Abstract
Background
Although systemic inflammation is an important feature of the cancer cachexia, studies on the association between systemic inflammation and prognostic of cancer cachexia are limited. The objective of this study is to evaluate whether the neutrophil‐to‐lymphocyte ratio (NLR) is associated with outcome and quality of life for patients with cancer cachexia and investigated any interaction between NLR and the clinical parameters.
Methods
This is a multicentre cohort study of 2612 cancer patients suffering from cachexia diagnosed between June 2012 and December 2019. The main parameters measured were overall survival (OS) time and all‐cause mortality. The association between NLR and all‐cause mortality was evaluated using hazard ratios (HRs) and the restricted cubic spline model with a two‐sided P‐value. Optimal stratification was used to solve threshold points. We also evaluated the cross‐classification of NLR for each variable of survival.
Results
Of the 2612 participants diagnosed with cancer cachexia, 1533 (58.7%) were male, and the mean (SD) age was 58.7 (11.7) years. Over a median follow‐up of 4.5 years, we observed 1189 deaths. The overall mortality rate for patients with cancer cachexia during the first 12 months was 30.2% (95%CI: 28.4%–32.0%), resulting in a rate of 226.07 events per 1000 patient‐years. An increase in NLR had an inverted L‐shaped dose–response association with all‐cause mortality. The optimal cut‐off point for NLR as a predictor of mortality in cancer patients with cachexia was 3.5. An NLR of 3.5 or greater could independently predict OS (HR, 1.51, 95%CI: 1.33–1.71). These associations were consistent across subtypes of cancer. Several potential effect modifiers were identified including gender, BMI, tumour type, KPS score and albumin in content. Increasing NLRs were independently associated with a worsening in the majority of EORTC QLQ‐C30 domains. Elevated baseline NLR was associated with low response and poor survival in patients treated with immunotherapy.
Conclusions
The baseline NLR status was found to be a significant negative prognostic biomarker for patients with cachexia; this effect was independent of other known prognostic factors.
Keywords: Systemic inflammation, Cachexia, Neutrophil‐to‐lymphocyte ratio, Prognostic
Introduction
Identifying cancer patients with cachexia, who are at high risk of adverse clinical outcomes and premature mortality, is a clinical priority. This has led to an exploration of various biomarkers that could be associated with a clinical outcome, thereby helping tailor therapies for cancer patients. Neutrophil‐to‐lymphocyte ratio (NLR) is a measure of systemic inflammation and has been received increased attention across cancer types and cachexia. 1 , 2 Cachexia has been known to be a serious side symptom of cancer. In recent years, there has been extensive research into the aetiology and treatment of cancer cachexia. The presence of cachexia could prevent patients from receiving the main cancer treatment, be unresponsive to the treatment or exhibit deteriorated clinical performance and decreased overall survival (OS). 3 , 4 NLR and other systemic inflammation‐related blood biomarkers (such as platelet‐to‐lymphocyte ratio, C‐reactive protein and lymphocyte‐to‐monocyte ratio) are commonly measured and used to predict clinical outcome for patients with cancer. 5 , 6 , 7 Although NLR is considered a measure of the immune system, it has been traditionally thought to be associated with disease control and treatment response to checkpoint inhibitors, and it is an important biomarker across therapeutic classes and molecular subgroups of cancer. 8 , 9 , 10
Recent studies about bile acid metabolism and IL‐20 in cachexia highlight systemic inflammation as an important driver of disease progression in cancer patients. 11 , 12 The systemic inflammatory response to the tumour has profound effects on the host's metabolism and nutritional status. 13 Cancer promotes the release of inflammatory cytokines and the formation of an inflammatory micro‐environment that subsequently enhances tumour aggressiveness or reduces treatment response. 14 Although the exact mechanisms by which this process leads to poorer cancer outcomes remain uncertain, NLR and other biomarkers could function as surrogate markers for potentially pro‐inflammatory tumour micro‐environments. 15
Systemic inflammation can be easily evaluated using existing clinical data; however, these biomarkers are not only important prognostic indicators but could also be improved by clinical intervention. NLR functions as an inexpensive marker of systemic inflammation; a high NLR has been proven as a prognostic factor for various advanced solid tumours. 1 The independent associations of the NLR with survival in cachexia patients are not well studied. This study aimed to evaluate pooled analyses of clinical parameters to determine whether the NLR is associated with an outcome and the quality of life for patients with cachexia. We also evaluated whether there was any interaction between the NLR and clinical parameters.
Methods
Study population and design
This multicentre cohort study included 12 792 patients aged 18–95 years, who were pathologically diagnosed with solid malignant cancer and enrolled at over 40 clinical centres across China between June 2012 and 31 December 2019. Patients with multiple hospitalizations for cancer treatments (all therapies, including surgery, chemotherapy, radiotherapy and others) were treated as one case; the baseline data from the first assessment were analysed. Patients with serious activate infections, continuous anti‐inflammatory use in the past 6 months or acquired immunodeficiency syndrome were excluded. Except patients who refused to participate in this study, there were no other specific exclusion criteria (Figure 1). The study was approved by the institutional review boards of all participating institutions; written informed consent was obtained from all participants.
Figure 1.
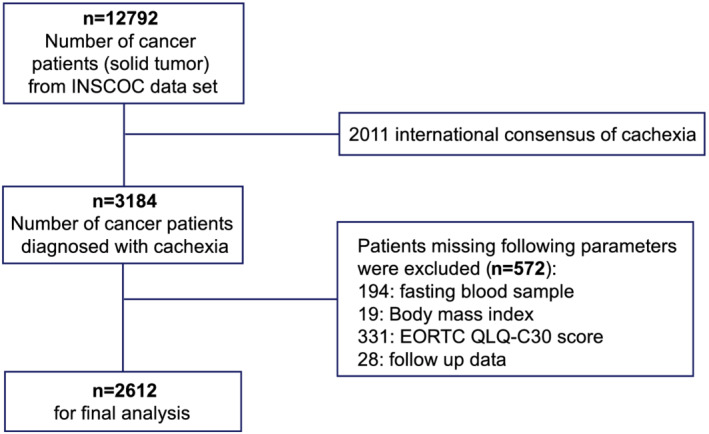
Flow chart of the study design.
Data collection and variable definition
Covariates and potential confounders were selected based on previously published studies. The demographic characteristics (gender, age, height and weight), family history, diet and lifestyle factors (smoking and alcohol history), disease information (tumour types, TNM stage, treatment method and nutritional intervention), body mass index (BMI) and comorbidities (hypertension and diabetes) were collected from the electronic medical record (EMR). Blood samples were collected after 10 h of fasting (prior to the surgery, or chemotherapy, radiotherapy, or other treatment). The following data were collected from the blood analysis: albumin and total bilirubin levels, as well as white blood cell, neutrophil, lymphocyte, red blood cell and platelet counts. In order to account for differences in location and/or scale of measurements between laboratories, all measurements were standardized. The TNM stage was defined as the 8th AJCC TNM classification system. The NLR was calculated as on the absolute neutrophil count divided by the absolute lymphocyte count. Baseline anthropometric measurements (mid‐arm circumference and hand grip strength), patient‐generated subjective nutrition assessment (PG‐SGA), Karnofsky Performance Status (KPS) and self‐reported symptoms (anorexia and reduced food intake) were also recorded by trained personnel.
Cachexia definition and assessment
The definition and classification of cancer cachexia were based on an international consensus with the following criteria 16 : (1) a weight loss >5% over past 6 months (in absence of simple starvation); or (2) BMI < 20 and any degree of weight loss >2%; or (3) appendicular skeletal muscle index consistent with sarcopenia and any degree of weight loss >2%. The assessment of skeletal muscle depletion was used by mid‐upper arm muscle area by anthropometry (men <32 cm2, women <18 cm2).
Outcome
In order to collect information on clinical outcomes, regular telephonic follow‐ups or outpatient visits were conducted for all patients. The OS time is defined as the interval between the first clinical assessment and the date of death, withdrawal from the study, final follow‐up (on 30 September 2019) or last contact, whichever came first. Our primary objective was to assess the relationship between NLR and OS and determine the recommended cut‐off value for cachexia patients. Our secondary objective was to assess the change in quality of life. The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ‐C30 Version 3.0) was used to assess the quality of life for cachexia patients on the day of admission. The QLQ‐C30 scale is a 30‐item questionnaire comprising functional assessment (physical, role, emotional, social and cognitive), symptom assessment (fatigue, nausea and vomiting and pain) and global health and quality‐of‐life assessment (dyspnoea, insomnia, appetite loss, constipation, diarrhoea and financial difficulties). 17
Statistical analysis
Descriptive statistics are presented as mean (± standard deviation [SD]), or in case of skewed distributions as median (interquartile range), or frequencies (percentages). Variables with skewed distributions were natural‐log transformed in order to meet the normality criteria. Pearson correlation analysis was used to test association between the NLR and nutrition‐related parameters. Restricted cubic spline regression was performed to evaluate the association between NLR and OS. Optimal cut‐off points for continues variables were determined using an outcome‐oriented method to maximize log‐rank statistics. For subsequent analysis, female and male patients were divided into normal and abnormal (high and low) groups based on various clinical parameters. Univariate and multivariate cox proportional OS hazard ratios (HRs) alone with 95% confidence intervals (95% CI) were computed for NLR and other major covariates. Trend tests were performed by assigning the median value to each quartile of the NLR and then modelling it as a continuous variable; Wald tests were used to assess statistical significance. The Kaplan–Meier method was used to estimate and plot survival endpoints, which were further tested using log‐rank analysis. Median OS was estimated using the Kaplan–Meier method. Interaction terms were used to investigate whether there was any association between NLR and the various clinical parameters. R software, Version 4.0.2, with RStudio, Version 1.2.5019 (R Foundation for Statistical Computing), was used for statistical analyses. All P‐values < 0.05 from the two‐sided test were considered statistically significant, except for the interaction analyses where P‐values < 0.10 were used.
Result
Patients with cachexia have a higher mean of NLR
Of the 12 792 cancer patients in this study, 3184 patients were diagnosed with cachexia. In total, 2612 cachexia patients were included in the analysis. Over a median follow‐up of 4.5 years, 1189 deaths were recorded. The overall mortality rate for cancer patients with cachexia at 12 months after the first assessment was 30.2% (95%CI 28.4%–32.0%), resulting in a rate of 226.07 events per 1000 patient‐years. The NLR was compared in patients with and without cachexia for each type and stages of cancer. As a group, cachexia patients exhibited significantly higher NLR than those in patients without cachexia (P < 0.05); the NLRs were remarkably higher for lung, colorectal, liver, pancreatic and cervical cancers (Figure 2). Notably, cachexia patients with non‐metastatic stage exhibited significantly lower NLR compared with those in cachexia patients with metastatic stage (Figure S1). The PG‐SGA and QLQ‐C30 results exhibited a weak positive association with NLR, whereas other clinical parameters were negatively associated with NLR (Figure S2).
Figure 2.
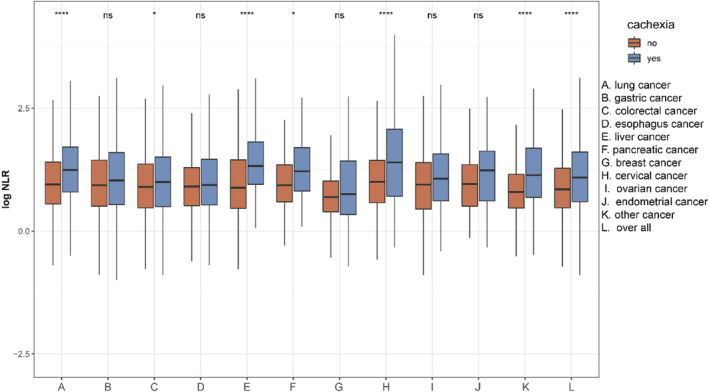
NLR (natural‐log transformation) in different cancer types stratified for patients with and without cachexia (ns P‐value > 0.05, *P‐value < 0.05, ****P‐value < 0.001).
Association of NLR with OS in patients with cachexia
Risk factors of all‐cause mortality were determined using univariate and multivariate cox regression analyses (Table S1). Univariate analysis revealed that the most morbid (or abnormal) conditions of baseline characteristics were associated with higher HRs. The multivariate analysis revealed that gender, age, tumour type, TNM stage, albumin level, total bilirubin level, NLR, red blood cell count, platelet count, KPS, hand grip strength, reported reduced food intake, anorexia and EORTC QLQ‐C30 score were the independent prognostic factors. The cut‐off for NLR associated with OS was 3.5 (Figure S3A). When analysed as a continuous variable, multivariate‐adjusted restricted cubic spline plot suggested that NLR had an inverted L‐shaped dose–response association with the all‐cause mortality risk in cachexia patients (Figure 3). Table 1 shows the results from the multivariate Cox regression models between NLR and OS. Continuous NLR was positively correlated with a worse prognosis (HR 1.22 per SD increase, 95%CI: 1.16–1.29) after adjusting for gender, age, BMI, tumour types, TNM stage, radiotherapy, chemotherapy, KPS, albumin level, total bilirubin level, red blood cell count, platelet count, hand grip strength, reduced food intake, anorexia and EORTC QLQ‐C30 score. NLR was divided into quartiles; unlike the first quartile, Q1 (< 1.83), the second (1.83–2.95), third (2.95–4.8) and fourth quartiles (>4.80) were all positively correlated with a worse prognosis (P for trend < 0.001). After adjusting for the confounding factors, HRs of all‐cause mortality (HR, 95% CI) were 1.3 (1.08–1.58), 1.58 (1.31–1.91) and 1.89 (1.56–2.30) for the second, third and fourth quartiles, respectively.
Figure 3.
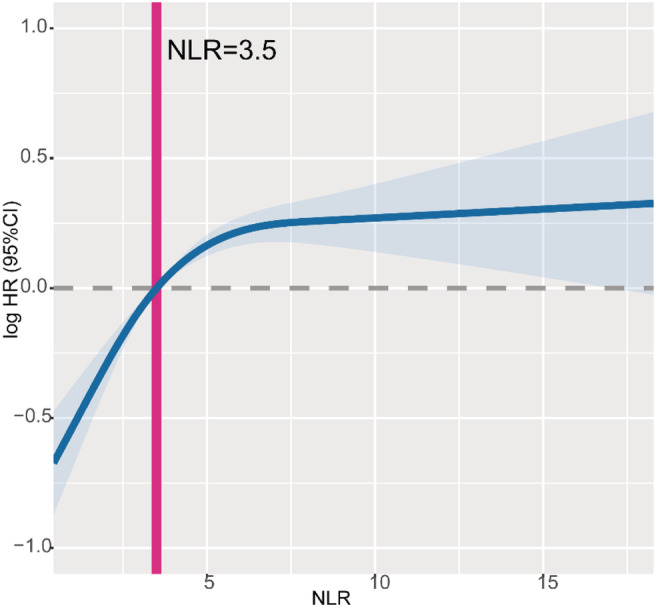
The association between NLR (continuous) and hazard ratio of overall survival. Splines is adjusted by gender, age, BMI, tumour type, TNM stage, radiotherapy, chemotherapy, KPS score, albumin level, total bilirubin level, red blood cell count, platelet count, hand grip strength, reported reduced food intake, reported anorexia and EORTC QLQ‐C30 score.
Table 1.
The association between NLR and HR in cachexia patients
| Neutrophil‐to‐lymphocyte ratio | Model A | Model B | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| As continuous (per SD) | 1.34 (1.27–1.40) | <0.001 | 1.22 (1.16–1.29) | <0.001 |
| By NLR cut‐off | ||||
| Low (<3.5) | Ref | Ref | ||
| High (≥3.5) | 1.91 (1.70–2.14) | <0.001 | 1.51 (1.33–1.71) | <0.001 |
| Interquartile | ||||
| Q1 (<1.83) | Ref | Ref | ||
| Q2 (1.8–2.95) | 1.49 (1.23–1.80) | <0.001 | 1.30 (1.08–1.58) | 0.007 |
| Q3 (2.95–4.80) | 1.96 (1.63–2.35) | <0.001 | 1.58 (1.31–1.91) | <0.001 |
| Q4 (≥4.80) | 2.77 (2.31–3.31) | <0.001 | 1.89 (1.56–2.30) | <0.001 |
| P for trend | <0.001 | <0.001 | ||
Model A: Adjusted for gender, age, BMI and TNM stage.
Model B: Adjusted for gender, age, BMI, tumour type, TNM stage, radiotherapy, chemotherapy, KPS score, albumin level, total bilirubin level, red blood cell count, platelet count, hand grip strength, reported reduced food intake, reported anorexia and EORTC QLQ‐C30 score.
BMI, body mass index; EORTC QLQ‐C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C‐30; KPS, Karnofsky Performance Status.
Patient demographics and disease characteristics stratified by NLR
Based on the NLR cut‐off, 1051 cachexia patients were diagnosed as the high NLR group. Kaplan–Meier curves and log‐rank test results indicated that the high NLR group exhibited a worse prognosis compared with that exhibited by the low NLR patients (Figure S3B). For most cancers, when only NLR was considered, KM curves and log‐rank tests demonstrated a significantly difference in OS distribution between cachexia patients with high and low NLR (Figure S4). There was no relationship between NLR and OS for pancreatic cancer patients. In the final model, high NLR was associated with lung, gastric, colorectal and cervix or endometrium cancer mortalities (Table S2). A comparison of the patients' demographic and clinical characteristics for the low and high NLR groups is presented in Table 2. High NLR was associated with male, older age, low BMI, certain tumour types (such as lung cancer), advanced TNM stage, radiotherapy recipient, low albumin level, high total bilirubin level, low red blood cell count, high platelet count, low KPS, low mid‐arm circumference, low hand grip strength, reported reduced food intake, reported anorexia and high PG‐SGA score. In particular, deteriorating KPS and increasing NLR were independently associated with a worsening in the majority of EORTC QLQ‐C30 domains (Table S3).
Table 2.
Demographic and clinical characteristics of cancer patients with cachexia stratified by NLR
| Characteristic | Overall n = 2612 | NLR low n = 1561 | NLR high n = 1051 | P‐value |
|---|---|---|---|---|
| Population characteristic | ||||
| Gender, male, n (%) | 1533 (58.7%) | 891 (57.1%) | 642 (61.1%) | 0.046 |
| Age, years, mean (SD) | 58.7 (11.7) | 58.1 (11.7) | 59.7 (11.8) | 0.001 |
| BMI, kg/m2, mean (SD) | 20.9 (3.3) | 21.0 (3.2) | 20.6 (3.3) | <0.001 |
| Hypertension, yes, n (%) | 423 (16.2%) | 240 (15.4%) | 183 (17.4%) | 0.183 |
| Diabetes, yes, n (%) | 206 (7.9%) | 115 (7.4%) | 91 (8.7%) | 0.260 |
| Smoke, yes, n (%) | 1196 (45.8%) | 708 (45.4%) | 488 (46.4%) | 0.616 |
| Alcohol, yes, n (%) | 613 (23.5%) | 363 (23.3%) | 250 (23.8%) | 0.789 |
| Clinical characteristic | ||||
| Tumor type, yes, n (%) | <0.001 | |||
| Lung cancer | 525 (20.1%) | 269 (17.2%) | 256 (24.4%) | |
| Gastroesophageal tumour | 801 (30.7%) | 517 (33.1%) | 284 (27.0%) | |
| Colorectal cancer | 625 (23.9%) | 405 (25.9%) | 220 (20.9%) | |
| Hepatic–biliary–pancreatic cancer | 149 (5.7%) | 69 (4.4%) | 80 (7.6%) | |
| Gynaecological and breast cancer | 320 (12.3%) | 195 (12.5%) | 125 (11.9%) | |
| Other cancer | 192 (7.4%) | 106 (6.8%) | 86 (8.2%) | |
| TNM stage, n (%) | <0.001 | |||
| I | 220 (8.4%) | 147 (9.4%) | 73 (6.9%) | |
| II | 547 (20.9%) | 343 (22.0%) | 204 (19.4%) | |
| III | 691 (26.5%) | 457 (29.3%) | 234 (22.3%) | |
| IV | 1154 (44.2%) | 614 (39.3%) | 540 (51.4%) | |
| Radiotherapy, yes, n (%) | 394 (15.1%) | 179 (11.5%) | 215 (20.5%) | <0.001 |
| Chemotherapy, yes, n (%) | 1327 (50.8%) | 832 (53.3%) | 495 (47.1%) | 0.002 |
| Immunotherapy, yes, n (%) | 95(3.6%) | 52(3.3%) | 43(4.1%) | 0.362 |
| Albumin, g/L, mean (SD) | 37.4 (5.6) | 38.6 (5.0) | 35.6 (5.8) | <0.001 |
| Total bilirubin, μmol/L, median (IQR) | 11.00(6.8) | 10.5(6.0) | 11.4(8.1) | <0.001 |
| WBC, 109/L, mean (SD) | 6.92 (3.4) | 5.73 (2.1) | 8.71 (4.0) | <0.001 |
| Neutrophil, 109/L, mean (SD) | 4.75 (3.0) | 3.37 (1.5) | 6.81 (3.5) | <0.001 |
| Lymphocyte, 109/L, mean (SD) | 1.47 (0.7) | 1.72 (0.7) | 1.09 (0.5) | <0.001 |
| NLR, ratio, mean (SD) | 3.96 (3.2) | 2.06 (0.8) | 6.79 (3.4) | <0.001 |
| RBC, 1012/L, median (IQR) | 4.08(0.8) | 4.16(0.8) | 3.95(0.9) | <0.001 |
| Platelet, 109/L, mean (SD) | 240 (103) | 233 (94.2) | 251 (114) | <0.001 |
| KPS score, mean (SD) | 83.1 (15.1) | 85.9 (12.5) | 78.8 (17.5) | <0.001 |
| MAC, cm, mean (SD) | 25.0 (3.7) | 25.2 (3.6) | 24.7 (3.9) | <0.001 |
| HGS, kg, mean (SD) | 23.4 (11.9) | 24.1 (11.7) | 22.5 (12.2) | 0.001 |
| Reduced intake, yes, n (%) | 703 (65.2%) | 974 (62.4%) | 729 (69.4%) | <0.001 |
| Anorexia, yes, n (%) | 673 (25.8%) | 323 (20.7%) | 350 (33.3%) | <0.001 |
| PG‐SGA, mean (SD) | 9.45 (4.6) | 8.62 (4.15) | 10.7 (4.9) | <0.001 |
| EORTC QLQ‐C30, mean (SD) | 52.4(11.3) | 50.3 (9.7) | 55.6 (12.8) | <0.001 |
| Parenteral nutrition, yes, n (%) | 402 (15.4%) | 225 (14.4%) | 177 (16.8%) | 0.103 |
| Enteral nutrition, yes, n (%) | 510 (19.5%) | 282 (18.1%) | 228 (21.7%) | 0.025 |
Data are represented as mean (SD), median (interquartile range) or number (%). For NLR, low < 3.5, high ≥ 3.5.
BMI, body mass index; EORTC QLQ‐C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C‐30; HGS, hand grip strength; IQR, interquartile range; KPS, Karnofsky Performance Status; MAC, mid‐arm circumference; NLR, neutrophil‐to‐lymphocyte ratio; PG‐SGA, patient‐generated subjective nutrition assessment; RBC, red blood cell; WBC, white blood cell.
Stratified analyses by potential effect modifiers
Stratified analyses were conducted to evaluate the relationship between NLR and the HR of OS in various subgroups (Figure 4 and Table S4). Overall, high NLR was associated with increased mortality risk consistently across each subgroup of cachexia patients. Although similar trends were observed for patients with a BMI greater than 24, early TNM stage (I or II) and a KPS greater than 70, they were not statistically significant (P > 0.05). Moreover, interaction analyses revealed that elevated NLR was significantly associated with increased all‐cause mortality risk for the following factors: gender, BMI, tumour type, KPS score and albumin level (all P for interaction < 0.1). No other variable had any obvious influence on the association between NLR (<3.5 vs. ≥3.5) and all‐cause mortality. The NLR and other covariates were then cross‐classified in order to understand the differential effects of each (Table S5). For other covariates without any multiplicative interaction, an increased risk for high NLR patients with morbid (or abnormal) conditions of covariates can be attributed to an additive effect of two risk factors. As observed from the KM curves (Figure S4), cachexia patients with an NLR of 3.5 or greater had the worst survival when they were male, with a BMI < 18.5, with a metastatic TNM stage, suffering from lung cancer, with a KPS < 70 and had an abnormal albumin level (all log‐rank P < 0.001).
Figure 4.
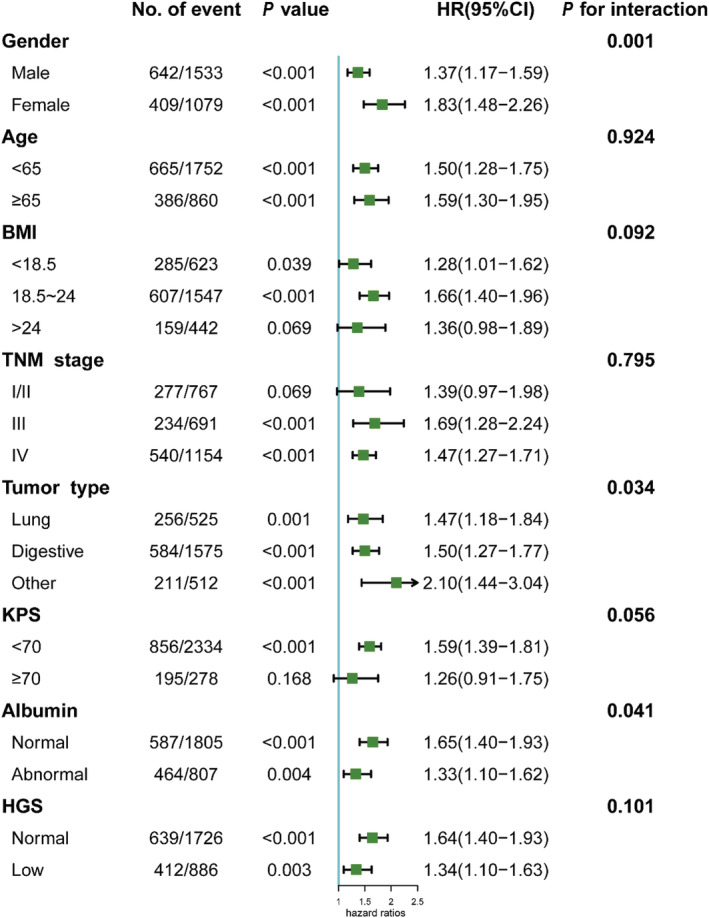
The association between NLR (stratified by cut‐offs 3.5) and hazard ratios of overall survival in various subgroups. Except the stratifying variable, the model is adjusted for gender, age, BMI, tumour type, TNM stage, radiotherapy, chemotherapy, KPS score, albumin level, total bilirubin level, red blood cell count, platelet count, hand grip strength, reported reduced food intake, reported anorexia and EORTC QLQ‐C30 score.
Sensitive analysis
Given the observation that the NLR was prognostic for patients with cachexia, a sensitivity analysis was investigated (Table 3). First, we used the cut‐off points of NLR previously reported in cancer patients(<3 vs. ≥3) and obtained an adjusted HR of 1.49 (95% CI: 1.31–1.69). Additionally, we divided the patients into three risk groups (<3, low‐risk group; 3–5, intermediate‐risk group; and ≥5, high‐risk group) and obtained an adjusted HR of 1.38(95% CI: 1.20–1.60) for intermediate‐risk group and 1.63(95% CI: 1.40–4.89) for high‐risk group (P for trend < 0.001). In particular, threshold effect analysis indicated a consistently increased HR with elevated NLR (≥5) (HR: 1.12, 95% CI: 1.01–1.25, P = 0.035) although HR was attenuated. We also conducted sensitivity analyses after excluding patients who lived less than 6 months from the first assessment (adjusted HR 1.38, 95% CI: 1.18–1.62) or patients with myelosuppression (adjusted HR 1.46, 95% CI: 1.27–1.68) and found that the results were similar with the analysis included these patients. Additionally, among 95 patients who received immunotherapy, patients with a baseline NLR < 3.5 had significantly better OS than that in patients with a baseline NLR ≥ 3.5 (Figure S6 and Table S6).
Table 3.
Thresholds and sensitivity analysis
| Neutrophil‐to‐lymphocyte ratio | HR (95% CI) a | P‐value | HR (95% CI) a | P‐value | ||
|---|---|---|---|---|---|---|
| Reference cut‐off points for cancer patients | ||||||
| Thresholds | ||||||
| <3 | Ref | |||||
| ≥3 | 1.49 (1.31–1.69) | <0.001 | ||||
| Thresholds | ||||||
| <3 | Ref | |||||
| 3–5 | 1.38 (1.20–1.60) | < 0.001 | ||||
| ≥5 | 1.63 (1.40–1.89) | < 0.001 | ||||
| P for trend | < 0.001 | |||||
| Thresholds effect analysis | ||||||
| ≥5 (as continuous, per SD) | 1.12 (1.01–1.25) | 0.035 | ||||
| Sensitive analysis | Excluding patients dying within 6 months | Without myelosuppression | ||||
| As continuous (per SD) | 1.15 (1.06–1.25) | 0.001 | 1.22 (1.14–1.30) | <0.001 | ||
| By NLR cut‐off | ||||||
| Low (<3.5) | Ref | Ref | ||||
| High (≥3.5) | 1.38 (1.18–1.62) | <0.001 | 1.46 (1.27–1.68) | <0.001 | ||
| Interquartile | ||||||
| Q1 (<1.83) | Ref | Ref | ||||
| Q2 (1.83–2.95) | 1.45 (1.15–1.81) | 0.001 | 1.33 (1.08–1.65) | 0.008 | ||
| Q3 (2.95–4.80) | 1.63 (1.29–2.05) | <0.001 | 1.58 (1.28–1.95) | <0.001 | ||
| Q4 (≥4.80) | 1.77 (1.38–2.27) | <0.001 | 1.91 (1.53–2.39) | <0.001 | ||
| P for trend | <0.001 | <0.001 | ||||
BMI, body mass index; EORTC QLQ‐C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C‐30; KPS, Karnofsky Performance Status.
The model was adjusted for gender, age, BMI, tumour type, TNM stage, radiotherapy, chemotherapy, KPS score, albumin level, total bilirubin level, red blood cell count, platelet count, hand grip strength, reported reduced food intake, reported anorexia and EORTC QLQ‐C30 score.
Discussion
In this study, we found that systemic inflammation has different manifestations in cachexia for different tumour types. We calculated the specific NLR cut‐off for cancer patients with cachexia. Using this cut‐off point, the baseline NLR was identified as an important prognostic biomarker. In this cohort of 2612 patients with cachexia, we found that the NLR at first assessment was weakly associated with the clinical parameters. Moreover, we found that NLR had an inverted L‐shape relationship with mortality risk among patients with cachexia. Although NLR increases with age and TNM stage, it could help identify high‐risk patient subgroups. Additionally, NLR was associated with improved survival when its baseline level was lower than the cut‐off value in patients who received immunotherapy.
Biomarkers for the degree of inflammation are not only easily obtainable in a clinical setting but also independently powerful prognostic indicators in cancer patients. 15 , 18 A ‘C SCANS’ study of 2470 patients with nonmetastatic colorectal cancer found that pre‐diagnosis inflammation was associated with at‐diagnosis sarcopenia; both overall and progression‐free survival were worse in the group with both sarcopenia and elevated NLR. 19 In contrast, few studies have individually investigated overall and progression‐free survival in patients with cancer cachexia. This is the first study conducted on hospitalized Chinese cancer patients suffering from cachexia. The findings of our analyses are consistent with the published literature; higher NLR was consistently associated with poor OS across all disease subgroups, tumour sites and disease stages. 1 Interestingly, data on other cancer patients suggested an association between NLR and response to chemotherapy or immunotherapies. 8 , 20 , 21 , 22
Systemic inflammation is a hallmark of cachexia and is hypothesized as a driving force that induces muscle wastages. 23 Our results suggest that there was a trend for the association of high NLR with metastatic disease; one possible explanation is that the systemic inflammation reflects the tumour burden or a process by the interaction of tumour cells with hosts. 24 , 25 In the current study, we found that all types of cancer showed a high NLR state in cachexia, though the differences were not statistically significant for some types of cancer (breast cancer; gastric cancer; oesophagus cancer; ovarian cancer and endometrial cancer). Patients with oesophageal and gastric cancer had a higher rate of cachexia mainly because of reduced intake caused by early satiety and dysphagia rather than hyper‐inflammatory responses. 26 In addition, female mounts stronger innate and adaptive immune responses than male at the cellular and individual level, which might explain the relatively low NLR levels of cachexia observed in certain tumours (breast cancer, ovarian cancer and endometrial cancer). 27 Notably, NLR may be a more meaningful therapeutic target to alter prognoses for patients in the earlier stage of the disease.
In this cohort, the NLR cut‐off point marking increased mortality was about 3.5, which is higher than that previously reported for cancer patients (NLR = 3). 15 Although the exact mechanism of cancer cachexia is unknown, systemic inflammation may have a prognostic role in this process. 28 A high NLR might result from a relatively increased neutrophil count or depleted lymphocyte count. In this scenario, the high NLR may indicate an impaired patient's immune response to malignancy. Although outside the scope of this analysis, this kind of inflammatory responses can change the tumour micro‐environment and facilitate progression and metastasis. 29 Neutrophils may act as tumour‐promoting leucocytes by producing TGF‐β and IL‐10, thereby inducing regulatory T‐cell pathways and matrix metalloproteinases in the tumour micro‐environment. 20 VEGF and interleukins could also be directly secreted by circulating neutrophils. 15
As reported in previous studies, the systemic inflammatory response is not only a prognostic factor but is also strongly linked to fatigue or subjective reduction in functional ability. 30 , 31 Alternatively, it is possible that a more advanced stage of cachexia, characterized by systemic inflammation, results in an objective or a subjective reduction in functional ability for the patient. 32 The systemic inflammatory response has been validated in the deterioration of quality of life in patients with cancer cachexia and could be independent of performance status. 24 Our results expand on previous evidence by reporting stratified analyses elucidating that NLR had different impacts on various clinical parameters. We found a significant interaction between NLR and gender, BMI, tumour type, KPS score and albumin level. The NLR also had a predictive value in this particular population.
Strength and limitation
To our knowledge, this is one of the largest studies to examine the relationship between biomarkers of systemic inflammation and cachexia and the only study to examine whether NLR is independently associated with the cachexia patient's survival. In addition, our results were also supported by the recent publication of ESMO guidelines. 33 Although this is a multicentre cohort study, it has some limitations. First, other markers of systemic inflammation such as platelet‐to‐lymphocyte ratio were not analysed in this study. Similarly, C‐reactive protein level was available for only few patients (less than 30%) and therefore not used. However, this did not lead to a bias because baseline characteristics of patients with and without CRP were similar (data not shown). Another limitation is the lack of serial NLR values for each patient during their follow‐up. But baseline NLR has undoubted prognostic value. As a dynamic marker, previously published series have suggested that normalizing a high baseline NLR value after clinical intervention could be associated with improved clinical outcomes. 34 , 35 Furthermore, other covariates and some unmeasured or measured confounders could influence the NLR, the therapy method, the mortality and the results of our analyses. Finally, a limitation of this study is lack of universality; therefore, the findings of this study could not reliably be extrapolated to other populations. Because it is difficult to find external validation to our results in publicly available resources, a better solution is to confirm these findings in other ethnic groups through international collaboration.
Conclusion
In conclusion, cancer patients with cachexia have generally higher levels of NLR than those without cachexia, and high NLR adversely affects the OS of the cancer patients, indicating NLR is an independent prognostic indicator for patients with cachexia. These results indicate the usefulness of determining the NLR in routine clinical practice to improve patient assessments, cachexia prognoses and interventions. Future studies are required to clarify whether reducing systemic inflammation could enhance OS for cachexia patients, thereby allowing longitudinal assessment of dynamic changes in NLR over time in association with treatment and response, as well as the identification of patients who can benefit the most.
Funding
This work was supported by the National Key Research and Development Program to Dr Han‐Ping Shi (No. 2017YFC1309200).
Author contributions
Dr Han‐Ping Shi had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Qi Zhang, Xi Zhang and Meng‐Meng Song contributed equally. Conceptualization: Han‐Ping Shi, Qi Zhang; data curation: Hong‐Xia Xu; formal analysis: Qi Zhang, Meng‐Meng Song, Xi Zhang; investigation: Qi Zhang, Meng‐Meng Song; methodology: Qi Zhang; project administration: Han‐Ping Shi, Chun‐Hua Song; supervision: Qi Zhang, Meng‐Meng Song, Xi Zhang, Jia‐Shan Ding, Guo‐Tian Ruan, Xiao‐Wei Zhang, Tong Liu, Ming Yang, Yi‐Zhong Ge, Meng Tang, Xiang‐Rui Li, Liang Qian; validation: Qi Zhang, Meng‐Meng Song, Xi Zhang; visualization: Qi Zhang, Meng‐Meng Song; writing–original draft: Qi Zhang; writing—review and editing: Qi Zhang.
Conflict of interest
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare they have no conflicts of interest.
Supporting information
Table S1. Univariate and multivariate cox regression analyses of factors associated with overall survival.
Table S2. Hazard ratio for overall survival in specific types of cancer cachexia patients with a high NLR (≥3.5).
Table S3. Relationship between EQRTC QLQ‐C30 and KPS and systemic inflammation.
Table S4. Associations between NLR (stratified by cut‐offs 3.5) and hazard ratios for overall survival in various subgroups.
Table S5. Multivariate models for NLR and other covariates.
Table S6. Hazard ratio for all‐cause mortality in cachexia patients with a high NLR (≥3.5) receiving immunotherapy.
Figure S1. NLRs (natural‐log transformed) for cancer patients with cachexia in different TNM stages.
Figure S2. Associations between the NLR and clinical parameters.
Figure S3. Overall survival of cachexia patients based on NLR cut‐off.
Figure S4. KM curves for OS in cachexia patients stratified by low and high NLR for multiple cancer types.
Figure S5. KM curves for OS in cachexia patients.
Figure S6. KM curves and the association between NLR (continuous) and hazard ratios for overall survival in cachexia patients treated with immunotherapy.
Acknowledgements
The authors would like to thank the INSCOC project members for their substantial work on data collecting and follow‐up.
Zhang Q., Song M.‐M., Zhang X., Ding J.‐S., Ruan G.‐T., Zhang X.‐W., Liu T., Yang M., Ge Y.‐Z., Tang M., Li X.‐R., Qian L., Song C.‐H., Xu H.‐X., and Shi H.‐P. (2021) Association of systemic inflammation with survival in patients with cancer cachexia: results from a multicentre cohort study, Journal of Cachexia, Sarcopenia and Muscle, 12, 1466–1476, 10.1002/jcsm.12761
Qi Zhang, Meng‐Meng Song and Xi Zhang contributed equally to this work.
Contributor Information
Hong‐Xia Xu, Email: hx_xu2015@163.com.
Han‐Ping Shi, Email: shihp@ccmu.edu.cn.
References
- 1. Templeton AJ, McNamara MG, Šeruga B, Vera‐Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil‐to‐lymphocyte ratio in solid tumors: a systematic review and meta‐analysis. J Natl Cancer Inst 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- 2. Barker T, Fulde G, Moulton B, Nadauld LD, Rhodes T. An elevated neutrophil‐to‐lymphocyte ratio associates with weight loss and cachexia in cancer. Sci Rep 2020;10:7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ravasco P, Monteiro‐Grillo I, Vidal PM, Camilo ME. Nutritional deterioration in cancer: the role of disease and diet. Clin Oncol (R Coll Radiol) 2003;15:443–450. [DOI] [PubMed] [Google Scholar]
- 4. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491–497. [DOI] [PubMed] [Google Scholar]
- 5. Templeton AJ, Ace O, McNamara MG, Al‐Mubarak M, Vera‐Badillo FE, Hermanns T, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta‐analysis. Cancer Epidemiol Biomarkers Prev 2014;23:1204–1212. [DOI] [PubMed] [Google Scholar]
- 6. Li Y, Zhong X, Cheng G, Zhao C, Zhang L, Hong Y, et al. Hs‐CRP and all‐cause, cardiovascular, and cancer mortality risk: a meta‐analysis. Atherosclerosis 2017;259:75–82. [DOI] [PubMed] [Google Scholar]
- 7. Gu L, Li H, Chen L, Ma X, Li X, Gao Y, et al. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systematic review and meta‐analysis. Oncotarget 2016;7:31926–31942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kazandjian D, Gong Y, Keegan P, Pazdur R, Blumenthal GM. Prognostic value of the lung immune prognostic index for patients treated for metastatic non‐small cell lung cancer. JAMA Oncol 2019;5:1481–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maymani H, Hess K, Groisberg R, Hong DS, Naing A, Piha‐Paul S, et al. Predicting outcomes in patients with advanced non‐small cell lung cancer enrolled in early phase immunotherapy trials. Lung Cancer 2018;120:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zer A, Sung MR, Walia P, Khoja L, Maganti M, Labbe C, et al. Correlation of neutrophil to lymphocyte ratio and absolute neutrophil count with outcomes with PD‐1 axis inhibitors in patients with advanced non‐small‐cell lung cancer. Clin Lung Cancer 2018;19:426–434.e1. [DOI] [PubMed] [Google Scholar]
- 11. Thibaut MM, Sboarina M, Roumain M, Pötgens SA, Neyrinck AM, Destrée F, et al. Inflammation‐induced cholestasis in cancer cachexia. J Cachexia Sarcopenia Muscle 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu SW, Pan HC, Hsu YH, Chang KC, Wu LW, Chen WY, et al. IL‐20 antagonist suppresses PD‐L1 expression and prolongs survival in pancreatic cancer models. Nat Commun 2020;11:4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Durham WJ, Dillon EL, Sheffield‐Moore M. Inflammatory burden and amino acid metabolism in cancer cachexia. Curr Opin Clin Nutr Metab Care 2009;12:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grenader T, Waddell T, Peckitt C, Oates J, Starling N, Cunningham D, et al. Prognostic value of neutrophil‐to‐lymphocyte ratio in advanced oesophago‐gastric cancer: exploratory analysis of the REAL‐2 trial. Ann Oncol 2016;27:687–692. [DOI] [PubMed] [Google Scholar]
- 16. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 17. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European organization for research and treatment of cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 18. Dell'Aquila E, Cremolini C, Zeppola T, Lonardi S, Bergamo F, Masi G, et al. Prognostic and predictive role of neutrophil/lymphocytes ratio in metastatic colorectal cancer: a retrospective analysis of the TRIBE study by GONO. Ann Oncol 2018;29:924–930. [DOI] [PubMed] [Google Scholar]
- 19. Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol 2017;3:e172319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grenader T, Nash S, Plotkin Y, Furuse J, Mizuno N, Okusaka T, et al. Derived neutrophil lymphocyte ratio may predict benefit from cisplatin in the advanced biliary cancer: the ABC‐02 and BT‐22 studies. Ann Oncol 2015;26:1910–1916. [DOI] [PubMed] [Google Scholar]
- 21. Sato H, Tsubosa Y, Kawano T. Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg 2012;36:617–622. [DOI] [PubMed] [Google Scholar]
- 22. Bartlett EK, Flynn JR, Panageas KS, Ferraro RA, Sta Cruz JM, Postow MA, et al. High neutrophil‐to‐lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with PD‐1 inhibitor monotherapy. Cancer 2020;126:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Castro GS, Correia‐Lima J, Simoes E, Orsso CE, Xiao J, Gama LR, et al. Myokines in treatment‐naïve patients with cancer‐associated cachexia. Clin Nutr 2020. [DOI] [PubMed] [Google Scholar]
- 24. Laird BJ, Fallon M, Hjermstad MJ, Tuck S, Kaasa S, Klepstad P, et al. Quality of life in patients with advanced cancer: differential association with performance status and systemic inflammatory response. J Clin Oncol 2016;34:2769–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McAllister SS, Weinberg RA. The tumour‐induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 2014;16:717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Birnstein E, Schattner M. Nutritional support in esophagogastric cancers. Surg Oncol Clin N Am 2017;26:325–333. [DOI] [PubMed] [Google Scholar]
- 27. Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, et al. Cancer immunotherapy efficacy and patients' sex: a systematic review and meta‐analysis. Lancet Oncol 2018;19:737–746. [DOI] [PubMed] [Google Scholar]
- 28. Bilir C, Engin H, Can M, Temi YB, Demirtas D. The prognostic role of inflammation and hormones in patients with metastatic cancer with cachexia. Med Oncol 2015;32:56. [DOI] [PubMed] [Google Scholar]
- 29. Shu Y, Qin M, Song Y, Tang Q, Huang Y, Shen P, et al. M2 polarization of tumor‐associated macrophages is dependent on integrin β3 via peroxisome proliferator‐activated receptor‐γ up‐regulation in breast cancer. Immunology 2020;160:345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr 2006;83:1345–1350. [DOI] [PubMed] [Google Scholar]
- 31. Dolan RD, Daly L, Sim WMJ, Fallon M, Ryan A, McMillan DC, et al. Comparison of the prognostic value of ECOG‐PS, mGPS and BMI/WL: Implications for a clinically important framework in the assessment and treatment of advanced cancer. Clin Nutr 2020;39:2889–2895. [DOI] [PubMed] [Google Scholar]
- 32. Peixoto da Silva S, Santos JMO, Costa ESMP, Gil da Costa RM, Medeiros R. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle 2020;11:619–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arends J, Strasser F, Gonella S, Solheim TS, Madeddu C, Ravasco P, et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chua W, Clarke SJ, Charles KA. Systemic inflammation and prediction of chemotherapy outcomes in patients receiving docetaxel for advanced cancer. Support Care Cancer 2012;20:1869–1874. [DOI] [PubMed] [Google Scholar]
- 35. Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer 2011;104:1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate and multivariate cox regression analyses of factors associated with overall survival.
Table S2. Hazard ratio for overall survival in specific types of cancer cachexia patients with a high NLR (≥3.5).
Table S3. Relationship between EQRTC QLQ‐C30 and KPS and systemic inflammation.
Table S4. Associations between NLR (stratified by cut‐offs 3.5) and hazard ratios for overall survival in various subgroups.
Table S5. Multivariate models for NLR and other covariates.
Table S6. Hazard ratio for all‐cause mortality in cachexia patients with a high NLR (≥3.5) receiving immunotherapy.
Figure S1. NLRs (natural‐log transformed) for cancer patients with cachexia in different TNM stages.
Figure S2. Associations between the NLR and clinical parameters.
Figure S3. Overall survival of cachexia patients based on NLR cut‐off.
Figure S4. KM curves for OS in cachexia patients stratified by low and high NLR for multiple cancer types.
Figure S5. KM curves for OS in cachexia patients.
Figure S6. KM curves and the association between NLR (continuous) and hazard ratios for overall survival in cachexia patients treated with immunotherapy.


