Thanks to the use of combination antiretroviral treatment (cART) people living with HIV (PLWH) have a similar life expectancy to HIV‐negative people. 1 , 2 However, PLWH may be at higher risk to develop chronic diseases associated with persistent immune activation despite virological control. 3 Physical activity has been demonstrated because to improve health parameters in PLWH. 4 We previously showed in a pilot study that a 12 week protocol consisting of three sessions per week of 60 min brisk walking at 65–75% of maximal heart rate with (strength‐walk group) 30 min resistance training or without (walk group), improved physical performance, lipid profile, and inflammatory markers. 5
We assessed body composition and bone health in a subset of 25 cART‐treated PLWH (Table S1) from this study [19/25 men, median age: 51 (Q1–Q3: 48–56) years; CD4+: 576 (463–701)], with particular attention to the indicators of osteosarcopenia. Percentage of fat mass (FM), fat free mass (FFM) at arms, limbs, and as total body, and bone mineral density (BMD), t‐score and z‐score at spine (L3/L4), femoral neck, and trochanter were measured by DEXA (Lunar Prodigy, version 8.8, GE, Medical System Madison, WI). The appendicular skeletal muscle mass (ASMMI) index was calculated to assess the presence of sarcopenia (women: ≤5.5 kg/m2; men: ≤7.0 kg/m2). 6 The bone t‐score was calculated to assess the presence of osteoporosis (−2.5) and osteopenia (−1 to −2.5). Bone remodelling biomarkers were measured in cryopreserved plasma samples by commercially available enzyme‐linked immunosorbent assays (R&D Systems Inc, Minneapolis, MN, USA). These included osteoprotegerin (OPG), receptor activator of NF‐kappaB ligand (RANKL), c‐terminal telopeptide (CTX), and bone alkaline phosphatase (BAP). Samples were analysed in batch at the end of the study and blindly with respect to group assignment. Quantitative variables were expressed as median, first, and third quartiles (Q1–Q3). Per cent changes between baseline (BL) and after 12 week of training W12 within each group were assessed by Wilcoxon signed rank test and between‐groups by Mann–Whitney test.
All participants completed the 12 week programme with a median adherence of 64% (Q1–Q3: 59–75%). Among all participants, significant W12 changes in ASMMI from BL were observed in both training groups, and in total, arms and legs FFM in the strength‐walk group, with changes from BL significantly larger in the strength‐walk than in the walk group for all measurements (Figure 1). According to ASMMI, eight participants (32%; three women and five men) had sarcopenia at baseline [women: 5.5 (5.4–5.5) kg/m2; men: 6.5 (6.4–6.7) kg/m2; age 52 (48–53)]. At Week 12, six of these eight participants normalized ASMMI, including two women [both in the walk group: 6.1 (6.0–6.1) kg/m2] and four men [two of the walk group and two of the strength‐walk group: 7.2 (7.1–7.2) kg/m2]. Neither significant W12 changes from BL nor significant change differences between groups were observed for BMD, t‐score, z‐score at spine, femoral neck, and trochanter. According to the t‐score, three participants were diagnosed with osteoporosis at BL (12%) and 20 (80%) with osteopenia. At the end of the training protocol, none of the participants improved the t‐score (Table 1). Significant W12 increases from BL were observed in CTX in both training groups. No significant changes were observed of BAP, OPG and RANKL level, and OPG/RANK ratio (Table 1).
Figure 1.
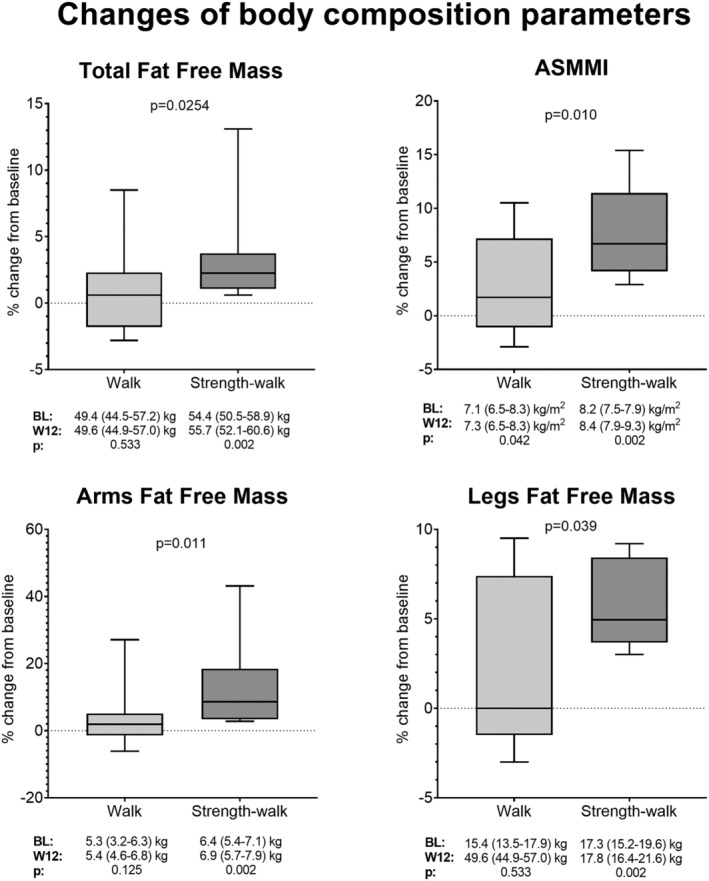
Changes of body composition parameters in people living with HIV following a 12 week physical activity protocol of walk or strength‐walk exercise. Data were assessed by Mann–Whitney test (differences between‐groups) and Wilcoxon signed rank test (changes between BL and W16 within‐groups, below graphs). Level of significance was set at 0.05. Box plots show median and interquartile ranges, and whiskers extend to the highest and lowest observations. ASMMI, appendicular skeletal muscle mass index, BL, baseline; W12, week 12.
Table 1.
Bone mineral density, t‐score, z‐score results, and bone remodelling biomarkers results
| Walk (n = 15) | Strength‐walk (n = 10) | |||||
|---|---|---|---|---|---|---|
| BL | W12 | P | BL | W12 | P | |
| Spine | ||||||
| BMD (g/cm2) | 61.5 (56.8–64.8) | 62.4 (57.5–65.0) | 0.135 | 62.2 (53.9–64.2) | 62.3 (54.5–64.6) | 0.109 |
| t‐score | −1.2 (−1.6 to –0.8) | −1.1 (−1.5 to –0.7) | 0.173 | −1.4 (−1.9 to –1.2) | −1.3 (1.8 to –0.9) | 0.141 |
| z‐score | −1.0 (−1.4 to –0.7) | −0.8 (−1.5 to –0.2) | 0.113 | −1.4 (−1.8 to –0.8) | −1.2 (−2.0 to –0.7) | 0.137 |
| Femoral neck | ||||||
| BMD (g/cm2) | 4.3 (3.7–5.1) | 4.0 (4.8–5.4) | 0.107 | 5.2 (4.3–5.9) | 5.2 (4.3–5.9) | 0.711 |
| t‐score | −1.6 (−1.9 to –0.4) | −1.5 (−1.8 to –0.3) | 0.201 | −0.9 (−1.0 to –0.5) | −0.8 (−1.1 to –0.4) | 0.468 |
| z‐score | −1.1 (−1.6 to –0.2) | −0.9 (−1.3 to –0.3) | 0.147 | −1.3 (−1.5 to –0.8) | −1.1 (−1.5 to –0.2) | 0.297 |
| Trocanther | ||||||
| BMD (g/cm2) | 8.3 (6.7–9.7) | 8.6 (6.8–9.8) | 0.229 | 9.7 (8.7–10.3) | 9.4 (8.7–10.6) | 0.642 |
| t‐score | −1.5 (−2–2 to –0.2) | −1.5 (−2.1 to –0.1) | 0.108 | −0.6 (−1.2 to –0.1) | −0.7 (−1.2 to –0.2) | 0.394 |
| z‐score | −0.9 (−1.5 to –0.1) | −1.0 (−1.7 to –0.1) | 0.688 | −0.4 (−0.9 to –0.1) | −0.8 (−1.3 to –0.2) | 0.203 |
| Bone remodelling biomarkers | ||||||
| OPG (pg/mL) | 1393 (1244–2418) | 1617 (1253–2240) | 0.107 | 1324 (957–1658) | 1243 (1123–1516) | 0.769 |
| RANKL (pg/mL) | 4.92 (3.23–7.78) | 4.96 (2.62–7.78) | 0.497 | 4.96 (2.62–5.88) | 4.36 (2.91–6.57) | 0.921 |
| OPG/RANKL | 325 (231–492) | 354 (195–433) | 0.978 | 228 (173–310) | 212 (169–306) | 0.193 |
| CTX (pg/mL) | 0.54 (0.32–0.84) | 0.76 (0.57–0.92) | 0.034 | 0.61 (0.36–0.67) | 0.76 (0.46–0.87) | 0.005 |
| BAP (ng/mL) | 8.44 (5.42–9.71) | 7.10 (5.29–9.02) | 0.124 | 8.63 (5.87–11.67) | 6.83 (6.12–10.09) | 0.185 |
BAP, bone alkaline phosphatase; BL, baseline; BMD, bone mineral density; CTX, c‐terninal telopeptide; OPG, osteoprotegerin; RANKL, receptor activator of NF‐kappaB ligand; W12, week 12.
Values are expressed as median (Q1–Q3). Data were assessed by Wilcoxon signed rank test (changes between BL and W12 within groups and by BMD).
The main finding of this study was a significant increase in muscle mass in PLWH including patients with sarcopenia. This is relevant because sarcopenia is an emerging health issue in HIV infection, 7 , 8 where both ageing and persistent immune activation may contribute to its development, in addition to other potential factors, like cART, risk behaviours, for example, smoking or use of drugs, and other co‐morbidities. 9 Although moderate aerobic activity alone was also associated in this study with increased appendicular muscle mass, the benefit of concurrent and resistance training was significantly superior, confirming that resistance exercise is relevant to counteract sarcopenia in PLWH. 10 , 11 We did not observe improvements of DEXA bone parameters following the 12 week moderate‐intensity exercise protocol. It is possible that this exercise intervention was too short to increase bone mineralization, relative to the length of a bone remodelling cycle. 12 , 13 On the other hand, we observed a significant increase of CTX plasma level in both training groups, with neither change of the classical bone formation marker BAP nor of the plasma OPG/RANKL concentration ratio as additional index of bone resorption. The increase of CTX plasma levels following exercise may thus represent a feedback stimulus for the reparative activity of the bone‐remodelling unit. 14
Our study has some limitations. First, we did not include a non‐exercise control group. Second, the low sample size did not allow drawing firm conclusions on the efficacy of the two training protocols on the studied parameters. Finally, this was a relatively short‐duration study.
In conclusions, PLWH following a physical activity protocol based on the combination of resistance and moderate aerobic training is likely to improve to improve total and appendicular muscle mass. Our study provides information to design larger interventional controlled studies to assess the efficacy of exercise protocols in increasing muscle mass and potentially reducing sarcopenia in PLWH.
Funding
This work was supported by unrestricted grants from AbbVie and ViiV Healthcare to Associazione Solidarietà AIDS (ASA) and Associazione Nazionale Lotta all'AIDS (ANLAIDS), Italy. This study was supported also by the Italian Ministry of Health (Ricerca Corrente).
Conflict of interest
The authors have no conflict of interest to declare.
Supporting information
Table S1. Participants' characteristics at baseline.
Table Note. Values are either expressed as number of participants (%) or as median (Q1‐Q3). Data were compared between groups by Mann–Whitney and Fisher Exact tests. P values were not significant for all parameters. a: chronic treatment, with no changes during the training period or the 6 weeks before; BMI: Body Mass Index; VACS: Veterans Aging Cohort Study Risk index (this index includes: i) age; ii) laboratory tests: white blood cell count, HIV‐1 RNA, hemoglobin, platelets, AST, ALT, creatinine; iii) liver fibrosis (FIB‐4): composed of AST, ALT, platelets and age; iv) impaired renal function (eGFR): composed of age, gender, race and creatinine; v) HCV status: if the patient ever had a positive antibody test or detectable virus prior the study); NRTI: nucleoside reverse transcriptase inhibitors; NNRTI; non‐nucleoside reverse transcriptase inhibitors; HDL‐C: High density lipoprotein cholesterol; SBP: systolic blood pressure. *Among the inclusion criteria were either objective evidence of lipodystrophy, as established by the visiting physician, or of at least one of the Adult Treatment Panel III definition criteria of the metabolic syndrome.
Bonato M., Galli L., Bossolasco S., Bertocchi C., Balconi G., Borderi M., Viale P., Pavei G., Merati G., La Torre A., Lazzarin A., Banfi G., and Cinque P. (2021) Benefits of a 12 week physical activity programme on muscle and bone health in people living with HIV, Journal of Cachexia, Sarcopenia and Muscle, 12, 1613–1616, 10.1002/jcsm.12824
References
- 1. Kaplan‐Lewis E, Aberg JA, Lee M. Aging with HIV in the ART era. Semin Diagn Pathol 2017;34:384–397. [DOI] [PubMed] [Google Scholar]
- 2. Bloch M, John M, Smith D, Rasmussen TA, Wright E. Managing HIV‐associated inflammation and ageing in the era of modern ART. HIV Med 2020;21:2–16. [DOI] [PubMed] [Google Scholar]
- 3. Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol 2013; 10.1016/B978-0-12-407707-2.00002-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ozemek C, Erlandson KM, Jankowski CM. Physical activity and exercise to improve cardiovascular health for adults living with HIV. Prog Cardiovasc Dis 2020;63:178–183. [DOI] [PubMed] [Google Scholar]
- 5. Bonato M, Galli L, Passeri L, Longo V, Pavei G, Bossolasco S, et al. A pilot study of brisk walking in sedentary combination antiretroviral treatement (cART)‐ treated patients: benefit on soluble and cell inflammatory markers. BMC Infect Dis 2017;17:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cruz‐Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393:2636–2646. [DOI] [PubMed] [Google Scholar]
- 7. Grant PM, Kitch D, McComsey GA, Collier AC, Bartali B, Koletar SL, et al. Long‐term body composition changes in antiretroviral‐treated HIV‐infected individuals. AIDS 2016;30:2805–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hawkins KL, Brown TT, Margolick JB, Erlandson KM. Geriatric syndromes: new frontiers in HIV and sarcopenia. AIDS 2017;31:S137–S146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonato M, Turrini F, Galli L, Banfi G, Cinque P. The role of physical activity for the management of sarcopenia in people living with HIV. Int J Environ Res Public Health 2020;17: 10.3390/ijerph17041283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonato M, Turrini F, DE Zan V, Meloni A, Plebani M, Brambilla E, et al. A mobile application for exercise intervention in people living with HIV. Med Sci Sports Exerc 2020;52:425–433. [DOI] [PubMed] [Google Scholar]
- 11. Gomes‐Neto M, Conceição CS, Oliveira Carvalho V, Brites C. A systematic review of the effects of different types of therapeutic exercise on physiologic and functional measurements in patients with HIV/AIDS. Clinics 2013;68:1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shojaa M, Von Stengel S, Schoene D, Kohl M, Barone G, Bragonzoni L, et al. Effect of exercise training on bone mineral density in post‐menopausal women: a systematic review and meta‐analysis of intervention studies. Front Physiol 2020;11: 10.3389/fphys.2020.00652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Banfi G, Lombardi G, Colombini A, Lippi G. Bone metabolism markers in sports medicine. Sports Med 2010;40:697–714. [DOI] [PubMed] [Google Scholar]
- 14. Johansson H, Odén A, Kanis JA, McCloskey EV, Morris HA, Cooper C, et al. A meta‐analysis of reference markers of bone turnover for prediction of fracture. Calcif Tissue Int 2014;94:560–567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Participants' characteristics at baseline.
Table Note. Values are either expressed as number of participants (%) or as median (Q1‐Q3). Data were compared between groups by Mann–Whitney and Fisher Exact tests. P values were not significant for all parameters. a: chronic treatment, with no changes during the training period or the 6 weeks before; BMI: Body Mass Index; VACS: Veterans Aging Cohort Study Risk index (this index includes: i) age; ii) laboratory tests: white blood cell count, HIV‐1 RNA, hemoglobin, platelets, AST, ALT, creatinine; iii) liver fibrosis (FIB‐4): composed of AST, ALT, platelets and age; iv) impaired renal function (eGFR): composed of age, gender, race and creatinine; v) HCV status: if the patient ever had a positive antibody test or detectable virus prior the study); NRTI: nucleoside reverse transcriptase inhibitors; NNRTI; non‐nucleoside reverse transcriptase inhibitors; HDL‐C: High density lipoprotein cholesterol; SBP: systolic blood pressure. *Among the inclusion criteria were either objective evidence of lipodystrophy, as established by the visiting physician, or of at least one of the Adult Treatment Panel III definition criteria of the metabolic syndrome.


