Abstract
Background
Nutritional impairment is common in cancer patients and is associated with poor outcomes. Only few studies focused on cachexia. We assessed the prevalence of cachexia in older cancer patients, identified associated risk factors, and evaluated its impact on 6 month overall mortality.
Methods
A French nationwide cross‐sectional survey (performed in 55 geriatric oncology clinics) of older cancer patients aged ≥70 referred for geriatric assessment prior to treatment choice and initiation. Demographic, clinical, and nutritional data were collected. The first outcome was cachexia, defined as loss of more than 5% of bodyweight over the previous 6 months, or a body mass index below 20 kg/m2 with weight loss of more than 2%, or sarcopenia (an impaired Strength, Assistance with walking, Rise from chair, Climb stairs and Falls score) with weight loss of more than 2%. The second outcome was 6 month overall mortality.
Results
Of the 1030 patients included in the analysis [median age (interquartile range): 83 (79–87); males: 48%; metastatic cancer: 42%; main cancer sites: digestive tract (29%) and breast (16%)], 534 [52% (95% confidence interval: 49–55%)] had cachexia. In the multivariate analysis, patients with breast (P < 0.001), gynaecologic (P < 0.001), urinary (P < 0.001), skin (P < 0.001), and haematological cancers (P = 0.006) were less likely to have cachexia than patients with colorectal cancer. Patients with upper gastrointestinal tract cancers (including liver and pancreatic cancers; P = 0.052), with previous surgery for cancer (P = 0.001), with metastases (P = 0.047), poor performance status (≥2; P < 0.001), low food intake (P < 0.001), unfeasible timed up‐and‐go test (P = 0.002), cognitive disorders (P = 0.03) or risk of depression (P = 0.005), were more likely to have cachexia. At 6 months, 194 (20.5%) deaths were observed. Cachexia was associated with 6 month mortality risk (adjusted hazard ratio = 1.49; 95% confidence interval: 1.05–2.11) independently of age, in/outpatient status, cancer site, metastatic status, cancer treatment, dependency, cognition, and number of daily medications.
Conclusions
More than half of older patients with cancer managed in geriatric oncology clinics had cachexia. The factors associated with cachexia were upper gastrointestinal tract cancer, metastases, poor performance status, poor mobility, previous surgery for cancer, cognitive disorders, a risk of depression, and low food intake. Cachexia was independently associated with 6 month mortality.
Keywords: Cancer, Elderly, Cachexia, Malnutrition, Screening, Prognostic value, Nutritional support
Introduction
Cancer is one of the leading causes of morbidity and mortality worldwide. Age is a major risk factor for cancer; indeed, more than half of all cancer patients are 70 or more years old. 1 , 2 The ongoing increase in life expectancy means that this proportion will increase. It now appears to be essential to take account of age‐related features when managing patients with cancer. 3 , 4 Indeed, the prevalence of malnutrition, mood disorders, and functional and cognitive impairments increase with age, and are associated with poor outcomes in older patients with cancer. 3 Consequently, the International Society of Geriatric Oncology recommends performing a geriatric assessment as a comprehensive health appraisal for guiding targeted geriatric interventions and selecting appropriate cancer treatments. 4 In France, the National Cancer Institute created geriatric oncology coordination units in order to implement the routine geriatric assessment of older patients with cancer. 5
Nutritional alterations are common in patients with cancer, and especially so in older people. 6 , 7 The term ‘cachexia’ is now preferred to ‘malnutrition’ for cancer patients because of the complexity of catabolic pathway activation and the prominence of muscle loss (sarcopenia, in the presence or absence of fat mass loss) in the pathophysiology of cancer‐related nutritional alterations. 8 , 9
Cachexia is currently defined as either (i) loss of more than 5% of the patient's stable body weight over the previous 6 months, (ii) a body mass index (BMI) below 20 kg/m2 and weight loss of more than 2%, or (iii) low muscle mass consistent with sarcopenia and weight loss of more than 2%. 9 Nutritional impairment is associated with a worse response to treatment, greater treatment toxicity, early withdrawal or discontinuation, 10 , 11 , 12 , 13 , 14 more frequent infections, 15 , 16 prolonged hospitalization, 17 and lower survival rates. 18 , 19 , 20 Low muscle mass (assessed using computerized tomography scan measurements of muscle mass) has also been associated with poor survival and treatment response in older patients with cancer. 21 , 22 , 23 , 24 However, the definition of sarcopenia involves two aspects: muscle quantity (the muscle mass estimated on imaging) and muscle quality (i.e. strength and functionality). The recent revised European consensus on the definition and diagnosis of sarcopenia 25 considers that muscle quality is a better predictor of poor outcomes than muscle mass. This expert working group recommended the use of the Strength, Assistance with walking, Rise from chair, Climb stairs and Falls (SARC‐F) questionnaire for screening sarcopenia because of the instrument's low cost, convenience, and quickness of administration. 26 The SARC‐F is a five‐item self‐questionnaire on the patient's limitations in strength, walking, rising from a chair, climbing stairs, and the number of falls in the past year. 27 It is a validated screening tool of sarcopenia and has been shown to predict adverse outcomes in large cohorts of older patients. 28 The SARC‐F score is also a highly specific screening tool of low muscle strength. 29
Over the last 10 years, several observational studies have evaluated malnutrition or isolated sarcopenia in older patients with cancer. 20 , 22 , 23 , 24 , 30 However, cachexia per se (i.e. a combination of weight loss, low BMI, and low muscle mass) has been overlooked. Most of these studies were performed in a single centre, had a small sample size, and used different nutritional assessment tools. The frequencies of malnutrition and low muscle mass (measured on a CT scan) in these studies ranged from 3% to 83% and from 38% to 62%, respectively, 20 , 22 , 23 , 24 , 30 depending on the definition used, the tumour site, and the cancer stage.
No prospective, multicentre, studies of cachexia have focused on older patients with cancer. Only two single‐centre study of small number of patients reported a cachexia prevalence of 62% (n = 100) 31 and 39% (n = 33) 32 among older patients with cancer. In one study using structural equation modelling approach, where cachexia was defined as a latent variable, it was associated with poor overall survival. 33
Thus, cachexia in older patients with cancer is a public health issue that need to be anticipated, diagnosed rapidly, and managed effectively. However, this condition is not often considered in clinical practice, and specific risk factors have yet to be well characterized. 30
First objectives were to evaluate the prevalence of cachexia prior to treatment initiation in cancer patients aged 70 or over, identify any associated risk factors with cachexia, and describe the implementation of nutritional support in this population. Second objective was to study the association between cachexia and 6 month overall mortality.
Materials and methods
Study design and patients
We first performed a nationwide cross‐sectional survey of French hospitals participating in the national network of geriatric oncology clinics (55 centres). The survey was conducted over a 6 week period (from 1 November to 14 December) in 2017 and again in 2018. The eligibility criteria were as follows: age ≥ 70, histologically confirmed cancer, referral to the geriatric oncology clinic by an oncologist, a radiation therapist or a surgeon for geriatric assessment prior to the choice and implementation of an anticancer strategy or a new therapeutic modality (chemotherapy, targeted therapy, hormone therapy, immunotherapy, radiotherapy, or surgery). Patients were not included if they were unable to understand information about the study and/or give their consent to participation. For the present analysis, only patients with complete weight loss and SARC‐F data were included. Additionally, patients from 48 centres participated in the longitudinal study, for whom survival data were recorded at 6 months of follow‐up.
All participants provided informed consent prior to inclusion in the study. The study was approved by the local independent ethics committee (CCP Ile de France XI, Paris, France; reference: IDRCB 2017‐A01397‐46, 17035). The study was registered at ClinicalTrials.gov (NCT03390816) and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
For the purposes of the present analysis, we included patients with available data on the baseline BMI, weight loss within the previous 6 months, and SARC‐F score.
Data collection
Demographic data (age, gender, family situation, and in/out patients), cancer‐related data [site, metastasis status (distant localization), and any cancer treatment in the previous 12 months], and Eastern Cooperative Oncology Group Performance Status (ECOG‐PS) were recorded at the first geriatric oncology consultation, as part of the geriatric assessment.
Geriatric data in the following seven domains were assessed during a geriatric assessment, using a standardized case report form: functional status, mobility, cognitive status, mood, comorbidities, polypharmacy, and nutritional status. Dependency was defined as loss of self‐sufficiency for one or more of six activities of daily living (ADL). 34 Mobility was assessed with the walking timed ‘up‐and‐go’ (TUG) test. 35 Impaired mobility was defined as a TUG test completion time of more than 20 s or inability to perform the test. Sarcopenia was assessed using the five‐item SARC‐F. Patients with an abnormal total score of 4 or more were classified as having sarcopenia. 27 Cognitive impairment was defined as a Mini Mental State Examination score below 24 out of 30 36 or the presence of a physician‐diagnosed cognitive disorder. Risk of depressed mood was defined as a mini‐Geriatric Depression Scale (GDS) score of 1 or more out of 4. 37 Comorbidities were assessed using the updated Charlson comorbidity index, 38 and cancer and metastatic statuses were coded accordingly. For each patient, the number of daily prescribed drugs was recorded. The following comorbidities were recorded: depression during the previous 12 months, chronic heart failure, dementia, chronic lung disease, rheumatic disease, liver disease, kidney failure (creatinine clearance < 30 mL/min), diabetes with chronic complications, and hemiplegia.
Nutritional status was assessed using the bodyweight at baseline, usual bodyweight before cancer disease, loss of weight over the previous 6 months, BMI, upper arm circumference, good dental health (yes/no), food intake (≤2/3 or >2/3 of last meal), and dysgueusia (yes/no). Two laboratory parameters [serum C‐reactive protein (CRP) and albumin levels] were recorded. High serum CRP was defined as ≥10 mg/L and low albumin, as <35 g/L.
The final planned treatment decision could consist on curative or palliative treatment or supportive care alone.
The first outcome was cachexia, defined as fulfilment of one or more of the following criteria: (i) loss of more than 5% of the patient's stable bodyweight over the previous 6 months, (ii) a BMI below 20 kg/m2 and weight loss of more than 2%, or (iii) sarcopenia (abnormal SARC‐F score ≥ 4/10), and weight loss of more than 2%. 9 The second outcome was 6 month mortality, defined as the time from evaluation to death within 6 months or to the last follow‐up for censored patients. Vital status was determined by telephoning the patients or their family or by extracting data from medical records.
The potential risk factors for cachexia included the above‐mentioned sociodemographic and oncologic parameters, five validated geriatric tests (ADL, TUG, Mini Mental State Examination, mini‐GDS, and the updated Charlson comorbidity index), cognitive disorders, depression, polypharmacy, and nutritional parameters (dental health, dysgueusia, and food intake).
Statistical analyses
Prevalence of cachexia was estimated along with its 95% confidence interval (CI) in the total population and by cancer site. The patient characteristics were presented for the total study population and according to cachexia. Quantitative variables were described as the median (interquartile range) and qualitative variables were described as the number (percentage). A univariate analysis was performed to assess associations between cachexia and the various factors studied; the results were expressed as a crude odds ratio [95% CI]. Factors associated (P < 0.2) with cachexia in the univariate analysis were included in a multivariable logistic regression model. A stepwise backward procedure was used to sequentially remove factors (based on P < 0.05) and identify those independently associated with cachexia. Interaction terms were tested, and potential confounding factors were investigated.
In a sensitivity analysis, missing values of explanatory variables were imputed by applying a 10‐fold multiple imputations with chained equations and combining the estimates according to Rubin's rules.
Six‐month overall survival was calculated by the Kaplan–Meier method and compared across cachectic and non‐cachectic groups by the log‐rank test. Univariate and multivariate Cox proportional hazards analyses were conducted after 6 months of follow‐up. Crude and adjusted hazard ratios were estimated along with their 95% CIs. Known prognostic factors for mortality were studied. 18 , 19 , 21 Recursive partitioning analysis 39 was carried out to determine optimal threshold for continuous variables (i.e. age ≥ 85 was the optimal value with the greatest difference in outcome). Discriminative performance of the final model was evaluated by Harrell's C‐index. The proportional hazard assumption was tested using Schoenfeld residual plots and the Grambsch–Therneau test, and it was retained.
All analyses were performed using Stata software (Version 15, StataCorp, USA). The threshold for statistical significance was set to P < 0.05. All tests were two‐tailed.
Results
Characteristics of the study population
Among 1241 patients assessed for eligibility, 1030 patients from 55 participating geriatric oncology clinics were included in the cross‐sectional study analysis (Figure 1). When compared with the population not included in this analysis (n = 211), the analysed population featured a lower proportion of hospitalized patients (34% vs. 47%, respectively; P < 0.001) and of patients with metastases (40% vs. 49%, respectively; P = 0.03). There were no other significant differences in demographic, clinical, and geriatric characteristics between included and non‐included patient populations.
Figure 1.
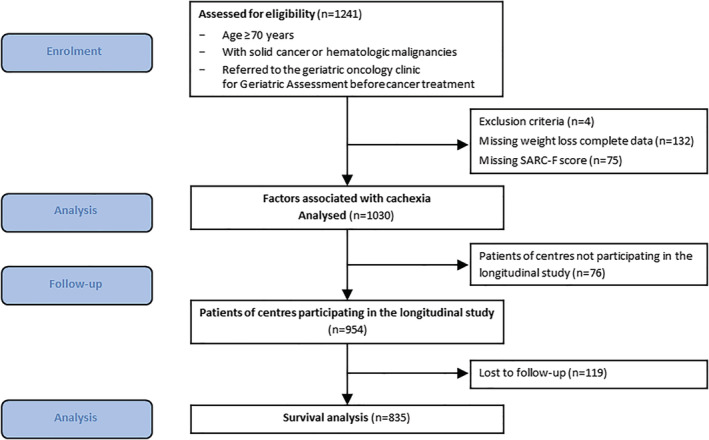
Study flow chart.
Of the 954 patients from the participating centres in the longitudinal study, 835 patients had follow‐up data available and were used for the survival analysis (Figure 1). When compared with the population not included in this analysis (n = 119), the analysed population featured a lower proportion of hospitalized patients (23% vs. 36%, respectively; P = 0.008) and of patients with supportive care alone (8% vs. 21%; P < 0.001); analysed patients were less likely to present cognitive disorders (36% vs. 52%, P = 0.004) and depression risk (42% vs. 55%; P = 0.02) There were no other significant differences in demographic and clinical characteristics between included and non‐included patients.
The study population's general and nutritional characteristics are summarized respectively in Tables 1 and 2. The median (interquartile range) age was 83 [79–87] years, 52% of the patients were female, and 42% had metastases. The main cancer sites were the digestive tract (29%) and the breast (16%). The most frequent comorbidities were rheumatologic disease (20.2%), renal disease (18.2%), chronic lung disease (14.4%), diabetes (13.1%), and congestive heart failure (12.9%). Thirteen per cent of patients were underweight (BMI < 20 kg/m2), 47% had lost more than 5% of their bodyweight in the previous 6 months, and 38% of patients had sarcopenia (i.e. an abnormal SARC‐F score).
Table 1.
Demographic and clinical characteristics (n = 1030)
| Features | Total (N = 1030) | No cachexia a (N = 496) | With cachexia a (N = 534) | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Age in years, median (IQR) | 83 | 79–87 | 83 | 79–87 | 83 | 79–87 |
| Gender, female | 537 | 52.1 | 282 | 56.9 | 255 | 47.8 |
| In/outpatient status | ||||||
| Hospitalization | 355 | 34.6 | 132 | 26.7 | 223 | 41.8 |
| Consultation | 672 | 65.4 | 362 | 73.3 | 310 | 58.2 |
| Cancer type | ||||||
| Breast | 167 | 16.2 | 118 | 23.8 | 49 | 9.2 |
| Colorectal | 157 | 15.2 | 59 | 11.9 | 98 | 18.4 |
| Upper gastrointestinal tract b | 144 | 14.0 | 34 | 6.9 | 110 | 20.6 |
| Lung | 105 | 10.2 | 38 | 7.7 | 67 | 12.6 |
| Gynaecological | 97 | 9.4 | 57 | 11.5 | 40 | 7.5 |
| Urinary tract | 91 | 8.8 | 51 | 10.3 | 40 | 7.5 |
| Prostate | 81 | 7.9 | 40 | 8.1 | 41 | 7.7 |
| Haematological c | 55 | 5.3 | 27 | 5.4 | 28 | 5.2 |
| Skin | 44 | 4.3 | 27 | 5.4 | 17 | 3.2 |
| Head and neck | 39 | 3.8 | 18 | 3.6 | 21 | 3.9 |
| Other d | 50 | 4.9 | 27 | 5.4 | 23 | 4.3 |
| Metastasis e (missing data n = 8) | 407 | 42.1 | 166 | 35.7 | 241 | 48.0 |
| Current therapy (missing data n = 18) | ||||||
| Surgery | 302 | 29.8 | 185 | 37.4 | 117 | 22.6 |
| Chemotherapy | 492 | 48.6 | 217 | 43.8 | 275 | 53.2 |
| Radiotherapy | 245 | 24.2 | 130 | 26.3 | 115 | 22.2 |
| Targeted therapy | 75 | 7.4 | 39 | 7.9 | 36 | 7.0 |
| Hormone therapy | 128 | 12.6 | 81 | 16.4 | 47 | 9.1 |
| Immunotherapy | 38 | 3.8 | 24 | 4.8 | 14 | 2.7 |
| Supportive care | 98 | 9.7 | 27 | 5.5 | 71 | 13.7 |
| Prior therapy f (missing data n = 1) | 318 | 30.9 | 143 | 28.8 | 175 | 32.8 |
| Surgery | 173 | 54.4 | 72 | 50.4 | 101 | 57.7 |
| Chemotherapy | 89 | 27.9 | 32 | 22.4 | 57 | 32.6 |
| Radiotherapy | 48 | 15.1 | 18 | 12.6 | 30 | 17.1 |
| Targeted therapy | 15 | 4.7 | 3 | 2.1 | 12 | 6.9 |
| Hormone therapy | 64 | 20.1 | 34 | 23.8 | 30 | 17.4 |
| Immunotherapy | 7 | 2.2 | 3 | 2.1 | 4 | 2.3 |
| Poor ECOG‐PS (≥2) (missing data n = 29) | 440 | 44 | 139 | 29 | 301 | 57.7 |
| Dependency (ADL ≤ 5/6) (missing data n = 8) | 343 | 33.6 | 115 | 23.3 | 228 | 43.1 |
| Timed up and go test (missing data n = 86) | ||||||
| ≤20 s | 587 | 62.2 | 330 | 71.3 | 257 | 53.4 |
| >20 s | 250 | 26.5 | 114 | 24.6 | 136 | 28.3 |
| Unable to perform the test | 107 | 11.3 | 19 | 4.1 | 88 | 18.3 |
| Cognitive impairment g (missing data n = 84) | 361 | 38.2 | 145 | 30.9 | 216 | 45.3 |
| Impaired mini‐GDS (risk of depression: ≥1/4) (missing data n = 94) | 400 | 42.7 | 146 | 32.6 | 254 | 52 |
| Updated Charlson comorbidity index, median (IQR) (missing data n = 37) | 5 | 3–7 | 4 | 2–6 | 6 | 3–7 |
| Number of daily prescribed drugs, median (IQR) (missing data n = 8) | 6 | 3–9 | 6 | 3–8 | 6 | 4–9 |
ADL, activities of daily living; ECOG‐PS, Eastern Cooperative Oncology Group Performance Status; GDS, Geriatric Depression Scale; MMSE, Mini Mental State Examination.
Cachexia was defined as the presence of one or more of the following criteria: weight loss >5% over the previous 6 months; or BMI < 20 kg/m2 and weight loss >2%; or abnormal SARC‐F score (≥4/10) and weight loss >2%.
Oesophagus, stomach, liver, and pancreas
Haematological malignancies included Hodgkin lymphoma (n = 2), non‐Hodgkin lymphoma (n = 19), myelodysplastic syndromes (n = 9), acute leukaemia (n = 5), myeloma (n = 2), chronic lymphocytic leukaemia (n = 2) and Waldenström's macroglobulinaemia (n = 1).
Sarcoma (n = 17), unknown origin (n = 11), thyroid (n = 4), other (n = 18).
Excluding haematological malignancies.
Cancer treatment in previous 12 months.
Impaired MMSE score (<24) or physician‐diagnosed cognitive disorder.
Table 2.
Nutritional characteristics (N = 1030)
| Features | Total (N = 1030) | No cachexia a (N = 496) | With cachexia a (N = 534) | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| BMI (kg/m2) | ||||||
| <20 | 132 | 12.8 | 20 | 4.0 | 112 | 21.0 |
| 20–24.9 | 429 | 41.7 | 179 | 36.1 | 250 | 46.9 |
| 25–29.9 | 307 | 29.8 | 197 | 39.7 | 110 | 20.6 |
| ≥30 | 161 | 15.6 | 100 | 20.2 | 61 | 11.4 |
| Weight loss within previous 6 months | ||||||
| Minimal (≤5%) | 549 | 53.3 | 496 | 100.0 | 53 | 9.9 |
| Moderate (>5% to <10%) | 213 | 20.7 | 0 | 0.0 | 213 | 39.9 |
| Severe (≥10%) | 268 | 26.0 | 0 | 0.0 | 268 | 50.2 |
| Sarcopenia, SARC‐F score ≥ 4 | 389 | 37.8 | 112 | 22.6 | 277 | 51.9 |
| Upper arm circumference (missing data n = 42) | ||||||
| <21 cm | 66 | 6.7 | 11 | 2.3 | 55 | 10.8 |
| 21–22 cm | 120 | 12.1 | 41 | 8.5 | 79 | 15.6 |
| >22 cm | 802 | 81.2 | 429 | 89.2 | 373 | 73.6 |
| Poor dental health status (missing data n = 17) | 221 | 21.8 | 91 | 18.6 | 130 | 24.8 |
| Low food intake (≤2/3 of last meal) (missing data n = 17) | 236 | 23.3 | 46 | 9.4 | 190 | 36.3 |
| Dysgueusia (missing data n = 16) | 127 | 12.5 | 44 | 9.0 | 83 | 15.8 |
| Low serum albumin (<35 g/L) (missing n = 325) | 280 | 39.7 | 85 | 26.6 | 195 | 50.6 |
| High serum CRP (≥10 mg/L) (missing data n = 391) | 338 | 52.9 | 109 | 38.9 | 229 | 63.8 |
| Nutritional support (missing data n = 20) | 393 | 38.9 | 98 | 20.3 | 295 | 55.9 |
| Fortified diet | 204 | 51.9 | 53 | 54.1 | 151 | 51.2 |
| Oral nutritional supplements | 323 | 82.2 | 77 | 78.6 | 246 | 83.4 |
| Enteral nutrition | 18 | 4.6 | 0 | 0.0 | 18 | 6.1 |
| Parenteral nutrition | 17 | 4.3 | 3 | 3.1 | 14 | 4.7 |
BMI, body mass index; CRP, C‐reactive protein; SARC‐F, Strength, Assistance with walking, Rise from chair, Climb stairs and Falls score.
Cachexia was defined as the presence of one or more of the following criteria: weight loss >5% over the previous 6 months; or BMI < 20 kg/m2 and weight loss >2%; or abnormal SARC‐F score (≥4/10) and weight loss >2%.
Prevalence of cachexia
Cachexia was present in 534 patients [51.8% (95% CI = 48.7–54.9%)]. The prevalence of cachexia was 62.8% among hospitalized patients (n = 223), 46.1% among outpatients (n = 310), 68.4% among patients with poor ECOG‐PS (≥2) (n = 301), 39.2% among patients with good PS (0–1) (n = 220), 46.9% among patients with localized cancer (n = 284), and 59.1% among patients with metastatic cancer (n = 246).
The prevalence of cachexia by tumour site was as follows: 76.4% (n = 110) in patients with upper gastrointestinal tract cancers (liver, pancreatic, oesophageal, and gastric cancers), 63.8% (n = 67) in lung cancer, 62.4% (n = 98) in colorectal cancer, 53.9% (n = 21) in head and neck cancer, 50.9% (n = 28) in haematological cancers, 50.6% (n = 41) in prostate cancer, 44% (n = 40) in urinary cancers, 41.2% (n = 40) in gynaecological cancers (uterus, ovary, cervical, vulval, and vaginal), and 29.3% (n = 49) in breast cancer (Figure 2).
Figure 2.
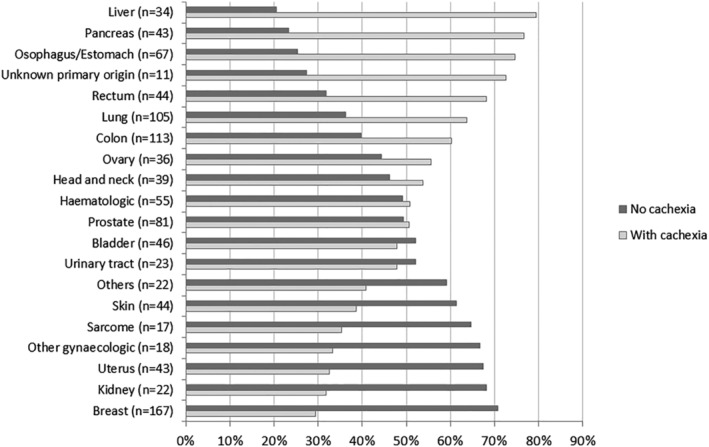
Prevalence of cachexia by cancer site.
Factors associated with cachexia
In a univariate analysis (Table 3), the following patients were more likely to have cachexia: hospitalized patients, patients with upper gastrointestinal tract cancers (compared with patients with colorectal cancer) and those with metastases, poor ECOG‐PS (≥2), dependency, impaired mobility, previous cancer treatment, poor dental health, dysgueusia, low food intake, cognitive impairment, risk of depression, or a high updated Charlson comorbidity index. Conversely, female patients and patients with urinary tract, breast, gynaecological, skin, or other cancer types (compared with patients with colorectal cancer) were less likely to have cachexia.
Table 3.
Univariate and multivariate analyses of factors associated with cachexia
| Features | Univariate analysis (N = 1030) | Multivariate analysis (N = 780) | ||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | P value | OR | (95% CI) | P value | |
| Age | 1.00 | (0.98–1.02) | 0.822 | ― | ||
| Gender, ref. female | 0.69 | (0.54–0.89) | 0.004 | ― | ||
| Family situation, ref. single | 1.00 | 0.263 | ― | |||
| Married/cohabiting | 1.57 | (0.95–2.58) | 0.075 | ― | ||
| Divorced | 1.24 | (0.62–2.46) | 0.544 | ― | ||
| Widowed | 1.35 | (0.81–2.25) | 0.246 | ― | ||
| Hospitalized patients vs. outpatients | 1.97 | (1.51–2.57) | <0.0001 | ― | ||
| Cancer site, ref. colorectal | 1.00 | <0.0001 | 1.00 | <0.0001 | ||
| Breast | 0.25 | (0.16–0.40) | <0.0001 | 0.22 | (0.12–0.39) | <0.0001 |
| Upper gastrointestinal tract a | 1.95 | (1.18–3.22) | 0.009 | 1.94 | (0.99–3.78) | 0.052 |
| Lung | 1.06 | (0.64–1.77) | 0.820 | 0.99 | (0.51–1.93) | 0.983 |
| Gynaecological | 0.42 | (0.25–0.71) | 0.001 | 0.27 | (0.13–0.53) | <0.0001 |
| Urinary tract | 0.47 | (0.28–0.80) | 0.005 | 0.29 | (0.14–0.58) | <0.0001 |
| Prostate | 0.62 | (0.36–1.07) | 0.081 | 0.55 | (0.27–1.14) | 0.106 |
| Haematological | 0.62 | (0.34–1.16) | 0.136 | 0.31 | (0.13–0.71) | 0.006 |
| Skin | 0.38 | (0.19–0.75) | 0.006 | 0.21 | (0.09–0.53) | <0.0001 |
| Head and neck | 0.70 | (0.35–1.43) | 0.328 | 0.56 | (0.23–1.37) | 0.207 |
| Other b | 0.51 | (0.27–0.98) | 0.042 | 0.18 | (0.07–0.41) | <0.0001 |
| Metastasis (yes vs. no) | 1.66 | (1.29–2.13) | <0.0001 | 1.44 | (1.01–2.05) | 0.047 |
| Cancer treatment in previous 12 months | 1.00 | 0.165 | ― | |||
| Surgery | 1.37 | (0.99–1.91) | 0.060 | 2.12 | (1.33–3.37) | 0.001 |
| Chemotherapy | 1.73 | (1.10–2.72) | 0.017 | ― | ||
| Radiotherapy | 1.58 | (0.87–2.87) | 0.133 | ― | ||
| Targeted therapy | 3.78 | (1.06–13.5) | 0.040 | ― | ||
| Hormone therapy | 0.81 | (0.49–1.34) | 0.412 | ― | ||
| Immunotherapy | 1.24 | (0.28–5.57) | 0.779 | |||
| ECOG‐PS (≥2 vs. 0–1) | 3.33 | (2.56–4.33) | <0.0001 | 2.57 | (1.70–3.88) | <0.0001 |
| TUG, ref. ≤20 s | 1.00 | <0.0001 | 1.00 | 0.007 | ||
| >20 s | 1.53 | (1.14–2.06) | 0.005 | 1.02 | (0.66–1.57) | 0.931 |
| Unable to perform the test | 5.95 | (3.53–10.0) | <0.0001 | 3.19 | (1.54–6.61) | 0.002 |
| Dependency (ADL ≤ 5/6) | 2.49 | (1.90–3.26) | <0.0001 | ― | ||
| Poor dental health | 1.44 | (1.07–1.95) | 0.017 | ― | ||
| Dysgueusia | 1.91 | (1.29–2.81) | 0.001 | ― | ||
| Food intake (≤2/3 vs. >2/3 of last meal) | 5.48 | (3.85–7.79) | <0.0001 | 3.67 | (2.28–5.91) | <0.0001 |
| Cognitive impairment | 1.85 | (1.42–2.41) | <0.0001 | 1.50 | (1.04–2.16) | 0.030 |
| Risk of depression (mini‐GDS ≥ 1/4) | 2.25 | (1.72–2.93) | <0.0001 | 1.66 | (1.17–2.35) | 0.005 |
| Updated Charlson comorbidity index | 1.13 | (1.07–1.18) | <0.0001 | ― | ||
| Number of daily prescribed medications | 1.02 | (0.99–1.06) | 0.174 | ― | ||
ADL, activities of daily living; ECOG‐PS, Eastern Cooperative Oncology Group Performance Status; GDS, Geriatric Depression Scale; MMSE, Mini Mental State Examination; OR, odds ratio; TUG, timed up and go test.
Oesophagus, stomach, liver, and pancreas.
Sarcoma (n = 17), unknown origin (n = 11), thyroid (n = 4), and other (n = 18).
In the multivariate analysis (Table 3), factors independently associated with higher risk of cachexia were as follows: metastases, previous cancer surgery, poor ECOG‐PS, unfeasible TUG test, low food intake, cognitive impairment, and risk of depression. Association between upper gastrointestinal tract cancer and cachexia was borderline significant: P = 0.052. In contrast, urinary tract, breast, gynaecological, haematological, skin, and other cancers were independently associated with lower risk of cachexia.
In a sensitivity analysis, when we included serum CRP status in the multivariate analysis (n = 486 patients with complete data), we found that an elevated serum CRP level (≥10 mg/L) was associated with cachexia [adjusted OR (95% CI) = 1.69 (1.01–2.64); P = 0.020].
Multiple imputation for missing data yielded similar results (n = 1030; data not shown).
Nutritional support
Nutritional support was implemented for 393 patients (39% of the total study population). Among patients with cachexia, 55.2% (n = 295/534) received nutritional support: at least one nutritional support modality was implemented for 170 patients (57.6%); this mainly corresponded to oral nutritional supplements (n = 124, 73%). Two nutritional support modalities were implemented in 116 patients (39.3%)—mainly oral nutritional supplements and a fortified diet (n = 107, 92.2%). Nine patients (3%) received enteral or parenteral nutrition in addition to a fortified diet and oral nutritional supplements.
Survival analysis
The median follow‐up time was 6.1 months (range 0.03–30.3). The 6 month overall survival was 74.7% (95% CI 71.5–77.7%).
The 6 month overall survival rate for patients without cachexia was 84.5% (80.3–87.9%) and 66.1% (61.2–70.5%) for patients with cachexia. Kaplan–Meier curves for overall 6 month survival according to cachexia are presented in Figure 3. Patients with and without cachexia showed significant differences in overall survival (log‐rank P < 0.0001).
Figure 3.
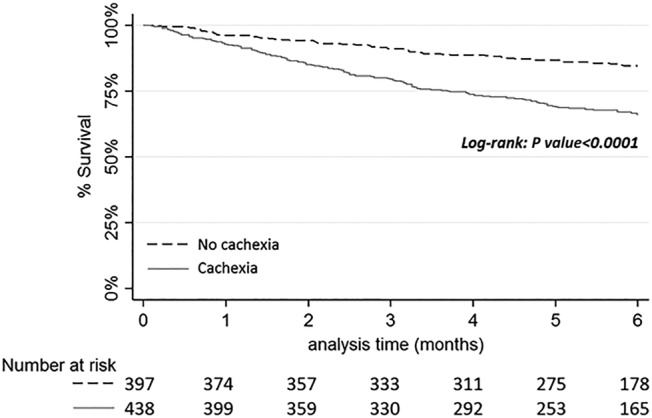
Kaplan–Meier curves of overall 6 month survival according to cachexia.
In the univariate analysis, most variables were significantly associated with 6 month overall survival (Table 4). Briefly, factors associated with greater overall mortality were older age, cachexia, hospitalization at inclusion, metastatic cancer, upper digestive tract, pancreas and liver cancers, lung cancer, haematological malignancies and other cancers (compared with colorectal cancer), palliative treatment and supportive care (compared with curative cancer treatment), poor performance status, impaired mobility, dependency, a greater index of comorbidities, a greater number of prescribed medications, cognitive impairment, and risk of depression.
Table 4.
Univariate and multivariate analysis of factors associated with 6 months overall survival
| Features | Univariate analysis (N = 835) | Multivariate analysis (N = 756) | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | P value | aHR | (95% CI) | P value | |
| Age ≥ 85 years | 1.47 | (1.11–1.95) | 0.008 | 1.39 | (1.01–1.92) | 0.046 |
| Gender, ref. female | 0.85 | (0.64–1.12) | 0.244 | — | ||
| Cachexia | 2.46 | (1.80–3.35) | <0.001 | 1.49 | (1.05–2.11) | 0.024 |
| Nutritional support a | 2.49 | (1.87–3.31) | <0.001 | — | ||
| Cancer site, ref. colorectal | <0.001 | <0.001 | ||||
| Head and neck | 1.52 | (0.63–3.67) | 0.351 | 1.85 | (0.72–4.80) | 0.204 |
| Upper digestive tract/Pancreas and liver | 2.86 | (1.63–5.04) | <0.001 | 2.74 | (1.49–5.02) | 0.001 |
| Prostate | 1.37 | (0.66–2.82) | 0.394 | 0.85 | (0.38–1.88) | 0.680 |
| Urinary tract | 1.57 | (0.79–3.15) | 0.201 | 1.69 | (0.82–3.49) | 0.159 |
| Lung | 3.11 | (1.71–5.64) | <0.001 | 2.14 | (1.12–4.10) | 0.022 |
| Breast | 0.54 | (0.25–1.19) | 0.126 | 0.67 | (0.29–1.54) | 0.342 |
| Gynaecologic | 1.76 | (0.91–3.42) | 0.095 | 2.01 | (0.98–4.13) | 0.058 |
| Hematologic | 2.31 | (1.14–4.69) | 0.02 | 1.50 | (0.68–3.30) | 0.314 |
| Skin | 1.55 | (0.67–3.60) | 0.304 | 1.43 | (0.59–3.44) | 0.425 |
| Other | 4.83 | (2.55–9.17) | <0.001 | 3.39 | (1.65–6.98) | 0.001 |
| Metastasis (Yes vs. No) | 2.19 | (1.64–2.92) | <0.001 | 1.51 | (1.04–2.20) | 0.028 |
| Previous anticancer treatment (<12 months) | 1.06 | (0.79–1.43) | 0.698 | — | ||
| Treatment, ref. curative | ||||||
| Palliative treatment | 3.45 | (2.46–4.83) | <0.001 | 2.27 | (1.52–3.39) | <0.001 |
| Supportive care | 6.00 | (3.77–9.54) | <0.001 | 2.75 | (1.63–4.65) | <0.001 |
| ECOG‐PS (≥2 vs. 0–1) | 2.93 | (2.17–3.95) | <0.001 | — | ||
| TUG, ref. ≤20 s | <0.001 | — | ||||
| >20 s | 1.56 | (1.10–2.21) | 0.012 | |||
| Not able to do the test | 3.35 | (2.28–4.92) | <0.001 | |||
| Impaired ADL (≤5/6) | 2.64 | (1.99–3.50) | <0.001 | 1.63 | (1.17–2.28) | 0.004 |
| Updated Charlson index | 1.20 | (1.14–1.27) | <0.001 | — | ||
| Number of daily prescribed medications | 1.09 | (1.05–1.13) | <0.001 | 1.05 | (1.00–1.09) | 0.051 |
| Hospitalized patients vs. outpatients | 2.26 | (1.71–3.00) | <0.001 | 1.40 | (1.01–1.94) | 0.043 |
| Risk of depression (mini‐GDS ≥ 1/4) | 1.95 | (1.45–2.63) | <0.001 | — | ||
| Cognitive impairment | 2.13 | (1.59–2.87) | <0.001 | 1.70 | (1.23–2.34) | 0.001 |
ADL, activities of daily living; aHR, adjusted hazard ratios; ECOG‐PS, Eastern Cooperative Oncology Group Performance Status; HR, hazard ratios; mini‐GDS, mini‐Geriatric Depression Scale; TUG, timed up and go test.
Multivariate model not adjusted for nutritional support because of collinearity with cachexia.
Nutritional support was strongly correlated with cachexia (Cramer's V = 0.38; P < 0.0001), and they had similar HR [2.49 (95% CI: 1.87–3.31) and 2.46 (1.80–3.35), respectively] (Table 4), suggesting that both variables measure the same clinical dimension. Therefore, nutritional support was not included in the multivariate analysis.
In the multivariate analysis (Table 4), after adjusting for age, inpatient status, anticancer treatment, ADL, number of prescribed medications, cognitive impairment, cancer site, and metastatic status, cachectic patients had a higher risk of 6 month mortality than non‐cachectic patients (adjusted hazard ratio = 1.49; 95% CI: 1.05–2.11). Models had good discrimination (Harrell's C‐index: 0.76). Replacing ADL from the model by ECOG‐PS or TUG—both strongly correlated with ADL (Cramer's V > 0.50), showed similar results for cachexia: adjusted hazard ratio = 1.48; 1.04–2.12; P = 0.031 and 1.56; 1.09–2.23; P = 0.016, respectively.
Discussion
The present prospective multicentre cross‐sectional study is the first to have focused on cachexia in older patients with cancer managed in geriatric oncology clinics. We found that the prevalence of this condition was high (51.8%) in our study population. Furthermore, the prevalence of cachexia depended on the cancer site; higher frequencies were observed in patients with upper gastrointestinal tract cancer. Previous surgery, poor ECOG‐PS (PS ≥ 2), low BMI (<20), low food intake (≤2/3 of last meal), low mobility, a risk of depression (mini‐GDS ≤ 1/4), and impaired cognitive function were independently associated with an elevated risk of cachexia. Overall, 38.9% of the total study population and only 55.9% of the patients with cachectic received nutritional support. Cachexia was significantly associated with 6 month mortality, independently of age, in/outpatient status, cancer site, metastatic status, cancer treatment, functional status, cognition, and number of daily medications.
Cachexia has a key role in the pathogenesis of frailty. 40 These common syndromes in older people partly overlap and are characterized by loss of fat‐free mass, in particular loss of skeletal muscle. In the present study, we adapted an international consensus definition of cachexia. Instead of using the muscle mass measured on CT to identify patient with sarcopenia, we used the SARC‐F score. Even though the SARC‐F was not designed as a diagnostic tool, it has a very high specificity and is relevant and easy to use in routine clinical practice. Furthermore, the SARC‐F reliably is correlated with sarcopenia and low muscle strength. 28 , 29 , 41 The SARC‐F measures muscle quality more than it does quantity, which is a better way of predicting adverse outcomes. 25
Concerning prevalence of cachexia, Dunne et al. 31 reported a similar prevalence (62%) in a cohort of patient with a mean age of 79.9 years (range: 66–95 years). Surprisingly, this study found no statistically significant differences in prevalence of cachexia by cancer type or stage, but probably suffered from a small patient number (n = 100), a heterogeneous cohort (with previously and not previously treated patients) and a monocentric recruitment. Cachexia was not associated with altered GDS, fatigue, performance status, or falls; only an instrumental ADL score (≥1 impairment) was significantly associated with cachexia in Dunne et al.'s univariate analysis. No other studies have looked for associated factors with cachexia, but many have looked for associated factors with malnutrition. Indeed, in most of the past studies, the terms malnutrition and cachexia have been used interchangeably. Nowadays, definitions are better differentiated in terms of pathophysiology and consensual definitions for both terms have been established. There is a recent consensus to prefer the term cachexia in cancer patients, because weight loss in this context is not only due to a low food intake but also to catabolic pathways and muscle loss. 9
Several studies have looked at malnutrition in older patients with cancer. 7 , 42 , 43 The prevalence ranged from 32% to almost 45%. This prevalence depends of the cancer site. Similarly, the prevalence in our study was lowest in patients with prostate and breast cancers and highest in patients with upper digestive (oesophageal/gastric) cancer and pancreatic cancer. In one of this study, malnutrition was strongly associated with poor PS and impaired mobility. 42 Other studies in older patients with cancer have found a relationship between malnutrition and poor PS 44 , 45 or between malnutrition and functional impairment, a prolonged hospital stay or discharge to a skilled nursing facility. 46 , 47
In the present study, only 55.9% of the patients with cachexia received nutritional support. Unfortunately for the patients, this value is in line with the literature data. Indeed, the prevalence of nutritional support in malnourished patients with cancer ranges from as little as 30% to no more than 60%. 48 , 49 , 50 , 51 Several studies have identified barriers to the use of nutritional support in cancer patients and have demonstrated that a lack of provider (practitioner) awareness, knowledge, and training is one of the main problems. 52 , 53 Greater awareness and better training for practitioners might therefore improve the implementation of nutritional support for patients with cancer.
In our longitudinal study, 20.5% of patients were dead at 6 months of follow‐up. Cachexia was independently associated with higher 6 month mortality after adjustment for relevant prognostic factors. In the Dunne et al.'s study, 80% (80/100) of patients died in the 5 years of follow‐up and cachexia was also associated with overall mortality after adjustment for cancer type and stage. 31 A more recent study, also reported that cachexia, defined by the Fearon criteria, was associated with overall mortality, with a 3.2 years of follow‐up both in older patients without cancer (n = 67) and with cancer (n = 33) (adjusted for age, sex, albumin, and CRP). 32 These two studies displayed longer follow‐up but suffered from small effectives and monocentric recruitment. Finally, the study of Pamoukdjian et al. found an association between cachexia and 6 month mortality, although cachexia was defined as a latent variable using observed variables related to nutrition and inflammation. 33
Our nationwide cohort survey is the largest yet to have reported on cachexia in older patient with cancer. We were able to identify independent factors significantly associated with cachexia; this might help clinicians not only to screen for cachexia but also to actively treat or mitigate these associated factors. It is of particular importance because cachexia is independently associated with overall 6 month mortality in our study. One limitation of the present study was our use of a clinical definition of sarcopenia, rather than a CT measurement of muscle mass. This choice might bring a sur‐estimation of the cachexia prevalence. However, the use of this definition is highly relevant regarding prognostic value and clinical applicability. 25 , 26 , 28 , 29 , 41 Furthermore, 211 patients were not included in the cross‐sectional analysis because of missing data. However, they did not greatly differ from the analysed cohort in terms of demographic and clinical characteristics. In addition, only 48 of 55 centres participated in the longitudinal study regarding 6 month mortality. These centres did not record follow‐up information, which should not create a systematic bias. During follow‐up, 119 patients of 954 were lost at follow‐up (12.5%). Data concerning factors associated with mortality must be viewed with caution. Lastly, there may have been selection bias because all the study patients had been referred to a geriatrician; these patients were probably frailer than patients who were not referred and thus not included in the study.
Our study can help practitioners to identify high risk older patients for cachexia that could benefit from multimodal approach including nutritional support.
In conclusion, more than half of older patients with cancer managed in geriatric oncology clinics had cachexia. Upper gastrointestinal tract cancer, metastases, poor performance status, poor mobility, previous cancer surgery, cognitive disorders, risk of depression, and low food intake were positively associated with cachexia. Nutritional support was implemented only in just over half of the patients with cachexia. Cachexia was significantly associated with 6 month mortality after adjustment of relevant prognostic factors.
Funding
This study was supported by Société Francophone d'OncoGeriatrie (SoFOG).
Author contributions
JP, CMT, and EP wrote the manuscript. CMT and FCP performed statistical analyses. DH, RG, GA, CF, MG, ALC, RB, EL, PBR, ACW, CG, FP, LDD, VA, CC, HSL, PAN, HM, ML, and OG included patients in the study. All authors read and critically revised the manuscript.
Conflict of interest
Elena Paillaud declares one paid congress by Nutricia. Anne Laure Couderc declares paid oral communications by BMS, Ferring et Nutricia. All other authors declare no potential conflicts of interest.
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle. 54 The authors thank the Société Francophone d'OncoGeriatrie for assistance and funding and the team of Clinical Research Unit of Henri‐Mondor Hospital (Laetitia Grégoire, project coordinator, Pierre‐André Natella, pharmacist, Aroua Zribi and Hayat Medjenah Clinical Research Assistant and Mylene Allain, Data‐manager). We thank David Fraser for editing the manuscript. The authors thanks the PACAN platform (“Personnes âgées et Cancer”) for funding the publication fees.
Poisson J., Martinez‐Tapia C., Heitz D., Geiss R., Albrand G., Falandry C., Gisselbrecht M., Couderc A.‐L., Boulahssass R., Liuu E., Boudou‐Rouquette P., Chah Wakilian A., Gaxatte C., Pamoukdjian F., de Decker L., Antoine V., Cattenoz C., Solem‐Laviec H., Guillem O., Medjenah H., Natella P. A., Canouï‐Poitrine F., Laurent M., and Paillaud E. (2021) Prevalence and prognostic impact of cachexia among older patients with cancer: a nationwide cross‐sectional survey (NutriAgeCancer), Journal of Cachexia, Sarcopenia and Muscle, 12, 1477–1488, 10.1002/jcsm.12776
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2. Arnold M, Karim‐Kos HE, Coebergh JW, Byrnes G, Antilla A, Ferlay J, et al. Recent trends in incidence of five common cancers in 26 European countries since 1988: analysis of the European Cancer Observatory. Eur J Cancer 2015;51:1164–1187. [DOI] [PubMed] [Google Scholar]
- 3. Caillet P, Laurent M, Bastuji‐Garin S, Liuu E, Culine S, Lagrange J‐L, et al. Optimal management of elderly cancer patients: usefulness of the Comprehensive Geriatric Assessment. Clin Interv Aging 2014;9:1645–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wildiers H, Heeren P, Puts M, Topinkova E, Janssen‐Heijnen MLG, Extermann M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sifer‐Rivière L, Saint‐Jean O, Gisselbrecht M, Cudennec T, Girre V, Programme d'OncoGériatrie de l'Ouest Parisien (POGOP) . What the specific tools of geriatrics and oncology can tell us about the role and status of geriatricians in a pilot geriatric oncology program. Ann Oncol 2011;22:2325–2329. [DOI] [PubMed] [Google Scholar]
- 6. Song C, Cao J, Zhang F, Wang C, Guo Z, Lin Y, et al. Nutritional risk assessment by scored patient‐generated subjective global assessment associated with demographic characteristics in 23,904 common malignant tumors patients. Nutr Cancer 2019;71:50–60. [DOI] [PubMed] [Google Scholar]
- 7. Lacau St Guily J, Bouvard É, Raynard B, Goldwasser F, Maget B, Prevost A, et al. NutriCancer: a French observational multicentre cross‐sectional study of malnutrition in elderly patients with cancer. J Geriatr Oncol 2018;9:74–80. [DOI] [PubMed] [Google Scholar]
- 8. Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr 2008;27:793–799. [DOI] [PubMed] [Google Scholar]
- 9. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 10. Laurent M, Paillaud E, Tournigand C, Caillet P, Le Thuaut A, Lagrange J‐L, et al. Assessment of solid cancer treatment feasibility in older patients: a prospective cohort study. Oncologist 2014;19:275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer 2012;118:3377–3386. [DOI] [PubMed] [Google Scholar]
- 13. Aaldriks AA, Maartense E, le Cessie S, Giltay EJ, Verlaan HACM, van der Geest LGM, et al. Predictive value of geriatric assessment for patients older than 70 years, treated with chemotherapy. Crit Rev Oncol Hematol 2011;79:205–212. [DOI] [PubMed] [Google Scholar]
- 14. Kim JW, Kim YJ, Lee K‐W, Chang H, Lee J‐O, Kim K‐I, et al. The early discontinuation of palliative chemotherapy in older patients with cancer. Support Care Cancer 2014;22:773–781. [DOI] [PubMed] [Google Scholar]
- 15. Paillaud E, Herbaud S, Caillet P, Lejonc J‐L, Campillo B, Bories P‐N. Relations between undernutrition and nosocomial infections in elderly patients. Age Ageing 2005;34:619–625. [DOI] [PubMed] [Google Scholar]
- 16. Schneider SM, Veyres P, Pivot X, Soummer A‐M, Jambou P, Filippi J, et al. Malnutrition is an independent factor associated with nosocomial infections. Br J Nutr 2004;92:105–111. [DOI] [PubMed] [Google Scholar]
- 17. Fukuta A, Saito T, Murata S, Makiura D, Inoue J, Okumura M, et al. Impact of preoperative cachexia on postoperative length of stay in elderly patients with gastrointestinal cancer. Nutrition 2019;58:65–68. [DOI] [PubMed] [Google Scholar]
- 18. Soubeyran P, Fonck M, Blanc‐Bisson C, Blanc J‐F, Ceccaldi J, Mertens C, et al. Predictors of early death risk in older patients treated with first‐line chemotherapy for cancer. J Clin Oncol 2012;30:1829–1834. [DOI] [PubMed] [Google Scholar]
- 19. Ferrat E, Paillaud E, Laurent M, Le Thuaut A, Caillet P, Tournigand C, et al. Predictors of 1‐year mortality in a prospective cohort of elderly patients with cancer. J Gerontol A Biol Sci Med Sci 2015;70:1148–1155. [DOI] [PubMed] [Google Scholar]
- 20. Zhang X, Tang T, Pang L, Sharma SV, Li R, Nyitray AG, et al. Malnutrition and overall survival in older adults with cancer: a systematic review and meta‐analysis. J Geriatr Oncol 2019;10:874–883. [DOI] [PubMed] [Google Scholar]
- 21. Camus V, Lanic H, Kraut J, Modzelewski R, Clatot F, Picquenot JM, et al. Prognostic impact of fat tissue loss and cachexia assessed by computed tomography scan in elderly patients with diffuse large B‐cell lymphoma treated with immunochemotherapy. Eur J Haematol 2014;93:9–18. [DOI] [PubMed] [Google Scholar]
- 22. Broughman JR, Williams GR, Deal AM, Yu H, Nyrop KA, Alston SM, et al. Prevalence of sarcopenia in older patients with colorectal cancer. J Geriatr Oncol 2015;6:442–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sasaki S, Oki E, Saeki H, Shimose T, Sakamoto S, Hu Q, et al. Skeletal muscle loss during systemic chemotherapy for colorectal cancer indicates treatment response: a pooled analysis of a multicenter clinical trial (KSCC 1605‐A). Int J Clin Oncol 2019;24:1204–1213. [DOI] [PubMed] [Google Scholar]
- 24. Mayr R, Gierth M, Zeman F, Reiffen M, Seeger P, Wezel F, et al. Sarcopenia as a comorbidity‐independent predictor of survival following radical cystectomy for bladder cancer. J Cachexia Sarcopenia Muscle 2018;9:505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bauer J, Morley JE, Schols AMWJ, Ferrucci L, Cruz‐Jentoft AJ, Dent E, et al. Sarcopenia: a time for action. An SCWD position paper. J Cachexia Sarcopenia Muscle 2019;10:956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC‐F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016;7:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woo J, Leung J, Morley JE. Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc 2015;16:247–252. [DOI] [PubMed] [Google Scholar]
- 29. Bahat G, Yilmaz O, Kılıç C, Oren MM, Karan MA. Performance of SARC‐F in regard to sarcopenia definitions, muscle mass and functional measures. J Nutr Health Aging 2018;22:898–903. [DOI] [PubMed] [Google Scholar]
- 30. Caillet P, Liuu E, Raynaud Simon A, Bonnefoy M, Guerin O, Berrut G, et al. Association between cachexia, chemotherapy and outcomes in older cancer patients: a systematic review. Clin Nutr 2017;36:1473–1482. [DOI] [PubMed] [Google Scholar]
- 31. Dunne RF, Roussel B, Culakova E, Pandya C, Fleming FJ, Hensley B, et al. Characterizing cancer cachexia in the geriatric oncology population. J Geriatr Oncol 2019;10:415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zopf Y, Schink K, Reljic D, Herrmann HJ, Dieterich W, Kiesswetter E, et al. Assessing cachexia in older patients: different definitions—but which one is the most practical for clinical routine? Arch Gerontol Geriatr 2020;86:103943, 10.1016/j.archger.2019.103943 [DOI] [PubMed] [Google Scholar]
- 33. Pamoukdjian F, Laurent M, Martinez‐Tapia C, Rolland Y, Paillaud E, Canoui‐Poitrine F. Frailty parameters, morbidity and mortality in older adults with cancer: a structural equation modelling approach based on the Fried phenotype. J Clin Med 2020;9: 10.3390/jcm9061826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 35. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 36. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 37. D'Ath P, Katona P, Mullan E, Evans S, Katona C. Screening, detection and management of depression in elderly primary care attenders. I: the acceptability and performance of the 15 item Geriatric Depression Scale (GDS15) and the development of short versions. Fam Pract 1994;11:260–266. [DOI] [PubMed] [Google Scholar]
- 38. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 39. van Putten W. CART: stata module to perform classification and regression tree analysis. 2006.
- 40. Cruz‐Jentoft AJ, Kiesswetter E, Drey M, Sieber CC. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res 2017;29:43–48. [DOI] [PubMed] [Google Scholar]
- 41. Ida S, Kojima Y, Hamaoka S, Urawa N, Araki J, Kaneko R, et al. Validity of Japanese version of SARC‐F questionnaire in patients with chronic liver disease. J Gastroenterol Hepatol 2019;34:947–953. [DOI] [PubMed] [Google Scholar]
- 42. Zhang X, Pang L, Sharma SV, Li R, Nyitray AG, Edwards BJ. Prevalence and factors associated with malnutrition in older patients with cancer. J Geriatr Oncol 2019;10:763–769. [DOI] [PubMed] [Google Scholar]
- 43. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491–497. [DOI] [PubMed] [Google Scholar]
- 44. Huisman MG, Veronese G, Audisio RA, Ugolini G, Montroni I, de Bock GH, et al. Poor nutritional status is associated with other geriatric domain impairments and adverse postoperative outcomes in onco‐geriatric surgical patients—a multicentre cohort study. Eur J Surg Oncol 2016;42:1009–1017. [DOI] [PubMed] [Google Scholar]
- 45. Attar A, Malka D, Sabaté JM, Bonnetain F, Lecomte T, Aparicio T, et al. Malnutrition is high and underestimated during chemotherapy in gastrointestinal cancer: an AGEO prospective cross‐sectional multicenter study. Nutr Cancer 2012;64:535–542. [DOI] [PubMed] [Google Scholar]
- 46. Badgwell B, Stanley J, Chang GJ, Katz MHG, Lin HY, Ning J, et al. Comprehensive geriatric assessment of risk factors associated with adverse outcomes and resource utilization in cancer patients undergoing abdominal surgery. J Surg Oncol 2013;108:182–186. [DOI] [PubMed] [Google Scholar]
- 47. Jacobsen EL, Brovold T, Bergland A, Bye A. Prevalence of factors associated with malnutrition among acute geriatric patients in Norway: a cross‐sectional study. BMJ Open 2016;6:e011512, 10.1136/bmjopen-2016-011512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr 2014;38:196–204. [DOI] [PubMed] [Google Scholar]
- 49. Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer‐related malnutrition. Clin Nutr 2017;36:1187–1196. [DOI] [PubMed] [Google Scholar]
- 50. Li Z, Chen W, Li H, Zhao B, Chinese Oncology Nutrition Survey Group . Nutrition support in hospitalized cancer patients with malnutrition in China. Asia Pac J Clin Nutr 2018;27:1216–1224. [DOI] [PubMed] [Google Scholar]
- 51. Planas M, Álvarez‐Hernández J, León‐Sanz M, Celaya‐Pérez S, Araujo K, García de Lorenzo A, et al. Prevalence of hospital malnutrition in cancer patients: a sub‐analysis of the PREDyCES® study. Support Care Cancer 2016;24:429–435. [DOI] [PubMed] [Google Scholar]
- 52. Martin L, de van der Schueren MAE, Blauwhoff‐Buskermolen S, Baracos V, Gramlich L. Identifying the barriers and enablers to nutrition care in head and neck and esophageal cancers: an international qualitative study. JPEN J Parenter Enteral Nutr 2016;40:355–366. [DOI] [PubMed] [Google Scholar]
- 53. Cotogni P, Pedrazzoli P, De Waele E, Aprile G, Farina G, Stragliotto S, et al. Nutritional therapy in cancer patients receiving chemoradiotherapy: should we need stronger recommendations to act for improving outcomes? J Cancer 2019;10:4318–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


