Abstract
Background
Diagnostic criteria for sarcopenia have not been established in Chinese. This study established criteria based on the L3‐skeletal muscle index (L3‐SMI) and assessed its value for outcomes predicting in cirrhotic Chinese patients.
Methods
Totally 911 subjects who underwent a CT scan at two centres were enrolled in Cohort 1 (394 male and 417 female subjects, aged 20–80 years). The data of those subjects younger than 60 years (365 male and 296 female subjects) were used to determine the reference intervals of the L3‐SMI and its influencing factors. Cohort 2 consisted of 480 patients (286 male and 184 female patients) from three centres, and their data were used to investigate the prevalence of sarcopenia and evaluate the value of L3‐SMI for predicting the prognosis and complications of cirrhosis.
Results
Age and sex had the greatest effects on the L3‐SMI (P < 0.001). The L3‐SMI scores were clearly higher in male patients than in female patients (52.94 ± 8.41 vs. 38.91 ± 5.65 cm2/m2, P < 0.001) and sharply declined in subjects aged ≥ 60 years. Based on the mean −1.28 × SD among adults aged < 60 years, the L3‐SMI cut‐off value for sarcopenia was 44.77 cm2/m2 in male patients and 32.50 cm2/m2 in female patients. Using these values, 22.5% of the cirrhotic patients (28.7% of male patients and 11.9% of female patients) were diagnosed with sarcopenia. Compared with non‐sarcopenia individuals, sarcopenia patients had lower body mass index (21.28 ± 3.01 vs. 24.09 ± 3.39 kg/m2, P < 0.001) and serum albumin levels (31.54 ± 5.93 vs. 32.93 ± 5.95 g/L, P = 0.032), longer prothrombin times (16.39 ± 3.05 vs. 15.71 ± 3.20 s, P = 0.049), higher total bilirubin concentrations (41.33 ± 57.38 vs. 32.52 ± 31.48 μmol/L, P = 0.039), worse liver function (Child–Pugh score, 8.05 ± 2.11 vs. 7.32 ± 2.05, P = 0.001), higher prevalence of cirrhosis‐related complications (81.82% vs. 62.24%, P < 0.001) and mortality (30.68% vs. 11.22%, P < 0.001). Overall survival was significantly lower in the sarcopenia group [risk ratio (RR) = 2.643, 95% confidence interval (CI) 1.646–4.244, P < 0.001], accompanied with an increased cumulative incidence of ascites (RR = 1.827, 95% CI 1.259–2.651, P = 0.002), spontaneous bacterial peritonitis (RR = 3.331, 95% CI 1.404–7.903, P = 0.006), hepatic encephalopathy (RR = 1.962, 95% CI 1.070–3.600, P = 0.029), and upper gastrointestinal varices (RR = 2.138, 95% CI 1.319–3.466, P = 0.002). Subgroup analysis showed sarcopenia shortened the survival of the patients with Model For End‐Stage Liver Disease score > 14 (RR = 4.310, 95% CI 2.091–8.882, P < 0.001) or Child–Pugh C (RR = 3.081, 95% CI 1.516–6.260, P = 0.002).
Conclusions
Sarcopenia is a common comorbidity of cirrhosis and can be used to predict cirrhosis‐related complications and the prognosis.
Keywords: Sarcopenia, L3 skeletal muscle index, Malnutrition, Nutritional screening, Nutritional assessment, Diagnostic criteria, Liver function, Cirrhosis‐related complications, Survival, Prognosis prediction
Introduction
Liver cirrhosis, the end stage for most chronic liver diseases, is the 14th leading cause of death worldwide, resulting in approximately 1.3 million deaths annually. 1 , 2 Based on large populations of hepatitis B virus (HBV)‐infected and hepatitis C virus (HCV)‐infected people and increasing numbers of patients with alcoholic liver disease and non‐alcoholic fatty liver disease, the morbidity and mortality due to cirrhosis are still increasing in China, which impose a substantial public health burden. 2 , 3 The prognosis in cirrhotic patients varies widely, with the reported 1 year mortality rate ranging from 1% to 57%, depending on the occurrence of clinical decompensating events. 1 Therefore, it is important to establish an accurate assessment system for the early prediction of decompensating events and prognosis.
Malnutrition is a common comorbidity in patients with liver cirrhosis. 4 Because of the different tools used for nutritional screening and assessment, the reported prevalence of malnutrition in cirrhotic patients varies substantially from 10% to 100%. 4 , 5 Sarcopenia, a complex syndrome characterized by progressive decreases in skeletal muscle mass, strength, and function, has now been integrated into the definition of malnutrition and used to determine protein‐energy malnutrition in cirrhotic patients. 5 It has been well documented that there is a close correlation between single cross‐sectional magnetic resonance imaging (MRI) or computed tomography (CT) data and body composition. Thus, the data obtained from single cross‐sectional CT/MRI images are commonly used to evaluate the body muscle and fat content. 6 Considering the convenience of acquiring CT and MRI imaging data at the level of lumbar vertebrae 3 (L3), the L3 skeletal muscle index (L3‐SMI), defined as the muscle area at the level of L3 (cm2) normalized for height in meters squared (m2), which reflects the muscle mass, has been recommended for the assessment of sarcopenia. 6 , 7 , 8 Due to the critical impact of race on skeletal muscle mass and strength, the European Working Group on Sarcopenia in Older People and the International Working Group on Sarcopenia suggested that the diagnosis of sarcopenia should be based on local population norms. 8 , 9 Because the aetiology of cirrhosis and characteristics of cirrhotic patients are markedly different in China than in Western populations, it is inappropriate to apply the criteria established in Western populations to Chinese subjects.
Some recent studies documented that a low L3‐SMI value might be a relatively strong predictor of a poor prognosis and was associated with a high risk of cirrhosis‐related complications, such as hepatic encephalopathy (HE), ascites, spontaneous bacterial peritonitis (SBP), and hepatorenal syndrome (HRS). 9 , 10 , 11 Because of the close correlation between sarcopenia and the prognosis of liver disease, Japanese scholars constructed the first global assessment criteria for sarcopenia specializing liver disease in 2016. 11 However, the diagnostic criteria for sarcopenia have not been established in Chinese subjects, and there is still a lack of research on sarcopenia in the Chinese population with cirrhosis to date. In this study, we aimed to establish the diagnostic criteria for sarcopenia in the Chinese population based on the L3‐SMI and explore the value of malnutrition for the prediction of complications and outcomes in Chinese patients with cirrhosis.
Materials and methods
Study population
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. The study protocol was reviewed and approved by the Institutional Ethics Committee of Shanghai Changzheng Hospital (2016SL018). Informed consent was obtained from all patients for being included in the study.
Cohort 1
To determine the reference intervals of the L3‐SMI and other indexes and investigate the factors influencing these nutrition‐related imaging parameters, 1236 subjects who underwent a CT scan in the outpatient or physical examination department of two centres from March 2018 to May 2018 were recruited. Eligibility criteria included (i) age 20–80 years; (ii) normal limb function; and (iii) available abdominal CT or chest CT scans from which an adequate image at the L3 level could be obtained. The exclusion criteria were as follows: (i) age < 20 or >80 years; (ii) confirmed or strongly suspected diagnosis of malignant tumours; (iii) liver cirrhosis or liver dysfunction; (iv) chronic renal and/or respiratory insufficiency or severe heart disease; (v) paralysis, long‐term bedridden status or other bodily dysfunction; (vi) severe burns or trauma; (vii) hyperthyroidism, hypothyroidism, tuberculosis and any other diseases that can affect the basic metabolism; (viii) diseases or conditions that cause intestinal nutrient absorption disorders, such as inflammatory bowel diseases, or gastrointestinal surgery; (ix) treatment with glucocorticoids or immunosuppressive agents; (x) pregnancy or lactation; and (xi) other diseases affecting nutritional status or influencing anthropometry and grip strength tests. Considering the deterministic effect of age on sarcopenia, only individuals aged <60 years were included to determine the cut‐off values for the imaging parameters; the data of all the enrolled respondents were used to identify the factors influencing the above parameters.
Cohort 2
To investigate the prevalence of sarcopenia in Chinese patients with cirrhosis and evaluate the value of sarcopenia for the prediction of prognosis and complications, 480 inpatients with liver cirrhosis were enrolled at three centres from January 2013 to December 2017. Eligibility criteria were age ranging from 18 to 80 years, with a clinical diagnosis of liver cirrhosis on the basis of typical clinical manifestations, laboratory tests, imaging characteristics, and/or representative pathology results on liver biopsy. Decompensation of the disease was defined as a Child–Pugh score greater than 7 lasting for at least 1 month or at least one episode of severe complications, including ascites, SBP, oesophageal and gastric variceal bleeding, and HE. All patients underwent an abdominal CT examination during hospitalization, and CT images at the L3 level were obtained. The exclusion criteria included the following: (i) liver transplantation; (ii) confirmed or strongly suspected diagnosis of malignant tumours; (iii) chronic renal and/or respiratory insufficiency or severe heart disease; (iv) diseases or conditions that cause intestinal nutrient absorption disorders, such as inflammatory bowel diseases, or gastrointestinal surgery; and (v) pregnancy or lactation.
CT scan and assessment of L3‐SMI, L3‐SMD, and other indexes
Computed tomography scans were performed with a multislice spiral CT scanner (Brilliance 256‐slice spiral CT scanner, Philips Medical Systems, the Netherlands or uCT780 256‐slice spiral CT scanner, United Imaging Medical Systems, Shanghai, China) with a collimating reconstruction thickness of 1 mm and an interval of 1 mm according to the standard operating procedures. All subjects were placed in the supine position and told to hold their breath to reduce breathing and movement artefacts during scanning.
Two independent radiologists analysed the CT images with SliceOmatic V5.0 software (Rev‐8, Tomovision, Montreal, Quebec, Canada). In accordance with a previous study, muscle tissues were identified on CT images based on Hounsfield unit (HU) thresholds ranging from −29 to +150, and subcutaneous and intermuscular adipose tissue was identified with HU thresholds ranging from −190 to −30. Additionally, tissue with HU thresholds ranging from −150 to −50 were considered visceral adipose tissue. The skeletal muscle at the L3 level contains the psoas major, erector spinalis, quadratus psoas, external abdominal oblique and internal abdominal oblique on the right and left sides, and the transverse abdominis. The sum of the relevant tissue area and the average density at the L3 level cross‐section was automatically calculated by the software, including the skeletal muscle area, visceral adipose tissue area, subcutaneous adipose tissue area, skeletal muscle density, visceral adipose tissue density, subcutaneous adipose tissue density, and intermuscular adipose tissue density. The L3‐SMI was calculated as follows: the skeletal muscle area at the L3 level divided by the square of the height (cm2/m2).
Anthropometric measurements and clinical assessments
All participants in Cohort 1 underwent an assessment, including a detailed record of their medical history and a physical examination. Age, height, weight, and body mass index (BMI) were collected. Anthropometric variables were measured according to the standard operating procedures, including waist circumference, biceps circumference, triceps skinfold thickness, abdominal skinfold thickness, subscapular skinfold thickness, dominant hand grip strength, and non‐dominant hand grip strength.
The basic characteristics of patients with cirrhosis in Cohort 2 were obtained from a standardized case collection system. All patients were followed up for 2 years or until death. The investigated complications were HE, ascites, SBP, oesophageal and gastric variceal bleeding, HRS, hepatocellular carcinoma, and so on. The laboratory parameters, including total bilirubin (TBil), direct bilirubin, indirect bilirubin, albumin, prothrombin time (PT) and platelet count, were recorded. The Child–Pugh score and classification and the Model For End‐Stage Liver Disease (MELD) score were determined at the first admission and the end of follow‐up.
Data collection
Data were collected by investigators at each centre and monitored by two independent inspectors and analysed by statistical experts at the Second Military Medical University of China. All authors vouch for the completeness and veracity of the data as well as data analyses.
Statistical analysis
The statistical analysis was performed using SPSS 22.0 (IBM SPSS, Chicago, IL). Continuous variables are shown as the means ± standard deviations (SDs), whereas categorical variables are expressed as numbers and percentages. Normally distributed continuous parameters were compared with Student's t‐tests, and non‐normally distributed continuous parameters were compared with the Mann–Whitney U test. The comparison of categorical parameters was determined by two‐tailed χ 2 tests or Fisher's exact tests. Univariate analysis and multiple linear regression were performed to analyse the factors influencing the L3‐SMI. The relationship between two variables was evaluated with the Pearson correlation coefficient. Normative reference data of the L3‐SMI were calculated using the formula: 90% lower = mean −1.28 × SD. The receiver operating characteristic (ROC) curve was generated to verify the value of the L3‐SMI for the diagnosis of sarcopenia. Cox proportional hazards models were used to compare the time with death or a breakthrough episode of complications between two groups with a two‐sided test. The Kaplan–Meier method was used to analyse survival in Cohort 2 and to estimate the proportions of patients experiencing complications at successive time points during the follow‐up. The time‐dependent ROC curves of L3‐SMI, MELD, and Child–Pugh scores for prognosis prediction were revealed in patients with liver cirrhosis and area under the ROC for in each time point (3, 6, 9, 12, 15, 18 and 21, & 24 months) was carried out to compare the value of above parameters. A P value < 0.05 was regarded as statistically significant.
Results
Factors influencing the L3‐SMI in general Chinese adults
A total of 1236 subjects were recruited, and 911 subjects who met the inclusion criteria and did not meet the exclusion criteria were enrolled in the analysis of the factors affecting L3‐SMI in the general Chinese population. This cohort included 394 male and 417 female participants aged from 20 to 80 years (Table 1 ). Univariate analysis showed that age, sex, height, weight, waist circumference, biceps circumference, triceps skinfold thickness, subscapular skinfold thickness, abdominal skinfold thickness, dominant hand grip strength, and non‐dominant hand grip strength influenced the L3‐SMI. Multiple linear regression analysis showed that age, sex, height, weight, biceps circumference, and triceps skinfold thickness were strongly associated with the L3‐SMI (Table S1 ). Among all the factors, age and sex had the strongest effect on the L3‐SMI. The L3‐SMI scores were clearly higher in males than in females and sharply declined in subjects aged ≥ 60 years (Tables 1 and S2 ).
Table 1.
Characteristics of the general Chinese population in Cohort 1
| Group | Male (n = 494) | Female (n = 417) | P value |
|---|---|---|---|
| Age (years) (mean ± SD) | 47.00 ± 15.54 | 48.62 ± 14.65 | 0.108 |
| Height (m) (mean ± SD | 1.72 ± 0.06 | 1.60 ± 0.05 | <0.001 |
| Weight (kg) (mean ± SD) | 72.21 ± 11.18 | 57.57 ± 8.12 | <0.001 |
| Body mass index (kg/m2) | 24.36 ± 3.27 | 22.42 ± 3.02 | <0.001 |
| Anthropometry | |||
| Waist circumference (cm) (mean ± SD) | 87.61 ± 9.48 | 78.66 ± 8.70 | <0.001 |
| Biceps circumference (cm) (mean ± SD) | 27.92 ± 2.49 | 25.85 ± 2.38 | <0.001 |
| Triceps skinfold thickness (mm) (mean ± SD) | 12.43 ± 5.77 | 18.36 ± 5.74 | <0.001 |
| Abdominal skinfold thickness (mm) (mean ± SD) | 24.43 ± 7.63 | 23.38 ± 5.94 | 0.032 |
| Subscapular skinfold thickness (mm) (mean ± SD) | 20.18 ± 6.90 | 21.12 ± 6.68 | 0.040 |
| Dominant hand grip strength (kg) (mean ± SD) | 41.77 ± 8.59 | 25.69 ± 4.60 | <0.001 |
| Non‐dominant hand grip strength (kg) (mean ± SD) | 38.81 ± 8.22 | 23.73 ± 4.70 | <0.001 |
| Image indexes | |||
| Area | |||
| L3‐SMA (cm2) | 153.26 ± 24.20 | 102.25 ± 13.76 | <0.001 |
| L3‐IMATA (cm2) | 10.82 ± 7.16 | 10.38 ± 7.05 | 0.354 |
| L3‐VATA (cm2) | 129.23 ± 73.95 | 76.71 ± 49.27 | <0.001 |
| L3‐SATA (cm2) | 123.88 ± 60.01 | 138.85 ± 54.01 | <0.001 |
| Indexes | |||
| L3‐SMI (cm2/m2) | 52.94 ± 8.41 | 38.91 ± 5.65 | <0.001 |
| L3‐IMATI (cm2/m2) | 3.77 ± 2.63 | 3.98 ± 2.78 | 0.232 |
| L3‐VATI (cm2/m2) | 44.69 ± 25.61 | 29.35 ± 19.05 | <0.001 |
| L3‐SATI (cm2/m2) | 42.78 ± 20.47 | 53.04 ± 21.70 | <0.001 |
| Density | |||
| L3‐SMD (Hu) | 42.79 ± 6.07 | 37.26 ± 6.77 | <0.001 |
| L3‐IMATD (Hu) | −64.90 ± 5.21 | −63.79 ± 4.44 | 0.001 |
| L3‐VATD (Hu) | −96.41 ± 7.65 | −94.06 ± 7.52 | <0.001 |
| L3‐SATD (Hu) | −100.53 ± 7.71 | −103.74 ± 5.27 | <0.001 |
| Ratio | |||
| L3‐IMATA/L3‐SMA | 0.07 ± .062 | 0.11 ± 0.08 | <0.001 |
| L3‐VATA/L3‐SMA | 0.84 ± 0.49 | 0.75 ± 0.48 | <0.001 |
| L3‐SATA/L3‐SMA | 0.80 ± 0.36 | 1.36 ± 0.50 | <0.001 |
| L3‐VATA/L3‐SATA | 1.06 ± 0.52 | 0.55 ± 0.34 | <0.001 |
IMATA, intermuscular adipose tissue area; IMATD, intermuscular adipose tissue density; IMATI, intermuscular adipose tissue index; SATA, subcutaneous adipose tissue area; SATD, subcutaneous adipose tissue density; SATI: subcutaneous adipose tissue index; SMA: skeletal muscle area; SMD: skeletal muscle density; SMI: skeletal muscle index; VATA: visceral adipose tissue area; VATD: visceral adipose tissue density; VATI: visceral adipose tissue index.
Determination of the cut‐off value of the L3‐SMI for the diagnosis of sarcopenia in general Chinese adults
According to previous studies, age is the most important factor affecting sarcopenia in the general population. Our study also found that the L3‐SMI scores sharply declined in subjects aged ≥ 60 years. Thus, subjects younger than 60 years, including 365 male and 296 female subjects, were enrolled to determine the reference intervals for the L3‐SMI (Table S3 ). Based on the mean −1.28 × SD among adults aged < 60 years, the cut‐off value for the L3‐SMI for the diagnosis of sarcopenia was 44.77 cm2/m2 in male patients and 32.50 cm2/m2 in female patients. Based on this cut‐off value, among the individuals over 60 years old, 37.9% (49/129) of the male patients and 17.3% (21/121) of the female patients were diagnosed with sarcopenia.
Prevalence of skeletal sarcopenia in Chinese patients with cirrhosis
In total, 480 patients (286 male and 184 female patients) with complete clinical data were enrolled in Cohort 2 to investigate the prevalence of skeletal muscle malnutrition in Chinese patients with liver cirrhosis and clarify the relationship between skeletal muscle malnutrition and cirrhosis‐related complications. The basic characteristics were listed in Table 2 . Based on the cut‐off values determined in this study, the overall prevalence of skeletal sarcopenia in Chinese patients with cirrhosis was 29.1% (86/296) in male patients and 12.0% (22/184) in female patients. Altogether, 22.5% (108/480) of the patients with cirrhosis had skeletal sarcopenia. These data suggest that sarcopenia is a common comorbidity in cirrhotic patients, and male patients are more likely to suffer from sarcopenia than female patients (P < 0.001).
Table 2.
Baseline characteristics of cirrhotic patients in Cohort 2
| Group | Male(n = 296) | Female (n = 184) | P value |
|---|---|---|---|
| Age (years) (mean ± SD) | 51.93 ± 11.27 | 58.45 ± 12.08 | <0.001 |
| Height (m) (mean ± SD | 171.01 ± 4.95 | 160.02 ± 5.05 | <0.001 |
| Weight (kg) (mean ± SD) | 69.32 ± 10.41 | 59.15 ± 10.39 | <0.001 |
| Body mass index (kg/m2) | 23.68 ± 3.27 | 23.08 ± 3.86 | 0.089 |
| Aetiology | 0.031 | ||
| HBV (%) | 149 (50.34) | 59 (32.07) | |
| Alcohol (%) | 60 (20.27) | 1 (0.54) | |
| Autoimmune (%) | 1 (0.34) | 38 (20.65) | |
| Budd–Chiari (%) | 6 (2.03) | 6 (3.26) | |
| HCV (%) | 4 (1.35) | 1 (0.54) | |
| Schistosomiasis (%) | 5 (1.69) | 7 (3.80) | |
| Others (%) | 49 (16.55) | 71 (38.59) | |
| Combined (%) | 22 (7.43) | 1 (0.54) | |
| Serum indexes | |||
| RBC (1012/L) | 3.54 ± 0.82 | 3.41 ± 0.68 | 0.059 |
| WBC (109/L) | 4.44 ± 2.89 | 4.76 ± 7.63 | 0.516 |
| Haemoglobin (g/L) | 104.21 ± 29.86 | 97.80 ± 22.76 | 0.013 |
| Neutrophil (109/L) | 2.74 ± 2.34 | 3.64 ± 12.66 | 0.235 |
| PLT (109/L) | 109.04 ± 138.34 | 124.95 ± 153.93 | 0.241 |
| HCT (%) | 31.35 ± 7.91 | 29.71 ± 6.42 | 0.018 |
| TBil (μmol/L) | 34.99 ± 36.28 | 33.70 ± 42.84 | 0.724 |
| DBil (μmol/L) | 15.24 ± 27.20 | 14.17 ± 29.05 | 0.685 |
| Albumin (g/L) | 32.81 ± 6.11 | 32.32 ± 5.75 | 0.379 |
| ALT (U/L) | 45.60 ± 110.05 | 29.10 ± 22.39 | 0.045 |
| AST (U/L) | 52.97 ± 96.53 | 44.96 ± 34.35 | 0.280 |
| GGT (U/L) | 82.66 ± 144.63 | 59.93 ± 74.00 | 0.049 |
| Scr (μmol/L) | 73.83 ± 50.23 | 56.92 ± 25.31 | <0.001 |
| PT (s) | 16.08 ± 3.31 | 15.52 ± 2.92 | 0.061 |
| INR | 1.36 ± 0.34 | 1.31 ± 0.25 | 0.093 |
| K+ (mmol/L) | 3.90 ± 0.72 | 3.92 ± 2.31 | 0.892 |
| Na+ (mmol/L) | 136.52 ± 20.07 | 138.26 ± 15.11 | 0.310 |
| Cl− (mmol/L) | 103.26 ± 15.54 | 104.29 ± 11.83 | 0.442 |
| Child–Pugh score | 7.50 ± 2.14 | 7.45 ± 2.0 | 0.789 |
| Child–Pugh (%) | 0.410 | ||
| A | 112 (37.84) | 70 (38.04) | |
| B | 129 (43.58) | 88 (47.83) | |
| C | 55 (18.58) | 26 (14.13) | |
| MELD score | 11.75 ± 4.36 | 11.82 ± 3.60 | 0.851 |
| Complications | |||
| Ascites (%) | 189 (63.85) | 120 (65.22) | 0.755 |
| HE (%) | 26 (8.78) | 20 (10.87) | 0.451 |
| SBP (%) | 12 (4.54) | 9 (4.89) | 0.664 |
| UGIB (%) | 170 (57.43) | 102 (55.43) | 0.668 |
| AKI/HRS (%) | 6 (2.03) | 2 (1.09) | 0.435 |
| PVT (%) | 84 (28.38) | 48 (26.09) | 0.586 |
AKI, acute kidney injury; ALT, glutamic‐pyruvic transaminase; AST, glutamic‐oxalacetic transaminase; DBil, direct bilirubin; GGT, gamma‐glutamyl transferase; HCT, hematocrit; HE, hepatic encephalopathy; HRS, hepatorenal syndrome; TBil: total bilirubin; INR, international normalized ratio; MELD, model for end‐stage liver disease; PLT, blood platelet; PT, prothrombin time; PVT, portal venous thrombosis; RBC, red blood cell; SBP, spontaneous bacterial peritonitis; UGIB, upper gastrointestinal bleeding; WBC, white blood cell.
Sarcopenia is related to worse liver function and higher incidences of ascites, SBP, and HRS
Univariate analysis showed that the L3‐SMI was positively correlated with BMI (P < 0.001), the serum albumin level (P = 0.009) and the ALT level (P = 0.023) and was negatively correlated with the presence of ascites and a history of SBP (P = 0.003). Further multivariate analysis confirmed the strong correlations between the L3‐SMI and BMI, ascites, SBP, and the ALT level (Supporting Information, Table S4 ). Based on the cut‐off values of the L3‐SMI obtained in Cohort 1, all patients with cirrhosis were divided into the sarcopenia group and the non‐sarcopenia group. As shown in Table 3 , compared with those without sarcopenia, patients with sarcopenia had lower BMI values (21.28 ± 3.01 vs. 24.09 ± 3.39 kg/m2, P < 0.001), red blood cell counts (3.30 ± 0.79 vs. 3.54 ± 0.76 × 1012/L, P = 0.004), haematocrit levels ([29.37 ± 7.40]% vs. [31.11 ± 7.38]%, P = 0.032), albumin levels (31.54 ± 5.93 vs. 32.93 ± 5.95 g/L, P = 0.032), longer PT (16.39 ± 3.05 vs. 15.71 ± 3.20 s, P = 0.049) and higher TBil concentrations (41.33 ± 57.38 vs. 32.52 ± 31.48 μmol/L, P = 0.039) and international normalized ratios (1.38 ± 0.28 vs. 1.32 ± 0.31, P = 0.049). The average Child–Pugh score was significantly higher in the sarcopenia group than in the non‐sarcopenia group (8.05 ± 2.11 vs. 7.32 ± 2.05, P = 0.001).
Table 3.
Baseline characteristics is compared between sarcopenia group and non‐sarcopenia group in Cohort 2
| Non‐sarcopenia (n = 372) | Sarcopenia (n = 108) | P value | |
|---|---|---|---|
| Age, years (mean ± SD) | 53.88 ± 11.95 | 56.31 ± 12.07 | 0.063 |
| Gender (male/female) | 210/162 | 86/22 | <0.001 |
| Body mass index (kg/m2) | 24.09 ± 3.39 | 21.28 ± 3.01 | <0.001 |
| Child–Pugh score | 7.32 ± 2.05 | 8.05 ± 2.11 | 0.001 |
| MELD score | 11.40 ± 3.85 | 12.14 ± 4.50 | 0.094 |
| Serum index | |||
| RBC (1012/L) | 3.54 ± 0.76 | 3.30 ± 0.79 | 0.004 |
| PLT (109/L) | 111.67 ± 120.10 | 127.10 ± 208.23 | 0.032 |
| HCT (%) | 31.11 ± 7.38 | 29.37 ± 7.40 | 0.032 |
| Haemoglobin (g/L) | 102.98 ± 27.46 | 97.52 ± 27.40 | 0.069 |
| TBil (μmol/L) | 32.52 ± 31.48 | 41.33 ± 57.38 | 0.039 |
| DBil (μmol/L) | 13.08 ± 21.83 | 20.93 ± 42.43 | 0.010 |
| Albumin (g/L) | 32.93 ± 5.95 | 31.54 ± 5.93 | 0.033 |
| Scr (μmol/L) | 65.83 ± 44.51 | 72.25 ± 37.73 | 0.176 |
| PT (s) | 15.71 ± 3.20 | 16.39 ± 3.05 | 0.049 |
| INR | 1.32 ± 0.31 | 1.38 ± 0.28 | 0.039 |
| Complications | |||
| Ascites (%) | 212/372 (56.66) | 87/108 (80.56) | <0.001 |
| Grade | <0.001 | ||
| 0 | 160 | 21 | |
| 1 | 116 | 41 | |
| 2 | 30 | 9 | |
| 3 | 66 | 37 | |
| HE (%) | 33/372 (8.87) | 13/108 (12.04) | 0.326 |
| SBP (%) | 11/372 (2.96) | 10/108 (9.26) | 0.005 |
| UGIB (%) | 213/372 (57.26) | 59/108 (54.63) | 0.628 |
| AKI/HRS (%) | 3/372 (0.81) | 5/108 (4.63) | 0.006 |
| PVT (%) | 102/372 (27.42) | 30/108 (27.78) | 0.942 |
| L3 body composition parameters index | |||
| L3‐IMATI (cm2/m2) | 3.47 ± 2.90 | 2.54 ± 1.90 | 0.002 |
| L3‐VATI (cm2/m2) | 31.95 ± 22.43 | 22.59 ± 19.05 | <0.001 |
| L3‐SATI (cm2/m2) | 41.35 ± 23.59 | 24.66 ± 18.83 | <0.001 |
| L3‐SMD (HU) | 40.13 ± 8.69 | 37.71 ± 8.62 | 0.011 |
| Myosteatosis% | 94/372 (25.3) | 53/108 (49.1) | <0.001 |
AKI, acute kidney injury; ALT, glutamic‐pyruvic transaminase; AST, glutamic‐oxalacetic transaminase; DBil, direct bilirubin; GGT, gamma‐glutamyl transferase; HCT, haematocrit; HE, hepatic encephalopathy; HRS, hepatorenal syndrome; IMATI, intermuscular adipose tissue index; INR, international normalized ratio; MELD, model for end‐stage liver disease; PLT, blood platelet; PT, prothrombin time; PVT, portal venous thrombosis; RBC, red blood cell; SATI, subcutaneous adipose tissue index; SBP, spontaneous bacterial peritonitis; SMD, skeletal muscle density; SMI, skeletal muscle index; TBil, total bilirubin; UGIB, upper gastrointestinal bleeding; VATI, visceral adipose tissue index; WBC, white blood cell.
To avoid the interference of the aetiology distribution deviations on the results, we divided the patients in the Cohort 2 into HBV and non‐HBV group and reanalysed the difference between sarcopenia and non‐sarcopenia subjects in the both group (Tables S5 – S6 ). The data showed that the sarcopenia patients in HBV group had lower BMI (21.40 ± 2.70 vs. 23.98 ± 3.01 kg/m2, P < 0.001), red blood cell counts (3.27 ± 0.77 vs. 3.61 ± 0.85 × 1012/L, P = 0.24), haematocrit levels ([28.27 ± 7.02]% vs. [31.66 ± 8.14]%, P = 0.18), serum levels of haemoglobin (92.97 ± 26.58 vs. 105.66 ± 30.30 g/L, P = 0.18) and albumin (31.00 ± 4.77 vs. 33.48 ± 5.62 g/L, P = 0.14), higher Child–Pugh scores (8.08 ± 2.71 vs. 7.28 ± 2.00, P = 0.029), serum levels of TBil (46.19 ± 84.53 vs. 31.49 ± 20.80 μmol/L, P = 0.045), direct bilirubin (25.96 ± 63.91 vs. 11.28 ± 12.15 μmol/L, P = 0.006), PT (17.22 ± 2.88 vs. 15.68 ± 2.54 s, P = 0.001) and international normalized ratio (1.46 ± 0.29 vs. 1.31 ± 0.22, P = 0.001) compared with those non‐sarcopenia individuals. Additionally, there were poorer Child–Pugh scores (8.04 ± 2.13 vs. 7.35 ± 2.03, P = 0.016), lower serum albumin concentrations (31.79 ± 1.46 vs. 32.48 ± 3.20 g/L, P = 0.044), more morbidity of ascites (85.71% vs. 56.93%, P < 0.001), and SBP (85.71% vs. 56.93%, P < 0.001) in the sarcopenia patients than that in the non‐sarcopenia patients in the non‐HBV group (Tables S7 – S8 ).
Sarcopenia is an indicator of the occurrence of complications and prognosis in cirrhotic patients
All 480 cirrhotic patients in Cohort 2 were followed until death or for at least 2 years after enrolment. In total, 30.68% (27/88) of the patients in the sarcopenia group and 11.22% (44/392) of the individuals in the non‐sarcopenia group died within the 2 year follow‐up period (P < 0.001). Sarcopenia significantly elevated the mortality in patients with liver cirrhosis [risk ratio (RR) = 2.643, 95% confidence interval (CI) 1.646–4.244, P < 0.001]. As shown in Figure 1 , log‐rank analysis showed that overall survival was significantly lower in the sarcopenia group than in the non‐sarcopenia group (P < 0.001). Additionally, 81.82% (72/88) of the patients in the sarcopenia group experienced episodes of the breakthrough of complications during the 2 year follow‐up period, while only 62.24% (244/392) of the patients in the non‐sarcopenia group experienced complications (P < 0.001). Cox proportional hazards models analysis showed that sarcopenia markedly increased the cumulative incidence of ascites (RR = 1.827, 95% CI 1.259–2.651, P = 0.002), SBP (RR = 3.331, 95% CI 1.404–7.903, P = 0.006), HE (RR = 1.962, 95% CI 1.070–3.600, P = 0.029), and upper gastrointestinal varices (RR = 2.138, 95% CI 1.319–3.466, P = 0.002). We also analysis the value of the widely used prognostic scores systems, MELD, and Child–Pugh scores, for the cirrhosis‐related prognosis prediction. Log‐rank analysis showed that higher MELD score and worse Child–Pugh class suggested poorer overall survival (both P < 0.001, Figure S1 ). Cirrhotic patients with MELD score > 14 had higher mortality within the 2 year follow‐up period compared with those with MELD score ≤ 14 (RR = 3.436, 95% CI 2.145–5.506, P < 0.001). Moreover, the mortality increased along with the deterioration of Child–Pugh class (RR = 3.497, 95% CI 2.467–4.959, P < 0.001). Although time‐dependent ROC analyses showed the area under the ROC for L3‐SMI was lower than those for MELD and Child–Pugh scores at each time point (Figure S2 ), the 2 year expected survival rate of the patients with sarcopenia was significantly shortened in those individuals with MELD score > 14 (RR = 4.310, 95% CI 2.091–8.882, P < 0.001) or Child–Pugh C (RR = 3.081, 95% CI 1.516–6.260, P = 0.002, Figure 2 ).
Figure 1.
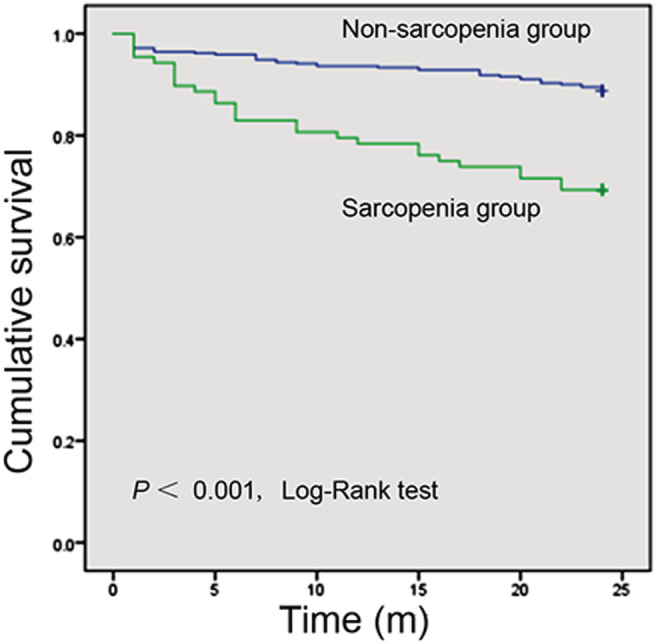
Kaplan–Meier estimates of overall survival in the sarcopenia group compared with that in the non‐sarcopenia group, according to Cohort 2.
Figure 2.
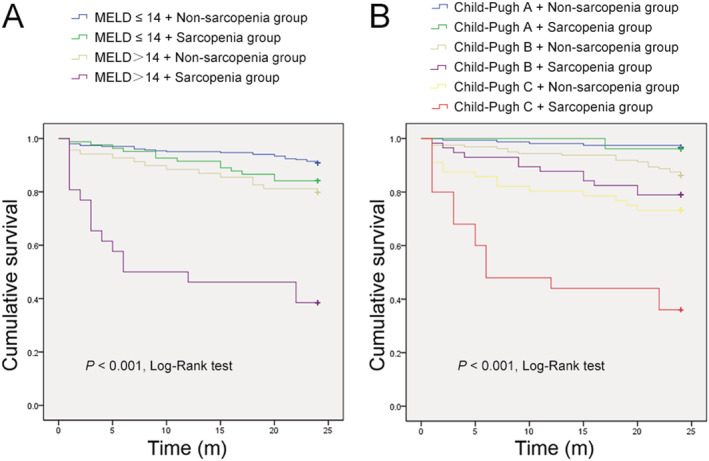
Kaplan–Meier estimates of overall survival in subgroups. (A) Kaplan–Meier estimates of overall survival in the sarcopenia group compared with that in the non‐sarcopenia group, according to subgroups discriminated with MELD score. (B) Kaplan–Meier estimates of overall survival in the sarcopenia group compared with that in the non‐sarcopenia group, according to subgroups discriminated with Child–Pugh class.
Discussion
Considering the possible correlation between malnutrition and poor prognosis in cirrhotic patients, it is crucial to establish an appropriate nutritional assessment to determine the impact of nutritional status on cirrhosis. 12 , 13 L3‐SMI, an indicator of sarcopenia, has been established to quantitatively assess muscle mass reduction and is recognized as an objective and quantifiable parameter that is useful for the evaluation of nutritional status. In this study, we determined the factors influencing the L3‐SMI, established the cut‐off value for the L3‐SMI in general Chinese adults younger than 60 years and defined sarcopenia as an L3‐SMI score < 44.77 cm2/m2 in male patients and <32.50 cm2/m2 in female patients. Moreover, our current study confirmed that sarcopenia was not only related to poor liver function in cirrhotic patients but also could be used to predict a poor prognosis and episodes of cirrhosis‐related complications.
Malnourishment has been shown to be a strong predictor of morbidity and mortality in patients with advanced decompensated chronic liver diseases. However, even among studies performed in the same population, there has been substantial variability and poor consistency among the nutrition assessment results obtained with different nutritional assessment tools. 12 Therefore, it is clear that the accuracy of the evaluation of malnutrition depends on the appropriateness of the nutritional assessment tools. Sarcopenia has been considered the primary nutritional consequence of liver cirrhosis. 12 , 13 Compared with other evaluation tools, including anthropometry, dual‐energy X‐ray absorptiometry, bioelectrical impedance analysis, biochemical tests, and the subjective global assessment, the L3‐SMI has the advantages of being objective, quantifiable, accurate, and non‐invasive, and it has been shown to be a relatively better protein‐calorie malnourishment and sarcopenia indicator. In addition, as routine follow‐up assessments for patients with cirrhosis, CT and MRI scans have become common. Thus, L3‐SMI has good accessibility and repeatability and is suitable for follow‐up monitoring. Moreover, the images at the L3 level based on CT or MRI scan can be used to assess the amount of intramuscular fat, which accumulating evidence suggests is important for the assessment of the quality of muscle and the associations with clinical outcomes. Therefore, L3‐SMI is a valid and useful tool for the assessment of malnutrition in cirrhosis‐related clinical practice. Because CT scans are more common and less expensive than MRI scans in China, we selected L3‐SMI based on CT scans to determine muscle mass and function in this study.
The guidelines suggest that the reference values for nutritional indicators might vary based on ethnicity. 14 To date, Chinese diagnostic criteria for sarcopenia based on the L3‐SMI have not been established. In the current study, we analysed the distributions of the L3‐SMI values in 911 subjects and investigated its influencing factors. To our knowledge, this is by far the largest investigation of sarcopenia among the general Chinese population. The cut‐off values for L3‐SMI established in this study were 44.77 cm2/m2 in male patients and 32.50 cm2/m2 in female patients. These values were significantly lower than those in Western populations but were close to those in the Japanese and Korean populations, which further verifies the deterministic influence of ethnicity on muscle characteristics and provides a reference for subsequent research in China. 12 , 13 , 14 , 15 , 16 , 17
It is generally believed that age and sex are the most important factors influencing L3‐SMI in the general population. In the current study, the L3‐SMI value in female patients was significantly higher than that in male patients and sharply declined in subjects aged ≥60 years, which were consistent with the previous reports. 12 , 13 , 14 , 15 , 16 , 17 In addition, our data confirmed the correlation between the L3‐SMI and anatomical indicators such as height, weight, biceps circumference, and triceps skinfold thickness. Grip strength is believed to reflect muscle strength and skeletal muscle function. However, it is worth noting that a reduction in muscle mass was only moderately related to a decline in grip strength in this study. Our study showed that 75 subjects with reduced L3‐SMI scores had normal grip strength, and 21 subjects with decreased grip strength had normal L3‐SMI scores. This finding highlights the inconsistent changes in muscle mass and function, and future research should be carried out to explore the impact of such inconsistencies on the outcomes of chronic liver diseases.
Sarcopenia is a common complication of liver cirrhosis. 10 Numerous factors induce cirrhosis‐related sarcopenia, including reduced nutrient intake and synthesis, abnormal protein metabolism, inadequate physical activity, abnormal myostatin and insulin‐like growth factor 1 levels, and altered intestinal flora. 10 , 18 , 19 Although it has been reported that sarcopenia is related to the severity of liver disease as estimated by the Child–Pugh score, the diagnosis of sarcopenia was based on dual‐energy X‐ray absorptiometry or biochemical tests in the majority of early studies. 20 A recent study in patients with HCV infections showed that the L3‐SMI was not significantly reduced in patients without cirrhosis but was negatively correlated with the Child–Pugh score in male patients with HCV‐related cirrhosis. 21 In our study, the L3‐SMI was negatively correlated with the Child–Pugh and MELD scores. The prevalence of sarcopenia identified by a low L3‐SMI score was 14.29% (26/182) in patients with Child–Pugh class A, 26.27% (57/217) in those with Child–Pugh B, and 30.86% (25/81) in those with Child–Pugh C. This observation further confirms that sarcopenia is common in patients with cirrhosis and worsens with the progression of liver disease.
Several systematic reviews and meta‐analyses have shown that sarcopenia is accompanied by relatively poor clinical outcomes and higher mortality in patients with cirrhosis, especially in those awaiting or undergoing liver transplantation. 22 , 23 , 24 Compared with those without sarcopenia, cirrhotic patients with sarcopenia had a higher incidence of HE, an increased risk of serious infections, prolonged hospitalizations, worse quality of life, and worse survival after liver transplantation. In fact, some investigators suggested that the impact of sarcopenia on the prognosis of cirrhotic patients is independent of the MELD score and the presence of portal hypertension. 25 , 26 Interestingly, the modification of the MELD score by inclusion of sarcopenia (MELD‐Sarcopenia) improved the prediction of mortality in patients with cirrhosis. 27 Moreover, some other studies showed that compared with the Child–Pugh score, the SMI has a higher area under the receiver operating characteristic curve for predicting the prognosis in male cirrhotic patients, and the ALBI‐SMM grading system, a scoring system combining albumin, bilirubin, and SMI, was a better prognostic predictor than albumin, bilirubin, or SMI alone. 28 , 29 Consistent with these previous studies, the current study confirmed that sarcopenia can be used to predict the occurrence of complications and prognosis in patients with cirrhosis. Patients with sarcopenia have increased risks of ascites, SBP, HE, and upper gastrointestinal varices, and sarcopenia shortened the survival of the patients with Child–Pugh class C or MELD score > 14. Therefore, incorporating sarcopenia into the widely used predictive strategies (e.g. MELD or Child–Pugh scores) might help to better identify the patients with poor prognosis.
There were some limitations in this study. The aetiology distributions in Cohort 2 were not perfectly consistent with that in the whole population with liver cirrhosis, which might lead to some skewing. To avoid the interference of these distribution deviations on the results, the difference between sarcopenia and non‐sarcopenia individuals were reanalysed in the HBV and non‐HBV patients respectively. The results were similar to those presented in whole population of Cohort 2.
In summary, we determined the cut‐off value for the L3‐SMI for the diagnosis of sarcopenia in Chinese adults, which can serve as a reference for subsequent research in China. Using a cut‐off of 44.77 cm2/m2 in male patients and 32.50 cm2/m2 in female patients for L3‐SMI, 22.5% of cirrhotic Chinese patients were diagnosed with skeletal sarcopenia. Patients with sarcopenia had poor liver function and a high prevalence of cirrhosis‐related complications. This evidence suggests that sarcopenia can be used to predict poor clinical outcomes and survival in patients with cirrhosis.
Funding
This work was supported by the Science and Technology Guidance Plan of Shanghai Science and Technology Commission (19411970500), the Outstanding Youth Medical Talents Project of Shanghai “Rising Stars of Medical Talent” Youth Development Program (SHWJRS2021‐99), the Pilot Talent Plan of Shanghai East Hospital (2019lhrcjh), the Top‐Level Clinical Discipline Project of Shanghai Pudong (PWYgf2018‐04), the grants from the National Natural Science Foundation Committee of China (81770600, 82070616) and the Grant from Shanghai Municipal Health Commission (201840233).
Author contributions
Z.X., X.W.F., and Y.H. designed the study. Z.C.Q., S.Z.W., W.L.F., Z.L.Y., J.S.M., and S.P.M. collected the data. Y.J.J., L.Y.Y., and T.W. analysed the data. Z.X. wrote the paper. Z.C.Q. and X.W.F. revised the manuscript.
Conflict of interest
The authors have no conflict of interest regarding this study.
Supporting information
Table S1. Univariate analysis and multiple linear regression analysis of the impact factors of L3‐SMI based on the general Chinese population in Cohort 1.
Table S2. Distribution of L3‐SMI according to age.
Table S3. Characteristics of population younger than 60 years in Cohort 1.
Table S4. Univariate analysis and multiple linear regression analysis of the impact factors of L3‐SMI based on cirrhotic patients in Cohort 2.
Table S5. Univariate analysis and multiple linear regression analysis of the impact factors of L3‐SMI based on cirrhotic patients with HBV in Cohort 2.
Table S6. Univariate analysis and multiple linear regression analysis of the impact factors of L3‐SMI based on non‐HBV cirrhotic patients in Cohort 2.
Table S7. Baseline characteristics is compared between sarcopenia group and non‐sarcopenia group in the cirrhotic patients with HBV.
Table S8. Baseline characteristics is compared between sarcopenia group and non‐sarcopenia group in the Non‐HBV cirrhotic patients.
Figure S1. Kaplan–Meier estimates of overall survival, according to Cohort 2.
A. Kaplan–Meier estimates of overall survival in patients with MELD score ≤14 compared with that in subjects with MELD score>14. B. Kaplan–Meier estimates of overall survival in patients with distinct Child‐Pugh class.
Figure S2. The time‐dependent ROC curves of L3‐SMI, MELD and Child‐Pugh scores for prognosis prediction in patients with liver cirrhosis, according to Cohort 2.
Acknowledgements
The authors would like to thank the patients and their families for their contribution to this study.
Zeng X., Shi Z.‐W., Yu J.‐J., Wang L.‐F., Luo Y.‐Y., Jin S.‐M., Zhang L.‐Y., Tan W., Shi P.‐M., Yu H., Zhang C.‐Q., and Xie W.‐F. (2021) Sarcopenia as a prognostic predictor of liver cirrhosis: a multicentre study in China, Journal of Cachexia, Sarcopenia and Muscle, 12, 1948–1958, 10.1002/jcsm.12797.
Xin Zeng, Zhi‐Wen Shi, Jia‐Jun Yu, and Li‐Fen Wang contributed equally to this work.
Contributor Information
Hong Yu, Email: yuhongphd@163.com.
Chun‐Qing Zhang, Email: 13583188661@163.com.
Wei‐Fen Xie, Email: weifenxie@medmail.com.cn.
References
- 1. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749–1761. [DOI] [PubMed] [Google Scholar]
- 2. Li M, Wang ZQ, Zhang L, Zheng H, Liu DW, Zhou MG. Burden of cirrhosis and other chronic liver diseases caused by specific etiologies in China, 1990‐2016: findings from the Global Burden of Disease Study 2016. Biomed Environ Sci 2020;33:1–10. [DOI] [PubMed] [Google Scholar]
- 3. Zhang L, Fan ZF, Liu DW, Zhou MG, Wang ZQ, Li M. Trend analysis on the disease burden related to cirrhosis and other chronic liver diseases caused by hepatitis B, in China, from 1990 to 2016. Zhonghua Liu Xing Bing Xue Za Zhi 2020;41:173–177. [DOI] [PubMed] [Google Scholar]
- 4. Lautz HU, Selberg O, Körber J, Bürger M, Müller MJ. Protein‐calorie malnutrition in liver cirrhosis. Clin Investig 1992;70:478–486. [DOI] [PubMed] [Google Scholar]
- 5. Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl 2012;18:1209–1216. [DOI] [PubMed] [Google Scholar]
- 6. Beaudart C, McCloskey E, Bruyère O, Cesari M, Rolland Y, Rizzoli R, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr 2016;16:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schweitzer L, Geisler C, Pourhassan M, Braun W, Glüer CC, Bosy‐Westphal A, et al. What is the Clin best reference site for a single MRI slice to assess whole‐body skeletal muscle and adipose tissue volumes in healthy adults? Am J Clin Nutr 2015;102:58–65. [DOI] [PubMed] [Google Scholar]
- 8. Yoo T, Lo WD, Evans DC. Computed tomography measured psoas density predicts outcomes in trauma. Surgery 2017;162:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghufran A. Nutrition in chronic liver disease: a point‐of‐care review. Nutr Clin Pract 2020;35:211–217. [DOI] [PubMed] [Google Scholar]
- 10. Bunchorntavakul C, Reddy KR. Review article: malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment Pharmacol Ther 2020;51:64–77. [DOI] [PubMed] [Google Scholar]
- 11. Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 2016;46:951–963. [DOI] [PubMed] [Google Scholar]
- 12. Ferreira LG, Anastácio LR, Lima AS, Correia MI. Assessment of nutritional status of patients waiting for liver transplantation. Clin Transplant 2011;25:248–254. [DOI] [PubMed] [Google Scholar]
- 13. Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis 2012;16:95–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rush EC, Freitas I, Plank LD. Body size, body composition and fat distribution: comparative analysis of European, Maori, Pacific Island and Asian Indian adults. Br J Nutr 2009;102:632–641. [DOI] [PubMed] [Google Scholar]
- 15. Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 16. Van Rijssen LB, van Huijgevoort NC, Coelen RJ, Tol JA, Haverkort EB, Nio CY, et al. Skeletal muscle quality is associated with worse survival after pancreatoduodenectomy for periampullary, nonpancreatic cancer. Ann Surg Oncol 2017;24:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park H, Oh DY, Kim TY, Lee KH, Han SW, Im SA, et al. Skeletal muscle depletion to predict survival of patients with advanced biliary tract cancer undergoing palliative chemotherapy. Oncotarget 2017;8:79441–79452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vasques J, Guerreiro CS, Sousa J, Pinto M, Cortez‐Pinto H. Nutritional support in cirrhotic patients with sarcopenia. Clin Nutr ESPEN 2019;33:12–17. [DOI] [PubMed] [Google Scholar]
- 19. Ebadi M, Bhanji RA, Mazurak VC, Montano‐Loza AJ. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol 2019;54:845–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roongpisuthipong C, Sobhonslidsuk A, Nantiruj K, Songchitsomboon S. Nutritional assessment in various stages of liver cirrhosis. Nutrition 2001;17:761–765. [DOI] [PubMed] [Google Scholar]
- 21. Fukui A, Kawabe N, Hashimoto S, Kamei H, Yoshioka K. Skeletal muscle mass depletion in patients with hepatitis C virus infection. Eur J Gastroenterol Hepatol 2019;31:59–66. [DOI] [PubMed] [Google Scholar]
- 22. van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, IJzermans JNM. Systematic review and meta‐analysis of the impact of computed tomography‐assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant 2016;16:2277–2292. [DOI] [PubMed] [Google Scholar]
- 23. Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol 2016;65:1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tandon P, Raman M, Mourtzakis M, Merli M. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology 2017;65:1044–1057. [DOI] [PubMed] [Google Scholar]
- 25. Kalafateli M, Mantzoukis K, Choi Yau Y, Mohammad AO, Arora S, Rodrigues S, et al. Malnutrition and sarcopenia predict post‐liver transplantation outcomes independently of the model for end‐stage liver disease score. J Cachexia Sarcopenia Muscle 2017;8:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kang SH, Jeong WK, Baik SK, Cha SH, Kim MY. Impact of sarcopenia on prognostic value of cirrhosis: going beyond the hepatic venous pressure gradient and MELD score. J Cachexia Sarcopenia Muscle 2018;9:860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Vugt JLA, Alferink LJM, Buettner S, Gaspersz MP, Bot D, Darwish Murad S, et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol 2018;68:707–714. [DOI] [PubMed] [Google Scholar]
- 28. Nishikawa H, Enomoto H, Ishii A, Iwata Y, Miyamoto Y, Ishii N, et al. Comparison of prognostic impact between the Child‐Pugh score and skeletal muscle mass for patients with liver cirrhosis. Nutrients 2017;9:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nishikawa H, Enomoto H, Yoh K, Iwata Y, Sakai Y, Kishino K, et al. Combined albumin‐bilirubin grade and skeletal muscle mass as a predictor in liver cirrhosis. J Clin Med 2019;8:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate analysis and multiple linear regression analysis of the impact factors of L3‐SMI based on the general Chinese population in Cohort 1.
Table S2. Distribution of L3‐SMI according to age.
Table S3. Characteristics of population younger than 60 years in Cohort 1.
Table S4. Univariate analysis and multiple linear regression analysis of the impact factors of L3‐SMI based on cirrhotic patients in Cohort 2.
Table S5. Univariate analysis and multiple linear regression analysis of the impact factors of L3‐SMI based on cirrhotic patients with HBV in Cohort 2.
Table S6. Univariate analysis and multiple linear regression analysis of the impact factors of L3‐SMI based on non‐HBV cirrhotic patients in Cohort 2.
Table S7. Baseline characteristics is compared between sarcopenia group and non‐sarcopenia group in the cirrhotic patients with HBV.
Table S8. Baseline characteristics is compared between sarcopenia group and non‐sarcopenia group in the Non‐HBV cirrhotic patients.
Figure S1. Kaplan–Meier estimates of overall survival, according to Cohort 2.
A. Kaplan–Meier estimates of overall survival in patients with MELD score ≤14 compared with that in subjects with MELD score>14. B. Kaplan–Meier estimates of overall survival in patients with distinct Child‐Pugh class.
Figure S2. The time‐dependent ROC curves of L3‐SMI, MELD and Child‐Pugh scores for prognosis prediction in patients with liver cirrhosis, according to Cohort 2.


