 (1.5–4.8) | 2.9 (1.6–5.4) | 3.1 (1.6–4.6) | 0.8 |
| Albumin (g/dL) | 3.7 ± 0.4 | 3.7 ± 0.3 | 3.7 ± 0.6 | 0.9 |
| Calcium (mg/dL) | 9.2 ± 0.3 | 9.3 ± 0.3 | 9.2 ± 0.4 | 0.7 |
| Phosphorus (mg/dL) | 3.6 ± 0.5 | 3.6 ± 0.6 | 3.7 ± 0.5 | 0.6 |
| PTH (pmol/L) | 106.8 ± 65.2 | 98.6 ± 57.3 | 115.1 ± 72.6 | 0.4 |
| CRP (mg/dL) | 0.05 (0.03–0.16) | 0.12 (0.03–0.35) | 0.05 (0.03–0.11) | 0.1 |
| IL‐6 (pg/mL) | 2.5 (1.8–4.5) | 2.6 (2.1–4.6) | 2.3 (1.5–4.2) | 0.2 |
| Total cholesterol (mg/dL) | 197.2 ± 37.1 | 194.6 ± 38.8 | 199.7 ± 36.0 | 0.6 |
| LDL cholesterol (mg/dL) | 108.0 ± 28.9 | 109.3 ± 30.6 | 106.7 ± 27.6 | 0.8 |
| HDL cholesterol (mg/dL) | 50.9 ± 14.4 | 49.8 ± 16.5 | 51.9 ± 12.2 | 0.6 |
| Triglyceride (mg/dL) | 141.5 ± 52.8 | 136.4 ± 51.2 | 146.7 ± 55.1 | 0.5 |
| BNP (pg/mL) | 47.7 (26.3–82.2) | 47.5 (29.6–64.2) | 47.9 (23.5–84.5) | 0.6 |
| hANP (pg/mL) | 47.7 (27.0–68.9) | 50.1 (27.1–64.7) | 47.0 (26.5–68.5) | 0.9 |
| Free carnitine (μmol/L) | 50.0 ± 10.8 | 48.1 ± 10.9 | 51.9 ± 10.6 | 0.2 |
| Acylcarnitine (μmol/L) | 17.2 ± 5.1 | 16.1 ± 4.6 | 18.4 ± 5.3 | 0.1 |
| AC/FC | 0.35 ± 0.12 | 0.34 ± 0.11 | 0.36 ± 0.13 | 0.6 |
AC/FC, acylcarnitine to free carnitine ratio; BMI, body mass index; BNP, brain natriuretic peptide; CCVD, cerebrovascular/cardiovascular disease; Cl, clearance; Cr, creatinine; CrCl, creatinine clearance; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; GNRI, geriatric nutritional risk index; hANP, human atrial natriuretic peptide; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance; IL‐6, interleukin‐6; LDL, low‐density lipoprotein; L‐FABP, liver‐type fatty acid‐binding protein; Na, sodium; nPCR, normalized protein catabolism rate; PTH, parathyroid hormone.
At baseline, a significant positive relationship was observed between ISWT and various KDQOL subscales: symptoms/problems, effects of kidney disease, work status, and KDCS (r = 0.38, P = 0.01; r = 0.38, P = 0.01; r = 0.31, P = 0.04; and r = 0.33, P = 0.02, respectively), as well as various SF‐36 subscales, including physical functioning, physical role functioning, bodily pain, emotional role functioning, physical component summary, and RCS (r = 0.70, P < 0.001; r = 0.49, P < 0.001; r = 0.29, P < 0.05; r = 0.35, P = 0.02; r = 0.69, P < 0.001; and r = 0.30, P = 0.04, respectively), as shown in Table 3. Additionally, handgrip and quadriceps strength and serum albumin were positively correlated with ISWT {β‐coefficient = 6.38 [95% confidence interval (CI), 1.10 to 11.65], P = 0.02; β‐coefficient = 5.28 [95% CI, 2.31 to 8.26], P < 0.001; β‐coefficient = 69.31 [95% CI, 2.56 to 136.05], P < 0.05, respectively}, whereas natural log (ln)‐transformed CRP, IL‐6, BMI, waist circumference, LESMI, and ECW/ICW were negatively correlated with ISWT, after adjusting for sex and age [β‐coefficient = −23.22 (95% CI, −42.43 to −4.02), P = 0.02; β‐coefficient = −58.51 (95% CI, −94.60 to −22.43), P = 0.003; β‐coefficient = −9.84 (95% CI, −16.07 to −3.61), P = 0.004; β‐coefficient = −3.43 (95% CI, −5.74 to −1.13), P = 0.006; β‐coefficient = −31.67 (95% CI, −59.33 to −4.01), P = 0.03; and β‐coefficient = −1.14 × 103 (95% CI, −1.98 × 103 to −2.92 × 102), P = 0.01, respectively], as presented in Table 4.
Table 3.
Correlations of health‐related quality of life scores with aerobic capacity
| Variables | ISWT (m) | |
|---|---|---|
| r | P‐value | |
| KDQOL | ||
| Symptoms/problems | 0.38 | 0.01 |
| Effects of kidney disease | 0.38 | 0.01 |
| Burden of kidney disease | 0.01 | 0.9 |
| Work status | 0.31 | 0.04 |
| Cognitive function | 0.21 | 0.2 |
| Quality of social interaction | 0.13 | 0.4 |
| Sleep | 0.07 | 0.6 |
| Social support | 0.13 | 0.4 |
| Overall health rating | −0.092 | 0.5 |
| KDCS | 0.33 | 0.02 |
| SF‐36 | ||
| Physical functioning | 0.70 | <0.001 |
| Physical role functioning | 0.49 | <0.001 |
| Bodily pain | 0.29 | <0.05 |
| General health | 0.02 | 0.9 |
| Vitality | 0.19 | 0.2 |
| Social functioning | 0.13 | 0.4 |
| Emotional role functioning | 0.35 | 0.02 |
| Mental health | 0.17 | 0.3 |
| PCS | 0.69 | <0.001 |
| MCS | −0.21 | 0.2 |
| RCS | 0.30 | 0.04 |
ISWT, incremental shuttle walking test; KDCS, kidney disease component summary; KDQOL, Kidney Disease Quality of Life; MCS, mental component summary; PCS, physical component summary; RCS, role/social component summary; SF‐36, Medical Outcomes Study 36‐Item Short‐Form Health Survey.
Table 4.
Results of linear regression analyses of aerobic capacity
| Variables | ISWT (m) a | ||
|---|---|---|---|
| β‐coefficient | 95% CI | P‐value | |
| Muscle strength | |||
| Handgrip strength (kg) | 6.38 | 1.10 to 11.65 | 0.02 |
| Quadriceps strength (kg) | 5.28 | 2.31 to 8.26 | 0.001 |
| Renal outcomes | |||
| Average Cr and urea Cl (mL/min/1.73 m2) | 2.11 | −2.22 to 6.44 | 0.4 |
| Ln urine albumin (mg/day) | −3.07 | −20.21 to 14.07 | 0.7 |
| Ln urine L‐FABP (μg/day) | 4.02 | −20.38 to 28.43 | 0.8 |
| Albumin (g/L) | 69.31 | 2.56 to 136.05 | <0.05 |
| Ln CRP (mg/L) | −23.22 | −42.43 to −4.02 | 0.02 |
| Ln IL‐6 (pg/mL) | −58.51 | −94.60 to −22.43 | 0.003 |
| Haemoglobin A1c (%) | −27.55 | −82.76 to 27.65 | 0.3 |
| Glycated albumin (%) | −11.56 | −26.27 to 3.15 | 0.1 |
| Ln HOMA‐IR | −3.52 | −9.49 to 2.45 | 0.2 |
| Anthropometric data | |||
| BMI (kg/m2) | −9.84 | −16.07 to −3.61 | 0.004 |
| Waist circumference (cm) | −3.43 | −5.74 to −1.13 | 0.006 |
| Leg circumference (cm) | −6.90 | −15.14 to 1.34 | 0.2 |
| SMI (kg/m2) | −24.57 | −49.20 to 0.057 | 0.06 |
| UESMI (kg/m2) | −1.76 | −106.47 to 102.96 | 0.9 |
| LESMI (kg/m2) | −31.67 | −59.33 to −4.01 | 0.03 |
| ECW/ICW | −1.14 × 103 | −1.98 × 103 to −2.92 × 102 | 0.01 |
| Phase angle | 20.31 | −5.92 to 73.71 | 0.1 |
| Arterial stiffness/BMD | |||
| baPWV(m/s) | 0.90 | −9.01 to 10.80 | 0.9 |
| BMD (g/cm2) | −114.73 | −254.24 to 24.79 | 0.1 |
| Exploratory outcomes | |||
| Free carnitine (μmol/L) | −1.56 | −4.58 to 1.46 | 0.3 |
| AC/FC | 28.22 | −224.50 to 280.95 | 0.8 |
AC/FC, acylcarnitine to free carnitine ratio; baPWV, brachial‐ankle pulse wave velocity; BMD, bone mineral density; BMI, body mass index; CI, confidence interval; Cl, clearance; Cr, creatinine; CRP, C‐reactive protein; ECW/ICW, extracellular water to intracellular water ratio; HOMA‐IR, homeostasis model assessment of insulin resistance; IL‐6, interleukin‐6; ISWT, incremental shuttle walking test; LESMI, lower extremity skeletal mass index; L‐FABP, liver‐type fatty acid‐binding protein; Ln, natural log transformed; SMI, skeletal mass index; UESMI, upper extremity skeletal mass index.
ISWT (m): adjusted for sex and age.
Effect of the home‐based exercise programme on aerobic capacity
At baseline, no significant differences were observed in ISWT between control and exercise groups (P = 0.9; 363.9 ± 131.6 and 371.3 ± 143.5 m, respectively). However, intervention improved ISWT significantly more in the exercise group compared with the control group (39.4 ± 54.6 vs. −21.3 ± 46.1 m, P < 0.001) (Table 5).
Table 5.
Effect of the 6 month exercise programme on aerobic capacity and subgroup analysis
| Groups | Control | Exercise | Between‐group differences a | P‐value b | P for interaction c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Baseline | Final | Δ6 months | N | Baseline | Final | Δ6 months | ||||
| Overall | 23 | 363.9 ± 131.6 | 342.6 ± 114.9 | −21.3 ± 46.1 | 23 | 371.3 ± 143.5 | 410.7 ± 153.5 | 39.4 ± 54.6 | 60.7 ± 14.9 | <0.001 | — |
| Subgroups | |||||||||||
| Age | 0.5 | ||||||||||
| <65 years | 5 | 404.0 ± 187.4 | 374.0 ± 131.6 | −30.0 ± 68.9 | 5 | 534.0 ± 102.1 | 540.0 ± 102.1 | 6.0 ± 62.7 | 36.0 ± 41.7 | 0.39 | |
| ≥65 years | 18 | 352.8 ± 116.3 | 333.9 ± 112.4 | −18.9 ± 40.0 | 18 | 326.1 ± 119.4 | 374.8 ± 141.0 | 48.7 ± 50.2 | 67.5 ± 15.1 | <0.001 | |
| Sex | 0.07 | ||||||||||
| Male | 16 | 411.9 ± 105.7 | 378.1 ± 98.2 | −33.8 ± 48.6 | 17 | 401.2 ± 124.1 | 446.2 ± 126.0 | 45.0 ± 59.8 | 78.8 ± 18.9 | <0.001 | |
| Female | 7 | 254.3 ± 123.9 | 261.4 ± 115.1 | 7.14 ± 22.9 | 6 | 286.7 ± 172.5 | 310.0 ± 191.0 | 23.3 ± 35.6 | 16.2 ± 16.3 | 0.32 | |
| Diabetes | 0.4 | ||||||||||
| Yes | 7 | 357.1 ± 127.5 | 335.7 ± 114.9 | −21.4 ± 41.0 | 7 | 347.1 ± 168.8 | 369.4 ± 179.3 | 22.3 ± 76.1 | 43.7 ± 32.7 | 0.18 | |
| No | 16 | 366.9 ± 137.3 | 345.6 ± 118.5 | −21.3 ± 49.4 | 16 | 381.9 ± 135.8 | 428.9 ± 143.4 | 46.9 ± 43.2 | 68.1 ± 16.4 | <0.001 | |
| CCVD | 0.9 | ||||||||||
| Yes | 5 | 280.0 ± 105.4 | 264.0 ± 102.1 | −16.0 ± 13.4 | 7 | 310.0 ± 143.3 | 353.7 ± 123.0 | 43.7 ± 68.7 | 59.7 ± 31.5 | 0.06 | |
| No | 18 | 387.2 ± 130.9 | 364.4 ± 110.9 | −22.8 ± 51.9 | 16 | 398.1 ± 139.6 | 435.6 ± 162.2 | 37.5 ± 49.7 | 60.3 ± 17.4 | 0.001 | |
| Smoking | 0.7 | ||||||||||
| Yes | 10 | 378.0 ± 124.3 | 369.0 ± 125.1 | −9.0 ± 42.8 | 11 | 370.0 ± 92.7 | 415.5 ± 112.6 | 45.5 ± 55.0 | 54.5 ± 21.7 | 0.01 | |
| No | 13 | 353.1 ± 140.9 | 322.3 ± 106.9 | −30.8 ± 47.9 | 12 | 372.5 ± 182.7 | 406.3 ± 188.6 | 33.8 ± 56.1 | 64.6 ± 20.9 | 0.002 | |
CCVD, cerebrovascular/cardiovascular disease; ISWT, incremental shuttle walking test.
Differences of ΔISWT in the exercise group as compared with that in the control group. Values are given as mean ± standard error.
P‐value indicates significant difference in ΔISWT between the exercise and control groups.
P‐value for the difference over time between the two subgroups (interaction).
Subgroup analyses revealed a significant between‐group difference in ΔISWT of 78.8 ± 18.9 m for men (P < 0.001) and 16.2 ± 16.3 m (P = 0.3) for women but only near‐significant difference (P for interaction = 0.07) between genders (Table 4). Between‐group differences in ΔISWT in patients ≥65 and <65 years were 67.5 ± 15.1 m (P < 0.001) and 36.0 ± 41.7 m (P = 0.4), respectively. Although there was no statistical interaction between age groups (P = 0.5), larger ISWT improvement in patients ≥65 years suggested that there was less room for improvement in patients <65 years with longer baseline ISWT distance compared with that of patients ≥65 years, and the intensity of exercise intervention in this trial was insufficient to improve exercise capacity in patients <65 years. Between‐group differences in ΔISWT for patients with and without diabetes were 43.7 ± 32.7 m (P = 0.2) and 68.1 ± 16.4 m (P < 0.001), respectively, without significant interactions (P = 0.5 and 0.4, respectively). Similarly, subgroup analysis by smoking and cerebrovascular/cardiovascular disease showed non‐significant interactions (P = 0.7 and 0.9, respectively).
In sub‐analyses among participants in the exercise group, ISWT tended to improve in the patients with HA to AE compared with those with LA to AE (38.7 ± 20.7 m, P = 0.08) (Table S2). Although a statistical difference was not found in the change of ISWT between the patients with HA and LA to RE (P = 0.9), ISWT was almost significantly improved in the participants with HA to AE + RE than those with LA to AE + RE (42.7 ± 20.6 m, P = 0.05) (Tables S3 and S5). Additionally, ISWT significantly improved in the patients with higher number of daily steps than those with lower number of daily steps (66.6 ± 24.3 m, P = 0.01) (Table S5).
Effect of the home‐based exercise programme on health‐related quality of life
At baseline, no significant differences were observed in any HRQOL subscales between the control and exercise groups. As presented in Table 6, after intervention, among KDQOL domains, work status, quality of social interaction, and KDCS outcomes were significantly better (12.70 ± 5.76, P = 0.03; 5.97 ± 2.59, P = 0.03; and 4.81 ± 1.71, P = 0.007, respectively), whereas symptoms/problems and sleep only tended to be better (4.33 ± 2.50, P = 0.09 and 6.52 ± 3.40, P = 0.06, respectively) in the exercise group. Among the domains in SF‐36, there was no significant improvement, but mental health and RCS tended to be better in the exercise group (6.67 ± 3.87, P = 0.09 and 4.37 ± 2.60, P = 0.1, respectively).
Table 6.
Effect of the 6 month exercise programme on health‐related quality of life
| Variables | Control (n = 23) | Exercise (n = 23) | 6 month ANCOVAs | |||
|---|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | Between‐group differences a | P for interaction | |
| KDQOL | ||||||
| Symptoms/problems | 84.8 ± 10.3 | 81.1 ± 13.0 | 82.7 ± 15.4 | 84.0 ± 11.8 | 4.33 ± 2.50 | 0.09 |
| Effects of kidney disease | 89.4 ± 10.3 | 86.2 ± 13.9 | 91.4 ± 7.3 | 91.2 ± 8.8 | 3.19 ± 2.50 | 0.2 |
| Burden of kidney disease | 64.9 ± 24.2 | 59.5 ± 26.2 | 69.6 ± 15.0 | 60.9 ± 19.3 | −1.86 ± 5.48 | 0.7 |
| Work status | 63.0 ± 27.0 | 50.0 ± 39.9 | 71.7 ± 29.5 | 71.7 ± 29.5 | 12.70 ± 5.76 | 0.03 |
| Cognitive function | 84.6 ± 17.8 | 83.8 ± 18.6 | 87.8 ± 10.4 | 88.7 ± 10.3 | 2.35 ± 2.82 | 0.4 |
| Quality of social interaction | 87.8 ± 11.7 | 82.9 ± 14.5 | 91.0 ± 9.6 | 91.3 ± 8.4 | 5.97 ± 2.59 | 0.03 |
| Sleep | 63.6 ± 19.9 | 60.3 ± 16.8 | 65.2 ± 16.3 | 67.8 ± 14.8 | 6.52 ± 3.40 | 0.06 |
| Social support | 78.3 ± 22.2 | 76.8 ± 23.4 | 81.9 ± 24.1 | 85.5 ± 16.1 | 7.13 ± 5.23 | 0.2 |
| Overall health rating | 60.9 ± 14.4 | 56.3 ± 16.3 | 60.7 ± 12.3 | 56.3 ± 16.3 | 4.71 ± 3.26 | 0.2 |
| KDCS | 77.1 ± 10.9 | 72.6 ± 11.8 | 80.2 ± 8.4 | 80.1 ± 7.6 | 4.81 ± 1.71 | 0.007 |
| SF‐36 | ||||||
| Physical functioning | 77.8 ± 16.1 | 77.4 ± 19.8 | 81.5 ± 11.9 | 81.3 ± 15.0 | 0.20 ± 3.10 | 0.9 |
| Physical role functioning | 80.4 ± 25.2 | 73.6 ± 20.6 | 79.3 ± 19.7 | 80.7 ± 19.5 | 7.37 ± 5.68 | 0.2 |
| Bodily pain | 74.7 ± 23.8 | 73.9 ± 24.7 | 70.3 ± 23.3 | 70.7 ± 24.9 | −0.74 ± 6.34 | 0.9 |
| General health | 48.5 ± 15.4 | 48.2 ± 16.6 | 51.0 ± 12.7 | 51.2 ± 14.1 | 0.98 ± 3.05 | 0.8 |
| Vitality | 60.3 ± 18.3 | 61.1 ± 18.1 | 62.8 ± 12.8 | 64.1 ± 15.8 | 1.88 ± 4.60 | 0.7 |
| Social functioning | 84.8 ± 19.6 | 76.8 ± 23.4 | 92.4 ± 10.5 | 85.5 ± 16.1 | 5.76 ± 4.61 | 0.2 |
| Emotional role functioning | 77.5 ± 23.4 | 77.9 ± 22.8 | 83.0 ± 18.4 | 84.1 ± 18.8 | 3.00 ± 5.11 | 0.6 |
| Mental health | 73.7 ± 16.7 | 70.4 ± 17.7 | 77.8 ± 14.0 | 79.3 ± 12.6 | 6.67 ± 3.87 | 0.09 |
| PCS | 41.5 ± 8.4 | 41.2 ± 11.8 | 40.5 ± 8.9 | 40.3 ± 11.7 | 0.13 ± 2.33 | 0.9 |
| MCS | 51.1 ± 9.6 | 51.3 ± 7.8 | 52.2 ± 6.2 | 52.7 ± 6.6 | 0.83 ± 1.75 | 0.6 |
| RCS | 49.0 ± 13.4 | 46.9 ± 10.5 | 52.0 ± 8.2 | 52.4 ± 8.5 | 4.37 ± 2.60 | 0.1 |
ANCOVA, analysis of covariance; KDCS, kidney disease component summary; KDQOL, Kidney Disease Quality of Life; MCS, mental component summary; PCS, physical component summary; RCS, role/social component summary; SF‐36, Medical Outcomes Study 36‐Item Short‐Form Health Survey.
Differences of the serial changes in the exercise group as compared with that in the control group. Values are given as mean ± standard error.
Effect of the home‐based exercise programme on secondary outcomes
At baseline, leg circumference, SMI, and LESMI were significantly higher in the exercise group (P = 0.02, 0.03, and 0.03, respectively), but otherwise not significant. Table 7 shows that among renal outcomes, reduction of ln urinary L‐FABP excretion was significantly greater in the exercise group (−0.579 ± 0.217, P = 0.008), but there were no significant group differences in combined urea and creatinine clearance and ln urinary albumin excretion (1.38 ± 1.72, P = 0.4 and −0.24 ± 0.18, P = 0.2, respectively). Additionally, reduction of ln CRP was significantly greater in the exercise group compared with the control group (−1.13 ± 0.35, P = 0.003), whereas group difference for ln IL‐6 did not reach statistical significance (−0.258 ± 0.177, P = 0.2). Quadriceps strength also showed insignificant group difference (2.97 ± 2.01, P = 0.1). There were no significant group differences in handgrip strength, biochemical metabolic parameters, and anthropometric data including BMI, SMI, ECW/ICW, arterial stiffness, and BMD. Among exploratory outcomes, reduction of AC/FC was significantly greater in the exercise group compared with the control group (−0.058 ± 0.024, P = 0.01).
Table 7.
Effect of the 6 month exercise programme on secondary outcomes
| Variables | Control (n = 23) | Exercise (n = 23) | 6 month ANCOVAs | |||
|---|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | Between‐group differences a | P for interaction | |
| Renal outcomes | ||||||
| Average Cr and urea Cl (mL/min/1.73 m2) | 23.5 ± 5.8 | 22.1 ± 5.8 | 25.7 ± 7.5 | 25.3 ± 9.5 | 1.38 ± 1.72 | 0.4 |
| Ln urine albumin (mg/day) | 5.58 ± 1.74 | 5.76 ± 1.81 | 5.76 ± 2.10 | 5.70 ± 9.5 | −0.24 ± 0.18 | 0.2 |
| Ln urine L‐FABP (μg/day) | 2.62 ± 1.08 | 3.18 ± 1.24 | 2.95 ± 1.52 | 2.90 ± 1.56 | −0.579 ± 0.217 | 0.008 |
| Albumin (g/L) | 3.73 ± 0.26 | 3.71 ± 0.25 | 3.72 ± 0.56 | 3.73 ± 0.52 | 0.031 ± 0.081 | 0.7 |
| Ln CRP (mg/L) | 0.18 ± 1.58 | 0.57 ± 1.47 | −0.62 ± 1.23 | −0.85 ± 0.96 | −1.13 ± 0.35 | 0.003 |
| Ln IL‐6 (pg/mL) | 1.15 ± 0.71 | 1.31 ± 0.82 | 0.94 ± 0.80 | 0.92 ± 0.65 | −0.258 ± 0.177 | 0.2 |
| Haemoglobin A1c (%) | 6.1 ± 0.8 | 6.1 ± 0.8 | 6.0 ± 0.6 | 6.3 ± 1.8 | 0.27 ± 0.36 | 0.5 |
| Glycated albumin (%) | 16.0 ± 2.9 | 15.8 ± 2.6 | 14.8 ± 2.4 | 14.8 ± 2.4 | −0.10 ± 0.40 | 0.8 |
| Ln HOMA‐IR | 1.15 ± 0.89 | 1.01 ± 1.08 | 1.07 ± 0.80 | 0.97 ± 0.87 | −0.014 ± 0.234 | 0.9 |
| Muscle strength | ||||||
| Handgrip strength (kg) | 25.3 ± 7.7 | 26.2 ± 5.9 | 30.0 ± 8.6 | 29.3 ± 8.5 | −0.76 ± 0.77 | 0.3 |
| Quadriceps strength (kg) | 25.2 ± 9.9 | 23.6 ± 8.7 | 29.3 ± 12.8 | 29.5 ± 12.1 | 2.97 ± 2.01 | 0.1 |
| Anthropometric data | ||||||
| BMI (kg/m2) | 23.0 ± 4.3 | 23.4 ± 4.4 | 24.7 ± 4.6 | 24.7 ± 4.8 | −0.27 ± 0.32 | 0.4 |
| Waist circumference (cm) | 89.9 ± 12.5 | 90.1 ± 12.4 | 95.5 ± 11.2 | 94.0 ± 11.2 | −0.56 ± 2.27 | 0.8 |
| Leg circumference (cm) | 33.7 ± 3.6 | 34.6 ± 3.43 | 36.6 ± 4.3 | 36.8 ± 4.9 | −0.56 ± 0.55 | 0.3 |
| SMI (kg/m2) | 7.70 ± 1.29 | 7.78 ± 1.53 | 8.74 ± 1.77 | 8.89 ± 1.84 | 0.122 ± 0.274 | 0.7 |
| UESMI (kg/m2) | 1.27 ± 0.28 | 1.26 ± 0.24 | 1.43 ± 0.39 | 1.42 ± 0.43 | 0.038 ± 0.066 | 0.6 |
| LESMI (kg/m2) | 6.43 ± 1.10 | 6.52 ± 1.44 | 7.31 ± 1.52 | 7.48 ± 1.58 | 0.119 ± 0.271 | 0.7 |
| ECW/ICW | 0.662 ± 0.028 | 0.661 ± 0.034 | 0.672 ± 0.040 | 0.673 ± 0.049 | 0.004 ± 0.010 | 0.7 |
| Phase angle | 4.90 ± 0.71 | 4.91 ± 0.66 | 4.89 ± 0.91 | 4.85 ± 1.00 | −0.052 ± 0.141 | 0.7 |
| Arterial stiffness/BMD | ||||||
| baPWV (m/s) | 17.9 ± 3.8 | 17.4 ± 3.0 | 16.8 ± 2.9 | 16.4 ± 2.8 | −48.6 ± 68.1 | 0.7 |
| BMD (g/cm2) | 1.17 ± 0.19 | 1.17 ± 0.20 | 1.26 ± 0.29 | 1.26 ± 0.30 | −0.009 ± 0.032 | 0.8 |
| Exploratory outcomes | ||||||
| Free carnitine (μmol/L) | 48.1 ± 10.9 | 48.9 ± 8.4 | 51.9 ± 10.6 | 53.4 ± 11.0 | 2.19 ± 2.23 | 0.3 |
| AC/FC | 0.34 ± 0.11 | 0.39 ± 0.10 | 0.36 ± 0.13 | 0.33 ± 0.08 | −0. 058 ± 0.024 | 0.01 |
AC/FC, acylcarnitine to free carnitine ratio; ANCOVAs, analyses of covariance; baPWV, brachial‐ankle pulse wave velocity; BMD, bone mineral density; BMI, body mass index; CI, confidence interval; Cl, clearance; Cr, creatinine; CRP, C‐reactive protein; ECW/ICW, extracellular water to intracellular water ratio; HOMA‐IR, homeostasis model assessment of insulin resistance; IL‐6, interleukin‐6; LESMI, lower extremity skeletal mass index; L‐FABP, liver‐type fatty acid‐binding protein; Ln, natural log transformed; SMI, skeletal mass index; UESMI, upper extremity skeletal mass index.
Differences of the serial changes in the exercise group as compared with that in the control group. Values are given as mean ± standard error.
In sub‐analyses among participants in the exercise group, no statistically significant differences were found in handgrip and quadriceps strength, between patients with HA and LA to AE, RE, and AE + RE sessions and between patients with higher number of steps per day and those with lower (Tables S2–S5).
Discussion
To the best of our knowledge, this study was the first RCT to describe the positive effects of exercise specifically in patients with Stage 4 CKD. The multifaceted benefits of exercise programmes for patients with Stage 3 CKD (eGFR around 40–50 mL/min/1.73 m2) were almost universal. 5 , 6 , 7 , 8 However, the effect of exercise exclusively in patients with severe CKD, especially with eGFR around 20 mL/min/1.73 m2, has not been described, although few studies partly recruited severe CKD patients (mean eGFR < 30 mL/min/1.73 m2), showing improved ISWT, muscle strength, and malnutrition, inflammation, and atherosclerosis syndrome. 9 , 10 , 27 The present trial recruited only Stage 4 CKD patients (mean eGFR, 23.2 mL/min/1.73 m2) with mean age of 73 years, compared with mean age around 50 years in previous trials. These populations seemed far more at risk of death and induction of dialysis than those with Stage 2–3 CKD. In our high‐risk population, we noted better outcomes in aerobic capacity (ISWT), several HRQOL domains, serum CRP, AC/FC, and urinary L‐FABP levels in the exercise group relative to the control group.
Incremental shuttle walking test is more objective than the 6MWT and has been increasingly used as an aerobic capacity outcome in pre‐dialysis CKD and dialysis patients. 28 , 29 , 30 Additionally, a strong correlation between ISWT and VO2peak, the gold standard measured by general cardiopulmonary exercise test in pre‐dialysis CKD patients, has been shown. 31 Greenwood et al. recently reported that improvement in ISWT with renal rehabilitation programme was associated with lower morbidity and mortality in pre‐dialysis and dialysis patients with CKD. 32 Therefore, ISWT improvement in our exercise group might reduce their morbidity and mortality in long‐term follow‐up.
Additionally, there were better outcomes in several KDQOL‐SF subscales in the exercise group, consistent with results of previous exercise intervention on patients with pre‐dialysis CKD, although the combinations of improved subscales differ among trials. 5 , 6 , 25 , 33 Quality of life is known to decrease even at early stages of CKD, and strong correlations between ISWT and HRQOL subscales were also seen in our study population, whereas another study on pre‐dialysis patients showed that increased handgrip strength was significantly associated with increased HRQOL scores. 34 Especially in elderly CKD patients similar to our study, HRQOL should be considered an important outcome similar to other outcomes including death and dialysis induction.
Unfortunately, we could not demonstrate the beneficial effect of exercise programme on renal function, assessed with combined urea and creatinine clearance, as previously shown in patients with Stage 3–4 CKD using eGFR calculated from serum creatinine and cystatin C. 7 This discrepancy might be caused by different study populations, although different exercise programme prescription, markers of renal function, or drugs including antihypertensive agents cannot be discounted.
On the contrary, the exercise programme produced significantly better outcomes regarding urinary L‐FABP and some positive, though non‐significant, impact on urinary albumin, which might attenuate decline in renal function. L‐FABP is 14 kDa and expressed in human proximal tubules. Its urinary excretion reflects various stresses on proximal tubules and correlates with both severity of tubulointerstitial injury and urinary proteins. 35 In clinical practice, urinary L‐FABP reflected severity, and predicted progression, of non‐diabetic and diabetic CKD. 21 , 36 , 37 Moreover, urinary L‐FABP was useful in early detection of acute kidney injury after cardiac surgery. 38 Improved urinary L‐FABP with exercise was also seen in our previous RCT post hoc analysis of peritoneal dialysis patients, suggesting that L‐FABP may be a more sensitive marker of reno‐protection after exercise. 39
Both serum CRP and IL‐6 were independently associated with decreased ISWT, suggesting that systemic inflammation in advanced CKD is related to the reduced ability to perform exercise. However, a beneficial effect of exercise was observed on serum CRP and IL‐6, though the group difference for serum IL‐6 did not reach statistical significance, probably due to large IL‐6 variation suggesting larger sample size required to detect statistically significant difference. These findings were consistent with previous trials in patients with advanced CKD, 10 , 40 suggesting possible reduced all‐cause and cardiovascular mortality, as both serum CRP and IL‐6 are well‐established predictors of these outcomes in patients with CKD. 41
As a novel finding, the significantly greater reduction in the exercise group of AC/FC, a marker of carnitine deficiency, indicates improved mitochondrial β‐oxidation, which decreases with CKD progression. 22 , 42 Mitochondrial dysfunction is not only an important cause of uraemic sarcopenia 3 but is also associated with steeper eGFR decline. 43 Further studies are needed to clarify the associations between exercise intervention and carnitine profile, mitochondrial β‐oxidation in CKD, and associated clinical outcomes.
This trial has some limitations. First, the 6 month follow‐up period prevented assessing whether exercise can improve all‐cause and cardiovascular mortality or delay introduction of renal replacement therapy. On the other hand, the higher exercise capacity including the distance in 6MWT or handgrip strength was strongly associated with lower all‐cause mortality in patients with CKD, 44 as well as higher cardiorespiratory fitness was associated with considerable improvement in survival in several populations. 45 Additionally, improvement in ISWT by renal rehabilitation contributed significantly to lower risk of death or combined events including death, cerebrovascular accident, myocardial infarction, and heart failure hospitalization in patients with CKD. 32 Therefore, improvement in ISWT by home‐based exercise programme observed in our trial might lead to better clinical outcomes including survival. At the same time, the level of training at which a difference in exercise capacity became significant was unknown, because of the absence of pre‐specified interim analysis. Therefore, future trials with longer follow‐up and larger samples with interim analyses are necessary. Second, we were unable to recruit adequate patients (estimated sample size, 60) to detect a statistically significant group difference in ISWT. This was partly because of high patient co‐morbidity. Although recruitment rate was comparable with previous trials, many patients were excluded based on the American College of Sports Medicine and Japanese Circulation Society guidelines. 23 , 46 However, the number of analysed patients of 46 coincided with the minimal required sample size before inflation, considering estimated attrition of 30%, and eventually, statistical difference in ISWT was demonstrated in our study. Third, due partly to the nature of the home‐based exercise programme in this trial, HR was not monitored for all patients during home‐based exercise, and their RPE was not reported after each home‐based exercise session by postcards. Therefore, compliance to the prescribed exercise intensity might lead to differences in the changes of ISWT and/or muscular strength. On the other hand, it was considered rather difficult for patients to monitor and report their HR with %peak HR, to report their RPE using Borg scale, and to assess %1‐RM during their home‐based exercise sessions. On the contrary, it is one of the most significant strengths in our trial that we have shown that such a simple and highly feasible home‐based exercise programme can improve various outcomes in patients with Stage 4 CKD. Finally, because our intervention included both AE and RE, it was difficult to know which intervention, or if both, contributed to the results. Further trials with head‐to‐head comparison of AE and RE will be necessary to answer this question, while the trial in patients with kidney transplantation examined this and concluded that both AE and RE interventions appeared to be feasible and clinically beneficial in this patient population, 47 and another trial in patients with non‐dialysis CKD compared the combination of AE and RE with AE only and demonstrated that the addition of RE to AE conferred greater benefits on exercise capacity than AE alone. 27 From these findings, and also considering the independent effects of AE and RE on cardiorespiratory fitness and muscle strength, it is assumed that performing both AE and RE was the most beneficial and reasonable. In our trial, we also found that higher adherence to both AE and RE and higher number of daily steps were relevant to improvement in ISWT from sub‐analyses among patients in the exercise group, although caution is needed to interpret these results due to the nature of the sub‐analyses in which randomization was broken with potential for bias.
In conclusion, this is the first study to indicate the beneficial effects of a 6 month home‐based exercise programme involving AE and RE in patients with Stage 4 CKD. The programme may improve aerobic capacity, various HRQOL subscales, systemic inflammation, urinary biomarkers, and mitochondrial function, without adverse effects.
Funding
We have no disclosure and financial support.
Author contributions
K.U., K.A., K. Muraoka, and S.W. contributed in the research idea and study design; K.A., K. Muraoka, T.N., T.O., M.Y., A.H., H.M., H.T., and S.W. in data acquisition; K.U. and K. Miyashita in data analysis/interpretation and statistical analysis; and S.W. and H.I. in supervision or mentorship. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest
None declared.
Supporting information
Table S1. The postcard (exercise check sheet) to assess patient adherence to prescribed home‐based exercise sessions and the number of steps per day.
Table S2. Comparison of change in exercise capacity between patients with high adherence and low adherence to home‐based aerobic exercise (AE) in the exercise group.
Table S3. Comparison of change in exercise capacity between patients with high adherence and low adherence to home‐based resistance exercise (RE) in the exercise group.
Table S4. Comparison of change in exercise capacity between patients with high adherence and low adherence to both home‐based aerobic exercise (AE) and resistance exercise (RE) in the exercise group.
Table S5. Comparison of change in exercise capacity between patients with high and low number of steps per day in the exercise group.
Data S1. The clinical trial protocol.
Acknowledgements
The authors thank all the participants for their dedication to this research project. The authors wish to acknowledge Dr Hideaki Nakaya for valuable technical assistance. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 48 The study protocol (for details, see Data S1) was reviewed and approved by the ethics committee of Keio University Hospital (approval number: 20180125) and adhered to the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants. The study was registered in a public trial registry (UMIN‐CTR, number: UMIN0000034855).
Uchiyama K., Adachi K., Muraoka K., Nakayama T., Oshida T., Yasuda M., Hishikawa A., Minakuchi H., Miyashita K., Tokuyama H., Wakino S., and Itoh H. (2021) Home‐based aerobic exercise and resistance training for severe chronic kidney disease: a randomized controlled trial, Journal of Cachexia, Sarcopenia and Muscle, 12, 1789–1802, 10.1002/jcsm.12775.
References
- 1. Fahal IH. Uraemic sarcopenia: aetiology and implications. Nephrol Dial Transplant 2014;29:1655–1665. [DOI] [PubMed] [Google Scholar]
- 2. Mak RH, Ikizler AT, Kovesdy CP, Raj DS, Stenvinkel P, Kalantar‐Zadeh K. Wasting in chronic kidney disease. J Cachexia Sarcopenia Muscle 2011;2:9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uchiyama K, Wakino S, Irie J, Miyamoto J, Matsui A, Tajima T, et al. Contribution of uremic dysbiosis to insulin resistance and sarcopenia. Nephrol Dial Transplant 2020, Epub ahead of print;35:1501–1517. [DOI] [PubMed] [Google Scholar]
- 4. Pereira RA, Cordeiro AC, Avesani CM, Carrero JJ, Lindholm B, Amparo FC, et al. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant 2015;30:1718–1725. [DOI] [PubMed] [Google Scholar]
- 5. Headley S, Germain M, Wood R, Joubert J, Milch C, Evans E, et al. Short‐term aerobic exercise and vascular function in CKD stage 3: a randomized controlled trial. Am J Kidney Dis 2014;64:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Craenenbroeck AH, van Craenenbroeck EM, van Ackeren K, Vrints CJ, Conraads VM, Verpooten GA, et al. Effect of moderate aerobic exercise training on endothelial function and arterial stiffness in CKD stages 3‐4: a randomized controlled trial. Am J Kidney Dis 2015;66:285–296. [DOI] [PubMed] [Google Scholar]
- 7. Greenwood SA, Koufaki P, Mercer TH, MacLaughlin HL, Rush R, Lindup H, et al. Effect of exercise training on estimated GFR, vascular health, and cardiorespiratory fitness in patients with CKD: a pilot randomized controlled trial. Am J Kidney Dis 2015;65:425–434. [DOI] [PubMed] [Google Scholar]
- 8. Ikizler TA, Robinson‐Cohen C, Ellis C, Headley SAE, Tuttle K, Wood RJ, et al. Metabolic effects of diet and exercise in patients with moderate to severe CKD: a randomized clinical trial. J Am Soc Nephrol 2018;29:250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castaneda C, Gordon PL, Uhlin KL, Levey AS, Kehayias JJ, Dwyer JT, et al. Resistance training to counteract the catabolism of a low‐protein diet in patients with chronic renal insufficiency: a randomized, controlled trial. Ann Intern Med 2001;135:965–976. [DOI] [PubMed] [Google Scholar]
- 10. Castaneda C, Gordon PL, Parker RC, Uhlin KL, Roubenoff R, Levey AS. Resistance training to reduce the malnutrition‐inflammation complex syndrome of chronic kidney disease. Am J Kidney Dis 2004;43:607–616. [DOI] [PubMed] [Google Scholar]
- 11. Uchiyama K, Washida N, Morimoto K, Muraoka K, Kasai T, Yamaki K, et al. Home‐based aerobic exercise and resistance training in peritoneal dialysis patients: a randomized controlled trial. Sci Rep 2019;9:2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohashi Y, Tai R, Aoki S, Mizuiri S, Ogura T, Tanaka Y, et al. The associations of malnutrition and aging with fluid volume imbalance between intra‐ and extracellular water in patients with chronic kidney disease. J Nutr Health Aging 2015;19:986–993. [DOI] [PubMed] [Google Scholar]
- 13. Ohashi Y, Joki N, Yamazaki K, Kawamura T, Tai R, Oguchi H, et al. Changes in the fluid volume balance between intra‐ and extracellular water in a sample of Japanese adults aged 15–88 yr old: a cross‐sectional study. Am J Physiol Renal Physiol 2018;314:F614–F622. [DOI] [PubMed] [Google Scholar]
- 14. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:300–307.e2. [DOI] [PubMed] [Google Scholar]
- 15. Jensky‐Squires NE, Dieli‐Conwright CM, Rossuello A, Erceg DN, McCauley S, Schroeder ET. Validity and reliability of body composition analysers in children and adults. Br J Nutr 2008;100:859–865. [DOI] [PubMed] [Google Scholar]
- 16. Faria SL, Faria OP, Cardeal MD, Ito MK. Validation study of multi‐frequency bioelectrical impedance with dual‐energy X‐ray absorptiometry among obese patients. Obes Surg 2014;24:1476–1480. [DOI] [PubMed] [Google Scholar]
- 17. Cha K, Chertow GM, Gonzalez J, Lazarus JM, Wilmore DW. Multifrequency bioelectrical impedance estimates the distribution of body water. J Appl Physiol 1995;79:1316–1319. [DOI] [PubMed] [Google Scholar]
- 18. Fürstenberg A, Davenport A. Comparison of multifrequency bioelectrical impedance analysis and dual‐energy X‐ray absorptiometry assessments in outpatient hemodialysis patients. Am J Kidney Dis 2010;57:123–129. [DOI] [PubMed] [Google Scholar]
- 19. Almond A, Siddiqui S, Robertson S, Norrie J, Isles C. Comparison of combined urea and creatinine clearance and prediction equations as measures of residual renal function when GFR is low. QJM 2008;101:619–624. [DOI] [PubMed] [Google Scholar]
- 20. Chronic Kidney Disease Prognosis Consortium , Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: a collaborative meta‐analysis. Lancet 2010;375:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamijo A, Sugaya T, Hikawa A, Yamanouchi M, Hirata Y, Ishimitsu T, et al. Clinical evaluation of urinary excretion of liver‐type fatty acid‐binding protein as a marker for the monitoring of chronic kidney disease: a multicenter trial. J Lab Clin Med 2005;145:125–133. [DOI] [PubMed] [Google Scholar]
- 22. Yoshihisa A, Watanabe S, Yokokawa T, Misaka T, Sato T, Suzuki S, et al. Associations between acylcarnitine to free carnitine ratio and adverse prognosis in heart failure patients with reduced or preserved ejection fraction. ESC Heart Fail 2017;4:360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riebe D, Ehrman JK, Liguori G, Magal M. ACSM's Guideline for Exercise Testing and Prescription: Tenth Edition. Philadelphia: Wolters Kluwer; 2017. [Google Scholar]
- 24. Yamagata K, Hoshino J, Sugiyama H, Hanafusa N, Shibagaki Y, Komatsu Y, et al. Clinical practice guideline for renal rehabilitation: systematic reviews and recommendations of exercise therapies in patients with kidney diseases. Ren Replace Ther 2019;5:28. [Google Scholar]
- 25. Mustata S, Groeneveld S, Davidson W, Ford G, Kiland K, Manns B. Effects of exercise training on physical impairment, arterial stiffness and health‐related quality of life in patients with chronic kidney disease: a pilot study. Int Urol Nephrol 2011;43:1133–1141. [DOI] [PubMed] [Google Scholar]
- 26. Howden EJ, Leano R, Petchey W, Coombes JS, Isbel NM, Marwick TH. Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Nephrol 2013;8:1494–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Watson EL, Gould DW, Wilkinson TJ, Xenophontos S, Clarke AL, Vogt BP, et al. Twelve‐week combined resistance and aerobic training confers greater benefits than aerobic training alone in nondialysis CKD. Am J Physiol Renal Physiol 2018;314:F1188–F1196. [DOI] [PubMed] [Google Scholar]
- 28. Greenwood SA, Lindup H, Taylor K, Koufaki P, Rush R, Macdougall IC, et al. Evaluation of a pragmatic exercise rehabilitation programme in chronic kidney disease. Nephrol Dial Transplant 2012;27:iii126–iii134. [DOI] [PubMed] [Google Scholar]
- 29. Wilund KR, Tomayko EJ, Wu PT, Ryong Chung H, Vallurupalli S, Lakshminarayanan B, et al. Intradialytic exercise training reduces oxidative stress and epicardial fat: a pilot study. Nephrol Dial Transplant 2010;25:2695–2701. [DOI] [PubMed] [Google Scholar]
- 30. Uchiyama K, Washida N, Muraoka K, Morimoto K, Kasai T, Yamaki K, et al. Exercise capacity and association with quality of life in peritoneal dialysis patients. Perit Dial Int 2019;39:66–73. [DOI] [PubMed] [Google Scholar]
- 31. Wilkinson TJ, Xenophontos S, Gould DW, Vogt BP, Viana JL, Smith AC, et al. Test–retest reliability, validation, and “minimal detectable change” scores for frequently reported tests of objective physical function in patients with non‐dialysis chronic kidney disease. Physiother Theory Pract 2019;35:565–576. [DOI] [PubMed] [Google Scholar]
- 32. Greenwood SA, Castle E, Lindup H, Mayes J, Waite I, Grant D, et al. Mortality and morbidity following exercise‐based renal rehabilitation in patients with chronic kidney disease: the effect of programme completion and change in exercise capacity. Nephrol Dial Transplant 2019;34:618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rossi AP, Burris DD, Lucas FL, Crocker GA, Wasserman JC. Effects of a renal rehabilitation exercise program in patients with CKD: a randomized, controlled trial. Clin J Am Soc Nephrol 2014;9:2052–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsai YC, Chen HM, Hsiao SM, Chen CS, Lin MY, Chiu YW, et al. Association of physical activity with cardiovascular and renal outcomes and quality of life in chronic kidney disease. PLoS One 2017;12:e0183642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yokoyama T, Kamijo‐Ikemori A, Sugaya T, Hoshino S, Yasuda T, Kimura K. Urinary excretion of liver type fatty acid binding protein accurately reflects the degree of tubulointerstitial damage. Am J Pathol 2009;174:2096–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hikawa A, Hirano N, et al. Urinary fatty acid‐binding protein as a new clinical marker of the progression of chronic renal disease. J Lab Clin Med 2004;143:23–30. [DOI] [PubMed] [Google Scholar]
- 37. Araki S, Haneda M, Koya D, Sugaya T, Isshiki K, Kume S, et al. Predictive effects of urinary liver‐type fatty acid‐binding protein for deteriorating renal function and incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathy. Diabetes Care 2013;36:1248–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matsui K, Kamijo‐Ikemori A, Sugaya T, Yasuda T, Kimura K. Usefulness of urinary biomarkers in early detection of acute kidney injury after cardiac surgery in adults. Circ J 2012;76:213–220. [DOI] [PubMed] [Google Scholar]
- 39. Uchiyama K, Washida N, Morimoto K, Muraoka K, Nakayama T, Adachi K, et al. Effects of exercise on residual renal function in patients undergoing peritoneal dialysis: a post‐hoc analysis of a randomized controlled trial. Ther Apher Dial 2020, Epub ahead of print;24:668–676. [DOI] [PubMed] [Google Scholar]
- 40. Viana JL, Kosmadakis GC, Watson EL, Bevington A, Feehally J, Bishop NC, et al. Evidence for anti‐inflammatory effects of exercise in CKD. J Am Soc Nephrol 2014;25:2121–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tripepi G, Mallamaci F, Zoccali C. Inflammation markers, adhesion molecules, and all‐cause and cardiovascular mortality in patients with ESRD: searching for the best risk marker by multivariate modeling. J Am Soc Nephrol 2005;16:S83–S88. [DOI] [PubMed] [Google Scholar]
- 42. Afshinnia F, Rajendiran TM, Soni T, Byun J, Wernisch S, Sas KM, et al. Impaired β‐oxidation and altered complex lipid fatty acid partitioning with advancing CKD. J Am Soc Nephrol 2018;29:295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang F, Sun L, Sun Q, Liang L, Gao X, Li R, et al. Associations of plasma amino acid and acylcarnitine profiles with incident reduced glomerular filtration rate. Clin J Am Soc Nephrol 2018;13:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roshanravan B, Robinson‐Cohen C, Patel KV, Ayers E, Littman AJ, de Boer IH, et al. Association between physical performance and all‐cause mortality in CKD. J Am Soc Nephrol 2013;24:822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation 2016;134:e653–e699. [DOI] [PubMed] [Google Scholar]
- 46. JCS Joint Working Group . Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ J 2014;78:2022–2093. [DOI] [PubMed] [Google Scholar]
- 47. Greenwood SA, Koufaki P, Mercer TH, Rush R, O'Connor E, Tuffnell R, et al. Aerobic or resistance training and pulse wave velocity in kidney transplant recipients: a 12‐week pilot randomized controlled trial (the Exercise in Renal Transplant [ExeRT] trial). Am J Kidney Dis 2015;66:689–698. [DOI] [PubMed] [Google Scholar]
- 48. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The postcard (exercise check sheet) to assess patient adherence to prescribed home‐based exercise sessions and the number of steps per day.
Table S2. Comparison of change in exercise capacity between patients with high adherence and low adherence to home‐based aerobic exercise (AE) in the exercise group.
Table S3. Comparison of change in exercise capacity between patients with high adherence and low adherence to home‐based resistance exercise (RE) in the exercise group.
Table S4. Comparison of change in exercise capacity between patients with high adherence and low adherence to both home‐based aerobic exercise (AE) and resistance exercise (RE) in the exercise group.
Table S5. Comparison of change in exercise capacity between patients with high and low number of steps per day in the exercise group.
Data S1. The clinical trial protocol.


