Abstract
Background
Falls cause considerable morbidity and mortality in older people. It is unclear how vitamin D supplementation affects falls risk, particularly when taken at high doses. We sought to determine whether monthly high‐dose vitamin D supplementation reduces risk and incidence of falls.
Methods
We used data from the randomized, double‐blind, placebo‐controlled D‐Health Trial conducted in Australia. Between February 2014 and May 2015, 21 315 participants aged 60–84 years were randomized (1:1) to monthly doses of either 60 000 IU of colecalciferol or placebo for a maximum of 5 years. People who reported a history of osteomalacia, sarcoidosis, hyperparathyroidism, hypercalcaemia or kidney stones or who were taking >500 IU/day supplementary vitamin D were ineligible. Each year, we collected blood samples from ~450 randomly sampled participants from each trial arm and measured 25‐hydroxyvitamin D [25(OH)D]. Falls, a prespecified tertiary outcome, were ascertained using annual surveys and, for a subset of participants, 3‐month falls diaries. The primary outcome for this analysis was any fall in the month before completing an annual survey. As part of our process to maintain blinding, we used random samples of participants (surveys, n = 16 000; diaries, n = 2400), with equal numbers per group. Participants with no outcome data were excluded. Following an intention‐to‐treat approach, we analysed outcomes using logistic, ordinal and negative binomial regression. Registration: Australian New Zealand Clinical Trials Registry (ACTRN12613000743763); registered 4 July 2013.
Results
Mean treatment duration was 4.3 years (standard deviation [SD] = 1.4 years). Mean serum 25(OH)D concentrations during the trial were 114.8 (SD 30.3) nmol/L and 77.5 (SD 25.2) nmol/L in the vitamin D and placebo groups, respectively. Survey and diary analytic sets included 15 416 and 2200 participants, respectively; approximately half were randomized to vitamin D (surveys: 50.1%; diaries: 50.4%). Vitamin D had no effect on falling in the past month (odds ratio [OR] 1.02, 95% confidence interval [CI] 0.95–1.10). There was an interaction with body mass index (BMI) (P‐interaction = 0.001); vitamin D increased risk in participants with BMI < 25 kg/m2 (OR 1.25, 95% CI 1.09–1.43), but there was no effect in those with BMI ≥ 25 kg/m2 (OR 0.95, 95% CI 0.87–1.04). Analyses of diary data were consistent with these findings. The incidence of hypercalcaemia and kidney stones did not differ between groups.
Conclusions
Monthly high‐dose vitamin D supplementation did not reduce risk of falling. A possible increased risk of falling with vitamin D supplementation in people with normal BMI warrants further investigation.
Keywords: Randomized controlled trial, Vitamin D, Falls, Bolus dose
Introduction
Falls cause substantial morbidity and mortality and impose a considerable burden on health systems. The Global Burden of Disease Study 2017 ranked falls as the 18th leading cause of disability‐adjusted life years and the second leading cause of death due to unintentional injury. 1 Ageing is associated with increased falls risk, 1 and older people experience a decrease in daily functional status following an injurious fall. 2
Vitamin D deficiency, as measured by serum 25‐hydroxyvitamin D [25(OH)D] concentration, is associated with muscle weakness 3 , 4 and increased falls risk. 5 The vitamin D receptor (VDR) has been identified in skeletal muscle, 6 and myocyte deletion of the VDR in mice results in sarcopenia and reduced muscle function. 7 There is evidence that vitamin D supplementation acts to improve balance 8 and increase muscle strength. 8 , 9
It is thus plausible that vitamin D supplementation might protect against falling; however, despite many randomized controlled trials (RCTs) of vitamin D supplementation, it remains unclear if this is the case. A 2009 meta‐analysis estimated that supplementing elderly people with 700–1000 IU per day of vitamin D reduced risk of falling by 19% (7 RCTs, n = 1921, relative risk [RR] 0.81, 95% confidence interval [CI] 0.71–0.92). 10 However, a 2018 meta‐analysis found supplementation had little effect (37 RCTs, n = 34 144, RR 0.97, 95% CI 0.93–1.02), 11 and the large (n = 25 871) Vitamin D and Omega‐3 Trial (VITAL) subsequently reported that supplementation with 2000 IU/day over 5 years did not alter the risk of having ≥2 falls per year (odds ratio [OR] 0.97, 95% CI 0.90–1.05). 12 The recently published Study To Understand Fall Reduction and Vitamin D in You (STURDY) found that although supplementation with 1000 IU/day did not reduce the time to first fall or death over 2 years, it potentially increased risk of serious falls, when compared with supplementation with 200 IU/day. 13
In addition to uncertainty about vitamin D supplementation's overall effect on risk of falling, the effect of dosing regimen is also unclear. Three RCTs found intermittent, high doses of vitamin D supplementation significantly increased falls risk. 14 , 15 , 16 However, a 2015 meta‐analysis of RCTs of intermittent, high‐dose vitamin D supplementation performed in older people found no effect (8 RCTs, n = 18 572, RR 1.02, 95% CI 0.96–1.08). 17 Likewise, the large (n = 5056) Vitamin D Assessment (ViDA) Trial found that monthly doses of 100 000 IU vitamin D did not increase risk of falling. 18
The D‐Health Trial is a population‐based RCT of vitamin D supplementation. 19 The objective of the current analysis was to determine whether long‐term supplementation with monthly high‐dose (60 000 IU) vitamin D affects risk of falling in older people. Our a priori hypothesis was that randomization to vitamin D supplementation would reduce the risk and incidence of falling.
Methods
Trial design
We have published detailed trial methods. 19 Briefly, the D‐Health Trial was a double‐blind, randomized, placebo‐controlled trial in older Australians. Between February 2014 and May 2015, we randomized 21 315 participants (1:1 ratio) to monthly doses of either 60 000 IU of colecalciferol (vitamin D3) or placebo. The primary outcome is all‐cause mortality. Secondary outcomes are total cancer incidence and colorectal cancer incidence. Falls is one of many pre‐specified tertiary outcomes. 20 The QIMR Berghofer Medical Research Institute Human Research Ethics Committee approved the trial. All participants gave written or online consent to participate.
Participants
Using the Commonwealth electoral roll as the sampling frame, we invited Australians (excluding people from the Northern Territory) aged 60–79 years to participate. We also sought volunteers aged 60–84 years. Participants with a self‐reported history of osteomalacia, sarcoidosis, hyperparathyroidism, hypercalcaemia or kidney stones or who were taking >500 IU/day of supplementary vitamin D were ineligible.
Randomization
We used computer‐generated permuted block randomization, stratified by age (60–64, 65–69, 70–74, 75 + years), sex and state of residence (New South Wales, Queensland, South Australia, Tasmania, Victoria, Western Australia). Study staff and investigators did not have access to the allocation sequence.
Intervention
Lipa Pharmaceuticals Pty. Ltd. manufactured the active and placebo gel capsules (identical in appearance). We mailed blister packs of 12 tablets at baseline and annually thereafter and instructed participants to take one tablet on the first day of each month. Reminders were sent via text message, landline message and/or email. Participants took study tablets for a maximum of 5 years.
Baseline characteristics
Participants completed a questionnaire at baseline that asked about personal, socio‐demographic and lifestyle factors, history of illness and intake of vitamin D ( Methods S1). We did not measure baseline serum 25(OH)D as the study was not powered for subgroup analyses, and we could therefore not justify the high cost; rather, we predicted whether de‐seasonalized baseline serum 25(OH)D concentration was <50 nmol/L using blood samples collected throughout the trial from a randomly selected subset of participants randomized to the placebo. The model was constructed using a training dataset (80% of the samples) and validated using the remaining 20% of samples (sensitivity, 0.64; specificity, 0.72; positive predictive value, 0.23; negative predictive value, 0.94; area under the receiver operating curve, 0.71) ( Methods S2). 21
Ascertaining falls
Annual surveys
Every 12 months after randomization, we sent annual surveys to participants, including those who had withdrawn and agreed to continue surveys. Each survey asked participants to report whether they had fallen during the previous month. From annual survey 3 (commencing early 2017) onwards, we also asked participants to report the number of times they had fallen in the previous year (none, 1–2, 3–4, 5–9, ≥10 times) and included a definition of a fall (‘fall means unintentionally coming to rest on the ground, floor or other lower level’).
Falls diaries
Between October 2016 and March 2018 (mean time from randomization 2.9 years; standard deviation [SD]: 0.5 years; range: 2.1–3.8 years), we invited 2615 randomly selected participants who remained enrolled to complete a falls diary. We sampled within strata defined by randomization group, age, sex and state; eligibility was restricted to participants aged ≥65 years at the time of sampling; and we sampled equal proportions of men and women. For 3 months, participants recorded whether they had fallen on each day. For each fall that occurred, we asked participants to indicate what injuries they suffered.
Primary outcome
The primary outcome for this analysis is the occurrence of falling in the month prior to completing an annual survey, using a repeated measures approach to incorporate responses from all five annual surveys.
Secondary outcomes
Secondary outcomes include (1) falling at least once in the month prior to individual annual surveys (each year analysed separately); (2) the number of falls in the year prior to an annual survey (using an upper category of ‘≥3’ due to small numbers); (3) falling at least once while keeping a falls diary; and (4) the incidence rate (IR) of falls experienced while keeping a falls diary.
Compliance monitoring
On each annual survey, we asked participants to report the number of study tablets taken during the previous year; this was used to calculate overall compliance ( Methods S3). We also asked about consumption of off‐trial supplements containing vitamin D (including multivitamins with vitamin D) during the previous month. In addition, each year, we measured serum 25(OH)D concentrations in a random sample of ~450 participants from each trial arm.
Sample size and blinding
Because the primary outcome of the trial (all‐cause mortality) has not yet been analysed, it was important that the current analysis did not ‘unblind’ D‐Health researchers. No analysts or investigators have access to original D‐Health data. An external researcher from QIMR Berghofer's statistics department (not an author of this manuscript) prepared datasets for these analyses, following the approach previously described. 20 Random samples of 16 000 and 2400 participants were taken for the analysis of surveys and diaries, respectively, with equal numbers per treatment arm. For the latter, we sampled from all participants invited to complete diaries. Study arms were randomly mapped to 0 and 1, and new person identification codes were generated. After the main results were finalized, the authors were advised which of Group 0 and Group 1 was the placebo group. In this way, blinding was maintained for this analysis and for the trial overall. In Methods S4, we describe how we will remain blinded when conducting future analyses.
Eligibility for current analyses
From the subset of 16 000 participants, we excluded anyone who did not answer the falls question on ≥1 survey (Table S1 shows missing data patterns). From the sample of falls diary participants, we excluded anyone who did not return a diary.
Adverse events
We asked participants to contact us if they experienced any adverse event. We coded adverse events using the Medical Dictionary for Regulatory Activities (MedDRA) and classified the relationship with the intervention as unrelated, unlikely, possible or probable. We also captured diagnoses of hypercalcaemia and kidney stones in annual surveys.
Statistical analyses
We performed analyses in SAS Version 9.4 (SAS Institute, Inc., Cary, NC) and used R Version 3.6.1 22 to produce Figures 2 and S1. Most analyses were pre‐specified; Methods S5 describes all data‐driven analyses. Hypothesis tests, when performed, were two‐sided; we used a statistical significance level of P < 0.05, with no adjustment for multiple testing.
Figure 2.
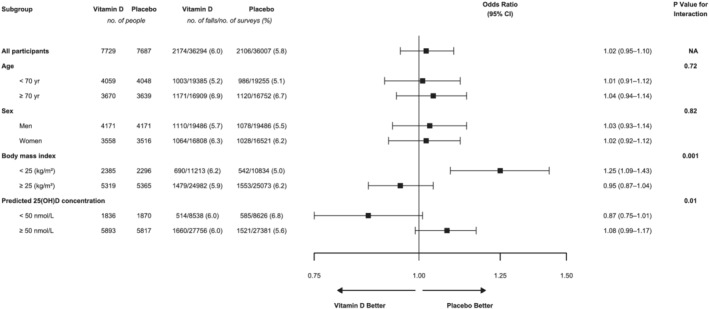
Effect of vitamin D supplementation on risk of falling in the past month for all participants and within participant subgroups: analysis of annual survey data using a repeated measures approach to incorporate responses from all five annual surveys. ORs compare vitamin D to placebo and were estimated using logistic regression with adjustment for randomization stratification variables of age, sex and state of residence at baseline. Each annual survey asked participants to report whether or not they had experienced a fall during the previous month. The numbers under ‘no. people’ show the number of participants who answered the falls question on at least one annual survey. Generalized estimating equations with an exchangeable correlation matrix used to account for participant clustering. P‐value from Wald test of significance of interaction term. Abbreviation: CI, confidence interval.
We used chi‐squared tests to compare baseline characteristics of participants who (1) were and were not included in the analytic populations and (2) completed the trial (i.e. took tablets for their full planned intervention period) in each randomization group. Associations between selected baseline characteristics and falls were analysed using regression models ( Methods S6).
Our analysis of the effect of vitamin D supplementation on falls followed a modified intention‐to‐treat approach because we excluded participants who were missing all outcome data (surveys: 3.7%; diaries: 8.3%) rather than imputing their outcomes. We used logistic regression to analyse the risk of experiencing > = 1 fall based on either survey or diary data. For survey data, we incorporated responses from all surveys using generalized estimating equations (GEE) with an exchangeable correlation matrix to account for participant clustering. To analyse the number of falls in the year prior to an annual survey, we used ordinal logistic regression. The incidence of falls recorded in diaries and the associated incidence rate ratio (IRR) were estimated using negative binomial regression. We compared the percentage of falls recorded in diaries that resulted in an injury using GEE logistic regression. All analyses were adjusted for the randomization stratification variables of age, sex and state of residence. Analyses of diary data were additionally adjusted for baseline body mass index (BMI), physical activity and time outdoors, because these variables showed some imbalance between randomization groups in the diary analytic set.
We used unadjusted Poisson regression to analyse adverse events for all 16 000 participants included in the random sample.
Interactions and subgroup analyses
In pre‐specified subgroup analyses, 20 we investigated whether the effect of supplementation was modified by the following baseline characteristics: age (<70 years; ≥70 years); sex; BMI (<25 kg/m2; ≥25 kg/m2); and predicted de‐seasonalized baseline serum 25(OH)D concentration (<50 nmol/L; ≥50 nmol/L).
For each factor, we report the P‐value for the interaction term (between randomization group and the factor of interest) based on either the Wald or likelihood ratio statistic. We also calculated effect estimates within each stratum of a factor.
To assist with interpretation of results, in exploratory analyses among participants with BMI < 25 kg/m2, we excluded anyone with BMI < 18.5 kg/m2 ( Methods S5).
Results
Trial profile
Detailed baseline characteristics have been published previously. 19 Five people withdrew their consent after randomization, and their data have been deleted. The mean age of the remaining 21 310 participants was 69.3 (SD 5.5) years, and 46% (n = 9780) were women. All participants had finished the intervention by 1 February 2020. The mean treatment duration was 4.3 (SD 1.4) years (range: 0.0–5.0).
Participants (current analysis)
Analyses of surveys and diaries included 15 416 (96.4%) and 2200 (91.7%) of participants, respectively (Figure 1); approximately half were randomized to vitamin D (surveys: 50.1%; diaries: 50.4%). The survey analytic population was very similar to the entire D‐Health cohort with respect to age (69.2 [SD 5.5] years) and sex (46% [n = 7074] women). By design, the diary analytic population was older (72.1 [SD 3.6] years) and had a higher percentage of women (51% [n = 1120]). The mean BMI was approximately 28 (SD 5) kg/m2 for both analytic sets. Fully adjusted analyses of diary data included 2093 (95%) participants with complete data for BMI, physical activity and time outdoors.
Figure 1.
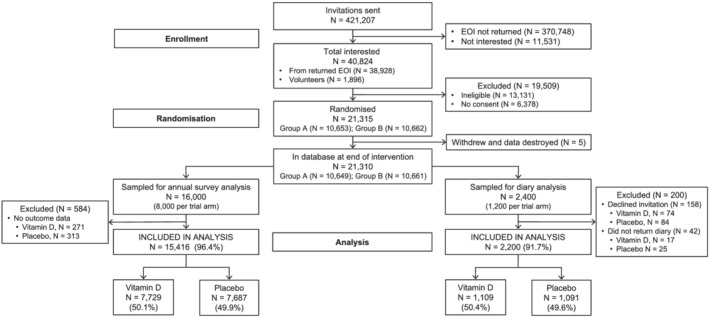
Participant flow for analyses of data from annual surveys and falls diaries (CONSORT flow diagram).
Treatment allocation was not associated with being excluded (randomized to vitamin D [included vs. excluded]: 50% vs. 46% for both surveys [P = 0.08] and diaries [P = 0.2]). Although some baseline characteristics differed between those who were and were not included in analyses (Table S2), there was very good balance between the study groups in the analytic populations, with the exception of small differences in BMI, time outdoors and physical activity for participants who completed diaries (Table 1).
Table 1.
Baseline characteristics according to randomization group for people included in analyses of data from annual surveys and falls diaries.
| n (%) | ||||
|---|---|---|---|---|
| Annual surveys | Falls diaries | |||
| Characteristic | Vitamin D (n = 7,729) | Placebo (n = 7687) | Vitamin D (n = 1109) | Placebo (n = 1091) |
| Age (years) | ||||
| 60–64 | 1934 (25.0) | 1924 (25.0) | 1 (0.1) | 0 (0.0) |
| 65–69 | 2125 (27.5) | 2124 (27.6) | 346 (31.2) | 354 (32.4) |
| 70–74 | 2103 (27.2) | 2066 (26.9) | 473 (42.7) | 427 (39.1) |
| ≥75 | 1567 (20.3) | 1573 (20.5) | 289 (26.1) | 310 (28.4) |
| Sex | ||||
| Men | 4171 (54.0) | 4171 (54.3) | 533 (48.1) | 547 (50.1) |
| Women | 3558 (46.0) | 3516 (45.7) | 576 (51.9) | 544 (49.9) |
| Body mass index (kg/m2) | ||||
| <18.5 | 56 (0.7) | 44 (0.6) | 13 (1.2) | 9 (0.8) |
| 18.5 to <25 | 2329 (30.2) | 2252 (29.4) | 352 (31.9) | 297 (27.3) |
| 25 to <30 | 3208 (41.6) | 3320 (43.3) | 438 (39.6) | 496 (45.6) |
| ≥30 | 2111 (27.4) | 2045 (26.7) | 302 (27.3) | 286 (26.3) |
| Missing | 25 | 26 | 4 | 3 |
| Highest qualification obtained | ||||
| None | 770 (10.1) | 731 (9.6) | 125 (11.4) | 137 (12.7) |
| School or intermediate cert. | 1311 (17.2) | 1257 (16.5) | 221 (20.1) | 181 (16.8) |
| Higher school or leaving cert. | 1049 (13.7) | 1114 (14.7) | 138 (12.5) | 150 (13.9) |
| Apprenticeship or cert. | 2494 (32.7) | 2595 (34.1) | 341 (31.0) | 352 (32.6) |
| University degree or higher | 2006 (26.3) | 1904 (25.0) | 275 (25.0) | 259 (24.0) |
| Missing | 99 | 86 | 9 | 12 |
| Living alone | ||||
| No | 6150 (80.0) | 6144 (80.3) | 865 (78.2) | 839 (77.1) |
| Yes | 1542 (20.0) | 1504 (19.7) | 241 (21.8) | 249 (22.9) |
| Missing | 37 | 39 | 3 | 3 |
| Alcohol consumption (drinks/week) | ||||
| <1 | 1798 (24.1) | 1822 (24.6) | 267 (25.1) | 277 (26.4) |
| 1 to 7 | 3324 (44.6) | 3295 (44.5) | 493 (46.3) | 468 (44.6) |
| >7 to 14 | 1393 (18.7) | 1330 (17.9) | 189 (17.7) | 176 (16.8) |
| >14 | 945 (12.7) | 965 (13.0) | 116 (10.9) | 128 (12.2) |
| Missing | 269 | 275 | 44 | 42 |
| Smoking history | ||||
| Never | 4298 (56.0) | 4174 (54.8) | 634 (57.4) | 624 (57.8) |
| Ex‐smoker | 3103 (40.4) | 3112 (40.8) | 438 (39.7) | 422 (39.1) |
| Current | 273 (3.6) | 336 (4.4) | 32 (2.9) | 34 (3.1) |
| Missing | 55 | 65 | 5 | 11 |
| Physical activity (METs/week) | ||||
| Low (<18) | 2463 (32.8) | 2485 (33.3) | 337 (31.4) | 376 (35.1) |
| Moderate (18 to <45) | 2502 (33.4) | 2470 (33.1) | 359 (33.5) | 336 (31.4) |
| High (≥45) | 2534 (33.8) | 2504 (33.6) | 377 (35.1) | 358 (33.5) |
| Missing | 230 | 228 | 36 | 21 |
| Time outdoors (hours/week) | ||||
| Low (<8) | 2462 (32.7) | 2513 (33.4) | 312 (29.0) | 372 (34.9) |
| Moderate (8 to <18) | 2480 (32.9) | 2503 (33.3) | 387 (35.9) | 344 (32.3) |
| High (≥18) | 2595 (34.4) | 2510 (33.4) | 378 (35.1) | 350 (32.8) |
| Missing | 192 | 161 | 32 | 25 |
| Chronic pain | ||||
| No | 6006 (78.0) | 5970 (77.9) | 869 (78.6) | 863 (79.4) |
| Yes | 1691 (22.0) | 1697 (22.1) | 237 (21.4) | 224 (20.6) |
| Missing | 32 | 20 | 3 | 4 |
| Self‐rated overall health | ||||
| Excellent or very good | 4239 (55.8) | 4209 (55.5) | 628 (57.8) | 603 (55.9) |
| Good | 2740 (36.1) | 2741 (36.1) | 384 (35.3) | 398 (36.9) |
| Fair or poor | 621 (8.2) | 638 (8.4) | 75 (6.9) | 77 (7.1) |
| Missing | 129 | 99 | 22 | 13 |
| Self‐rated quality of life | ||||
| Excellent or very good | 5083 (67.2) | 5088 (67.6) | 739 (68.6) | 732 (68.3) |
| Good | 2046 (27.0) | 1994 (26.5) | 285 (26.5) | 296 (27.6) |
| Fair or poor | 435 (5.8) | 444 (5.9) | 53 (4.9) | 44 (4.1) |
| Missing | 165 | 161 | 32 | 19 |
| Self‐rated fair or poor memory | ||||
| No | 5655 (73.4) | 5579 (72.8) | 805 (72.8) | 792 (72.9) |
| Yes | 2045 (26.6) | 2089 (27.2) | 301 (27.2) | 295 (27.1) |
| Missing | 29 | 19 | 3 | 4 |
| Predicted 25(OH)D concentration (nmol/L) | ||||
| < 50 | 1,836 (23.8) | 1,870 (24.3) | 257 (23.2) | 276 (25.3) |
| ≥50 | 5,893 (76.2) | 5,817 (75.7) | 852 (76.8) | 815 (74.7) |
cert., certificate; METs, metabolic equivalent tasks.
Falls outcomes over time
The percentage of participants reporting a fall in the month prior to each annual survey ranged from 3.6% (Year 1) to 7.6% (Year 4). There was a sharp increase from Year 2 (3.9%) to Year 3 (7.2%), corresponding to when the definition of falling was first included in the survey. The distribution of number of falls in the year prior to a survey was very stable across all years in which it was collected (Years 3–5). Approximately 75% of participants reported no falls in the previous year, and just over 20% reported falling once or twice; the percentage who reported falling ≥3 times ranged from 3.5% to 3.7%.
Baseline factors associated with falling
Analyses of survey data showed that older age, worse overall health, worse quality of life, chronic pain and fair or poor memory were strongly associated with increased risk of falling ( Tables S3 and S4). In addition, falls were more common among women, and participants with BMI ≥ 30 kg/m2, and in those who lived alone or had ever smoked. After adjustment for age, sex, BMI and physical activity, predicted baseline serum 25(OH)D concentration was not associated with falling. In analyses of diary data, women and participants with poorer memory were at greater risk of falling ( Tables S5 and S6).
Effect of vitamin D supplementation on risk and incidence of falling
Vitamin D had little effect on the risk of falling at least once (surveys: OR 1.02, 95% CI 0.95–1.10; diaries: fully adjusted OR [AOR] 1.07, 95% CI 0.84–1.36) (Tables 2 and S7) or on the risk of falling 1–2 or ≥3 times in the year prior to separate annual surveys ( Table S8). Of those participants who fell while keeping a diary, most experienced a single fall (vitamin D, n = 116 [73%]; placebo, n = 123 [80%]); in each group, two participants experienced the maximum of four falls over 3 months ( Table S9). There was little difference in IRs between the treatment groups (Table 2). Estimates from analyses of diary data adjusted for age, sex and state only (but restricted to participants with complete data) were not meaningfully different ( Table S10).
Table 2.
Effect of vitamin D supplementation on risk of falling and incidence of falls
| No./total no. (%) or incidence rate | |||
|---|---|---|---|
| Vitamin D | Placebo | OR/IRR (95% CI) a | |
| Annual survey data (n = 15 416) | n = 7729 | n = 7687 | |
| Any fall in previous month b | 2174/36 294 (6.0) | 2,106/36,007 (5.8) | 1.02 (0.95–1.10) |
| Diary data (N = 2093) c | n = 1045 | n = 1048 | |
| Any fall over 3 months | 159/1045 (15.2) | 153/1048 (14.6) | 1.07 (0.84–1.36) |
| Number of falls, IR per 1000 PYAR d | 820 | 728 | 1.13 (0.89–1.43) |
CI, confidence interval; IRR, incidence rate ratio; OR, odds ratio; PYAR, person‐years at risk.
Estimates compare vitamin D to placebo. ORs estimated using logistic regression; generalized estimating equations with an exchangeable correlation matrix used to account for participant clustering when analysing annual survey data. IRR estimated using negative binomial regression. All estimates adjusted for age, sex and state of residence. Analyses of diary data additionally adjusted for body mass index, physical activity and time outdoors at baseline.
Data analysed using a repeated measures approach to incorporate responses from all five annual surveys. Numerator is the total number of falls; denominator is the total number of surveys.
Results presented for people with complete data for all covariates included in the model. Minimally adjusted estimates (adjusted for age, sex and state) were not meaningfully different (see Table S10).
Goodness‐of‐fit chi‐squared test (based on the deviance) showed that the model was adequate.
Effect of vitamin D supplementation within participant subgroups
We found significant and consistent interaction effects between randomization group and BMI (surveys: P = 0.001; diaries: P = 0.02). In participants with BMI < 25 kg/m2, vitamin D supplementation increased the risk of falling (surveys: OR 1.25, 95% CI 1.09–1.43 [Figure 2]; diaries: AOR 1.72, 95% CI 1.09–2.73 [Table 3]). In contrast, there was no effect in participants with BMI ≥ 25 kg/m2. Analysis of incidence showed a similar pattern (Table 3). For number of falls experienced during a year, there was no clear interaction with BMI except in Year 5 ( Table S8).
Table 3.
Diary data: fully adjusted estimates of the effect of vitamin D supplementation on risk of falling and incidence of falls over a 3‐month period within participant subgroups
| No. fallers/total no. people (%) | IR per 1000 PYAR | |||||||
|---|---|---|---|---|---|---|---|---|
| Vitamin D | Placebo | OR (95% CI) a | P‐int b | Vitamin D | Placebo | IRR (95% CI) a | P‐int b | |
| Age (years) | 0.13 | 0.32 | ||||||
| <70 | 45/329 (13.7) | 56/341 (16.4) | 0.81 (0.52–1.25) | 674 | 733 | 0.92 (0.61–1.38) | ||
| ≥70 | 114/716 (15.9) | 97/707 (13.7) | 1.24 (0.92–1.67) | 839 | 677 | 1.24 (0.93–1.65) | ||
| Sex | 0.79 | 0.69 | ||||||
| Men | 70/509 (13.8) | 69/533 (12.9) | 1.11 (0.77–1.59) | 760 | 648 | 1.17 (0.82–1.68) | ||
| Women | 89/536 (16.6) | 84/515 (16.3) | 1.05 (0.76–1.46) | 886 | 816 | 1.09 (0.80–1.48) | ||
| Body mass index (kg/m2) | 0.02 | 0.17 | ||||||
| <25 | 60/345 (17.4) | 34/298 (11.4) | 1.72 (1.09–2.73) | 888 | 607 | 1.46 (0.94–2.28) | ||
| ≥25 | 99/700 (14.1) | 119/750 (15.9) | 0.88 (0.66–1.18) | 758 | 755 | 1.00 (0.76–1.32) | ||
| Predicted 25(OH)D concentration (nmol/L) | 0.72 | 0.59 | ||||||
| <50 | 45/235 (19.1) | 44/256 (17.2) | 1.12 (0.70–1.80) | 895 | 719 | 1.25 (0.82–1.90) | ||
| ≥50 | 114/810 (14.1) | 109/792 (13.8) | 1.04 (0.78–1.39) | 777 | 715 | 1.09 (0.82–1.44) | ||
CI, confidence interval; IR(R), incidence rate (ratio); OR, odds ratio; P‐int, P‐value for interaction; PYAR, person‐years at risk.
ORs and IRRs (comparing vitamin D to placebo) were estimated using logistic and negative binomial regression, respectively. All estimates adjusted for baseline values of age, sex, state of residence, body mass index, physical activity and time outdoors; analyses were restricted to people who had complete data for all these covariates (n = 2093). Minimally adjusted estimates (age, sex, and state) were not meaningfully different (see Table S10).
P‐values from Wald test or likelihood ratio test of significance of interaction term.
When we excluded participants with BMI < 18.5 kg/m2 from the ‘normal’ BMI stratum, survey estimates were largely unaltered (all outcomes; data not shown); however, the AOR for having ≥1 fall while keeping a diary decreased from 1.72 to 1.60 (95% CI 1.00–2.57). In exploratory analyses, we found that effect estimates were generally higher in participants with BMI between 22.5 and <25 kg/m2 compared with participants with BMI between 18.5 and <22.5 kg/m2 ( Table S11).
Based on survey data, vitamin D appeared to be protective in participants with predicted baseline serum 25(OH)D concentration <50 nmol/L and harmful in participants with predicted concentration ≥50 nmol/L (P‐interaction = 0.01) (Figure 2). However, this was not seen for analyses of diaries (Table 3).
There was little evidence of effect modification by age or sex ( Tables S12–S14).
Falls resulting in injuries (falls diaries)
In both groups, 9% of participants had ≥1 injurious fall over 3 months ( Table S9). The percentage of falls resulting in an injury was lower in the vitamin D group (52% vs. 58%; AOR 0.75, 95% CI 0.48–1.17). There were eight fractures in total (four per group), with one participant in the vitamin D group experiencing two factures from separate falls ( Table S9).
Participant retention and compliance
The mean treatment duration for participants included in these analyses was higher than for the entire D‐Health cohort (surveys: 4.5 [SD 1.2] years; diaries: 4.9 [SD 0.3] years), with little difference between randomization groups. Of the 15 416 participants included in the analysis of survey data, 82% (n = 12 654) took tablets for their full planned intervention period (i.e. completed the trial), with slightly higher study completion in the vitamin D group (83% vs. 81%) ( Table S15). With the exception of smoking status, baseline characteristics of participants who completed the trial did not differ between randomization groups ( Table S16). Of the 2762 participants who did not complete the intervention, 10% (n = 281) ceased taking tablets due to death. Trial completion was very high (94%) among participants who completed a falls diary. The vast majority of participants reported taking ≥80% of their study tablets (surveys: ~85%; diaries: 98%) ( Table S15). Compliance with study tablets decreased over time ( Table S17).
For each year of the trial, the percentage of participants who reported taking no off‐trial supplementary vitamin D was ~80% and ~75% for the vitamin D and placebo groups, respectively ( Table S18). Intake was ≤500 IU/day for most participants who took off‐trial supplementary vitamin D.
There was a clear separation in the distribution of measured serum 25(OH)D concentrations between the groups throughout the intervention (Figure S1). The mean serum 25(OH)D concentration of the 2205 blood samples from 1956 participants randomized to vitamin D was 114.8 (SD 30.3) nmol/L; it was 77.5 (SD 25.2) nmol/L for the 2236 blood samples taken from 1987 participants randomized to placebo. In the vitamin D group, the mean decreased with increasing BMI category (<25 kg/m2: 121.0 [SD 32.6] nmol/L; 25–<30 kg/m2: 114.3 [SD 29.6] nmol/L; >30 kg/m2: 106.9 [SD 26.3] nmol/L). Only 13% of samples from the placebo group had serum 25(OH)D concentration < 50 nmol/L.
Adverse events
Participants randomized to vitamin D had a lower incidence of adverse events of any type and any severity (IR per 1000 person‐years at risk [PYAR] 59 vs. 64, IRR 0.92; 95% CI 0.86–0.97). Table S19 shows adverse events according to MedDRA System Organ Classes. IRs (per 1000 PYAR; vitamin D vs. placebo) did not differ materially between groups for adverse events (of any type) classified as possibly or probably related to the intervention (IRR 0.91, 95% CI 0.80–1.04), hypercalcaemia (IR 1.5 vs. 1.6, IRR 0.91, 95% CI 0.62–1.33) or kidney stones (IR 3.3 vs. 3.1, IRR 1.08, 95% CI 0.83–1.41). The total number of falls reported to study staff on an ad hoc basis was very similar between groups (vitamin D, n = 86; placebo, n = 84) ( Table S20). Although the percentage of falls resulting in hospitalization was higher in participants randomized to vitamin D (45.3%) compared with placebo (40.5%), the IRs for falls resulting hospitalization were similar (IR 1.1 vs. 1.0) ( Table S20).
Discussion
We found that, overall, long‐term monthly supplementation with 60 000 IU of vitamin D3 had no effect on risk of falling in older Australians. However, in participants with baseline BMI < 25 kg/m2, the risk of falling was higher among those randomized to vitamin D.
The percentage of participants who reported falling is consistent with expectations. When a definition of falling was included in the surveys, ~7.5% of D‐Health participants reported falling in the previous month, and 25% reported ≥1 fall over the previous 12 months. There are limited Australian data with which to compare the risk of falling, as only falls resulting in hospitalization are routinely reported. 23 However, our findings are closely aligned with those of the ViDA Trial participants (mean age, 66 years), among whom 6% reported falling in the 4 weeks prior to baseline assessment. 18 Our annual figures are also broadly consistent with the finding that 29% of people in the United States aged ≥65 years fell at least once during 2014. 24 As expected, 25 , 26 we found that factors associated with poorer health and physical decline were also strongly associated with increased risk of falling.
Our finding of no overall effect is consistent with recent meta‐analyses. 11 , 17 The subsequently published VITAL Trial (the largest RCT of high‐dose vitamin D supplementation to date) that used the same daily equivalent dose as D‐Health also found no effect on the risk of having two or more falls per year. 12
Concerns have been raised about the safety of intermittent, high‐dose vitamin D supplementation, 27 with increased falls risk seen in three previous RCTs that used bolus doses of vitamin D3. 14 , 15 , 16 All were performed in populations selected to be at increased risk of falling (elderly women at high risk of fracture, 14 people with a history of falling 15 and long‐term care residents 16 ), two had very small samples (n < 70 per group), and only one included a placebo group. 14 The largest of these (n = 2256) found that annual doses of 500 000 IU over 3–5 years increased incidence of falls (IRR 1.15, 95% CI, 1.02–1.30); baseline BMI was not reported. 14 The other two supplemented participants with monthly doses over 1 year and reported increased risk 15 and incidence 16 in the high‐dose group (≥1 fall, 60 000 IU vs. 24 000 IU: 67% vs. 48%; 100 000 IU vs. standard dose [equivalent to 400–1000 IU/day]: IRR 2.33, 95% CI 1.49–3.63). Differences in age, sex and intervention mean that these results cannot be readily compared with those from the D‐Health Trial.
Despite evidence of increased risk in these studies, the 2018 meta‐analysis found no effect of bolus dosing on risk of falling. 11 The New Zealand ViDA Trial was the largest RCT of intermittent, high‐dose vitamin D3 supplementation included. It had a similar study design to the D‐Health Trial and found that monthly doses of 100 000 IU over 2.5–4.2 years had no effect on falls (hazard ratio [HR] 0.99, 95% CI 0.92–1.07). 18
The 2018 meta‐analysis did not find any interaction between vitamin D supplementation and BMI, although the BMI cut‐point used was higher (30 kg/m2) than in our analysis and dosing regimen was not considered. 11 In contrast to our results, VITAL found a suggestion that vitamin D supplementation reduced falls in participants with BMI < 25 kg/m2 (≥2 falls per year: OR 0.88, 95% CI 0.76–1.02), although the interaction with BMI was not significant (p = 0.07). The ViDA study did not test for interaction with BMI.
To the best of our knowledge, our study is the first to suggest that intermittent supplementation with high‐dose vitamin D may increase the risk of falling in people with BMI < 25 kg/m2 while having little effect in people with BMI ≥ 25 kg/m2. Whereas subgroup analyses should be viewed with caution, the results were consistent across analyses of surveys and diaries, suggesting that this may be a true effect. Exploratory analyses demonstrated that results were not driven by outcomes among ‘underweight’ participants. Explanations for a possible deleterious effect of high‐dose vitamin D are currently speculative. 28 Supraphysiological doses of vitamin D have the potential to saturate the vitamin D binding protein, leading to displacement of 1,25(OH)D and 25(OH)D into the circulation in the days after supplementation. 29 It is plausible that this effect would be greater in people with lower body fat, and we showed that those with normal BMI achieved the highest levels of serum 25(OH)D. However, it is not clear why this would increase risk of falling.
In analyses of survey data, there was a suggestion that supplementation might be protective in participants with lower predicted baseline serum 25(OH)D concentrations and harmful for those with higher predicted concentrations. The inverse association between BMI and serum 25(OH)D concentrations makes it difficult to disentangle the influence of these factors. Because this effect was not seen in analyses of diary data, the model we developed to predict baseline serum 25(OH)D concentrations was only moderately reliable, 21 and the predicted values have random error that has not been accounted for here, these results should be interpreted cautiously. The lack of measured baseline 25(OH)D concentrations is a weakness of this study.
An exploratory analysis of adverse events reported on an ad hoc basis, performed after unblinding, suggested that, compared with the placebo group, a greater percentage of falls experienced by participants randomized to vitamin D resulted in hospitalization. However there was no meaningful difference in the IRs for falls resulting in hospitalization, and analyses of diary data (collected systematically) showed that participants in both groups were equally likely to experience at least one injurious fall over 3 months. Nevertheless, the result is consistent with the findings of the small (n = 647) STURDY trial performed in older adults with elevated risk of falling. 13 In a comparison of daily supplementation with 1000 IU vs. 200 IU, the higher dose had no benefit overall (HR 0.94, 95% CI 0.76–1.15) and actually appeared to increase the risk of serious falls (HR 1.87, 95% CI 1.03–3.41) and falls requiring hospitalization (HR 2.48, 95% CI 1.13–5.46). 13
Although using falls diaries allowed us to collect accurate information, estimates based on these data might be biased. Despite adjusting analyses of diaries for characteristics that showed some imbalance between trial arms, residual confounding remains a possibility.
Vitamin D testing is widespread in Australia, 30 and D‐Health participants who suspected that they had been randomized to placebo may have decided to withdraw or start taking off‐trial vitamin D supplementation in doses higher than permitted by the study protocol. We only collected blood from participants who remained in the trial at the time the blood collection was due, and ~20% of participants invited to give blood declined (mostly because of lack of easy access to a pathology collection centre). Nevertheless, the clear and sustained separation in the distribution of serum 25(OH)D concentrations between the trial arms suggests that contamination of the placebo group was not a serious problem.
The D‐Health Trial has several strengths. Excellent retention, compliance and survey completion rates, combined with the long duration of the intervention, increase our confidence that our main null finding of no effect is robust. All researchers remained blinded to treatment allocation until all analyses of falls outcomes were completed, and we have taken careful steps to ensure that blinding was maintained for the trial overall.
Our results are likely to be generalizable to the general older Australian population, despite the D‐Health cohort being, on average, somewhat healthier 19 ; the mean 25(OH)D concentration in the placebo group in the D‐Health Trial was reasonably similar to that in the Australian Health Survey (78 vs. 69 nmol/L). 31 However, we cannot extrapolate from our results to populations with a high prevalence of vitamin D deficiency. Because it is ethically questionable to perform a placebo‐controlled trial of vitamin D supplementation in people who are vitamin D deficient, we recommend high‐quality observational studies be undertaken; this will help elucidate the relationship between serum 25(OH)D concentrations and falls risk.
In conclusion, our results do not support the use of monthly high‐dose vitamin D supplementation for falls prevention. The evidence of increased risk in people with BMI < 25 kg/m2 warrants further investigation, ideally through reanalysis of existing trials. In the meantime, caution should be used in prescribing high‐dose vitamin D to older adults in the normal BMI range and who are vitamin D replete.
Funding
The D‐Health Trial is funded by National Health and Medical Research Council (NHMRC) project grants (GNT1046681 and GNT1120682). PMW and DCW are supported by fellowships from the NHMRC (GNT1173346 and GNT1155413). DSAM is supported by a Metro North Clinician Research Fellowship and a Queensland Advancing Clinical Research Fellowship. HP is supported by a University of Queensland PhD Scholarship.
Author contributions
MW and REN developed the statistical analysis plan and wrote the original draft. MW analysed the data. MW, REN, DSAM, DRE, BKA, PRE, GH, MGK, HP, JCvdP, PMW and DCW revised the manuscript. REN, BKA, CB, BDR, PRE, DRE, MGK, DSAM, RLO'C, JCvdP, AJV, PMW and DCW were involved in design of the trial protocol. CB, BDR, MW, HP and REN curated data during the trial. All authors read and approved the final manuscript.
Conflict of interest
Penelope Webb has funding from AstraZeneca for an unrelated study of ovarian cancer. Peter Ebeling reports grants and other from Amgen, other from Sanofi, grants and other from Novartis, grants from Eli‐Lilly and grants from Alexion. Mary Waterhouse, Emma Sanguineti, Catherine Baxter, Briony Duarte Romero, Donald McLeod, Dallas English, Bruce Armstrong, Gunter Hartel, Michael Kimlin, Rachel O'Connell, Hai Pham, Jolieke van der Pols, Alison Venn, David Whiteman and Rachel Neale declare that they have no conflict of interest.
Supporting information
Table S1. Patterns of missing annual survey data.
Table S2. Baseline characteristics according to inclusion in the analyses of annual surveys and falls diaries.
Table S3. Associations between selected baseline characteristics and risk of falling in the month prior to completing an annual surveya.
Table S4. Associations between selected baseline characteristics and number of falls in the year prior to completing an annual survey – complete case analysisa,b.
Table S5. Associations between selected baseline characteristics and falling at least once over 3 months (analysis of diary data)a.
Table S6. Associations between selected baseline characteristics and number of falls experienced over 3 months (analysis of diary data using ordinal logistic regression)a,b.
Table S7. Total number of times participants reported falling at least once in the month prior to an annual survey according to randomisation group; results stratified by the total number of responses provided.
Table S8. Effect of vitamin D supplementation on risk of falling 1–2 or ≥3 times in the year prior to separate annual surveys; results from ordinal logistic regression a,b.
Table S9. Summary of falls outcomes over a 3‐month period (diary data).
Table S10. Minimally adjusted estimates of the effect of vitamin D supplementation on falling over a 3‐month period; analyses of diary dataa.
Table S11. Effect of vitamin D supplementation on risk of falling and incidence of falls within selected strata of body mass indexa.
Table S12. Summary statistics by randomisation group for falls in month prior to annual surveys for all participants and within subgroups.
Table S13. Effect of vitamin D supplementation on falling in the month prior to completing each annual survey ‐ all participants and within subgroupsa.
Table S14. Total number of times participants reported falling at least once in the month prior to an annual survey according to randomisation group; results within participant subgroups and stratified by the total number of responses provided.
Table S15. Retention and compliance with study tablets according to randomisation group, within subsets included in the analysis.
Table S16. Baseline characteristics according to randomisation group restricted to participants included in the analysis of annual surveys who completed the triala.
Table S17. Compliance with study tablets over time according to randomisation group, within the subset of participants included in the analysis of annual surveys.
Table S18. Intake of vitamin D from off‐trial supplements according to randomisation group, within the subset of participants included in the analysis of annual surveys.
Table S19. Adverse events according to according to MedDRA System Organ Class and randomisation group for all participants included in the random subset of size n = 16,000a.
Table S20. Falls reported to study staff on an ad hoc basis; results calculated using data for all participants included in the random subset of size n = 16,000a
Figure S1. Boxplots of serum 25(OH)D concentrations by year of blood draw according to randomisation group.
Acknowledgements
We would like to acknowledge the D‐Health Trial staff and members of the Data and Safety Monitoring Board (Patricia Valery, Ie‐Wen Sim, Kerrie Sanders). We also extend our thanks to the D‐Health Trial participants who committed to this research. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 32
Waterhouse M., Sanguineti E., Baxter C., Duarte Romero B., McLeod D. S. A., English D. R., Armstrong B. K., Ebeling P. R., Hartel G., Kimlin M. G., O'Connell R. L., Pham H., van der Pols J. C., Venn A. J., Webb P. M., Whiteman D. C., and Neale R. E. (2021) Vitamin D supplementation and risk of falling: outcomes from the randomized, placebo‐controlled D‐Health Trial, Journal of Cachexia, Sarcopenia and Muscle, 12, 1428–1439, 10.1002/jcsm.12759
References
- 1. James SL, Lucchesi LR, Bisignano C, Castle CD, Dingels ZV, Fox JT, et al. The global burden of falls: global, regional and national estimates of morbidity and mortality from the global burden of disease study 2017. Inj Prev 2020;26:i3–i11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tinetti ME, Williams CS. The effect of falls and fall injuries on functioning in community‐dwelling older persons. J Gerontol A Biol Sci Med Sci 1998;53:M112–M119. [DOI] [PubMed] [Google Scholar]
- 3. Bouillon R, van Schoor NM, Gielen E, Boonen S, Mathieu C, Vanderschueren D, et al. Optimal vitamin D status: a critical analysis on the basis of evidence‐based medicine. J Clin Endocrinol Metab 2013;98:E1283–E1304. [DOI] [PubMed] [Google Scholar]
- 4. Ensrud KE, Blackwell TL, Cauley JA, Cummings SR, Barrett‐Connor E, Dam TT, et al. Circulating 25‐hydroxyvitamin D levels and frailty in older men: the osteoporotic fractures in men study. J Am Geriatr Soc 2011;59:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Snijder MB, van Schoor NM, Pluijm SM, van Dam RM, Visser M, Lips P. Vitamin D status in relation to one‐year risk of recurrent falling in older men and women. J Clin Endocrinol Metab 2006;91:2980–2985. [DOI] [PubMed] [Google Scholar]
- 6. Girgis CM, Mokbel N, Cha KM, Houweling PJ, Abboud M, Fraser DR, et al. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25‐hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 2014;155:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Girgis CM, Cha KM, So B, Tsang M, Chen J, Houweling PJ, et al. Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function. J Cachexia Sarcopenia Muscle 2019;10:1228–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muir SW, Montero‐Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta‐analysis. J Am Geriatr Soc 2011;59:2291–2300. [DOI] [PubMed] [Google Scholar]
- 9. Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta‐analysis of randomized controlled trials. J Clin Endocrinol Metab 2014;99:4336–4345. [DOI] [PubMed] [Google Scholar]
- 10. Bischoff‐Ferrari HA, Dawson‐Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: a meta‐analysis of randomised controlled trials. BMJ 2009;339:b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta‐analysis, and trial sequential analysis. Lancet Diabetes Endocrinol 2018;6:847–858. [DOI] [PubMed] [Google Scholar]
- 12. LeBoff MS, Murata EM, Cook NR, Cawthon P, Chou SH, Kotler G, et al. VITamin D and omegA‐3 TriaL (VITAL): effects of vitamin D supplements on risk of falls in the US population. J Clin Endocrinol Metab 2020;105:2929–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Appel LJ, Michos ED, Mitchell CM, Blackford AL, Sternberg AL, Miller ER 3rd, et al. The effects of four doses of vitamin D supplements on falls in older adults: a response‐adaptive, randomized clinical trial. Ann Intern Med 2021;174:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high‐dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010;303:1815–1822. [DOI] [PubMed] [Google Scholar]
- 15. Bischoff‐Ferrari HA, Dawson‐Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, et al. Monthly high‐dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med 2016;176:175–183. [DOI] [PubMed] [Google Scholar]
- 16. Ginde AA, Blatchford P, Breese K, Zarrabi L, Linnebur SA, Wallace JI, et al. High‐dose monthly vitamin D for prevention of acute respiratory infection in older long‐term care residents: a randomized clinical trial. J Am Geriatr Soc 2017;65:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng YT, Cui QQ, Hong YM, Yao WG. A meta‐analysis of high dose, intermittent vitamin D supplementation among older adults. PLoS ONE 2015;10:e0115850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khaw KT, Stewart AW, Waayer D, Lawes CMM, Toop L, Camargo CA, et al. Effect of monthly high‐dose vitamin D supplementation on falls and non‐vertebral fractures: secondary and post‐hoc outcomes from the randomised, double‐blind, placebo‐controlled ViDA trial. Lancet Diabetes Endocrinol 2017;5:438–447. [DOI] [PubMed] [Google Scholar]
- 19. Neale RE, Armstrong BK, Baxter C, Duarte Romero B, Ebeling P, English DR, et al. The D‐health trial: a randomized trial of vitamin D for prevention of mortality and cancer. Contemp Clin Trials 2016;48:83–90. [DOI] [PubMed] [Google Scholar]
- 20. Waterhouse M, English DR, Armstrong BK, Baxter C, Duarte Romero B, Ebeling PR, et al. A randomized placebo‐controlled trial of vitamin D supplementation for reduction of mortality and cancer: statistical analysis plan for the D‐health trial. Contemp Clin Trials Commun 2019;14:100333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Waterhouse M, Baxter C, Duarte Romero B, McLeod DS, English DR, Armstrong BK, et al. Predicting deseasonalised serum 25 hydroxy vitamin D concentrations in the D‐health trial: an analysis using boosted regression trees. Contemp Clin Trials 2021;104:106347. [DOI] [PubMed] [Google Scholar]
- 22. Team RC . R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2017.
- 23. AIHW: Pointer S . Trends in hospitalised injury due to falls in older people, 2007–08 to 2016–17. Injury research and statistics series no. 126. Cat. no. INJCAT 206. Canberra: AIHW; 2019.
- 24. Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years ‐ United States, 2014. MMWR Morb Mortal Wkly Rep 2016;65:993–998. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization . WHO global report on falls prevention in older age. 2007.
- 26. Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community‐dwelling older people: a systematic review and meta‐analysis. Epidemiology 2010;21:658–668. [DOI] [PubMed] [Google Scholar]
- 27. Sanders KM, Seibel MJ. Therapy: new findings on vitamin D‐3 supplementation and falls ‐ when more is perhaps not better. Nat Rev Endocrinol 2016;12:190–191. [DOI] [PubMed] [Google Scholar]
- 28. Sanders KM, Nicholson GC, Ebeling PR. Is high dose vitamin D harmful? Calcif Tissue Int 2013;92:191–206. [DOI] [PubMed] [Google Scholar]
- 29. Heaney RP, Armas LA. Quantifying the vitamin D economy. Nutr Rev 2015;73:51–67. [DOI] [PubMed] [Google Scholar]
- 30. Gordon L, Waterhouse M, Reid IR, Neale RE. The vitamin D testing rate is again rising, despite new MBS testing criteria. Med J Aust 2020;213:155. [DOI] [PubMed] [Google Scholar]
- 31. Australian Bureau of Statistics . Australian Health Survey: biomedical results for nutrients, 2011–12. 2014. https://www.abs.gov.au/statistics/health/health‐conditions‐and‐risks/australian‐health‐survey‐biomedical‐results‐nutrients/latest‐release#data‐download (accessed 28 Oct 2020).
- 32. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patterns of missing annual survey data.
Table S2. Baseline characteristics according to inclusion in the analyses of annual surveys and falls diaries.
Table S3. Associations between selected baseline characteristics and risk of falling in the month prior to completing an annual surveya.
Table S4. Associations between selected baseline characteristics and number of falls in the year prior to completing an annual survey – complete case analysisa,b.
Table S5. Associations between selected baseline characteristics and falling at least once over 3 months (analysis of diary data)a.
Table S6. Associations between selected baseline characteristics and number of falls experienced over 3 months (analysis of diary data using ordinal logistic regression)a,b.
Table S7. Total number of times participants reported falling at least once in the month prior to an annual survey according to randomisation group; results stratified by the total number of responses provided.
Table S8. Effect of vitamin D supplementation on risk of falling 1–2 or ≥3 times in the year prior to separate annual surveys; results from ordinal logistic regression a,b.
Table S9. Summary of falls outcomes over a 3‐month period (diary data).
Table S10. Minimally adjusted estimates of the effect of vitamin D supplementation on falling over a 3‐month period; analyses of diary dataa.
Table S11. Effect of vitamin D supplementation on risk of falling and incidence of falls within selected strata of body mass indexa.
Table S12. Summary statistics by randomisation group for falls in month prior to annual surveys for all participants and within subgroups.
Table S13. Effect of vitamin D supplementation on falling in the month prior to completing each annual survey ‐ all participants and within subgroupsa.
Table S14. Total number of times participants reported falling at least once in the month prior to an annual survey according to randomisation group; results within participant subgroups and stratified by the total number of responses provided.
Table S15. Retention and compliance with study tablets according to randomisation group, within subsets included in the analysis.
Table S16. Baseline characteristics according to randomisation group restricted to participants included in the analysis of annual surveys who completed the triala.
Table S17. Compliance with study tablets over time according to randomisation group, within the subset of participants included in the analysis of annual surveys.
Table S18. Intake of vitamin D from off‐trial supplements according to randomisation group, within the subset of participants included in the analysis of annual surveys.
Table S19. Adverse events according to according to MedDRA System Organ Class and randomisation group for all participants included in the random subset of size n = 16,000a.
Table S20. Falls reported to study staff on an ad hoc basis; results calculated using data for all participants included in the random subset of size n = 16,000a
Figure S1. Boxplots of serum 25(OH)D concentrations by year of blood draw according to randomisation group.


