Abstract
Background
Fasting is attracting an increasing interest as a potential strategy for managing diseases, including metabolic disorders and complementary cancer therapy. Despite concerns of clinicians regarding protein catabolism and muscle loss, evidence‐based clinical data in response to long‐term fasting in healthy humans are scarce. The objective of this study was to measure clinical constants, metabolic, and muscular response in healthy men during and after a 10 day fast combined with a physical activity programme.
Methods
Sixteen men (44 ± 14 years; 26.2 ± 0.9 kg/m2) fasted with a supplement of 200–250 kcal/day and up to 3 h daily low‐intensity physical activity according to the peer‐reviewed Buchinger Wilhelmi protocol. Changes in body weight (BW) and composition, basal metabolic rate (BMR), physical activity, muscle strength and function, protein utilization, inflammatory, and metabolic status were assessed during the 10 day fast, the 4 days of food reintroduction, and at 3 month follow‐up.
Results
The 10 day fast decreased BW by 7% (−5.9 ± 0.2 kg, P < 0.001) and BMR by 12% (P < 0.01). Fat mass and lean soft tissues (LST) accounted for about 40% and 60% of weight loss, respectively, −2.3 ± 0.18 kg and −3.53 ± 0.13 kg, P < 0.001. LST loss was explained by the reduction in extracellular water (44%), muscle and liver glycogen and associated water (14%), and metabolic active lean tissue (42%). Plasma 3‐methyl‐histidine increased until Day 5 of fasting and then decreased, suggesting that protein sparing might follow early proteolysis. Daily steps count increased by 60% (P < 0.001) during the fasting period. Strength was maintained in non‐weight‐bearing muscles and increased in weight‐bearing muscles (+33%, P < 0.001). Glycaemia, insulinemia, blood lipids, and blood pressure dropped during the fast (P < 0.05 for all), while non‐esterified fatty acids and urinary beta‐hydroxybutyrate increased (P < 0.01 for both). After a transient reduction, inflammatory cytokines returned to baseline at Day 10 of fasting, and LST were still lower than baseline values (−2.3% and −3.2%, respectively; P < 0.05 for both).
Conclusions
A 10 day fast appears safe in healthy humans. Protein loss occurs in early fast but decreases as ketogenesis increases. Fasting combined with physical activity does not negatively impact muscle function. Future studies will need to confirm these first findings.
Keywords: Long‐term fasting, Body composition, Muscle function, Apelin, Energy metabolism, Lipids
Introduction
Until early humans were able to store food, they were subjected to seasonal cycles of food abundance and scarcity associated with recurrent periods of fasting, which likely selected adaptive mechanisms for survival. Fasting for religious purposes has been practiced for centuries. 1 Fasting periods from 2 to 21 days or more, so called long‐term fasting, have also been used for decades for preventive and therapeutic purposes in specialized centres. 1 At the beginning of the 20th century, Benedict et al 2 observed a normal‐weight subject fasting safely up to 31 days and documented among other a drop in basal metabolic rate (BMR). 3 Cahill later observed that, after a quick depletion of liver glycogen, free fatty acids are mobilized from adipose tissue and partly transformed into ketone bodies, whereas glucose is produced by the liver using body proteins as substrate. 4 In the 1960s, total fasting was introduced in hospitals to treat morbid obesity during periods lasting from 6 up to 382 days. 5 Despite reports of successful treatments, one person died 6 after 30 weeks of fasting, probably of protein depletion. This led to the development of liquid protein diets, which in turn caused the death of 44 persons. 7 Finally, the composition of protein supplemented formula was regulated, and the duration of the procedure reduced to 3–4 weeks.
Fasting regimens have regained major attention and are relayed by the media for prevention and treatment of metabolic, chronic inflammatory, neurologic, and oncological diseases. 8 Clinical studies have been reporting beneficial effects of intermittent fasting and chronic calorie restriction (CR). 9 For example, 2 years of moderate CR (25%) decreases multiple cardiometabolic risk factors in non‐obese adults. 10 Other studies of shorter duration reported beneficial health effects of alternate day fasting and time‐restricted eating. 9 , 11 However, valid concerns still exist in the medical community about possible protein catabolism and muscle loss.
Although a recent observational study reported no harmful effects during fasting programmes lasting up to 21 days, 12 this was a cross‐sectional observational study, and the fundamental physiological responses of humans to long‐term fasting clearly need more evidence‐based clinical data. Here, we investigated the physiological responses during a 10 day fast, subsequent food reintroduction (RF) and at 3 months follow‐up (Fup) in 16 healthy men. Body composition, muscle strength, energy metabolism, lipid profile, hepatic markers, and inflammatory status were measured. Self‐perceived satiety and well‐being were also assessed. To do so, we used a peer‐reviewed protocol 13 consisting in a fasting programme combined with physical activity and mindfulness programme to improve fitness and reduce stress.
Materials and methods
Participants
Sixteen healthy males above 18 years and of body mass index below 32 kg/m2 were recruited (Supporting Information, Figure S1). Exclusion criteria included any history of smoking, cachexia, anorexia nervosa, advanced kidney, liver, or cerebrovascular insufficiencies as described before. 13 The study was approved by the medical council Baden‐Württemberg (application number: F‐2016‐090) and registered at the German Clinical Trials Register (DRKS‐ID: DRKS00011165). A signed informed written consent was obtained from all participants.
Study design
This longitudinal non‐controlled intervention was conducted between November and December 2016 at the Buchinger Wilhelmi Clinic in Überlingen, Germany. Following a screening visit, eligible participants were enrolled in a 10 day fast followed by a 4 day period of progressive RF. Data were collected at baseline, during the 10 day fast, the 4 day RF, and after 3 months of Fup. At Fup, subjects arrived the evening prior to a morning test session performed in the same conditions as prior to the fast.
Fasting protocol
All subjects remained under daily supervision of nurses and physicians. Following their admission in the clinic, participants received a standardized vegetarian dinner. On the next day (i.e. baseline period), participants were given a 600 kcal vegetarian diet divided in three meals. To initiate the 10 day fasting period, the intestinal tract was emptied with the intake of a laxative (20–40 g of NaSO4 in 500 mL of water). During fasting, all subjects received a portion of 20 g of honey each morning and had to drink 2–3 L of water or non‐caloric herbal tea. An organic freshly squeezed fruit juice (250 mL) was served at noon and a vegetable broth (250 mL) in the evening. Total daily calorie intake was 200–250 kcal/day (Table S1). During the fasting period, an enema was applied every other day in order to remove intestinal remnants and desquamated mucosal cells. Food was reintroduced during 3 days, progressively increasing from 800 to 1600 kcal/day.
Fasting was combined with a low‐to‐moderate intensity programme of physical activity led by certified trainers. The daily programme consisted in a 1 h gymnastic group class including whole‐body stretching and yoga, and outdoor walks for 30 min in the morning and 90 min in the afternoon.
Clinical outcomes
After waking up and voiding, body weight, waist circumference, blood pressure, and heart rate were measured by a nurse.
Body composition, markers of protein and muscle utilization
Body composition at baseline and Fup was assessed by whole bio‐impedance spectroscopy (BIS, SFB7 system, Impedimed, USA). Measurements were performed in the morning when the subject woke up but before getting up (no fluid shift). We used the dual tab electrodes provided by Impedimed, which guarantees equidistance between the electrodes. These adhesive electrodes were placed on the left side hand and foot, as instructed by Impedimed. A pen mark was left on the hand and foot to avoid analytical variations due to electrodes positioning. We carried out three series of measurements composed of 10 measurements with 1 s interval. The impedance meter was calibrated daily with the calibration resistor provided by Impedimed. We used the manufacturer's default values to derive body composition (i.e. body density of 1.049, hydration constant of 0.732, and body proportion of 4.30). Raw data were exported to a computer by the manufacturer software. Lean soft tissues (LST) were calculated using the default setting proposed by the manufacturer (Cole–Cole model between 10 to 500 kHz), and the three values were averaged. Fat mass (FM) was calculated as body mass minus LST.
During the fasting period of this study, the sum of changes in lean metabolically active tissues, extracellular water, and glycogen plus associated water was referred as the loss in LST. We assumed that bone mineral density remained constant. Because of fasting‐specific changes in hydration of lean tissues, measurement of LST by BIS during fasting could not be accurately assessed. Because protein intake was virtually zero, we rather estimated the change in LST by using the 10 day cumulated loss of nitrogen excretion in 24 h urine measured during fasting, extracellular water loss, and muscle and liver glycogen loss and its associated water loss. Urinary nitrogen was measured at the core facility of the INRA – UMR Pegase (Saint‐Gilles, France) by an automated Dumas technique system (sensitivity < 20 ppm, precision < 0.05%). Loss of metabolically active tissues was estimated on the basis that it contains 33 g N/kg. 14 Glycogen loss and its associated water loss in liver (90% depletion) and skeletal muscle (40% depletion) were estimated based on previous publications that used liver percutaneous and muscle needle biopsy techniques to measure changes in glycogen stores in liver and skeletal muscle from fasting humans. 15 , 16 Muscle mass was estimated as 57% of LST measured by BIS at baseline, 17 and we assumed that 1% of total muscle mass was composed of glycogen at baseline. 16 Extracellular water loss (essentially plasma volume and water loss linked to enemas) was accounted for by the BIS measurement. FM loss was calculated as the difference between body weight loss and LST loss.
To assess the validity of our calculations and assumptions, we compared total energy expenditure (TEE) during fasting calculated by two independent methods: one based on energy intake and body composition changes 18 as explained above and one based on 3D accelerometry data. 19 From energy intake and changes in body composition, TEE during fasting was estimated at 2731 ± 185 kcal/day. Estimation of TEE from 3D‐accelerometry was 2675 ± 64 kcal/day with a spearman correlation coefficient between the two estimates of 0.53 (P = 0.06) and a non‐significant mean difference of −56 ± 165 kcal/day (P = 0.74). These results suggest that our calculations and assumptions to assess body composition changes during fasting were appropriate.
Plasma 3‐methyl histidine, a marker of muscle breakdown when protein intake is zero, was measured using the human 3MH ELISA kit by Finetest (%CV intra‐assay <8%, %CV inter‐assay 10%, sensitivity 3.75 nmol/mL, Wuhan, China).
Physical performance and muscle biomechanical properties
Submaximal work rate was determined with an incremental exercise tolerance test on a cycle ergometer (Sana Couch 250 L, Ergosana, Bitz, Germany). Because measurements were performed early in the mornings on Day 5 of fasting and Day 4 of RF, they in fact correspond to four full days of fasting (F04) and three full days of RF (RF3), respectively. Following a 1 min warm‐up, the test was initiated at 75 W, which was increased by 25 W every minute until subjective exhaustion level was attained. This happened sometimes under or above the estimated maximum theoretical aerobic capacity [determined as (2.8 * BW) minus 10% per decade over 30 years old]. 20 Time needed for the volunteers to go up 40 stairs at their normal pace was also used as an indicator of fitness.
Muscle physical and biomechanical properties were further measured using the non‐invasive Myoton system (Myoton AS, Tallinn, Estonia). Tonus (intrinsic tension at the cellular level; high tonus is associated with fatigue), stiffness (biomechanical resistance to contraction; high stiffness leads to an inefficient economy of movement), and elasticity (the capacity to recover its initial shape after contraction) were measured. This was performed on brachioradialis (forearm) and rectus femoris (thigh) muscles in the early mornings at baseline, Days 5 (F04) and 10 (F09) of fasting, Day 4 of RF (RF3), and at Fup. Following these measurements, grip strength was assessed using a Camry 200 LBS/90 KGS digital hand dynamometer (Camry Scale, City Industry, USA). Maximal contraction was recorded on the first measurements of 10 reps separated by 1 min interval. The 10 reps were used to measure fatigability. We used a TKK5002 Back A dynamometer device (Takei Corp. Japan) in a position that minimizes the contribution of the arms and back and maximizes the contribution of the legs to the strength measurements. Subjects stood on a platform connected to a chain hold by hands through an ergonomic holder connected to the dynamometer (0 to 300 kg, sensitivity 1 kg). The chain was set so that the legs were one‐third bend with straight arms aligned with the back. Subjects performed three successive maximal isometric pull up with only the use of the legs. The three values were averaged.
Physical activity
Time spent sedentary and physically active was determined using a tri‐axial accelerometer (ActiGraph GT3X+; ActiGraph, Pensacola, USA). Participants were instructed to wear the accelerometer at their right hip at all time, except for water‐activities, for 1 week prior entering the clinic, throughout the fasting protocol, and for 1 week at Fup. After identification of sleep and non‐wear time, an automatic activity‐recognition algorithm 21 was used to determine time spent in different postures and activities and physical activity energy expenditure per minute. 19 Sedentary behaviour was defined as the time spent awake in lying, reclining, or sitting postures. Cutpoints of 1.5–3 METs and >3METs were used for light intensity activity and moderate‐to‐very vigorous activity, respectively. Valid data comprised at least 3 days with a minimum of 10 h wear‐time during waking time.
Basal metabolic rate and index of substrate use
Basal metabolic rate was determined at baseline and on the mornings of Days 5 (F04) and 10 (F09) of fasting, Day 4 of RF (RF3), and at Fup. Subjects were awakened at 7 a.m., and BMR was measured for 30 min using a mask connected to a Fitmate open indirect calorimetry system (Cosmed, Rome, Italy) measuring O2 consumption while subjects were resting, awake, and at thermo‐neutrality. BMR was adjusted on LST.
Because the Fitmate can measure O2 consumption only, respiratory quotient could not be calculated. To overcome this limitation and obtain an index of shift in substrate use during fasting, we measured 13CO2 enrichment in breath samples. Lipids being naturally depleted in 13C, any shift in the mix being oxidized towards lipids is indicated by a decrease in 13CO2 in breath. Subjects blew in an air‐sealed tube at baseline and every other day of fasting. Breath 13C enrichment was measured by a Gas‐Bench system (Thermo, Germany) interfaced to a delta V IRMS (Thermo, Germany). Data are presented as delta per mill enrichment vs. the international PDB standard (IEAE, Vienna, Austria).
Clinical blood parameters
Fasting blood samples were collected in the morning after BMR measurements at baseline, on Days 2 (F01), 5 (F04), and 10 (F09) of fasting; Days 2 of RF (RF1) and 4 (RF3); and at Fup. Liver enzymes (serum GGT, ASAT, and ALAT), kidney parameters (urea and creatinine), lipid parameters (total cholesterol, triglycerides, HDL‐C, LDL‐C, and LDL‐C/HDL‐C ratio), electrolytes, glucose, CRP, and TSH were measured using classical clinical lab assays, as previously described. 12 Inflammatory parameters (IFNg, TNFa, IL6, IL10, and MCP1) and metabolic hormones (insulin, PP, PYY, GLP1, leptin, ghrelin, IGF‐1, and IGF‐BP1) were measured using the multiplex technology by Merck (KGaA, Darmstadt, Germany) at the Cytometry and Immunobiology core facility of the Cochin Institute (INSERM U1016) in Paris, France. Information on the %CV intra & inter‐assay, accuracy, and sensitivity can be found on the Merckmillipore website (www.merckmillipore.com). Blood non‐esterified fatty acids (NEFA) was measured by the Wako Diagnostics assay (%CV intra‐assay 0.61%, %CV inter‐assay 4.91%, and accuracy 100%, France). Blood myostatin was measured by the Eurobio Scientific assay (%CV intra‐assay 10.4%, %CV inter‐assay 12%, Sensitivity 0.37 ng/mL, France). Blood apelin was measured by the Sigma Aldrich assay (%CV intra & intra‐assay 10%, sensitivity 5.84 ng/mL, France). Free urinary cortisol was assessed using the Diametra test (%CV intra‐assay 6.5%, %CV inter‐assay 7.2%, sensitivity 2.96 ng/ml, Darmstadt, Germany) in 24 h urine samples collected at the same time points than urinary nitrogen. In the same sample, ketone bodies were measured by the Cayman assay (%CV intra‐assay 3.7%, %CV inter‐assay 3.0%, Michigan, USA).
Well‐being and perceived hunger profile
Participants self‐reported daily their physical and emotional well‐being on numeric rating scales from 0 (very bad) to 10 (excellent). The German version of the Well‐being Index (WHO‐5) was used to measure positive psychological well‐being. 22 The WHO‐5 refers to the feelings over the last 2 weeks. In this survey, we shortened the period to the last 24 h. Self‐perceived hunger and desired food consumption were assessed by using a 100‐mm visual analogue scale. 23
Data and statistical analyses
Data analyses were carried out using SAS 9.4 (SAS Institute, Inc., Cary, North Carolina). We used linear mixed‐effects models accounting for repeated measurements among subjects to test changes over time of the different outcomes. Based on the Akaike's information criterion, a heterogeneous compound symmetry or a spatial power variance–covariance structure was selected for the estimation of the within‐subject errors. The effects of fasting and of RF were tested using a joint F‐test. Pre‐specified post‐hoc differences between Fup and baseline were further tested with a Bonferroni adjustment to account for multiple comparisons. As the data were mainly exploratory, no additional adjustment was made for multiple outcomes testing. Significance was set up at 0.05 for time effect. Data are presented as lsmean ± SE.
Results
Study participants
Baseline clinical and metabolic subject's characteristics are shown in Table 1. One subject did not come back at Fup, and data for 15 instead of 16 subjects are therefore presented. For body composition, data of only 12 subjects are shown at Fup due to a defect of the SFB7 instrument on the last day of the study. Otherwise, all participants completed the study without deviating from the protocol and no serious adverse event was reported. Participants took part in the daily physical activity sessions, which increased daily steps number by 60% (+5514 ± 884 steps/day) and shifted time spent standing and sitting towards time spent walking (Table S2) during the fasting period compared with both baseline and Fup. Time spent in moderate‐intensity physical activities increased at the expense of time spent in light‐intensity activities.
Table 1.
Baseline data of the subjects
| Variable | Baseline characteristics (n = 16) |
|---|---|
| Age (years) | 44 ± 14 |
| Weight (kg) | 85.7 ± 2.5 |
| BMI (kg/m2) | 26.2 ± 0.9 |
| Waist circumference (cm) | 92.7 ± 2.1 |
| FM (%) | 17.5 ± 1.77 |
| LST (kg) | 70.8 ± 1.9 |
| Fasting glucose (mM) | 4.7 ± 0.1 |
| Fasting insulin (pg/mL) | 84.6 ± 15.3 |
| HbA1C (%) | 5.23 ± 0.27 |
| HOMA index | 2.04 ± 1.3 |
| NEFA (μM) | 472 ± 43.7 |
| TG (mg/dL) | 122.3 ± 14.4 |
| HDL (mg/dL) | 53.8 ± 3.5 |
| LDL (mg/dL) | 150.4 ± 10.2 |
| LDL/HDL | 3.0 ± 0.3 |
Data are means ± SE.
BMI, body mass index; FM, fat mass; HDL, high‐density lipoprotein; HOMA, homeostatic model assessment; LDL, low‐density lipoprotein; LST, lean soft tissues; NEFA, non‐esterified fatty acids; TG, triglycerides.
Changes in anthropometric parameters
Body mass was reduced by 5.9 ± 0.2 kg after the 10 day fast. This effect was maintained during RF. Three months after the end of fasting, body weight had increased but was still significantly lower than before fasting (Figure 1A). Waist circumference was reduced by 4.4 ± 0.4 cm during fasting. It did not increase during the RF period but had returned to baseline 3 months later (Figure 1B). Both systolic and diastolic blood pressure decreased during fasting but had returned to baseline at Fup (Figure 1C–D).
Figure 1.
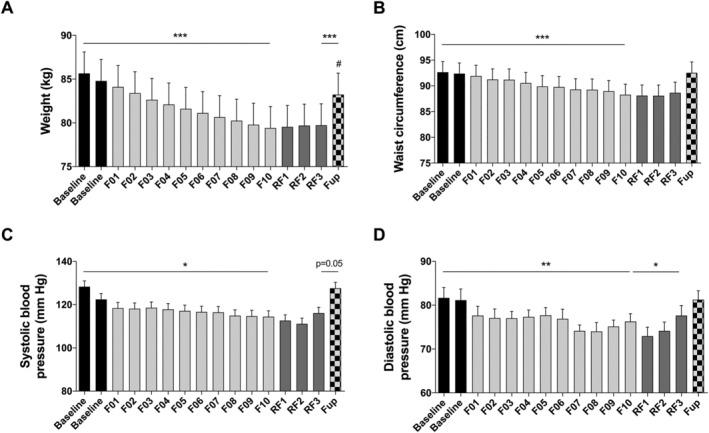
Changes in body weight (A), waist circumference (B), systolic (C), and diastolic (D) blood pressures before and during the 10 day fasting (n = 16) and after 3 months of Fup (n = 15). Data are lsmeans ± SE. F, fasting days; Fup, follow‐up; RF, food reintroduction days. *P < 0.05, **P < 0.01, ***P < 0.001 as indicated by the lines for the whole effect of fasting, the whole effect of RF or the effect of Fup vs. the end of fasting. #P < 0.05 vs. baseline during Fup.
Changes in body composition and protein oxidation
Weight loss during fasting was explained by changes in both FM and LST (−2.34 ± 0.18 kg and −3.53 ± 0.13 kg, respectively, after 10 days of fasting Figure 2A).
Figure 2.
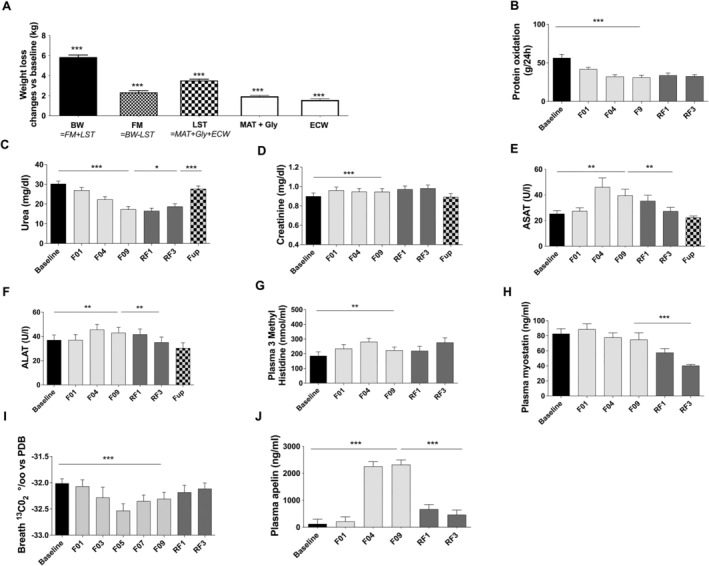
Changes in body weight (BW), fat mass (FM), and lean soft tissues (LST) at F09 vs. baseline. LST change is estimated as the loss of metabolically active tissues (MAT), glycogen (Gly), and extracellular water (ECW) (A). Measurement of protein oxidation (B), urea excretion (C), creatinine excretion (D), plasma ASAT (E), plasma ALAT (F), plasma 3 methyl histidine (G), myostatin (H), 13CO2 of expired air (I) and plasma apelin (J) before and during the 10 day fasting (n = 16) and after 3 months of Fup (n = 15). Data are lsmeans ± SE. ALAT, alanine amino transferase; ASAT, aspartate amino transferase; F, fasting days; Fup, follow‐up; RF, food reintroduction days. *P < 0.05, **P < 0.01, ***P < 0.001 as indicated by the lines for the whole effect of fasting, the whole effect of RF or the effect of Fup vs. the end of fasting. ###P < 0.01 vs. baseline during Fup.
FM loss accounted for 40 ± 2.1% of changes in total body weight. LST loss accounted for 60.7 ± 2.1% of body weight loss, explained for 44% (−1.6 ± 0.1 kg) by extracellular water loss (which includes loss of faeces by enemas), for 14% (0.50 ± 0.01 kg) by early glycogen loss and its associated water, and for 42% by metabolic active (liver, kidneys, heart, intestine, muscles, etc.) tissues (1.5 ± 0.1 kg representing 25% of weight loss). However, during the 10 days of fasting, protein oxidation (total N excretion) was not constant. It dropped by 41 ± 7% on F05 and then remained stable until the end of the 10 day fast (Figure 2B). A gradual 41 ± 5% decrease in plasma urea (Figure 2C) was concomitantly noted. These effects were maintained during RF but baseline values were re‐established at Fup. Creatinine, ASAT, and ALAT increased during fasting but returned to baseline value during the RF and Fup periods (Figure 2D–F). 3‐Methyl‐histidine, a marker of skeletal muscle breakdown, transiently increased during the first 4 days of fasting (Figure 2G) and returned thereafter to baseline values. Myostatin, which inhibits muscle mass growth, remained stable during fasting but dropped significantly during RF (Figure 2H). These data suggest a transient increase in proteolysis and muscle utilization during early fasting, followed by a protein sparing phase. A decrease in 13CO2 enrichment in breath was concomitantly observed, indicating a greater reliance on fat as fuel (Figure 2I). This was accompanied by a drastic increase in plasma apelin concentration at F04, known to stimulate muscle fat oxidation, that returned to baseline values during RF (Figure 2J).
Changes in basal metabolic rate
Basal metabolic rate decreased gradually by 12% during fasting (Figure 3A ). BMR values during RF and Fup remained lower than baseline values, but the difference was not significant. BMR drop during fasting remained significant after adjustment for changes in LST (Figure 3B). By comparing baseline and Fup data (n = 12), we observed that body mass and LST, but not FM, remained lower at Fup (Figure 3C). BMR and LST‐adjusted BMR were lower (Figure 3D) compared with baseline, but this did not reach significance. Plasma TSH concentration remained unchanged (Figure 3E ).
Figure 3.
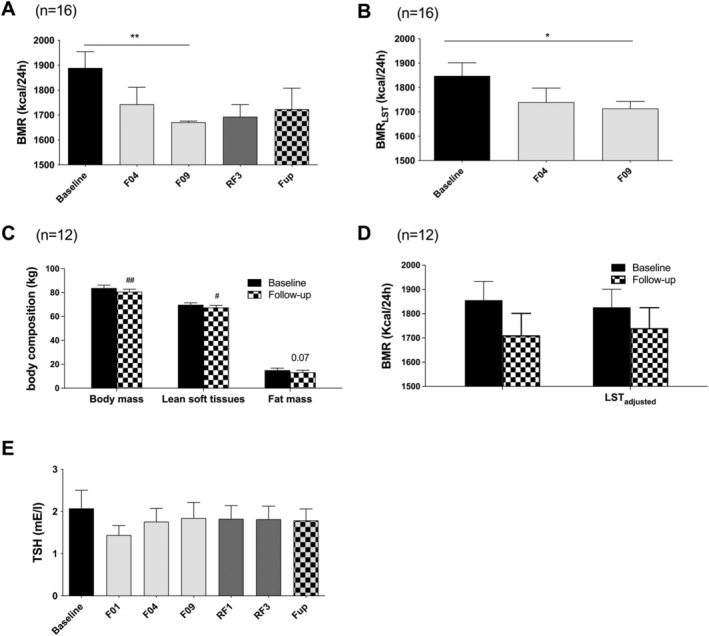
Changes in raw BMR (A) and BMR adjusted on LST calculated from changes in urinary nitrogen excretion (B) on 16 subjects during the whole fasting and 15 subjects at Fup. The figure also reports the changes in body composition assessed by impedancemetry at baseline and Fup (n = 12 subjects, C) used to adjust BMR at Fup (D). TSH concentrations are presented on panel (E). Data are lsmeans ± SE. BMR, basal metabolic rate; F, fasting days; Fup, follow‐up; LST, lean soft tissues; RF, food reintroduction days, TSH, thyroid stimulated hormone. *P < 0.05, **P < 0.01 as indicated by the lines for the whole effect of fasting, the whole effect of RF or the effect of Fup vs. the end of fasting. #P < 0.05, ##P < 0.01 vs. baseline.
Changes in fitness, skeletal muscle function, and biomechanical properties
We assessed changes in muscle function and physical properties. Maximal grip strength and fatigability remained unchanged during fasting (Figure 4A–C). Conversely leg strength was increased at F04 and remained so even at Fup (Figure 4D). Submaximal work power‐to‐weight ratio developed on a cycle ergometer increased after 4 days of fasting and was still higher during RF compared with baseline (Figure 4E). These improvements were not associated to any changes in muscle physical properties (stiffness, tonus, and elasticity), neither on the forearm muscle involved in grip strength nor on the thigh muscle (Figure 4F–K). The time to climb 40 steps was not affected by fasting but tended to be reduced during RF. This reduction reached significance at Fup (Figure 4L).
Figure 4.
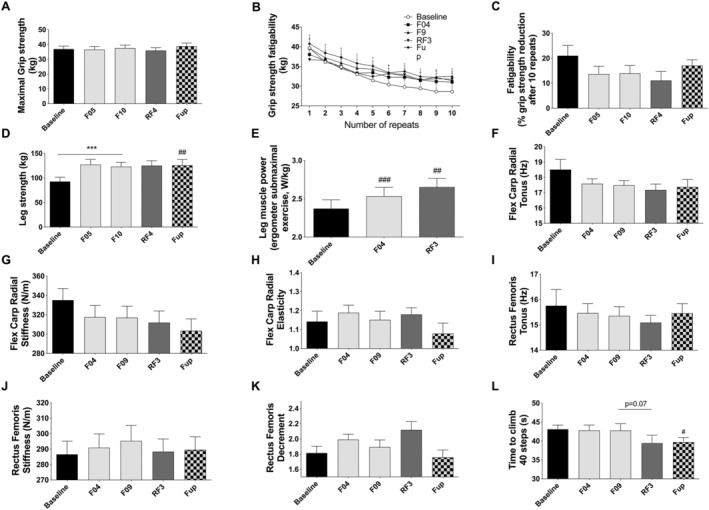
Changes in maximal grip strength (A) and fatigability expressed in kg after 10 repeats (B) or as a percent of strength reduction after 10 repeats (C), leg strength (D), leg power during a submaximal exercise on an ergometer (E), skeletal muscle flexor carpi radialis tonus (F), stiffness (G) and decrement (H), rectus femoris tonus (I), stiffness (J) and decrement (K), and time to climb 40 steps (L) before and during the 10 day fasting (n = 16) and after 3 months of Fup (n = 15). Data are lsmeans ± SE. F, fasting days; Fup, follow‐up; RF, food reintroduction days. ***P < 0.001 as indicated by the lines for the whole effect of fasting, the whole effect of RF or the effect of Fup vs. the end of fasting. #P < 0.05, ##P < 0.01, ###P < 0.001 vs. baseline.
Changes in plasma lipids, glucose, and insulin concentration
Total cholesterol, high‐density and low‐density lipoproteins and triglycerides were all reduced during fasting and RF (Figure 5A–E). As expected, plasma NEFA (Figure 5F) and urine beta‐hydroxybutyrate (Figure 5G) increased during fasting (2.5‐folds and 6‐folds, respectively) and dropped at RF. While NEFA rose linearly during the 10 days of fasting, beta‐hydroxybutyrate plateaued at F04. Glycaemia decreased by 15% during fasting but remained within physiological range (4.0 ± 0.1 mM at F10) and immediately returned to basal value during RF (Figure 5H). Plasma insulin dropped rapidly by 59% during fasting to reach 34.6 ± 6.2 pg/mL at F10. It progressively increased during RF (Figure 5I). GGT was reduced during the fasting and RF periods (Figure 5J). As expected, plasma prealbumin was reduced during fasting but still within normal range at F09 (Figure 5K). All these metabolic variables went back to baseline values at Fup.
Figure 5.
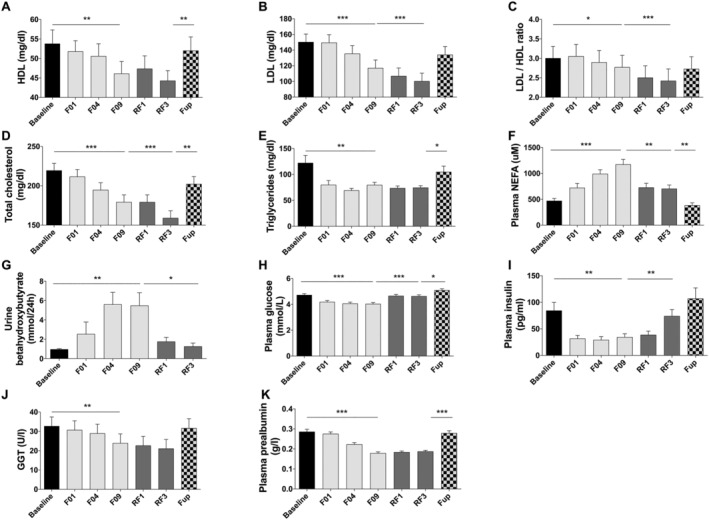
Changes in HDL (A), LDL (B), LDL/HDL ratio (C), total cholesterol (D), triglycerides (E), plasma NEFA (F), urine hydroxybutyrate (G), plasma glucose (H), plasma insulin (I), GGT (J), and plasma prealbumin (K) before and during the 10 day fasting (n = 16) and after 3 months of Fup (n = 15). Data are lsmeans ± SE. F, fasting days, Fup, follow‐up; GGT, gamma‐glutamyl transferase; HDL, high density lipoprotein; LDL, low density lipoprotein; NEFA, non‐esterified fatty acids; RF, food reintroduction days. *P < 0.05, **P < 0.01, ***P < 0.001 as indicated by the lines for the whole effect of fasting, the whole effect of RF or the effect of Fup vs. the end of fasting.
Changes in inflammatory markers
CRP remained unchanged during the intervention (Figure 6A). Pro‐inflammatory cytokines (IFNg, TNFa, IL6, and IL10) showed very consistent changes. All dropped on F01 and then gradually came back to baseline over the course of the 10 day fasting. Only IL10 remained lower during fasting, but significance was not reached. All these markers increased significantly during RF but returned to baseline values at Fup (Figure 6B–E). A trend to decrease was observed for MCP1 that did not reach significance (Figure 6F).
Figure 6.
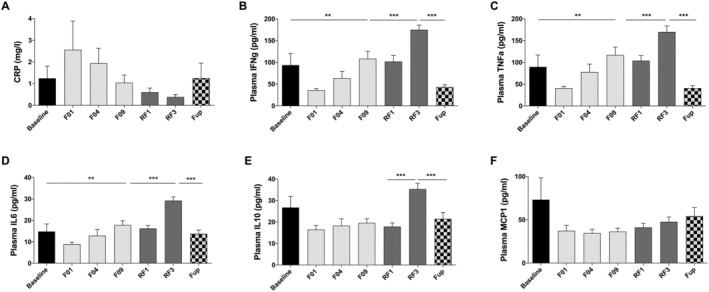
Changes in CRP (A), IFNg (B), TNFa (C), IL6 (D), IL10 (E), and MCP1 (F) before and during the 10 day fasting (n = 16) and after 3 months of Fup (n = 15). Data are lsmeans ± SE. CRP, C‐reactive protein; F, fasting days; Fup, follow‐up; IL, interleukin; INFg, interferon gamma; MCP, monocyte chemoattractant protein; RF, food reintroduction days. **P < 0.01, ***P < 0.001 as indicated by the lines for the whole effect of fasting, the whole effect of RF or the effect of Fup vs. the end of fasting.
Changes in fluid and electrolytes
While total body water, intracellular fluids, and blood volume remained stable throughout fasting, transient increases in haematocrit and haemoglobin concentration were observed after 1 day of fasting. They decreased back to basal value on F04 (Figure S2A–E). All these parameters returned to baseline values 3 months later, except for intracellular fluids that were slightly but significantly lower, in line with the reduced LST. A slight decrease in plasma sodium concentration, within a normal physiological range, was also noticed during fasting. Baseline values were re‐established with RF (Figure S2F).
Changes in satiety and appetite‐related regulatory hormones
Circulating levels of orexigenic (i.e. ghrelin) or anorexigenic (i.e. PP, PYY, and GLP‐1) hormones remained unchanged during the study (Figure 7A–D). Plasma leptin concentration was reduced during fasting and RF, and this lower level was still maintained at Fup (Figure 7E). IGF1 was reduced with fasting while IGF‐binding protein was increased and returned to basal values during the RF period (Figure 7F–G). Perceived hunger was either unchanged or transiently reduced during fasting and re‐increased to baseline during RF (Figure 7H–I).
Figure 7.
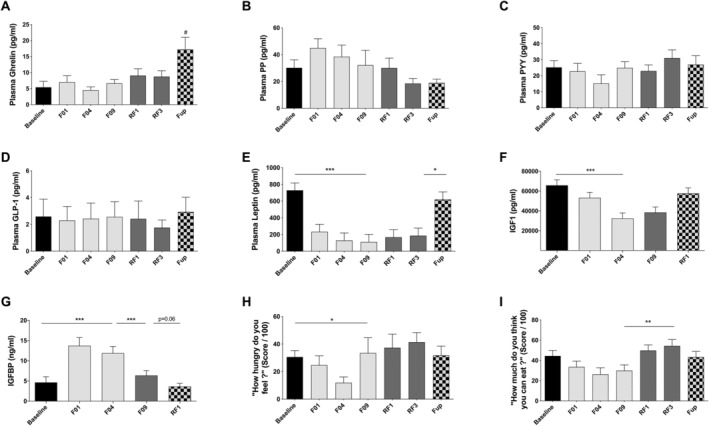
Changes in plasma ghrelin (A), plasma PP (B), PYY (C), GLP‐1 (D), leptin (E), IGF1 (F), IGFBP1 (G), and two question on satiety derived from visual scale: ‘how hungry do you feel’ (H) and ‘how much do you think you can eat’ (I) before and during the 10 day fasting (n = 16) and after 3 months of Fup (n = 15). Data are lsmeans ± SE. F, fasting days; Fup, follow‐up; GLP‐1, glucagon like peptide 1; IGF1, insulin growth factor 1; IGFBP1, insulin growth factor binding protein; PYY, polypeptide YY; RF, food reintroduction days. *P < 0.05, **P < 0.01, ***P < 0.001 as indicated by the lines for the whole effect of fasting, the whole effect of RF or the effect of Fup vs. the end of fasting. #P < 0.05 vs. baseline during Fup.
Changes in general well‐being
No significant changes in general well‐being scales were observed (Figure S3A–C). Urinary cortisol concentration, a marker of stress, was 36% lower on the last day of fasting compared with baseline and remained low during RF (Figure S3D).
Discussion
Many studies have been focusing on the effects of CR and intermittent fasting, but only few recent ones considered long‐term fasting in non‐obese healthy humans. 1 , 9 By studying clinical physiology during a 10 day fasting protocol combined with a physical activity programme in healthy men we provided new data to the field.
One major concern regarding long‐term fasting in humans is related to the decrease in LST. Lignot and Le Maho demonstrated in a number of animal species that three phases occur in response to fasting. 24 During the first 24 to 48 h, glycogen stores are depleted, and body cells rely upon fat and proteins (around 70–30% respectively) (Phase 1). The fuel mix then shifts towards lipids, and a protein sparing mechanism is set up (Phase 2) that can last from weeks to months in humans according to initial FM. When fat stores reach 10% of body weight, protein oxidation increases massively (Phase 3).
In most fasting studies, humans stay in Phase 2 although in extremely malnourished or anorectic patients Phase 3 can be reached. 25 As suggested by CR studies, one could expect muscle function to be preserved if fasting adaptations were selected during evolution. Our results support this hypothesis. First, we observed a major shift towards lipid and ketone metabolism. 24 Second, apelin, known to increase lipid oxidation at the muscle level, 26 raised 18‐fold. Third, nitrogen excretion dropped and remained stable during fasting, limiting protein breakdown to 25% of total weight loss.
We showed that weight loss during fasting was rather due to the loss of LST than FM (60% vs. 40%, respectively). An important part of LST loss was due to extracellular water loss and glycogen depletion in liver and muscle during fasting. Our results are in agreement with a previous 20 day fasting study without enemas in subjects with obesity that resulted in a loss of 50% LST and 50% FM. 27
We cannot specifically ascertain how much muscle mass breakdown explained the LST decrease. However, plasma 3‐methylhistidine, a good marker of muscle mass utilization when protein intake is zero, as well as other proteolysis markers such as ASAT and creatinine, suggest that there was only a transient increase in skeletal muscle proteolysis in the first days of fasting. Animal studies demonstrated that protein sparing occurs during prolonged fasting in skeletal muscles. 24 Rat models showed that protein utilization during CR is mainly due to a loss in metabolically active organs such as kidneys, liver, and intestine. 28 In contrast to the observed increase in BMR during short‐term fasting, which can be expected to contribute to increased protein utilization, 29 BMR decreased during the present study, up to 12% after 10 days. This drop remained significant even after adjustment for LST loss.
Muscle function was maintained or improved, suggesting that changes in protein breakdown did not negatively impact muscle function in this context. Importantly, because our fasting protocol included daily physical activity, we are unable to evaluate the impact of fasting per se on muscle strength and performance. One study showed that 8 weeks of intermittent fasting does not attenuate the muscle adaptations to a resistance exercise training programme. 30 In our study, we did not notice changes in muscle physical properties, suggesting that the biochemical properties of skeletal muscles were not affected. We rather observed an improvement in muscle function, as shown by an increase in leg muscle power and strength while grip strength remained unchanged. The specific improvement observed in weight‐bearing muscles is likely due to the adherence to the daily physical activity programme that led to a 60% increase in daily steps and a shift from time spent sitting/standing to time spent walking.
While hunger decreased during fasting, presumably in relation with the fully compensated acidosis linked to ketogenesis, 31 an increase was measured on the last fasting day, likely as a psychogenic anticipation of RF. The increase in the orexigenic hormone ghrelin at Fup may correspond to a compensatory mechanism to restore the initial body weight after weight loss because LST and BMR had remained lower compared with baseline. However, body weight of the subjects remained under baseline values at Fup.
We observed that fasting reduced cardiometabolic risk factors. Blood pressure, plasma glucose, insulin, and lipids decreased, even if baseline values were still in the normal range and returned to baseline at Fup. These results support the beneficial metabolic adaptions to long‐term fasting initially reported in a previous study. 12 , 32 Similar observations were made in healthy patients and patients with overweight or type 2 diabetes, 33 and after CR, alternate day fasting and intermittent fasting. 9 , 11 , 34
Low‐grade inflammation is a key feature of cardiometabolic diseases including insulin resistance. 35 We observed that all inflammatory markers decreased, even if MCP‐1 showed only a trend, and went slowly back to baseline on the last day of fasting. Previous studies showed that fasting reduces inflammation and oxidative stress, 36 , 37 alternate day fasting reduces TNFa in overweight asthma patients, 38 and CR decreases other pro‐inflammatory cytokines. 39
By contrast, CRP tended to increase in the first days of fasting, concomitantly with a transient rise in ALAT and ASAT that could be associated with apoptosis occurring in the liver during fasting. It is well‐known that liver and other organs transiently shrink in volume during fasting. 40 Interestingly, pro‐inflammatory cytokines increased on Day 4 of RF. This is in line with a study describing the pro‐inflammatory status during RF following fasting. 41
The physical and emotional well‐being of participants was not negatively affected by the fasting protocol, and even tended to improve, which corroborates a recent study in 1422 subjects who followed the same fasting protocol. 12 In contrast with other studies showing an increase in cortisol after 2 to 10 fasting days, 42 we documented a decrease in cortisol levels.
Limitations include the small sample size and the absence of a parallel control group that would have allowed to delineate the specific role of the fasting protocol from the potential influence of the environment and holistic care of the participants on the measured outcomes. Changes in energy substrates would have also been better characterized by using stable isotopes and indirect calorimetry. Determining the contribution of the different metabolically active organs to LST loss is required to fully understand the balance between energy and protein sparing. Yet changes in different body compartments were estimated using assumptions from previously published studies and were not directly measured. Nevertheless, this first set of results lays the foundation for future mechanistic and clinical studies. Finally, future studies should include women and a longer Fup period.
In conclusion, these data show that a 10 day fasting programme combined with physical activity according to a peer‐reviewed protocol is safe. As observed in animals, humans similarly trigger protein sparing mechanisms limiting the initial loss in skeletal muscle proteins. Fasting combined with physical activity did not affect the effects of training on muscle performance. However, given the popularity of fasting seen in public media, we obviously urge extreme caution when applying fasting in underweight or older individuals, as well as in patients with eating disorders, patients who are suffering from higher severity or end‐stage chronic diseases and those taking medication that may be impacted by fasting, or patients undergoing chemotherapy who are more vulnerable to sarcopenia.
Conflict of interest
Claire Laurens, Anthony Damiot, Isabelle Chery, Anne‐Laure Le Maho, Alexandre Zahariev, Yvon Le Maho, Audrey Bergouignan, Guillemette Gauquelin‐Koch, Chantal Simon, and Stephane Blanc declare that they have no conflict of interest. Françoise Wilhelmi de Toledo is managing director of Amplius GmbH, a company that coordinates the scientific documentation for the Buchinger Wilhelmi Clinics and is co‐worker of the Buchinger Wilhelmi Clinic. Franziska Grundler is co‐worker at the Buchinger Wilhelmi Clinic.
Funding
This study was funded by the CNRS and Amplius GmbH, Überlingen, Germany. We further received a grant by the Laboratoire Therascience (Monaco, France) to fund hormonal and metabolic assays. Claire Laurens is a fellow of the French Space Agency. No additional external funding received for this study.
Authors' contributions
Françoise Wilhelmi de Toledo, Chantal Simon, Stephane Blanc, and Yvon Le Maho conceived and conceptualized the study. Franziska Grundler and Isabelle Chery were responsible for the project administration and coordinated study conduction and data collection. All authors actively participated to the collection of data. Chantal Simon realized the statistical analyses of the data. Claire Laurens and Franziska Grundler wrote the draft manuscript. All authors contributed to the revision and editing of the final manuscript.
Supporting information
Table S1. Nutrient composition of the diet during the 10‐day fast.
Figure S1. Study Flow Chart.
Figure S2. Changes in total body water (A), intracellular water (B), blood volume (C), hematocrit (D), hemoglobin (E) and sodium (F) before and during the 10‐day fast (n = 16) and after 3‐months of Fup (n = 15). F: fasting days, RF: food reintroduction days, Fup: Follow‐up. *p < 0.05, **p < 0.01, ***p < 0.001 as indicated by the lines for the whole effect of fasting, the whole effect of food RF or the effect of Fup versus the end of the fast. ##p < 0.01 versus baseline during Fup.
Figure S3. Changes in physical well‐being (A), emotional well‐being (B), WHO well‐being index (C) and free urinary cortisol (D), before and during the 10‐day fast (n = 16) and after 3‐months of Fup (n = 15). VAS: visual analog scale, WHO: world health organization, F: fasting days, RF: food reintroduction days, Fup: Follow‐up. **p < 0.01 as indicated by the lines for the whole effect of fasting.
Acknowledgements
We thank the study subjects for their participation. We thank Charlotte Cuerc and Anne Mialon for performing blood metabolites at the hospital of Lyon and Stefan Drinda for performing the ergometer tests. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 43
Laurens C., Grundler F., Damiot A., Chery I., Le Maho A.‐L., Zahariev A., Le Maho Y., Bergouignan A., Gauquelin‐Koch G., Simon C., Blanc S., and Wilhelmi de Toledo F. (2021) Is muscle and protein loss relevant in long‐term fasting in healthy men? A prospective trial on physiological adaptations, Journal of Cachexia, Sarcopenia and Muscle, 12, 1690–1703, 10.1002/jcsm.12766
Claire Laurens and Franziska Grundler equally contributed to this work.
Clinical Trial Registry: DRKS00011165.
References
- 1. Wilhelmi de Toledo F, Grundler F, Sirtori CR, Ruscica M. Unravelling the health effects of fasting: a long road from obesity treatment to healthy life span increase and improved cognition. Ann Med 2020;52:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benedict FG, Goodall HW, Ash JE, Langfeld HS, Kendall AI, Higgins HL. A Study of Prolonged Fasting. Carnegie institution of Washington; 1915. [Google Scholar]
- 3. Ditschuneit H, Faulhaber J, Beil I, Pfeiffer E. Metabolic changes in zero‐diet. Internist 1970;11:176. [PubMed] [Google Scholar]
- 4. Cahill GF Jr. Starvation in man. N Engl J Med 1970;282:668–675. [DOI] [PubMed] [Google Scholar]
- 5. Stewart W, Fleming LW. Features of a successful therapeutic fast of 382 days' duration. Postgrad Med J 1973;49:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garnett E, Barnard D, Ford J, Goodbody R, Woodehouse M. Gross fragmentation of cardiac myofibrils after therapeutic starvation for obesity. Lancet 1969;293:914–916. [DOI] [PubMed] [Google Scholar]
- 7. Frank A, Graham C, Frank S. Fatalities on the liquid‐protein diet: an analysis of possible causes. Int J Obes (Lond) 1981;5:243–248. [PubMed] [Google Scholar]
- 8. Johnstone A. Fasting for weight loss: an effective strategy or latest dieting trend? Int J Obes (Lond) 2015;39:727–733. [DOI] [PubMed] [Google Scholar]
- 9. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med 2019;381:2541–2551. [DOI] [PubMed] [Google Scholar]
- 10. Das SK, Roberts SB, Bhapkar MV, Villareal DT, Fontana L, Martin CK, et al. Body‐composition changes in the Comprehensive Assessment of Long‐term Effects of Reducing Intake of Energy (CALERIE)‐2 study: a 2‐y randomized controlled trial of calorie restriction in nonobese humans. Am J Clin Nutr 2017;105:913–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, et al. Effect of alternate‐day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med 2017;177:930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilhelmi de Toledo F, Grundler F, Bergouignan A, Drinda S, Michalsen A. Safety, health improvement and well‐being during a 4 to 21‐day fasting period in an observational study including 1422 subjects. PLoS ONE 2019;14:e0209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilhelmi de Toledo F, Buchinger A, Burggrabe H, Holz G, Kuhn C, Lischka E, et al. Fasting therapy—an expert panel update of the 2002 consensus guidelines. Forsch Komplementmed 2013;20:434–443. [DOI] [PubMed] [Google Scholar]
- 14. Forbes GB, Drenick EJ. Loss of body nitrogen on fasting. Am J Clin Nutr 1979;32:1570–1574. [DOI] [PubMed] [Google Scholar]
- 15. Nilsson LH, Hultman E. Liver glycogen in man—the effect of total starvation or a carbohydrate‐poor diet followed by carbohydrate refeeding. Scand J Clin Lab Invest 1973;32:325–330. [DOI] [PubMed] [Google Scholar]
- 16. Hultman E, Bergström J, Roch‐Norlund A. Glycogen storage in human skeletal muscle. In Pernow B, Saltin B, eds. Muscle Metabolism During Exercise Advances in Experimental Medicine and Biology. Boston, MA: Springer; 1971. p 273–288. [Google Scholar]
- 17. Bahat G, Tufan A, Tufan F, Kilic C, Akpinar TS, Kose M, et al. Cut‐off points to identify sarcopenia according to European Working Group on Sarcopenia in Older People (EWGSOP) definition. Clin Nutr 2016;35:1557–1563. [DOI] [PubMed] [Google Scholar]
- 18. Votruba SB, Blanc S, Schoeller DA. Pattern and cost of weight gain in previously obese women. Am J Physiol Endocrinol Metab 2002;282:E923–E930. [DOI] [PubMed] [Google Scholar]
- 19. Garnotel M, Bastian T, Romero‐Ugalde HM, Maire A, Dugas J, Zahariev A, et al. Prior automatic posture and activity identification improves physical activity energy expenditure prediction from hip‐worn triaxial accelerometry. J Appl Physiol (1985) 2018;124:780–790. [DOI] [PubMed] [Google Scholar]
- 20. Platen P. Beurteilung der körperlichen Leistungsfähigkeit. In Rost R, ed. Lehrbuch der Sportmedizin Deutscher Ärzteverlag Köln. Deutscher Ärzteverlag Köln; 2001. [Google Scholar]
- 21. Bastian T, Maire A, Dugas J, Ataya A, Villars C, Gris F, et al. Automatic identification of physical activity types and sedentary behaviors from triaxial accelerometer: laboratory‐based calibrations are not enough. J Appl Physiol (1985) 2015;118:716–722. [DOI] [PubMed] [Google Scholar]
- 22. Bech P. Measuring the dimension of psychological general well‐being by the WHO‐5. Quality of Life Newsletter 2004:15–16.
- 23. Bergouignan A, Legget KT, De Jong N, Kealey E, Nikolovski J, Groppel JL, et al. Effect of frequent interruptions of prolonged sitting on self‐perceived levels of energy, mood, food cravings and cognitive function. Int J Behav Nutr Phys Act 2016;13:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lignot JH, Le Maho Y. A history of modern research into fasting, starvation, and inanition. In Comparative Physiology of Fasting, Starvation, and Food Limitation; 2012;Chapter 2. p 7–21. [Google Scholar]
- 25. Rigaud D, Hassid J, Meulemans A, Poupard AT, Boulier A. A paradoxical increase in resting energy expenditure in malnourished patients near death: the king penguin syndrome. Am J Clin Nutr 2000;72:355–360. [DOI] [PubMed] [Google Scholar]
- 26. Attane C, Foussal C, Le Gonidec S, Benani A, Daviaud D, Wanecq E, et al. Apelin treatment increases complete fatty acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin‐resistant mice. Diabetes 2012;61:310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Owen OE, Smalley KJ, D'Alessio DA, Mozzoli MA, Dawson EK. Protein, fat, and carbohydrate requirements during starvation: anaplerosis and cataplerosis. Am J Clin Nutr 1998;68:12–34. [DOI] [PubMed] [Google Scholar]
- 28. Weindruch R, Sohal RS. Caloric intake and aging. N Engl J Med 1997;337:986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zauner C, Schneeweiss B, Kranz A, Madl C, Ratheiser K, Kramer L, et al. Resting energy expenditure in short‐term starvation is increased as a result of an increase in serum norepinephrine. Am J Clin Nutr 2000;71:1511–1515. [DOI] [PubMed] [Google Scholar]
- 30. Tinsley GM, Moore ML, Graybeal AJ, Paoli A, Kim Y, Gonzales JU, et al. Time‐restricted feeding plus resistance training in active females: a randomized trial. Am J Clin Nutr 2019;110:628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marliss EB, Aoki TT, Unger RH, Soeldner JS, Cahill GF. Glucagon levels and metabolic effects in fasting man. J Clin Invest 1970;49:2256–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grundler F, Mesnage R, Michalsen A, Wilhelmi de Toledo F. Blood pressure changes in 1610 subjects with and without antihypertensive medication during long‐term fasting. J Am Heart Assoc 2020;9:e018649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li C, Sadraie B, Steckhan N, Kessler C, Stange R, Jeitler M, et al. Effects of a one‐week fasting therapy in patients with type‐2 diabetes mellitus and metabolic syndrome—a randomized controlled explorative study. Exp Clin Endocrinol Diabetes 2017;125:618–624. [DOI] [PubMed] [Google Scholar]
- 34. Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, et al. Ten‐hour time‐restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab 2020;31:92–104, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen L, Chen R, Wang H, Liang F. Mechanisms linking inflammation to insulin resistance. Int J Endocrinol 2015;2015:508409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab 2014;19:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grundler F, Mesnage R, Goutzourelas N, Tekos F, Makri S, Brack M, et al. Interplay between oxidative damage, the redox status, and metabolic biomarkers during long‐term fasting. Food Chem Toxicol 2020;145:111701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med 2007;42:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meydani SN, Das SK, Pieper CF, Lewis MR, Klein S, Dixit VD, et al. Long‐term moderate calorie restriction inhibits inflammation without impairing cell‐mediated immunity: a randomized controlled trial in non‐obese humans. Aging 2016;8:1416–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wasselin T, Zahn S, Maho YL, Dorsselaer AV, Raclot T, Bertile F. Exacerbated oxidative stress in the fasting liver according to fuel partitioning. Proteomics 2014;14:1905–1921. [DOI] [PubMed] [Google Scholar]
- 41. Traba J, Kwarteng‐Siaw M, Okoli TC, Li J, Huffstutler RD, Bray A, et al. Fasting and refeeding differentially regulate NLRP3 inflammasome activation in human subjects. J Clin Invest 2015;125:4592–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakamura Y, Walker BR, Ikuta T. Systematic review and meta‐analysis reveals acutely elevated plasma cortisol following fasting but not less severe calorie restriction. Stress 2016;19:151–157. [DOI] [PubMed] [Google Scholar]
- 43. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Nutrient composition of the diet during the 10‐day fast.
Figure S1. Study Flow Chart.
Figure S2. Changes in total body water (A), intracellular water (B), blood volume (C), hematocrit (D), hemoglobin (E) and sodium (F) before and during the 10‐day fast (n = 16) and after 3‐months of Fup (n = 15). F: fasting days, RF: food reintroduction days, Fup: Follow‐up. *p < 0.05, **p < 0.01, ***p < 0.001 as indicated by the lines for the whole effect of fasting, the whole effect of food RF or the effect of Fup versus the end of the fast. ##p < 0.01 versus baseline during Fup.
Figure S3. Changes in physical well‐being (A), emotional well‐being (B), WHO well‐being index (C) and free urinary cortisol (D), before and during the 10‐day fast (n = 16) and after 3‐months of Fup (n = 15). VAS: visual analog scale, WHO: world health organization, F: fasting days, RF: food reintroduction days, Fup: Follow‐up. **p < 0.01 as indicated by the lines for the whole effect of fasting.


