Abstract
Background
Gut microbiota dysbiosis and sarcopenia commonly occur in the elderly. Although the concept of the gut–muscle axis has been raised, the casual relationship is still unclear. This systematic review analyses the current evidence of gut microbiota effects on muscle/sarcopenia.
Methods
A systematic review was performed in PubMed, Embase, Web of Science, and The Cochrane Library databases using the keywords (microbiota* OR microbiome*) AND (sarcopen* OR muscle). Studies reporting the alterations of gut microbiota and muscle/physical performance were analysed.
Results
A total of 26 pre‐clinical and 10 clinical studies were included. For animal studies, three revealed age‐related changes and relationships between gut microbiota and muscle. Three studies focused on muscle characteristics of germ‐free mice. Seventy‐five per cent of eight faecal microbiota transplantation studies showed that the recipient mice successfully replicated the muscle phenotype of donors. There were positive effects on muscle from seven probiotics, two prebiotics, and short‐chain fatty acids (SCFAs). Ten studies investigated on other dietary supplements, antibiotics, exercise, and food withdrawal that affected both muscle and gut microbiota. Twelve studies explored the potential mechanisms of the gut–muscle axis. For clinical studies, 6 studies recruited 676 elderly people (72.8 ± 5.6 years, 57.8% female), while 4 studies focused on 244 young adults (29.7 ± 7.8 years, 55.4% female). The associations of gut microbiota and muscle had been shown in four observational studies. Probiotics, prebiotics, synbiotics, fermented milk, caloric restriction, and exercise in six studies displayed inconsistent effects on muscle mass, function, and gut microbiota.
Conclusions
Altering the gut microbiota through bacteria depletion, faecal transplantation, and various supplements was shown to directly affect muscle phenotypes. Probiotics, prebiotics, SCFAs, and bacterial products are potential novel therapies to enhance muscle mass and physical performance. Lactobacillus and Bifidobacterium strains restored age‐related muscle loss. Potential mechanisms of microbiome modulating muscle mainly include protein, energy, lipid, and glucose metabolism, inflammation level, neuromuscular junction, and mitochondrial function. The role of the gut microbiota in the development of muscle loss during aging is a crucial area that requires further studies for translation to patients.
Keywords: Gut microbiota, Muscle, Gut–muscle axis, Sarcopenia, Aging, Function
Introduction
Sarcopenia is characterized by a progressive loss of muscle mass, function, and physical performance during aging, and the disease has now become a global threat. 1 , 2 In fact, the incidence of sarcopenia has reached up to 5–13% in 60–70 years old population and 11–50% in those at 80 years or above. 3 With these rapidly increasing numbers, the diagnosis of sarcopenia was further updated by the European Working Group on Sarcopenia in Older People (EWGSOP2) and Asian Working Group for Sarcopenia (AWGS) in 2019. 4 , 5 The latest cut‐off points of muscle mass, strength, and physical performance were reported (Table 1).
Table 1.
Cut‐off points used to diagnose sarcopenia
| Test | EWGSOP2 (2019) 4 | AWGS (2019) 5 |
|---|---|---|
| Grip strength |
Men < 27 kg Women < 16 kg (as a key characteristic) |
Men < 28 kg Women < 18 kg |
| Appendicular skeletal muscle mass (ASM) |
Men < 20 kg Women < 15 kg |
/ |
| ASM/height2 |
Men < 7.0 kg/m2 Women < 5.5 kg/m2 |
Dual‐energy X‐ray absorptiometry: Men < 7.0 kg/m2 Women < 5.4 kg/m2 or bioelectrical impedance analysis: Men < 7.0 kg/m2 Women < 5.7 kg/m2 |
| Gait speed | ≤0.8 m/s | <1.0 m/s |
| Short Physical Performance Battery (SPPB) | ≤8 point score | ≤9 point score |
| Timed Up and Go | ≥20 s | / |
| 400 m walk test | Non‐completion or ≥6 min for completion | / |
| 5‐time chair stand test | >15 s | ≥12 s |
There is currently convincing evidence that sarcopenic individuals have higher risk of falls, fractures, and all‐cause mortality. 6 , 7 , 8 In 2019, two studies showed the annual cost to exceed $2315.7 in the USA and £2707 in the UK for a single sarcopenic patient hospitalized. 9 , 10 It is also estimated that with a reduction in the prevalence of sarcopenia by 10%, the US healthcare system would save $1.1 billion annually. 11 Therefore, public policies are urgently required to cope with these at‐risk patients. 4 As of now, there is still a lack of a single effective therapeutic drug targeting sarcopenia. 12 Resistance exercise is the primary non‐pharmacological approach to improve muscle mass and function, 13 but elderly patients often have poor adherence 14 and lack physical fitness for long‐term sustainability. 15 The increment of protein intake, essential amino acids and their derivatives, antioxidant supplements, polyunsaturated fatty acids, minerals, and vitamin D appears to be beneficial to muscle mass and function in the elderly. 16 , 17 , 18 , 19 , 20 However, the beneficial effects of exercise and nutrients were mainly observed in healthy older population, and more evidence of these strategies in frail and malnourished older population are needed. In addition, new insights of the mechanisms underlying muscle loss should be considered.
The human gut microbiota consists of 10–100 trillion microorganisms, with Bacteroidetes and Firmicutes predominantly in adults. 21 The complex ecosystem plays a vital role in intestinal immune and endocrine functions, energy homeostasis, nutritional status, and health maintenance. 22 In fact, the gut microbiota serves an intermediate role by breaking down carbohydrates, proteins, and lipids to supply energy for the host. 23 To regulate tissues beyond the gastrointestinal tract, microbial products can traverse past the intestinal barrier or can be further metabolized via other organs to enter the circulatory system. 24 , 25 For instance, lipopolysaccharide (LPS) and trimethylamine‐N‐oxide induce a pro‐inflammatory status, while short‐chain fatty acids (SCFAs) and bile acids regulate host metabolism. 26
More importantly, advanced age not only affects the muscle but also causes gut microbiome dysbiosis, 27 with altered microbial diversity and predominant bacteria, and lower beneficial bacterial metabolites. 28 , 29 Gut microbiota‐derived micronutrients and metabolites can reach and act on muscle. 30 Therefore, the concept of the ‘gut–muscle axis’ has been raised to study this relationship. 31 Recent advances elucidated that interventions via the axis have the potential to reverse sarcopenic phenotype. 30 Lactobacillus and Bifidobacterium supplements notably enhanced muscle mass, strength, and endurance capacity in aged mice. 32 Clinical studies have also shown evidence that old people can benefit from the relevant pathways. 33 Therefore, interventions via the gut–muscle axis may be a novel target to delay age‐related muscle wasting and dysfunction. 31 Currently, there is a need to further understand the complex molecular mechanisms of this axis. The purpose of this systematic review was to characterize and analyse current studies to shed light on this topic:
Causal relationship: can the gut microbiota directly affect muscle mass and function?
Sarcopenia target: what kinds of bacteria and bacterial products are beneficial to muscle?
Mechanisms: how does the gut microbiome regulate muscle?
Methods
Search strategy
Four electronic databases, PubMed, Embase, Web of Science, and The Cochrane Library (date last accessed 17 September 2020), were screened. The following keywords were used in the search strategy: (microbiota* OR microbiome*) AND (sarcopen* OR muscle). Reference lists of related reviews were searched for additional studies. This review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 34
Search criteria
The inclusion criteria were (i) clinical/pre‐clinical original studies, (ii) study on gut microbiota and its effects on skeletal muscle/sarcopenia, and (iii) study reporting muscle mass and function indicators. The exclusion criteria were (i) review articles, (ii) conference and abstract publication, (iii) study not involving the gut microbiota or muscle/sarcopenia, (iv) athletes and patients/animal models with other diseases rather than age‐related sarcopenia, (v) lack of baseline characteristics data, and (vi) non‐English article.
Selection of studies
Two reviewers screened titles and abstracts of articles obtained through the search strategy followed by full text for eligible criteria independently. Any disagreements were decided and solved by consensus.
Data extraction
Data extraction was performed by two reviewers. The following data were extracted from pre‐clinical studies: author, year, animal model, assessment methodology, intervention, endpoints, outcome measures (muscle mass and mechanisms, physical performance, description of gut microbiota, and relationships), and key findings. For clinical studies, the extracted data were author, year, demographics (age and sex), sample size, intervention, follow‐up duration, assessment methodology, outcome measures (muscle mass, physical performance, description of gut microbiota, and relationships), and key findings.
Data analysis
Due to the heterogeneity in both clinical and pre‐clinical studies, a qualitative review was performed. The outcomes among groups that showed significantly statistical differences were displayed (P < 0.05). Clinical data representation was shown by mean ± standard deviation or median (interquartile range), and P value if applicable.
Results
A total of 3224 studies were extracted through electronic database search (Figure 1). After removing duplicates, 2282 studies were identified for title and abstract screening. Among these studies, 116 full texts were reviewed, and 36 studies (26 pre‐clinical trials and 10 clinical trials) satisfied all inclusion and exclusion criteria. Supporting Information, Table S1 shows the summary for pre‐clinical studies, and Table S2 shows the clinical studies.
Figure 1.
Study search and selection process.
Pre‐clinical studies
Animal models
There were 26 (4 non‐interventional and 22 interventional) animal studies. 32 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 A total of 22 (84.6%) studies were mice (1 day to 20 months) using C57BL/6 in 13, 32 , 35 , 36 , 37 , 39 , 40 , 41 , 42 , 44 , 45 , 57 , 58 , 59 BALB/C in 2, 38 , 54 institute of cancer research (ICR) in 5, 48 , 49 , 50 , 52 , 53 senescence accelerated mouse prone 8 (SAMP8) in 1, 47 and Kunming (KM) in 1. 55 Four (15.4%) studies were rats (30 days to 24 months) using Wistar rats. 43 , 46 , 51 , 56
Study designs
Three studies observed age‐related changes in gut microbiome and muscle, 32 , 43 , 44 and another one study focused on gene knockout animals to explore the alterations of their gut bacteria, skeletal muscle mass, and functions. 45 Three studies showed the influence of gut microbiota depletion on muscle and/or functions. 35 , 39 , 40 A total of 22 studies had interventional groups with microbiota transplantation in 9 studies, 36 , 37 , 38 , 39 , 40 , 41 , 42 , 58 , 59 supplement intake in 13 studies, 32 , 39 , 46 , 47 , 48 , 49 , 50 , 52 , 53 , 54 , 55 , 56 , 58 antibiotics administration in 4 studies, 39 , 57 , 58 , 59 exercise training in 2 studies, 50 , 51 and food withdrawal in 1 study. 51 Please refer to Table S1.
Outcome assessments of the gut microbiota
Gut microbiota in faecal samples was analysed in 16 studies, 32 , 36 , 37 , 38 , 41 , 42 , 43 , 44 , 45 , 49 , 50 , 51 , 54 , 57 , 58 , 59 and 5 studies extracted microbiome from the caecum, 32 , 52 , 53 , 55 , 56 colon, 56 or small intestine. 56 High‐throughput 16s rRNA sequencing were performed on diverse platforms in 16 studies, 32 , 36 , 37 , 38 , 41 , 43 , 44 , 45 , 49 , 51 , 52 , 53 , 55 , 57 , 58 , 59 with amplified hypervariable region 3–4 (V3–V4), 32 , 38 , 49 , 51 , 52 , 53 , 55 , 59 V3, 36 V4, 37 , 41 , 45 , 58 V1 and V3, 57 or V4–V6. 43 Two studies utilized metagenomic sequencing, 42 , 44 and two used quantitative polymerase chain reaction (PCR) to quantify the bacteria. 58 , 59 The abundance or relative abundance of microbiota was studied on phylum, class, order, family, genus, or species levels in 18 studies. 32 , 36 , 37 , 38 , 41 , 42 , 43 , 44 , 45 , 49 , 51 , 52 , 53 , 55 , 56 , 57 , 58 , 59 Five studies assessed the concentrations of SCFAs in faeces by 1H nuclear magnetic resonance spectroscopy and gas or liquid chromatography. 42 , 50 , 54 , 56 , 58 Alpha diversity was presented in 9 studies, 32 , 36 , 38 , 41 , 43 , 45 , 51 , 58 , 59 and beta diversity was evaluated in 11 studies. 32 , 36 , 38 , 41 , 43 , 44 , 45 , 51 , 52 , 55 , 59
Outcome assessments of muscle and function
Among studies that assessed muscle mass, 16 studies reported the weights of gastrocnemius, soleus, tibialis anterior, quadriceps, extensor digitorum longus, and/or triceps, 32 , 35 , 39 , 42 , 43 , 45 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 57 , 58 , 59 and 7 studies measured lean body mass through magnetic resonance (MR) and dual‐energy X‐ray absorptiometry (DXA). 36 , 37 , 41 , 43 , 45 , 47 , 56 Twelve studies evaluated size and types of myofibres, muscle metabolites, and target genes of muscle growth and atrophy. 32 , 37 , 38 , 39 , 40 , 44 , 45 , 47 , 48 , 51 , 57 , 59
Physical performance was conducted in 19 studies. 32 , 35 , 39 , 40 , 41 , 42 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 52 , 53 , 54 , 55 , 58 , 59 Nine studies performed exhaustive swimming test, 32 , 35 , 48 , 49 , 50 , 52 , 53 , 54 , 55 and five studies tested with treadmill running task to reveal anti‐fatigue properties. 39 , 41 , 46 , 58 , 59 The measurement of locomotion was performed in six studies. 39 , 40 , 42 , 44 , 45 , 46 Muscle strength were assessed in 13 studies, using forelimb grip strength test, 32 , 39 , 41 , 44 , 47 , 48 , 49 , 50 , 52 , 53 , 54 four‐limb hanging test, 47 or ex vivo muscle contractile function. 59 Serum fatigue‐related markers were reported in seven studies. 48 , 49 , 50 , 52 , 53 , 54 , 55
Key findings
Relation between gut microbiota and muscle mass/function
In the process of aging, decreased muscle mass and/or function were compatible with gut microbiota composition changes in two studies of mice and one of rats. 32 , 43 , 44 Although certain bacteria genera were correlated with muscle mass in old rats, 43 Langille et al. observed that neither frailty index nor age were good predictors of microbiome alterations. 44 Ghrelin‐null (Ghrl −/−) mice had microbial dysbiosis when young and had reduced muscle mass and physical function when aged. 45 Two studies showed that gut microbiota depletion directly induced muscle atrophy. 35 , 39 For physical performance, one study reported germ‐free (GF) mice were more fatigable, 35 and another study found an attenuated muscle strength. 39 Bacteroides fragilis gnotobiotic mice showed higher function and muscle mass compared with GF mice. 35 However, one study comparing GF with conventionalized mice indicated the absence of microbiota improved locomotor activity. 40 To explore the direct relationship of muscle and gut microbiota, six out of eight studies (75%) using microbiome‐transplanted models indicated the muscle phenotypes of recipient mice were similar to their donors. 37 , 38 , 39 , 41 , 58 , 59 Four studies showed replicated muscle mass characteristics of colonized mice donated by lean or obese pigs, 38 healthy or malnourished children, 37 pathogen‐free (PF) mice, 39 and conventionally raised mice. 59 Functional abilities, including grip strength, locomotor activity, and/or endurance capacity, were also transmitted to the recipients in four studies. 39 , 41 , 58 , 59 However, two studies did not find differences in either muscle mass or function after microbiome transplant. 36 , 42 One study revealed that gavaging faecal matter diluted from lean or obese human did not affect muscle mass of colonized mice. 36 GF mice transplanted microbiota from old compared with young mice also showed comparable muscle mass and locomotion between groups. 42
Interventions targeting the gut microbiota
Six studies reported seven probiotic supplements Saccharomyces boulardii (SB), 46 Lactobacillus casei LC122 (LC122), 32 Bifidobacterium longum BL986 (BL986), 32 Lactobacillus paracasei PS23 (LPPS23), 47 Lactobacillus salivarius SA‐03 (SA‐03), 49 Lactobacillus plantarum TWK10 (LP10), 48 and Bifidobacterium longum OLP‐01 (OLP‐01) 50 were beneficial to muscle growth 32 , 47 , 48 and function. 32 , 46 , 47 , 48 , 49 , 50 The majority of SCFAs were acetate, propionate, and butyrate, which were in favour of maintaining muscle mass and strength in GF mice. 39 Infusion of acetate improved functional ability in antibiotic‐treated mice, but butyrate did not have the benefits. 58 Dietary supplements that altered gut microbiome had different effects on muscle. Declining muscle mass was found in animals fed with diets based on low microbiome‐accessible carbohydrate (LMC), soy protein, or whey protein. 56 , 58 A single administration of inulin with colonization had no extra benefits on muscle mass but could improve anti‐fatigue capacity in mice. 58 Curcumin as prebiotics ameliorated gut microbiome composition, muscle mass, and functions. 52 High microbiome‐accessible carbohydrate (HMC) diet, oyster polypeptide (OP), and kefir composed of lactic acid bacteria could also improve muscle mass and/or performance by shifting intestinal microbes. 52 , 53 , 55 , 58 Beef extract (BE) had microbiota‐independent effects on muscle function promotion. 54 Consistent results of four studies showed that antibiotics induced gut microbiota dysbiosis, muscle atrophy, and poor functions. 39 , 57 , 58 , 59 One study reported that a 3 day mild exercise did not affect gut microbiota and muscle mass in rats. 51 Another study showed a 6 week exercise course increased the amount of faecal butyrate and had a positive effect on muscle function. 50 Short‐term food withdrawal altered microbial composition, but not muscle mass. 51
Mechanisms involved in regulation of muscle
The mechanisms of the gut–muscle axis have been investigated in 12 animal studies. 32 , 37 , 38 , 39 , 40 , 44 , 45 , 47 , 48 , 51 , 57 , 59 The muscle fibre features (size and types) could be transferred from the pig to mice. 38 High‐dose LP10 supplements increased the slow‐twitch fibres of young mice, 48 while antibiotic reduced the size of myofibres. 57
The absence of the gut microbiota induced the degradation of branched‐chain amino acids (BCAA) in muscle. In the BCAA pathway, serum corticosterone concentrations, gene expression of Kruppel‐like factor 15 (KLF15), branched‐chain aminotransferase 2 (BCAT2), and branched‐chain‐keto acid dehydrogenase (BCKDH) were increased, while BCKDH kinase (BCKDK) was reduced. The quantities of alanine and glycine were higher in GF mice. 39 Muscle amino acid concentrations increased in the mice transplanted by undernourished compared with the healthy donors. 37 The functional annotation of gut microbiota indicated the creatine degradation in aging mice, which was related to muscle atrophy. 44
Germ‐free mice also had increased phosphorylation of the adenosine 5′‐monophosphate‐activated protein kinase (AMPK) in muscle, 39 , 40 which resisted obesity by promoting fatty acid oxidation. The expression of forkhead box O3 (FoxO3) and its downstream Atrogin‐1 and muscle RING‐finger protein‐1 (Murf‐1) were significantly up‐regulated in GF mice. These could be reversed by microbiota transplantation. Lower levels of myosin heavy chain genes, insulin‐like growth factor 1 gene (IGF1), myoblast determination protein 1 (MyoD), and myogenin in GF mice also had adverse effects on muscle growth. 39 SCFAs attenuated muscle loss of GF mice via regulating Atrogin‐1 and MyoD. 39 Two studies reported that antibiotic‐treated mice had higher expression of FoxO3 and other muscle atrophy genes, 39 , 57 but another study did not find any changes of muscle‐related genes after treatment. 59 The measured muscle genes in Ghrl −/− mice did not change, although there was lower normalized lean mass. 45
Mitochondrial dysfunction occurred in the GF mice, accompanied with decreased mitochondrial DNA (mtDNA) content, succinate dehydrogenase (SDH) activity, expression of peroxisome proliferator‐activated receptor γ coactivator 1 alpha (PGC1α), mitochondrial transcription factor A (TFAM), and cytochrome oxidase subunits of complex IV. 39 LC122 and BL986 supplements increased the expression of salt inducible kinase 1 (SIK1) and/or PGC1α4, as well as attenuated inflammatory cytokines [tumour necrosis factor (TNF), interleukin (IL)‐6, and IL‐1β], which improved the muscle protein synthesis. 32 The old mice supplied with LPPS23 had more mtDNA copy number, higher expression of superoxide dismutase (SOD), glutathione peroxidase (GPx), PGC1α, nuclear respiratory factor 1 (NRF1), and TFAM in muscle. LPPS23 also alleviated the muscle inflammation by increasing IL‐10 level. 47 Exercise combined with food withdrawal altered gut microbiota composition, as well as increased the expression of PGC‐1α, IGF1 receptor, carnitine palmitoyl transferase (CPT)1b, CPT2, and uncoupling protein 3 (UCP3) in muscle. 51
The damaged neuromuscular junction (NMJ) in GF mice was reflected in reduced gene of acetylcholine receptor (AChR) subunits, Rapsyn, and increased muscle‐specific kinase (MuSK). 39 After the GF mice transplanted microbes by PF mice, the expression of Rapsyn and low‐density lipoprotein receptor‐related protein 4 (Lrp4) genes were elevated. 39 To further study the mechanisms of muscle function changes, seven studies analysed the fatigue‐related markers in serum, including lactate, ammonia, creatine kinase (CK), and blood urea nitrogen (BUN). 48 , 49 , 50 , 52 , 53 , 54 , 55 Compared with the vehicle groups, supplement with LP10, SA‐03, OLP‐01, curcumin, kefir, BE, or OP selectively reduced the concentrations of these markers after exercising and enhanced the endurance capacity of animals. 48 , 49 , 50 , 52 , 53 , 54 , 55
Clinical studies
There were 10 clinical studies in which 5 were observational studies (N = 35–338) 60 , 61 , 62 , 63 , 64 and 5 were interventional studies (N = 17–54). 33 , 65 , 66 , 67 , 68 In total, 920 participants were included. A total of 676 (73.5%) were elderly participants (72.8 ± 5.6 years, 57.8% female) in 6 studies, 33 , 60 , 61 , 64 , 66 , 68 and 244 (26.5%) were young adults (29.7 ± 7.8 years, 55.4% female) in 4 studies. 62 , 63 , 65 , 67 The inclusion criteria of subjects were based on physical performance, muscle mass and strength, exercise, dietary, and smoking habits. One study referred to the sarcopenia recommendations of Foundation for the National Institutes of Health (FNIH) 69 to confirm muscle mass cut‐off points and only used short physical performance battery as the functional test. 60 For two studies, one or several items to diagnose frailty syndrome were used as the inclusion criteria. 33 , 68
To evaluate muscle mass, four studies used DXA 60 , 61 , 62 , 63 and four studies used bioelectrical impedance analysis (BIA). 64 , 66 , 67 , 68 One studied muscle protein degradation‐related amino acids. 65 Nine studies performed functional tests, muscle strength assessment, habitual physical ability, questionnaires, and endurance and fatigue‐related serum markers as physical performance results. 33 , 60 , 61 , 62 , 63 , 64 , 66 , 67 , 68 The gut microbiota of faecal samples was assessed by 16s rRNA sequencing in six studies, 60 , 61 , 62 , 63 , 64 , 66 shotgun metagenomics in one study, 65 and quantitative PCR in two studies. 63 , 64 The abundance of microbiota was classified by order, family, genus, and species in seven studies. 60 , 61 , 62 , 63 , 64 , 65 , 66 The concentrations of gut microbial metabolites were analysed by untargeted metabolomics and targeted SCFAs using gas chromatography–mass spectrometer in one study. 61 Alpha and beta diversity analyses were shown in five 60 , 61 , 62 , 63 , 65 and four studies, 61 , 62 , 63 , 65 respectively.
Key findings
The summary of the 10 clinical studies is described in Table S2. Four observational studies investigated the relationship between muscle mass, functions, and gut microbiota through the comparison of older people with or without sarcopenia (N = 35), 60 with high or low physical fitness (N = 207), 61 and sedentary or active adults (N = 109, N = 40). 62 , 63 Two studies revealed the discrepancies in the relative abundance of gut microbiome that were strongly correlated with age‐related muscle loss and poor physical performance. 60 , 61 The correlation of microbes and muscle mass was also found in young women. 63 Active individuals with greater muscle mass had a higher diversity of gut microbiota. 62 One observational study compared old people drinking fermented milk with different frequency and found no differences in muscle mass and function (N = 338). 64 One non‐controlled longitudinal study found caloric restriction (CR) changed muscle protein breakdown markers in blood without significant impact on gut bacteria (N = 41). 65 One non‐randomized comparative trial (N = 29) found the relative abundance of Bacteroides to be significantly increased in sedentary women after 12 weeks of aerobic exercise, which was positively correlated with improved walking performance. 66 In three randomized controlled trials, prebiotics, probiotics, and synbiotic were supplied separately with inconsistent results. 33 , 67 , 68 Prebiotics Darmocare Pre®, which consist of inulin and fructooligosaccharides (FOS), significantly enhanced the grip strength and endurance capacity in frail elderly people (N = 50). 33 Similarly, in young healthy adults (N = 54) who took LP10 daily, an increased muscle mass and a dose‐dependent higher anti‐fatigue capacity were observed. 67 In a small‐sized study (N = 17), the long‐term intake of synbiotic (FOS, Lactobacillus strains, and Bifidobacterium lactis) did not ameliorate fat‐free mass or muscle strength in the elderly. 68
Interventions bridging animal and human
The positive effects of LP10 on muscle mass and anti‐fatigue properties had successfully been verified in both young animal and human. 48 , 67 Although LC122, BL986, 32 and LPPS23 47 improved muscle mass and function in aged animal models, the synbiotic that contain Lactobacillus and Bifidobacterium strains, as well as fermented milk containing L. casei strain Shirota, had no effects on muscle status in elderly people. 64 , 68 Prebiotics including inulin played a positive role in physical performance of microbiota‐reduced mice and frail elderly people. 33 , 58
Discussion
In this review, 36 studies were included. Among the 26 animal studies, mice were mostly used because of their similar genome to humans and advantages to study mechanisms of diseases, which is necessary for further clinical translation. 70 Three studies investigated the changes of gut microbiota and muscle during aging. 32 , 43 , 44 However, the causality of the gut–muscle axis could not be verified by observational studies. Transplantation, 36 , 37 , 38 , 39 , 40 , 41 , 42 , 58 , 59 prebiotics, 52 , 58 probiotics, 32 , 46 , 47 , 48 , 49 , 50 and bacterial products 39 , 53 , 58 directly influence the gut microbiota, which were better choices to study the effects of bacteria on host organs. Antibiotics, 39 , 57 , 58 , 59 exercise, 50 , 51 food withdrawal, 51 gene knockout, 45 and other nutrition supplements 54 , 55 , 56 might regulate muscle and gut microbes simultaneously, but whether the gut bacteria are mediators in muscle regulation remains unclear.
16s rRNA and metagenomic sequencing are the two most utilized microbial analysis approach. 16s rRNA sequencing is cost‐effective and required low biomass, and metagenomics is more accurate to identify species level and annotate functional pathways. 71 Quantitative PCR using universal bacterial primers or special strains primers could quantify the bacteria load or actual abundance. 58 , 59 Microbiota‐derived metabolites not limited to SCFAs were important to be measured. 42 , 50 , 54 , 56 , 58 Alpha and beta diversity were calculated to present the variability within a sample and between samples. The latter could also justify whether faecal microbiota was successfully transplanted from donors to recipients. 42 A total of 24 out of 26 (92.3%) studies measured muscle mass and/or muscle‐related factors. Compared with muscle weight, the lean body mass could be measured by MR and DXA. 32 , 35 , 36 , 37 , 39 , 41 , 42 , 43 , 45 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 56 , 57 , 58 , 59 Animal models had the advantages to study mechanisms of muscle via histomorphology, gene and protein expression, and metabolites. 32 , 37 , 38 , 39 , 40 , 44 , 45 , 47 , 48 , 51 , 57 , 59 Endurance and fatigue‐related serum metabolites, grip strength, and locomotion tests displayed the anti‐fatigue capacity, maximum force, and voluntary activity in 19 studies (73.1%). 32 , 35 , 39 , 40 , 41 , 42 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 52 , 53 , 54 , 55 , 58 , 59
Age‐related changes of gut microbiota and muscle status occurred together, but the causal correlations had not been clearly investigated. There is growing evidence suggesting the gut microbiota is involved in regulating muscle physiology in various muscle wasting diseases. 43 , 72 , 73 , 74 Sarcopenia has a high incidence in the elderly and may be affected by gut microbiota dysbiosis. In this review, three animal studies focused on the links of age‐related changes in gut microbiota and muscle/function. 32 , 43 , 44 One study investigated aged Wistar rats characterized by low muscle mass. 43 A variation in gut microbiome were also observed. 43 16s rRNA‐based operational taxonomic units of aged rats were correlated with decreased muscle mass and increased fat mass. 43 According to the results of metagenomic functional content, the gut microbiota played an important role in vitamin synthesis, lipid metabolism, growth, and immune‐related factors to regulate muscle phenotype. 43 Another study did not evaluate muscle mass directly but found frail mice had a higher creatine degradation functionality assessed by microbiome sequencing, 44 which may suppress myogenesis. 75 One study reported old mice had poor physical performance, lower muscle mass, as well as distinctive bacterial functions, such as reduced antigen processing and presentation, mineral absorption, and bile secretion. 32 Gene knockout mice, such as Ghrl −/− mice, had a special microbiome composition towards pro‐inflammation status, but whether it contributes to the muscle degeneration during aging is still unknown. 45 After transplanting the gut bacteria from young and old C57BL/6 donor mice, there were no differences in the muscle mass and function of young GF mice. Surprisingly, the recipients colonized with old mice faeces had higher faecal butyrate levels and triggered the prolongevity signalling pathways. 42 However, other studies showed microbiome from aged mice did not have positive effects on recipients. 76 , 77 Therefore, whether gut microbiota dysbiosis accelerates age‐related muscle wasting requires more research into its mechanisms.
Three animal studies analysed the effects of gut microbiota depletion on skeletal muscle. 35 , 37 , 40 The absence of gut microbiota reduces muscle mass, but the changes of various functions are inconsistent. Compared with PF mice, GF condition in C57BL/6 mice was significantly associated with lower muscle mass, especially lower gastrocnemius weight in two studies. 35 , 39 However, there were inconsistent roles of the gut microbiota for functional ability. One study revealed reduced locomotor activity in GF mice compared with conventionalized mice. 39 Another study was the opposite, which may be due to the higher adaptability of GF mice when fed with special diets, such as high‐fat, sugar‐rich diet. 40 A declined muscle strength of GF mice was also revealed in another study. 39 Whether the absence of gut microbiota reduced anti‐fatigue capacity is still controversial in two studies, because swimming and running endurance tests might lead to the slightly different results. 35 , 39
Muscle phenotypes, including muscle mass and function characteristics, can be transferred from donors to recipients, although it is not always successful. Faecal microbiota transplantation is a novel therapy for intestinal disorders and has been clinically applied to patients with recurrent Clostridium difficile infection. 78 To find the causality between gut microbiota and muscle, eight transplantation studies were analysed. 36 , 37 , 38 , 39 , 41 , 42 , 58 , 59 There is a widespread use of GF 35 , 37 , 38 , 39 , 40 , 41 , 42 and antibiotic‐induced gut microbiota depletion 36 , 59 mice models to perform transplant experiments. After transplanting faecal microbiota to recipient mice, six studies demonstrated characteristics of muscle mass and/or function could be transferred through gut microbiota. 37 , 38 , 39 , 41 , 58 , 59 Faecalibacterium prausnitzii were predominant in higher muscle mass recipient mice donated by healthy infants, while Clostridium neonatale were predominant in recipients donated by malnourished and underweight infants. 37 F. prausnitzii has anti‐inflammatory roles and can be a next‐generation probiotic. 79 Colonization with mono‐microbiome B. fragilis 35 or cohousing with PF mice 39 restored muscle mass and function for GF mice. The potential mechanisms of reversal may be due to increased muscle mitochondrial oxidative and glucose metabolism. 39 Natural reseeding antibiotic‐treated mice affected muscle mass slightly. The caecum was hypertrophic in antibiotic‐treated mice, and no differences were found in muscle mass normalized by caecum‐free body weight between antibiotic‐treated and reseeding groups. 59 However, reseeding improved physical performance through microbiome‐driven glucose homeostasis. 59 The combination of prebiotics and faeces transplantation from HMC‐diet‐fed mice enhanced the endurance of LMC‐diet‐fed mice, which indicated that HMC diet might benefit muscle function via gut microbiota. 58 One study investigated Rongchang pigs (RP) that had lower lean mass, and Yorkshire pigs (YP) characterized by higher fibre diameter and cross‐sectional area. GF mice transplanted with faeces from RP and YP displayed similar muscle characteristics with their pig donors. 38 One study transplanted gut microbiota from high or low function human to GF mice and found only the characteristic of muscle strength was replicated in mice. 41 The muscle mass and endurance capacity of colonized mice remained the same. 41 Kundu et al. indicated GF recipient mice colonized by old and young mice donors had similar muscle mass at Week 8, but the related genes were not reported. 42 Another study compared recipient mice donated by lean or obese humans, but no differences in muscle mass were found. 36 Because the muscle mass of two groups of donors was not shown, whether this indictor had differences was unclear. 36 In summary, GF and antibiotic‐treated mice could both replicate the muscle status of donors of different species after microbiota transplantation. To improve the efficiency, it is necessary to analyse the muscle phenotypes in the donors and whether the recipients are successfully colonized by gut microbiota in the future gut–muscle transplanting studies.
A variety of interventions have been raised to target gut microbiota. Probiotics, prebiotics, SCFAs, bacteria, and dietary supplements effectively enhanced muscle mass and/or physical performance. Antibiotics induced muscle loss and dysfunction and reduced gut microbiota. The role of gut microbiota in muscle changes by antibiotic, exercise, and food withdrawal was unclear. Probiotics LC122, BL986, and LPPS23 prevented the age‐related loss of muscle mass and strength, as well as gut microbiota dysbiosis in old C57BL/6 mice and SAMP8 mice. 32 , 47 The anti‐aging ability of probiotics was attributed to the regulation of inflammation levels (IL‐6, TNF‐α, MCP‐1, and IL‐1β), muscle mitochondrial function, and biogenesis and ultimately modulated the expression of muscular genes. 32 , 47 Animal and cell studies revealed the positive effects of LP10 and Lactobacillus curvatus CP2998 on muscle whereby the suppression of glucocorticoid receptor (GR) activation and the improvement of glucose utilization. 48 , 80 OLP‐01, SA‐03, and SB were also favourable to physical fitness, but the roles in muscle weight gain remained uncertain. 46 , 49 Probiotics offered from kefir fermented milk reduced the Firmicutes/Bacteroidetes ratio, which increased muscle mass, strength, and endurance in mice. 53 Gut microbiota can degrade prebiotics to remodel the community of bacteria. 81 Okamoto et al. found inulin combined with microbial transplantation only improved endurance, but not muscle mass in LMC‐diet mice. 58 It could be interpreted that only a single‐time inulin supplement was difficult to benefit muscle growth. A high dose of nano‐bubble curcumin altered the community of gut bacteria, including the higher Firmicutes/Bacteroidetes ratio, which played a positive role in muscle. 52 Provision of prebiotic‐enriched diet to gestating mice enhanced the muscle growth of offspring. 82 Unexpectedly, only three animal studies focused on prebiotics and muscle, and the efficiency of specific prebiotic to increase muscle mass is unclear. 52 , 58 , 82 A cocktail of SCFAs reduced the expression of Atrogin‐1 and increased MyoD, muscle mass, and function in GF mice. 39 Additionally, SCFAs switched the energy metabolism of myotubes from oxidative phosphorylation to glycolysis. 39 Subcutaneous infusion of acetate restored anti‐fatigue capacity of antibiotic‐treated mice but did not rescue muscle mass. 58 Similar outcomes were not found in butyrate infusion. 58 However, 5% butyrate fed diet prevented muscle loss in sarcopenic mice via the improvement of mitochondrial function. 83 The administration route and different animal models might influence the results. Besides, the effects of acetate, propionate, and butyrate should be investigated to illustrate the signalling pathways that SCFAs improved muscle status. 25 Higher muscle mass, endurance capacity, concentrations of SCFAs, bacterial diversity, and lower Firmicutes/Bacteroidetes ratio were found in HMC‐diet‐fed mice. 58 An observational study that included older adult men identified higher levels of butyrate‐producing bacteria and gene counts for butyrate production in subjects with higher values of lean mass, physical function, and dietary fibre density (grams of fibre consumed per 100 calories). 84 Nutrient supplements OP and BE improved muscle function but not mass. 54 , 55 BE also aided GF mice to enhance endurance capacity, and so it had microbiota‐independent effect on function. 54 Diets based on soy and whey protein induced muscle loss in rats compared with placebo diet. 56 However, human studies indicated protein supplements increased muscle mass and performance through the gut–muscle axis. 85 , 86 , 87 The better reaction of human subjects may be due to their frail conditions. Long‐term use of antibiotics destroy systemic microbiome community, and geriatric population were most often affected. 88 Four studies discovered that antibiotic induced muscle atrophy and/or fatiguability in mice. 39 , 57 , 58 , 59 The regulations of myogenetic factors, muscular adipogenesis, and oxidative metabolic capacity supported the above changes. 39 , 57 , 58 One study did not find any differences in muscle‐related protein expression after antibiotic treatment, and microbiota structure was not significantly variable. 59 Indeed, muscle mass was also reduced in antibiotic‐treated GF mice, indicating antibiotic had microbiota‐independent effects on muscle. 57 The perspective of antibiotic–gut–muscle highway has been challenged based on existing research. Using antibiotic‐induced pseudo GF animal models to study muscle should be the last resort. Exercise‐related studies only showed minimal changes in muscle mass, although the physical performance and gut microbiota had changed. 50 , 51 Unlike bacteria and their products, antibiotics, exercise, and nutrition supplements regulated muscle and gut microbiota together. To investigate the roles of gut microbiota in the process, transplantation of faeces from treated mice to disease models may be a solution. 89
The mechanisms of the gut–muscle axis were discussed. GF conditions, transplantation, SCFAs, probiotics, prebiotics, and other supplements could affect the protein synthesis and degradation balance, energy, glucose, and lipid metabolism, mitochondria, NMJ, metabolites, and various molecular pathways in muscle. The gut microbiota not only alters muscle mass and function but also the myofibre size and types. 38 , 48 , 57 The up‐regulation of BCAA catabolism in GF mice was one factor of muscle atrophy. 39 It was intriguing that the increased concentrations of several amino acids in muscle of the mice colonized with undernourished donor faeces might be stored for oxidation and energy production. 37 The aging gut microbiota might also degrade the creatine of old mice. 44 Exogenous creatine supplements could reverse the age‐related muscle loss. 90 The gut microbiome modified metabolic pathways of fatty acids and glucose to maintain energy balance of the host. 39 , 40 The functional annotation showed microbiome and the hosts had an interaction of immune response, vitamin, lipid, and glucose metabolisms, which might regulate muscle growth. 32 , 41 , 43 As for the protein synthesis and degradation, myogenin, MyoD, FoxO3 pathway, and its downstream E3 ubiquitin ligases genes were also regulated by gut microbiota and derived metabolites. 39 , 80 Mitochondria dysfunction was found in GF mice and aged mice and could be prevented by probiotics. 32 , 39 , 47 , 51 Age‐related low‐grade inflammation and NMJ dysfunction were causative factors of sarcopenia. 91 , 92 Probiotics reduced the systemic pro‐inflammatory cytokines in old mice, and the bacteria colonization improved the NMJ function of GF mice. 39 , 47 Except for damaged NMJ, the serum fatigue factors also affected the physical performance of mice and human. Probiotics and other bacterial supplements improved the ability of liver to remove metabolic wastes, increased the glycogen in liver and muscle, and enhanced the cardiopulmonary function via increased maximal oxygen uptake. 48 , 49 , 50 , 52 , 53 , 54 , 55 , 67 Although gene knockout, exercise, antibiotic, and food withdrawal influenced signalling pathways in muscle, it is still unclear whether it is modulated by microbiota.
In vitro studies also showed microbial products (quorum sensing molecules, LPS) directly affected myogenesis. 93 , 94 LPS activated toll‐like receptor 4 (TLR4) and induced muscle catabolism. 95 Two gut microbial metabolites, indoxyl sulfate (IS) and p‐cresyl sulfate, are increased during aging and play a role in muscle. 96 IS, known as a uraemic sarcopenia biomarker, was inversely associated with muscle mass and physical activity. 97 , 98 In vivo and in vitro studies showed that IS accelerated muscle atrophy through elevating inflammation level, oxidative stress, excess antioxidative response, and damaged muscular mitochondrion. 97 , 99 , 100 , 101 , 102 , 103 Putrefactive bacteria‐produced p‐cresyl sulfate also led to poor muscle status. It caused insulin resistance and increased the muscle lipid content. 104 The current known mechanisms of how gut microbiota affect muscle mass are shown in Figure 2. Besides, how other gut microbial metabolites impact muscle and sarcopenia needs further studies, such as amino acids, bile acids, and their derivatives. 105 , 106
Figure 2.
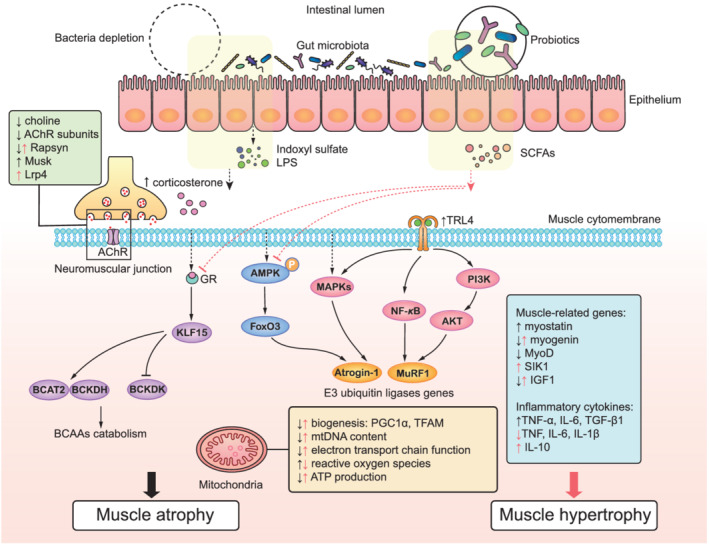
The molecular signalling pathways of gut microbiota and skeletal muscle: the noxious bacterial metabolites (indoxyl sulfate and LPS) and absence of gut microbiota (black) induced muscle atrophy. 39 , 94 , 95 , 97 , 99 , 100 , 102 Indoxyl sulfate and LPS caused muscle atrophy and inflammation by activating the PI3K/AKT, NF‐κB, and MAPKs (p38, JNK, ERK) signalling pathways to up‐regulate Atrogin‐1/MAFbx and MuRF1 genes encoding E3 ubiquitin ligases, and inflammatory cytokines. AMPK–FoxO3–Atrogin‐1/MuRF1 cascade and BCAAs catabolism were activated in the bacteria depletion condition. The expressions of IGF1, myogenin, and MyoD were reduced, and myostatin was increased. NMJ function and mitochondrial function were widely impaired. Supplements with probiotics, SCFAs, or germ‐free transplanted models showed (red) the suppression of GR and excessive AMPK activation, attenuated inflammation levels, mitochondrial and NMJ function repair, as well as increased the expressions of muscle growth‐related genes (IGF1, myogenin, SIK1) to maintain muscle mass and functions. 32 , 39 , 47 , 80 For abbreviations of the main text, please see Table S3.
Current clinical translation
In this review, 10 clinical studies were included. A total of 26.5% participants were young adults, and 73.5% were old people. None of the aging studies focused on sarcopenia or strictly followed the recommended guidelines of sarcopenia diagnosis. 33 , 60 , 61 , 64 , 66 , 68 DXA, BIA, functional tests, and physical activity level were recorded to divide subjects into different groups or used to observe the effects of treatments. The methodology of gut microbiota analysis in human and animal studies was similar.
Gut microbiota dysbiosis is related to lower muscle mass and poor physical function, and exercise‐induced improvement of function is associated with alterations of gut microbiome. Four observational studies found the gut microbiome was separated between different groups of people, such as old subjects with or without physical frailty and sarcopenia, 60 low or high physical fitness, 61 and sedentary or active young people. 62 , 63 Several bacteria could be biomarkers to identify old individuals under the conditions of reduced muscle mass and poor functions (Table S2). 60 , 61 Another observational study indicated that Lactobacilli, F. prausnitzii, and Bacteroides/Prevotella ratio declined sharply and Enterobacteriaceae increased in the frail old people. 107 Active adults had higher muscle mass, functions, and different predominant bacteria when compared with sedentary individuals. 62 , 63 One interventional study reported aerobic exercise did not increase muscle mass compared with trunk muscle training but changed microbiota and physical performance in the old women. 66 Moreover, the abundance of Bacteroides was shown positively associated with 6 min walk test. 66 CR modulated the blood amino acids related to skeletal muscle protein breakdown, but the composition of microbiota did not change. 65 One animal study showed food withdrawal increased the variation of the microbiome but did not influence muscle mass. 51
In this context, only three studies provided bacterial supplements to the young 67 and old 33 , 68 people. The benefits of taking supplements for muscle/sarcopenia are conflicting, which may depend on the type, dose, intake duration, and target populations. Six‐week LP10 intake dose‐dependently increased the endurance capacity of young individuals, in the aspects of physical performance and serum indicators during and after exercise. 67 After proteome and microbiota analysis in mice, LP10 improved energy utilization via the promotion of peroxisomal fatty acid oxidation in liver and more butyrate‐producing bacteria. 108 The anti‐inflammation effect of Lactobacilli was assumed to protect against muscle atrophy, which had been studied in cachexia mouse models. 109 One study focused on physical frailty in old subjects and revealed that prebiotics (inulin and FOS) significantly improved their physical performance, but without changes in serum TNF‐α. 33 Discoveries of another study suggested that long‐term provision of synbiotic had no extra benefit to muscle as well as inflammatory status in older people. 68 One observational study separated older adults ingesting fermented milk into three groups (0–2, 3–5, and 6–7 days/week) and showed similar muscle mass and physical activity although increased abundance of Lactobacillus was detected in the high‐frequency groups. 64 High‐quality randomized controlled trials are required. Due to a single study with each intervention, the effect of diverse supplements remains difficult to draw an overall conclusion. There is a lack of effective bacterial supplements to help old subjects gain muscle mass. Therefore, we urgently need novel strategies to improve the efficiency of bacterial products, such as hydrogel, genetically modified bacteria, and programmed inhibitor cells. 110 , 111 , 112
The understanding of the gut–muscle axis is still insufficient. Bacteria correlation with muscle mass is shown in Table 2. The current knowledge of primary bacteria that maintain muscles includes Lactobacillus and Bifidobacterium, while the bacteria negatively affecting muscle mass are uncertain due to the lack of interventional studies. Other conflicting results depend on the level of microbiota, interventions, experimental subjects, and their counterparts. The alterations of gut microbiota impacted the muscle through various pathways, which were partially overlap to the pathogenesis of sarcopenia (Figure 2). It is important to identify specific gut bacteria or metabolites that can discriminate old individuals with or without sarcopenia based on a larger sample size. These bacteria can potentially regulate age‐related muscle disorder and be targeted.
Table 2.
The abundance of gut bacteria correlated with muscle mass
| Study | Bacteria positively associated with muscle mass gain, or abundant in higher muscle mass rodents/human | Bacteria negatively associated with muscle mass gain, or abundant in lower muscle mass rodents/human |
|---|---|---|
| Castro‐Mejía et al. 2020 61 | Bifidobacterium adolescentis, Christensenella | Enterobacterales |
| Castellanos et al. 2020 62 | Coprococcus, Blautia, Eubacterium | Unclassified Bacteroides, Parabacteroides |
| Chen et al. 2020 52 | Firmicutes, Lactobacillaceae, Lactobacillus, Proteobacteria | Bacteroidetes, Clostridiales, Allobaculum |
| Picca et al. 2020 60 | Barnesiellaceae, Christensenellaceae, Slackia, Eubacterium | Oscillospira, Ruminococcus, Peptostreptococcaceae, Bifidobacteriaceae, Dialister, Pyramidobacter, Eggerthella |
| Chen et al. 2019 47 | Lactobacillus paracasei PS23 a | / |
| Okamoto et al. 2019 58 | Bacteroidetes, Prevotella, S24–7 | Firmicutes, Actinobacteria, Lactococcus, Allobaculum |
| Huang et al. 2019 67 | Lactobacillus plantarum TWK10 a | / |
| Ni et al. 2019 32 | Lactobacillus casei LC122 a , Bifidobacterium longum BL986 a , Epsilonbacteraeota, Actinobacteria, Spirochaetes, Bifidobacterium, Lactobacillus | Verrucomicrobia, Epsilonbacteraeota, Muribaculum, Parasutterella |
| Hsu et al. 2018 53 | Lactobacillus fermentum DSM 32784 (LF26) a , L. helveticus DSM 32787 (LH43) a , L. paracasei DSM 32785 (LPC12) a , L. rhamnosus DSM 32786 (LRH10) a , Streptococcus thermophilus DSM 32788 (ST30) a , Bacteroidetes, Bacteroidales, Bacteroidia, Ruminococcaceae, Clostridia, Clostridiales, Rikenellaceae | Firmicutes |
| Manickam et al. 2018 57 | / | Proteobacteria, Erysipelotrichales |
| Bressa et al. 2017 63 | Bifidobacterium, Faecalibacterium prausnitzii, Roseburia hominis, Akkermansia muciniphila, Faecalibacterium, Coprococcus, Lachnospiraceae unclassified 1, Ruminococcae unclassified 1 | Barnesiellaceae, Odoribacteraceae, Ruminococcus |
| Siddharth et al. 2017 43 | Lachnospiraceae insertae sedis, Lachnobacterium, Barnesiella | Thermotalea, Sutterella |
| Chen et al. 2016 48 | Lactobacillus plantarum TWK10 a | / |
| Blanton et al. 2016 37 | Faecalibacterium prausnitzii a , Clostridium nexile a , Clostridium symbiosum a , Ruminococcus gnavus a , Dorea formicigenerans a | Clostridium neonatale, Enterococcus species |
| Yan et al. 2016 38 | Bacteroidetes, Proteobacteria, Prevotella | Firmicutes, Fusobacteria, Actinobacteria, Roseburia, Ruminococcus, Blautia |
| Hsu et al. 2015 35 | Bacteroides fragilis a | / |
The effects of bacteria on muscle mass that had been proven in interventional studies.
Strengths and limitations
This is the first systematic review focusing on the gut–muscle axis, especially on sarcopenia. This review includes both clinical and pre‐clinical studies of gut microbiota, muscle, and/or physical performance in available databases, with a reproducible search approach. Our study highlights the key findings and issues under exploration in this field via summarizing and analysing current literatures. Moreover, we explained potential mechanisms with current evidence. There are several limitations of this review. We excluded athletes and patients with metabolic disorders, cancer, and secondary muscle wasting diseases because of discrepancies in mechanisms. Furthermore, due to the limited eligible studies, there was heterogeneity. More well‐designed mechanism research and clinical practice of the gut–muscle axis are required in the future.
Conclusions
The role of the gut–muscle axis is vital in both animals and humans. Absence of the gut microbiota was harmful to muscle growth, but its influences on physical performance were inconsistent. The gut microbiota can directly affect muscle phenotypes through faecal transplantation and supplements. Probiotics Lactobacillus, Bifidobacterium, and Saccharomyces strains, prebiotics inulin and curcumin, SCFAs, and some nutrient supplements (kefir, HMC diet, and OP) enhanced muscle mass or function by shifting the microbial community. Current potential pathways included protein synthesis and degradation, mitochondrial function, NMJ function, inflammation, energy, glucose, and lipid metabolism. As for sarcopenia, L. casei LC122, L. paracasei PS23, and B. longum BL986 supplements restored muscle loss in old rodents, but the causality of age‐related gut microbiota dysbiosis and muscle atrophy has not yet been proven. Whether antibiotic or exercise‐induced changes in muscle mass relies on the gut–muscle axis remains unclear.
Conflict of interest
None to declare.
Supporting information
Table S1. Characteristics and key finding of pre‐clinical studies.
Table S2. Characteristics and key finding of clinical studies.
Table S3. Abbreviation list.
Acknowledgements
There were no sources of funding. All authors certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 113
Liu C., Cheung W. H., Li J., Chow S. K.‐H., Yu J., Wong S. H., Ip M., Sung J. J. Y., and Wong R. M. Y. (2021) Understanding the gut microbiota and sarcopenia: a systematic review, Journal of Cachexia, Sarcopenia and Muscle, 12, 1393–1407, 10.1002/jcsm.12784.
References
- 1. Rosenberg IH. Epidemiologic and methodologic problems in determining nutritional status of older persons: summary comments. Am J Clin Nutr 1989;50:1231–1233. [PubMed] [Google Scholar]
- 2. Beaudart C, Rizzoli R, Bruyère O, Reginster J‐Y. Sarcopenia: burden and challenges for public health. Arch Public Health 2014;72:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle 2010;1:129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:300–307, e2. [DOI] [PubMed] [Google Scholar]
- 6. Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle 2019;10:485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wong RMY, Wong H, Zhang N, Chow SKH. The relationship between sarcopenia and fragility fracture—a systematic review. Osteoporos Int 2019;30:541–553. [DOI] [PubMed] [Google Scholar]
- 8. Kelley GA, Kelley KS. Is sarcopenia associated with an increased risk of all‐cause mortality and functional disability? Exp Gerontol 2017;96:100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goates S, Du K, Arensberg MB, Gaillard T, Guralnik J, Pereira SL. Economic impact of hospitalizations in US adults with sarcopenia. J Frailty Aging 2019;8:93–99. [DOI] [PubMed] [Google Scholar]
- 10. Pinedo‐Villanueva R, Westbury LD, Syddall HE, Sanchez‐Santos MT, Dennison EM, Robinson SM, et al. Health care costs associated with muscle weakness: a UK population‐based estimate. Calcif Tissue Int 2019;104:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Janssen I, Shepard D, Katzmarzyk P, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 2004;52:80–85. [DOI] [PubMed] [Google Scholar]
- 12. Kwak J, Kwon K‐S. Pharmacological interventions for treatment of sarcopenia: current status of drug development for sarcopenia. Ann Geriatric Med Res 2019;23:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cruz‐Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393:2636–2646. [DOI] [PubMed] [Google Scholar]
- 14. Hawley‐Hague H, Horne M, Campbell M, Demack S, Skelton DA, Todd C. Multiple levels of influence on older adults' attendance and adherence to community exercise classes. Gerontologist 2014;54:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Milanovic Z, Pantelic S, Trajkovic N, Sporis G, Kostic R, James N. Age‐related decrease in physical activity and functional fitness among elderly men and women. Clin Interv Aging 2013;8:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beaudart C, Dawson A, Shaw SC, Harvey NC, Kanis JA, Binkley N, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int 2017;28:1817–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamei Y, Hatazawa Y, Uchitomi R, Yoshimura R, Miura S. Regulation of skeletal muscle function by amino acids. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Dronkelaar C, van Velzen A, Abdelrazek M, van der Steen A, Weijs PJM, Tieland M. Minerals and sarcopenia; the role of calcium, iron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: a systematic review. J Am Med Dir Assoc 2018;19:6–11, e3. [DOI] [PubMed] [Google Scholar]
- 19. Yoshimura Y, Wakabayashi H, Yamada M, Kim H, Harada A, Arai H. Interventions for treating sarcopenia: a systematic review and meta‐analysis of randomized controlled studies. J Am Med Dir Assoc 2017;18:553e1–553e16. [DOI] [PubMed] [Google Scholar]
- 20. Robinson SM, Reginster JY, Rizzoli R, Shaw SC, Kanis JA, Bautmans I, et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin Nutr (Edinburgh, Scotland) 2018;37:1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bakhtiar SM, LeBlanc JG, Salvucci E, Ali A, Martin R, Langella P, et al. Implications of the human microbiome in inflammatory bowel diseases. FEMS Microbiol Lett 2013;342:10–17. [DOI] [PubMed] [Google Scholar]
- 22. Delzenne NM, Cani PD. Interaction between obesity and the gut microbiota: relevance in nutrition. Annu Rev Nutr 2011;31:15–31. [DOI] [PubMed] [Google Scholar]
- 23. Oliphant K, Allen‐Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by‐products and their impact on host health. Microbiome 2019;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krautkramer KA, Fan J, Backhed F. Gut microbial metabolites as multi‐kingdom intermediates. Nat Rev Microbiol 2020. [DOI] [PubMed] [Google Scholar]
- 25. Frampton J, Murphy KG, Frost G, Chambers ES. Short‐chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat Metab 2020;2:840–848. [DOI] [PubMed] [Google Scholar]
- 26. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 2021;19:55–71. [DOI] [PubMed] [Google Scholar]
- 27. O'Toole PW, Jeffery IB. Gut microbiota and aging. Science (New York, NY) 2015;350:1214–1215. [DOI] [PubMed] [Google Scholar]
- 28. Ticinesi A, Nouvenne A, Cerundolo N, Catania P, Prati B, Tana C, et al. Gut microbiota, muscle mass and function in aging: a focus on physical frailty and sarcopenia. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salazar N, Arboleya S, Fernandez‐Navarro T, de Los Reyes‐Gavilan CG, Gonzalez S, Gueimonde M. Age‐associated changes in gut microbiota and dietary components related with the immune system in adulthood and old age: a cross‐sectional study. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Picca A, Fanelli F, Calvani R, Mule G, Pesce V, Sisto A, et al. Gut dysbiosis and muscle aging: searching for novel targets against sarcopenia. Mediators Inflamm 2018;2018:7026198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bindels LB, Delzenne NM. Muscle wasting: the gut microbiota as a new therapeutic target? Int J Biochem Cell Biol 2013;45:2186–2190. [DOI] [PubMed] [Google Scholar]
- 32. Ni Y, Yang X, Zheng L, Wang Z, Wu L, Jiang J, et al. Lactobacillus and Bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol Nutr Food Res 2019;63:e1900603. [DOI] [PubMed] [Google Scholar]
- 33. Buigues C, Fernandez‐Garrido J, Pruimboom L, Hoogland AJ, Navarro‐Martinez R, Martinez‐Martinez M, et al. Effect of a prebiotic formulation on frailty syndrome: a randomized, double‐blind clinical trial. Int J Mol Sci 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hsu YJ, Chiu CC, Li YP, Huang WC, Huang YT, Huang CC, et al. Effect of intestinal microbiota on exercise performance in mice. J Strength Cond Res 2015;29:552–558. [DOI] [PubMed] [Google Scholar]
- 36. Rodriguez DM, Benninghoff AD, Aardema NDJ, Phatak S, Hintze KJ. Basal diet determined long‐term composition of the gut microbiome and mouse phenotype to a greater extent than fecal microbiome transfer from lean or obese human donors. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science (New York, NY) 2016;351:aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yan H, Diao H, Xiao Y, Li W, Yu B, He J, et al. Gut microbiota can transfer fiber characteristics and lipid metabolic profiles of skeletal muscle from pigs to germ‐free mice. Sci Rep 2016;6:31786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lahiri S, Kim H, Garcia‐Perez I, Reza MM, Martin KA, Kundu P, et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med 2019;11:eaan5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet‐induced obesity in germ‐free mice. Proc Natl Acad Sci U S A 2007;104:979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fielding RA, Reeves AR, Jasuja R, Liu C, Barrett BB, Lustgarten MS. Muscle strength is increased in mice that are colonized with microbiota from high‐functioning older adults. Exp Gerontol 2019;127:110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kundu P, Lee HU, Garcia‐Perez I, Tay EXY, Kim H, Faylon LE, et al. Neurogenesis and prolongevity signaling in young germ‐free mice transplanted with the gut microbiota of old mice. Sci Transl Med 2019;11:eaau4760. [DOI] [PubMed] [Google Scholar]
- 43. Siddharth J, Chakrabarti A, Pannérec A, Karaz S, Morin‐Rivron D, Masoodi M, et al. Aging and sarcopenia associate with specific interactions between gut microbes, serum biomarkers and host physiology in rats. Aging 2017;9:1698–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Langille MGI, Meehan CJ, Koenig JE, Dhanani AS, Rose RA, Howlett SE, et al. Microbial shifts in the aging mouse gut. Microbiome 2014;2:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu CS, Wei Q, Wang H, Kim DM, Balderas M, Wu G, et al. Protective effects of ghrelin on fasting‐induced muscle atrophy in aging mice. J Gerontol A Biol Sci Med Sci 2020;75:621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Soares ADN, Wanner SP, Morais ESS, Hudson ASR, Martins FS, Cardoso VN. Supplementation with Saccharomyces boulardii increases the maximal oxygen consumption and maximal aerobic speed attained by rats subjected to an incremental‐speed exercise. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen L‐H, Huang S‐Y, Huang K‐C, Hsu C‐C, Yang K‐C, Li L‐A, et al. Lactobacillus paracasei PS23 decelerated age‐related muscle loss by ensuring mitochondrial function in SAMP8 mice. Aging‐Us 2019;11:756–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen Y‐M, Wei L, Chiu Y‐S, Hsu Y‐J, Tsai T‐Y, Wang M‐F, et al. Lactobacillus plantarum TWK10 supplementation improves exercise performance and increases muscle mass in mice. Nutrients 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee MC, Hsu YJ, Ho HH, Hsieh SH, Kuo YW, Sung HC, et al. Lactobacillus salivarius subspecies salicinius SA‐03 is a new probiotic capable of enhancing exercise performance and decreasing fatigue. Microorganisms 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang WC, Hsu YJ, Huang CC, Liu HC, Lee MC. Exercise training combined with Bifidobacterium longum OLP‐01 supplementation improves exercise physiological adaption and performance. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Giacco A, Delli Paoli G, Simiele R, Caterino M, Ruoppolo M, Bloch W, et al. Exercise with food withdrawal at thermoneutrality impacts fuel use, the microbiome, AMPK phosphorylation, muscle fibers, and thyroid hormone levels in rats. Physiol Rep 2020;8:e14354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen YM, Chiu WC, Chiu YS, Li T, Sung HC, Hsiao CY. Supplementation of nano‐bubble curcumin extract improves gut microbiota composition and exercise performance in mice. Food Funct 2020;11:3574–3584. [DOI] [PubMed] [Google Scholar]
- 53. Hsu Y‐J, Huang W‐C, Lin J‐S, Chen Y‐M, Ho S‐T, Huang C‐C, et al. Kefir supplementation modifies gut microbiota composition, reduces physical fatigue, and improves exercise performance in mice. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hsu TH, Chiu CC, Wang YC, Chen TH, Chen YH, Lee YP, et al. Supplementation with beef extract improves exercise performance and reduces post‐exercise fatigue independent of gut microbiota. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xiao M, Lin L, Chen H, Ge X, Huang Y, Zheng Z, et al. Anti‐fatigue property of the oyster polypeptide fraction and its effect on gut microbiota in mice. Food Funct 2020;11:8659–8669. [DOI] [PubMed] [Google Scholar]
- 56. Wroblewska B, Juskiewicz J, Kroplewski B, Jurgonski A, Wasilewska E, Zlotkowska D, et al. The effects of whey and soy proteins on growth performance, gastrointestinal digestion, and selected physiological responses in rats. Food Funct 2018;9:1500–1509. [DOI] [PubMed] [Google Scholar]
- 57. Manickam R, Oh HYP, Tan CK, Paramalingam E, Wahli W. Metronidazole causes skeletal muscle atrophy and modulates muscle chronometabolism. Int J Mol Sci 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Okamoto T, Morino K, Ugi S, Nakagawa F, Lemecha M, Ida S, et al. Microbiome potentiates endurance exercise through intestinal acetate production. Am J Physiol Endocrinol Metab 2019;316:E956–e66. [DOI] [PubMed] [Google Scholar]
- 59. Nay K, Jollet M, Goustard B, Baati N, Vernus B, Pontones M, et al. Gut bacteria are critical for optimal muscle function: a potential link with glucose homeostasis. Am J Physiol Endocrinol Metab 2019;317:E158–E171. [DOI] [PubMed] [Google Scholar]
- 60. Picca A, Ponziani FR, Calvani R, Marini F, Biancolillo A, Coelho‐Junior HJ, et al. Gut microbial, inflammatory and metabolic signatures in older people with physical frailty and sarcopenia: results from the BIOSPHERE study. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Castro‐Mejía JL, Khakimov B, Krych L, Bulow J, Bechshoft RL, Hojfeldt G, et al. Physical fitness in community‐dwelling older adults is linked to dietary intake, gut microbiota, and metabolomic signatures. Aging Cell 2020;19:e13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Castellanos N, Diez GG, Antunez‐Almagro C, Bailen M, Bressa C, Gonzalez Soltero R, et al. A critical mutualism–competition interplay underlies the loss of microbial diversity in sedentary lifestyle. Front Microbiol 2020;10:3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bressa C, Bailén‐Andrino M, Pérez‐Santiago J, González‐Soltero R, Pérez M, Montalvo‐Lominchar MG, et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017;12:e0171352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Aoyagi Y, Amamoto R, Park S, Honda Y, Shimamoto K, Kushiro A, et al. Independent and interactive effects of habitually ingesting fermented milk products containing Lactobacillus casei strain Shirota and of engaging in moderate habitual daily physical activity on the intestinal health of older people. Front Microbiol 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zou H, Wang D, Ren H, Cai K, Chen P, Fang C, et al. Effect of caloric restriction on BMI, gut microbiota, and blood amino acid levels in non‐obese adults. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Morita E, Yokoyama H, Imai D, Takeda R, Ota A, Kawai E, et al. Aerobic exercise training with brisk walking increases intestinal bacteroides in healthy elderly women. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Huang WC, Lee MC, Lee CC, Ng KS, Hsu YJ, Tsai TY, et al. Effect of Lactobacillus plantarum TWK10 on exercise physiological adaptation, performance, and body composition in healthy humans. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Valentini Neto J, de Melo CM, Lima Ribeiro SM. Effects of three‐month intake of synbiotic on inflammation and body composition in the elderly: a pilot study. Nutrients 2013;5:1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chinwalla AT, Cook LL, Delehaunty KD, Fewell GA, Fulton LA, Fulton RS, et al. Initial sequencing and comparative analysis of the mouse genome. Nature 2002;420:520–562. [DOI] [PubMed] [Google Scholar]
- 71. Liu YX, Qin Y, Chen T, Lu M, Qian X, Guo X, et al. A practical guide to amplicon and metagenomic analysis of microbiome data. Protein Cell 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bindels LB, Beck R, Schakman O, Martin JC, De Backer F, Sohet FM, et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS ONE 2012;7:e37971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hatter JA, Kouche YM, Melchor SJ, Ng K, Bouley DM, Boothroyd JC, et al. Toxoplasma gondii infection triggers chronic cachexia and sustained commensal dysbiosis in mice. PLoS ONE 2018;13:e0204895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Das Neves RX, Roy S, Dzutsev A, Huang A, Smith L, Difilippantonio S, et al. Germ free mice accelerate cachexia‐associated cancer. Cancer Res 2017;77. [Google Scholar]
- 75. Farshidfar F, Pinder MA, Myrie SB. Creatine supplementation and skeletal muscle metabolism for building muscle mass—review of the potential mechanisms of action. Curr Protein Pept Sci 2017;18:1273–1287. [DOI] [PubMed] [Google Scholar]
- 76. Lee J, Venna VR, Durgan DJ, Shi H, Hudobenko J, Putluri N, et al. Young versus aged microbiota transplants to germ‐free mice: increased short‐chain fatty acids and improved cognitive performance. Gut Microbes 2020;1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lee J, d'Aigle J, Atadja L, Quaicoe V, Honarpisheh P, Ganesh BP, et al. Gut microbiota‐derived short‐chain fatty acids promote poststroke recovery in aged mice. Circ Res 2020;127:453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Staley C, Khoruts A, Sadowsky MJ. Contemporary applications of fecal microbiota transplantation to treat intestinal diseases in humans. Arch Med Res 2017;48:766–773. [DOI] [PubMed] [Google Scholar]
- 79. Martin R, Miquel S, Benevides L, Bridonneau C, Robert V, Hudault S, et al. Functional characterization of novel Faecalibacterium prausnitzii strains isolated from healthy volunteers: a step forward in the use of F. prausnitzii as a next‐generation probiotic. Front Microbiol 2017;8:1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Katsuki R, Sakata S, Nakao R, Oishi K, Nakamura Y. Lactobacillus curvatus CP2998 prevents dexamethasone‐induced muscle atrophy in C2C12 myotubes. J Nutr Sci Vitaminol 2019;65:455–458. [DOI] [PubMed] [Google Scholar]
- 81. Davani‐Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Desbuards N, Gourbeyre P, Haure‐Mirande V, Darmaun D, Champ M, Bodinier M. Impact of perinatal prebiotic consumption on gestating mice and their offspring: a preliminary report. Br J Nutr 2012;107:1245–1248. [DOI] [PubMed] [Google Scholar]
- 83. Walsh ME, Bhattacharya A, Sataranatarajan K, Qaisar R, Sloane L, Rahman MM, et al. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015;14:957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Barger K, Langsetmo L, Orwoll ES, Lustgarten MS. Investigation of the diet‐gut‐muscle axis in the osteoporotic fractures in men study. J Nutr Health Aging 2020;24:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chalé A, Cloutier GJ, Hau C, Phillips EM, Dallal GE, Fielding RA. Efficacy of whey protein supplementation on resistance exercise–induced changes in lean mass, muscle strength, and physical function in mobility‐limited older adults. J Gerontology: Ser A 2013;68:682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lustgarten MS, Price LL, Chalé A, Fielding RA. Metabolites related to gut bacterial metabolism, peroxisome proliferator‐activated receptor‐alpha activation, and insulin sensitivity are associated with physical function in functionally‐limited older adults. Aging Cell 2014;13:918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ren G, Zhang J, Li M, Tang Z, Yang Z, Cheng G, et al. Gut microbiota composition influences outcomes of skeletal muscle nutritional intervention via blended protein supplementation in posttransplant patients with hematological malignancies. Clin Nutr (Edinburgh, Scotland) 2020. [DOI] [PubMed] [Google Scholar]
- 88. Beckett CL, Harbarth S, Huttner B. Special considerations of antibiotic prescription in the geriatric population. Clin Microbiol Infect 2015;21:3–9. [DOI] [PubMed] [Google Scholar]
- 89. Zhang XY, Chen J, Yi K, Peng L, Xie J, Gou X, et al. Phlorizin ameliorates obesity‐associated endotoxemia and insulin resistance in high‐fat diet‐fed mice by targeting the gut microbiota and intestinal barrier integrity. Gut microbes 2020;12:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Candow DG, Forbes SC, Chilibeck PD, Cornish SM, Antonio J, Kreider RB. Effectiveness of creatine supplementation on aging muscle and bone: focus on falls prevention and inflammation. J Clin Med 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age‐related sarcopenia. Front Physiol 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bao Z, Cui C, Chow SK, Qin L, Wong RMY, Cheung WH. AChRs degeneration at NMJ in aging‐associated sarcopenia—a systematic review. Front Aging Neurosci 2020;12:597811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. De Spiegeleer A, Elewaut D, Van Den Noortgate N, Janssens Y, Debunne N, Van Langenhove S, et al. Quorum sensing molecules as a novel microbial factor impacting muscle cells. Biochim et Biophys Acta Molecular Basis Dis 1866;2020:165646. [DOI] [PubMed] [Google Scholar]
- 94. Song J, Wang C, Long D, Li Z, You L, Brand‐Saberi B, et al. Dysbacteriosis‐induced LPS elevation disturbs the development of muscle progenitor cells by interfering with retinoic acid signaling. FASEB J 2020. [DOI] [PubMed] [Google Scholar]
- 95. Doyle A, Zhang G, Abdel Fattah EA, Eissa NT, Li YP. Toll‐like receptor 4 mediates lipopolysaccharide‐induced muscle catabolism via coordinate activation of ubiquitin‐proteasome and autophagy‐lysosome pathways. FASEB J 2011;25:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Viaene L, Thijs L, Jin Y, Liu Y, Gu Y, Meijers B, et al. Heritability and clinical determinants of serum indoxyl sulfate and p‐cresyl sulfate, candidate biomarkers of the human microbiome enterotype. PLoS ONE 2014;9:e79682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sato E, Mori T, Mishima E, Suzuki A, Sugawara S, Kurasawa N, et al. Metabolic alterations by indoxyl sulfate in skeletal muscle induce uremic sarcopenia in chronic kidney disease. Sci Rep 2016;6:36618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Saoi M, Li A, McGlory C, Stokes T, von Allmen MT, Phillips SM, et al. Metabolic perturbations from step reduction in older persons at risk for sarcopenia: plasma biomarkers of abrupt changes in physical activity. Metabolites 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Enoki Y, Watanabe H, Arake R, Sugimoto R, Imafuku T, Tominaga Y, et al. Indoxyl sulfate potentiates skeletal muscle atrophy by inducing the oxidative stress‐mediated expression of myostatin and atrogin‐1. Sci Rep 2016;6:32084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Changchien CY, Lin YH, Cheng YC, Chang HH, Peng YS, Chen Y. Indoxyl sulfate induces myotube atrophy by ROS‐ERK and JNK‐MAFbx cascades. Chem Biol Interact 2019;304:43–51. [DOI] [PubMed] [Google Scholar]
- 101. Jheng JR, Chen YS, Ao UI, Chan DC, Huang JW, Hung KY, et al. The double‐edged sword of endoplasmic reticulum stress in uremic sarcopenia through myogenesis perturbation. J Cachexia Sarcopenia Muscle 2018;9:570–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Thome T, Salyers ZR, Kumar RA, Hahn D, Berru FN, Ferreira LF, et al. Uremic metabolites impair skeletal muscle mitochondrial energetics through disruption of the electron transport system and matrix dehydrogenase activity. Am J Physiol Cell Physiol 2019;317:C701–C713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Enoki Y, Watanabe H, Arake R, Fujimura R, Ishiodori K, Imafuku T, et al. Potential therapeutic interventions for chronic kidney disease‐associated sarcopenia via indoxyl sulfate‐induced mitochondrial dysfunction. J Cachexia Sarcopenia Muscle 2017;8:735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Koppe L, Pillon NJ, Vella RE, Croze ML, Pelletier CC, Chambert S, et al. p‐Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol 2013;24:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lavelle A, Sokol H. Gut microbiota‐derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2020;17:223–237. [DOI] [PubMed] [Google Scholar]
- 106. Kobayashi Y, Hara N, Sugimoto R, Mifuji‐Moroka R, Tanaka H, Eguchi A, et al. The associations between circulating bile acids and the muscle volume in patients with non‐alcoholic fatty liver disease (NAFLD). Intern Med 2017;56:755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. van Tongeren SP, Slaets JP, Harmsen HJ, Welling GW. Fecal microbiota composition and frailty. Appl Environ Microbiol 2005;71:6438–6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chen YM, Liao CC, Huang YC, Chen MY, Huang CC, Chen WC, et al. Proteome and microbiota analysis highlight Lactobacillus plantarum TWK10 supplementation improves energy metabolism and exercise performance in mice. Food Sci Nutr 2020;8:3525–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Varian BJ, Goureshetti S, Poutahidis T, Lakritz JR, Levkovich T, Kwok C, et al. Beneficial bacteria inhibit cachexia. Oncotarget 2016;7:11803–11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhang S, Ermann J, Succi MD, Zhou A, Hamilton MJ, Cao B, et al. An inflammation‐targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci Transl Med 2015;7:300ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Praveschotinunt P, Duraj‐Thatte AM, Gelfat I, Bahl F, Chou DB, Joshi NS. Engineered E. coli Nissle 1917 for the delivery of matrix‐tethered therapeutic domains to the gut. Nat Commun 2019;10:5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ting SY, Martinez‐Garcia E, Huang S, Bertolli SK, Kelly KA, Cutler KJ, et al. Targeted depletion of bacteria from mixed populations by programmable adhesion with antagonistic competitor cells. Cell Host Microbe 2020;28:313–321, e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics and key finding of pre‐clinical studies.
Table S2. Characteristics and key finding of clinical studies.
Table S3. Abbreviation list.


