Abstract
Background
Cancer cachexia is known to adversely affect the clinical course in patients with malignant lymphoma. The cachexia index (CXI) is a potential biomarker of cancer cachexia, and its implications for the prognosis and treatment outcome of lung cancer and aggressive lymphoma has been assessed in previous studies.
Methods
A total of 267 patients diagnosed with diffuse large B‐cell lymphoma who were treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP) immunochemotherapy were retrospectively reviewed. The CXI was calculated as the skeletal muscle index (SMI) × serum albumin/neutrophil–lymphocyte ratio (NLR). Although previous studies measured the SMI using the muscles of the L3 vertebral level, the present study used both the L3 vertebral muscles and the pectoralis muscles (PM) at the T4 vertebral level to measure the SMI. Depending on the type of muscles used, the CXI was termed the L3‐CXI or PM‐CXI. Using sex‐specific cutoff values for CXI, the patients were categorized as follows: (i) high‐CXI group (high L3‐CXI and high PM‐CXI), (ii) intermediate‐CXI group (high L3‐CXI and low PM‐CXI), and (iii) low‐CXI group (low L3‐CXI and low PM‐CXI).
Results
Complete responses to R‐CHOP were obtained in 145/173 (83.8%), 25/36 (69.4%), and 27/57 (47.4%) patients in the high‐CXI, intermediate‐CXI, and low‐CXI groups, respectively (P < 0.001). Treatment‐related anaemia (15.6%, 30.6%, and 26.3%, P = 0.038), thrombocytopenia (21.4%, 36.1%, and 43.9%, P < 0.001), febrile neutropenia (23.7%, 44.4%, and 36.8%, P = 0.022), and any nonhaematologic toxicity (31.2%, 44.4%, and 54.4%, P = 0.001) of Grade 3 or more were more common in the lower CXI groups than in the higher‐CXI groups. Early treatment discontinuation for reasons other than lymphoma progression also occurred more frequently in the low‐CXI group (24/57, 42.1%) compared with the intermediate‐CXI (5/36, 13.9%) and high‐CXI (18/173, 10.4%) groups (P < 0.001). Median overall survival in the high‐CXI, intermediate‐CXI, and low‐CXI groups was not reached, 50.6 months, and 14.5 months, respectively (p < 0.001). Multivariable analysis showed that low CXI was an independent negative prognostic factor for overall survival (hazard ratio 2.103, 95% confidence interval 1.278–3.460, P = 0.003).
Conclusions
We suggest that in patients with diffuse large B‐cell lymphoma, the CXI is a biomarker for cancer cachexia that can predict survival, treatment response, treatment‐related toxicity, and compliance with R‐CHOP. Patients were more clearly stratified by this new CXI category compared with the classifications described in previous studies.
Keywords: Cachexia, Sarcopenia, Serum albumin, Lymphoma, large B‐cell, diffuse, Prognosis, Biomarkers
Introduction
Cancer cachexia, which is diagnosed based on involuntary weight loss, low body mass index (BMI), and/or the presence of sarcopenia, is a representative systemic manifestation of aggressive lymphoma. 1 , 2 Currently, cachexia is not used as a prognostic factor in standard prognostic systems for malignant lymphoma. 3 , 4 , 5 , 6 However, several biomarkers and indices of cachexia have been suggested to be related to the clinical outcome of patients with malignant lymphoma. 7 , 8 , 9 , 10 , 11 , 12 , 13 For example, Karmali et al. reported that patients diagnosed with diffuse large B‐cell lymphoma (DLBCL) or mantle cell lymphoma and who had a low cachexia index (CXI) had a poor prognosis compared with those with a high CXI. 9 The CXI, consisting of the skeletal muscle index (SMI), serum albumin level, and neutrophil–lymphocyte ratio (NLR), was first developed by Jafri et al. to estimate the prognosis of patients with non‐small‐cell lung cancer. 14 These studies used the SMI of the L3 vertebral level muscles (L3‐SMI) to calculate the CXI. Recently, we reported the prognostic impact of CXI in patients with small‐cell lung cancer. In that study, the pectoralis muscles were used to determine the SMI (PM‐SMI) because the L3‐SMI was difficult to measure on the chest computed tomography (CT) performed during the staging work‐up for small‐cell lung cancer. 15 Given that abdomen and chest CT scans are routinely obtained as part of the initial staging work‐up for DLBCL, we planned to evaluate the clinical impact of the CXI calculated using both L3‐SMI and PM‐SMI and to develop a new classification for CXI in DLBCL patients.
Methods
Study population
We retrospectively screened all patients diagnosed with DLBCL in a single institution between January 2004 and March 2020. Among these, we included all patients who were treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP) immunochemotherapy as induction therapy. Inclusion criteria were age ≥18 years and availability of the data required to calculate CXI measured within one (laboratory test) or two (CT scans) weeks before the initiation of R‐CHOP. Patients with double primary cancers and active infection and in whom the enhanced International Prognostic Index designed using the National Comprehensive Cancer Network database (NCCN‐IPI) 4 could not be calculated were excluded. As a result, a total of 267 patients were included in the analysis. The study was performed in accordance with the ethical standards in the 1964 Declaration of Helsinki and its later amendments and approved by the Institutional Review Board of Gyeongsang National University Hospital.
Calculation of cachexia index
The CXI was measured using the following formula: [SMI, (cm2/m2) × serum albumin (g/dL)]/NLR. 14 NLR was calculated as absolute neutrophil count/absolute lymphocyte count. The pretreatment laboratory values measured at the time closest to the initiation of R‐CHOP were used. To calculate the SMI, the cross‐sectional area of the psoas, paraspinal, and abdominal wall muscles at the L3 vertebral level and the pectoralis major and minor muscles at the T4 vertebral level were measured by a radiologist with 13 years' experience. 12 , 16 The region of interest was drawn manually at the outermost border of the muscles. Then, CT histogram analysis (using the ‘X section’ analysis tool, Advantage Window 4.4; GE Healthcare) was used to calculate the area of muscles ranging from −29 to 100 Hounsfield units. Finally, the SMI was calculated by normalizing the total area of the L3 region muscles and the average area of the bilateral pectoralis muscles to patient height in meters squared (cm2/m2). Based on the type of muscle measured, CXI was categorized as L3‐CXI or PM‐CXI.
Assessments
Patient demographics, symptoms, performance status (PS), laboratory and radiological findings, and treatment history were reviewed from electronic medical records. As BMI (kg/m2) was classified according to Asian criteria, underweight was defined as BMI < 18.5 kg/m2. 17 Bulky disease was defined as any tumour mass with diameter ≥7.5 cm. 18 Tumour staging was determined according to the Lugano modification of the Ann Arbor staging system. 19 Disease in the central nervous system, gastrointestinal tract, liver, lung, or bone marrow was considered to indicate the presence of extranodal disease. 4 The NCCN‐IPI was stratified by age, serum lactate dehydrogenase (LDH), Ann Arbor stage, the presence or absence of extranodal disease, and the Eastern Cooperative Oncology Group (ECOG) PS. Cell‐of‐origin subtype was evaluated from the available data using the Hans criteria. 20 Treatment response was assessed as complete response (CR), partial response, no response or stable disease, or progressive disease using the Lugano response criteria. 19 Relative dose intensity, defined as the percentage of dose actually delivered relative to the planned dose, of cyclophosphamide and doxorubicin was calculated to classify the patients who required dose adjustment. Early treatment discontinuation was defined when the treatment was prematurely terminated because of causes other than disease progression or refractory disease, such as treatment toxicity or unintentional loss to follow‐up. Treatment‐related mortality (TRM) was defined as death unrelated to lymphoma progression that occurred within 30 days of the last cycle of R‐CHOP or death that was definitely related to R‐CHOP, regardless of the date of death. Treatment‐related toxicities were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Statistical analyses
Using a time‐dependent receiver operating characteristic curve, sex‐specific cutoff values for L3‐CXI and PM‐CXI were defined as the values maximizing the Youden index (sensitivity plus specificity) to predict 5 year survival. 21 Patients were stratified into high/low L3‐CXI and PM‐CXI groups using these cutoffs. Then, they were recategorized into high‐CXI, intermediate‐CXI, and low‐CXI groups as described later. To evaluate correlations between variables, a χ 2 test for trend, a Spearman's rank correlation test, and linear regression analysis were used, as appropriate. Progression‐free survival (PFS) was defined as the time between initiation of R‐CHOP and disease progression, death, or last visit. Overall survival (OS) was measured as the time between initiation of R‐CHOP and death or last visit. Survival was estimated using Kaplan–Meier plots with a log‐rank test. Forest plots were constructed to perform subgroup analyses for OS. For multivariable analyses of survival, variables with P values <0.10 were included in a Cox regression model, which was internally validated by 1000‐bootstrap resampling. A two‐sided P value <0.05 was considered significant. All statistical analyses were conducted using R version 4.0.5 (R Foundation for Statistical Computing, Austria) and STATA version 16.1 (College Station, USA).
Results
Association between L3‐CXI and PM‐CXI
The median (interquartile range) values of L3‐CXI and PM‐CXI were 80.59 (44.90–122.74) and 8.17 (4.12–13.92), respectively, in men and 55.50 (27.35–95.43) and 4.96 (2.67–8.97), respectively, in women. There was a strong positive correlation between L3‐CXI and PM‐CXI (R 2 = 0.854; Figure 1).
Figure 1.
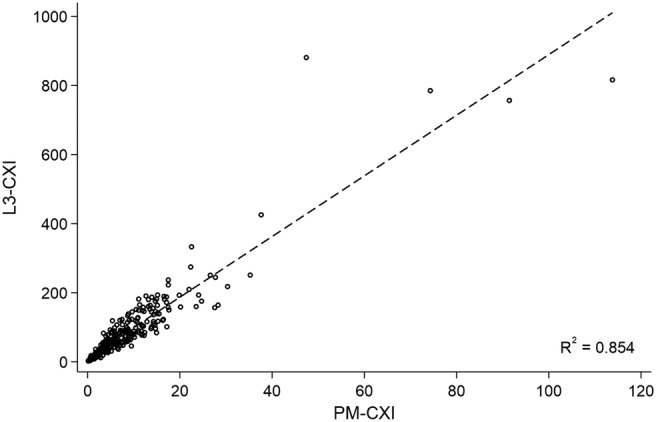
Linear regression between L3‐CXI and PM‐CXI. Abbreviations: CXI cachexia index, PM pectoralis muscle.
The cutoff values for L3‐CXI and PM‐CXI were determined to be 40.43 and 5.60, respectively, in men and 23.06 and 3.43, respectively, in women (Figure S1). Based on these cutoff values, 209 and 58 patients had high and low L3‐CXI and 174 and 93 patients had high and low PM‐CXI, respectively. Only one of the 267 patients had a low L3‐CXI and a high PM‐CXI: This patient was excluded from further analyses. The remaining 266 patients were recategorized into three groups as follows: (i) high‐CXI group (n = 173, high L3‐CXI and high PM‐CXI), (ii) intermediate‐CXI group (n = 36, high L3‐CXI and low PM‐CXI), and (iii) low‐CXI group (n = 57, low L3‐CXI and low PM‐CXI).
Comparison of baseline characteristics
The baseline characteristics of the three CXI groups are listed in Table 1. Patients in the low‐CXI group were older than those in the other groups (median ages, 62 vs. 67 vs. 73 years in the high‐CXI, intermediate‐CXI, and low‐CXI groups, respectively). The low‐CXI group had poorer PS than the higher CXI groups (ECOG PS grades 2–3, 19.1% vs. 33.3% vs. 54.4%), and also had more frequent B‐symptoms (12.7% vs. 19.4% vs. 31.6%) and a higher proportion of Ann Arbor Stages III–IV disease (50.3% vs. 63.9% vs. 77.2%), normalized LDH > 3 (5.2% vs. 16.7% vs. 29.8%), and high NCCN‐IPI (11.0% vs. 16.7% vs. 56.1%) than the higher CXI groups. Median BMIs did not differ significantly between the CXI groups.
Table 1.
Baseline characteristics
| High CXI (n = 173) | Intermediate CXI (n = 36) | Low CXI (n = 57) | P | |
|---|---|---|---|---|
| Age | ||||
| Median (IQR), years | 62 (49–70) | 67 (58–74.5) | 73 (64–76) | <0.001 |
| ≤40 years | 20 (11.6) | 2 (5.6) | 2 (3.5) | <0.001 |
| 41–60 years | 65 (37.6) | 10 (27.8) | 8 (14.0) | |
| 61–74 years | 67 (38.7) | 15 (41.7) | 25 (43.9) | |
| ≥75 years | 21 (12.1) | 9 (25.0) | 22 (38.6) | |
| Sex | ||||
| Male | 98 (56.7) | 19 (52.8) | 33 (57.9) | 0.951 |
| Female | 75 (43.4) | 17 (47.2) | 24 (42.1) | |
| ECOG PS | ||||
| 0–1 | 140 (80.9) | 24 (66.7) | 26 (45.6) | <0.001 |
| 2–3 | 33 (19.1) | 12 (33.3) | 31 (54.4) | |
| B‐symptoms | ||||
| Absent | 151 (87.3) | 29 (80.6) | 39 (68.4) | 0.001 |
| Present | 22 (12.7) | 7 (19.4) | 18 (31.6) | |
| Median BMI (IQR), kg/m2 | 23.4 (21.5–25.5) | 22.7 (21.4–25.1) | 22.7 (20.0–24.8) | 0.137 |
| Bulky disease | ||||
| Non‐bulky | 138 (79.8) | 31 (86.1) | 46 (80.7) | 0.739 |
| Bulky | 35 (20.2) | 5 (13.9) | 11 (19.3) | |
| Ann Arbor stage | ||||
| I–II | 86 (49.7) | 13 (36.1) | 13 (22.8) | <0.001 |
| III–IV | 87 (50.3) | 23 (63.9) | 44 (77.2) | |
| Extranodal disease | ||||
| Absent | 68 (39.3) | 14 (38.9) | 15 (26.3) | 0.098 |
| Present | 105 (60.7) | 22 (61.1) | 42 (73.7) | |
| LDH, normalized | ||||
| Normal | 88 (50.9) | 11 (30.6) | 9 (15.8) | <0.001 |
| >1 to ≤3 | 76 (43.9) | 19 (52.8) | 31 (54.4) | |
| >3 | 9 (5.2) | 6 (16.7) | 17 (29.8) | |
| NCCN‐IPI | ||||
| Low | 23 (13.3) | 1 (2.8) | 1 (1.8) | <0.001 |
| Low‐intermediate | 79 (45.7) | 10 (27.8) | 5 (8.8) | |
| High‐intermediate | 52 (30.1) | 19 (52.8) | 19 (33.3) | |
| High | 19 (11.0) | 6 (16.7) | 32 (56.1) | |
| Cell‐of‐origin (n = 174) | ||||
| GCB | 27 (24.8) | 3 (15.8) | 16 (34.8) | 0.266 |
| Non‐GCB | 82 (75.2) | 16 (84.2) | 30 (65.2) | |
Abbreviations: BMI, body mass index; CXI, cachexia index; ECOG PS, Eastern Cooperative Oncology Group performance status; GCB, germinal center B‐cell; IQR, interquartile range; LDH, lactate dehydrogenase; NCCN‐IPI, National Comprehensive Cancer Network‐International Prognostic Index.
Data are presented as number of patients (%) except median age and BMI.
Treatment response
The CR, progressive disease, and nonresponder (no response or stable disease, progressive disease, and not available) rates were 83.8%, 8.7%, and 7.5% in the high‐CXI group, 69.4%, 27.8%, and 2.8% in the intermediate‐CXI group, and 47.4%, 35.1%, and 17.5% in the low‐CXI group. The CR rates were significantly lower in the low‐CXI group (P < 0.001; Table 2). When the patients completed treatment as scheduled without dose adjustment, the CR rate was highest in the high‐CXI group (92.4% vs. 64.7% vs. 75.0% in the high‐CXI, intermediate‐CXI, and low‐CXI groups, respectively, P = 0.007). For patients in whom a cyclophosphamide and/or doxorubicin dose adjustment of greater than 25% was required during the scheduled treatment period, the CR rate was lower in the lower CXI groups (93.8%, 66.7%, and 50.0% in the high‐CXI, intermediate‐CXI, and low‐CXI groups, respectively, P = 0.031). There were no differences in CR rates between three groups for patients in whom the treatment was discontinued early. These patients had a very low CR rate regardless of the CXI.
Table 2.
Complete response rate according to compliance for treatment
| High CXI (n = 173) | Intermediate CXI (n = 36) | Low CXI (n = 57) | P | |
|---|---|---|---|---|
| CR in all patients | 145/173 (83.8) | 25/36 (69.4) | 27/57 (47.4) | <0.001 |
| CR in patients who completed treatment without DA | 85/92 (92.4) | 11/17 (64.7) | 9/12 (75.0) | 0.007 |
| CR in patients who completed treatment with DA | ||||
| DA, any degree | 56/63 (88.9) | 12/14 (85.7) | 15/21 (71.4) | 0.065 |
| DA ≥ 75% a | 41/47 (87.2) | 8/8 (100.0) | 13/17 (76.5) | 0.386 |
| DA < 75% b | 15/16 (93.8) | 4/6 (66.7) | 2/4 (50.0) | 0.031 |
| CR in patients who early discontinued treatment c | 4/18 (22.2) | 2/5 (40.0) | 3/24 (12.5) | 0.400 |
Abbreviations: CR, complete response; CXI, cachexia index; DA, dose adjustment.
Relative dose intensity of cyclophosphamide and doxorubicin ≥75%.
Relative dose intensity of cyclophosphamide and/or doxorubicin <75%.
Not due to disease progression.
Data are presented as number of patients (%).
Survival
The median duration of follow‐up was 56.6 months. For both types of CXI, PFS and OS were markedly longer in patients with a high CXI than in those with a low CXI (Figure 2). When survival was compared between the CXI groups, there was a clear separation between the survival curves for the three groups. In the high‐CXI, intermediate‐CXI, and low‐CXI groups, the median PFS was not reached, 49.4 months, and 10.3 months, respectively (P < 0.001; Figure 3A). The 5 year PFS rates were 67.0%, 46.7%, and 23.7% in the high‐CXI, intermediate‐CXI, and low‐CXI groups, respectively. The median OS was not reached, 50.6 months, and 14.5 months in the high‐CXI, intermediate‐CXI, and low‐CXI groups, respectively (P < 0.001; Figure 3B). The 5 year OS rates were 70.1%, 48.9%, and 21.4% in the high‐CXI, intermediate‐CXI, and low‐CXI groups, respectively. Subgroup analyses showed clear differences in OS between the high‐ and low‐CXI groups for nearly all subgroups. The intermediate prognosis observed in the intermediate‐CXI group was generally maintained on subgroup analyses (Figure S2). Multivariable analyses showed that, as well as underweight BMI and higher NCCN‐IPI, low CXI was an independent negative prognostic indicator for PFS (hazard ratio 1.904, 95% confidence interval 1.189–3.051, P = 0.007) and OS (hazard ratio 2.103, 95% confidence interval 1.278–3.460, P = 0.003) (Table 3). The prognostic value of the CXI remained robust after internal validation with bootstrap resampling (Table S1).
Figure 2.
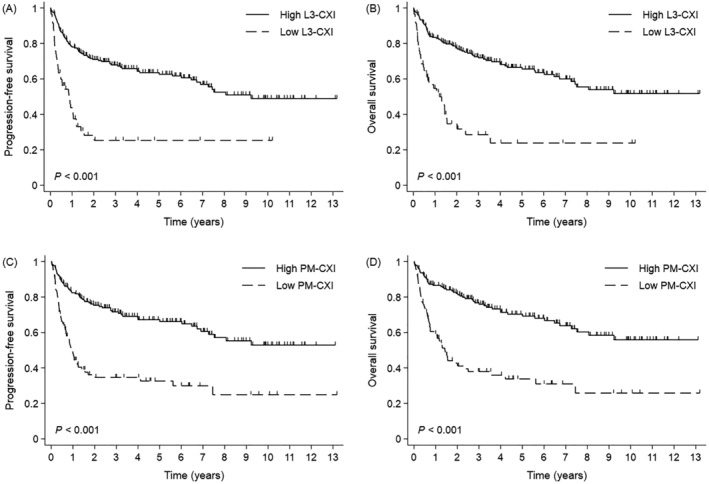
Survival outcomes according to the type and level of CXI. (A) Progression‐free survival and (B) overall survival in the high and low L3‐CXI groups. (C) Progression‐free survival and (D) overall survival in the high and low PM‐CXI groups. Abbreviations: CXI, cachexia index; PM, pectoralis muscle.
Figure 3.
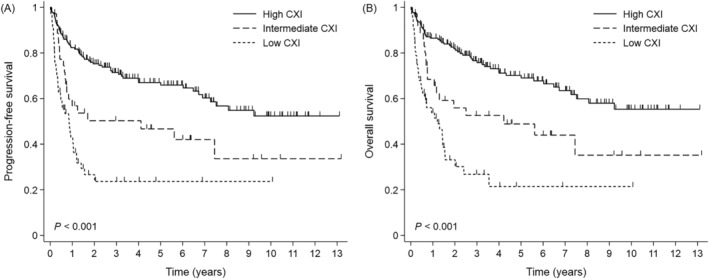
Survival outcomes according to the level of combined CXI. (A) Progression‐free survival and (B) overall survival. Abbreviations: CXI, cachexia index.
Table 3.
Cox regression for progression‐free and overall survival
| Progression‐free survival | Overall survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Sex | ||||||||||||
| Male | Ref. | Ref. | ||||||||||
| Female | 0.813 | 0.563–1.175 | 0.271 | 0.778 | 0.528–1.148 | 0.207 | ||||||
| B‐symptoms | ||||||||||||
| Absent | Ref. | Ref. | Ref. | Ref. | ||||||||
| Present | 2.263 | 1.513–3.385 | <0.001 | 1.277 | 0.827–1.971 | 0.269 | 2.200 | 1.443–3.356 | <0.001 | 1.246 | 0.792–1.958 | 0.341 |
| BMI | ||||||||||||
| ≥18.5 kg/m2 | Ref. | Ref. | Ref. | Ref. | ||||||||
| <18.5 kg/m2 | 1.956 | 1.023–3.739 | 0.042 | 2.291 | 1.153–4.549 | 0.018 | 1.965 | 0.993–3.890 | 0.052 | 2.463 | 1.197–5.072 | 0.014 |
| Bulky disease | ||||||||||||
| Non‐bulky | Ref. | Ref. | ||||||||||
| Bulky | 0.776 | 0.470–1.282 | 0.323 | 0.760 | 0.447–1.293 | 0.311 | ||||||
| NCCN‐IPI | ||||||||||||
| Low to low‐intermediate | Ref. | Ref. | Ref. | Ref. | ||||||||
| High‐intermediate | 4.483 | 2.722–7.382 | <0.001 | 3.619 | 2.149–6.094 | <0.001 | 4.350 | 2.585–7.320 | <0.001 | 3.490 | 2.026–6.011 | <0.001 |
| High | 9.477 | 5.616–15.995 | <0.001 | 7.432 | 4.188–13.188 | <0.001 | 9.702 | 5.603–16.799 | <0.001 | 7.387 | 4.026–13.554 | <0.001 |
| CXI | ||||||||||||
| High | Ref. | Ref. | Ref. | Ref. | ||||||||
| Intermediate | 1.990 | 1.198–3.304 | 0.008 | 1.627 | 0.958–2.764 | 0.071 | 2.066 | 1.222–3.492 | 0.007 | 1.716 | 0.990–2.974 | 0.054 |
| Low | 4.053 | 2.671–6.149 | <0.001 | 1.904 | 1.189–3.051 | 0.007 | 4.403 | 2.837–6.835 | <0.001 | 2.103 | 1.278–3.460 | 0.003 |
Abbreviations: BMI, body mass index; CI, confidence interval; CXI, cachexia index; HR, hazard ratio; NCCN‐IPI, National Comprehensive Cancer Network‐International Prognostic Index.
Treatment‐related toxicity
Table 4 presents the data concerning treatment‐related toxicities. Anaemia, thrombocytopenia, and febrile neutropenia of Grades 3–4 occurred less frequently in the high‐CXI group (15.6%, 21.4%, and 23.7%) than in the intermediate‐CXI (30.6%, 36.1%, and 44.4%) and low‐CXI (26.3%, 43.9%, and 36.8%) groups (P = 0.038, P < 0.001, and p = 0.022, respectively). Nonhaematologic toxicities of Grade 3 or more were also less common in the high‐CXI group (31.2%) than in the intermediate‐CXI (44.4%) and low‐CXI (54.4%) groups (P = 0.001). The TRM rate did not differ between the three CXI groups. Early treatment discontinuation was very common in the low‐CXI group (42.1%) compared with the high‐CXI (10.4%) and intermediate‐CXI (13.9%) groups (P < 0.001).
Table 4.
Treatment‐related toxicity
| High CXI (n = 173) | Intermediate CXI (n = 36) | Low CXI (n = 57) | P | |
|---|---|---|---|---|
| Haematologic toxicity, Grade ≥3 | ||||
| Anaemia | 27 (15.6) | 11 (30.6) | 15 (26.3) | 0.038 |
| Thrombocytopenia | 37 (21.4) | 13 (36.1) | 25 (43.9) | <0.001 |
| Neutropenia | 130 (75.1) | 31 (86.1) | 46 (80.7) | 0.261 |
| Febrile neutropenia | 41 (23.7) | 16 (44.4) | 21 (36.8) | 0.022 |
| Non‐haematologic toxicity, Grade ≥3 | 54 (31.2) | 16 (44.4) | 31 (54.4) | 0.001 |
| Treatment‐related mortality | 12 (6.9) | 2 (5.6) | 8 (14.0) | 0.130 |
| Early treatment discontinuation | 18 (10.4) | 5 (13.9) | 24 (42.1) | <0.001 |
Abbreviations: CXI, cachexia index.
Data are presented as number of patients (%).
Discussion
This study confirms the findings of Karmali et al. that low CXI is associated with worse survival in patients with DLBCL. 9 A similar trend was observed in the analyses of treatment response, treatment‐related toxicity, and intolerance to treatment. Although the low‐CXI groups also had worse baseline characteristics, such as older age, poor PS, advanced stage disease, and high LDH, the CXI remained independently prognostic in multivariable analyses adjusting for the NCCN‐IPI, which includes all these confounding factors. Subgroup analyses also showed consistent trends.
The major difference between the present study and previous studies of aggressive lymphoma and cancer cachexia 9 is the muscle type used to measure the SMI employed to calculate the CXI. A previous study used L3‐SMI alone; this is an established index for assessing sarcopenia and cancer cachexia. 1 In the present study, which used both L3‐SMI and PM‐SMI, we observed a close linear correlation between L3‐CXI and PM‐CXI. In addition, the combined use of L3‐CXI and PM‐CXI could identify patients who had an intermediate prognosis. For patients with a high L3‐CXI, those with a low PM‐CXI (intermediate‐CXI group) showed worse survival than those with a high PM‐CXI (high‐CXI group). This suggests that measurement of SMI at more than one site may be better for calculating the CXI, particularly when both chest and abdomen CT scans are performed during the initial staging work‐up for patients with DLBCL. Another difference in the present study was the method used to determine the cutoff values of CXI. The previous study defined cachectic patients as those with a CXI less than the median value, but did not take account of the patient's sex. 9 Because the CXI is directly proportional to the SMI and the SMI cutoff used to determine sarcopenia differs according to sex, 1 the present study used sex‐specific cutoff values for CXI obtained using a time‐dependent receiver operating characteristic curve analysis. Although the present study analysed a larger number of patients than the previous study, the type of muscle used to measure the CXI and the method for determining the cutoff value of CXI should be validated in a prospective study.
Cancer cachexia is known to increase a patient's susceptibility to chemotherapy‐related toxicities and to negatively affect their responsiveness to chemotherapy. 22 , 23 In this study, the CXI effectively addresses these clinical implications of cancer cachexia. The worse survival seen in the low‐CXI group is probably related to the low CR rate. For patients who completed the scheduled treatment regardless of dose adjustment, the CR rate was about 90% in the high‐CXI group and 75% or less in the low‐CXI group. The patients who experienced early discontinuation of treatment (not due to disease progression) uniformly showed a very low CR rate, and the proportion of such patients was much higher in the low‐CXI group. This indicates that the poor prognosis with a low CR rate observed in the low‐CXI group is related to both primary resistance of the tumour cells and intolerance to treatment.
Sarcopenia (low SMI), malnutrition (hypoalbuminaemia), and systemic inflammation (high NLR) are key components of cancer cachexia, each of which is also related to worse clinical outcomes in patients with DLBCL. Sarcopenia is associated with shorter survival, reduced response to treatment, increased treatment‐related toxicities, frequent dose reduction, and early termination of treatment. 13 , 24 , 25 , 26 , 27 Hypoalbuminaemia is a predictive factor for shorter survival, decreased relative dose intensity of anthracycline and cyclophosphamide, and increased risk of TRM. 28 , 29 , 30 , 31 , 32 High NLR is also correlated with shorter survival, lower CR rate, advanced stage, higher LDH level, and increased risk of depression. 32 , 33 , 34 , 35 , 36 , 37 The CXI comprehensively assesses these clinical parameters, and therefore may more effectively overcome the bias caused by confounding factors affecting the clinical outcomes of the patients than any single parameter of cancer cachexia.
The main limitation of this study is its retrospective nature, which may have caused a selection bias, as indicated by the imbalance in baseline characteristics between the CXI groups, and raises the risk of insufficient data collection. To overcome these problems, we applied multivariable and subgroup analyses for survival to adjust for confounding factors and used mostly objective data, such as laboratory tests, radiological findings, and treatment processes. The frequency of B‐symptoms and nonhaematologic treatment‐related toxicities may be slightly underestimated. Another caution is that as the CXI is a novel index for cancer cachexia, and although an internal validation was performed in this study, external validation is needed before applying the CXI to clinical practice. The study could not assess the clinical implications for the one patient with low L3‐CXI and high PM‐CXI, or of any changes in CXI during the follow‐up period. These factors should be clarified in future prospective studies.
This study shows that the CXI may be useful as a biomarker for cancer cachexia that can predict survival, treatment response, treatment‐related toxicity, and compliance with immunochemotherapy in patients with DLBCL. In addition to the support for the results of previous studies, 9 , 14 , 15 this study suggested a revised method to measure the CXI and developed a novel CXI category to stratify cancer patients more clearly according to their clinical outcome. We anticipate that cachectic patients with DLBCL who are identified based on their CXI could benefit by participating in clinical trials for cancer cachexia and from supportive care including nutritional support to improve their compliance with chemotherapy and their prognosis.
Conflict of interest
The authors declared no potential conflicts of interest.
Supporting information
Figure S1. Receiver operating characteristic curves for the prediction of 5‐year survival according to the CXI and sex.
Figure S2. Forest plots for subgroup analyses of overall survival.
Table S1. Internal validation for overall survival by 1000 bootstrap resampling.
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guideline for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle. 38
Go S.‐I., Park M. J., Park S., Kang M. H., Kim H.‐G., Kang J. H., Kim J. H., and Lee G.‐W. (2021) Cachexia index as a potential biomarker for cancer cachexia and a prognostic indicator in diffuse large B‐cell lymphoma, Journal of Cachexia, Sarcopenia and Muscle, 12, 2211–2219, 10.1002/jcsm.12837
Se‐Il Go and Mi Jung Park equally contributed to this work.
References
- 1. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 2. Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non‐Hodgkin lymphoma. Lancet 2017;390:298–310. [DOI] [PubMed] [Google Scholar]
- 3. International Non‐Hodgkin's Lymphoma Prognostic Factors Project . A predictive model for aggressive non‐Hodgkin's lymphoma. N Engl J Med 1993;329:987–994. [DOI] [PubMed] [Google Scholar]
- 4. Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby‐Thompson A, et al. An enhanced International Prognostic Index (NCCN‐IPI) for patients with diffuse large B‐cell lymphoma treated in the rituximab era. Blood 2014;123:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Federico M, Bellei M, Marcheselli L, Luminari S, Lopez‐Guillermo A, Vitolo U, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol 2009;27:4555–4562. [DOI] [PubMed] [Google Scholar]
- 6. Geisler CH, Kolstad A, Laurell A, Raty R, Jerkeman M, Eriksson M, et al. The Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive first‐line immunochemotherapy and autologous stem cell transplantation (ASCT). Blood 2010;115:1530–1533. [DOI] [PubMed] [Google Scholar]
- 7. Burkart M, Schieber M, Basu S, Shah P, Venugopal P, Borgia JA, et al. Evaluation of the impact of cachexia on clinical outcomes in aggressive lymphoma. Br J Haematol 2019;186:45–53. [DOI] [PubMed] [Google Scholar]
- 8. Go SI, Park S, Kang MH, Kim HG, Kim HR, Lee GW. Clinical impact of prognostic nutritional index in diffuse large B cell lymphoma. Ann Hematol 2019;98:401–411. [DOI] [PubMed] [Google Scholar]
- 9. Karmali R, Alrifai T, Fughhi IAM, Ng R, Chukkapalli V, Shah P, et al. Impact of cachexia on outcomes in aggressive lymphomas. Ann Hematol 2017;96:951–956. [DOI] [PubMed] [Google Scholar]
- 10. Camus V, Lanic H, Kraut J, Modzelewski R, Clatot F, Picquenot JM, et al. Prognostic impact of fat tissue loss and cachexia assessed by computed tomography scan in elderly patients with diffuse large B‐cell lymphoma treated with immunochemotherapy. Eur J Haematol 2014;93:9–18. [DOI] [PubMed] [Google Scholar]
- 11. Lanic H, Kraut‐Tauzia J, Modzelewski R, Clatot F, Mareschal S, Picquenot JM, et al. Sarcopenia is an independent prognostic factor in elderly patients with diffuse large B‐cell lymphoma treated with immunochemotherapy. Leuk Lymphoma 2014;55:817–823. [DOI] [PubMed] [Google Scholar]
- 12. Go SI, Park MJ, Song HN, Kim HG, Kang MH, Lee HR, et al. Prognostic impact of sarcopenia in patients with diffuse large B‐cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Cachexia Sarcopenia Muscle 2016;7:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iltar U, Sozel H, Sozel YK, Atas U, Yucel OK, Salim O, et al. Prognostic impact of the psoas muscle index, a parameter of sarcopenia, in patients with diffuse large B‐cell lymphoma treated with rituximab‐based chemoimmunotherapy. Leuk Lymphoma 2021;62:1098–1106. [DOI] [PubMed] [Google Scholar]
- 14. Jafri SH, Previgliano C, Khandelwal K, Shi R. Cachexia index in advanced non‐small‐cell lung cancer patients. Clin Med Insights Oncol 2015;9:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Go SI, Park MJ, Lee GW. Clinical significance of the cachexia index in patients with small cell lung cancer. BMC Cancer 2021;21:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009;15:2920–2926. [DOI] [PubMed] [Google Scholar]
- 17. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163. [DOI] [PubMed] [Google Scholar]
- 18. Held G, Murawski N, Ziepert M, Fleckenstein J, Poschel V, Zwick C, et al. Role of radiotherapy to bulky disease in elderly patients with aggressive B‐cell lymphoma. J Clin Oncol 2014;32:1112–1118. [DOI] [PubMed] [Google Scholar]
- 19. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B‐cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275–282. [DOI] [PubMed] [Google Scholar]
- 21. Kamarudin AN, Cox T, Kolamunnage‐Dona R. Time‐dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol 2017;17:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martin A, Freyssenet D. Phenotypic features of cancer cachexia‐related loss of skeletal muscle mass and function: lessons from human and animal studies. J Cachexia Sarcopenia Muscle 2021;12:252–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sadeghi M, Keshavarz‐Fathi M, Baracos V, Arends J, Mahmoudi M, Rezaei N. Cancer cachexia: diagnosis, assessment, and treatment. Crit Rev Oncol Hematol 2018;127:91–104. [DOI] [PubMed] [Google Scholar]
- 24. Besutti G, Massaro F, Bonelli E, Braglia L, Casali M, Versari A, et al. Prognostic impact of muscle quantity and quality and fat distribution in diffuse large B‐cell lymphoma patients. Front Nutr 2021;8:620696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo J, Cai P, Li P, Cao C, Zhou J, Dong L, et al. Body composition as a predictor of toxicity and prognosis in patients with diffuse large B‐cell lymphoma receiving R‐CHOP immunochemotherapy. Curr Oncol 2021;28:1325–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Go SI, Kim HG, Kang MH, Park S, Lee GW. Prognostic model based on the geriatric nutritional risk index and sarcopenia in patients with diffuse large B‐cell lymphoma. BMC Cancer 2020;20:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chu MP, Lieffers J, Ghosh S, Belch A, Chua NS, Fontaine A, et al. Skeletal muscle density is an independent predictor of diffuse large B‐cell lymphoma outcomes treated with rituximab‐based chemoimmunotherapy. J Cachexia Sarcopenia Muscle 2017;8:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei X, Zheng J, Zhang Z, Liu Q, Zhan M, Huang W, et al. Consecutive hypoalbuminemia predicts inferior outcome in patients with diffuse large B‐cell lymphoma. Front Oncol 2020;10:610681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi JH, Kim TM, Kim HJ, Koh SA, Mun YC, Kang HJ, et al. Multicenter retrospective analysis of clinical characteristics, treatment patterns, and outcomes in very elderly patients with diffuse large B‐cell lymphoma: the Korean Cancer Study Group LY16‐01. Cancer Res Treat 2018;50:590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miura K, Konishi J, Miyake T, Makita M, Hojo A, Masaki Y, et al. A host‐dependent prognostic model for elderly patients with diffuse large B‐cell lymphoma. Oncologist 2017;22:554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jung SH, Yang DH, Ahn JS, Kim YK, Kim HJ, Lee JJ. Serum lactate dehydrogenase with a systemic inflammation score is useful for predicting response and survival in patients with newly diagnosed diffuse large B‐cell lymphoma. Acta Haematol 2015;133:10–17. [DOI] [PubMed] [Google Scholar]
- 32. Castro D, Beltran B, Quinones MDP, Pachas C, Huerta Y, Lalupu K, et al. Clinical, inflammatory and immunohistochemical features in a cohort of Peruvian patients with diffuse large B‐cell lymphoma. Leuk Res 2021;102:106513. [DOI] [PubMed] [Google Scholar]
- 33. Keam B, Ha H, Kim TM, Jeon YK, Lee SH, Kim DW, et al. Neutrophil to lymphocyte ratio improves prognostic prediction of International Prognostic Index for patients with diffuse large B‐cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma 2015;56:2032–2038. [DOI] [PubMed] [Google Scholar]
- 34. Wang J, Zhou X, Liu Y, Li Z, Li X. Prognostic significance of neutrophil‐to‐lymphocyte ratio in diffuse large B‐cell lymphoma: a meta‐analysis. PLoS ONE 2017;12:e0176008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beltran BE, Paredes S, Cotrina E, Sotomayor EM, Castillo JJ. The impact of the neutrophil:lymphocyte ratio in response and survival of patients with de novo diffuse large B‐cell lymphoma. Leuk Res 2018;67:82–85. [DOI] [PubMed] [Google Scholar]
- 36. Beltran BE, Villela L, Torres MA, Otero V, Fiad L, Pena C, et al. A multi‐institutional validation of the prognostic value of the neutrophil‐to‐lymphocyte ratio in patients with diffuse large B‐cell lymphoma: a study from the Latin American Group of Lymphoproliferative Disorders (GELL). Clin Lymphoma Myeloma Leuk 2020;20:637–646. [DOI] [PubMed] [Google Scholar]
- 37. Yi K, Lan H, Zhang Y, Liu L, Jin S, Mou X, et al. The neutrophil–lymphocyte ratio and immunosuppressive acidic protein may be useful parameters for predicting depression among middle‐aged patients with diffuse large B cell lymphoma. Ann Palliat Med 2021;10:3299–3306. [DOI] [PubMed] [Google Scholar]
- 38. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Receiver operating characteristic curves for the prediction of 5‐year survival according to the CXI and sex.
Figure S2. Forest plots for subgroup analyses of overall survival.
Table S1. Internal validation for overall survival by 1000 bootstrap resampling.


