Abstract
Sarcopenia, characterized by loss of skeletal muscle mass, quality, and strength, has become a common hallmark of ageing and many chronic diseases. Diabetes mellitus patients have a higher prevalence of sarcopenia, which greatly aggravates the metabolic disturbance and compromises treatment response. Preclinical and clinical studies have shown differential impacts of anti‐diabetic drugs on skeletal muscle mass, strength, and performance, highlighting the importance of rational therapeutic regimen from the perspective of sarcopenia risk. In this review, we provide an update on the regulation of muscle mass and quality by major anti‐diabetic drugs, focusing primarily on emerging data from clinical studies. We also discuss the underlying mechanisms and clinical implications for optimal selection of anti‐diabetic drugs to reduce the risk of sarcopenia. In view of the lifelong use of anti‐diabetic drugs, we propose that a better understanding of the sarcopenia risk and interventional strategies is worthy of attention in future studies.
Keywords: Sarcopenia, Skeletal muscle, Diabetes, Anti‐diabetic drugs, Lean mass
Introduction
Sarcopenia is a muscle‐wasting syndrome characterized by progressive and generalized degenerative loss of skeletal muscle mass (SMM), quality, and strength occurring during normal ageing. 1 In addition to the suffering from muscle loss, sarcopenia patients are at higher risks for falls, bone fracture, and metabolic diseases, morbidities that greatly reduce their quality of life. 2 Understanding the causal factors and molecular mechanisms for sarcopenia is therefore highly needed for the prevention of this debilitating condition. To date, multiple pathophysiological mechanisms have been defined, which largely involve the change of hormone levels or their response, the disturbance of proteostasis and mitochondrial function under inflammatory stimuli, and abnormal differentiation and proliferation of myo‐satellite cells. 3 , 4 , 5
The increasing prevalence of sarcopenia is also observed in patients with chronic diseases, among which diabetes mellitus has been reported as a common risk factor for the exacerbation of sarcopenia. Older people with diabetes are twice more likely to develop sarcopenia than those without diabetes. 6 , 7 The strength and resistance of skeletal muscle decreased significantly in type 2 diabetes mellitus (T2DM) patients. 8 , 9 Poor glycaemic control in patients with diabetes could further lead to low muscle mass. 10 Because skeletal muscle is the largest tissue in the body that plays a remarkable role in energy and metabolic homeostasis, the loss of muscle mass and function exerts a negative impact on glycaemic control, forming a vicious cycle with the metabolic disturbance (Figure 1). Therefore, improving muscle quality and decreasing the sarcopenia risk are of exceptional importance for the clinical care of diabetic patients.
Figure 1.
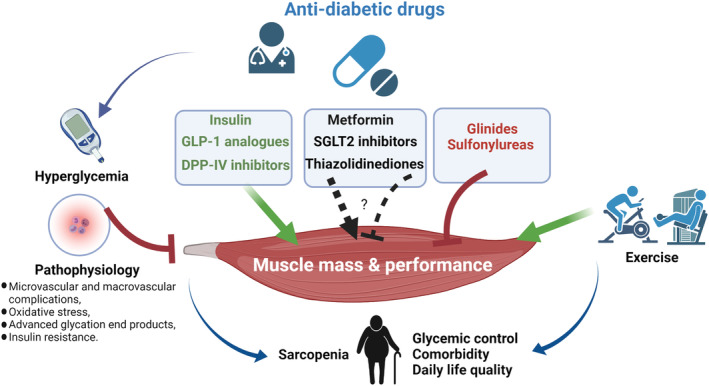
Anti‐diabetic drugs are involved in the regulation of muscle mass and performance in diabetic patients. Pathophysiological factors of diabetic patients, such as metabolic disturbance, chronic inflammation, insulin resistance, and vascular complications, are detrimental factors for normal muscle quality and function. Poor glycaemic control is another risk factor for muscle atrophy and loss. In addition to the regulation of blood glucose, anti‐diabetic drugs exert differential impacts on muscle mass and performance (green: generally protective; black: controversial; red: generally detrimental), which are important for the rationale design of clinical therapies. Moreover, lifestyle factors such as exercise (e.g. resistance training) have interactive impacts with anti‐diabetic drugs on modulating sarcopenia risk. Evidence‐based integration of these approaches are desirable to reduce sarcopenic risk and improve diabetic pathology. DPP‐IV, dipeptidyl peptidase IV; GLP‐1, glucagon‐like peptide‐1; SGLT2, sodium‐glucose co‐transporter 2.
As lifelong treatment with anti‐diabetic drugs is needed for most diabetic patients, the impact of these drugs on skeletal muscle quality is an important consideration in clinical regimen design. Accumulating studies in recent years have uncovered differential impacts of major anti‐diabetic drugs on the skeletal muscle or body composition, which carries huge implications for the rational use of anti‐diabetic drugs in the clinic. In this review, we summarize representative preclinical and clinical studies investigating the impact of anti‐diabetic drugs on muscle function and discuss potential mechanisms. We highlight the clinical implications and open questions for rational drug regimen with the aim to reduce the risk of sarcopenia and improve clinical benefits.
Prevalence and pathophysiological characteristics of sarcopenia in diabetic patients
Diabetic patients are at increased risk of physical disability, including disability of mobility and instrumental activities of daily living. Epidemiological data have reported that T2DM is related to poor muscle strength and function, with an accelerated rate of decline in muscle quality and strength in older individuals of up to 30%. 6 The main manifestation of muscle dysfunction in patients with diabetes is insufficient strength of quadriceps femoris, 11 which leads to walking disorder. 8 The grip strength and endurance of the upper limbs also decrease in diabetic patients, causing disability and much compromise in life quality. 12 Not surprisingly, patients with T2DM are at greater risk of developing sarcopenia, as shown by several studies from different countries. 13 , 14 The mechanisms underlying this link have been reviewed elsewhere. 15
In terms of the features of sarcopenia in the diabetic population, an early study specifically showed that leg lean mass and appendicular SMM were significantly lower in older men with T2DM. 16 Nevertheless, individuals with sarcopenia could pose normal or obese body weight, 17 and sarcopenic obesity is a clinical condition in which lean body mass decreases and fat mass (FM) increases, the prevalence of which reaches 18.7% in T2DM patients. Studies are therefore increasingly paying attention to the change of both FM and lean mass as a measure of body composition in diabetic patients. 18 Low muscle mass was independently associated with all‐cause mortality in patients with T2DM. Sarcopenia is also reported to increase the complications of diabetes such as infection. 19 Therefore, the preservation of SMM is important to protect patients with T2DM from increased mortality risk. 20 Pharmacological intervention in combination with lifestyle changes are effective means in the clinical management of diabetes, suggesting the need to clarify their impacts on sarcopenia.
Effects of anti‐diabetic drugs on the muscle
To gain a systematic understanding of the impact of commonly used anti‐diabetic drugs on sarcopenia, we performed a systematic research of preclinical and clinical studies in MEDLINE, using the searching items as follows: (‘sarcopenia’ OR ‘skeletal muscle mass’ OR ‘muscle mass’ OR ‘lean mass’ OR ‘body composition’ OR ‘muscle strength’) AND (‘antidiabetic drugs’ OR ‘glucose‐lowering drugs’ OR ‘metformin’ OR ‘thiazolidinediones’ OR ‘pioglitazone’ OR ‘rosiglitazone’ OR ‘sulfonylureas’ OR ‘DPP‐4 inhibitors’ OR ‘GLP‐1 receptor agonists’ OR ‘SGLT2 inhibitors’ OR ‘insulin’). Most of the results from representative clinical trials were reported in the past 20 years.
Insulin
Insulin therapy has been a cornerstone in the clinical development of glucose‐lowering agents. 21 In addition to the effect on glycaemic homeostasis, insulin is a potent stimulatory factor for muscle protein synthesis. The mechanisms by which insulin enhances muscle protein anabolism are not yet completely understood, although they largely involve increased initiation of mRNA translation, microvascular recruitment, blood flow, and amino acid delivery to skeletal muscle and reduced protein degradation. 22 Nonetheless, in consideration of insulin resistance, endothelial dysfunction, and diabetic microangiopathy, the positive effect of insulin on muscle mass may be compromised in T2DM individuals.
Indeed, reduction in endogenous insulin secretion is an independent risk factor of sarcopenia in men with T2DM. 23 A previous study in Japanese patients has shown that insulin therapy could attenuate the decline of muscle strength in the lower extremities but not in the upper extremities, 24 supporting the clinical use of insulin to reduce sarcopenia risk in T2DM patients. More recently, a longitudinal study of insulin therapy with changes in muscle parameters showed that insulin preserved muscle mass, but not muscle function as assessed by hand grip strength. 25 However, it is noteworthy that several early clinical studies examining insulin therapy and body composition have shown that insulin‐induced weight gain is attributed to increase in both fat and fat‐free mass (FFM). 26 , 27 , 28 Typically, in patients with T2DM, weight gain largely reflects increase in trunk FM. Therefore, the effect of insulin therapy on skeletal mass may vary depending on the types of diabetes, and it remains to be determined whether the trend to central obesity partly offsets other benefits of insulin therapy in T2DM.
Sulfonylureas and glinides
Sulfonylureas and glinides are insulin secretion stimulating drugs that work via inhibition of ATP‐sensitive K+ (KATP) channel. The KATP channel is an octameric complex composing of inwardly rectifying K+ channels (Kir6.1 and Kir6.2) and sulfonylurea receptor subunits (SUR1, SUR2A, and SUR2B) in a tissue‐dependent manner. 29 In skeletal muscle, the Kir6.2/SUR2A subunits constitute the main KATP channel complex, while other SUR subunits are expressed in different types of muscle. 30 Preclinical data have linked KATP channel blockers to muscle atrophy. For example, Tricarico et al. found that down‐regulation of SUR1/Kir6.2, and possibly other KATP channel subtypes, led to atrophic signalling in slow‐twitch and fast‐twitch skeletal muscles in rats. 31 In vitro experiments showed that down‐regulation of KATP channel induced by an antibody targeting the pyruvate kinase, which is functionally coupled to the Kir6.2 subunit, resulted in skeletal muscle fibre atrophy and cell death. 32 Furthermore, glibenclamide was found to enhance caspase‐3 activity in slow‐twitch muscle and reduce the ratio of protein concentration to muscle weight. 31 Given the intimate relationship between KATP channel and skeletal muscle homeostasis, the suppressive effect of sulfonylureas and glinides on the KATP channel raises the possibility that these drugs may cause adverse effect on SMM and function. A previous database‐searching study investigating atrophy‐related signals associated with the use of the sulfonylureas and glinides reported that, in an 8 month period, muscle atrophy was found in 0.27% of the glibenclamide reports, 12 times the incidence of the total reports for all drugs not related with sulfonylureas or glinides. 33 It is suggested that drug‐induced atrophy can be explained by the KATP channel blockade and the enhancement of the mitochondrial succinic dehydrogenase activity. Recently, a post hoc analysis showed that 24 week treatment of glimepiride induced none significant decrease in fat and bone‐free mass (FBFM) in T2DM patients. 34 These findings suggest that drugs such as glibenclamide and glimepiride should be used with high caution to the patients that have high propensity of sarcopenia.
Metformin
As the first‐line oral medication for T2DM, metformin improves insulin resistance and hyperinsulinaemia through multiple mechanisms, which predominantly involve the activation of AMP‐activated protein kinase (AMPK) signalling pathway. 35 The metabolic benefits of metformin are attributed to actions on multiple tissues typically including the liver, intestine, adipose tissue, as well as the muscle. 36 The glycaemic decrease during metformin treatment is more often accompanied by weight loss, and many studies have consistently associated long‐term metformin use with decreased FM. 37 , 38 , 39 The impact of metformin on lean mass in T2DM patients, however, remains controversial. Musi et al. demonstrated that FFM did not change significantly after metformin treatment for 10 weeks. 40 Similar result was also found in a clinical study of 29 participants with newly diagnosed T2DM over a period of up to 6 months. 39 In contrast, a multicentre longitudinal cohort study recruiting ambulatory men aged over 65 years showed that men treated with metformin had significantly less total or appendicular lean mass loss than those with untreated diabetes or diabetes treated without metformin. 41 The authors speculated that this effect may be explained by up‐regulation of peroxisome proliferator‐activated receptor‐γ coactivator 1α (PGC1α) stimulated by AMPK. In line with this, it was reported that, in non‐diabetic subjects, the administration of metformin for 2 months could increase the lean mass and water content. 38 Together, these findings, despite the inconsistencies on the impact on body composition, highlight the need to consider the impact of skeletal mass and performance when designing metformin‐based therapy for diabetes. Considering the fact that metformin may induce appetite suppression and inhibition of intestinal oligopeptide absorption, 42 the risk of sarcopenia with clinical metformin therapy should be born in mind, especially in women with T2DM and elder patients.
Thiazolidinediones
Thiazolidinediones (TZDs), typically including rosiglitazone and pioglitazone, are frequently prescribed for diabetic patients to enhance insulin sensitivity in the muscle, liver, and adipose tissue via activation of peroxisome proliferator‐activated receptor gamma (PPAR‐γ). TZDs, as insulin sensitizing agents, may play an active role in maintaining SMM and function, as shown by preclinical studies. In particular, studies with skeletal muscle cells revealed that rosiglitazone reduced apoptosis through a PPAR‐γ‐dependent mechanism. 43 It was also reported that rosiglitazone potently inhibits inflammatory mediator‐induced nuclear factor‐kappa B (NF‐κB) transcription, which could attenuate protein degradation in cultured skeletal muscle myotubes. 44 There are mixed data from clinical studies in this aspect. An early multicentre longitudinal study by Lee et al. reported that TZD may reduce the loss of muscle mass in patients with impaired fasting glucose or diabetes. 41 However, the ACT NOW trial has shown that the lean body mass in the legs was significantly lower after 33.6 month pioglitazone treatment in subjects with prediabetes, with significant increase in body weight and no change in overall lean body mass. 45 Indeed, earlier case reports showed acute rhabdomyolysis in T2DM patients after pioglitazone or troglitazone treatment. 46 , 47 Therefore, caution is needed when prescribing TZDs to patient with skeletal muscle problem.
Numerous studies have shown that TZDs reduce muscle lipid content by diminishing fatty acid (FA) uptake, elevating FA oxidation, and increasing FA transport capacity from muscle into subcutaneous adipose tissue. 48 A randomized cross‐over study found that, although there was no change in total body weight or total fat after pioglitazone use in non‐diabetic patients for 4 months, the visceral/subcutaneous adipose tissue ratio was decreased by 16%. 49 This was aligned with another finding that pioglitazone significantly improved the whole‐body aerobic capacity and skeletal muscle FA metabolism in patients with metabolic syndrome. 50 As intermuscular and intramyocellular lipid overload may cause insulin resistance, skeletal muscle wasting, and dysfunction, 51 the lipid‐lowering effect of TZDs is theoretically useful to strengthen muscle content and function. More well‐designed clinical trials are therefore needed to clarify the effects of TZDs on sarcopenia in T2DM patients.
Glucagon‐like peptide‐1 analogues
Glucagon‐like peptide‐1 (GLP‐1) is a naturally occurring incretin hormone secreted from intestinal L‐cells and exerts a number of potentially anti‐hyperglycaemic actions including enhancement of glucose‐dependent insulin secretion, restoration of the glucose sensitivity of pancreatic β‐cells, and suppression of glucagon release. 52 GLP‐1 analogues, such as exenatide and liraglutide, produce many of the glucoregulatory actions observed with endogenous GLP‐1. Several studies have reported that GLP‐1 or GLP‐1 analogues can cause weight loss in animals and humans. 53 , 54 The mechanisms are likely attributed to decreased energy intake (e.g. delayed gastric emptying, gastric secretion, and motility), appetite sensation, increased energy expenditure, and perception of satiety. Weight loss induced by GLP‐1 analogues treatment primarily comes from reductions in FM rather than lean mass, as already demonstrated in some observational studies. 55 , 56 , 57 , 58 Specifically, it was shown that the relative total body FM was reduced by 2.3%, while the relative total body lean mass was increased by 2.3% following a 12 week liraglutide treatment in obese T2DM patients with metformin. 59 Similar results were found in another study. 60 Interestingly, Li and colleagues also found significant correlations between weight loss and increases in both plasma atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) levels, suggesting that liraglutide‐induced change of body composition might be associated with changes in the NP system.
By studying the effect of hypoxia on sarcopenia, it was found that GLP‐1 is the strongest predictor of FFM loss, suggesting that GLP‐1 analogues (such as exendin‐4) can be used to reduce sarcopenia risks. 61 Perna et al. evaluated liraglutide in overweight and obese elderly patients with T2DM. After 24 weeks of treatment, it was found that the reduction of body weight was mainly due to the decrease of FM, and liraglutide could prevent the degradation of muscle protein and maintain the stability of skeletal muscle. 62 However, in another study involving 21 T2DM patients undergoing haemodialysis, addition of dulaglutide to insulin therapy significantly decreased FM and SMM, suggesting the sarcopenia risk of this drug. 63 A recent meta‐analysis of randomized controlled trials also indicated that semaglutide was associated with both weight loss and FFM decrease, prompting the need to understand drug‐specific effects on sarcopenic parameters. 64
Dipeptidyl peptidase IV inhibitors
Dipeptidyl peptidase IV (DPP‐IV) inhibitors such as sitagliptin, vildagliptin, and saxagliptin increase endogenous GLP‐1 to achieve glucose‐lowering effect. While DPP‐IV inhibitors do not increase the weight of T2DM patients, studies have found that it has the potential to improve skeletal muscle injury. As mentioned earlier, movement disorders in people with diabetes mainly occur in the lower limb, especially in the quadriceps femoris. In a retrospective study, Bouchi et al. confirmed the protective effect of DPP‐IV inhibitor on muscle dysfunction in T2DM patients, especially on lower limb muscle, indicating a promise of DPP‐IV inhibitors in clinical intervention of muscle loss. 65 In a clinical study of 80 elderly patients with T2DM, treatment with DPP‐IV inhibitors (vildagliptin 50 mg bid or sitagliptin 100 mg/day or saxagliptin 5 mg/day) showed better sarcopenic parameters (FFM, SMM, muscle strength, and gait speed) compared with sulfonylureas treatment. 66 These clinical benefits are supported by studies from animals. For example, Bianchi et al. showed that PKF275‐055 (a vildagliptin analogue) could partially improve the damage of white fibre muscle induced by streptozotocin in type 1 diabetic rats. 67 Enoki et al. found that teneligliptin had the potential to treat muscle dysfunction in chronic renal disease (CKD) mice, and it was suggested that teneligliptin could not only indirectly play a cellular protective role through GLP‐1 but also directly act on muscle atrophy induced by CKD. 68 In addition, Giannocco et al. found that sitagliptin could up‐regulate the displacement and expression of GLUT4 in myocardium and skeletal muscle of spontaneously hypertensive rats. 69
Interestingly, FDA has warned that high‐dose DPP‐IV inhibitors can induce acute toxicity in monkeys, including the increase in creatine kinase (CK) activity, the pathological elevation of which is commonly observed with muscular dystrophy. In particular, high dose (160 mg/kg) of vildagliptin was used in the experiments with cynomolgus monkey. 70 It was found that some animals had extremely high CK activity (more than 40 000 U/L), and anatomical findings of skeletal muscle necrosis and intramuscular bleeding in extremities. However, the researchers also suggest that the acute toxicity caused by vildagliptin appears to be unique to monkeys, and it is uncertain whether it will occur in human. Therefore, more research is needed to determine the effects of DPP‐IV inhibitors on skeletal muscle in clinical use.
A recent study has found that in metformin‐treated T2DM, a protein preload has the capacity to enhance the efficacy of vildagliptin to slow gastric emptying, increase plasma intact incretins, and reduce postprandial glycaemia, indicating that vildagliptin has the potential to reduce the risk of weight gain in patients with T2DM. 71 However, Flock et al. proposed that vildagliptin could increase lipid storage in adipose tissue and reduce fat in muscle and liver, 72 and a large number of studies have shown that DPP‐IV inhibitors have no significant effect on the body weight of human or rodent. 72 , 73 Future studies are therefore warranted to systematically evaluate the effect of DPP‐IV inhibitors on body composition especially the fat and lean mass.
Sodium‐glucose co‐transporter 2 inhibitors
Sodium‐glucose co‐transporter 2 (SGLT2) inhibitors selectively inhibit SGLT2 to reduce proximal tubular glucose reabsorption, thus increasing urinary sugar excretion to reduce blood glucose concentration. Currently, the representative drugs used include dapagliflozin, canagliflozin, empagliflozin, ipragliflozin, tofogliflozin, and luseogliflozin. 74 SGLT2 inhibitors have a well‐confirmed effect to induce weight loss, and about 90% of weight loss is due to a decrease in FM. 75 , 76 , 77 Some other studies have also shown that the use of SGLT2 inhibitors in T2DM patients reduces FM by two‐thirds and lean mass by one‐third. 78 , 79 Typically, both ipragliflozin and canagliflozin reduce the weight of FM and lean mass. 80 , 81 However, a randomized controlled trial found that dapagliflozin significantly reduced subcutaneous and visceral abdominal fat after 24 weeks of treatment but had no effect on lean tissue. 82 Sasaki et al. found that SMM in T2DM patients treated with luseogliflozin did not change significantly until 36 weeks after treatment, while bone mineral content (BMC) decreased only briefly after 12 weeks, and then remained unchanged. 83
In line with these clinical findings, when diet‐induced obese (DIO) rats were treated with tofogliflozin, there was no significant change in bone mass and lean mass. 84 Interestingly, Naznin et al. found that treatment with canagliflozin for 8 weeks could induce body weight loss in mice, characterized by decreased mass of visceral and subcutaneous fat. 85 Despite these findings, there are still concerns that SGLT2 inhibitors may lead to muscle and bone mass loss, osteoporosis, and decreased body function. 86 Therefore, although most of current studies suggest that SGLT2 inhibitors have no adverse effects on skeletal muscle, 87 , 88 , 89 , 90 , 91 more studies are needed to explore the effects of SGLT2 inhibitors on muscle.
Interaction of anti‐diabetic drugs with lifestyle factors
Lifestyle intervention is commonly recommended as a major anti‐diabetic strategy, prompting the investigation of their interactive impacts with anti‐diabetic drugs on the muscle performance and sarcopenia risk. A randomized controlled trial showed that the insulin sensitizer rosiglitazone has a beneficial effect on resting and blood pressure (BP) response to exercise in men with cardiovascular disease and T2D, especially in those with an exaggerated BP response to exercise. 92 Resistance training is believed as an alternative way to increase total lean mass and improve functional performance. 93 An intriguing observation is that pioglitazone and resistance training exert synergistic effects on muscle power in older overweight and obese women but not in men, 94 supporting a combinatory strategy to decrease sarcopenia risks.
In consistence, in obese elderly, combined aerobic and resistance exercise for 6 months is superior to improve muscle protein synthesis and myocellular quality, thereby maintaining muscle mass during weight‐loss therapy. 95 Therefore, future studies aiming to understand anti‐diabetic drug–lifestyle factor interactions are highly valuable to offer novel approaches to strengthen the mass function of diabetic patients and reduce sarcopenia risk.
Rational use of anti‐diabetic drugs to reduce the risk of sarcopenia
The high prevalence and detrimental impacts of sarcopenia in diabetic patients make it a priority to strengthen the muscle quality and function in clinical treatment and care of this population. In addition to the various pathophysiological factors, the impact of major anti‐diabetic drugs on the muscle is attracting attention, especially given their daily exposure. 96 As summarized earlier, there are still controversies over the effects of metformin, TZDs, and SGLT2 inhibitors on the muscle, necessitating more investigations into the clinical impacts in an individualized manner. Nevertheless, almost all studies have proven that sulfonylureas and glinides have adverse effects on skeletal muscle, suggesting the need to circumvent the use of these drugs in diabetes patients with sarcopenia. In addition, GLP‐1 analogues and DPP‐IV inhibitors seem to be favourable in protecting muscles, not only rarely leading to loss of muscle mass but also promoting muscle contractility and improving muscle injury (Table 1). Lastly, although studies have shown that insulin can also increase muscle mass in patients with T2DM, the weight gain effect of insulin cannot be ignored, which should be carefully considered in the treatment of T2DM patients.
Table 1.
Summary of representative clinical findings on the regulation of sarcopenic parameters by major anti‐diabetic drugs
| Anti‐diabetic drugs | Mechanism of action on muscle | Study design | Effect on muscle mass/performance | Methods/criteria for sarcopenic assessment | Effect on body weight | Effect on fat mass | Refs |
|---|---|---|---|---|---|---|---|
| Insulin | Increasing protein synthesis in muscle | Retrospective observational study | Attenuate the decline of muscle strength in the lower extremities | SMI and GS were used to assess sarcopenia | Weight increase | Increase of both FM and FFM | 24 |
| Population‐based KORA‐Age study | Preserve muscle mass, but not muscle function | SMI, hand grip strength, a timed up and go test | 25 | ||||
| Sulfonylureas and glinides (glibenclamide, repaglinide) | Inhibiting ATP‐sensitive potassium channel and increasing caspase‐3 activity in the skeletal muscle | Database‐searching study | Muscle atrophy was found in 0.27% of glibenclamide reports within 8 months | 33 | |||
| Post hoc analysis | 24 week treatment of glimepiride induced none significant decrease in muscle mass in T2DM patients | FBFM | 34 | ||||
| Metformin | Increasing AMPKα2 activity in the skeletal muscle; inhibiting mTORC1 | Randomized clinical trial in newly diagnosed T2DM patients | Significant decrease in per cent body fat and body fat mass | 39 | |||
| Multicentre longitudinal cohort study | Insulin sensitizers may attenuate muscle loss | Total lean and appendicular lean mass was derived from dual X‐ray absorptiometry scans | 41 | ||||
| Placebo‐controlled clinical trial (850 mg of metformin or a placebo twice a day for 2 months) | Increase in lean weight | Body mass index and waist/hip ratio | 38 | ||||
| Thiazolidinedione (rosiglitazone, pioglitazone) | Activating PPAR‐γ; decreasing muscle lipid content and increasing muscle mass; inducing oxidative stress | A multicentre longitudinal cohort study | Attenuate muscle loss | Total lean and appendicular lean mass was derived from dual X‐ray absorptiometry scans | No change/sight decrease/increase in FM | 41 | |
| Older (65–79 years) non‐diabetic overweight/obese men and women undergoing weight‐loss training | Pioglitazone increased visceral fat loss but did not reduce skeletal muscle loss | Lean mass was measured using dual X‐ray absorptiometry | 93 | ||||
| GLP‐1 analogues (exenatide, liraglutide) | Increasing insulin secretion; decreasing the loss of FFM; decreasing protein degradation | T2DM patients treated with metformin and other oral anti‐diabetic drugs except for thiazolidinediones | Total lean mass was significantly reduced from baseline | Weight loss | Decrease in FM | 59 | |
| Perspective study carried out in overweight and obese T2DM patients (metformin and liraglutide) | Induce an increase in SMI and preserve the muscular tropism | SMI | 62 | ||||
| T2DM patients on haemodialysis, who had been treated with insulin and newly added teneligliptin or dulaglutide | Dulaglutide significantly decreased SMM | SMM | 63 | ||||
| DPP‐IV inhibitor (sitagliptin, vildagliptin, saxagliptin) | Increasing GLP‐1 concentration and decreasing muscle lipid content | Retrospective observational study with 105 T2DM patients | Prevent the progressive loss of muscle mass with ageing in patients with T2DM. | SMI | No significant effect | Unclear | 65 |
| Elderly T2DM patients | Induce better sarcopenic parameters | Fat‐free mass, skeletal muscle mass, and related indices, muscle strength, and gait speed | 66 | ||||
| SGLT2 inhibitor (dapagliflozin, canagliflozin, tofogliflozin, luseogliflozin) | Inhibiting inflammatory cytokines, macrophage aggregation; increasing muscle contractility and muscle mass | Post hoc analysis of a prospective, single‐centre, open‐label, single‐arm study with patients receiving ipragliflozin for 24 weeks | Lean mass was significantly decreased in ipragliflozin group but not the ipragliflozin + metformin group | Weight loss | Decrease in FM | 80 | |
| Single‐arm, single‐centre, open‐label study | A significant reduction in lean mass | 81 |
DPP‐IV, dipeptidyl peptidase IV; FBFM, fat and bone‐free mass; FFM, fat‐free mass; FM, fat mass; GLP‐1, glucagon‐like peptide‐1; GS, grip strength; SGLT2, sodium‐glucose co‐transporter 2; SMI, skeletal muscle index; SMM, skeletal muscle mass; T2DM, type 2 diabetes mellitus.
Despite the advances, controversy and inconsistence still exist in present findings, especially those from the clinical setting. It is clear that the impact of anti‐diabetic drugs on muscle mass and performance is complex and multiple mechanisms are involved. Given the existence of various confounding factors, more well‐designed clinical studies are expected to validate the effect of specific drugs and shed more light into the mechanisms. In clinical trials seeking to answer these questions, the measurement of sarcopenic parameters (FFM, SMM, muscle strength, and gait speed) should be comprehensive and concomitant examination of the change of body fat percentage is highly desirable. 18 To give more conclusive insights, more attention should be paid to methods and indices for sarcopenia evaluation in future clinical trials.
In addition to glycaemic control, clinicians can put muscle quality evaluation in the work of routine diagnosis and treatment, especially for those patients who show clear weight loss after drug intervention. These prospective measures may reduce the risk of sarcopenia as well as provide rich database for future mining. Specifically, the nutritional and metabolic status of diabetic patients are important for managing the risk of sarcopenia. In a multicentre study of 588 Japanese patients with T2DM, it was found that poor glycaemic control was significantly associated with SMM or gait speed in Japanese patients. 97 A recent survey in T2DM patients with or without sarcopenia revealed that sarcopenia patients with T2DM had worse glucose metabolism and nutritional status. 98 Therefore, the importance of nutritional and metabolic control for the prevention of muscle decline and sarcopenia is highlighted. To decrease the risk of sarcopenia, optimization of nutritional therapy is undoubtedly needed in diabetes care. Moreover, exercise training represents an intervention that can attenuate or even reverse the process of muscle wasting by tipping the balance of protein degradation towards protein synthesis. 99 Exercise intervention programmes should therefore be incorporated to nutritional and pharmacological therapy to strengthen muscle mass, strength, and functional capacity of diabetic patients, especially those at higher risk of sarcopenia.
Conclusions
In summary, we review the basic and clinical studies on the effects of anti‐diabetic drugs on skeletal muscle. The differential impacts of anti‐diabetic drugs on muscle quality highlight that the choice of anti‐diabetic drugs should take the risk of sarcopenia into consideration, in addition to comprehensive consideration of glycaemic status and cardiovascular complications. Because diabetic patients are more likely to develop sarcopenia, which has a detrimental effect on glycaemic control and life quality, it is clear that clinicians should take the basic muscle mass of patients as a routine reference index for individual drug administration. However, unambiguous clinical data to guide the rational choice of anti‐diabetic drugs are still inadequate at present, with conflicting results reported from different studies. With more clinical evidence gained in clarifying the link between anti‐diabetic drugs and sarcopenia, the clinical benefits of anti‐diabetic treatment and care are expected to be improved in the near future.
Conflict of interest
The authors declare that they have no competing interests.
Funding
This work was financially supported by the National Natural Science Foundation of China (81503121), the Project for Major New Drug Innovation and Development (2020ZX09201015), and Jiangsu Provincial Medical Youth Talent Project (QNRC2016811).
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 100 The authors are grateful to BioRender for figure sources (https://biorender.com).
Zhang X., Zhao Y., Chen S.‐b., and Shao H. (2021) Anti‐diabetic drugs and sarcopenia: emerging links, mechanistic insights, and clinical implications, Journal of Cachexia, Sarcopenia and Muscle, 12, 1368–1379, 10.1002/jcsm.12838
Contributor Information
Xueli Zhang, Email: xlzhang1225@126.com.
Hua Shao, Email: gycsh@163.com.
References
- 1. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xie WQ, He M, Yu DJ, Wu YX, Wang XH, Lv S, et al. Mouse models of sarcopenia: classification and evaluation. J Cachexia Sarcopenia Muscle 2021;12:538–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wiedmer P, Jung T, Castro JP, Pomatto LCD, Sun PY, Davies KJA, et al. Sarcopenia—molecular mechanisms and open questions. Ageing Res Rev 2021;65:101200. [DOI] [PubMed] [Google Scholar]
- 4. Abbatecola AM, Paolisso G, Fattoretti P, Evans WJ, Fiore V, Dicioccio L, et al. Discovering pathways of sarcopenia in older adults: a role for insulin resistance on mitochondria dysfunction. J Nutr Health Aging 2011;15:890–895. [DOI] [PubMed] [Google Scholar]
- 5. Riuzzi F, Sorci G, Arcuri C, Giambanco I, Bellezza I, Minelli A, et al. Cellular and molecular mechanisms of sarcopenia: the S100B perspective. J Cachexia Sarcopenia Muscle 2018;9:1255–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009;32:1993–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park SW, Goodpaster BH, Strotmeyer ES, Kuller LH, Broudeau R, Kammerer C, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care 2007;30:1507–1512. [DOI] [PubMed] [Google Scholar]
- 8. Volpato S, Bianchi L, Lauretani F, Lauretani F, Bandinelli S, Guralnik JM, et al. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care 2012;35:1672–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes 2006;55:1813–1818. [DOI] [PubMed] [Google Scholar]
- 10. Sugimoto K, Tabara Y, Ikegami H, Takata Y, Kamide K, Ikezoe T, et al. Hyperglycemia in non‐obese patients with type 2 diabetes is associated with low muscle mass: The Multicenter Study for Clarifying Evidence for Sarcopenia in Patients with Diabetes Mellitus. J Diabetes Investig 2019;10:1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalyani RR, Tra Y, Yeh HC, Egan JM, Ferrucci L, Brancati FL. Quadriceps strength, quadriceps power, and gait speed in older U.S. adults with diabetes mellitus: results from the National Health and Nutrition Examination Survey, 1999–2002. J Am Geriatr Soc 2013;61:769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah S, Sonawane P, Nahar P, Buge K, Vaidya S. Are we ignoring diabetic disability: a cross sectional study of diabetic myopathy. Indian J Med Sci 2011;65:186–192. [PubMed] [Google Scholar]
- 13. Wang T, Feng X, Zhou J, Gong H, Xia S, Wei Q, et al. Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre‐sarcopenia in Chinese elderly. Sci Rep 2016;6:38937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010;33:1497–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu CN, Tien KJ. The impact of antidiabetic agents on sarcopenia in type 2 diabetes: a literature review. J Diabetes Res 2020;2020:9368583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leenders M, Verdijk LB, van der Hoeven L, Adam JJ, van Kranenburg J, Nilwik R, et al. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc 2013;14:585–592. [DOI] [PubMed] [Google Scholar]
- 17. Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr 2012;31:583–601. [DOI] [PubMed] [Google Scholar]
- 18. Fukuoka Y, Narita T, Fujita H, Morii T, Sato T, Sassa MH, et al. Importance of physical evaluation using skeletal muscle mass index and body fat percentage to prevent sarcopenia in elderly Japanese diabetes patients. J Diabetes Investig 2019;10:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Weng S, Huang L, Shen X, Zhao F, Yan S. Association of sarcopenia with a higher risk of infection in patients with type 2 diabetes. Diabetes Metab Res Rev 2021;e3478. [DOI] [PubMed] [Google Scholar]
- 20. Miyake H, Kanazawa I, Tanaka KI, Sugimoto T. Low skeletal muscle mass is associated with the risk of all‐cause mortality in patients with type 2 diabetes mellitus. Ther Adv Endocrinol Metab 2019;10:2042018819842971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nauck MA, Wefers J, Meier JJ. Treatment of type 2 diabetes: challenges, hopes, and anticipated successes. Lancet Diabetes Endocrinol 2021;9:525–544. [DOI] [PubMed] [Google Scholar]
- 22. Zheng C, Liu Z. Vascular function, insulin action, and exercise: an intricate interplay. Trends Endocrinol Metab 2015;26:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tanaka K, Kanazawa I, Sugimoto T. Reduction in endogenous insulin secretion is a risk factor of sarcopenia in men with type 2 diabetes mellitus. Calcif Tissue Int 2015;97:385–390. [DOI] [PubMed] [Google Scholar]
- 24. Bouchi R, Fukuda T, Takeuchi T, Nakano Y, Murakami M, Minami I, et al. Insulin treatment attenuates decline of muscle mass in Japanese patients with type 2 diabetes. Calcif Tissue Int 2017;101:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferrari U, Then C, Rottenkolber M, Selte C, Seissler J, Conzade R, et al. Longitudinal association of type 2 diabetes and insulin therapy with muscle parameters in the KORA‐Age study. Acta Diabetol 2020;57:1057–1063. [DOI] [PubMed] [Google Scholar]
- 26. Salle A, Guilloteau G, Ryan M, Bouhanick B, Ritz P. Effect of insulin treatment on the body composition of Type 2 diabetic patients. Diabet Med 2004;21:1298–1303. [DOI] [PubMed] [Google Scholar]
- 27. Sinha A, Formica C, Tsalamandris C, Panagiotopoulos S, Hendrich E, DeLuise M, et al. Effects of insulin on body composition in patients with insulin‐dependent and non‐insulin‐dependent diabetes. Diabet Med 1996;13:40–46. [DOI] [PubMed] [Google Scholar]
- 28. Birkeland KI, Hanssen KF, Urdal P, Berg K, Vaaler S. A long‐term, randomized, comparative study of insulin versus sulfonylurea therapy in type 2 diabetes. J Intern Med 1994;236:305–313. [DOI] [PubMed] [Google Scholar]
- 29. Zhang H, Hanson A, de Almeida TS, Emfinger C, McClenaghan C, Harter T, et al. Complex consequences of Cantu syndrome SUR2 variant R1154Q in genetically modified mice. JCI Insight 2021;6:e145934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tricarico D, Mele A, Lundquist AL, Desai RR, George AL Jr, Conte Camerino D. Hybrid assemblies of ATP‐sensitive K+ channels determine their muscle‐type‐dependent biophysical and pharmacological properties. Proc Natl Acad Sci U S A 2006;103:1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tricarico D, Mele A, Camerino GM, Bottinelli R, Brocca L, Frigeri A, et al. The KATP channel is a molecular sensor of atrophy in skeletal muscle. J Physiol 2010;588:773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mele A, Buttiglione M, Cannone G, Vitiello F, Camerino DC, Tricarico D. Opening/blocking actions of pyruvate kinase antibodies on neuronal and muscular KATP channels. Pharmacol Res 2012;66:401–408. [DOI] [PubMed] [Google Scholar]
- 33. Mele A, Calzolaro S, Cannone G, Cetrone M, Conte D, Tricarico D. Database search of spontaneous reports and pharmacological investigations on the sulfonylureas and glinides‐induced atrophy in skeletal muscle. Pharmacol Res Perspect 2014;2:e00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ishii S, Nagai Y, Kato H, Fukuda H, Tanaka Y. Effect of the dipeptidyl peptidase‐4 inhibitor sitagliptin on muscle mass and the muscle/fat ratio in patients with type 2 diabetes. J Clin Med Res 2020;12:122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vancura A, Bu P, Bhagwat M, Zeng J, Vancurova I. Metformin as an anticancer agent. Trends Pharmacol Sci 2018;39:867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Foretz M, Guigas B, Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol 2019;15:569–589. [DOI] [PubMed] [Google Scholar]
- 37. Aghili R, Malek M, Valojerdi AE, Banazadeh Z, Najafi L, Khamseh ME. Body composition in adults with newly diagnosed type 2 diabetes: effects of metformin. J Diabetes Metab Disord 2014;13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rodriguez‐Moctezuma JR, Robles‐Lopez G, Lopez‐Carmona JM, Gutierrez‐Rosas MJ. Effects of metformin on the body composition in subjects with risk factors for type 2 diabetes. Diabetes Obes Metab 2005;7:189–192. [DOI] [PubMed] [Google Scholar]
- 39. Wang H, Ni Y, Yang S, Li H, Li X, Feng B. The effects of gliclazide, metformin, and acarbose on body composition in patients with newly diagnosed type 2 diabetes mellitus. Curr Ther Res Clin Exp 2013;75:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, et al. Metformin increases AMP‐activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 2002;51:2074–2081. [DOI] [PubMed] [Google Scholar]
- 41. Lee CG, Boyko EJ, Barrett‐Connor E, Miljkovic I, Hoffman AR, Everson‐Rose SA, et al. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care 2011;34:2381–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hindlet P, Barraud C, Boschat L, Farinotti R, Bado A, Buyse M. Rosiglitazone and metformin have opposite effects on intestinal absorption of oligopeptides via the proton‐dependent PepT1 transporter. Mol Pharmacol 2012;81:319–327. [DOI] [PubMed] [Google Scholar]
- 43. Meshkani R, Sadeghi A, Taheripak G, Zarghooni M, Gerayesh‐Nejad S, Bakhtiyari S. Rosiglitazone, a PPARγ agonist, ameliorates palmitate‐induced insulin resistance and apoptosis in skeletal muscle cells. Cell Biochem Funct 2014;32:683–691. [DOI] [PubMed] [Google Scholar]
- 44. Remels AH, Langen RC, Gosker HR, Russell AP, Spaapen F, Voncken JW, et al. PPARγ inhibits NF‐κB‐dependent transcriptional activation in skeletal muscle. Am J Physiol Endocrinol Metab 2009;297:E174–E183. [DOI] [PubMed] [Google Scholar]
- 45. Bray GA, Smith SR, Banerji MA, Tripathy D, Clement SC, Buchanan TA, et al. Effect of pioglitazone on body composition and bone density in subjects with prediabetes in the ACT NOW trial. Diabetes Obes Metab 2013;15:931–937. [DOI] [PubMed] [Google Scholar]
- 46. Slim R, Ben Salem C, Zamy M, Biour M. Pioglitazone‐induced acute rhabdomyolysis. Diabetes Care 2009;32:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yokoyama M, Izumiya Y, Yoshizawa M, Usuda R. Acute rhabdomyolysis associated with troglitazone. Diabetes Care 2000;23:421–422. [DOI] [PubMed] [Google Scholar]
- 48. Hu S, Yao J, Howe AA, Menke BM, Sivitz WI, Spector AA, et al. Peroxisome proliferator‐activated receptor gamma decouples fatty acid uptake from lipid inhibition of insulin signaling in skeletal muscle. Mol Endocrinol 2012;26:977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zanchi A, Tappy L, Le KA, Bortolotti M, Theumann N, Halabi G, et al. Pioglitazone improves fat distribution, the adipokine profile and hepatic insulin sensitivity in non‐diabetic end‐stage renal disease subjects on maintenance dialysis: a randomized cross‐over pilot study. PLoS ONE 2014;9:e109134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yokota T, Kinugawa S, Hirabayashi K, Suga T, Takada S, Omokawa M, et al. Pioglitazone improves whole‐body aerobic capacity and skeletal muscle energy metabolism in patients with metabolic syndrome. J Diabetes Investig 2017;8:535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tamilarasan KP, Temmel H, Das SK, Al Zoughbi W, Schauer S, Vesely PW, et al. Skeletal muscle damage and impaired regeneration due to LPL‐mediated lipotoxicity. Cell Death Dis 2012;3:e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol 2019;15:226–237. [DOI] [PubMed] [Google Scholar]
- 53. Wang G, Wu P, Qiu Y, Dong X, Wang Y, Chi Y, et al. Effect of beinaglutide treatment on weight loss in Chinese patients with type 2 diabetes mellitus and overweight/obesity. Arch Endocrinol Metab 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP‐1 receptor agonists: established and emerging indications. Lancet 2021;398:262–276. [DOI] [PubMed] [Google Scholar]
- 55. Bradley DP, Kulstad R, Racine N, Shenker Y, Meredith M, Schoeller DA. Alterations in energy balance following exenatide administration. Appl Physiol Nutr Metab 2012;37:893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, et al. Effect of 12‐week dulaglutide therapy in Japanese patients with biopsy‐proven non‐alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res 2017;47:1206–1211. [DOI] [PubMed] [Google Scholar]
- 57. Rondanelli M, Perna S, Astrone P, Grugnetti A, Solerte SB, Guido D. Twenty‐four‐week effects of liraglutide on body composition, adherence to appetite, and lipid profile in overweight and obese patients with type 2 diabetes mellitus. Patient Prefer Adherence 2016;10:407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hong JY, Park KY, Kim BJ, Hwang WM, Kim DH, Lim DM. Effects of short‐term exenatide treatment on regional fat distribution, glycated hemoglobin levels, and aortic pulse wave velocity of obese type 2 diabetes mellitus patients. Endocrinol Metab (Seoul) 2016;31:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li CJ, Yu Q, Yu P, Yu TL, Zhang QM, Lu S, et al. Changes in liraglutide‐induced body composition are related to modifications in plasma cardiac natriuretic peptides levels in obese type 2 diabetic patients. Cardiovasc Diabetol 2014;13:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Diaz‐Soto G, de Luis DA, Conde‐Vicente R, Izaola‐Jauregui O, Ramos C, Romero E. Beneficial effects of liraglutide on adipocytokines, insulin sensitivity parameters and cardiovascular risk biomarkers in patients with Type 2 diabetes: a prospective study. Diabetes Res Clin Pract 2014;104:92–96. [DOI] [PubMed] [Google Scholar]
- 61. Wandrag L, Siervo M, Riley HL, Khosravi M, Fernandez BO, Leckstrom CA, et al. Does hypoxia play a role in the development of sarcopenia in humans? Mechanistic insights from the Caudwell Xtreme Everest Expedition. Redox Biol 2017;13:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Perna S, Guido D, Bologna C, Solerte SB, Guerriero F, Isu A, et al. Liraglutide and obesity in elderly: efficacy in fat loss and safety in order to prevent sarcopenia. A perspective case series study. Aging Clin Exp Res 2016;28:1251–1257. [DOI] [PubMed] [Google Scholar]
- 63. Yajima T, Yajima K, Takahashi H, Yasuda K. The effect of dulaglutide on body composition in type 2 diabetes mellitus patients on hemodialysis. J Diabetes Complications 2018;32:759–763. [DOI] [PubMed] [Google Scholar]
- 64. Ida S, Kaneko R, Imataka K, Okubo K, Shirakura Y, Azuma K, et al. Effects of antidiabetic drugs on muscle mass in type 2 diabetes mellitus. Curr Diabetes Rev 2021;17:293–303. [DOI] [PubMed] [Google Scholar]
- 65. Bouchi R, Fukuda T, Takeuchi T, Nakano Y, Murakami M, Minami I, et al. Dipeptidyl peptidase 4 inhibitors attenuates the decline of skeletal muscle mass in patients with type 2 diabetes. Diabetes Metab Res Rev 2018;34. [DOI] [PubMed] [Google Scholar]
- 66. Rizzo MR, Barbieri M, Fava I, Desiderio M, Coppola C, Marfella R, et al. Sarcopenia in elderly diabetic patients: role of dipeptidyl peptidase 4 inhibitors. J Am Med Dir Assoc 2016;17:896–901. [DOI] [PubMed] [Google Scholar]
- 67. Bianchi R, Cervellini I, Porretta‐Serapiglia C, Oggioni N, Burkey B, Ghezzi P, et al. Beneficial effects of PKF275‐055, a novel, selective, orally bioavailable, long‐acting dipeptidyl peptidase IV inhibitor in streptozotocin‐induced diabetic peripheral neuropathy. J Pharmacol Exp Ther 2012;340:64–72. [DOI] [PubMed] [Google Scholar]
- 68. Enoki Y, Watanabe H, Arake R, Fujimura R, Ishiodori K, Imafuku T, et al. Potential therapeutic interventions for chronic kidney disease‐associated sarcopenia via indoxyl sulfate‐induced mitochondrial dysfunction. J Cachexia Sarcopenia Muscle 2017;8:735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Giannocco G, Oliveira KC, Crajoinas RO, Venturini G, Salles TA, Fonseca‐Alaniz MH, et al. Dipeptidyl peptidase IV inhibition upregulates GLUT4 translocation and expression in heart and skeletal muscle of spontaneously hypertensive rats. Eur J Pharmacol 2013;698:74–86. [DOI] [PubMed] [Google Scholar]
- 70. Hoffmann P, Martin L, Keselica M, Gunson D, Skuba E, Lapadula D, et al. Acute toxicity of vildagliptin. Toxicol Pathol 2017;45:76–83. [DOI] [PubMed] [Google Scholar]
- 71. Wu T, Little TJ, Bound MJ, Borg M, Zhang X, Deacon CF, et al. A protein preload enhances the glucose‐lowering efficacy of vildagliptin in type 2 diabetes. Diabetes Care 2016;39:511–517. [DOI] [PubMed] [Google Scholar]
- 72. Flock G, Baggio LL, Longuet C, Drucker DJ. Incretin receptors for glucagon‐like peptide 1 and glucose‐dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes 2007;56:3006–3013. [DOI] [PubMed] [Google Scholar]
- 73. Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Liraglutide, a long‐acting glucagon‐like peptide‐1 analog, reduces body weight and food intake in obese candy‐fed rats, whereas a dipeptidyl peptidase‐IV inhibitor, vildagliptin, does not. Diabetes 2007;56:8–15. [DOI] [PubMed] [Google Scholar]
- 74. Scheen AJ. Sodium‐glucose cotransporter type 2 inhibitors for the treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2020;16:556–577. [DOI] [PubMed] [Google Scholar]
- 75. Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC. Comparison of empagliflozin and glimepiride as add‐on to metformin in patients with type 2 diabetes: a 104‐week randomised, active‐controlled, double‐blind, phase 3 trial. Lancet Diabetes Endocrinol 2014;2:691–700. [DOI] [PubMed] [Google Scholar]
- 76. Bolinder J, Ljunggren O, Johansson L, Wilding J, Langkilde AM, Sjostrom CD, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 2014;16:159–169. [DOI] [PubMed] [Google Scholar]
- 77. Blonde L, Stenlof K, Fung A, Xie J, Canovatchel W, Meininger G. Effects of canagliflozin on body weight and body composition in patients with type 2 diabetes over 104 weeks. Postgrad Med 2016;128:371–380. [DOI] [PubMed] [Google Scholar]
- 78. Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012;97:1020–1031. [DOI] [PubMed] [Google Scholar]
- 79. Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA‐SU): 52 week results from a randomised, double‐blind, phase 3 non‐inferiority trial. Lancet 2013;382:941–950. [DOI] [PubMed] [Google Scholar]
- 80. Nagai Y, Fukuda H, Kawanabe S, Nakagawa T, Ohta A, Tanaka Y. Differing effect of the sodium‐glucose cotransporter 2 inhibitor ipragliflozin on the decrease of fat mass vs. lean mass in patients with or without metformin therapy. J Clin Med Res 2019;11:297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Koike Y, Shirabe SI, Maeda H, Yoshimoto A, Arai K, Kumakura A, et al. Effect of canagliflozin on the overall clinical state including insulin resistance in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2019;149:140–146. [DOI] [PubMed] [Google Scholar]
- 82. Lundkvist P, Sjostrom CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once‐daily and exenatide once‐weekly dual therapy: a 24‐week randomized, placebo‐controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab 2017;19:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sasaki T, Sugawara M, Fukuda M. Sodium‐glucose cotransporter 2 inhibitor‐induced changes in body composition and simultaneous changes in metabolic profile: 52‐week prospective LIGHT (Luseogliflozin: the Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J Diabetes Investig 2019;10:108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Suzuki M, Takeda M, Kito A, Fukazawa M, Yata T, Yamamoto M, et al. Tofogliflozin, a sodium/glucose cotransporter 2 inhibitor, attenuates body weight gain and fat accumulation in diabetic and obese animal models. Nutr Diabetes 2014;4:e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Naznin F, Sakoda H, Okada T, Tsubouchi H, Waise TM, Arakawa K, et al. Canagliflozin, a sodium glucose cotransporter 2 inhibitor, attenuates obesity‐induced inflammation in the nodose ganglion, hypothalamus, and skeletal muscle of mice. Eur J Pharmacol 2017;794:37–44. [DOI] [PubMed] [Google Scholar]
- 86. Jackuliak P, Kuzma M, Payer J. Effect of antidiabetic treatment on bone. Physiol Res 2019;68:S107–S120. [DOI] [PubMed] [Google Scholar]
- 87. Schork A, Saynisch J, Vosseler A, Jaghutriz BA, Heyne N, Peter A, et al. Effect of SGLT2 inhibitors on body composition, fluid status and renin‐angiotensin‐aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovasc Diabetol 2019;18:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tobita H, Sato S, Miyake T, Ishihara S, Kinoshita Y. Effects of dapagliflozin on body composition and liver tests in patients with nonalcoholic steatohepatitis associated with type 2 diabetes mellitus: a prospective, open‐label, uncontrolled study. Curr Ther Res Clin Exp 2017;87:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yamamoto C, Miyoshi H, Ono K, Sugawara H, Kameda R, Ichiyama M, et al. Ipragliflozin effectively reduced visceral fat in Japanese patients with type 2 diabetes under adequate diet therapy. Endocr J 2016;63:589–596. [DOI] [PubMed] [Google Scholar]
- 90. Seino Y, Yabe D, Sasaki T, Fukatsu A, Imazeki H, Ochiai H, et al. Sodium‐glucose cotransporter‐2 inhibitor luseogliflozin added to glucagon‐like peptide 1 receptor agonist liraglutide improves glycemic control with bodyweight and fat mass reductions in Japanese patients with type 2 diabetes: a 52‐week, open‐label, single‐arm study. J Diabetes Investig 2018;9:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yokono M, Takasu T, Hayashizaki Y, Mitsuoka K, Kihara R, Muramatsu Y, et al. SGLT2 selective inhibitor ipragliflozin reduces body fat mass by increasing fatty acid oxidation in high‐fat diet‐induced obese rats. Eur J Pharmacol 2014;727:66–74. [DOI] [PubMed] [Google Scholar]
- 92. Piche ME, Laberge AS, Brassard P, Arsenault BJ, Bertrand OF , Despres JP, et al. Rosiglitazone lowers resting and blood pressure response to exercise in men with type 2 diabetes: a 1‐year randomized study. Diabetes Obes Metab 2018;20:1740–1750. [DOI] [PubMed] [Google Scholar]
- 93. Shea MK, Nicklas BJ, Marsh AP, Houston DK, Miller GD, Isom S, et al. The effect of pioglitazone and resistance training on body composition in older men and women undergoing hypocaloric weight loss. Obesity (Silver Spring) 2011;19:1636–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Marsh AP, Shea MK, Vance Locke RM, Miller ME, Isom S, Miller GD, et al. Resistance training and pioglitazone lead to improvements in muscle power during voluntary weight loss in older adults. J Gerontol A Biol Sci Med Sci 2013;68:828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Colleluori G, Aguirre L, Phadnis U, Fowler K, Armamento‐Villareal R, Sun Z, et al. Aerobic plus resistance exercise in obese older adults improves muscle protein synthesis and preserves myocellular quality despite weight loss. Cell Metab 2019;30:261–273, e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Massimino E, Izzo A, Riccardi G, Della Pepa G. The impact of glucose‐lowering drugs on sarcopenia in type 2 diabetes: current evidence and underlying mechanisms. Cell 2021;10:1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sugimoto K, Ikegami H, Takata Y, Katsuya T, Fukuda M, Akasaka H, et al. Glycemic control and insulin improve muscle mass and gait speed in type 2 diabetes: the MUSCLES‐DM study. J Am Med Dir Assoc 2021;22:834–838, e831. [DOI] [PubMed] [Google Scholar]
- 98. He Q, Wang X, Yang C, Zhuang X, Yue Y, Jing H, et al. Metabolic and nutritional characteristics in middle‐aged and elderly sarcopenia patients with type 2 diabetes. J Diabetes Res 2020;2020:6973469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bowen TS, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle 2015;6:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


