Abstract
This study aimed to assess the impact of the pharmacist-led intervention on perioperative antibiotic prophylaxis by standardizing the cephalosporin intradermal skin test in the orthopedic department.
A pre-and postintervention study was conducted among patients in the Orthopedics Department at the Beijing Chao-Yang Hospital in China. Use of intradermal skin test, perioperative antibacterial prophylaxis, and cost of care were compared between the preintervention population (admitted from 6/1/2018 to 8/31/2018) and postintervention population (admitted from 1/1/2019 to 3/31/2019). Logistic regression and generalized linear regression were used to assess the intervention impact.
425 patients from the preintervention period and 448 patients from the postintervention period were included in the study. After the implementation of the pharmacist intervention program, there was a decrease in the utilization of intradermal skin tests, from 95.8% to 16.5% (P < .001). Patients were more likely to have cephalosporin as prophylactic antimicrobials (OR = 5.28, P < .001) after the implementation. The cost of antimicrobials was significantly reduced by $150.21 (P < .001) for each patient.
Pharmacist-involved intervention can reduce the utilization of cephalosporins skin tests and decrease the prescription of unnecessary high-cost antimicrobials.
Keywords: anti-bacterial agents, antibiotic prophylaxis, antimicrobial stewardship, orthopedics
1. Introduction
Cephalosporins are currently one of the most commonly prescribed antimicrobials due to their broad spectrum of antimicrobial activity, low toxicity profile, and good pharmacoeconomic properties worldwide.[1] According to global guidelines, cephalosporins are recommended for the prevention of surgical site infections as the first-line prophylaxis for a variety of surgical procedures.[2]
Nevertheless, beta-lactam antimicrobials including penicillin and cephalosporins are the most common class of drugs suspected when drug hypersensitivity reactions occur.[3] The prevalence of beta-lactam antimicrobials allergy goes from 0.7% to 10% in the general population.[4] Anaphylactic and anaphylactoid reactions occurring during anesthesia are also concerns of medical practitioners since they are usually unpredictable and may be potentially life-threatening even when appropriately treated. Several cases of perioperative anaphylaxis caused by cephalosporins have been reported.[5,6] In a 2 years survey of such reactions in France, penicillin and cephalosporins were identified as the offending agents in 33 and 31 cases, respectively, from 518 cases of perioperative anaphylaxis.[7]
However, overdiagnosis of beta-lactam allergy is a significant and growing public health problem. Once an individual is considered allergic to beta-lactam antimicrobials, it was rarely questioned, re-evaluated, or verified. Even worse, due to lack of knowledge about beta-lactam allergy, some physicians developed a prescribing habit to cross off all beta-lactam antimicrobials for patients with a history of allergy.[8,9,10] Moreover, in China, in order to avoid allergic reactions during perioperative, to reduce the medical risks or conflicts between doctors and patients, the intradermal skin tests of cephalosporins were conducted on all patients regardless of drug allergic history and the false-positive rate was high.[11,12]
For years, pharmaceutical care has been a key strategy to improve healthcare safety. Pharmaceutical care is a collaborative care approach which implies all the actors of the medication circuit in order to prevent and correct drug-related problems that can lead to adverse drug events.[13] One way to improve medication safety in hospitals is to integrate clinical pharmacists into the medication process. According to available data, the integration of a clinical pharmacist in multi-professional teams during admission, hospitalization and discharge can significantly reduce drug-related problem, costs and increases efficacy of drug therapy.[14] In terms of antimicrobial stewardship, pharmacists are acknowledged as health care professionals specializing in pharmacotherapy outcomes and management, responsibility is to conserve the effectiveness of antimicrobials and to promote appropriate antibiotic use.[15]
Fluoroquinolones, clindamycin, vancomycin, and other broad-spectrum antimicrobials as alternatives have led to worse clinical outcomes, increased incidence of serious antibiotic-resistant infections, and prolonged hospitalizations.[16] In 2016, the Infectious Diseases Society of America antimicrobial stewardship guidelines emphasized the burden of antibiotic allergy and recommended that the assessment of antibiotic allergy should be included in antimicrobial stewardship programs.[17] This study aimed to assess the impact of the pharmacist intervention on perioperative antibiotic prophylaxis by standardizing the cephalosporin intradermal skin test in orthopedic department.
2. Methods
2.1. Settings and study population
The study was a pre-post intervention study conducted in the Orthopedics Department at the Beijing Chao-Yang Hospital, Capital Medical University. Changes in perioperative antibiotic prophylaxis were compared between 425 patients from the preintervention period (from June 1, 2018, to August 31, 2018) and 448 patients from the postintervention period (from January 1, 2019, to March 31, 2019).
All patients admitted to the hospital for the following orthopedic procedures were included in this study: repair and plastic operations on joint structures, reduction of fracture and dislocation, operations on muscle, tendon, fascia, and bursa, incision and excision of joint structures, operations on the spinal cord and spinal canal structures, and other operation on bones except facial bones. Patients were excluded if they had any of the listed conditions:
-
1.
patients had no surgical indications after evaluation;
-
2.
only clean orthopedic procedures were needed without the use of prophylaxis;
-
3.
patients had a diagnosis of infectious disease such as staphylocoelitis;
-
4.
patients had preoperative infections and were using antimicrobials;
-
5.
patients had grade III open fractures with extensive soft tissue damage and crushing;
-
6.
patients were transferred to the surgical intensive care unit after operations.
2.2. Interventions
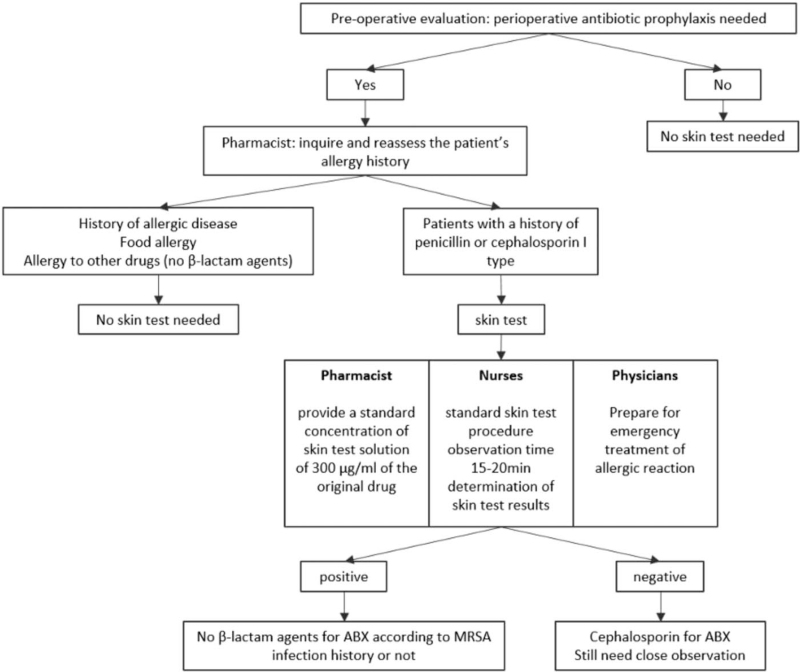
Pharmacists who participated in antimicrobial stewardship projects for perioperative prophylaxis developed the following interventions:
-
1.
joining the treatment team and participating in ward rounds;
-
2.
conducting medication reconciliation;
-
3.
inquiring and reassessing the patient's allergy history;
-
4.
providing a standard concentration of skin test solution by the pharmacy intravenous admixture services with the concentration of 300 μg/mL of the original drug;
-
5.
providing training to all the medical staff of the orthopedics department on limiting intradermal skin test only to the patients with allergic history using the standard concentration solution provided by pharmacy intravenous admixture services.
The detailed intervention process flow is presented in Figure 1.
Figure 1.
Intervention Flow Chart.
2.3. Data source
Patients’ demographic information, diagnosis, clinical outcomes, and antimicrobial data were manually retrieved from the electronic health record system of the hospital. Surgical procedures were recorded using the International Classification of Diseases, 9th version, Procedure Codes (ICD -9-PCS).
The following data were collected:
-
1.
demographics like patient gender and age;
-
2.
admission and discharge diagnosis and case mix index (CMI) which indicates patients’ condition severity;
-
3.
procedure type according to the ICD-9-PCS and duration of surgery;
-
4.
allergy information including food, drug, and disease allergic history, the results of intradermal skin tests, and the allergic reactions after medication;
-
5.
perioperative antibiotic prophylaxis data such as antimicrobial agents, duration and adverse effects;
-
6.
pharmacoeconomic indicators such as total drug cost and antibiotic cost.
The protocol was approved by the Drug and Therapeutics Committee and the Ethics Committee of Beijing Chao-Yang Hospital, Capital Medical University (Approval number: 2017-11-28-3).
2.4. Data analysis
The data were analyzed using SPSS software (version 24.0; SPSS, Inc, Chicago, IL). The Mann–Whitney tests were conducted for the numerical measures with non-normal distributions. Pearson Chi-Squared tests were used for categorical variables. P < .05 was considered to indicate statistical significance.
3. Results
During the pre-and post-intervention periods, a total of 1611 patients were admitted to the Orthopedic Department. Of 784 patients in the pre-intervention period, 21 were excluded for not having surgical indications, 230 for undergoing clean orthopedic procedures without the use of prophylaxis, 8 for having infectious diseases, 39 for having preoperative infections and using antimicrobials, 6 for having grade III open fractures with extensive soft tissue damage and crushing, 55 for being admitted to the surgical intensive care unit after the operation. In the postintervention period, 9 out of 827 patients were excluded for not having surgical indications, 258 for undergoing clean orthopedic procedures without the use of prophylaxis, 3 for having infectious diseases, 54 for having preoperative infections and using antimicrobials, 3 for having Grade III open fractures with extensive soft tissue damage and crushing, and 52 for being admitted to the surgical intensive care unit. In the end, a total of 873 patients were included in this study, of which 425 were from the pre-intervention period and 448 were from the postintervention period.
There was no significant difference between pre-and postintervention populations in gender, length of stay, and CMI. Postintervention population is slightly older than the preintervention population. The demographic and procedural characteristics of the 2 populations are shown in Table 1.
Table 1.
Demographic and procedural data by pre- and postintervention groups.
| Characteristics | Preintervention (n = 425) | Postintervention (n = 448) | P |
| Female, frequency (%) | 224 (57.4%) | 239 (53.3%) | .247 |
| Age, mean (SE) | 52.4 (0.897) | 58.0 (0.824) | .047 |
| Type of procedure, frequency (%) | <.001 | ||
| Repair and plastic operations on joint structures | 159 (37.4%) | 184 (41.1%) | |
| Other operation on bones, except facial bones | 105 (24.7%) | 91 (20.3%) | |
| Reduction of fracture and dislocation | 103 (24.2%) | 97 (21.7%) | |
| Operations on muscle, tendon, fascia, and bursa, except hand | 27 (6.4%) | 16 (3.6%) | |
| Incision and excision of joint structures | 22 (5.2%) | 12 (2.7%) | |
| Operations on spinal cord and spinal canal structures | 9 (2.1%) | 41 (9.2%) | |
| Others | 0 (0%) | 7 (1.6%) | |
| Length of stay | 11.47 (0.332) | 11.01 (0.283) | .293 |
| CMI, mean (SE) | 1.50 (0.034) | 1.55 (0.033) | .317 |
In Table 2, patients’ allergic information was compared between the 2 populations. Asthma is the most common history allergy which represents 1.6% and 0.4% in pre and postintervention groups. The top 3 most common previous drug allergy history were penicillins (25, 5.9% in preintervention; 17, 3.8% in postintervention), sulfonamides (10, 2.4% in preintervention; 15, 3.3% in postintervention) and cephalosporins (3, 0.7% in preintervention; 5, 1.1% in postintervention).
Table 2.
Allergic data pre-and postintervention.
| Preintervention (n = 425) | Postintervention (n = 448) | |
| Previous allergic disease history | ||
| Asthma | 7 (1.6%) | 2 (0.4%) |
| Allergic rhinitis | 0 (0.0%) | 2 (0.4%) |
| Urticaria | 1 (0.2%) | 1 (0.2%) |
| Anaphylactoid purpura | 0 (0.0%) | 1 (0.2%) |
| Food allergy | 1 (0.2%) | 1 (0.2%) |
| Previous drug allergic history | ||
| Penicillins | 25 (5.9%) | 17 (3.8%) |
| Sulfonamides | 10 (2.4%) | 15 (3.3%) |
| Cephalosporins | 3 (0.7%) | 5 (1.1%) |
| Tetanus antitoxin | 2 (0.5%) | 2 (0.4%) |
| Chinese patent medicine | 2 (0.5%) | 0 (0.0%) |
| SMZCo | 1 (0.2%) | 0 (0.0%) |
| Macrolides | 1 (0.2%) | 1 (0.2%) |
| Furazolidone | 0 (0.0%) | 1 (0.2%) |
| Metoclopramide | 1 (0.2%) | 1 (0.2%) |
| Ephedrine | 1 (0.2%) | 0 (0.0%) |
| Nitroglycerin | 1 (0.2%) | 0 (0.0%) |
| Iodine | 1 (0.2%) | 0 (0.0%) |
| Alcohol | 0 (0.0%) | 2 (0.4%) |
The number of patients receiving intradermal skin tests decreased from 95.8% (407) in pre- to 16.5% (74) in postintervention (P < .001). In the postintervention period, there was an increase in the use of cephalosporin for perioperative antibacterial prophylaxis from 83.5% (355) to 96.6% (433) (P < .001). On the contrary, there was a decrease in the use of suboptimal antimicrobials for prophylaxis, such as vancomycin from 8.5% (36) to 1.6% (7) (P < .001), clindamycin from 8.5% (36) to 1.6% (7) (P < .001), etc. From the cost perspective, although there was no significant difference in the total drug expenditure per patient (stands for the cost of all therapeutic drugs used during each patient's hospitalization, including antibacterial drugs, analgesics, anticoagulants, and treatment drugs for chronic diseases [if the patient has underlying chronic diseases]) (P = .211) between the 2 groups, the average cost of antimicrobials decreased from $49.7 to $44.2 (P = .024) with antimicrobials cost proportion dropped from 16.8% to 13.8% (P < .001). Findings of utilization and cost of intradermal skin tests and perioperative antibacterial prophylaxis were summarized in Table 3.
Table 3.
Intradermal skin test, perioperative antibacterial prophylaxis, and cost data pre-and postintervention.
| Preintervention (n = 425) | Postintervention (n = 448) | P | |
| Patients with intradermal skin test | 407 (95.8%) | 74 (16.5%) | <.001 |
| POABP | <.001 | ||
| Cephalosporin | 355 (83.5%) | 433 (96.6%) | <.001 |
| Vancomycin | 36 (8.5%) | 7 (1.6%) | <.001 |
| Clindamycin | 28 (6.6%) | 4 (0.9%) | <.001 |
| Levofloxacin | 6 (1.4%) | 4 (0.9%) | .537 |
| Cost | |||
| Total drugs per patient ($) | 349.2 (191.5, 577.8) | 375.9 (237.6, 567.8) | .211 |
| Antimicrobials per patient ($) | 49.7 (32.1, 82.8) | 44.2 (28.7, 71.6) | .024 |
| Antibiotic cost percentage | 16.8% (11.4%, 25.1%) | 13.8% (8.6%, 21.4%) | <.001 |
Table 4 presents the results from logistic regressions estimating the likelihood of patients getting intradermal skin test and the usage of prophylactic antimicrobials, controlling for patients’ age, gender, length of inpatient stay, and CMI. Compared to preintervention population, postintervention population was significantly less likely to undergo an intradermal skin test (OR: 0.008, 95% CI: 0.005–0.014). Patients in postintervention group had a 5.28-fold higher likelihood (95% CI: 2.95–9.43) of having cephalosporin as prophylactic antimicrobials compared with who were in preintervention group. We also examined the impact of the intervention on the total drug cost and the cost of antimicrobials, adjusted for patients’ age, gender, length of inpatient stay, and CMI. There was no difference in the total drug cost between pre- and postintervention groups. On average, patients in the postintervention group had lower antimicrobials cost and lower antimicrobials cost percentage than patients in the preintervention group, by $150.21 (95% CI: −225.00–−75.43) and 3.2% (95% CI: −4.7%–−1.7%), respectively.
Table 4.
Impact of the intervention on intradermal skin test, prophylactic antibiotics, and drug costs.
| Effect size∗ (95% confidence intervals) | P | |
| Patients with intradermal skin tests, OR | 0.008 (0.005, 0.014) | <.001 |
| Cephalosporin as POABP, OR | 5.28 (2.95, 9.43) | <.001 |
| Cost | ||
| Total drugs per patient ($), Coef | –7.34 (–165.61, 150.93) | .928 |
| Antibiotics cost per patient ($), Coef | –150.21 (–225.00, –75.43) | <.001 |
| Antibiotics cost percentage, Coef | –3.2% (–4.7%, –1.7%) | <.001 |
4. Discussion
At the G20 in Hangzhou China in 2017, antimicrobial resistance has been recognized as one of the top 5 factors impacting the world economy. China has carried out a 3-year special rectification activity on the clinical application of antimicrobials nationwide since 2011. In 2016, China launched the national action plan to Contain Antimicrobial Resistance (2016–2020). Several studies have demonstrated that the pharmacists’ participation could promote the reasonable use of antimicrobials both in preventive and therapeutic applications.[18,19,20] Especially in the perioperative prophylactic application of antimicrobials, the perioperative-dose timing, the selection, the dosing, and the duration of prophylaxis have been greatly improved. However, we are faced with another problem of over-utilization of intradermal skin tests for penicillin and cephalosporins in inpatient settings.
In 2012, penicillin and cephalosporin were the top 2 antimicrobials sold in China, making up nearly 70% of antibacterial drug market.[21] However, the annual report of national adverse drug reaction monitoring of China showed that a total of 1.4 million adverse drug reaction events were identified in 2017, among which about 508 thousand cases were related to antimicrobial agents, with cephalosporins ranked the first. The top 3 systems and organs affected by adverse drug reactions were skin and appendages disorders (24.2%), body as whole-general disorders (18.6%), and resistance mechanism disorders (11.4%). Unfortunately, a lot of physicians still lack knowledge on the safe use of β-lactams.[22] Because there are no clear regulations in the Chinese pharmacopeia and the medicine specification, 3 types of situations could happen in practice, administering without intradermal skin test, substituting of cefazolin or penicillin intradermal skin test, and ordering drug intradermal skin test.[11] In our hospital, the physicians are accustomed to conducting routine original drug intradermal skin tests before using cephalosporins to avoid allergy. However, in some circumstances, due to false-positive outcomes of the intradermal skin test, physicians could not use first-line antimicrobials and had to choose suboptimal alternatives, which led to increased treatment costs and increased risk of antibiotic resistance.
The penicillin skin tests have been proved for the diagnosis and indications of IgE-mediated hypersensitivity to penicillin.[23,24] Whereas, although cephalosporin intradermal skin tests are widely used in China, Korea, and other Asian countries,[25] there is still much controversy. Some researchers claimed it could accurately predict immediate hypersensitivity.[26] But some scholars had the opposite opinions, S.-Y. Yoon developed a prospective study by conducting intradermal skin tests with 4 different generations of cephalosporins. 74 (5.2%) out of 1421 patients were positive to at least 1 cephalosporin but none of the responders had immediate hypersensitivity reactions after a challenge dose of the same or different cephalosporin. Therefore, inferred the positive predictive value is 0%.[27]
Our study is one of the earliest study to examine the impact of eliminating unnecessary penicillin skin-test through an pharmacists-participated intervention in the antibiotic stewardship program. In our study, pharmacists participated in the clinical practice, evaluated patients’ allergic history, and recommended that intradermal skin tests should not be performed on patients without a drug allergic history. Patients with allergic history should conduct the intradermal skin test of the original drug with a standard 300 μg/mL concentration solution which was dissolved at pharmacy intravenous admixture service. The intervention avoided unnecessary intradermal skin tests, substantially reduced the prescription of suboptimal antimicrobials, and significantly lowered the antimicrobial expenditure.
Although our study showed promising results in population with orthopedics surgery, we recognized that our results are not representative of the general inpatient population, and we also acknowledge that our approach may not be applicable to patients with non-elective surgeries during emergent situations. Another outcome that we were not able to evaluate is the false positive results of the patients with positive skin tests. Because those patients did not go through cephalosporins therapy. Further research will be needed to assess the false-positive rate and innovative antibiotic stewardship intervention is needed to identify and un-label false-positive cases.
5. Conclusion
Pharmacist involved intervention can reduce the utilization of cephalosporins skin tests and decrease the prescription of unnecessary higher cost antimicrobials such as vancomycin, clindamycin, and levofloxacin as the perioperative prophylaxis.
Author contributions
Formal analysis: Xiao Sun.
Investigation: Xiaojia Yu, Ye Su, Zhaoyuan You.
Supervision: Lihong Liu.
Validation: Huaguang Wang.
Writing – original draft: Hong Zhou.
Writing – review & editing: Zhuoling An.
Footnotes
Abbreviation: CMI = case mix index.
How to cite this article: Zhou H, Liu L, Sun X, Wang H, Yu X, Su Y, You Z, An Z. The impact of pharmacist intervention on prophylactic antibiotics use in orthopedic surgery at a hospital in China. Medicine. 2021;100:52(e28458).
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Drug and Therapeutics Committee and the Ethics Committee of Beijing Chao-Yang Hospital, Capital Medical University (Approval number: 2017-11-28-3). Written informed consent was obtained from individual or guardian participants.
The authors have no funding and conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Adjusted for age, gender, length of inpatient stay, CMI.
References
- [1].Yang MS, Kang DY, Seo B, et al. Incidence of cephalosporin-induced anaphylaxis and clinical efficacy of screening intradermal tests with cephalosporins: a large multicenter retrospective cohort study. Allergy 2018;73:1833–41. [DOI] [PubMed] [Google Scholar]
- [2].Global Guidelines for the Prevention of Surgical Site Infection. Geneva: World Health Organization; 2018. [PubMed] [Google Scholar]
- [3].Warrington R, Silviu-Dan F, Wong T. Drug allergy. Allergy Asthma Clin Immunol 2018;14:60.Published 2018 Sep 12. doi:10.1186/s13223-018-0289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Doña I, Barrionuevo E, Blanca-Lopez N, et al. Trends in hypersensitivity drug reactions: more drugs, more response patterns, more heterogeneity. J Investig Allergol Clin Immunol 2014;24:143–53. [PubMed] [Google Scholar]
- [5].Culp JA, Palis RI, Castells MC, et al. Perioperative anaphylaxis in a 44-year-old man. Allergy Asthma Proc 2007;28:602–5. [DOI] [PubMed] [Google Scholar]
- [6].Sengupta A, Kohli JK. Antibiotic prophylaxis in cesarean section causing anaphylaxis and intrauterine fetal death. J Obstet Gynaecol Res 2008;34:252–4. [DOI] [PubMed] [Google Scholar]
- [7].Mertes PM, Laxenaire MC, Alla F. Groupe d’Etudes des Réactions Anaphylactoïdes Peranesthésiques. Anaphylactic and anaphylactoid reactions occurring during anesthesia in France in. Anesthesiology 2003;99:536–45. [DOI] [PubMed] [Google Scholar]
- [8].Rubin R. Overdiagnosis of penicillin allergy leads to costly, inappropriate treatment. JAMA 2018;320:1846–8. [DOI] [PubMed] [Google Scholar]
- [9].Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol 2014;133:790–6. [DOI] [PubMed] [Google Scholar]
- [10].Macy E. Penicillin and beta-lactam allergy: epidemiology and diagnosis. Curr Allergy Asthma Rep 2014;14:476.doi:10.1007/s11882-014-0476-y. [DOI] [PubMed] [Google Scholar]
- [11].Yao Y, Zhou R, Wang Y. Fatal adverse effects of injected ceftriaxone sodium in China. Pharmacoepidemiol Drug Saf 2012;21:1197–201. [DOI] [PubMed] [Google Scholar]
- [12].Blanca M, Romano A, Torres MJ, et al. Update on the evaluation of hypersensitivity reactions to betalactams. Allergy 2009;64:183–93. [DOI] [PubMed] [Google Scholar]
- [13].Leguelinel-Blache G, Castelli C, Roux-Marson C, et al. Impact of collaborative pharmaceutical care on in-patients’ medication safety: study protocol for a stepped wedge cluster randomized trial (MEDREV study). Trials 2018;19:19.Published 2018 Jan 8. doi:10.1186/s13063-017-2412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Blassmann U, Morath B, Fischer A, et al. Arzneimitteltherapiesicherheit im Krankenhaus: einbindung von Stationsapothekern zur Reduktion von arzneimittelbezogenen Problemen im stationären Medikationsprozess Medication safety in hospitals: integration of clinical pharmacists to reduce drug-related problems in the inpatient setting. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2018;61:1103–10. [DOI] [PubMed] [Google Scholar]
- [15].Maeda M, Miyake T, Inose R, et al. Bibliometric analysis of pharmacist's research on antimicrobial stewardship in Japan: an interrupted time series analysis on the implementation of the certification system for infection control pharmacists. J Pharm Health Care Sci 2021;7:38.Published 2021 Nov 1. doi:10.1186/s40780-021-00223-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Torres MJ, Adkinson NF, Caubet JC, et al. Controversies in drug allergy: beta-lactam hypersensitivity testing published correction appears in J Allergy Clin Immunol Pract. 2019 Mar;7(3):1096. J Allergy Clin Immunol Pract 2019;7:40–5. [DOI] [PubMed] [Google Scholar]
- [17].Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the infectious diseases society of America and the society for healthcare epidemiology of America. Clin Infect Dis 2016;62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhou Y, Ma LY, Zhao X, Tian SH, Sun LY, Cui YM. Impact of pharmacist intervention on antibiotic use and prophylactic antibiotic use in urology clean operations. J Clin Pharm Ther 2015;40:404–8. [DOI] [PubMed] [Google Scholar]
- [19].Tang ZQ, Jiang RH, Xu HB. Effectiveness of pharmaceutical care on treatment outcomes for patients with first-time pulmonary tuberculosis in China. J Clin Pharm Ther 2018;43:888–94. [DOI] [PubMed] [Google Scholar]
- [20].Shi QP, Ding F, Liu Y, et al. Pharmacists promote rational use of antibiotic prophylaxis in Type I incision operations via application of drug use evaluation. Int J Clin Pharmacol Ther 2013;51:704–10. [DOI] [PubMed] [Google Scholar]
- [21].Li Y, Shen S, Geng W, et al. The antibiotics market under the policy guidance. Shanghai Yiyao 2013;34:30–4. [Google Scholar]
- [22].Prematta T, Shah S, Ishmael FT. Physician approaches to beta-lactam use in patients with penicillin hypersensitivity. Allergy Asthma Proc 2012;33:145–51. [DOI] [PubMed] [Google Scholar]
- [23].Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract 2013;1:258–63. [DOI] [PubMed] [Google Scholar]
- [24].Macy E. The clinical evaluation of penicillin allergy: what is necessary, sufficient and safe given the materials currently available? Clin Exp Allergy 2011;41:1498–501. [DOI] [PubMed] [Google Scholar]
- [25].Lee SH, Park HW, Kim SH, et al. The current practice of skin testing for antibiotics in Korean hospitals. Korean J Intern Med 2010;25:207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Torres MJ, Blanca M, Fernandez J, et al. Diagnosis of immediate allergic reactions to beta-lactam antibiotics. Allergy 2003;58:961–72. [DOI] [PubMed] [Google Scholar]
- [27].Yoon SY, Park SY, Kim S, et al. Validation of the cephalosporin intradermal skin test for predicting immediate hypersensitivity: a prospective study with drug challenge. Allergy 2013;68:938–44. [DOI] [PubMed] [Google Scholar]