Abstract
Background:
Data are conflicting on whether proton pump inhibitors (PPIs) diminish the efficacy of clopidogrel. We investigated individual PPIs and adverse cardiovascular events in postpercutaneous coronary intervention (PCI) patients on dual antiplatelet therapy with clopidogrel.
Methods:
We searched Ovid-MEDLINE, EMBASE, and Cochrane from inception to March 2020 to identify studies that evaluated the efficacy and safety of clopidogrel added PPIs versus clopidogrel only in post-PCI patient. We extracted data from 28 studies for major adverse cardiovascular endpoints (MACE), myocardial infarction (MI), cardiovascular death, and gastrointestinal bleeding. Risk ratios (RR) and hazard ratios (HR) were pooled separately.
Results:
Data were extracted on 131,412 patients from the 28 studies included. Concomitant use of PPI with clopidogrel was associated with increased risk of MACE (RR 1.30; 95% confidence interval [CI] 1.15–1.48; P < .001) and MI (RR 1.43; 95% CI 1.25–1.64; P < .001). Random-effects meta-analyses with individual PPIs demonstrated an increased risk of MACE in those taking pantoprazole (RR 1.31; 95% CI 1.07–1.61, P = .01) or lansoprazole (RR 1.35; 95% CI 1.19–1.54, P < .0001) compared with patients not on PPIs. Likewise, in adjusted analyses, the pooled HR of adjusted events for MACEs showed that the increased risk of MACEs was similar for 4 classes of PPIs but not for rabeprazole (HR: 1.32; 95% CI 0.69–2.53, P = .40).
Conclusion:
The post-PCI patients on dual antiplatelet therapy with clopidogrel in the PPI group were associated with higher risk of MACE and MI. Although the results for rabeprazole were not robust, it was the only PPI that did not yield a significantly increased risk of MACE.
Keywords: clopidogrel, meta-analysis, percutaneous coronary intervention, proton pump inhibitor
1. Introduction
One concern with using proton pump inhibitors (PPIs) is the potential interaction with the clopidogrel's antiplatelet effect, a prodrug requiring activation by cytochrome P450 isoenzymes; the literature includes contrasting findings on this issue.[1] PPIs are competitive inhibitors of cytochrome P450. Moreover, individual PPIs have different effects on CYP metabolism and thus may adversely affect clopidogrel metabolism and subsequent cardiovascular (CV) outcomes to varying degrees.[2]
Compared with the numerous studies showing interactions between overall PPIs and clopidogrel, meta-analyses for the interactions between clopidogrel and individual PPIs in patients on dual antiplatelet therapy (DAPT) are scarce,[3,4,5] and previous meta-analyses only include articles published until 2014. Pending dedicated clinical trials aimed at addressing the question of the optimal PPI for the prevention of gastrointestinal (GI) bleeding after a percutaneous coronary intervention (PCI), an updated meta-analysis about this drug–drug interaction could be insightful. Recently, many studies have been published based on the CV outcomes observed in patients treated with clopidogrel and a PPI. Therefore, this study aimed to determine the association between individual PPIs and adverse CV events in post-PCI patients on DAPT with clopidogrel.
2. Materials and methods
This systematic review and meta-analysis fully adhered to the principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses[6] checklist. The study was registered with the International Prospective Register of Systematic Reviews, number CRD42020160651. The study protocol was approved by the institutional review board of Wonkwang University Hospital [IRB No. WMCSB 201912-100-1912101].
2.1. Ethical issues
This systematic review does not require ethical approval or informed consent because there was no direct contact with individual patients, and only previously published data were included in the review.
2.2. Data sources and searches
MEDLINE (through PubMed), EMBASE, the Cochrane Central Register of Controlled Trials, and relevant websites were searched for pertinent published or unpublished studies using keywords associated with clopidogrel and PPIs (from inception to March 22, 2020). A MEDLINE search strategy (Data S1, Supplemental Digital Content) was developed and modified for the other 2 databases as appropriate. Bibliographies of systematic and narrative reviews were also manually searched. No restrictions were imposed on language, study period, or sample size.
2.3. Study selection
We selected studies meeting the following prespecified criteria: studies comparing the outcomes of PPI versus no PPI in post-PCI patients receiving DAPT therapy consisting of aspirin and clopidogrel; studies with a full-text English language paper published in a peer-reviewed journal; and studies showing data for either one or more of the following outcomes: a major adverse cardiovascular endpoints (MACE) defined as a composite ischaemic endpoint: composite of cardiac and noncardiac death, nonfatal myocardial infarction (MI), and target vessel revascularisation; MI; CV death; or GI bleeding. We accepted the definitions of MACE, CV death, MI, and GI bleeding used in each study. Studies with the following characteristics were excluded from the meta-analysis: interactions with PPIs initially studied using a single antiplatelet agent; clopidogrel used for any other indications, such as peripheral vascular disease or stroke; concomitant use of anticoagulation drugs; significant difference between groups in baseline analysis or no mention of baseline characteristic analyses; and abstracts, letters, reviews, and meta-analyses. Randomised controlled trials (RCTs) and nonrandomised controlled trials (non-RCTs) were included. Eligible non-RCTs were adjusted appropriately for baseline differences between groups with or without the use of PPIs (propensity score-based matching or multivariate adjustment). Non-RCTs without multivariable or propensity score-based adjustment, and single-arm studies without comparison or control groups were not included. Two authors (DYL and BJK) screened titles and abstracts using the described search strategy; disagreements were resolved by 3 researchers (DYL, BJK, and JSK).
2.4. Data extraction, synthesis, and quality assessment
Two authors (DYL and BJK) independently used a standardised form to extract information on study characteristics, design, number of patients, regimen of the administered PPIs, and modifiers. The rates of CV mortality, MI, GI bleeding, and MACE transfusion were collected along with the definitions of outcomes used in each study. When additional information was required, the corresponding author was contacted. The primary outcome was MACE, which is a composite of CV death, MI, stroke, and revascularisation. Secondary outcomes were CV death, MI, and GI bleeding. The definitions of individual outcomes followed those defined in each trial. For all phases, discrepancies were resolved by discussion among 3 authors (DYL, BJK, and JSK).
The quality of eligible studies was assessed using the Cochrane Collaboration tool for assessing the risk of bias for RCTs, the Newcastle–Ottawa Scale (NOS),[7] and the strengthening the reporting of observational studies in epidemiology checklist for non-RCTs. We did not exclude individual studies from the analysis based on the thresholds of the NOS or strengthening the reporting of observational studies in epidemiology checklists.
2.5. Statistical analysis
The primary and secondary outcomes were analysed using a random-effects model with a generic inverse variance method. We calculated the risk ratio (RR)/relative risk with a 95% confidence interval (CI) using 2 × 2 tables from the original articles for MACE, MI, and CV deaths. In the case of reporting results as a hazard ratio (HR) only, the HR with a 95% CI was presented as a summary statistic. Primary literature with the effect size of RRs and HRs was summarised for abstraction. We used the definitions as reported in the included studies.
Heterogeneity was assessed using the I 2 test developed by Higgins, which measures the percentage of total variation across studies.[8,9] Publication bias was assessed using funnel plot asymmetry and Egger and Begg tests.[10] When visual asymmetry of the funnel plot was suspected, the trim-and-fill method was used to calculate an adjusted odds ratio. Subgroup analyses were performed according to the study design (RCT or non-RCT) and the use of individual PPI. Two-tailed P values <.05 were considered statistically significant. Statistical analysis was performed using Review Manager version 5.3.3 (RevMan; the Nordic Cochrane Centre, Copenhagen, Denmark) and R software version 3.4.2 (The R foundation for Statistical Computing).
3. Results
3.1. Search results
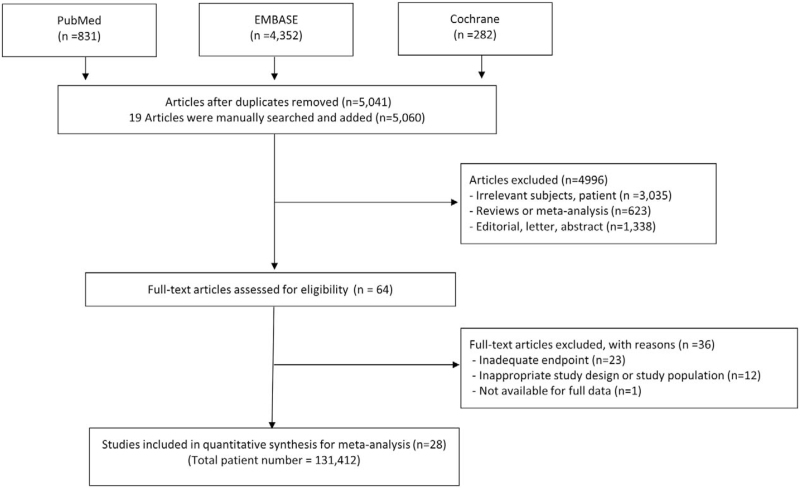
Figure 1 presents a flow diagram showing how relevant studies were identified. In total, 5465 candidate studies were identified after a search of 3 databases; 19 studies were added after a manual search. After excluding 424 duplicate studies, an additional 4996 studies were excluded during the initial screening through a review of titles and abstracts. The remaining 64 eligible studies were extracted for complete review. After careful evaluation with no disagreement between the 2 reviewers, 36 studies were excluded; 28 studies with a total of 131,412 patients met the inclusion criteria and were selected. The reasons for study exclusion during the final review were as follows: inadequate endpoints (n = 23), inappropriate study design or study population (n = 12), and full data not available (n = 1). The final 28 articles comprised 6 RCTs (Table 1) and 22 non-RCTs (Table 2).
Figure 1.
Flow chart of the study-selection process.
Table 1.
Summary of characteristics of randomized studies.
| No. of patients | Event number: MACE | |||||||||
| Author (yr) | Study design | Clinical setting (ACS/Mixed) | Follow-up (mo) | Nationality | Definition of composite outcome of MACE | PPI | PPI | Control | PPI | Control |
| Bhatt et al (2010)[17] | RCT | Mixed | 3.5 | Multinational, 15 countries | Composite of CV death, nonfatal MI, CABG, PCI, stroke) | O | 1876 | 1885 | 55 | 54 |
| Yano et al (2012)[13] | RCT | ACS | 12 | Japan | MI, revascularization, rehospitalization for ACS | O | 65 | 65 | 8 | 12 |
| Ng et al (2012)[14] | RCT | ACS | 4.5 | Hong Kong | CV mortality, nonfatal MI, stroke revascularization, revascularization | E | 163 | 148 | 7 | 5 |
| Zhang et al (2015)[15] | RCT | ACS | 6 | China | MI, revascularization, rehospitalization forACS | L | 53 | 51 | 7 | 5 |
| Wei et al (2016)[16] | RCT | ACS | 6 | China | cardiac death, MI, HF severe arrhythmia, angina | P | 117 | 80 | 48 | 33 |
| Ren et al (2011)[12] | RCT | ACS | 1 | China | CV mortality, nonfatal MI, strokerevascularization | O | 86 | 86 | 22 | 22 |
Table 2.
Summary characteristics of nonrandomized studies.
| No. of patients | Event number: MACE | |||||||||
| Author (year) | Study design | Clinical setting (ACS/Mixed) | Follow-up duration | Nationality | PPI | PPI use | Control | PPI use | Control | Definition of composite outcome of MACE |
| O’ Donoghue et al (2009) | Non-RCT | ACS | 15 | Multinational | O, P, E, L | 4529 | 9079 | 255 | 526 | Composite of CV mortality, nonfatal MI and stroke |
| Gaglia et al (2009) | Non-RCT | Mixed | 12 | USA | O, P, E, L, R | 318 | 502 | 6 | 5 | Composite of all-cause death MI revascularization, stent thrombosis |
| Rassen et al (2009) | Non-RCT | Mixed | 6 | USA | O, P, E, L, R | 3996 | 14,569 | 299 | 875 | MI |
| Ray et al (2010) | Non-RCT | Mixed | 12 | USA | O, P, E, L, R | 7226 | 8995 | 461 | 580 | Composite of CV mortality, nonfatal MI, stroke, sudden cardiac death |
| Stockl et al (2010) | Non-RCT | Mixed | 12 | USA | O, P, E, L, R | 1033 | 1033 | 133 | 94 | MI |
| Gupta et al (2010) | Non-RCT | Mixed | 50 | USA | O, P, R | 72 | 243 | 40 | 92 | CV death, all cause death TLR, TVF, MI |
| Kreutz et al (2010) | Non-RCT | Mixed | 12 | USA | O, P, E, L | 6828 | 9862 | 1710 | 1766 | Stroke, revascularization |
| Aihara et al (2012) | Non-RCT | Mixed | 24 | Japan | O, P, E | 500 | 500 | 22 | 21 | MI, CVA, all-cause death |
| Charlot et al (2010) | Non-RCT | ACS | 1 | Denmark | O, P, E, L | 6556 | 6556 | 1058 | 1506 | Composite of CV mortality, nonfatal MI, stroke |
| Tsai et al (2010) | Non-RCT | ACS | 12 | Taiwan | O, R | 1052 | 1325 | 121 | 62 | Hospitalization for MI, PCI, CABG, stroke |
| Evanchan et al (2010) | Non-RCT | Mixed | 12 | USA | O, P, E, L | 6828 | 9862 | 1710 | 1766 | MI |
| Rossini et al (2011) | Non-RCT | Mixed | 12 | Italy | O, P, L | 1158 | 170 | 87 | 10 | All-cause death, nonfatal MI, stent thrombosis stroke |
| Simon et al (2011) | Non-RCT | ACS | 12 | France | O, P, E, L, R | 1453 | 900 | 125 | 100 | Composite of all cause death mortality, nonfatal MI, stroke |
| Goodman et al (2012) | Non-RCT | ACS | 12 | Multinational | O, P, E, L, R | 3255 | 6021 | 398 | 611 | MI, stroke, CV death MI, stroke, Stent thrombosis |
| Burkard et al (2012) | Non-RCT | Mixed | 36 | Switzerland | O, P, E | 109 | 692 | 33 | 144 | Composite of nonfatal MI, death TVR |
| Chitose et al (2012) | Non-RCT | Mixed | 18 | Japan | NR | 331 | 939 | 11 | 32 | Composite of no fatal MI, stroke, CV death |
| Hokimoto et al (2014) | Non-RCT | Mixed | 18 | Japan | R | 50 | 124 | 5 | 10 | CV death, nonfatal MI, revascularization, ACS |
| Zou et al (2014) | Non-RCT | ACS | 12 | China | O, P, E | 6188 | 1465 | 860 | 155 | Composite of no fatal MI, TVR all cause death, CV death stent thrombosis |
| Shih et al (2014) | Non-RCT | Mixed | 4 | Taiwan | NR | 5430 | 5430 | 223 | 153 | MI |
| Weisz et al (2015) | Non-RCT | Mixed | 24 | USA, Germany | NR | 2162 | 6419 | 238 | 531 | Composite of no fatal MI, TVR CV death, all cause death |
| Gargiulo et al (2016) | Non-RCT | Mixed | 24 | Italy | L | 738 | 1232 | 85 | 113 | CVA, all-cause death nonfatal MI |
| Van Boxel et al (2010) | Non-RCT | Mixed | 12 | Netherlands | O, P, E | 5734 | 12,405 | 754 | 830 | Composite of ACS, stroke and/or all-cause mortality |
3.2. Risk of bias for included studies
The major characteristics of the 28 studies are summarised in Tables 1 and 2. The risk of bias for the RCTs and the NOS scores of the non-RCTs are shown in Table S1, Supplemental Digital Content and Table S2, Supplemental Digital Content, respectively. The 6 RCTs included 4675 patients, whereas the 22 non-RCTs included 126 708 patients. For the posthoc analyses of the RCTs,[11] the populations and outcomes of interest (DAPT plus PPI versus DAPT only) were not randomised; therefore, they were included in the analyses of the non-RCTs. Among the 6 RCTs, 1 did not report the method of random sequence generation[12,13,14] and allocation concealments[12,13,15] (Table S1, Supplemental Digital Content). All endpoints were objective findings (MACE, CV death, MI, GI bleeding) and the judgement of outcomes was not likely to be influenced by blinding. The NOS scores of the 22 included studies ranged from 6 to 9 stars (Table S2, Supplemental Digital Content). The risk of selection and comparability bias was considered low in all studies except for those by Goodman et al and Gargiulo et al, as these did not adjust for potential confounders because of the posthoc analysis of the RCT. Outcome bias was counted as moderate for studies that did not report data on patients lost to follow-up. All included studies were published between 2009 and 2016. The characteristics of the studies involved in the meta-analysis are summarised in Tables 1 and 2 according to the major outcome groups. Altogether, we found data for MACE in 24 publications, CV death in 11 publications, MI in 14 publications, and GI bleeding in 4 publications. Seventeen studies were performed in the USA and Europe, while 11 studies were conducted in Asia.
3.3. The overall effect of concomitant administration of any PPI and clopidogrel on MACEs in patients post-PCI compared to patients not on PPI therapy
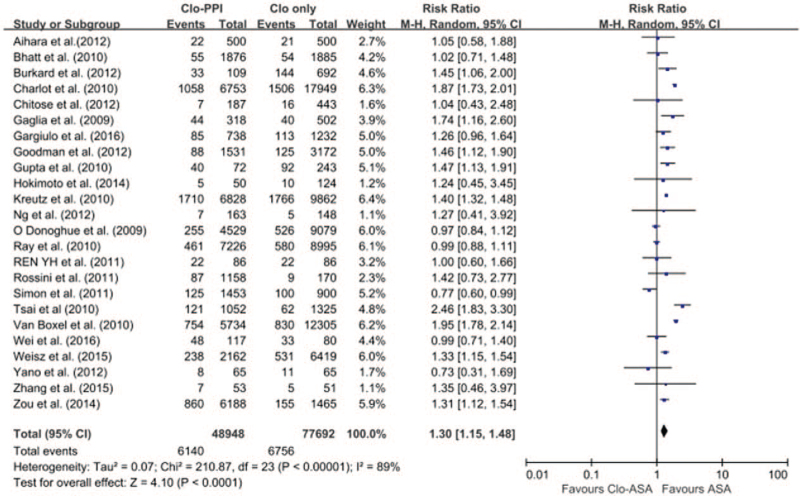
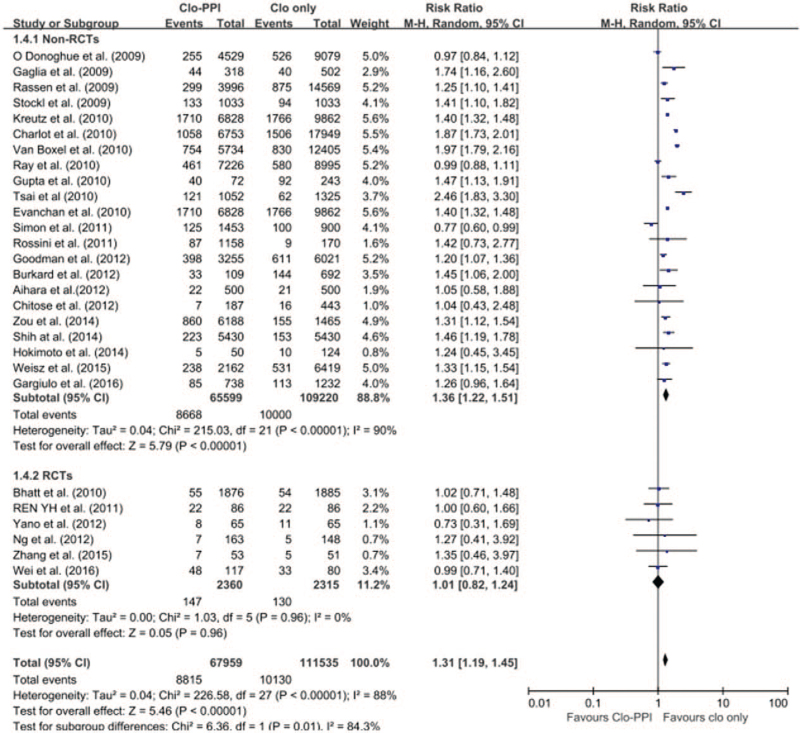
The pooled meta-analysis of 24 studies (6 RCTs[12,13,14,15,16,17] and 18 non-RCTs) revealed a higher risk of MACE in patients with any PPI (RR 1.30; 95% CI 1.15–1.48; I2 = 89.0%; P < .001) in a random-effects meta-analysis (Fig. 2). Stratification using RCT and non-RCTs showed that the risk of MACE in patients receiving any PPI and clopidogrel was significantly increased in the 18 non-RCTs with the random-effects model meta-analysis. However, in the 6 RCTs, there was no significant increased risk of MACE (non-RCTs: RR 1.36; 95% CI 1.22–1.51; I 2 = 90%, P < .001; RCTs: RR 1.01; 95% CI 0.82–1.24; I2 = 0; P = .96) (Fig. 3). Given the differences in the duration of follow-up both in the RCTs and non-RCTs, a meta-regression was conducted for the length of follow-up. MACE did not depend on the length of follow-up (SE = 0.014; 95% CI = −0.029 to 0.027; P = .93).
Figure 2.
Forest plot of the pooled meta-analysis of 28 studies (6 RCT and 22 non-RCT studies), exhibiting a higher risk of MACE in patients with any PPI versus those without PPI in a random effect meta-analysis. CI = confidence interval, MACE = major adverse cardiovascular endpoints, non-RCTs = nonrandomised observational studies, PPI = proton pump inhibitor, RCTs = randomised controlled trials.
Figure 3.
The risk of MACE in patients receiving any PPI versus those without PPI. By stratifying according to RCTs and non-RCTs, the risk of MACE in patients receiving any PPI and clopidogrel was significantly increased in 19 non-RCTs with random-effects model meta-analysis. However, in 6 RCTs, there was no significant increased risk of MACE. CI = confidence interval, MACE = major adverse cardiac events, non-RCTs = nonrandomised observational studies, PPI = proton pump inhibitor, RCT = randomised controlled trial.
Publication bias was not evident when the symmetrical funnel plot supported by Egger and Begg tests was used (Fig. S1A, Supplemental Digital Content).
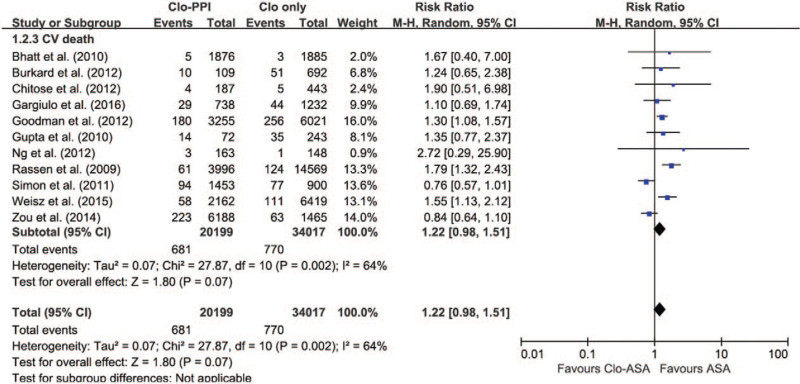
3.4. Cardiovascular death for any PPI compared to no PPI
Data on CV death were reported in 11 studies that included 54 216 patients; only 1 study was evaluated as an RCT.[17] A significant effect of concomitant clopidogrel and any PPI treatment on CV death was observed using a random-effects model analysis (Fig. 4; RR = 1.22; 95% CI 0.98–1.51; I 2 = 64%). The length of follow-up of the patients did not affect the risk for CV death based on the results of the 11 studies that were included (follow-up: SE = 0.015; 95% CI −0.045 to 0.014; P = .30).
Figure 4.
The risk of cardiovascular death in patients receiving any PPI versus those without PPI. CI = confidence interval, CV = cardiovascular, PPI = proton pump inhibitor.
A funnel plot inspection and Egger statistical test for CV death revealed significant asymmetry in the concomitant use of PPIs and clopidogrel. Since visual asymmetry of the funnel plot was suspected, the trim-and-fill method was used to calculate RR with 95% CIs using a random- effects model (RR = 1.45; 95% CI 1.24–1.69; I 2 = 61%; P < .0001) (Fig. S1B, Supplemental Digital Content). Unfortunately, the small amount of data prevented us from evaluating the risk of CV death for specific PPIs.
3.5. Myocardial infarction for any PPI compared to no PPI
Fourteen studies contained eligible data on MI in 71 268 patients. For the evaluation, 1 study was evaluated as an RCT.[17] The risk of MI was significantly higher in the PPI group (RR = 1.43; 95% CI 1.25–1.64; I 2 = 61%; P < .001) in a random-effects analysis (Fig. 5). For analyses excluding the 1 RCT,[17] there were no significant changes for the summary RR estimate (RR 1.45; 95% CI 1.26–1.67; I 2 = 62%; P = .002). Similar to MACE and CV death, MI did not depend on the length of follow-up based on the included 14 studies (follow-up: SE = 0.016; 95% CI −0.055 to 0.009; P = .17). There was no distinct discrepancy between the different follow-up durations. Publication bias was not evident from the visual examination of the symmetrical funnel plot supported by the Egger and Begg tests (Fig. S1C, Supplemental Digital Content).
Figure 5.
The risk of myocardial infarction in patients receiving any PPI versus those without PPI. CI = confidence interval, MI = myocardial infarction, PPI = proton pump inhibitor.
3.6. Gastrointestinal (GI) bleeding for any PPI compared to no PPI
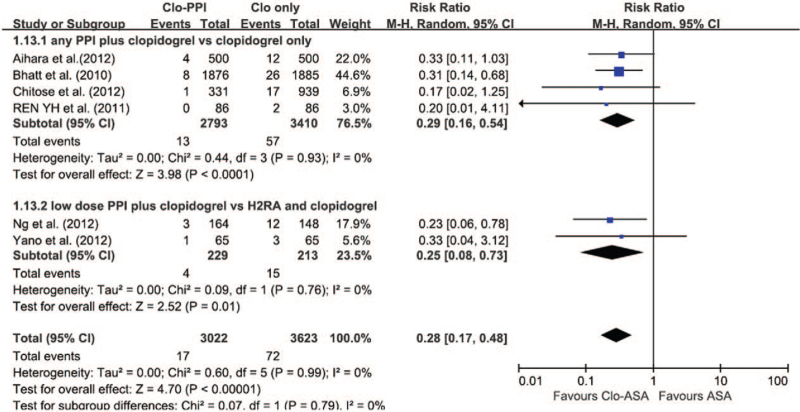
Unfortunately, the small amount of data prevented us from evaluating the risk of GI bleeding for specific PPIs. Random-effects meta-analysis suggested that PPI therapy with DAPT was superior to DAPT alone for preventing GI bleeding (RR 0.29; 95% CI 0.16–0.54; P < .0001; I 2 = 0%) (Fig. 6). The risk of GI bleeding with half-dose PPI co-therapy was also lower than that in the histamine 2 receptor antagonist treatment groups (RR 0.25; 95% CI 0.08–0.73; P = .01; I 2 = 0%).
Figure 6.
The risk of DAPT-related upper GI bleeding in patients receiving any PPI versus those without PPI. The risk of MACE with half-dose PPI co-therapy was also superior to that in the histamine 2 receptor antagonist (H2RA) treatment groups. The high-dose cutoff points were esomeprazole > 40 mg, omeprazole > 20 mg, pantoprazole > 40 mg, rabeprazole > 20 mg, and lansoprazole > 30 mg. CI = confidence interval, DAPT = dual antiplatelet therapy, GI = gastrointestinal, MACE = major adverse cardiac events, PPI = proton pump inhibitor.
3.7. Subgroup analyses for MACEs for individual PPIs compared to no PPI
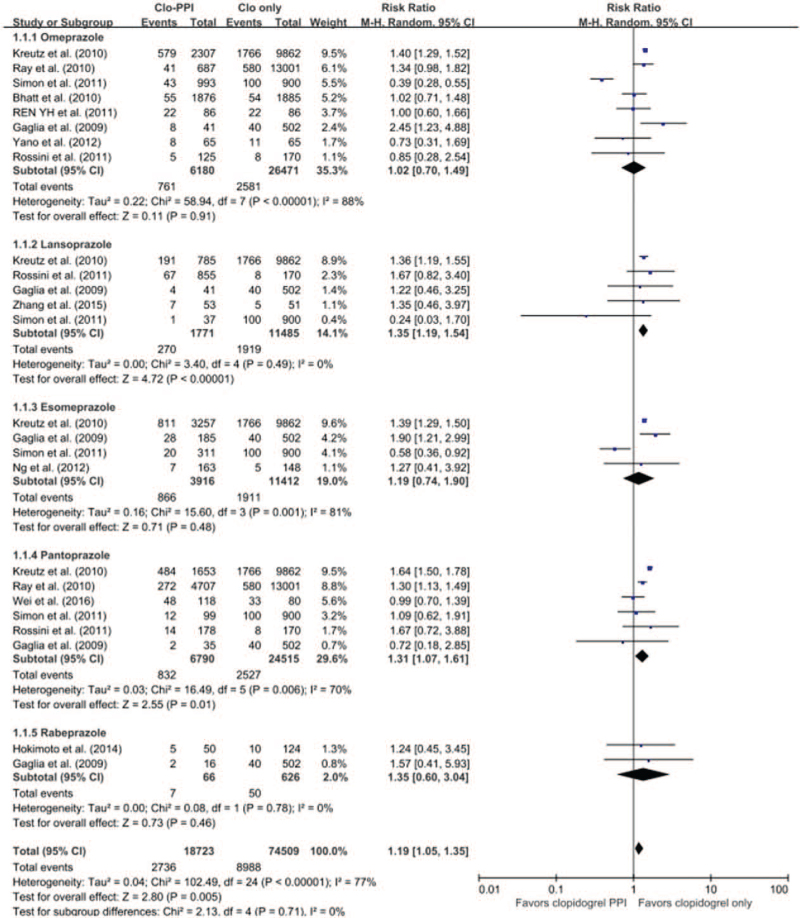
The risk of MACEs in patients receiving an individual PPI versus no PPI from the pooled meta-analysis of 13 studies (6 RCTs[12,13,14,15,16,17] and 7 non-RCTs) is shown in Figure 7.
Figure 7.
The risk of MACEs in patients receiving individual PPIs versus those without PPI from the pooled meta-analysis of 14 studies. The risk of MACE in patients receiving individual PPI versus those without PPI. CI = confidence interval, MACE = major adverse cardiac events, PPI = proton pump inhibitor.
Eight studies with omeprazole (3 RCTs[12,13,17] with 4063 patients and 5 non-RCTs with 28 588 patients) showed no significantly increased risk of adverse CV outcomes (RR 1.02; 95% CI 0.70–1.49; I 2 = 88%; P = .91). Stratification using RCTs (RR 0.98; 95% CI 0.74–1.30; I 2 = 0%; P = .88) and non-RCTs (RR 1.08; 95% CI 0.62–1.88; I 2 = 93%; P = .79) showed that the risk of MACE was not significantly increased in either type of study.
Five studies (1 RCT[15] with 104 patients and 4 non-RCTs with 13,152 patients) have been reported on lansoprazole. On average, the lansoprazole studies showed an overall increased risk (RR 1.35; 95% CI 1.19–1.54; I 2 = 0%; P < .001). For analyses excluding the 1 RCT,[15] there were no significant changes for the summary RR estimate (RR 1.34; 95% CI 1.02–1.73; I 2 = 12%; P = .02).
Four studies (1 RCT[14] with 311 patients and 3 non-RCTs with 15,017 patients) on esomeprazole showed no significantly increased risk (RR 1.19; 95% CI 0.74–1.90; I 2 = 81%; P = .48) in a random-effects model analysis. In an analysis of the 3 non-RCTs only, the result was similar (RR 1.17; 95% CI 0.68–2.01; I 2 = 87%; P = .48).
Six studies (1 RCT[18] with 198 patients and 5 non-RCTs with 31,107 patients) were reported on pantoprazole. These 6 studies showed a significantly increased risk of CV adverse events when the random-effects model analysis was used (RR 1.31; 95% CI 1.07–1.61; I 2 = 70%; P = .01). In analyses excluding the 1 RCT,[16] there were no significant changes for the summary RR estimate (RR 1.41; 95% CI 1.16–1.72; I 2 = 62%; P < .001).
There were 2 non-RCTs with 692 patients evaluating rabeprazole; the pooled RR was not statistically significant with a random-effects model analysis (RR 1.35; 95% CI 0.60–3.04; I 2 = 0%; P = .46).
To further explore individual studies, we repeated the entire analysis, excluding the study by Simon et al as seen in Fig. S1, Supplemental Digital Content all RRs with different PPIs were increased and there were evident individual study effects for MACEs.
A different pattern was present when studies that used adjusted HRs were pooled. The results of analysing the adjusted events for MACE with different PPIs are presented in Fig. S2, Supplemental Digital Content. A random-effects meta-analysis of the adjusted events for MACE from non-RCTs showed an overall increased risk of MACE for all PPIs except for rabeprazole (HR: 1.32; 95% CI 0.69–2.53; I 2 = 0; P = .40) in Fig. S2, Supplemental Digital Content.
Publication bias was not evident according to the symmetrical funnel plot supported by the Egger and Begg tests. Although most of the methods for the detection of publication bias were underpowered due to a small number of publications (<10), thorough statistical analyses were performed. Using different PPIs with clopidogrel did not suggest significant asymmetries when funnel plots and the Egger test (Egger test: P = .33, P = .80, P = .81, P = .22, and P = .97 for omeprazole, lansoprazole, esomeprazole, pantoprazole, and rabeprazole, respectively) were used (Fig. S3, Supplemental Digital Content).
4. Discussion
We performed an updated meta-analysis that investigated the safety and efficacy of PPIs with clopidogrel following PCI. The principal findings were as follows: although the study design affected the results of PPI use with clopidogrel, PPI as a class effect showed an increase in MACE and MI in post-PCI patients on DAPT; for the analysis of the specific effects of individual PPIs, we obtained inconsistent findings in the evaluation of the association between concomitant clopidogrel use and the risk of MACE; and confined to the analyses of the non-RCTs with adjusted HR, studies with individual PPIs, except for rabeprazole, showed a significantly increased risk of MACE.
Because the PPI-induced risk reduction clearly outweighs the possible adverse CV risk in patients with a high risk of GI bleeding, a combination of clopidogrel with PPIs is recommended in the ACC/ACG/AHA 2010 Expert Consensus Document[19] and the European Society of Cardiology 2017 guidelines.[20] However, current FDA recommendations warn against concomitant therapy of clopidogrel and omeprazole or esomeprazole, but not other PPIs[21]; hence, there is a need for further research to evaluate drug safety.
Melloni et al[22] indicated that large, well-conducted non-RCTs of PPIs[23,24] and RCTs of omeprazole[17] appeared to provide conflicting results regarding the effect of PPIs on CV outcomes when co-administered with DAPT. Additionally, 3 meta-analyses[3,4,5] that were conducted for the association of clopidogrel and individual PPIs in post-PCI patients showed inconsistent results and different inclusion criteria. Niu et al[3] and Kwok et al[4] found a significant association except for rabeprazole. Sherwood et al[5] included extensive, well-conducted non-RCTs presenting only adjusted HRs and found a significant association for all PPIs except omeprazole.
PPIs are metabolised mainly by CYP2C19 and inhibition of this enzyme is heterogeneous within the class of PPIs. PPIs are classified based on their binding affinity for CYP2C19, including those with high and low CYP2C19-inhibitory potential.[11] The hepatic metabolism of rabeprazole involves both CYP-mediated and non-CYP-mediated metabolism, with the latter taking the dominant role. Compared with the other PPIs, rabeprazole is a weaker competitive inhibitor for CYP2C19 and has a minimal effect on the metabolic pathway of clopidogrel.[25] Moreover, the plasma concentration of several PPIs, except rabeprazole, decreased by up to 40% in the case of postprandial medication.[26] We do not know how many enrolled patients took PPIs in a fasting state. Therefore, current meta-analyses with inconsistent findings should be interpreted with caution after considering correct adherence to PPI indications.
Even with the large, well-conducted non-RCTs of PPIs, there could be an unbalanced distribution of baseline characteristics, such as sicker patients in the PPI group. Similarly, patients on a PPI were 3 years older than those in the comparison group and also had a higher prevalence of CV disease, chronic kidney disease, and diabetes at baseline in Charlot et al study,[23] and patients on clopidogrel had a higher prevalence of Charlson comorbidity index at baseline in Bhurke et al study.[24] Given that PPIs are not expected to have significant interaction with clopidogrel, those findings support the hypothesis that using a PPI is not the cause of increased adverse results, but rather a marker of increased baseline characteristic risk. Considering that the COGENT study[17] and a study by Jensen et al[27] remain the only 2 large-scale RCTs exploring the interaction between thienopyridine and PPIs, we had no choice but to review and include several small- to medium-sized RCTs and non-RCTs with extensive review.
As with previous studies, concomitantly administered PPIs with clopidogrel had a protective effect on GI bleeding in our analysis. Moreover, Yano et al[13] and Ng et al[14] demonstrated that the use of low-dose PPIs did not lead to an increased risk of MACE with a sustaining protective effect against GI bleeding (the PPIs used in those studies were rabeprazole 10 mg, esomeprazole 20 mg, and omeprazole 10 mg). Therefore, low-dose PPIs can be an option for clinicians who are simultaneously concerned about the risk of clopidogrel–PPI interaction and the need to prevent GI bleeding.
Our confidence in the results of the current meta-analysis is strengthened by the following few points. First, the enrolment of a large population from a balanced database reflected real clinical practice. All included non-RCTs had adjusted outcomes except for previous studies by Goodman et al[28] and Gargiulo et al,[18] which were posthoc analyses of multicentre RCTs with no apparent baseline difference. Second, we also conducted subgroup analysis based on the modifiers. Compared with studies evaluating PPIs as a class, separate meta-analyses were rare because of a lack of subgroup reporting. We tried to discover which PPIs are safe in post-PCI patients by making the most of the existing studies published so far. Lastly, this study focused specifically on PCI patients with DAPT medication, in whom the effect of this drug–drug interaction could have a substantial impact because of the higher baseline risk of MACE outcomes in PCI patients.
Despite its strengths, the present study has several limitations. First, there was no large-scale RCT conducted on the interaction between DAPT with clopidogrel and PPIs except for the COGENT trial[24] discovered using our search strategy, and this study would seem to negate the clinical impact of the interaction. These results suggested that omeprazole in association with clopidogrel did not increase MACEs, although it did reduce GI bleeding. Second, though a few small-sized RCTs were enrolled in our meta-analysis, most of the patients in this study were from nested case-control or cohort studies. Therefore, detailed data on drugs, such as adherence to PPI, dose, and duration could not be fully assessed. Observational studies may be confounded by selection bias if they include sicker patients on PPI and thus are at higher risk for adverse CV outcomes. We used the RR or adjusted HR if provided, which was most likely different among studies. Moreover, the discrepancies among the results between the RCT and non-RCT studies may have weakened our results. Third, because of the relatively small number of studies on rabeprazole, more trials are needed in the future to better support the drug interaction. Lastly, there were slight differences in the MACE definitions and in the adjusting covariates used in the meta-analysis, which may have introduced some imprecision in our effect estimates.
In conclusion, our meta-analysis shows that there is evidence for a significant association between the risk of MACE and PPI use as a class effect in post-PCI patients on DAPT. Our subgroup analysis shows no consistent evidence of MACE among individual PPIs when used with clopidogrel except for rabeprazole. However, due to the small number of rabeprazole trials, more trials are needed in the future to support this conclusion.
Author contributions
Conceptualization: Dongyoung Lee, Je Sang Kim, Seung Yong Shin, Dong Bin Kim, Hyung Sik Ahn.
Data curation: Dongyoung Lee, Je Sang Kim, Beom Jin Kim.
Formal analysis: Dongyoung Lee, Je Sang Kim, Beom Jin Kim.
Investigation: Dongyoung Lee, Je Sang Kim, Beom Jin Kim, Seung Yong Shin, Dong Bin Kim.
Methodology: Dongyoung Lee, Je Sang Kim, Beom Jin Kim, Seung Yong Shin, Dong Bin Kim, Hyung Sik Ahn.
Supervision: Beom Jin Kim, Seung Yong Shin, Dong Bin Kim, Hyung Sik Ahn.
Writing – original draft: Je Sang Kim.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CV = cardiovascular, DAPT = dual antiplatelet therapy, GI = gastrointestinal, HRs = hazard ratios, MACE = major adverse cardiovascular endpoints, MI = myocardial infarction, non-RCTs = nonrandomised controlled trials, NOS = Newcastle–Ottawa Scale, PCI = percutaneous coronary intervention, PPI = proton pump inhibitor, RCTs = randomised controlled trials, RRs = risk ratios.
How to cite this article: Lee D, Kim JS, Kim BJ, Shin SY, Kim DB, Ahn HS. Influence of individual proton pump inhibitors on clinical outcomes in patients receiving clopidogrel following percutaneous coronary intervention. Medicine. 2021;100:52(e27411).
DL and JSK equally contributed to this work.
The authors have no funding and conflicts of interest to disclose.
All authors approved the final version of the article.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
CV = cardiovascular, MACE = major adverse cardiovascular endpoints, MI = myocardial infarction, PCI = percutaneous coronary intervention, RCT = randomised controlled trial.
CV = cardiovascular, MACE = major adverse cardiovascular endpoints, MI = myocardial infarction, non-RCTs = nonrandomised controlled trials, PPI = proton pump inhibitor.
References
- [1].Sudlow CL, Mason G, Maurice JB, WedderburnF CJ, HankeyF GJ. Thienopyridine derivatives versus aspirin for preventing stroke and other serious vascular events in high vascular risk patients. Cochrane Database Syst Rev 2009;2009:CD001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tantry US, Kereiakes DJ, Gurbel PA. Clopidogrel and proton pump inhibitors: influence of pharmacological interactions on clinical outcomes and mechanistic explanations. JACC Cardiovasc Interv 2011;4:365–80. [DOI] [PubMed] [Google Scholar]
- [3].Niu Q, Wang Z, Zhang Y, et al. Combination use of clopidogrel and proton pump inhibitors increases major adverse cardiovascular events in patients with coronary artery disease: a meta-analysis. J Cardiovasc Pharmacol Ther 2017;22:142–52. [DOI] [PubMed] [Google Scholar]
- [4].Kwok CS, Jeevanantham V, Dawn B, et al. No consistent evidence of differential cardiovascular risk amongst proton-pump inhibitors when used with clopidogrel: meta-analysis. Int J Cardiol 2013;167:965–74. [DOI] [PubMed] [Google Scholar]
- [5].Sherwood MW, Melloni C, Jones WS, et al. Individual proton pump inhibitors and outcomes in patients with coronary artery disease on dual antiplatelet therapy: a systematic review. J Am Heart Assoc 2015;4:e002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- [7].Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa: Ottawa Hospital Research Institute. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed March 1, 2017. [Google Scholar]
- [8].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Oxford: The Cochrane Collaboration; 2011. [Google Scholar]
- [10].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [11].O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009;374:989–97. [DOI] [PubMed] [Google Scholar]
- [12].Ren YH, Zhao M, Chen YD, et al. Omeprazole affects clopidogrel efficacy but not ischemic events in patients with acute coronary syndrome undergoing elective percutaneous coronary intervention. Chin Med J (Engl) 2011;124:856–61. [PubMed] [Google Scholar]
- [13].Yano H, Tsukahara K, Morita S, et al. Influence of omeprazole and famotidine on the antiplatelet effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes: a prospective, randomized, multicenter study. Circ J 2012;76:2673–80. [DOI] [PubMed] [Google Scholar]
- [14].Ng FH, Tunggal P, Chu WM, et al. Esomeprazole compared with famotidine in the prevention of upper gastrointestinal bleeding in patients with acute coronary syndrome or myocardial infarction. Am J Gastroenterol 2012;107:389–96. [DOI] [PubMed] [Google Scholar]
- [15].Zhang JR, Wang DQ, Du J, et al. Efficacy of clopidogrel and clinical outcome when clopidogrel is coadministered with atorvastatin and lansoprazole: a prospective, randomized, controlled trial. Medicine (Baltimore) 2015;94:e2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wei P, Zhang YG, Ling L, et al. Effects of the short-term application of pantoprazole combined with aspirin and clopidogrel in the treatment of acute STEMI. Exp Ther Med 2016;12:2861–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010;363:1909–17. [DOI] [PubMed] [Google Scholar]
- [18].Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the prolonging dual-antiplatelet treatment after grading stent-induced intimal hyperplasia study trial. Am Heart J 2016;174:95–102. [DOI] [PubMed] [Google Scholar]
- [19].Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol 2010;105:2533–49. [DOI] [PubMed] [Google Scholar]
- [20].Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–77. [DOI] [PubMed] [Google Scholar]
- [21].U.S. Food and Drug Administration. Interaction between esomeprazole/omeprazole and clopidogrel label change. Updated December 11, 2012. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm327922.htm. Accessed June 26, 2016. [Google Scholar]
- [22].Melloni C, Washam JB, Jones WS, et al. Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: systematic review. Circ Cardiovasc Qual Outcomes 2015;8:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Charlot M, Ahlehoff O, Norgaard ML, et al. Proton-pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: a nationwide cohort study. Ann Intern Med 2010;153:378–86. [DOI] [PubMed] [Google Scholar]
- [24].Bhurke SM, Martin BC, Li C, et al. Effect of the clopidogrel-proton pump inhibitor drug interaction on adverse cardiovascular events in patients with acute coronary syndrome. Pharmacotherapy 2012;32:809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ahn JH, Park Y, Bae JS, et al. Influence of rabeprazole and famotidine on pharmacodynamic profile of dual antiplatelet therapy in clopidogrel-sensitive patients: the randomized, prospective, PROTECT trial. Platelets 2020;31:329–36. [DOI] [PubMed] [Google Scholar]
- [26].Furuta K, Adachi K, Aimi M, et al. Effect of timing of proton pump inhibitor administration on acid suppression. Digestion 2016;93:111–20. [DOI] [PubMed] [Google Scholar]
- [27].Jensen BES, Hansen JM, Larsen KS, et al. Randomized clinical trial: the impact of gastrointestinal risk factor screening and prophylactic proton pump inhibitor therapy in patients receiving dual antiplatelet therapy. Eur J Gastroenterol Hepatol 2017;29:1118–25. [DOI] [PubMed] [Google Scholar]
- [28].Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation 2012;125:978–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.