Supplemental Digital Content is available in the text.
Keywords: big data, blood pressure, critical care
Abstract
OBJECTIVES:
Differences and biases between directly measured intra-arterial blood pressure and intermittingly measured noninvasive blood pressure using an oscillometric cuff method have been reported in adults and children. At the bedside, clinicians are required to assign a confidence to a specific blood pressure measurement before acting upon it, and this is challenging when there is discordance between measurement techniques. We hypothesized that big data could define and quantify the relationship between noninvasive blood pressure and intra-arterial blood pressure measurements and how they can be influenced by patient characteristics, thereby aiding bedside decision-making.
DESIGN:
A retrospective analysis of cuff blood pressure readings with associated concurrent invasive arterial blood pressure measurements (452,195 noninvasive blood pressure measurements).
SETTING:
Critical care unit at The Hospital for Sick Children, Toronto.
PATIENTS:
Six-thousand two-hundred ninety-seven patients less than or equal to 18 years old, hospitalized in a critical care unit with an indwelling arterial line.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Two-dimensional distributions of intra-arterial blood pressure and noninvasive blood pressure were generated and the conditional distributions of intra-arterial blood pressure examined as a function of the noninvasive systolic, diastolic, or mean blood pressure. Modification of these distributions according to age and gender were examined using a multilevel mixed-effects model. For any given combination of patient age and noninvasive blood pressure, the expected distribution of intra-arterial blood pressure readings exhibited marked variability at the population level and a bias that significantly depended on the noninvasive blood pressure value and age. We developed an online tool that allows exploration of the relationship between noninvasive blood pressure and intra-arterial blood pressure and the conditional probability distributions according to age.
CONCLUSIONS:
A large physiologic dataset provides clinically applicable insights into the relationship between noninvasive blood pressure and intra-arterial blood pressure measurements that can help guide decision-making at the patient bedside.
Critically ill patients present treatment challenges as they experience dynamic changes in their clinical condition and the associated risk over variable intervals of time. To facilitate an accurate assessment of patient condition, rate of change and to support prompt detection of deterioration, patients are continuously monitored using vital signs that are displayed on the bedside monitor. Blood pressure (BP) monitoring has a central role in the evaluation of the critically ill patient’s hemodynamic status. In particularly unstable patients or those at high risk of decline, intra-arterial BP (IABP) is used with the rationale that this provides a high fidelity and continuous measurement. This is accomplished via a catheter inserted in a peripheral or central artery and is considered the gold standard measurement (1). However, this method is not free from bias, requires an invasive procedure with associated risk, increased care complexity, morbidity and cost, and can be inaccurate and subject to artifacts. The commonly used alternative to invasive measurements in critically ill children is the noninvasive BP (NIBP) obtained by the oscillometric method (2) that depends on measuring oscillations in cuff pressure pulsatility during deflation with a known complex relationship between patient characteristics and estimation accuracy, both of the mean BP and more so for the systolic and diastolic pressures (3–5). Measured intermittently, this method has additional potential inaccuracies related to cuff size and position and pulse detection, for example.
Several studies have examined the agreement of NIBP measurements with invasive arterial BP in a variety of patient populations (6, 7) and even specifically critically ill infants and children (8) or those with specific background conditions (9). The largest study published so far in children comparing these measurements (10) reported analysis of ~50,000 measurements from 2,459 children and focused on quantifying the overall biases and precision. They did not find a relationship with patient age or a bias between NIBP and invasive measurements that was dependent on the NIBP value.
In this study, using a much larger dataset of observations, we hypothesized that there would be a quantifiable relationship between NIBP and IABP and that patient characteristics such as age and gender could modify these relationships in predictable ways. Recognizing that for a specific BP reading, there are many modifiers of both the IABP and NIBP, we are not attempting to validate or quantify the accuracy of either method. Rather, we sought to generate the conditional distribution of expected IABP for an arbitrary patient given their age and NIBP. In simple words, we wish to answer the following question, for example—given a 10-year-old critically ill patient with a systolic NIBP of 80 mm Hg, what is the exact probability that if we were to insert an arterial line, his IABP would be lower than 80 mm Hg (or any other number, depending on the clinical context). So, we aimed to create a data-driven tool that could help clinicians improve confidence in the acceptability of NIBP reading under certain circumstances and guide decision-making about the need for invasive monitoring.
MATERIALS AND METHODS
Patient Population and Inclusion Criteria
Patients were included in the analysis if they had concurrent IABP and NIBP measurements. Patients without IABP measurements were excluded. Using this criterion, the cohort was 6,297 patients less than or equal to 18 years old admitted to the critical care unit (CCU) at the Hospital for Sick Children between March 2013 and February 2020 inclusive. The Research Ethics Board of The Hospital for Sick Children (Toronto, ON, Canada) approved this study (Research Ethics Board 1000049114).
Data Capture
This study uses continuously recorded physiologic data: systolic, mean, and diastolic BP extracted by the bedside monitor (Intellivue MP70; Philips, Amsterdam, The Netherlands, Software Version J.10.50). IABP readings recorded from monitors were compared with NIBP recorded by the oscillometric method. For each patient, we used NIBP measurements if there was an associated invasive arterial line reading during the minute prior to the cuff measurement time. Data streaming through the monitor were captured at 1-second increments using the ViNES medical device connectivity platform (Baxter, Deerfield, IL) or at 5 seconds intervals by the Tracking, Trajectory, Triggering system (Etiometry, Boston, MA). Data were stored in a software system developed at the Hospital for Sick Children known as AtriumDB (11) on a secure institutional server. For each cuff measurement, we recorded the patient age (at the time of measurement), time of measurement, and statistics summarizing the heart rate and invasive BP (systolic, diastolic and mean) in the minute prior to the cuff-based measurement: average of the values within that minute window, minimum and maximum values, and total number of values recorded by the monitor during that minute.
In the CCU at SickKids NIBP is measured on almost every admission, with over 99% of patients having at least one NIBP reading recorded. IABP is recorded less frequently, with approximately 74% of admissions having invasive recordings at some stage during their admission. It is standard practice in our unit to crosscheck a NIBP reading with the invasive values at least once or twice per nurse shift (where a shift is 12 hr) but records show that simultaneous recordings are much more frequently obtained than this. The Philips MP70 monitor provides three different methods of collecting NIBP measurements; manual (which may be triggered by a clinician at any time), auto (which continually collects samples at a preset time interval), and STAT, which triggers a series of rapid-fire measurements over a 5-minute period.
Ensuring Quality of Cuff-Based and Arterial Line Derived Measurements
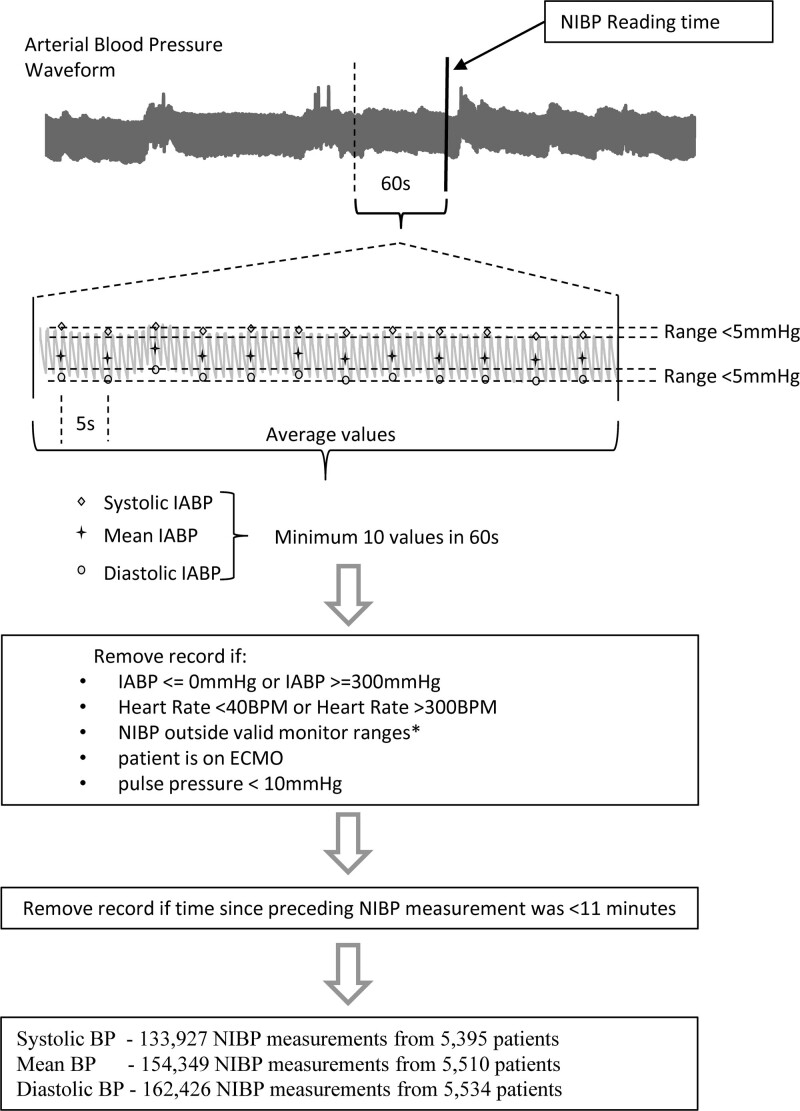
We implemented a sequential approach to exclude signals of poor quality (See Fig. 1 for analysis outline). Initially, we removed all measurements in which IABP readings were implausible as described in (12, 13) (≤ 0 mm Hg or ≥ 300 mm Hg) or had a pulse pressure of less than 10 mm Hg to exclude readings from suspected damped arterial lines. An invasively measured pulse pressure of 10 mm Hg was selected as a cutoff because, below this threshold, the NIBP and IABP pulse pressures were uncorrelated, suggesting that the invasive monitoring may have been compromised in some way in these situations. Measurements from patients who were on extracorporeal membrane oxygenation (ECMO) at the time of monitoring were excluded. Information about patients on ECMO was only readily available up to April 2, 2018; however, this covered almost 85% of the study period and only removed 3,400 NIBP measurements (approximately 0.7% of the dataset).
Figure 1.
Flowchart and conceptual figure showing the data processing pipeline. The analysis of the intra-arterial blood pressure (IABP) in the minute preceding an noninvasive blood pressure (NIBP) reading is shown. Note that we do not show here the 5 mm Hg range cutoff for mean blood pressure (BP) for clarity even though it was used in the actual analysis. Also, note that the time interval between successive IABP was either 1 or 5 seconds in our database, depending on the data ingestion method. BPM = beats per minute, ECMO = extracorporeal membrane oxygenation.
NIBP values that were outside the measurement range shown in Supplemental Table 1 (http://links.lww.com/CCX/A859) were excluded. As we did not know which monitor setting was in use at the time of recording, this setting was inferred from the patient age, with patients less than 6 weeks assumed to be using the neonatal setting, patients greater than 6 weeks and less than 12 years old to be assumed to be using the pediatric setting, and patients greater than 12 years old assumed to be using the adult setting. Finally, we attempted to exclude measurements that were captured when the heart rate of the patient was less than 40 beats per minute (BPM) or greater than 300 BPM, the range for valid NIBP measurement as defined in the Philips MP70 Manual (14). We only had ready access to hourly maximum and minimum heart rates for the period starting mid-2016 and covered approximately 50% of the dataset. The exact start date of the heart rate data was dependant on which bed the patient was in as this data collection method was rolled out gradually over a 6-month period beginning in February 2016 (11).
After this preprocessing, our dataset included 452,195 NIBP readings with associated concurrent IABP measurements. Then, for each comparison between a NIBP estimate and associated IABP systolic, mean, or diastolic reading, the following data inclusion criteria were used:
1) A NIBP measurement was included if the previous NIBP measurement was at least 11 minutes earlier (to avoid periods of rapid cycling)
2) The associated IABP (systolic, diastolic, or mean) had continuous measurement and at least 10 readings recorded from the minute prior to the cuff reading. The mean IABP measure in that minute was taken as the representative value for comparison
3) The associated IABP readings varied less than 5 mm Hg (maximum–minimum value) in a minute-sized window.
We examined the distribution of elapsed times between NIBP readings collected from patients who were also invasively monitored and found pronounced peaks at sampling periods of 1, 2, 5, 10, 15, 20, 30, 45, and 60 minutes. These fixed sampling frequencies are presets available in the auto mode of NIBP monitoring provided by the monitor. Use of an 11-minute threshold removed 46.4% of the NIBP readings but only removed 2.7% of the IABP data coverage in terms of elapsed monitoring time. The rationale for removing NIBP measurements that were so close in time was local unit practice to cycle the NIBP frequently in those cases that the bedside clinicians suspect a faulty IABP reading, and so to avoid introducing a bias. Of note, we also generated a 2D histogram of NIBP and IABP using data acquired from NIBP with larger minimum time differences between measurements (up to 5 hr) with similar qualitative results, albeit a much smaller sample size. We also acknowledge that the approach of comparing the NIBP reading to the mean corresponding IABP value of the minute prior to that measurement is not the standard method used when comparing concurrent measurements. The reasons to choose a mean of a minute of IABP data prior to the timing of NIBP measurements was that the relation between exact time of cuff inflation versus the recorded time is not clear. Also, in some cases where the cuff is located on the same limb in which the arterial line is placed taking exactly concurrent measurements might affect the invasive recorded values and moreover, the mere act of cuff inflation might cause the patient’s BP to change. Thus, we chose a minute prior to recorded cuff inflation time as the representative time window, and moreover ensured that the IABP at the time be relatively stable without fluctuations.
After these filtering measures, we had 133,927 samples from 5,395 patients comparing systolic NIBP to IABP and similarly 154,349 (5,510 patients), 162,426 (5,534 patients) for mean and diastolic pressures, respectively.
Statistical Analysis
Conditional distributions were estimated using histograms with a bin size of 1 mm Hg, and 1 × 1 mm Hg for 2D distributions, and by normalizing to the total observation count. Two-dimensional histograms were smoothed using a Gaussian filter using a filter size of 2 mm Hg (Matlab’s Image Processing toolbox; MathWorks, Natick, MA). Tests for normality of the data were performed using one-sample Kolmogorov-Smirnov test (Matlab’s Statistical toolbox; MathWorks).
To test the relative contributions of age, gender, patient identity, and NIBP on the observed variability of IABP given a noninvasive measurement, we used a hierarchical multilevel mixed-effects model (15–17). The constructed model treats the invasive BP as the dependent variable while the NIBP, age and sex are treated as independent variables. We used a cubic polynomial to capture the relationship between the two variables. The age is measured in days, and a log (base e) transformation is applied to account for the greater differences observed during development early in life. Sex is included as a binary variable. Because patients are repeatedly sampled, the correlation structure between the samples from each patient must be considered. Patient level deviations of IABP from their NIBP can occur due to differences in equipment, placement of arterial cannula, etc. We assume that such differences can approximately be captured by including a random intercept component in the model. More details on model development, assumptions and results can be found in the Online Supplementary Material (http://links.lww.com/CCX/A860).
RESULTS
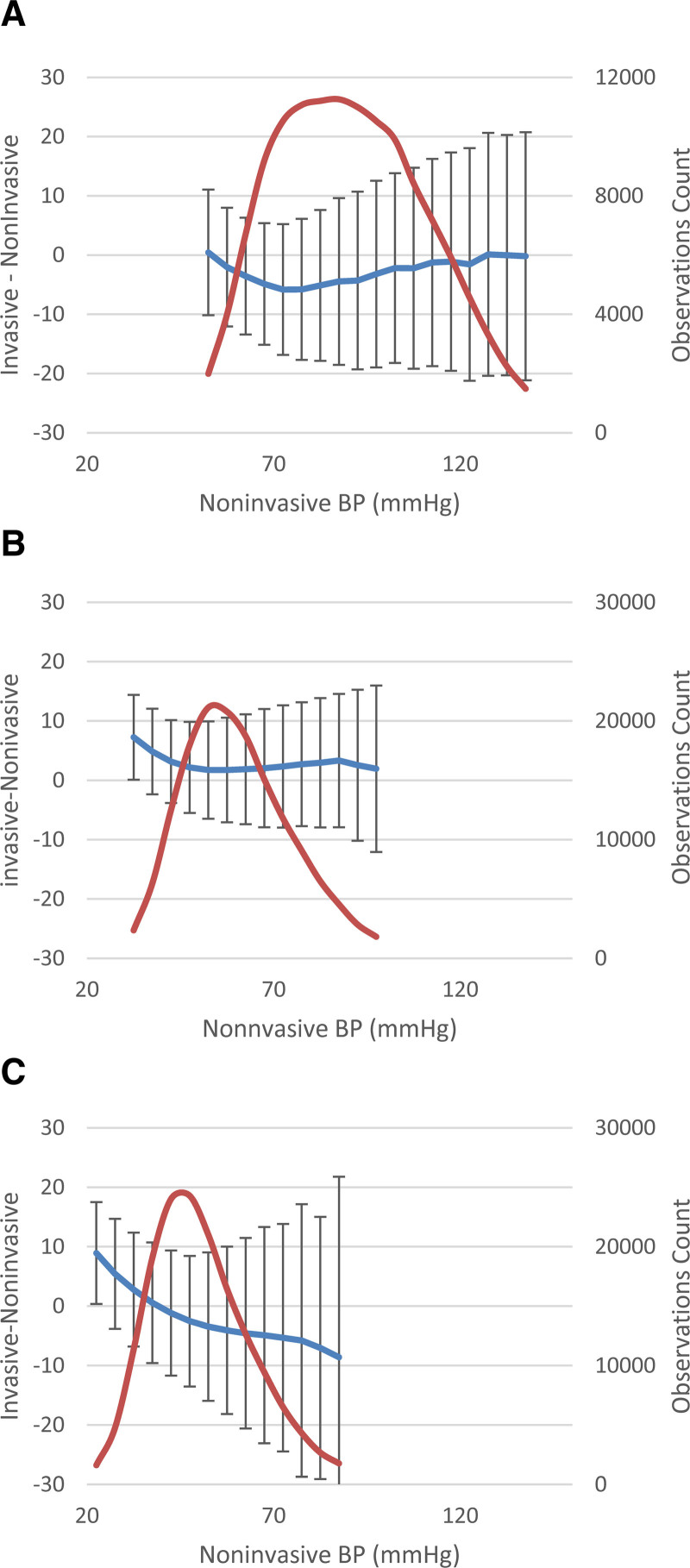
Figure 2 A–C shows histograms of the number of systolic, mean, and diastolic BP comparisons (observations) as a function of NIBP value. Also shown is the mean and sd of measurement differences (IABP–NIBP) as a function of NIBP value. Examination of this figure demonstrates that: the distribution of observed NIBP values for all ages is unimodal but nongaussian (Kolmogorov-Smirnov test; p < 10–20) and that the relationship between the NIBP value and the observed difference to the concurrently measured IABP value is nontrivial and depends on the NIBP value, with different types of dependencies observed for systolic, mean, and diastolic BP. For example, for diastolic BP—at lower NIBP values, the IABP tends to be higher while this relationship is opposite at higher levels. Examination of the error bars (one sd) shows that the variability is of a magnitude that is often larger than the mean difference. In what follows, we attempt to quantify these differences, their dependence on the age and gender of the patient, and their variability.
Figure 2.
Noninvasive blood pressure (NIBP) histograms and mean differences between intra-arterial blood pressure (IABP) and NIBP. X-axis is blood pressure (BP) (mm Hg, bin size 5 mm Hg), while on the right y-axis and red line, we show the number of NIBP samples included in the analyses in each BP bin. Left y-axis and blue line show the observed mean difference between IABP and NIBP for each BP bin. Error bars represent sd of the observed differences per bin. Only NIBP bin values with at least 1,000 measurements are shown in the figure. A, Systolic BP. B, Mean BP. C, Diastolic BP.
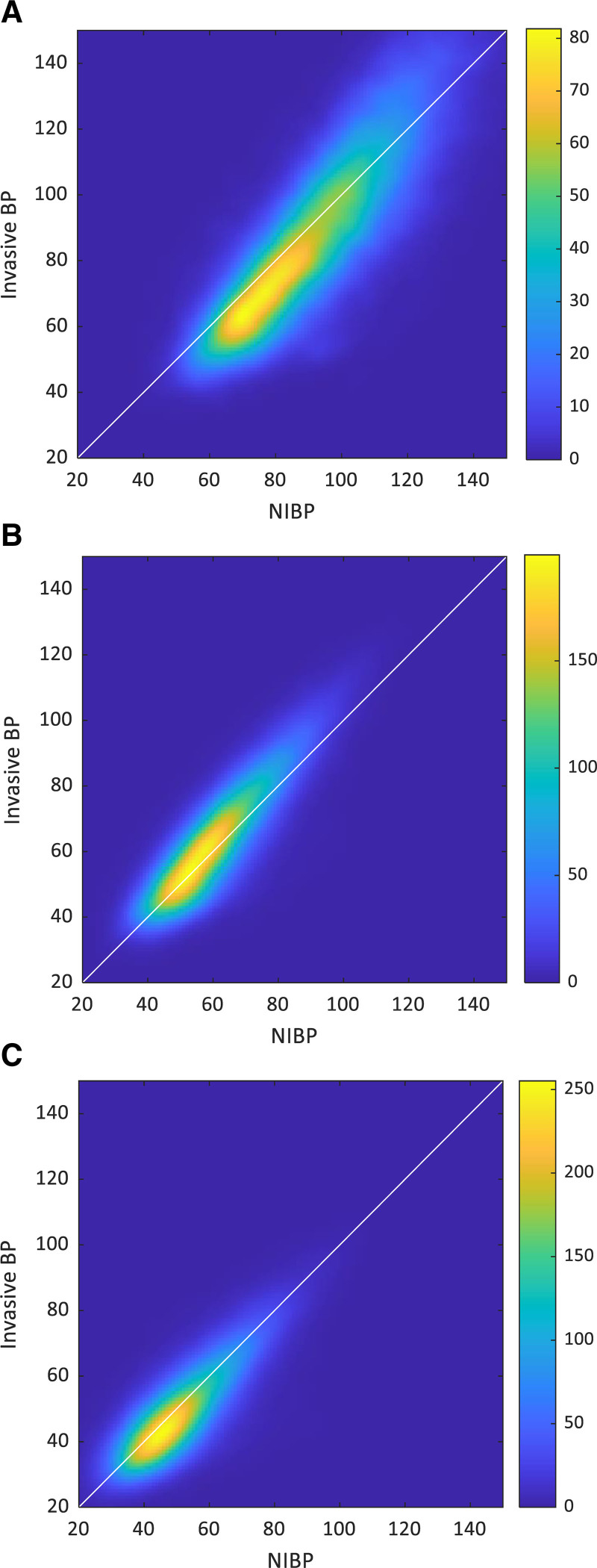
Figure 3 adds additional information by showing 2D histograms using color-coding, allowing appreciation of the bivariate distribution of NIBP and IABP. We opted to show these histograms rather than a Bland-Altman scatter plot due to the large number of points that would make it impossible to appreciate their density (over 100,000 per plot) and as our goal here is to study the joint and conditional distributions and not to validate one method nor determine its accuracy relative to the other. Panel A depicts this relationship for systolic BP demonstrating that for most NIBP values up to approximately 100 mm Hg, the associated IABP is lower than the NIBP, while for higher values, this relationship is less clear. Also, all panels demonstrate that for any given NIBP value, the associated distribution of invasive recordings is quite wide.
Figure 3.
Invasive and noninvasive blood pressure (NIBP) 2D histograms. X-axis and y-axis are NIBP and invasive blood pressure (BP) (mm Hg, bin size 1 mm Hg, Gaussian filter of size 2 mm Hg). Color code depicts sample count in each bin. A, Systolic BP. B, Mean BP. C, Diastolic BP.
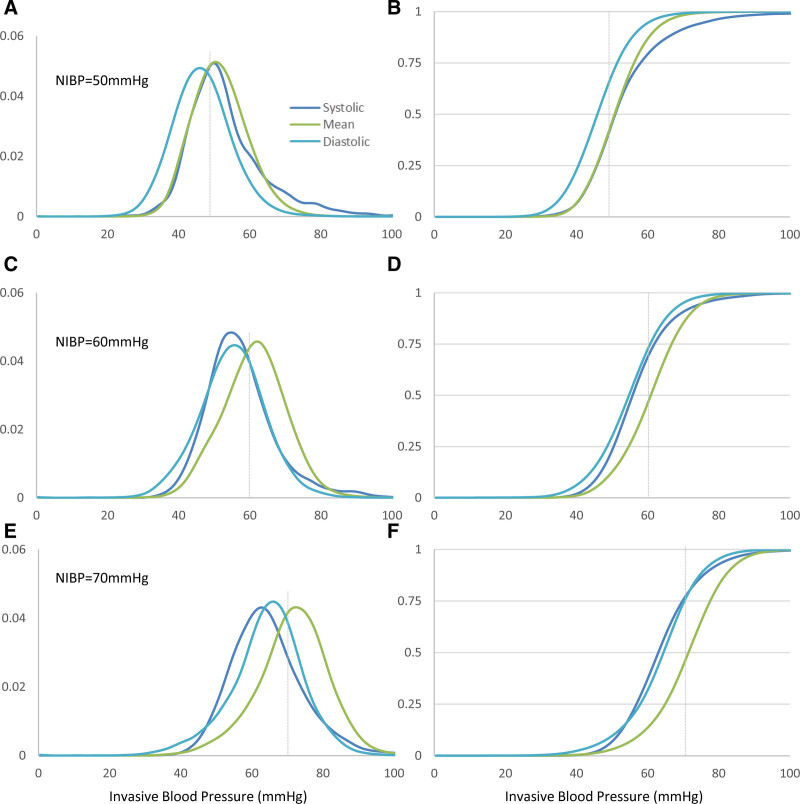
In Figure 4, we continue to explore these conditional distributions for NIBP values of 50, 60, and 70 mm Hg chosen arbitrarily as an example. For mean BP, NIBP, and IABP generally agree for this BP range, but for diastolic and systolic BP, NIBP readings share a complex relationship to IABP, that depends on the NIBP value. Using cumulative distributions such as those depicted in Figure 4, B, D, and F, it is possible to quantify this association: for example, given a recorded systolic NIBP of 70 mm Hg, there is a ~40% chance that the IABP is 60 mm Hg or below.
Figure 4.
Invasive blood pressure (BP) (systolic, mean, and diastolic) histograms conditional on measured noninvasive blood pressure (NIBP) and category (systolic, mean, and diastolic, respectively). Left-sided show the distribution, while the right-sided ones depict the cumulative distribution with grid lines at the 25th, 50th, and 75th percentiles. X-axis is observed invasive BP (mm Hg, bin size 1 mm Hg, for either systolic, mean, or diastolic as color-coded) while on the y-axis, we show the fraction of samples in each BP bin. A and B, NIBP 50 mm Hg. C and D, NIBP 60 mm Hg. E and F, NIBP 70 mm Hg.
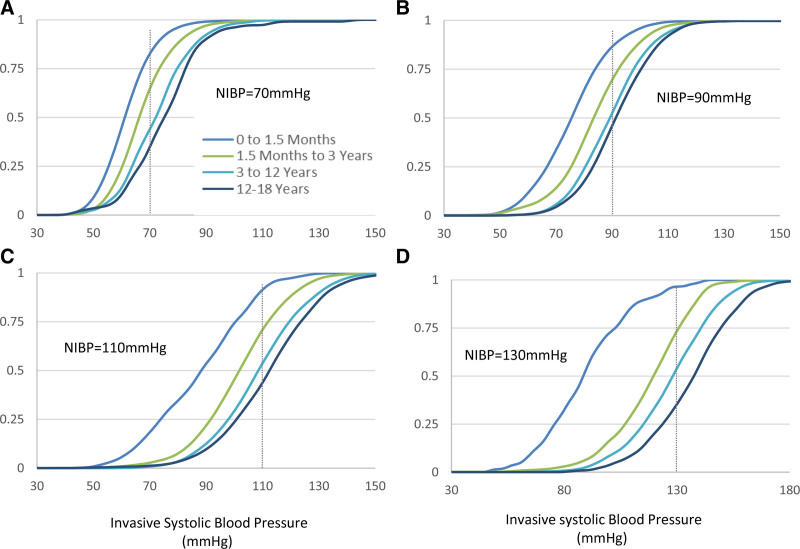
We sought to further explore the relationship between NIBP readings and the concurrently observed IABP by examining the substantial modifying effects of the patient’s age on the conditional distributions. Figure 5 demonstrates the extent of this modification on the cumulative distributions of invasively recorded systolic values as a function of the systolic NIBP pressure for four age groups. For example, given a systolic NIBP of 110 mm Hg, the median expected invasive value would be 88.5 mm Hg for infants 0–1.5 months old (interquartile range, 74.5–100.5 mm Hg), while it would be 113 mm Hg for children 12–18 years old (interquartile range, 99.5–123.5 mm Hg). These age-related modifications of the conditional distributions are even more pronounced for higher BPs.
Figure 5.
Cumulative systolic invasive blood pressure (BP) histograms for various measured noninvasive blood pressure (NIBP) pressures and different age groups. X-axis is systolic BP (mm Hg, bin size 1 mm Hg), while on the y-axis, we show the fraction of samples with a BP below the value in the x-axis. A, Systolic NIBP 70 mm Hg. B, Systolic NIBP 90 mm Hg. C, Systolic NIBP 110 mm Hg. D, Systolic NIBP 130 mm Hg. Age groups defined in legend at the bottom and same color coding for all panels.
We further examined the relative effects of NIBP recorded value, patient age (natural log transformation of age in days), identity, and their sex on the observed invasive BP using a multilevel mixed-effects regression model. This model shows that the observed IABP depends on the NIBP value in a complex fashion with an intercept (bias) and linear to cubic terms, all contributing significant effects (p < 10–15). Age as well was identified as a significant modifier (p < 10–15), while sex was not (p > 0.08). These results were observed for systolic, diastolic, and mean BPs. Of note, when examining the random effects, it seems that the variability incurred by the specific patient’s identity and all the additional unmodeled modifiers were of similar magnitude (sd of ~10 mm Hg for systolic BP and ~5–7 mm Hg for mean and diastolic BPs). Full details of the models and derived coefficients, together with a figure showing the conditional distributions of observed IABP as dependent on NIBP and age are given in the Online Supplementary Material (http://links.lww.com/CCX/A860).
We developed an online tool that allows exploration of these conditional distributions. The hyperlink to this tool is http://media.laussenlabs.ca/figures/bp-comparison/.
DISCUSSION
Our analysis of the distributions of observed invasive BPs associated with a given NIBP demonstrated a complex relationship that depends on the NIBP value and was different for systolic, mean, and diastolic BPs. There was a significant modifying effect of age but minimal effect of sex (in terms of effect size) on the conditional probabilities and an associated high variance. Of note, we have not attempted to validate either method or estimate its accuracy. Rather, we attempt to answer the simple question—for a given patient of a certain age, if we observe at the bedside a NIBP reading, can we quantify the conditional distribution of invasive arterial BP values?
Most clinicians will have a general sense of the uncertainty associated with any BP value recorded in a clinical setting, and the onus is on physicians to determine the clinical significance of any BP measurement. Despite the presence of this inherent uncertainty, which is compliant with the device testing procedures outlined in the American National Standards Institute/Association for the Advancement of Medical Instrumentation SP10-1992 standard (18), the complex relationship between systolic, mean, and diastolic NIBP and IABP can be estimated using large datasets. We chose a nonstandard retrospective approach capitalizing on the power of big data to allow us to appreciate these relationships, rather than validate one method against the other. In the associated interactive application, we allow the conditional relationships between IABP and NIBP according to patient age to be actively explored.
As this is a retrospective study on a very large dataset but with limited associated clinical information, we cannot determine the sources of the observed complex relationships nor the marked variability of the conditional distributions, even after accounting for age and NIBP value. These probably stem from device, the nature of the oscillometric estimation method, and patient-specific causes, some of them are detailed below.
Another common dilemma faced by clinicians in critical care environments is when to augment a patient monitoring strategy with additional, continuous and possibly more reliable data. In the case of BP measurement, the latter is commonly necessary when the NIBP measurements are predominantly outlying values, as is seen in hypertensive or hypotensive patients. Placement of catheter for invasive BP monitoring facilitates the continuous titration of therapy to normalize the BP in these patients. However, in less clear cases, clinicians may ponder the utility of placing an invasive catheter in an attempt to limit patient discomfort and other direct risks associated with invasive BP monitoring. These risks include injury to extremities, infection, and bleeding risks. Knowing the conditional relationship between NIBP and IABP may obviate the need for direct measurement. For example, using the results presented here and the associated application one might be able to answer questions with direct clinical implications such as—“for a 5-year-old child with a NIBP systolic measurements of 80 mm Hg, what are the chances of observing an invasive value of less than 70 mm Hg (or any other value) if we insert an arterial line?”.
This study has limitations given it is a single center, retrospective evaluation and may represent biases characteristic of the local environment (patients and equipment), but we believe that it is reasonable to assume that these relationships will be similar in other institutions. We could not consistently identify or correct for many environmental and patient-related factors that may impact the accuracy and/or validity of measurements that were included in our analysis. These factors may include either over- or under-damped arterial lines (19) but did try to exclude the former as detailed in the methods section. Our dataset also did not include any record of whether the monitor was set to neonatal, pediatric, or adult mode when the readings were collected nor information about the exact cuff size used, but we did attempt to account for these factors as described in the Methods section. Many factors relating to the collection of the NIBP readings were unknown including calibration (20), differences due to monitoring site (21), elevation of the monitored limb relative to the heart (which is usually rare in the pediatric critical care setting) (14), issues related to the body mass index of the patient (22), the impact of cuff inflation (23), and other sources of error that we cannot completely exclude (24). Additionally, our dataset may have included some BP values obtained from patients who were on ECMO. Any or all these factors could introduce unknown errors into the NIBP readings and are probably sources of the variability in the observed relationships.
It should be noted that while caregivers at the bedside have contextual information that may let them ignore an erroneous reading, our statistical models were constructed on data that were lacking this specific context, potentially allowing inclusion of some data that would have not been entered into the medical record. On the other hand, automatically obtained data are not affected by human-introduced bias and preferences. We did try to avoid erroneous readings by employing strict filtering techniques at the price of excluding many data points from analysis. The only patient variables we stratified against were age and sex. We did not attempt to do this by diagnosis, monitoring site, or treatment received at the time of measurement as we were interested in the relationship between IABP and NIBP measurements and not whether these measurements were influenced by other uncontrolled variables and plan to explore some of these effects in future work.
CONCLUSIONS
Quantifiable relationships exist between NIBP and IABP in critically ill patients that have the potential to provide clinicians with actionable insights in scenarios where decisions need to be made about the indication for and utility of invasive BP monitoring. These relationships are best understood as a distribution that can be personalized for an individual patient with the incorporation of data about patient age. Defining these relationships may be an important adjunct to effective patient monitoring and facilitate clinical decision-making.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
All authors have participated substantially in study conception and design, experiment conduct, drafting and revising of the article, and have approved the final article as submitted.
The authors have disclosed that they do not have any potential conflicts of interest.
This work was performed at The Hospital for Sick Children, 555 University Avenue, Toronto, ON M5G 1X8 Canada.
REFERENCES
- 1.Antonelli M, Levy M, Andrews PJ, et al. : Hemodynamic monitoring in shock and implications for management. International Consensus Conference, Paris, France, 27-28 April 2006. Intensive Care Med. 2007; 33:575–590 [DOI] [PubMed] [Google Scholar]
- 2.Forouzanfar M, Dajani HR, Groza VZ, et al. : Oscillometric blood pressure estimation: Past, present, and future. IEEE Rev Biomed Eng. 2015; 8:44–63 [DOI] [PubMed] [Google Scholar]
- 3.Mauck GW, Smith CR, Geddes LA, et al. : The meaning of the point of maximum oscillations in cuff pressure in the indirect measurement of blood pressure–part ii. J Biomech Eng. 1980; 102:28–33 [DOI] [PubMed] [Google Scholar]
- 4.Geddes LA, Voelz M, Combs C, et al. : Characterization of the oscillometric method for measuring indirect blood pressure. Ann Biomed Eng. 1982; 10:271–280 [DOI] [PubMed] [Google Scholar]
- 5.Ursino M, Cristalli C: A mathematical study of some biomechanical factors affecting the oscillometric blood pressure measurement. IEEE Trans Biomed Eng. 1996; 43:761–778 [DOI] [PubMed] [Google Scholar]
- 6.Picone DS, Schultz MG, Otahal P, et al. : Accuracy of cuff-measured blood pressure: Systematic reviews and meta-analyses. J Am Coll Cardiol. 2017; 70:572–586 [DOI] [PubMed] [Google Scholar]
- 7.Wax DB, Lin HM, Leibowitz AB: Invasive and concomitant noninvasive intraoperative blood pressure monitoring: Observed differences in measurements and associated therapeutic interventions. Anesthesiology. 2011; 115:973–978 [DOI] [PubMed] [Google Scholar]
- 8.Joffe R, Duff J, Garcia Guerra G, et al. : The accuracy of blood pressure measured by arterial line and non-invasive cuff in critically ill children. Crit Care. 2016; 20:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werther T, Aichhorn L, Baumgartner S, et al. : Discrepancy between invasive and non-invasive blood pressure readings in extremely preterm infants in the first four weeks of life. PLoS One. 2018; 13:e0209831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray S, Rogers L, Noren DP, et al. : Risk of over-diagnosis of hypotension in children: A comparative analysis of over 50,000 blood pressure measurements. Intensive Care Med. 2017; 43:1540–1541 [DOI] [PubMed] [Google Scholar]
- 11.Goodwin AJ, Eytan D, Greer RW, et al. : A practical approach to storage and retrieval of high-frequency physiological signals. Physiol Meas. 2020; 41:035008. [DOI] [PubMed] [Google Scholar]
- 12.Eytan D, Goodwin AJ, Greer R, et al. : Distributions and behavior of vital signs in critically ill children by admission diagnosis. Pediatr Crit Care Med. 2018; 19:115–124 [DOI] [PubMed] [Google Scholar]
- 13.Eytan D, Goodwin AJ, Greer R, et al. : Heart rate and blood pressure centile curves and distributions by age of hospitalized critically ill children. Front Pediatr. 2017; 5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.IntelliVue Patient Monitor: MP20/30, MP40/50, MP60/70/80/90, Release G. 0 With Software Revision G. 0x. xx (PHILIPS). 200. Available at: https://medaval.ie/docs/manuals/Philips-MP20-MP90-Manual.pdf. Accessed September 24, 2021
- 15.Hedeker D, Gibbons RD: Longitudinal Data Analysis. Vol. 451. John Wiley & Sons, 2006 [Google Scholar]
- 16.Bates D, Mächler M, Bolker B, et al. : Fitting linear mixed-effects models using lme4. J Stat Softw. 2014; 67:1–48 [Google Scholar]
- 17.Kunzetsova A, Brockhoff P, Christensen R: lmerTest package: Tests in linear mixed effect models. J Stat Softw. 2017; 82:1–26 [Google Scholar]
- 18.Stergiou GS, Alpert B, Mieke S, et al. : A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. Hypertension. 2018; 71:368–374 [DOI] [PubMed] [Google Scholar]
- 19.Rook W, Turner J, Clutton-Brock T: Analysis of damping characteristics of arterial catheter blood pressure monitoring in a large intensive care unit. South Afr J Crit Care. 2017; 33:8–10 [Google Scholar]
- 20.Balestrieri E, Rapuano S: Calibration of automated non invasive blood pressure measurement devices. In: Advances in Biomedical Sensing, Measurements, Instrumentation and Systems. Mukhopadhyay SC, Lay-Ekuakille A. (Eds). New York, New York, Springer, 2010, pp 281–304 [Google Scholar]
- 21.Siaron KB, Cortes MX, Stutzman SE, et al. : Blood pressure measurements are site dependent in a cohort of patients with neurological illness. Sci Rep. 2020; 10:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palatini P, Parati G: Blood pressure measurement in very obese patients: A challenging problem. J Hypertens. 2011; 29:425–429 [DOI] [PubMed] [Google Scholar]
- 23.Sheshadri V, Tiwari AK, Nagappa M, et al. : Accuracy in blood pressure monitoring: The effect of noninvasive blood pressure cuff inflation on intra-arterial blood pressure values. Anesth Essays Res. 2017; 11:169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balestrieri E, Daponte P, De Vito L, et al. : Oscillometric blood pressure waveform analysis: Challenges and developments. 2019 IEEE International Symposium on Medical Measurements and Applications (MeMeA). Istanbul, Turkey, IEEE, 2019, pp 1–6 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.