Abstract
Background
Recent evidence from cross‐sectional and longitudinal studies supports the hypothesis that sarcopenia is associated with worsening cognitive function. However, primary evidence largely comes from high‐income countries, whereas in low‐ and middle‐income countries, this association has been underexplored. This study aimed to estimate the longitudinal association between sarcopenia and mild cognitive impairment in a sample of older Mexican adults.
Methods
Data come from the three waves of the World Health Organization (WHO) Study on Global AGEing and Adult Health (SAGE) in Mexico (2009, 2014, 2017). Four hundred ninety‐six older adults, aged ≥50, were included. Sarcopenia was defined as having low muscle quantity and either/both slow gait speed and weak handgrip strength. Mild cognitive impairment was determined based on the recommendations of the National Institute on Aging‐Alzheimer's Association. Cognitive function was evaluated by a composite cognitive score of five different cognitive tests: immediate and delayed recall, forward and backward digit span and semantic verbal fluency. Three‐level mixed‐effects models (logistic and linear) were used to estimate the longitudinal associations between sarcopenia, mild cognitive impairment and cognitive function.
Results
The prevalence of mild cognitive impairment (8.9%, 12.9%, 16.0%) and sarcopenia (10.5%, 20.7%, 23.3%) showed a significant temporal increase for Waves 1, 2 and 3 (P‐value < 0.01, respectively). The presence of sarcopenia was significantly associated with mild cognitive impairment (OR = 1.74; CI95% 1.02, 2.96; P = 0.04) and worse cognitive function (β = −0.57; CI95% ‐0.93, −0.21; P < 0.01). We observed significant associations between sarcopenia and immediate verbal recall (β = −0.14; CI95% −0.28, −0.01; P = 0.04), delayed verbal recall (β = −0.12; CI95% −0.23, −0.01; P = 0.03) and semantic verbal fluency (β = −0.17; CI95% −0.28, −0.05; P = 0.01). The prevalence of mild cognitive impairment increased at an annual rate of 0.8% for non‐sarcopenic older adults, but nearly 1.5% for sarcopenic adults.
Conclusions
Significant longitudinal associations were observed between sarcopenia, mild cognitive impairment and cognitive function among older Mexican adults. Public health strategies, including policy research and clinical interventions, must be implemented in low‐ and middle‐income countries in order to reduce or delay the onset of sarcopenia and thus improve population‐level cognitive health among older adults.
Keywords: Sarcopenia; Mild cognitive impairment; Cognitive function; Older adults
Introduction
Sarcopenia is a complex geriatric syndrome characterized by progressive and generalized loss of skeletal muscle mass (SMM), muscle strength and/or physical performance. 1 Loss of total muscle mass begins in midlife and accelerates up to 10% per decade around the age of 65. 2 Sarcopenia has been associated with adverse health outcomes such as risk of falls and fractures, mental disorders, loss of independence, increasing mortality, healthcare costs and poor quality of life. 3 Due to these deleterious effects, sarcopenia has become a subject of increased focus in the clinical geriatrics and the ageing policy research.
Cognitive function (CF) naturally decreases as we age, and when declining at a normal pace, will not significantly affect the functional capacity of the older adults (OA) (i.e. basic and instrumental activities of the daily life). 4 However, when this decline is greater than expected, it can result in mild cognitive impairment (MCI) or dementia, leading to a loss of abilities and changes in behaviour. 5
The definition of sarcopenia varies according to the parameters and tools used to determine muscle mass, muscle strength and physical performance. A wide range of sarcopenia prevalence has been reported, ranging from 0 to 15% in healthy OA and 2 to 34% in geriatric outpatients. 6 In Mexico, prevalence ranges from 9.3 to 33.6%. 7 Like sarcopenia, the criteria for diagnosing MCI have been controversial. However, the inclusion of different conditions such as self‐reported cognition changes, cognitive impairment in one or more domains, functional independence and no dementia is widely accepted. 8 MCI has been observed between 3 and 22% worldwide, 8 whereas in Mexico the prevalence ranges between 6 and 16.5%. 9 , 10 It is projected that, in low‐ and middle‐income countries, MCI and sarcopenia will only continue to increase, 5 , 11 making it even more important to understand how these two conditions may be associated.
Ageing plays an important role for the development of sarcopenia and MCI. In addition, there are similar factors that predispose these conditions (sex, physical inactivity and other modifiable factors like diet or nutritional status). 12 , 13 These findings led to hypothesize on a common mechanism, for both conditions, that is enhanced by the role of hormones, chronic inflammation and oxidative stress. 14 , 15 Recent evidence seems to support the hypothesis that sarcopenia is associated with a worse CF and MCI, both in cross‐sectional and longitudinal studies. Peng et al., in the most recent review of cross‐sectional studies, evidenced a significant association between sarcopenia and MCI. 16 Evidence has not been conclusive from longitudinal data studies. In one study, OA with sarcopenia had lower scores on the Mini‐Mental State Examination (MMSE) at a 1‐year follow‐up when compared with baseline. 17 On the other hand, in two cohort studies with French women and Korean OA, no significant association was reported between sarcopenia and MCI. 18 , 19
Given that the evidence has not been conclusive, and the studies have been confined to high‐income countries, whereas in low‐ and middle‐income countries there are no studies that have explored this association, the aim of this study was to estimate the longitudinal association between sarcopenia and MCI as well as with CF in a sample of older Mexican adults.
Methods
Population and sample
We used data from three waves of the World Health Organization (WHO) Study on Global AGEing and Adult Health (SAGE) in Mexico. A multi‐country, longitudinal study, SAGE was based on nationally representative samples of individuals aged 50 + years. It has been conducted in six countries, namely, China, Ghana, India, Mexico, Russia and South Africa, with different geographic distributions, population sizes, income levels (low and medium) and demographic as well as epidemiological transition phases. To date, SAGE has three longitudinal measurements in Mexico—Wave 1 (2009), Wave 2 (2014) and Wave 3 (2017). Details on the study design have been published elsewhere. 20
Mexican sample
For the SAGE Mexican sample, data for Wave 1 (baseline) were collected between July and September 2009, with a total sample of 2312 respondents aged 50+. Wave 2 data were collected between July and October 2014 with a sample of 2516 individuals, and Wave 3 from August to November 2017 with 2407 participants. Overall, a response rate of 83% was observed along the three waves.
Subsample for the sarcopenia measurements
In Wave 1, measurements on physical performance (Short Physical Performance Battery [SPPB]) were obtained from a subsample of 650 OA. From these, 496 had the three follow‐up measurements completed. A 12% mortality and 11.6% attrition rates were observed. Excluded participants, when compared with the final sample, skewed older and had a higher prevalence of both frailty and multimorbidity (P < 0.05). The final analytical sample was composed of 496 OA aged 50+ (Figure 1).
Figure 1.
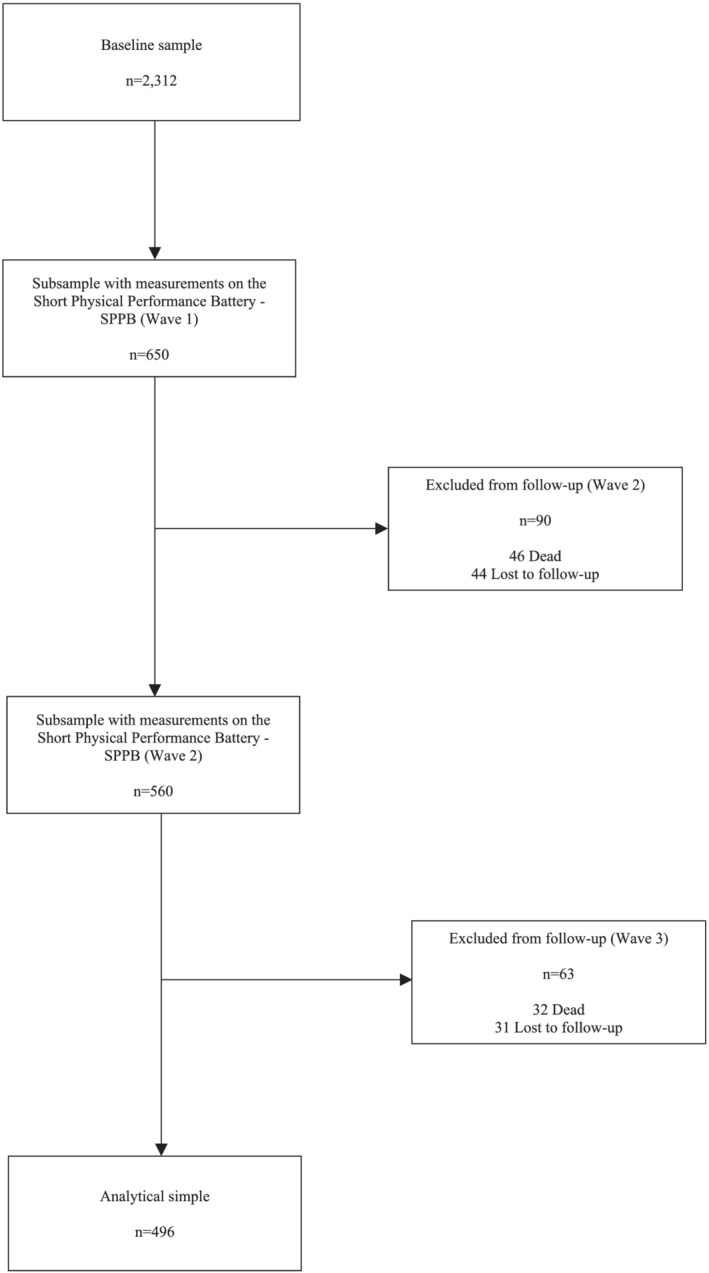
Study population and analytical sample.
Measurements
Height and weight were measured using stadiometers and calibrated electronic weighting scales. Grip strength was measured twice on both hands with the use of the hand dynamometer (Baseline Electronic Smedley Hand Dynamometer, Fabrication Enterprises, White Plains, NY, USA). A 4‐m time walk was used to measure the gait speed. Participants were asked to walk at a normal pace. Walking aids (like a cane) were allowed if the participant was more comfortable with it.
Definition of variables
Outcomes
Cognitive function
The SAGE study included five standard cognitive tests to examine the association between sarcopenia and cognition. These measures encompass different domains of CF: verbal learning and recall (immediate and delayed verbal recall), attention and working memory (forward and backward digit span) and executive function (verbal fluency). 21 A brief description for each test procedure is given below.
Immediate and delayed verbal recall. The interviewers read a list of 10 words and asked the participants to immediately recall and repeat as many words as they could in 1 min. Three trials of this assessment were performed. Upon completing the third trial, the interviewer administered the other cognitive tests, after which delayed recall ability was determined by asking subjects to remember the list of words.
Forward and backward digit span. In these tests, participants were required to repeat progressively longer series of numbers; the total score was recorded as the longest digit span repeated without error. The process was then performed with the OA repeating a new set of increasingly longer digit spans in reverse.
Semantic verbal fluency test. Consisted of naming as many animals (without using proper nouns) as possible in 1 min; the final score was correct responses minus errors.
According to a previous study with Mexico‐SAGE data, 22 composite z‐scores were calculated (to facilitate the comparison of cognitive test performance between individuals) for each cognitive test and to calculate an overall composite CF score. Specifically, z‐scores for each cognitive test were first computed (using the sample mean and adjustment for level of education and age), and these five z‐scores were then summed to generate an overall composite cognition z‐score.
Mild cognitive impairment
Based on recommendations from the National Institute of Aging‐Alzheimer's Association 23 and previous works with the Mexico‐SAGE data, an algorithm was used to generate the MCI variable. The OA who met all the following criteria were considered to have MCI:
Concern regarding a change in cognition. Participants were asked the following questions: ‘How would you best describe your memory at present?’ and/or ‘Compared to 12 months ago, would you say your memory is now better, the same or worse than it was then?’ to evaluate this item. Those OA who reported ‘bad’ or ‘very bad’ and ‘worse’ were considered to have concern with cognition.
Evidence of impairment in one or more cognitive domains were assessed (based on a <−1 SD cut‐off after adjustment for level of education and age) with immediate and delayed verbal recall, forward and backward digit span and verbal fluency tests.
Independence in basic activities of the daily life (BADL) evaluated with Katz scale. 24
Not demented participants who could not take the survey due to a severe cognitive impairment.
Main exposure
Sarcopenia
The presence of sarcopenia was defined, according to previous studies using the Mexico‐SAGE data, 10 as having low SMM, reflected by lower skeletal muscle mass index (SMI), and either or both slow gait speed and weak handgrip strength. Specifically, sarcopenia was determined according to the following criteria:
Low SMM. First, SMM was calculated as the appendicular skeletal muscle mass (ASM) based on the equation proposed by Lee et al. 25 The following specific equation was applied to our data: ASM = 0.244 * weight+7.8 * height+6.6 * sex (female = 0, male = 1) − 0.098 * age+race (White and Hispanic = 0, Black = 1.9, Asian = 1.6) − 3.3. Further, the SMI was obtained, dividing the ASM by the body mass index (BMI). 26 Then, low SMM (defined as the presence of low SMI) was established by the lowest quintile of the SMI based on sex‐stratified values.
Slow gait speed. It was defined as the lowest quintile of walking speed (m/s) based on height, age and sex‐stratified values. 27 , 28
Weak handgrip strength. Using the average value of the two handgrip measurements of the dominant hand, weak handgrip was defined as <30 kg for men and <20 kg for women. 29
Covariates
Potential confounders were identified through research into existing literature. These included sex (female = 1), age (5‐year categories from 50 to 79 years and 80+) and years of formal education (0–5, 6–8, 9–12 and 13 + years). Paid job (yes = 1) and marital status (coupled = 1) were included as dichotomous variables. Frailty status was assessed using a modified frailty phenotype based on the criteria proposed by Fried et al. that includes five components: weight loss, exhaustion, low physical activity, slow walking speed and weakness. 27 OA were considered frail if they met three or more of these criteria, prefrail if they met one or two and not frail or robust if they met none of the above criteria. Details of the application on this measurement of frailty in the SAGE sample have been published elsewhere. 30 Briefly, slow gait speed and weakness (low grip strength) were described above. Physical activity was assessed with the Global Physical Activity Questionnaire (GPAQ) classifying OA in three categories (low, moderate and high physical activity) based on reported time spent in moderate or vigorous activities during work, recreational/leisure time and transportation. Exhaustion was measured on a 5‐point Likert scale by asking respondents whether they had enough energy for daily activities. This criterion was considered present if the OA answered, ‘Not at all’ or ‘A little’. Weight loss was defined using the self‐report of unintentional weight loss (≥5 kg) in the past 6 months. We included a list of six chronic diseases contained in the SAGE study: Diabetes, stroke, hypercholesterolaemia and hypertriglyceridaemia were measured according to self‐reported medical diagnosis; depression was estimated through algorithms for symptomatology and self‐reported treatment; and hypertension was determined by either blood pressure measurement and/or self‐reported treatment. A detailed description of these diseases has been published elsewhere. 31 Anthropometric measures of weight and height were used to create the BMI (kg/m2). We also included the following lifestyle/behavioural variables: physical activity, tobacco use, alcohol consumption and daily vegetable and fruit intake. Tobacco use (never; ever smoked, no longer; current smoker, not daily; current smoker, daily), alcohol consumption (never; ever drinker, no longer; current drinker, low risk; current drinker, high risk) and vegetable consumption (daily portions: 0–2, 3–4, 5–6 and >7) were self‐reported. In all models, we include a categorical variable associated with the time of measurement (2009 was the reference category, 2014 and 2017).
Statistical analyses
Baseline characteristics are presented in percentages and means (standard deviation). We used chi‐square or t‐tests to compare sarcopenia groups regarding baseline socio‐demographic, health and lifestyle variables.
Given that all individuals aged 50 years or older within the same household were included in the Mexico‐SAGE study and that we have repeated measurements of cognition and sarcopenia, our data have a three‐level hierarchical structure: measurement occasions at Level 1, individuals at Level 2 and households at level 3. We then analysed the associations between MCI (also overall CF and its components) and sarcopenia using logistic and linear mixed‐effects regression models. Specifically, we fitted random intercept models including the subject and household IDs as random effects. Odds ratios and regression coefficients (95% confidence intervals [CI]) were reported.
Additional sensitivity analyses were performed. First, to consider the time‐varying independent variables in our analyses, we disaggregate the between‐ and within‐person effects in the random intercept models using the so‐called hybrid models (refs. 1 , 2 in Appendix S1). In this method, the association of outcome and time‐varying covariate is separated according to the between‐ and within‐person effects. For longitudinal data, the person‐mean‐centred time‐varying covariate serves as a within‐person predictor, representing the amount by which a person deviates from his or her average at each time point (within‐person effect) at Level 1. At Level 2, the associated mean of the person serves as a between‐person predictor, representing each person's average, pooling over all time points (between‐person effect). Second, we explore the associations between the specific components of sarcopenia (low muscle mass, slowness, weakness) and mild cognitive impairment as well as CF. In addition, possible combinations of these components were tested as well. All components and their combinations were modelled as time‐varying variables.
Differences were considered significant if P < 0.05. All statistical analyses were performed using Stata Version 16.1 software (StataCorp. 2020, College Station, TX.).
This study was conducted following the STROBE guidelines for reporting cohort studies (STROBE checklist is reported in Appendix S2) and the ethical guidelines for this journal (ref. 3 in Appendix S1).
Results
The baseline study sample included 496 OA, with a mean age of 65.5 years (SD = 7.3 years), 65.1% female, 26.7% with paid jobs, 64.9% married/cohabiting and 4.1 mean years of formal education (SD = 3.7). As for health characteristics, 78.1% reported the presence of at least one chronic condition, and 37.1% had multimorbidity, defined as the presence of two or more chronic diseases. Prevalence of pre‐frailty and frailty were 53.9% and 5.7%, respectively. For lifestyle variables, 21.7% performed a moderate level of physical activity, and 39.2%, a high level. Finally, 52% had never smoked, and 65.3% had never consumed alcohol.
The prevalence rates of MCI and sarcopenia for each wave of the study are shown in Table 1. Significant increases were observed for both variables across time. For MCI, a prevalence of 8.9, 12.9 and 16.0% was reported in Waves 1, 2 and 3, respectively. For sarcopenia, a prevalence of 10.5, 20.7 and 23.3%, was reported in Waves 1, 2 and 3.
Table 1.
Prevalence of MCI and sarcopenia by wave of the SAGE‐Mexico study
| Wave 1 | Wave 2 | Wave 3 | P‐value a | |
|---|---|---|---|---|
| 2009 | 2014 | 2017 | ||
| Mild cognitive impairment (MCI) | 8.9 | 12.9 | 16.0 | <0.01 |
| Sarcopenia | 10.5 | 20.7 | 23.3 | <0.01 |
P‐value for three‐level logistic models with random intercept for subjects and households.
Table 2 shows the distribution of the outcomes and the socio‐demographic characteristics of participants by sarcopenia status. OA with sarcopenia had lower scores in immediate verbal recall (P < 0.01), delayed verbal recall (P = 0.03), forward digit span (P = 0.05) and semantic verbal fluency (P < 0.01), as compared with those without sarcopenia. Also, participants with sarcopenia were, on average, older (P < 0.01) than their non‐sarcopenic counterparts. As for health characteristics, sarcopenic OA had a higher proportion of pre‐frail or frail individuals (P < 0.01). No significant differences were observed in other health‐related or lifestyle variables (Table 3).
Table 2.
Baseline socio‐demographic characteristics according to the presence of sarcopenia
| Sarcopenia a | |||
|---|---|---|---|
| No | Yes | P‐value b | |
| 444 | 52 | ||
| 89.5% | 10.5% | ||
| Outcomes | |||
| Mild cognitive impairment | 8.7 | 10.1 | 0.20 |
| Overall composite cognition score | 0.84 (3.00) | −0.86 (2.60) | <0.01 |
| Immediate verbal recall | 0.25 (0.85) | −0.11 (0.77) | <0.01 |
| Delayed Verbal recall | 0.16 (0.93) | −0.17 (0.98) | 0.03 |
| Forward digit spam | 0.15 (0.86) | −0.10 (0.82) | 0.05 |
| Backward digit spam | 0.10 (0.91) | −0.16 (0.97) | 0.07 |
| Semantic verbal fluency | 0.17 (0.87) | −0.27 (0.78) | <0.01 |
| Covariates | |||
| Sex (female = 1) | 66.6 | 55.1 | 0.11 |
| Age | |||
| 50–54 | 4.0 | 2.0 | |
| 55–59 | 19.9 | 0.0 | |
| 60–64 | 29.7 | 6.1 | |
| 65–69 | 22.9 | 14.3 | |
| 70–74 | 14.0 | 30.6 | |
| 75–79 | 7.5 | 32.7 | |
| 80+ | 2.1 | 14.3 | <0.01 |
| Years of education | |||
| 0–5 | 63.5 | 75.5 | |
| 6–8 | 23.0 | 16.3 | |
| 9–12 | 6.3 | 6.1 | |
| 13+ | 7.3 | 2.0 | 0.30 |
| Paid job | 26.9 | 18.4 | 0.20 |
| Marital status (older adult with couple) | 65.1 | 63.3 | 0.80 |
Cells are percentages or means (std. dev.).
P‐value for a chi‐square or t tests.
Table 3.
Baseline health and health‐related lifestyles characteristics according to the presence of sarcopenia
| Sarcopenia a | |||
|---|---|---|---|
| No | Yes | P‐value b | |
| 444 | 52 | ||
| 89.5% | 10.5% | ||
| Frailty status | |||
| Not frail | 41.8 | 20.4 | |
| Pre‐frail | 53.5 | 63.3 | |
| Frail | 4.7 | 16.3 | <0.01 |
| Depression | 10.8 | 6.1 | 0.22 |
| Diabetes | 15.9 | 10.2 | 0.23 |
| Stroke | 3.3 | 4.1 | 0.79 |
| Hypercholesterolaemia | 25.4 | 17.0 | 0.16 |
| Hypertriglycaemia | 15.9 | 6.4 | 0.02 |
| Hypertension | 64.9 | 73.5 | 0.21 |
| Body mass index | 28.68 (4.95) | 29.5 (5.19) | 0.30 |
| Physical activity | |||
| Low | 38.4 | 52.1 | |
| Moderate | 22.5 | 14.6 | |
| High | 39.1 | 33.3 | 0.16 |
| Tobacco use | |||
| Never | 65.6 | 67.4 | |
| Ever smoked, no longer | 19.7 | 12.2 | |
| Current smoker, not daily | 5.2 | 6.1 | |
| Current smoker, daily | 9.6 | 14.3 | 0.51 |
| Alcohol consumption | |||
| Never | 52.7 | 49.0 | |
| Ever drinker, no longer | 35.4 | 42.9 | |
| Current drinker (low risk) | 8.4 | 8.2 | |
| Current drinker (high risk) | 3.5 | 0.0 | 0.47 |
| Vegetable and fruit intake (daily portions) | |||
| 0–2 | 42.1 | 34.8 | |
| 3–4 | 39.0 | 52.2 | |
| 5–6 | 14.0 | 8.7 | |
| 7+ | 5.0 | 4.4 | 0.36 |
Cells are percentages or means (std. dev.).
P‐value for a chi‐square or t tests.
Table 4 depicts the results of the mixed‐effects regression models with and without covariates adjustment. The presence of sarcopenia was significantly associated with MCI (OR = 1.74; CI95% 1.02, 2.96; P = 0.04) and worse CF as assessed with the CCS (β = −0.57; CI95% −0.93, −0.21; P < 0.01). Significant associations were also observed between sarcopenia and immediate verbal recall (β = −0.14; CI95% −0.28, −0.01; P = 0.04), delayed verbal recall (β = −0.12; CI95% −0.23, −0.01; P = 0.03) and semantic verbal fluency (β = −0.17; CI95% −0.28, −0.05; P = 0.01).
Table 4.
Results of the three‐level logistic and linear mixed regression models
| Without covariates adjustment | With covariates adjustment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P‐value | OR | 95%CI | P‐value | |||||
| Mild cognitive impairment | 1.89 | 1.19 | ; | 3.00 | <0.01 | 1.74 | 1.02 | ; | 2.96 | 0.04 |
| β | 95%CI | P‐value a | β | 95%CI | P‐value b | |||||
| Composite cognition score | −0.78 | −1.15 | ; | −0.40 | <0.01 | −0.57 | −0.93 | ; | −0.21 | <0.01 |
| Immediate verbal recall | −0.26 | −0.39 | ; | −0.13 | <0.01 | −0.14 | −0.28 | ; | −0.01 | 0.04 |
| Delayed verbal recall | −0.24 | −0.35 | ; | −0.14 | <0.01 | −0.12 | −0.23 | ; | −0.01 | 0.03 |
| Forward digit span | −0.11 | −0.22 | ; | 0.01 | 0.05 | −0.08 | −0.19 | ; | 0.04 | 0.19 |
| Backward digit span | −0.12 | −0.24 | ; | 0.01 | 0.05 | −0.10 | −0.23 | ; | 0.03 | 0.12 |
| Semantic verbal fluency | −0.23 | −0.34 | ; | −0.12 | <0.01 | −0.17 | −0.28 | ; | −0.05 | 0.01 |
The results of the mixed regression models treating sarcopenia as a time‐varying variable are shown in Table 5. Overall, sarcopenia was significantly associated with all indicators except for immediate verbal recall (P = 0.92) and delayed verbal recall (P = 0.44) for the between‐person effects. Meanwhile, for within‐person effects, the only significant observed association was for the immediate verbal recall (P < 0.01). Complete results with all covariates (invariant and time varying) are shown in Appendix S3 and Table S1.
Table 5.
Results of the three‐level logistic and linear mixed regression models with sarcopenia as time‐varying exposure a
| Mild cognitive impairment | Composite cognition score | Immediate verbal recall | Delayed verbal recall | Forward digit span | Backward digit span | Semantic verbal fluency | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P‐value | β | 95%CI | P‐value | β | 95%CI | P‐value | β | 95%CI | P‐value | β | 95%CI | P‐value | β | 95%CI | P‐value | β | 95%CI | P‐value | |
| Sarcopenia | 1.74 | 1.02; 2.96 | 0.04 | −0.57 | −0.93; −0.21 | <0.01 | −0.14 | −0.28; −0.01 | 0.04 | −0.12 | −0.23; −0.01 | 0.03 | −0.08 | −0.19; 0.04 | 0.19 | −0.10 | −0.23; 0.03 | 0.12 | −0.17 | −0.28; −0.05 | 0.01 |
| Between‐person effect | 2.40 | 1.06; 5.41 | 0.03 | −0.77 | −0.15; −1.38 | 0.01 | −0.01 | −0.21; 0.19 | 0.92 | −0.06 | −0.23; 0.10 | 0.44 | −0.17 | −0.34; −0.01 | 0.04 | −0.18 | −0.37; 0.01 | 0.05 | −0.31 | −0.50; −0.13 | <0.01 |
| Within‐person effect | 1.35 | 0.60; 3.09 | 0.48 | −0.35 | −0.79; 0.09 | 0.12 | −0.23 | −0.41; −0.05 | 0.01 | −0.11 | −0.25; 0.03 | 0.12 | 0.03 | −0.11; 0.18 | 0.61 | 0.01 | −0.17; 0.18 | 0.94 | −0.06 | −0.21; 0.09 | 0.41 |
Regarding the results of the sarcopenia components and their combinations (Table 6), none were significantly associated with MCI or immediate verbal recall. However, significant associations were observed for several other indicators of CF. The composite cognition score was associated with slowness (β = −0.37, P = 0.03), low muscle mass (β = −0.63, P < 0.01), slowness/weakness (β = −0.81, P < 0.01) and weakness/low muscle mass (β = −0.75, P < 0.01) combinations. Delayed verbal recall with weakness (β = −0.10, P = 0.04) and slowness/weakness combination (β = −0.18, P = 0.03). The forward digit span was associated only with the combination of weakness/low muscle mass (β = −0.27, P < 0.01); meanwhile, the backward digit span with slowness (β = −0.14, P = 0.03), low muscle mass (β = −0.20, P = 0.02) and the combination of slowness/weakness (β = −0.22, P = 0.02). Finally, semantic verbal fluency was associated with slowness (β = −0.14, P = 0.01), weakness (β = −0.11, P = 0.04), low muscle mass (β = −0.18, P = 0.02) and the combinations of slowness/weakness (β = −0.24, P = 0.01) and slowness/low muscle mass (β = −0.20, P = 0.04). We observed similar results for the specific between‐person effects. Regarding within‐person effects, we found significant associations for composite cognition score with combinations of slowness/weakness (β = −0.69, P < 0.01) and weakness/low muscle mass (β = −0.58, P = 0.04); forward digit span with weakness/low muscle mass combination (β = −0.24, P = 0.02); and semantic verbal fluency with combinations of slowness/weakness (β = −0.27, P < 0.01) and slowness/low muscle mass (β = −0.28, P = 0.02).
Table 6.
Estimated associations of the sarcopenia components, mild cognitive impairment and cognitive function a
| Mild cognitive impairment | Composite cognition score | Immediate verbal recall | Delayed verbal recall | Forward digit span | Backward digit span | Semantic verbal fluency | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P‐value | β | 95%CI | P‐value | β | 95%CI | P‐value | β | 95%CI | P‐value | β | 95%CI | P‐value | β | 95%CI | P‐value | β | 95%CI | P‐value | |
| Sarcopenia components | |||||||||||||||||||||
| Slowness | 1.57 | 0.91; 2.70 | 0.11 | −0.37 | −0.71; −0.03 | 0.03 | −0.10 | −0.23; 0.03 | 0.12 | −0.02 | −0.12; 0–09 | 0.75 | −0.01 | −0.11; 0.10 | 0.94 | −0.14 | −0.26; −0.02 | 0.03 | −0.14 | −0.25; −0.03 | 0.01 |
| Between‐person effect | 1.90 | 0.78; 4.62 | 0.16 | −0.86 | −1.61; −0.11 | 0.02 | −0.08 | −0.33; 0.16 | 0.51 | −0.17 | −0.38; 0.03 | 0.09 | 0.01 | −0.19; 0.22 | 0.90 | −0.33 | −0.56; −0.10 | <0.01 | −0.24 | −0.47; −0.01 | 0.04 |
| Within‐person effect | 1.30 | 0.66; 2.55 | 0.45 | −0.25 | −0.62; 0.11 | 0.18 | −0.12 | −0.27; 0.03 | 0.12 | 0.05 | −0.07; 0.17 | 0.38 | 0.01 | −0.12; 0.14 | 0.88 | −0.07 | −0.22; 0.07 | 0.31 | −0.12 | −0.24; 0.01 | 0.06 |
| Weakness | 1.03 | 0.60; 1.75 | 0.92 | −0.21 | −0.52; 0.10 | 0.18 | −0.05 | −0.17; 0.07 | 0.42 | −0.10 | −0.20; −0.01 | 0.04 | −0.03 | −0.13; 0.07 | 0.61 | 0.05 | −0.07; 0.16 | 0.42 | −0.11 | −0.21; −0.01 | 0.04 |
| Between‐person effect | 1.67 | 0.70; 4.01 | 0.25 | −0.42 | −1.15; 0.30 | 0.26 | −0.20 | −0.43; 0.04 | 0.11 | −0.14 | −0.34; 0.06 | 0.16 | −0.08 | −0.28; 0.11 | 0.41 | 0.09 | −0.13; 0.31 | 0.45 | −0.11 | −0.33; 0.12 | 0.36 |
| Within‐person effect | 0.83 | 0.44; 1.58 | 0.57 | −0.11 | −0.45; 0.22 | 0.51 | 0.02 | −0.11; 0.16 | 0.73 | −0.09 | −0.20; 0.03 | 0.13 | −0.01 | −0.12; 0.11 | 0.90 | 0.06 | −0.08; 0.19 | 0.41 | −0.11 | −0.22; 0.01 | 0.07 |
| LSMM | 1.11 | 0.52; 2.37 | 0.78 | −0.63 | −1.10; −0.16 | <0.01 | −0.09 | −0.27; 0.08 | 0.30 | −0.09 | −0.24; 0.05 | 0.20 | −0.14 | −0.28; 0.01 | 0.07 | −0.20 | −0.36; −0.03 | 0.02 | −0.18 | −0.33; −0.03 | 0.02 |
| Between‐person effect | 1.22 | 0.43; 3.46 | 0.71 | −1.19 | −1.99; −0.38 | <0.01 | −0.12 | −0.38; 0.14 | 0.37 | −0.09 | −0.31; 0.13 | 0.43 | −0.34 | −.055; −0.12 | <0.01 | −0.24 | −0.48; 0.01 | 0.06 | −0.40 | −0.64; −0.15 | <0.01 |
| Within‐person effect | 1.13 | 0.35; 3.62 | 0.83 | −0.31 | −0.90; 0.27 | 0.29 | −0.04 | −0.28; 0.20 | 0.74 | −0.06 | −0.25; 0.13 | 0.52 | 0.01 | −0.19; 0.21 | 0.90 | −0.18 | −0.41; 0.04 | 0.11 | −0.04 | −0.24; 0.16 | 0.72 |
| Slowness + weakness | 1.43 | 0.64; 3.21 | 0.38 | −0.81 | −1.31; −0.32 | <0.01 | −0.09 | −0.28; 0.10 | 0.38 | −0.18 | −0.33; −0.02 | 0.03 | −0.11 | −0.27; 0.05 | 0.18 | −0.22 | −0.40; −0.04 | 0.02 | −0.24 | −0.41; −0.08 | <0.01 |
| Between‐person effect | 1.64 | 0.47; 5.78 | 0.44 | −0.94 | −2.01; 0.14 | 0.09 | −0.15 | −0.51; 0.20 | 0.40 | −0.24 | −0.54; 0.06 | 0.11 | 0.01 | −0.29; 0.30 | 0.97 | −0.35 | −0.69; −0.02 | 0.04 | −0.18 | −0.52; 0.15 | 0.28 |
| Within‐person effect | 1.27 | 0.46; 3.48 | 0.65 | −0.69 | −1.24; −0.14 | 0.01 | −0.07 | −0.29; 0.16 | 0.56 | −0.11 | −0.29; 0.07 | 0.22 | −0.11 | −0.30; 0.08 | 0.25 | −0.14 | −0.35; 0.08 | 0.21 | −0.27 | −0.45; −0.08 | <0.01 |
| Slowness + LSMM | 0.94 | 0.38; 2.34 | 0.90 | −0.43 | −0.99; 0.14 | 0.14 | −0.07 | −0.29, 0.15 | 0.52 | −0.12 | −0.29; 0.06 | 0.20 | −0.09 | −0.28; 0.09 | 0.31 | −0.01 | −0.21; 0.20 | 0.95 | −0.20 | −0.38; −0.01 | 0.04 |
| Between‐person effect | 1.55 | 0.38; 6.34 | 0.54 | −0.87 | −2.02; 0.28 | 0.14 | 0.05 | −0.33; 0.42 | 0.81 | −0.15 | −0.46; 0.17 | 0.35 | −0.18 | −0.50; 0.13 | 0.25 | −0.47 | −0.82; −0.12 | <0.01 | −0.04 | −0.40; 0.31 | 0.82 |
| Within‐person effect | 0.65 | 0.19; 2.17 | 0.48 | −0.30 | −0.96; 0.35 | 0.36 | −0.12 | −0.39; 0.14 | 0.36 | −0.08 | −0.30; 0.13 | 0.45 | −0.05 | −0.27; 0.17 | 0.66 | 0.23 | −0.03; 0.48 | 0.08 | −0.28 | −0.50; −0.05 | 0.02 |
| Weakness + LSMM | 1.50 | 0.64; 3.51 | 0.35 | −0.75 | −1.26; −0.25 | <0.01 | −0.15 | −0.35; 0.04 | 0.13 | −0.16 | −0.32; 0.01 | 0.06 | −0.27 | −0.44; −0.10 | <0.01 | −0.06 | −0.24; 0.13 | 0.55 | −0.14 | −0.31; 0.03 | 0.10 |
| Between‐person effect | 1.71 | 0.40; 7.33 | 0.47 | −1.23 | −2.35; −0.10 | 0.03 | −0.11 | −0.48; 0.25 | 0.54 | −0.29 | −0.60; 0.02 | 0.07 | −0.35 | −0.66; −0.05 | 0.02 | −0.04 | −0.39; 0.30 | 0.81 | −0.40 | −0.74; −0.06 | 0.02 |
| Within‐person effect | 1.46 | 0.48; 4.42 | 0.50 | −0.58 | −1.14; −0.01 | 0.04 | −0.16 | −0.39; 0.07 | 0.18 | −0.10 | −0.28; 0.09 | 0.31 | −0.24 | −0.43; −0.04 | 0.02 | −0.03 | −0.26; 0.19 | 0.76 | −0.05 | −0.24; 0.15 | 0.63 |
Figure 2 shows the trajectory of adjusted MCI prevalence, obtained from the three‐level logistic mixed model, according to the presence of sarcopenia. For both groups (non‐sarcopenic and sarcopenic), an overall increase in the prevalence of MCI was observed: 6.1% for non‐sarcopenic and 12.1% for sarcopenic OA. These amounts represent an annual increase rate of 0.8% for non‐sarcopenic OA and 1.5% for sarcopenic OA.
Figure 2.
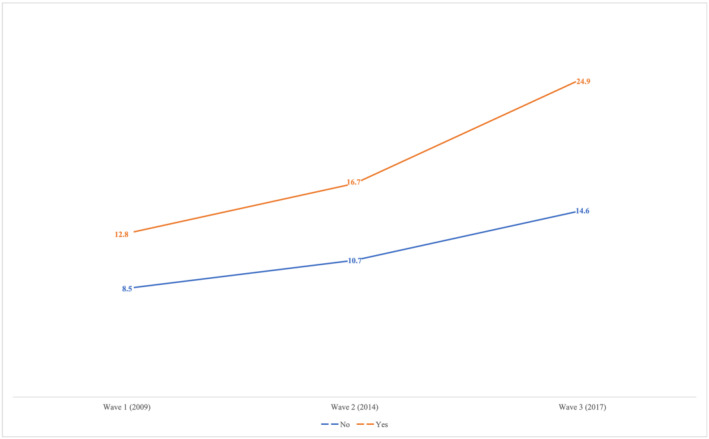
Longitudinal trajectory of mild cognitive impairment (MCI) according to the presence of sarcopenia. Note: MCI prevalence according to the three‐level logistic mixed model adjusted for covariates shown in Tables 2 and 3.
Discussion
Our findings show that sarcopenia is longitudinally associated with MCI and several CF domains in a sample of community‐dwelling older Mexican adults. Specifically, we observed that sarcopenia increases the odds of MCI and decreases the scores of the overall composite CF as well as immediate verbal recall, delayed verbal recall and semantic verbal fluency tests.
Previous research has explored the association between sarcopenia and MCI/CF in cross‐sectional and longitudinal studies with mixed results. In a recent systematic review and meta‐analysis that evaluated the association between sarcopenia and MCI/dementia across15 cross‐sectional studies, including 10 410 OA from Asia, Europe and America, a significant association was found between sarcopenia and MCI (pooled OR = 2.25; 95%CI: 1.70–2.97). 16 Among longitudinal studies, there have been no conclusive results. One study with 131 Japanese community‐dwelling OA reported a significant association between sarcopenia and cognitive decline (OR = 7.86; 95%CI: 1.53–40.5; P = 0.01). 17 Other longitudinal studies have explored the role of specific factors included in the sarcopenia diagnosis. Results from the Korean Longitudinal Study on Health and Aging, with a sample of 297 OA (>65), found a partial association between sarcopenia diagnosis and the incidence of MCI in 5‐year follow‐up. 19 Van Kan et al. explored changes in body composition (muscle and fat mass) assessed by dual‐energy X‐ray absorptiometry (DXA) related to CF in women >75 from a French cohort after 7‐year follow up. No association was found between percentage changes in muscle or fat, BMI or CF (P‐values: 0.27, 0.54 and 0.14, respectively). 18 There are several factors that could explain the observed differences: (a) use of distinct instruments to measure sarcopenia and MCI/CF; (b) although OA have been included, different age groups have been analysed; and (c) evaluation across different follow‐up periods. Future studies, including meta‐analysis, could help to determine the magnitude and directionality of the association between sarcopenia and MCI, trying to harmonize the measurements of sarcopenia and MCI/CF.
In our study, between‐person associations were predominant over within‐person associations, a result that has already been reported in a longitudinal study with a sample of Chinese OA in which the association of lifestyle factors with cognition was evaluated (ref. 4 in Appendix S1). This could be attributed to the fact that changes within individuals require a longer follow‐up time compared with changes between individuals. For example, and regarding sarcopenia, we found significant longitudinal differences between individuals for MCI and CF (global and for forward digit span, backward digit span and semantic verbal fluency) that did not were observed within individuals. Likely, 8 years is not enough to observe significant changes in these indicators for the same individuals followed over time. However, further studies are needed to confirm or discard this hypothesis with a longer follow‐up time.
As for the association between specific components of sarcopenia and MCI or the CF, prior studies have reported no conclusive results. A cross‐sectional study observed that sarcopenia was only associated with impairment in semantic verbal fluency test. However, when disaggregating for sarcopenia components, slowness and weakness, rather than low muscle mass, were associated with global CF and various cognitive domains (ref. 5 in Appendix S1). On the contrary, one study with older Mexican adults did not found significant associations for slowness and cognitive impairment (ref. 6 in Appendix S1). In our study, the results were mixed. We also found that slowness was associated with CF and weakness with the semantic verbal fluency test. Nonetheless, low muscle mass was also associated with overall CF, forward and backward digit span tests and semantic verbal fluency test. Additionally, we found that specific combinations of sarcopenia components were associated with several cognitive domains. All in all, the findings on the association between the components of sarcopenia and MCI or CF remain controversial; more longitudinal studies are needed to clarify what sarcopenia components could be determinant for the risk of MCI or a worse CF.
The specific mechanisms underlying the association between sarcopenia and MCI/CF have not been elucidated. However, some shared pathways have been suggested given that both conditions have multifactorial causes. First, elevated pro‐inflammatory cytokines and acute‐phase proteins like IL‐6, CRP and TNFα have influence in protein balance synthesis, inducing catabolic state and muscle loss. 15 Second, anabolic hormones like IGF‐1, insulin, growth hormone and steroid hormones are involved in protein metabolism. During ageing, these hormones decrease, leading to a reduction in lean body mass and reduced muscle strength. 32 Third, oxidative stress modulates transcription factors and kinases. This induces the accumulation of molecular damage that has been associated with protein breakdown, protein synthesis and apoptosis, subsequently leading to muscle fibre atrophy and fibre loss. 33 Evidence has shown that all these mechanisms are also associated with MCI or poorer CF. 14 , 34 Additionally, it has been hypothesized that physical inactivity could play a role on the association of sarcopenia and CF. One such hypothesis is that sarcopenia could lead to physical inactivity, even to disability, which in turn could result in MCI or diminished CF. 35 Another possible mechanism is that physical inactivity, potentially generated by sarcopenia, could decrease the expression of molecules related to neuronal plasticity and learning (brain‐derived neurotrophic factor and IGF‐1). 36
Our study has some strengths. To the best of our knowledge, this is the first study conducted in low‐ or middle countries to assess the longitudinal association of sarcopenia and CF among OA population. Additionally, we used a comprehensive set of cognitive tests in our study, broadening the specific cognitive domains that could be significant related to sarcopenia. There were also some limitations in our study. First, although we used the criteria proposed by the EWGSOP2 to define sarcopenia, we were not able to use the specific algorithm because we did not have the information from the SARC‐F. 37 Even so, the algorithm that we used in this study had already been applied in previous studies, including those that used SAGE data, to determine the prevalence of sarcopenia and its associated factors. 38 Second, we used predictive equations to estimate body composition, which could either under‐ or overestimate muscle quantity, specifically in those with higher BMI. However, Lee's equation has been validated shown to have a strong association with the magnetic resonance image as a reference method (R‐square = 0.86). 25 Third, although it is true that the application of the mixed‐effects models and explicit modelling of time‐varying covariates strengthen our results, it is necessary to consider that both sarcopenia and MCI can be reversible. This fact implies that ad hoc statistical models (e.g. Markov chains) should be used in future studies evaluating the longitudinal association between sarcopenia and MCI. Fourth, even though we used the criteria recommended by the National Institute on Aging and Alzheimer's Association to define MCI, it was not possible to distinguish individuals with probable or confirmed dementia. For this task, specialized clinical diagnoses are required, which is not feasible in population‐based epidemiological studies.
In conclusion, this study provides support for a longitudinal association between sarcopenia and MCI/CF in community‐dwelling OA. Sarcopenia compromises the health of OA through reduced mobility, increased risk of falls and bone fractures, disability, MCI and risk of death. 3 Therefore, relevant public health strategies should be targeted towards reducing or delaying the onset of sarcopenia through policy research and clinical interventions, specifically at early ages. In particular, it is important to identify the key mechanisms that could lead to sarcopenia, which in turn could help direct interventions in OA populations. 39 A recent systematic review and meta‐analysis of randomized control trials, for example, evidenced that exercise and nutrition interventions could be effective in sarcopenia treatment. 40 If a causal association between sarcopenia and MCI were established, these kinds of interventions could further help in the reduction of MCI and Alzheimer's disease.
Funding
SAGE is supported by the WHO and the US National Institute on Aging through interagency agreements (OGHA04034785, YA1323‐08‐CN‐0020 and Y1‐AG‐1005‐01) and a competitive grant (R01AG034479).
Conflict of interest
The authors declare no conflicts of interest. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.
Supporting information
Table S1. Results of the three‐level logistic and linear mixed regression models with covariatesa.
Salinas‐Rodríguez A., Palazuelos‐González R., Rivera‐Almaraz A., and Manrique‐Espinoza B. (2021) Longitudinal association of sarcopenia and mild cognitive impairment among older Mexican adults, Journal of Cachexia, Sarcopenia and Muscle, 12, 1848–1859, 10.1002/jcsm.12787
References
- 1. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997;127:990S–991S. [DOI] [PubMed] [Google Scholar]
- 2. Grimby G, Saltin B. The ageing muscle. Clin Physiol 1983;3:209–218. [DOI] [PubMed] [Google Scholar]
- 3. Beaudart C, Rizzoli R, Bruyère O, Reginster JY, Biver E. Sarcopenia: burden and challenges for public health. Arch Public Heal 2014;72:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harada CN, Love MCN, Triebel K. Normal cognitive aging. Clin Geriatr Med 2013;29:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. The Lancet, 2017;390(10113):2673–2734. [DOI] [PubMed] [Google Scholar]
- 6. Reijnierse EM, Trappenburg MC, Leter MJ, Blauw GJ, Sipilä S, Sillanpää E, et al. The impact of different diagnostic criteria on the prevalence of sarcopenia in healthy elderly participants and geriatric outpatients. Gerontology 2015;61:491–496. [DOI] [PubMed] [Google Scholar]
- 7. Manrique‐Espinoza B, Salinas‐Rodríguez A, Rosas‐Carrasco O, Gutiérrez‐Robledo LM, Avila‐Funes JA. Sarcopenia is associated with physical and mental components of health‐related quality of life in older adults. J Am Med Dir Assoc 2017;18:636.e1–636.e5. [DOI] [PubMed] [Google Scholar]
- 8. Sanford AM. Mild cognitive impairment. Clin Geriatr Med 2017;33:325–337. [DOI] [PubMed] [Google Scholar]
- 9. Manrique‐Espinoza B, Salinas‐Rodríguez A, Moreno‐Tamayo K, Acosta‐Castillo I, Sosa‐Ortiz AL, Gutiérrez‐Robledo LM, et al. Condiciones de salud y estado funcional de los adultos mayores en México. Salud Pública de México. 2013;55:s323–s331. 10.21149/spm.v55s2.5131 [DOI] [PubMed] [Google Scholar]
- 10. Salinas‐Rodríguez A, Rivera‐Almaraz A, Scott A, Manrique‐Espinoza B. Severity levels of disability among older adults in low‐ and middle‐income countries: results from the study on global ageing and adult health (SAGE). Front Med 2020;7:562963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brennan‐Olsen SL, Bowe SJ, Kowal P, Naidoo N, Quashie NT, Eick G, et al. Functional measures of sarcopenia: prevalence, and associations with functional disability in 10,892 adults aged 65 years and over from six lower‐ and middle‐income countries. Calcif Tissue Int 2019;105:609–618. [DOI] [PubMed] [Google Scholar]
- 12. Robinder Dhillon JSD, Hasni SM. Pathogenesis and management of sarcopenia. Clin Geriatr Med 2018;176:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morley JE. An overview of cognitive impairment. Clin Geriatr Med 2018;34:505–513. [DOI] [PubMed] [Google Scholar]
- 14. Ali SA, Begum T, Reza F. Hormonal influences on cognitive function. Malaysian J Med Sci 2018;25:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age‐related sarcopenia. Front Physiol 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peng TC, Chen WL, Wu LW, Chang YW, Kao TW. Sarcopenia and cognitive impairment: a systematic review and meta‐analysis. Clin Nutr 2020;39:2695–2701. [DOI] [PubMed] [Google Scholar]
- 17. Nishiguchi S, Yamada M, Shirooka H, Nozaki Y, Fukutani N, Tashiro Y, et al. Sarcopenia as a risk factor for cognitive deterioration in community‐dwelling older adults: a 1‐year prospective study. J Am Med Dir Assoc 2016;17:372.e5–372.e8. [DOI] [PubMed] [Google Scholar]
- 18. Abellan van Kan G, Cesari M, Gillette‐Guyonnet S, Dupuy C, Vellas B, Rolland Y. Association of a 7‐year percent change in fat mass and muscle mass with subsequent cognitive dysfunction: the EPIDOS‐Toulouse cohort. J Cachexia Sarcopenia Muscle 2013;4:225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moon JH, Moon JH, Kim KM, Choi SH, Lim S, Park KS, et al. Sarcopenia as a predictor of future cognitive impairment in older adults. The journal of nutrition, health & aging. 2016;20:496–502. 10.1007/s12603-015-0613-x [DOI] [PubMed] [Google Scholar]
- 20. Kowal P, Chatterji S, Naidoo N, Biritwum R, Fan W, Lopez Ridaura R, et al. Data resource profile: the World Health Organization study on global AGEing and adult health (SAGE). Int J Epidemiol 2012;41:1639–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cullen B, O'neill B, Evans JJ, Coen RF. A review of screening tests for cognitive impairment. J Neurol Neurosurg Psychiatry 2007;78:790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gildner TE, Salinas‐Rodríguez A, Manrique‐Espinoza B, Moreno‐Tamayo K, Kowal P. Does poor sleep impair cognition during aging? Longitudinal associations between changes in sleep duration and cognitive performance among older Mexican adults. Arch Gerontol Geriatr 2019;83:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7(3):270–279. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADLl: a standardized measure of biological and psychosocial function. JAMA 1963;185(12):914–919. [DOI] [PubMed] [Google Scholar]
- 25. Lee RC, Wang Z, Heo M, Ross R, Janssen I, Heymsfield SB. Total‐body skeletal muscle mass: development and cross‐validation of anthropometric prediction models. Am J Clin Nutr 2000;72:796–803. [DOI] [PubMed] [Google Scholar]
- 26. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol ‐ Ser A Biol Sci Med Sci 2014;69:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A Biol Sci Med Sci 2001;56:M146–M157. [DOI] [PubMed] [Google Scholar]
- 28. Capistrant BD, Glymour MM, Berkman LF. Assessing mobility difficulties for cross‐national comparisons: results from the world health organization study on global ageing and adult health. J Am Geriatr Soc 2014;62:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoogendijk EO, Rijnhart JJM, Kowal P, Pérez‐Zepeda MU, Cesari M, Abizanda P, et al. Socioeconomic inequalities in frailty among older adults in six low‐ and middle‐income countries: results from the WHO study on global AGEing and adult health (SAGE). Maturitas 2018;115:56–63. [DOI] [PubMed] [Google Scholar]
- 31. Arokiasamy P, Uttamacharya KP, Capistrant BD, Gildner TE, Thiele E, Biritwum RB, et al. Chronic noncommunicable diseases in 6 low‐ and middle‐income countries: findings from wave 1 of the World Health Organization's study on global ageing and adult health (SAGE). Am J Epidemiol 2017;185:414–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, et al. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol 2013;45:2288–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci 2010;11:1509–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Popa‐Wagner A, Mitran S, Sivanesan S, Chang E, Buga AM. ROS and brain diseases: the good, the bad, and the ugly. Oxid Med Cell Longev 2013;2013:963520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chou MY, Nishita Y, Nakagawa T, Tange C, Tomida M, Shimokata H, et al. Role of gait speed and grip strength in predicting 10‐year cognitive decline among community‐dwelling older people. BMC Geriatr 2019;19:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim S, Choi JY, Moon S, Park DH, Kwak HB, Kang JH. Roles of myokines in exercise‐induced improvement of neuropsychiatric function. Pflugers Arch Eur J Physiol 2019;471:491–505. [DOI] [PubMed] [Google Scholar]
- 37. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tyrovolas S, Koyanagi A, Olaya B, Ayuso‐Mateos JL, Miret M, Chatterji S, et al. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: a multi‐continent study. J Cachexia Sarcopenia Muscle 2016;7:312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kämpfen F, Wijemunige N, Evangelista B. Aging, non‐communicable diseases, and old‐age disability in low‐ and middle‐income countries: a challenge for global health. Int J Public Health 2018;63:1011–1012. [DOI] [PubMed] [Google Scholar]
- 40. Yoshimura Y, Wakabayashi H, Yamada M, Kim H, Harada A, Arai H. Interventions for treating sarcopenia: a systematic review and meta‐analysis of randomized controlled studies. J Am Med Dir Assoc 2017;18:553.e1–553.e16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Results of the three‐level logistic and linear mixed regression models with covariatesa.


