Abstract
Background
Advanced pancreatic ductal adenocarcinoma (PDAC) is characterized by progressive weight loss and nutritional deterioration. This wasting has been linked to poor survival outcomes, alterations in host defenses, decreased functional ability, and diminished health‐related quality of life (HRQOL) in pancreatic cancer patients. There are currently no standardized approaches to the management of pancreatic cancer cachexia. This study explores the feasibility and efficacy of enteral tube feeding of a peptide‐based formula to improve weight stability and patient‐reported outcomes (PROs) in advanced PDAC patients with cachexia.
Methods
This was a single‐institution, single‐arm prospective trial conducted between April 2015 and March 2019. Eligible patients were adults (>18 years) diagnosed with advanced or locally advanced PDAC and cachexia, defined as greater than 5% unexplained weight loss within 6 months from screening. The study intervention included three 28 day cycles of a semi‐elemental peptide‐based formula, administered through a jejunal or gastrojejunal feeding tube. The primary outcome was weight stability at 3 months (Cycle 3), defined as weight change less than 0.1 kg/baseline BMI unit from baseline. Secondary outcomes included changes in lean body mass, appendicular lean mass, bone mineral density, fat mass, and percent body fat, as measured with a DEXA scan, HRQOL (EORTC QLQC30) and NIH PROMIS PROs assessed at each cycle. Daily activity (steps, distance, active minutes, heart rate, and sleep) were remotely monitored using a wearable activity monitor (Fitbit) over the 3 month study period.
Results
Thirty‐six patients were screened for eligibility, 31 patients consented onto study and underwent jejunal tube placement, and 16 patients completed treatment: mean age 67 years (SD 9.3), 43.8% male. Among evaluable patients (n = 16), weight stability was achieved in 10 patients (62.5%), thus completing the trial early. Increases in lean body mass (1273.1, SD: 4078, P = 0.01) and appendicular lean mass (0.45, SD: 0.6, P = 0.02) were observed. Statistically significant improvements at Cycle 3 from baseline were also observed for QLQC30 role function [mean difference (MD): 20.1, P = 0.03], appetite (MD: 27.4, P = 0.02), and global health scores (MD: 13.3, P = 0.05) as well as for NIH PROMIS t‐scores for depression (MD: −10.4, P = 0.006) and pain interference (MD: −7.5, P = 0.05). Objectively monitored (Fitbit) activity levels increased, although statistical significance was not reached.
Conclusions
Our findings suggest that enteral nutrition support may improve weight stability, lean body mass, appendicular lean mass and PROs in PDAC patients with cachexia who completed treatment, representing a subsample of the study population. The feasibility and role of enteral feeding in routine care remain unclear, and larger and randomized controlled trials are warranted.
Keywords: Enteral nutrition, Cancer cachexia, Advanced pancreatic ductal adenocarcinoma, Patient‐reported outcomes, Weight stability, Lean body mass
Introduction
Advanced pancreatic adenocarcinoma is characterized by progressive weight loss and nutritional deterioration. 1 , 2 This wasting has been linked to poor survival outcomes, alterations in host defenses, decreased functional ability, and diminished quality of life. 3 , 4 , 5 There have been few studies of whether nutritional support improves outcomes for these patients, and the results have been inconsistent. 3 , 4 This is despite evidence that artificial nutrition can improve performance status and other outcomes in terminal cancer patients. 5
One option to provide nutritional support for patients with pancreatic cancer is through enteral tube feeding. 6 In randomized studies, it has shown benefit over other forms of artificial nutrition in patients following a pancreaticoduodenectomy. 6 , 7 Jejunal and gastrojejunal feeding are also accepted approaches for patients with compromised gastric emptying, a common feature of pancreatic cancer. 8 However, there is concern that enteral feeding may not be a suitable support therapy for cancer patients undergoing chemotherapy and there is a lack of evidence for its effectiveness in this patient population. Furthermore, there are few studies that have evaluated more contemporary nutritional formulations including peptide‐based diets.
Semi‐elemental formulas are peptide‐based containing a high percentage of medium chain triglycerides that are quick to absorb in the GI tract and can be utilized as energy without the need for pancreatic enzymes or bile salts. Similarly, peptides are easily absorbed in the GI tract, breaking down into its respective amino acids. It has been suggested that amino acids, such as leucine and valine, have a role in treating cachexia, potentially counteracting the characteristic protein hypercatabolism seen in patients with cachexia. 9 , 10
Our lack of understanding of pancreatic cancer cachexia extends to its management. Despite guidelines recommending nutritional support and dietary consult for malnourished cancer patients, 7 these are rarely done. 11 Studies of nutritional support and effects of feeding on outcomes in pancreatic cancer patients have been limited to the perioperative setting or retrospective in nature. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15
In this study, we prospectively evaluated advanced pancreatic adenocarcinoma patients with cachexia who received enteral feeding with a peptide‐based diet through a jejunal or gastrojejunal feeding tube. Our primary objective was to establish the feasibility and efficacy of enteral nutrition and its relationship to meaningful clinical outcomes.
Methods
Study design
We conducted a single‐institution, single‐arm prospective trial between April 2015 and March 2019 to evaluate the feasibility and efficacy of enteral tube feeding on weight stability in advanced pancreatic cancer patients with cachexia. All study activities were conducted with prior approval and oversight from the Cedars‐Sinai Medical Center Institutional Research Ethics Board. The study is registered in ClinicalTrials.gov (NCT02400398).
Participants
Patients were recruited from the Cedars‐Sinai Medical Center gastrointestinal oncology clinic by their medical oncologists after providing informed consent. Eligible patients were adults (>18 years) diagnosed with advanced or locally advanced pancreatic cancer and cachexia. Cachexia was defined as greater than 5% unexplained weight loss within 6 months prior to the screening visit. Patients were required to be candidates for enteral feeding or have a jejunal or gastrojejunal tube either previously placed or placed prior to study intervention initiation. Additionally, eligible patients had an ECOG performance status <2 and life expectancy >3 months. Excluded were patients with uncontrolled intercurrent illnesses, pregnancy, or current bowel obstruction (partial or total). Patients may have received prior anticancer treatment or previous resection of primary tumour. Patients were eligible for the Fitbit activity monitoring substudy if they had access to a smart phone (could also belong to a caregiver) and ambulatory at consent (walking aids were permitted). Patients with other implantable medical devices, such as a pacemaker, and allergies to rubber or steel were excluded, for safety purposes.
Intervention
The study intervention included three 28 day cycles of a peptide‐based formula (Peptamen), administered through a jejunal or gastrojejunal feeding tube. Peptamen 1.5 is a peptide‐based 100% whey, 70% MCT formula that includes approximately 150 Cal, 6.8 g protein, 18.8 g carbohydrate, and 5.6 g fat per 100 mL. Jejunal or gastrojejunal tube feeds were provided at the patient's home for the duration of the protocol and as directed by the nutritionist. Peptamen doses ranged from 40 to 85 mL/h over 8–20 h/day depending on dose and as determined by the nutritionist. Peptamen dosing was determined using the Mifflin St Jeor formula for estimating energy expenditure required, using a stress factor of 1.5 as validated for use in cancer patients 16 :
Men = Resting metabolic rate : 9.99 (weight in kg)+6.25 (height in cm) − 4.92 (age)+5.
Women = Resting metabolic rate : 9.99 (weight in kg)+6.25 (height in cm) − 4.92 (age) − 161.
Patients were permitted to supplement tube feeding with solid food throughout the day while on study, summarized at each cycle by the nutritionist using the 24 h food recall.
Primary outcome
The primary outcome was weight stability at 3 months (Cycle 3), defined as weight change less than 0.1 kg/baseline body mass index (BMI)‐unit. Weight was assessed in clinic at the end of each 28 day cycle in kg. BMI was assessed based on the patient's weight in kilograms divided by their height in meters squared.
Secondary outcomes
Secondary body composition measurements included changes in lean body mass, bone mineral density, fat mass, appendicular lean mass, and percent body fat, as measured with the DEXA scan at each cycle. Changes in inflammatory cytokines, gut hormones were also assessed at baseline and 3 months and will be reported in a separate paper. Clinic assessments of function also included hand grip strength, as measured by a dynamometer taking the average of three measures using the dominant hand, and gait speed (time to walk 15 ft). Objective measures of physical activity were remotely assessed using a wearable activity monitor (Fitbit Charge HR 2) 24 h/day over the 3 month study period as part of an optional substudy. Activity metrics included average daily steps, distance, active minutes, and sedentary time. Average resting heart rate and nighttime sleep duration were also assessed. Valid wear‐days were defined as at least 3 days of activity data for 10 consecutive hours per 24 h period preceding each timepoint.
Safety
Safety was assessed in all patients using the Common Terminology Criteria for Adverse Events v4.0 at each cycle and during follow‐up where grade (range 1–4) and attribution (unrelated, unlikely, possible, probable, or definite) were documented by the study clinician. Expected adverse events (AEs) included diarrhoea, abdominal pain, nausea, vomiting, aspiration pneumonitis dehydration, hyperglycaemia, hypokalaemia, hypophosphatemia, and abdominal gas and bloating. Patients were also monitored for refeeding syndrome, thus phosphorous, potassium, and magnesium levels were monitored throughout the study and patients were assessed for malabsorption by their treating oncologist.
Patient‐reported outcomes
Patient‐reported outcomes (PROs) were assessed at each timepoint and included the validated EORTC QLQ‐C30 questionnaire, which includes 30 questions across three functioning domains (physical, emotional, and role) and cancer symptoms, including fatigue, pain, nausea, and appetite loss. 9 Each question is rated on a 4‐point scale: ‘Not at all’, ‘A little’, ‘Quite a bit’, and ‘Very much’.
In 2016, the protocol was amended to include NIH PROMIS questionnaires and Fitbit activity data. NIH PROMIS short forms, ranging from 4 to 16 items, included pain, physical function, sleep disturbance, fatigue, and depression. Responses were on a 5‐point scale ranging from ‘Not at all’ to ‘Very much’ or ‘Without any difficulty’ to ‘Unable to do’, where higher scores represent worst outcomes, with the exception of physical function. 10 Surveys were self‐administered on paper or electronically through REDCap on Day 1 of each study cycle up to 3 months. NIH PROMIS scores were converted to T‐scores (mean = 50, SD = 10) standardized to a cancer population. Patient food intake (24 h food recall 11 ), taste, and smell alteration were assessed at baseline and each cycle up to Cycle 3.
Statistical analysis
The primary outcome of the study was the proportion of patients with favourable response to enteral tube feeding, defined as weight stability (weight change less than 0.1 kg/baseline BMI unit) at Cycle 3. Thus, 37 patients were needed to test the null hypothesis (proportion ≤ 0.2) against the alternative hypothesis (proportion ≥ 0.4) at 80% power and 5% significance. An early stopping point was defined as 14 or more patients with favourable response. Evaluable patients were defined as any patients who completed three cycles of Peptamen treatment.
Descriptive analyses were conducted for all study data at each timepoint where continuous measures were summarized as means (standard deviation) and medians (interquartile ranges) and the number (%) of participants were presented for categorical variables. Differences in outcomes at Cycle 3 from baseline were assessed using Mann–Whitney U test or independent samples t‐test (for continuous data including PROs) and Pearson's χ 2 test or Fisher's exact test for categorical outcomes. A P value < 0.05 indicated statistical significance. Fitbit activity metrics were summarized as 7 day averages preceding each cycle. Pearson correlation coefficients were calculated for continuous variables at each timepoint and for changes in continuous variables. Exploratory survival analysis was conducted using the Kaplan–Meier method, and multivariable proportional hazards regression models were used to assess survival differences among patients with weight stability. Analyses were conducted in Stata v.15 (Texas).
Results
Patient characteristics
A total of 36 patients were screened for eligibility. Of these, 31 consented onto study and underwent jejunal tube placement, and 16 patients completed all three cycles of tube feeding (Figure 1 ). Patients who did not complete treatment included nine patients who expired on study due to advanced disease, transferred to hospice (n = 1), non‐compliance (n = 1), complications (n = 2), or withdrew consent (n = 2). Two patients required tube exchanges. Patient characteristics are displayed in Table 1 . Evaluable patients (n=16) were on average 67 years of age (SD 9.3), 43.8% male and 56.3% non‐Hispanic white, had 62.5% and 37.5% ECOG performance status scores of 1 and 2, respectively, and had median BMI 22.3 kg/m2 (Table 1 ). Among evaluable patients, patients had a treatment history of gemcitabine and abraxane (n = 6), FOLFIRINOX (n = 3), FOLFIRI (n = 2), Veliparib + Gemcitabine with radiation (n = 2), or Gemcitabine with capecitabine (n = 1) or did not receive chemotherapy. Among these, three patients had a history of pancreatic cancer surgery. No statistically significant differences in weight change across chemotherapy regimens were observed. Tube feeding was supplemented with solid food taken orally, for all evaluable patients (Supporting Information, Table S1 ) as assessed with the 24 h food recall at each timepoint.
Figure 1.

Flow chart.
Table 1.
Patient characteristics
| Characteristics | Overall | Evaluable |
|---|---|---|
| N (%) or mean ± SD | N (%) or mean ± SD | |
| Total (N) | 31 | 16 |
| Age | 67.1 (10.9) | 67.0 (9.3) |
| Sex | ||
| Male | 12 (38.7) | 7 (43.8) |
| Female | 19 (61.3) | 9 (56.2) |
| Race/ethnicity | ||
| Non‐Hispanic White | 19 (61.3) | 9 (56.3) |
| African American | 2 (6.4) | 1 (6.3) |
| Asian/Pacific Islander | 7 (22.6) | 3 (18.8) |
| Hispanic/Latino | 7 (22.6) | 3 (18.8) |
| Other | 2 (6.4) | 0 (0.0) |
| Not reported | 1 (3.2) | 0 (0.) |
| BMI (kg/m2) | 21.5 (4.0) | 22.3 (4.6) |
| ECOG | ||
| 1 | 22 (73.3) | 10 (62.5) |
| 2 | 8 (26.7) | 6 (37.5) |
| Not reported | 1 | |
| Smoking history | ||
| Never | 16 (64) | 11 (68.8) |
| Past smoker | 9 (36) | 5 (31.2) |
| Unknown | 6 | 0 |
| CA19‐9 | 9852 (24 796) | 10 505 (30 554) |
| CRP | 37.6 (47.3) | 24.0 (39.9) |
| Total body bone mineral density | 1.08 (0.16) | 1.07 (0.17) |
| Total body fat mass (g) | 16 519 (7971) | 17 546 (9182) |
| Total percent body fat (%) | 28.5 (10.6) | 29.7 (11.7) |
| Lean body mass (g) | 40 267 (11 413) | 30 331 (10 991) |
| Appendicular lean mass (kg/m2) | 5.3 (1.1) | 5.2 (1.1) |
| BMC (g) | 2210 (650) | 2175 (575) |
| Physical activity (steps per day) | 1092 (679) | 1143 (710) |
| Hand grip strength (kg) | 18.6 (5.8) | 19.9 (6.0) |
Weight stability
Among 16 patients evaluable for the primary outcome, weight was stable or increased in 10 patients (62.5%): 4 patients maintained their weight, and 6 patients exhibited increases in weight, with an overall mean increase of 0.05 kg/BMI unit at Cycle 3. Figure 2 shows results from DEXA scans over time for men and women. Overall increases in average weight (+1.29 kg, SD: 5.8), BMI (+0.6 kg/m2, SD: 1.7), % body fat (−1.6%, SD:5%) and decreases in bone mineral density (−0.01, SD: 0.02), and body fat mass (−602 g, SD: 2794) were observed at Cycle 3, but did not reach statistical significance (P > 0.05). Statistically significant increases in lean body mass (+1273.1, SD: 4078, P = 0.01) and appendicular lean mass were observed in evaluable patients at cycle 3 (+0.45, SD: 0.62, P = 0.02) (Figure S1 ).
Figure 2.

DXA measurements over time. BMI, body mass index.
Secondary outcomes
In exploratory survival analysis, no statistically significant difference was observed in overall survival among evaluable patients with stable weight compared with those without [hazard ratio (HR): 1.31, 95% confidence interval (CI) 0.41–4.14, P = 0.64]. Median survival was 172 days overall (n = 36) and 316 days for evaluable patients (n = 16). CRP levels (mean difference: −9.77, SE 11.6, P = 0.4) and CA‐199 levels (mean difference: −875.9, SE: 2 = 10 993, P = 0.9), decreased at Cycle 3 from baseline, although differences were not statistically significant.
Patient‐reported outcomes
Health‐related quality of life outcomes and subscales from the EORTC QLQ‐C30 questionnaire improved over time (Table 2 , Figure S2 ). Specifically, statistically significant improvements were observed for role function, appetite, and global health scores (Table 2 , Figure S2 ). NIH PROMIS pain interference and depression t‐scores improved significantly at Cycle 3. Clinically significant improvements were also observed in NIH PROMIS physical function, fatigue, sleep disturbance, pain, social and emotional well‐being, as well as gastrointestinal symptoms (Figure 3). However, ability to taste and smell did not change significantly, although stronger sense of smell was reported in 43% of patients at Cycle 3 compared with 38% at baseline, and bitter taste was also stronger in 30% of patients at Cycle 3 compared with 25% at baseli (Table S2 ).
Table 2.
Mean change in patient‐reported outcomes, activity, and physical function over three cycles
| Variables | N | C1 | C2 | C3 | Mean differences | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Change C2–C1 | P value | Change C3–C1 | P value | ||
| NIH PROMIS a | |||||||||||
| Pain interference | 24 | 66.7 | 8.8 | 60.7 | 7.6 | 59.2 | 12.1 | −6.0 | 0.04 | −7.5 | 0.05 |
| Fatigue | 24 | 64.3 | 10.1 | 60.6 | 9.5 | 57.2 | 7.6 | −3.7 | 0.27 | −7.1 | 0.06 |
| Depression | 24 | 58.1 | 9.9 | 56.9 | 10.5 | 47.7 | 5.7 | −1.2 | 0.8 | −10.4 | 0.006 |
| Sleep disturbance | 24 | 51.0 | 4.0 | 52.8 | 3.2 | 50.0 | 2.99 | 1.8 | 0.16 | −1.47 | 0.5 |
| Physical function | 24 | 45.6 | 6.9 | 43.7 | 6.7 | 44.4 | 10.3 | −1.9 | 0.43 | 3.1 | 0.7 |
| QLQ‐C30 b | |||||||||||
| Physical function | 29 | 46.1 | 26.9 | 48.9 | 28.0 | 56.7 | 26.4 | 2.8 | 0.73 | 10.6 | 0.2 |
| Role function | 29 | 29.9 | 25.7 | 48.1 | 32.3 | 50.0 | 29.2 | 18.2 | 0.04 | 20.1 | 0.03 |
| Cognitive function | 29 | 62.6 | 24.7 | 60.2 | 33.4 | 71.4 | 29.5 | 2.4 | 0.4 | 8.8 | 0.3 |
| Emotional | 29 | 57.2 | 28.3 | 55.1 | 24.6 | 67.3 | 26.4 | 2.1 | 0.79 | 10.1 | 0.27 |
| Social | 29 | 37.9 | 31.1 | 41.7 | 32.9 | 47.6 | 33.9 | 3.8 | 0.69 | 9.7 | 0.32 |
| Global health | 29 | 30.7 | 19.4 | 43.1 | 20.7 | 44.0 | 20.8 | 12.4 | 0.04 | 13.3 | 0.05 |
| Subscales | |||||||||||
| Fatigue | 29 | 70.1 | 28.3 | 54.9 | 30.6 | 52.8 | 24.1 | −15.2 | 0.09 | −17.3 | 0.06 |
| Pain | 29 | 58.6 | 37.2 | 48.1 | 33.3 | 46.4 | 28.6 | −10.5 | 0.24 | −12.2 | 0.18 |
| Appetite loss | 29 | 72.6 | 32.8 | 45.1 | 42.4 | 45.2 | 38.4 | −27.5 | 0.02 | −27.4 | 0.02 |
| Constipation | 29 | 34.5 | 42.2 | 18.5 | 28.5 | 28.6 | 34.3 | −11.3 | 0.16 | −5.9 | 0.63 |
| Nausea/vomiting | 29 | 42.5 | 35.2 | 36.1 | 36.3 | 28.5 | 34.2 | −16.4 | 0.13 | −11.4 | 0.22 |
| Insomnia | 29 | 47.1 | 28.9 | 42.6 | 31.9 | 41.0 | 33.7 | −4.5 | 0.62 | −9.9 | 0.54 |
| Average daily Fitbit activity c | |||||||||||
| Steps per day | 15 | 1092 | 678.7 | 1160 | 980.7 | 1226 | 734 | 68 | 0.8 | 134 | 0.7 |
| Stairs | 13 | 0.36 | 0.8 | 0.5 | 0.4 | 0.68 | 0.48 | 0.12 | 0.7 | 0.31 | 0.3 |
| Heart rate | 11 | 72.9 | 7.3 | 74.9 | 7.7 | 70.3 | 10.3 | −3.6 | 0.59 | −4.7 | 0.6 |
| Restless sleep | 9 | 7.9 | 5.6 | 4.1 | 1.5 | 3.6 | 4.5 | −3.8 | 0.13 | −4.3 | 0.14 |
| Strength | |||||||||||
| Grip strength d | 19 | 17.9 | 5.4 | 19.5 | 6.2 | 15 | 19.0 | 1.6 | 0.4 | 1.1 | 0.6 |
Lower scores are better and negative changes indicate improvement, except for physical function (higher scores better, positive changes indicate improvement).
Higher scores are better for physical, role, emotional, social, and global health. Positive changes indicate improvement. Lower scores are better for subscales (pain, fatigue, appetite loss, constipation, nausea/vomiting, and insomnia). Negative changes indicate improvement.
From Fitbit optional substudy (n = 15). Steps available for all 15 patients. Missing heart rate/sleep data in three patients due to syncing errors.
Assessed with Jamar dynamometer. Computed using average across three measures of dominant hand.
Figure 3.
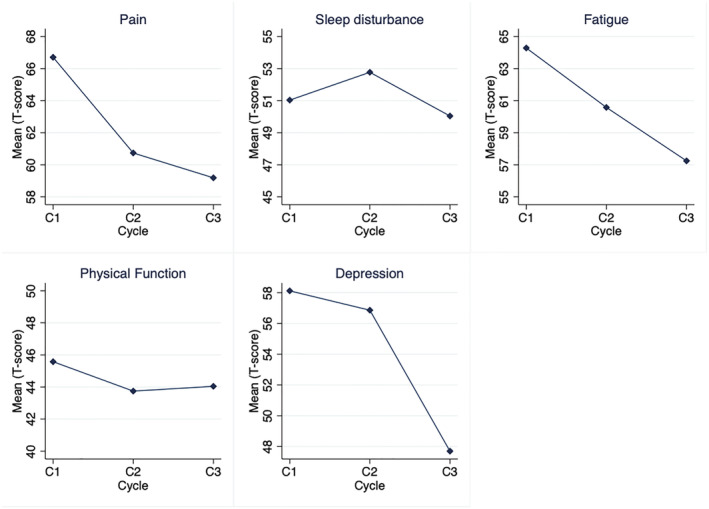
NIH PROMIS scales.
Improvements in objective measures of activity and physical function were observed in the subset of patients who wore Fitbit activity monitors (n = 15), all of whom had at least 7 days of available activity data prior to each cycle. Non‐statistically significant increases in the average number of daily steps taken, distance walked, stairs climbed, resting heart rate, or hand grip strength were observed (Table 2 ).
Pearson correlation coefficients for changes from baseline to Cycle 3 in DEXA body measurements, objective activity and functional measures, and PROs are shown in Figure 4 .
Figure 4.

Heat map of Pearson correlation coefficients between DEXA measurements, patient‐reported outcomes, and activity metrics in evaluable patients (n = 16). PROs, patient‐reported outcomes.
Strongest correlations were observed between changes at Cycle 3 from baseline in DEXA body fat mass and daily step counts (r = −0.87, P < 0.023), increased lean body mass and lower resting heart rate (r = −0.77, P < 0.0001), increased weight (kg/BMI unit) and higher cognitive function (r = 0.81, P = 0.027), increased weight and decreased fatigue levels (r = −0.78, P = 0.037), increased lean body mass and higher cognitive function (r = 0.95, P = 0.001), increased fat mass and improved hand grip strength (r = 0.66, P = 0.05), and increases in weight and decrease in patient‐reported physical function (r = −0.48, P = 0.066) (Figure 4 ). Increased patient‐reported physical function was also associated with higher levels of daily steps (r = 0.55, P > 0.5), improved sleep (r = 0.75, P > 0.5), lower grip strength (r = −0.78, P > 0.5), and improved quality of life (r = 0.62, P > 0.5) while decreases in QLQC30 fatigue scores were correlated with increased step counts (r = −0.80, P = 0.049), lower resting heart rate (r = 0.95, P = 0.21), decreased levels of pain (r = 0.30, P = 0.005) and increased hand grip strength (r = −0.17, P = 0.004).
Safety
Adverse events experienced during the study are presented in Table 3 . Among 31 patients evaluated for adverse events, 18 patients had at least one toxicity of any grade. All adverse events were expected where the majority of adverse events were grades 1–2 and deemed unrelated to the intervention. Adverse events that were possibly or probably related to the intervention included diarrhoea, hyperglycaemia, nausea or vomiting, hypokalaemia, and hypophosphatemia (Table 3 ). Expected grade 3 or 4 adverse events that were possibly or probably related to the intervention included hyperglycaemia, hypokalaemia, and hypophosphatemia, occurring in 14 patients. There were no serious adverse events reported.
Table 3.
Number of adverse events by grade occurring during study period (n = 31)
| Grade | All AEs | Possibly or probably related | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any | 1 | 2 | 3 | 4 | Any | 1 | 2 | 3 | 4 | |
| Adverse event | n | n | n | n | n | n | n | n | n | n |
| Abdominal pain | 11 | 2 | 6 | 3 | 0 | 3 | 1 | 2 | 0 | 0 |
| Bloating | 2 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 0 |
| Diarrhoea | 9 | 7 | 1 | 1 | 0 | 9 | 7 | 1 | 1 | 0 |
| Hyperglycaemia | 9 | 0 | 0 | 7 | 2 | 9 | 0 | 0 | 7 | 2 |
| Nausea | 8 | 3 | 3 | 2 | 0 | 4 | 2 | 2 | 0 | 0 |
| Vomiting | 9 | 7 | 2 | 0 | 0 | 5 | 4 | 1 | 0 | 0 |
| Hypokalaemia | 16 | 11 | 1 | 3 | 1 | 11 | 8 | 0 | 2 | 1 |
| Hypophosphatemia | 17 | 0 | 7 | 9 | 1 | 15 | 0 | 6 | 8 | 1 |
| Eye disorder—other | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Urinary frequency | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Haematuria | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Proteinuria | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anaemia haemoglobin | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Abdominal distension | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Dehydration | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
AE, adverse events.
Discussion
This study was designed to evaluate the feasibility and efficacy of enteral nutrition supplementation in pancreatic cancer patients with cachexia. Our findings suggest that enteral feeding resulted in weight stability, increased lean mass, appendicular lean mass, and improved HRQOL and PROs among patients who completed and tolerated the treatment. However, this was based on a small sample at a single institution, and it is unclear whether these findings support its feasibility and implementation into routine care. More research is needed to understand the role of enteral feeding and identify patients who benefit from it.
Unintentional weight loss and cancer cachexia remains highly prevalent among pancreatic cancer patients and is associated with poor survival outcomes and diminished health‐related quality of life. 1 , 2 To date, an approach to the treatment and management of pancreatic cancer cachexia has yet to be established. However, growing evidence supports the use of specialized nutrition treatments such has enteral or parenteral nutrition to control cachexia in cancer patients, as demonstrated in a randomized trial of artificial nutrition in patients following pancreaticduodenectomy. 2 , 12 Early nutrition support is also recommended in the European Society for Clinical Nutrition and Metabolism guidelines, 13 although not routinely recommended in North America. 2 Recent ASCO guidelines also recommend that enteral tube feeding or parental nutrition should not be routinely offered outside the context of a clinical trial. 14 These recommendations were based on two studies rated as low for evidence quality and specific to routine care.
A proportion of consented patients in this study did not complete therapy or ever received the jejunal tube placement. This was expected given a high number of patients with advanced disease, rapid changes in their condition, poor performance status, and increased symptom burden from concurrent treatments experienced in this population. These findings suggest that enteral tube feeding may not be tolerated or feasible in all patients and that benefits are greater among those who complete the intervention. Nutritional interventions are likely to have an even greater impact if they were used closer to the onset of weight loss as opposed to later stages of disease progression. 15 Regardless of whether patients are receiving enteral tube feeding, support and management of the patient's diet and discussions about the importance of nutrition should be part of a patient's care throughout their overall cancer treatment experience.
Of interest were the observed improvements in PROs over the three treatment cycles. Clinically significant improvements were observed for the QLQ‐C30 health‐related quality of life and its subscales, with statistically significant differences observed for role function, global health, and appetite subscales. Statistically significant improvements were also observed for NIH PROMIS pain interference, fatigue, and depression. Improvements in objective measurements of activity, including increased Fitbit‐assessed step counts, lower resting heart rate, and improved sleep quality, and increased clinic‐assessed hand grip strength and walking speed were observed, although not statistically significant. While this study did not include a control group, clinical practice and observational studies have seen that PROs and activity metrics remain stable and often decline over time in the absence of an intervention. Previous studies have also reported associations between weight loss and PROs, psychological distress, quality of life, and physical functioning. 16 , 17 , 18 , 19 We have also shown that physical function was ranked as highest among NIH PROMIS PROs in a survey of pancreatic cancer patients and physicians. 20 Thus, the use of nutritional support may provide additional benefit for the overall quality of life and physical function in this group of patients. These findings should be replicated in future trials and further explored in the presence of a control group.
Additionally, we observed statistically significant correlations between PROs, including physical function and fatigue, with objective measures including DEXA body composition metrics and Fitbit activity metrics, although these were evaluated as secondary outcomes and not powered for the analysis. However, these findings highlight the importance of incorporating PROs and activity metrics into clinical trials as meaningful outcomes in order to include the patient perspective and to demonstrate the effect of a treatment on a patient's well‐being and physical function. Furthermore, as functional assessment and body scan measurements are not routinely conducted often due to budget limitations and logistical issues, remote monitoring of daily activity and PROs may serve as a supplementary tool to monitor and assess a patient's physical function and performance status outside the clinic setting or when clinic assessments are not possible or missing. 21 , 22 , 23
We did not observe a significant difference in overall survival among evaluable patients with or without weight stability, possibly due to the severity of disease burden or other factors. Given the established association between cancer cachexia and poor survival outcomes, a larger study to evaluate the effect of nutritional support on survival outcomes in this population is warranted. 2
This study was limited by its small sample size, lack of randomization and lack of control group. We were unable to control for treatment type received and other potential confounding factors (e.g., stage and degree of weight loss), which may have impacted the patient outcomes and change in body weight. More research in a controlled setting is required to understand the effects of different treatment regimens on changes in patient body weight. Due to the aggressive nature of the disease, patient dropout was also high due to patient death or unwillingness to continue at the end stage of life, thus introducing potential bias due to missing data. Patient adherence to the recommended treatments and study activities was also challenging and may generate additional bias and missing data, especially for questionnaires and PROs.
Additional therapeutic approaches to the management of cancer cachexia are currently being explored in ongoing studies including the MENAC trial, a Multimodal Intervention for Cachexia in Advanced Cancer Patients Undergoing Chemotherapy (NCT02330926), the combination of Xilonix™, Onivyde® (nanoliposomal irinotecan) and 5‐fluourouracil in pancreatic cancer (NCT03207724), and the use of Pancreaze (pancrelipase) for patients with exocrine pancreatic insufficiency (NCT04098237) at our centre. There have also been increased organized efforts to advance and improve cancer outcomes through the National Cancer Institute and Cancer UK Grand Challenges, which includes a challenge to understand and reverse cachexia and declining performance status in cancer patients. Consortiums and conferences have also been established to help accelerate the development of anticachexia therapies and promote collaborative research in this field, including the International Cancer Society and Cancer Cachexia Conference.
In conclusion, findings from this study suggest that, in patients who complete treatment, enteral nutrition support may improve weight stability, PROs, and physical function in pancreatic cancer patients with cachexia. Given the high attrition rate and small, uncontrolled nature of the study, our findings cannot support the routine use of enteral nutrition in advanced pancreatic cancer patients. However, we did demonstrate feasibility of collecting detailed clinical, laboratory, psychometric, and biometric data over the course of the study in this setting, providing a comprehensive account of this population. Larger clinical trials are needed to validate these findings and further enhance our understanding of the role of nutritional management in pancreatic cancer.
Funding
This study was funded in part by the National Institutes of Health/National Center for Advancing Translational Sciences Grant UL1TR000124. Gillian Gresham is being funded in part by Pancreatic Cancer Action Network (20‐20‐GRES).
Conflicts of interest
The authors have no conflicts of interest to declare.
Supporting information
Table S1. Peptamen dosing and nutritional information among evaluable patients.
Figure S1. Change in appendicular lean mass by cycle.
Figure S2. Health related quality of life (EORTC QLQC30) subscale scores by cycle.
Table S2. Taste/Smell alteration.
Acknowledgements
The authors would like to acknowledge the study dietician Meghan Laszlo and Mindy Mamelak for conducting DEXA assessments and nutrition surveys. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 24
Gresham G., Placencio‐Hickok V. R., Lauzon M., Nguyen T., Kim H., Mehta S., Paski S., Pandol S. J., Osipov A., Gong J., Jamil L. H., Nissen N., Lo S. K., and Hendifar A. E. (2021) Feasibility and efficacy of enteral tube feeding on weight stability, lean body mass, and patient‐reported outcomes in pancreatic cancer cachexia, Journal of Cachexia, Sarcopenia and Muscle, 12, 1959–1968, 10.1002/jcsm.12799
References
- 1. Tan C, Jamil L, Yaffee P, Tuli R, Nissen N, Lo S, et al. Pancreatic cancer cachexia: a review of mechanisms and therapeutics. Front Physiol 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hendifar AE, Petzel MQ, Zimmers TA, Denlinger CS, Matrisian LM, Picozzi VJ, et al. Pancreas cancer‐associated weight loss. Oncologist 2019;24:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bachmann J, Heiligensetzer M, Krakowski‐Roosen H, Büchler MW, Friess H, Martignoni ME. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg 2008;12:1193–1201. [DOI] [PubMed] [Google Scholar]
- 4. Bachmann J, Büchler MW, Friess H, Martignoni ME. Cachexia in patients with chronic pancreatitis and pancreatic cancer: impact on survival and outcome. Nutr Cancer 2013;65:827–833. [DOI] [PubMed] [Google Scholar]
- 5. Davidson W, Ash S, Capra S, Bauer J, Group CCS . Weight stabilisation is associated with improved survival duration and quality of life in unresectable pancreatic cancer. Clin Nutr 2004;23:239–247. [DOI] [PubMed] [Google Scholar]
- 6. Lassen K, Coolsen MM, Slim K, Carli F, de Aguilar‐Nascimento JE, Schäfer M, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 2012;31:817–830. [DOI] [PubMed] [Google Scholar]
- 7. August DA, Huhmann MB, Directors ASfPaENASPENBo . A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr 2009;33:472–500. [DOI] [PubMed] [Google Scholar]
- 8. Coolsen MM, van Dam RM, van der Wilt AA, Slim K, Lassen K, Dejong CH. Systematic review and meta‐analysis of enhanced recovery after pancreatic surgery with particular emphasis on pancreaticoduodenectomies. World J Surg 2013;37:1909–1918. [DOI] [PubMed] [Google Scholar]
- 9. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 10. Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient‐Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self‐reported health outcome item banks: 2005‐2008. J Clin Epidemiol 2010;63:1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gersovitz M, Madden JP, Smiciklas‐Wright H. Validity of the 24‐hr. dietary recall and seven‐day record for group comparisons. J Am Diet Assoc 1978;73:48–55. [PubMed] [Google Scholar]
- 12. Huhmann MB, August DA. Review of American Society for Parenteral and Enteral Nutrition (ASPEN) Clinical Guidelines for Nutrition Support in Cancer Patients: nutrition screening and assessment. Nutr Clin Pract 2008;23:182–188. [DOI] [PubMed] [Google Scholar]
- 13. Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NE, et al. ESPEN expert group recommendations for action against cancer‐related malnutrition. Clin Nutr 2017;36:1187–1196. [DOI] [PubMed] [Google Scholar]
- 14. Roeland EJ, Bohlke K, Baracos VE, Bruera E, Del Fabbro E, Dixon S, et al. Management of cancer cachexia: ASCO guideline. J Clin Oncol 2020;38:2438–2453. [DOI] [PubMed] [Google Scholar]
- 15. Gärtner S, Krüger J, Aghdassi AA, Steveling A, Simon P, Lerch MM, et al. Nutrition in pancreatic cancer: a review. Gastroint Tumors 2015;2:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. LeBlanc TW, Nipp RD, Rushing CN, Samsa GP, Locke SC, Kamal AH, et al. Correlation between the international consensus definition of the Cancer Anorexia‐Cachexia Syndrome (CACS) and patient‐centered outcomes in advanced non‐small cell lung cancer. J Pain Symptom Manage 2015;49:680–689. [DOI] [PubMed] [Google Scholar]
- 17. Rhondali W, Chisholm GB, Daneshmand M, Allo J, Kang DH, Filbet M, et al. Association between body image dissatisfaction and weight loss among patients with advanced cancer and their caregivers: a preliminary report. J Pain Symptom Manage 2013;45:1039–1049. [DOI] [PubMed] [Google Scholar]
- 18. Moses AW, Slater C, Preston T, Barber MD, Fearon KC. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n‐3 fatty acids. Br J Cancer 2004;90:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prigerson HG, Bao Y, Shah MA, Paulk ME, LeeBlanc TW, Schneider BJ, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol 2015;1:778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guan M, Gresham G, Shinde A, Lapite I, Gong J, Placencio‐Hickok VR, et al. Priority rankings of patient‐reported outcomes for pancreatic ductal adenocarcinoma: a comparison of patient and physician perspectives. J Natl Compr Canc Netw 2020;18:1075–1083. [DOI] [PubMed] [Google Scholar]
- 21. Gresham G, Hendifar AE, Spiegel B, Neeman E, Tuli R, Rimel BJ, et al. Wearable activity monitors to assess performance status and predict clinical outcomes in advanced cancer patients. NPJ Digital Medicine 2018;1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gresham G, Schrack J, Gresham LM, Shinde AM, Hendifar AE, Tuli R, et al. Wearable activity monitors in oncology trials: current use of an emerging technology. Contemp Clin Trials 2018;64:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schrack JA, Gresham G, Wanigatunga AA. Understanding physical activity in cancer patients and survivors: new methodology, new challenges, and new opportunities. Cold Spring Harbor Mol Case Stud 2017;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Peptamen dosing and nutritional information among evaluable patients.
Figure S1. Change in appendicular lean mass by cycle.
Figure S2. Health related quality of life (EORTC QLQC30) subscale scores by cycle.
Table S2. Taste/Smell alteration.


