Abstract
Background:
Pulse radiofrequency (PRF) therapy is one of effective physical therapy modalities for treat temporomandibular disorders (TMD). This prospective randomized controlled trial aimed to evaluate the long-term treatment efficacy and patient satisfaction with PRF therapy in TMD.
Methods:
Eighty-six female patients with TMD were randomly assigned to either pulsed radiofrequency or placebo therapy in combination with other conventional treatments once a week for 12 weeks. A final analysis was performed 12 weeks after the completion of treatment. Clinical parameters and patient satisfaction were analyzed at baseline, 4, 8, and 12 weeks of intervention and at 24 weeks from baseline.
Results:
Pain intensity, comfortable and maximum mouth opening, and pain on capsule and masticatory muscle palpation were significantly improved after treatment in both groups. Notably, the pulsed radiofrequency group showed a significantly lower pain intensity at the final evaluation performed 3 months after the completion of treatment. Significantly more patients reported subjective pain improvement and satisfaction with treatment following intervention at baseline in the PRF group. Most patients did not report any discomfort following treatment in either group. However, significantly more patients in the PRF group reported a burning sensation with intervention.
Conclusion:
Long-term regular pulsed radiofrequency therapy was effective in significantly reducing TMD pain, and the effect was long-lasting following treatment completion. Pulsed radiofrequency therapy should be considered as a supportive physical therapy modality for TMD.
Keywords: orofacial pain, physical therapy, radiofrequency, rehabilitation, temporomandibular disorders
1. Introduction
Temporomandibular disorders (TMD) is a chronic musculoskeletal pain disorder with a prevalence as high as 38% of the general adult population and is one of the most common causes of orofacial pain, second only to tooth pain.[1] TMD shows a female predilection (2.24:1) with a peak incidence around 20 to 40 years of age.[2] The pain intensity of TMD is generally mild to moderate, and commonly associated symptoms include mouth opening limitation, otologic symptoms, cervical disorders, and headaches.[3] Conservative approaches are favored as first-line treatments for TMD, which include exercises, intraoral stabilization splints, physical therapy, and medication as single or combination therapy.[4] Various physical therapy modalities such as thermal moist heat, ultrasound, low-level laser, and transcutaneous electrical nerve stimulation have been applied with proven efficacy, leading to decreased pain and increased mandibular motion in 80% to 90% of the treated patients.[5] However, the level of evidence is considered low in many cases.[6,7]
Pulsed electromagnetic radio waves with a frequency between 250 kHz and 3 MHz are generally used for physical therapy targeting joint and muscle tissue to induce vasodilatation and muscle relaxation.[8] Although it is a relatively new technique, the effectiveness of pulsed radiofrequency therapy has been evaluated in several pain conditions, including spinal disorders, low back pain, and peripheral neuropathies.[9,10,11] Unlike conventional continuous radiofrequency, which results in ablation of the involved nerve and surrounding tissue due to an internal temperature increase as high as 60°C to 80°C, pulsed radiofrequency is less invasive with temperature increases less than 45°C, which is considered as the threshold for irreversible tissue damage when exposed for longer than 20 seconds.[12] Pulsed radiofrequency applies brief electrical stimulations separated by long resting phases which is delivered in oscillatory currents (range 2000–3000/s) transforming into thermal energy that converts into heat, which results in increased blood circulation and tissue metabolism leading to an overall analgesic effect while avoiding structural damage.[10,13] Although the exact mechanism of pulsed radiofrequency is yet to be fully elucidated, the proposed theories include inhibition of pain propagation by decreased microglial activity and increased c-fos expression in the dorsal horn.[14,15] In addition, pulsed radiofrequency may cause microscopic damage to C- and A-delta fibers while sparing larger non-nociceptive fibers.[16] Pulsed radiofrequency stimulation has also been reported to enhance descending pain inhibitory pathway activity.[17]
Despite the steady increase in studies showing the beneficial effect of pulsed radiofrequency on chronic pain conditions, the literature on the application of radiofrequency as a supportive treatment for TMD is sparse, with only two studies reporting on this specific patient group.[18,19] The results of such previous studies suggest the need for further investigations with improved study designs and larger sample sizes.
Therefore, the aim of this randomized controlled study was to investigate the effect of pulsed radiofrequency on a well-defined group of TMD patients in a longitudinal manner to evaluate the efficacy of pulsed radiofrequency in controlling TMD symptoms and to suggest a treatment regimen for its optimal application.
2. Methods
2.1. Study participants
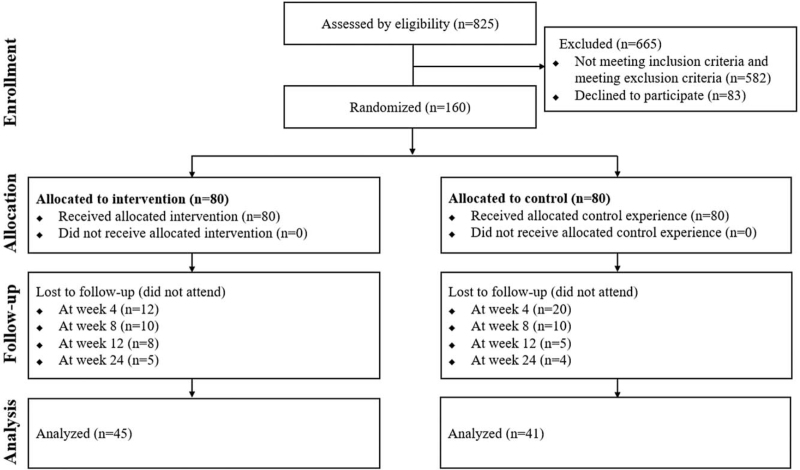
A total of 825 consecutive patients who visited the Orofacial Pain Clinic of Seoul National University Dental Hospital from August 2019 to October 2020 with the chief complaint of pain and dysfunction of the temporomandibular joint (TMJ) and surrounding area were evaluated. Among these, only Korean women aged 20 to 65 years who were diagnosed with TMD following the diagnostic criteria for temporomandibular disorders (DC/TMD) were included.[20] Exclusion criteria were systemic diseases including musculoskeletal, neurologic, liver, kidney, autoimmune, and endocrine diseases, primary sleep disorder, active inflammatory disease of any part of the body including the orofacial region, pregnancy, pacemaker implantation, heat- or photosensitivity, trauma history within the last 6 months, medication intake in the last 3 weeks that could affect treatment results, and diagnosis of pain disorder of any non-orofacial regions within the last 6 months. Among the 243 patients who met the inclusion criteria and did not meet the exclusion criteria, 83 declined to participate. The study followed the guidelines of the Declaration of Helsinki, and the research protocol was approved by the Institutional Review Board of the Seoul National University Dental Hospital (CDE 19002). All participants provided written informed consent. Based on previous research,[18] a sample size of 112 (56 per group) participants was initially calculated as two-sided, with an α-value of 0.05, and a desired power of 80%. The anticipated drop-out rate was 30% considering the current clinical situation, and 160 patients were enrolled as an adequate sample size, with 80 subjects randomized to either the intervention or control group.
2.2. Study design
A randomized controlled trial (Clinical Research Information Service trial registration number KCT0006080) was conducted on 160 female patients with TMD. The study had a double-blind design, with the participant and principal examiner (JHJ) blinded to the grouping until the end of the study. Patients were randomly assigned to two groups: pulsed radiofrequency intervention (n = 80) and placebo control (n = 80) groups based on a simple randomization procedure using Microsoft Excel 2019 software (Microsoft Corporation, Redmond, WA, USA). A non-duplicated random number table contained two columns: patient numbers (1–160) and Microsoft Excel random formula (“RAND”) (Fig. 1). A designated researcher (YJ) that was not involved in any of the evaluation processes conducted randomization and delivered the intervention accordingly. Thirty patients in the intervention group and 35 in the placebo group discontinued participation due to personal reasons. The final analysis was performed on a total of 86 female TMD patients who completed the entire study protocol (aged 20–61 yr; mean age 37.5 yr; standard deviation [SD] 13.8 yr).
Figure 1.
Flow diagram.
2.3. Clinical and radiological examinations
All patients completed questionnaires on demographic data, past medical and dental history, parafunctional habits, and pain-related parameters. Pain intensity was measured using a 10-point numeric rating scale, and pain duration was recorded in days. Clinical evaluations were performed based on DC/TMD. According to the DC/TMD axis I guidelines, patients with TMD were divided into four diagnostic subgroups: myalgia, arthralgia, headache attributed to TMD, and intra-articular joint disorders.[20] The patients were once again divided into three pain subgroups according to the main source of subjective pain: myogenous, arthrogenous, and mixed (both myogenous and arthrogenous) pain groups. The severity of pain-related disability was assessed using the Graded Chronic Pain scale and classified into two disability groups: low (grades 1 and 2) and high (grades 3 and 4) disability groups.[21] The DC/TMD axis II, including the Jaw Functional Limitation Scale-20,[22] Oral Behavior Checklist,[23] Generalized Anxiety Disorder-7,[24] Patient Health Questionnaire-9,[25] Patient Health Questionnaire-15,[26] and Symptom Checklist-90-Revision were administered for psychosocial assessment.[27]
Plain radiographs, including orthopantomograms, TMJ tomograms, and transcranial radiographs were taken for all patients to initially diagnose the presence of degenerative joint disease of the TMJ condyle. Cone beam computerized tomography images were taken only when crepitation was noticed on clinical examination, or degenerative bony changes were suspected on such radiographs. Degenerative joint disease was definitively diagnosed when condylar erosion, subchondral bone cyst, generalized sclerosis, or osteophyte was observed on cone beam computerized tomography.[28] The initial diagnosis of degenerative joint disease based on plain radiographs was maintained in all diagnosed patients after cone-beam computerized tomography evaluation. The radiographs were digitally generated using Orthopantomograph OP 100 (Instrumentariu Dental) and cone beam computerized tomography images were taken by DINNOVA 3 (HDXWILL, tube voltage 100 kVp, tube current 9 mA, exposure time 14 s, field of view; 20 cm × 14 cm, and 0.3 mm voxel size).
Clinical parameters of TMD included comfortable mouth opening range, maximum unassisted mouth opening range, response to palpation of the TMJ and masticatory muscle areas, and presence of joint noises. The comfortable mouth opening range was measured as the inter-incisal distance between the maxillary and mandibular incisal edges at the midline when patients opened their mouth until they felt any pain. The maximum unassisted mouth opening range was measured as the inter-incisal distance between the maxillary and mandibular incisal edges at the midline when patients opened their mouth as wide as possible, even if it was painful. The comfortable mouth opening range and maximum unassisted mouth opening range were recorded in millimeters. Positive responses to palpation of the TMJ and masticatory muscle areas were recorded when palpation of any of the four areas of both TMJs (lateral pole and around lateral pole) and 12 areas of the masticatory muscles on both sides (3 parts of the temporalis: anterior, middle, and posterior, 3 parts of the masseter: origin, body, and insertion) elicited subjective pain. The presence of joint noise was detected through palpation during opening, closing, lateral, and protrusive jaw movements.
Evaluation of the above clinical parameters was repeated at each session, and results were obtained at 4, 8, and 12 weeks of TMD treatment, and the final evaluation was used for analysis. The patients visited for the final post-treatment evaluation after 12 weeks of completing the 12-week treatment protocol. All evaluations were performed by a single orofacial pain specialist (JHJ).
2.4. Interventions
Both groups received treatment once a week for 12 weeks through 12 visits. At each visit, pulsed radiofrequency was applied for 10 minutes in the intervention group on both masticatory muscles with an energy unit (TRF-C1, ILOODA). Each session of pulsed radiofrequency used the same energy level (1.5 W), frequency (1 MHz), and duration (10 min) while being directly applied to the skin above the masseter muscle with two electrodes. The placebo control group received sham therapy on both masseter muscles with an identical unit without pulsed radiofrequency delivery. The sham device had an indistinguishable appearance, emitting the same visual light and auditory sound signals as the experimental unit.
Both groups also received identical conventional noninvasive TMD treatment, including counselling for behavioral therapy to correct parafunctional habits, occlusal stabilization splint, and physical therapy including thermal moist heat (80°C for 10 min), ultrasound (0.7 W/cm2 for 10 min), low-level laser (red diode laser at wave length 650 ± 20 nm for 10 min), and transcutaneous electrical nerve stimulation (10–15 MHz for 45 min). Medication, including anti-inflammatory drugs (aceclofenac 100 mg twice a day per os for 14 days) and muscle relaxants (cyclobenzaprine 10 mg before sleeping per os for 14 days) were also administered. The stabilization was constructed of hard resin and designed to cover all upper teeth with a flat occlusal surface in even contact with the functional cusps of all opposing teeth. All treatments were administered by a single orofacial pain specialist.
2.5. Outcomes
The primary outcomes were clinical parameters including pain intensity (numeric rating scale), comfortable and maximum unassisted mouth opening range (mms), pain on TMJ and masticatory muscle palpation, and presence of joint noises assessed at each visit. Secondary outcomes were the incidence of side effects and patient satisfaction levels with the intervention assessed using a questionnaire after the application of the intervention at each visit. The questionnaire was based on a 5-point Likert scale and included the following questions:
-
1)
Do you feel any discomfort at the application site after treatment? (1: very uncomfortable to 5: very comfortable) those who answered 1 and 2 were considered to have experienced discomfort with intervention,
-
2)
Is there an improvement in your TMD pain after treatment? (1: no improvement to 5: completely resolved) those who answered ≥2 were considered to have experienced improvement in TMD pain with intervention,
-
3)
How satisfied are you with your treatment results? (1: very unsatisfied to 5: very satisfied) those who answered 4 and 5 were considered as having experienced satisfaction with intervention,
-
4)
If you felt any discomfort after the treatment, how would you describe it? (1: tingling, 2: electrical, 3: burning, 4: stiffness, 5: itchy). Each type of discomfort was considered present if the patient reported it in any of the 12 treatment sessions.
2.6. Statistical analysis
The Kolmogorov–Smirnov test was used to test the normality of the data, and each test was selected accordingly. Demographic and clinical features of the participants according to group at baseline were analyzed using Student's t test and chi-square test. Repeated measures ANOVA and generalized estimating equation were conducted to analyze changes in clinical variables after intervention according to groups. Comparisons of satisfaction and side effects of intervention after intervention according to group were analyzed using a linear mixed effect regression model. All statistical analyses were performed using SPSS 25.0 software (IBM). The level of statistical significance was set at P < .05.
3. Results
3.1. Clinical characteristics and diagnostic criteria for TMD profile according to groups
Of the 160 patients, 95 patients (intervention: 50, control: 45) completed interventions, and 65 patients (intervention: 30, control: 35) discontinued the protocol due to personal reasons. After completion of the 12-week treatment protocol, nine patients (intervention: 5, control: 4) did not attend the final post-treatment evaluation in the total of 86 patients and 45 patients (mean age 36.7, SD 13.9 yr) in the intervention group and 41 patients (mean age 38.4, SD 13.8 yr) in the control group were included in the final analysis.
As shown in Table 1, there were no statistically significant differences in the demographic and clinical characteristics between the groups at baseline. Clinical and psychological characteristics based on DC/TMD and Symptom Checklist-90-Revision profiles, report rate of parafunctional habits, and incidence of degenerative joint disease on cone beam computerized tomography imaging did not differ between the groups.
Table 1.
Clinical characteristics at baseline.
| Variables | Intervention (n = 45) | Control (n = 41) | P |
| Age (yr)∗ | 36.7 (13.9) | 38.4 (13.8) | .603 |
| Pain duration (days)∗ | 49.6 (108.1) | 44.4 (66.0) | .811 |
| Pain intensity (NRS)∗ | 3.8 (2.4) | 4.6 (2.0) | .076 |
| Comfortable mouth opening∗ | 36.6 (10.9) | 38.1 (8.8) | .490 |
| Maximum mouth opening∗ | 43.3 (8.4) | 43.0 (8.1) | .830 |
| Pain on palpation: capsule† | 17/45 (38%) | 16/41 (39%) | .905 |
| Pain on palpation: muscle† | 26/45 (58%) | 28/41 (68%) | .314 |
| Noise† | 33/45 (73%) | 23/41 (56%) | .094 |
| DC/TMD diagnosis† | |||
| Myalgia | 26/45 (58%) | 28/41 (68%) | .314 |
| Arthralgia | 17/45 (38%) | 16/41 (39%) | .905 |
| Headache attributed to TMD | 11/45 (24%) | 12/41 (29%) | .614 |
| Intra-articular TMD | 40/45 (89%) | 30/41 (73%) | .061 |
| Degenerative joint disease† | 16/45 (36%) | 14/41 (34%) | .891 |
| Arthrogenous pain group† | 4/45 (9%) | 3/41 (8%) | 1.000 |
| Myogenous pain group† | 13/45 (29%) | 15/41 (37%) | .447 |
| Mixed pain group† | 13/45 (29%) | 13/41 (32%) | .776 |
| GCPS low disability† | 32/42 (76%) | 32/40 (80%) | .635 |
| GCPS high disability† | 6/42 (14%) | 6/40 (15%) | .962 |
| JFLS-20∗ | 33.6 (29.3) | 43.0 (28.1) | .242 |
| OBC∗ | 15.1 (8.7) | 15.8 (9.4) | .743 |
| GAD-7∗ | 4.0 (3.6) | 4.0 (4.8) | .978 |
| PHQ-9∗ | 5.5 (4.3) | 4.8 (5.5) | .518 |
| PHQ-15∗ | 5.7 (2.9) | 5.9 (3.5) | .794 |
| SCL-90R-somatization score∗ | 45.4 (6.4) | 45.0 (6.4) | .751 |
| SCL-90R-depression score∗ | 43.8 (8.5) | 43.4 (9.0) | .810 |
| SCL-90R-anxiety score∗ | 42.8 (6.6) | 42.6 (7.0) | .860 |
| Bruxism† | 12/45 (27%) | 8/41 (20%) | .433 |
| Clenching† | 22/45 (49%) | 20/41 (49%) | .992 |
| Tongue thrusting† | 8/45 (18%) | 2/41 (5%) | .093 |
| Hard food† | 15/45 (33%) | 15/41 (37%) | .752 |
| Unilateral chewing† | 30/45 (67%) | 27/41 (66%) | .937 |
| Unilateral sleeping† | 20/45 (44%) | 20/41 (49%) | .687 |
3.2. Long-term change in clinical parameters according to groups
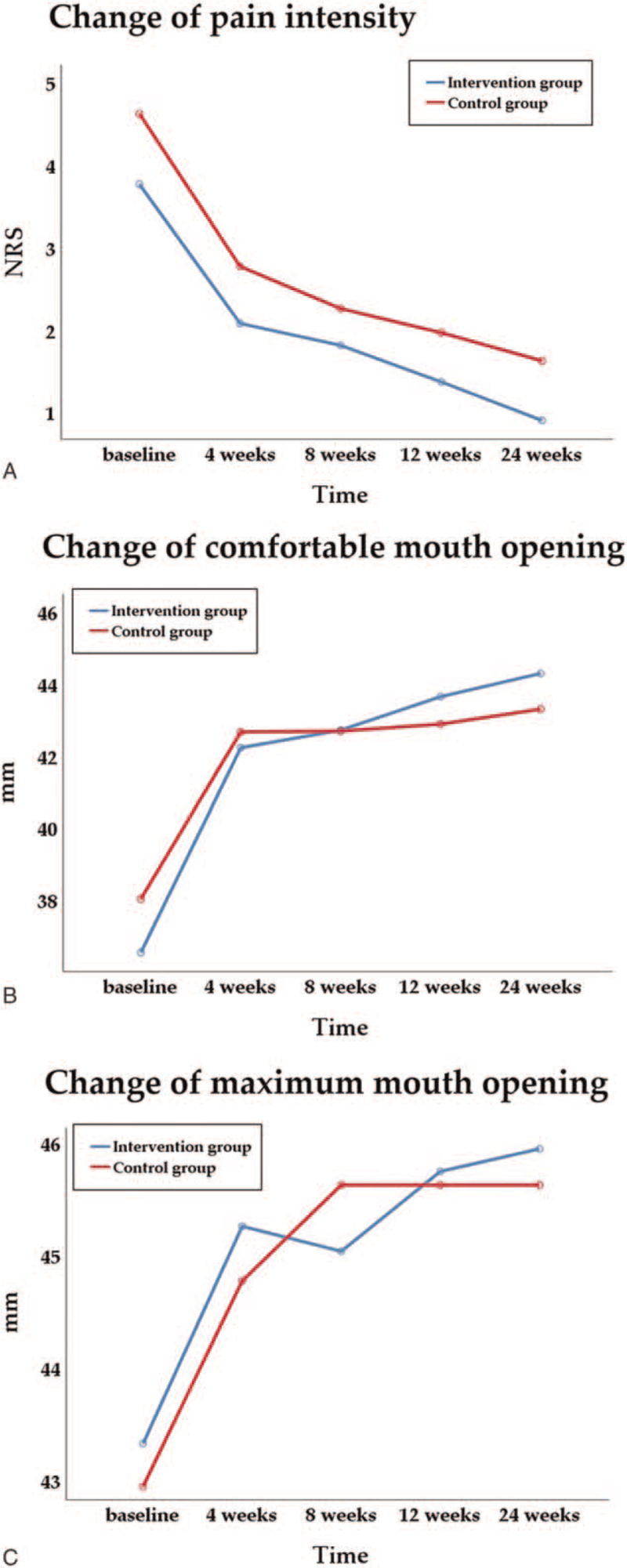
As shown in Table 2, clinical parameters such as pain intensity, comfortable mouth opening range, maximum unassisted mouth opening range, presence of pain on the capsule, and muscle palpation were significantly improved after treatment in both groups. Pain intensity significantly decreased from numeric rating scale 3.8 (SD 2.4) and 4.6 (SD 2.0) at baseline to 0.9 (SD 1.1) and 1.6 (SD 1.7) in the intervention group and the control group, respectively (Fig. 2A). The comfortable mouth opening range and maximum unassisted mouth opening range significantly increased from baseline to the final evaluation in both intervention groups (Fig. 2B and C). The presence of pain on the TMJ capsule and masticatory muscle palpation significantly improved from baseline to the final evaluation in both groups. Although the presence of joint noise did not show any significant change with treatment, there were significant differences between the groups as the sessions were repeated (P = .018). The comfortable mouth opening range and maximum unassisted mouth opening range were higher, and fewer patients reported pain on the TMJ capsule and masticatory muscle palpation in the intervention group at the final evaluation; however, the difference did not reach statistical significance.
Table 2.
Long-term change in clinical parameters.
| P | |||||||||
| Outcome | Groups | Baseline [1] | 4 weeks [2] | 8 weeks [3] | 12 weeks [4] | 24 weeks [5] | Time | Group | Time × Group |
| Pain intensity (NRS)† | Intervention | 3.8 (2.4) | 2.1 (1.8) | 1.8 (1.8) | 1.4 (1.5) | 0.9 (1.1) | <.001∗∗ | .026∗ | .786 |
| Control | 4.6 (2.0) | 2.8 (1.9) | 2.3 (1.7) | 2.0 (1.6) | 1.6 (1.7) | ||||
| Comfortable mouth opening (mm)† | Intervention | 35.6 (10.9) | 42.2 (9.0) | 42.7 (9.3) | 43.7 (8.5) | 44.3 (8.7) | <.001∗∗ | .986 | .398 |
| Control | 38.1 (8.8) | 42.7 (7.9) | 42.7 (7.7) | 42.9 (7.8) | 43.3 (7.7) | ||||
| Maximum mouth opening (mm)† | Intervention | 43.3 (8.4) | 45.3 (10.0) | 45.0 (7.1) | 45.8 (7.2) | 46.0 (7.3) | .001∗∗ | .920 | .772 |
| Control | 43.0 (8.1) | 44.8 (6.9) | 45.6 (6.0) | 45.6 (5.8) | 45.6 (6.2) | ||||
| Pain on palpation: capsule‡ | Intervention | 17/45 (38%) | 15/45 (33%) | 13/45 (29%) | 5/45 (11%) | 5/45 (11%) | <.001∗∗ | .386 | .138 |
| Control | 16/41 (39%) | 16/41 (39%) | 8/41 (20%) | 9/41 (22%) | 10/41 (24%) | ||||
| Pain on palpation: muscle‡ | Intervention | 26/45 (58%) | 18/45 (40%) | 18/45 (40%) | 16/45 (36%) | 14/45 (31%) | <.001∗∗ | .168 | .857 |
| Control | 28/41 (68%) | 23/41 (56%) | 20/41 (49%) | 20/41 (49%) | 18/41 (44%) | ||||
| Noise‡ | Intervention | 33/45 (72%) | 28/45 (62%) | 23/45 (51%) | 25/45 (56%) | 24/45 (53%) | .567 | .645 | .018∗ |
| Control | 23/41 (56%) | 27/41 (66%) | 28/41 (68%) | 26/41 (63%) | 26/41 (63%) | ||||
Figure 2.
Change in clinical outcomes. (A) Pain intensity (numeric rating scale), (B) comfortable mouth opening (mm), and (C) maximum mouth opening (mm).
Notably, there was a significant difference between groups in pain intensity at the final evaluation after completion of the 12-week treatment protocol (P = .026), with the intervention group showing a significantly lower pain intensity.
3.3. Side effect and satisfaction levels according to gorups
As shown in Table 3, very few patients reported discomfort with the intervention. Post-intervention pain improvement was significantly higher in the PRF group after the first treatment session (P < .001) and increased as the sessions were repeated (P < .001). Significantly more patients were satisfied with the treatment results in the PRF group after the first treatment session (P = .01). Satisfaction levels were consistently high in the PRF group, while satisfaction level increased with session in the control group.
Table 3.
Long-term change in satisfaction levels.
| P | |||||||||
| Outcomes | Groups | Baseline [1] | 4 weeks [2] | 8 weeks [3] | 12 weeks [4] | 24 weeks [5] | Time | Group | Time × Group |
| Discomfort after intervention | Intervention | 4.6 (0.6)0/45 (0%) | 4.3 (0.8)0/45 (0%) | 4.2 (0.9)0/45 (0%) | 4.3 (0.9)1/45 (2%) | 4.4 (0.7)0/45 (0%) | .571 | .986 | .305 |
| Control | 4.3 (0.8)0/41 (0%) | 4.6 (0.7)0/41 (0%) | 4.4 (0.8)1/41 (2%) | 4.4 (0.8)0/41 (0%) | 4.6 (0.7)0/41 (0%) | ||||
| Post-intervention pain improvement | Intervention | 1.3 (0.5)15/45 (33%) | 2.4 (1.0)37/45 (82%) | 2.6 (1.2)35/45 (78%) | 3.0 (1.2)40/45 (89%) | 3.0 (1.1)43/45 (96%) | <.001∗ | .166 | .263 |
| Control | 1.0 (0.2)1/41 (2%) | 2.2 (1.2)26/41 (63%) | 2.2 (1.2)27/41 (66%) | 2.8 (1.3)32/41 (78%) | 3.0 (1.1)38/41 (93%) | ||||
| Satisfied with intervention results | Intervention | 4.0 (0.7)35/45 (78%) | 4.2 (0.7)38/45 (84%) | 4.2 (0.8)36/45 (80%) | 4.4 (0.7)39/45 (87%) | 4.3 (0.7)39/45 (87%) | <.001∗ | .104 | .080 |
| Control | 3.6 (0.6)21/41 (51%) | 4.1 (0.8)29/41 (71%) | 4.2 (0.7)34/41 (83%) | 4.3 (0.7)35/41 (85%) | 4.3 (0.7)36/41 (88%) | ||||
There were no statistically significant differences in overall patient discomfort and satisfaction after intervention and post-intervention pain improvement between the groups.
Table 4 shows that burning sensation and stiffness was the most common type of discomfort in the pulsed radiofrequency and control group respectively. The incidence of most of types of discomfort such as tingling, electrical, stiffness, and itchy did not show any statistically significant differences between groups. However, burning sensation was significantly more common in the pulsed radiofrequency group (P = .001).
Table 4.
Type of discomfort with intervention.
| Intervention (n = 45) | Control (n = 41) | Estimated differences [95% CI] | |
| Tingling | 2/45 (4%) | 0/41 (0%) | 0.044 [−0.018, 0.107] |
| Electrical | 0/45 (0%) | 1/41 (2%) | −0.024 [−0.074, 0.025] |
| Burning | 13/45 (29%) | 1/41 (2%) | 0.264 [0.119, 0.410] |
| Stiffness | 10/45 (22%) | 5/41 (12%) | 0.100 [−0.061, 0.262] |
| Itchy | 1/45 (2%) | 2/41 (5%) | −0.027 [−0.106, 0.053] |
4. Discussion
The results of this prospective randomized placebo-controlled trial showed that supportive treatment with pulsed radiofrequency is efficacious in controlling TMD-related pain and symptoms, and this effect lasts for 3 months after completion of regular treatment.
Studies on the efficacy of pulsed radiofrequency in treating TMD are sparse, but the results of our study are in line with those of previous studies showing pulsed radiofrequency to be efficacious in reducing pain and improving function. The study by Al-Badawi[18] based on 40 TMD patients (20 intervention and 20 placebo) reported that those who received pulsed radiofrequency treatment experienced a significant decrease in pain intensity and increase in mouth opening and jaw movement range. The mean decrease in pain was 3.07 points on a numeric rating scale, which is roughly identical to the amount reported in our study (numeric rating scale 2.9 point decrease) after completing 12 weeks of treatment and final evaluation at 24 weeks. The mean increase in mouth opening in the Al-Badawi et al study was 6.75 mm for maximum unassisted mouth opening range while the amount from our study was 8.7 mm for comfortable mouth opening range and 2.7 mm for maximum unassisted mouth opening range. Such a variance in the amount of increase could be partly explained by the different characteristics of the study groups in each study. Although sub-analysis based on different DC/TMD axis I diagnoses was not performed, our study included patients from all four groups of DC/TMD, while the patient group of Al-Badawi et al exclusively comprised patients with arthralgia, restricting the general application of the results to TMD patients with myalgia. Another factor could be the difference in treatment regimens. The patients of Al-Badawi et al received a total of six sessions that took place every other day. A single session consisted of six 15 second treatments with 7 second intervals. The total treatment period was 2 weeks, with two additional evaluations occurring once a week after treatment completion. Compared to this, the total treatment exposure in our study was 120 minutes over 12 weeks, and the final evaluation was performed 12 weeks after the last treatment. However, owing to the lack of information regarding energy level and frequency, it is difficult to directly compare the treatment effects in both studies. The other latest study on pulsed radiofrequency treatment in TMD by Pihut[19] was based on 20 patients and 20 controls that were described as having a prominent myogenous pain component. Those in the intervention group received a total of 10 pulsed radiofrequency sessions at an energy level of 20 J with a frequency of 3 MHz, which lasted for 10 minutes. The results showed that masticatory muscle pain level showed a significant difference between the two groups with those that received pulsed radiofrequency, reporting a lower level. Although the treatment regimen differs in all three studies, the results collectively show that pulsed radiofrequency is an effective tool for controlling TMD pain regardless of the type. On the other hand, the three studies point out a lack of standard protocols to follow for optimal treatment results. Further studies based on better-defined patient groups and various pulsed radiofrequency protocols are necessary to establish guidelines for pulsed radiofrequency treatment. The exact level of energy, exposure time, wave frequency, treatment interval, and total session numbers were determined through such investigations.
In all three studies on pulsed radiofrequency treatment in TMD, the control group also showed a significant decrease in pain intensity. Unlike the Al-Badawi study,[18] the control patients in our study also showed a significant improvement in the mouth opening range. This improvement in symptoms even with sham treatment could be attributed to the placebo effect. For trials involving pain treatment, placebo is generally known to have a beneficial effect, with an average reduction of 6.5 mm on a 100 mm visual analog scale.[29] The amount of pain reduction in the placebo group in our study was 3.0, on a 0 to 10 numeric rating scale, which could roughly correspond to a 0.3 reduction on a 0 to 100 scale. Although direct comparison between numeric rating scale and visual analog scale results is irrational, the large amount of reduction that occurred in the placebo group could be explained as effects from other conventional treatments that were conducted in a standardized manner in both groups. To elucidate the sole effect of pulsed radiofrequency on TMD symptoms, future studies excluding other treatment modalities from the protocol should be conducted. However, such an approach may be limited because of ethical issues.
Pulsed radiofrequency is known to alleviate pain through various mechanisms, although the entire process is not fully understood. Unlike conventional belief, the role of the electrical field has been unveiled through recent studies in comparison to heat increase.[30,31] Radiofrequency is also known to result in neuromodulation and could eventually lead to alterations in gene expression related to nerve activity.[15] This suggests the possibility of a relatively longer-lasting pain relief period compared to conventional physical therapy modalities. The results of our study also showed that pain relief was evident up to 3 months after completing the treatment protocol. In addition, the difference in pain intensity between the pulsed radiofrequency and placebo groups was significant at the final evaluation, which was done at that point. The pain relief elicited by pulsed radiofrequency may take longer to reach a certain level, and after reaching that level, although the reversible effect may last longer than other treatment modalities that do not exert an influence on the molecular level. Additional investigations on neural activity and gene expression in both animal and human models on the effects of pulsed radiofrequency on factors related to TMD and orofacial pain are called upon.
Our study is the first to report on patient satisfaction with pulsed radiofrequency treatment in patients with TMD. The previous two studies solely focused on therapeutic effects, and one reported slight erythema and burning sensation in a few patients during pulsed radiofrequency application.[18] Studies reporting the side effects of pulsed radiofrequency, even with other body parts, are limited. Due to the difference in application site and technical details, it is difficult to directly compare the incidence of side effects between studies. However, the overall rates of such patient reporting are known to be low and usually transient. A study with chronic refractory cluster headache reported a transient increase in pain intensity in 36% of treated patients. However, the method applied in this study was percutaneous, and other reported side effects were more closely related to the nature of the approach taken.[32] Another study on radicular pain also reported a temporary increase in pain intensity following treatment that lasted less than a day without any major side effects.[33] The participants of our study also did not report any major side effects due to treatment, and less than 2% reported discomfort with the intervention, with no statistically significant difference between the groups. However, the method to evaluate side effects was limited to patient surveys on discomfort; therefore, a more extensive investigation on this issue should be considered in future studies. Most patients who reported discomfort described it as a burning sensation. This suggests the need to further adjust the treatment energy level to find the optimal efficacy with the least incidence of side effects. Future studies on the effect of pulsed radiofrequency in orofacial pain should consider patient satisfaction levels as an important component. Recently, patient satisfaction has been increasingly considered an important factor in patient-centered treatment. Better treatment outcomes are associated with patient satisfaction and are associated with higher treatment compliance.[34] The results of our study showed that the overall satisfaction level with conventional TMD treatment was high and those that received pulsed radiofrequency therapy rated their satisfaction level higher in the first treatment session compared to those in the placebo group.
The eligibility for pulsed radiofrequency should be carefully evaluated following a standardized procedure, although the treatment is noninvasive in nature with minimal side effects. Contraindications for the application of pulsed radiofrequency are not well-established but are known to include skin problems, including open wounds and local inflammation, metal implant installation, pregnancy, cancer, neurological, and thyroid disease.[19] On the other hand, there are studies that state that there are no contraindications for pulsed radiofrequency based on the fact that temperature increase is within the limits of tissue denaturation.[35] Another factor closely related to treatment success and high patient satisfaction is the psychological status of the patient.[30] This component should be carefully monitored before patient selection through systemic history taking and psychological evaluation based on reliable tools such as Symptom Checklist-90-Revision.[27] All participants in our study were carefully evaluated based on multiple validated tools, and the two groups did not show any difference in psychological aspects, supporting the fact that the difference in treatment outcome, side effect rate, and patient satisfaction levels were due to actual symptom improvement and not psychological differences. In addition to psychological aspects, the two groups did not show any difference in other factors including age, symptom duration at initial visit, pain origin, and various parafunctional habits that are well-known to affect symptom severity and treatment response. Therefore, the differences occurring in both groups following treatment could be explained as the effect of the intervention.
This study had several limitations. First, the study population was limited to female patients aged 20 to 61 years. Although this was done to exclude the effect of gender, the results may not apply to different gender and age groups. In addition, the study group was solely composed of Koreans, which may limit the generalizability of the results. Additional studies with diverse ethnic, gender, and age groups are necessary for more in-depth knowledge of the effect of pulsed radiofrequency in TMD. Second, the high dropout rate of study participation may have affected the final analysis results. Although specific reasons for follow-up loss were not verified, such subjects may have had certain characteristics. Although additional analysis based on the last observation forward approach did not show any significant differences in clinical outcomes compared to the final analysis of this study, a detailed investigation related to the cause of drop-out should be considered in future research. Finally, pulsed radiofrequency was applied in combination with other conventional treatment modalities including physical therapy, behavioral therapy, occlusal splint and pharmacological therapy. Most previous studies on the effect of pulsed radiofrequency on pain disorders have also investigated pulsed radiofrequency as an add-on therapy. However, future studies should consider the sole application of pulsed radiofrequency to truly establish the effect of pulsed radiofrequency on TMD symptoms.
The results of our study show that pulsed radiofrequency therapy is effective in successfully controlling TMD pain and related symptoms in combination with other TMD treatments. Interestingly, this effect was evident up to 3 months after the completion of treatment. Patient satisfaction was high, and the occurrence of side effects was low. Based on these results, pulsed radiofrequency could be considered as a supportive add-on therapy for TMD and related symptoms. To obtain optimal treatment results, well-designed future studies are needed with more diverse study samples while controlling various contributing factors of TMD to establish a standardized guideline for the application of pulsed radiofrequency in TMD. Further studies on other orofacial pain disorders are urgently needed.
Author contributions
Conceptualization: Ji Woon Park
Data curation: Jung Hwan Jo, Yewon Jang
Formal analysis: Jung Hwan Jo, Gehoon Chung, Ji Woon Park
Funding acquisition: Ji Woon Park, Jung Hwan Jo
Investigation: Jung Hwan Jo, Yewon Jang
Methodology: Gehoon Chung, Jin Woo Chung, Ji Woon Park
Project administration: Yewon Jang
Resources: Yewon Jang
Software: Jung Hwan Jo, Gehoon Chung
Supervision: Ji Woon Park
Validation: Gehoon Chung, Jin Woo Chung
Visualization: Jung Hwan Jo
Writing – original draft: Jung Hwan Jo, Yewon Jang
Writing – review & editing: Jin Woo Chung, Ji Woon Park
Footnotes
Abbreviations: DC/TMD = diagnostic criteria for temporomandibular disorders, GAD-7 = generalized anxiety disorder-7, GCPS = graded chronic pain scale, JFLS-20 = jaw functional limitation scale-20, NRS = numeric rating scale, OBC = oral behavior checklist, PHQ-15 = patient health questionnaire-15, PHQ-9 = patient health questionnaire-9, PRF = pulsed radiofrequency, SCL-90R = symptom checklist-90 revision, TMD = temporomandibular disorders, TMJ = temporomandibular joint.
How to cite this article: Jo JH, Jang Y, Chung G, Chung JW, Park JW. Long-term efficacy and patient satisfaction of pulsed radiofrequency therapy in temporomandibular disorders: A randomized controlled trial. Medicine 2021;100:52(e28441).
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant number: HI15C1535), and APC was funded by Seoul National University.
The authors declare no conflict of interest.
The datasets generated during and/or analyzed during the present study are not publicly available, but are available from the corresponding author on reasonable request.
DC TMD = Diagnostic criteria for temporomandibular disorders, GAD-7 = Generalized anxiety disorder-7, GCPS = Graded chronic pain scale, JFLS-20 = Jaw functional limitation scale-20, NRS = Numeric rating scale, OBC = Oral behavior checklist, PHQ-15 = Patient health questionnaire-15, PHQ-9 = Patient health questionnaire-9, SCL-90R = Symptom checklist-90 revision.
Results were obtained from student's t test: mean ± SD.
Results were obtained from chi-square test: number of subjects showing positive sign.
NRS, numeric rating scale.
Significant difference, P < .05.
Significant difference, P < .01.
Results were obtained from repeated measures ANOVA: mean (SD).
Results were obtained from generalized estimating equation: number of subjects showing positive sign.
Mean (SD) and number of subjects showing positive sign.
Results were obtained from linear mixed effect regression model.
Significant difference, P < .01.
CI = confidential interval.
References
- [1].Anastassaki Köhler A, Hugoson A, Magnusson T. Prevalence of symptoms indicative of temporomandibular disorders in adults: cross-sectional epidemiological investigations covering two decades. Acta Odontol Scand 2012;7:213–23. [DOI] [PubMed] [Google Scholar]
- [2].Bueno CH, Pereira DD, Pattussi MP, Grossi PK, Grossi ML. Gender differences in temporomandibular disorders in adult populational studies: a systematic review and meta-analysis. J Oral Rehabil 2018;45:720–9. [DOI] [PubMed] [Google Scholar]
- [3].Gauer RL, Semidey MJ. Diagnosis and treatment of temporomandibular disorders. Am Fam Physician 2015;91:378–86. [PubMed] [Google Scholar]
- [4].Shaffer SM, Brismée JM, Sizer PS, Courtney CA. Temporomandibular disorders. Part 2: conservative management. J Man Manip Ther 2014;22:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Martins-Júnior RL, Palma AJ, Marquardt EJ, Gondin TM, Kerber FC. Temporomandibular disorders: a report of 124 patients. J Contemp Dent Pract 2010;11:71–8. [PubMed] [Google Scholar]
- [6].McNeely ML, Armijo Olivo S, Magee DJ. A systematic review of the effectiveness of physical therapy interventions for temporomandibular disorders. Phys Ther 2006;86:710–25. [PubMed] [Google Scholar]
- [7].Paço M, Peleteiro B, Duarte J, Pinho T. The effectiveness of physiotherapy in the management of temporomandibular disorders: a systematic review and meta-analysis. J Oral Facial Pain Headache 2016;30:210–20. [DOI] [PubMed] [Google Scholar]
- [8].Górnicki M, Gala A, Pihut M. The mechanism of beneficial effect of radiofrequency therapy on masticatory muscles in temporomandibular disorders – a literature review. Folia Med Cracov 2020;60:75–83. [DOI] [PubMed] [Google Scholar]
- [9].Yang S, Chang MC. Efficacy of pulsed radiofrequency in controlling pain caused by spinal disorders: a narrative review. Ann Palliat Med 2020;9:3528–36. [DOI] [PubMed] [Google Scholar]
- [10].Yang S, Boudier-Revéret M, Chang MC. Use of pulsed radiofrequency for the treatment of discogenic back pain: a narrative review. Pain Pract 2021;21:594–601. [DOI] [PubMed] [Google Scholar]
- [11].Chang MC. Efficacy of pulsed radiofrequency stimulation in patients with peripheral neuropathic pain: a narrative review. Pain Physician 2018;21:E225–34. [PubMed] [Google Scholar]
- [12].Cosman ER, Jr, Cosman ER, Sr. Electric and thermal field effects in tissue around radiofrequency electrodes. Pain Med 2005;6:405–24. [DOI] [PubMed] [Google Scholar]
- [13].Chua NH, Vissers KC, Sluijter ME. Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications – a review. Acta Neurochir (Wien) 2011;153:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cho HK, Cho YW, Kim EH, Sluijter ME, Hwang SJ, Ahn SH. Changes in pain behavior and glial activation in the spinal; dorsal horn after pulsed radiofrequency current administration to the dorsal root ganglion in a rat model of lumbar disc herniation: laboratory investigation. J Neurosurg Spine 2013;19:256–63. [DOI] [PubMed] [Google Scholar]
- [15].Higuchi Y, Nashold BS, Jr, Sluijter M, Cosman E, Pearlstein RD. Exposure of the dorsal root ganglion in rats to pulsed radiofrequency currents activates dorsal horn lamina I and II neurons. Neurosurgery 2002;50:850–5. [DOI] [PubMed] [Google Scholar]
- [16].Erdine S, Bilir A, Cosman ER, Cosman ER, Jr. Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract 2009;9:407–17. [DOI] [PubMed] [Google Scholar]
- [17].Cho IT, Cho YW, Kwak SG, Chang MC. Comparison between ultrasound-guided interfascial pulsed radiofrequency and ultrasound-guided interfascial block with local anesthetic in myofascial pain syndrome of trapezius muscle. Medicine (Baltimore) 2017;96:e6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Al-Badawi EA, Mehta N, Forgione AG, Lobo SL, Zawawi KH. Efficacy of pulsed radio frequency energy therapy in temporomandibular joint pain and dysfunction. Cranio 2004;22:10–20. [DOI] [PubMed] [Google Scholar]
- [19].Pihut M, Górnicki M, Orczykowska M, Zarzecka E, Ryniewicz W, Gala A. The application of radiofrequency waves in supportive treatment of temporomandibular disorders. Pain Res Manag 2020. 6195601.1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache 2014;28:06–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain 1992;50:133–49. [DOI] [PubMed] [Google Scholar]
- [22].Ohrbach R, Larsson P, List T. The jaw functional limitation scale: development, reliability, and validity of 8-item and 20-item versions. J Orofac Pain 2008;22:219–30. [PubMed] [Google Scholar]
- [23].Markiewicz MR, Ohrbach R, McCall WD, Jr. Oral behaviors checklist: reliability of performance in targeted waking-state behaviors. J Orofac Pain 2006;20:306–16. [PubMed] [Google Scholar]
- [24].Lowe B, Decker O, Muller S. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care 2008;46:266–74. [DOI] [PubMed] [Google Scholar]
- [25].Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002;64:258–66. [DOI] [PubMed] [Google Scholar]
- [27].Derogatis LR. SCL-90-R: Administration, Scoring and Procedures Manual. Baltimore, MD, USA: Clinical Psychometric Research; 1977. [Google Scholar]
- [28].Ahmad M, Hollender L, Anderson Q, et al. Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD): development of image analysis criteria and examiner reliability for image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;107:844–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med 2001;344:1594–602. [DOI] [PubMed] [Google Scholar]
- [30].Van Zundert J, Raj P, Erdine S, van Kleef M. Application of radiofrequency treatment in practical pain management: state of the art. Pain Pract 2002;2:269–78. [DOI] [PubMed] [Google Scholar]
- [31].Shah RV, Racz GB. Pulsed mode radiofrequency lesioning of the suprascapular nerve for the treatment of chronic shoulder pain. Pain Physician 2003;6:503–6. [PubMed] [Google Scholar]
- [32].Lazzari ZT, Palmisani S, Hill B, Al-Kaisy A, Lambru G. A prospective case series of sphenopalatine ganglion pulsed radiofrequency therapy for refractory chronic cluster headache. Eur J Neurol 2020;27:1190–6. [DOI] [PubMed] [Google Scholar]
- [33].Shanthanna H, Chan P, McChesney J, Thabane L, Paul J. Pulsed radiofrequency treatment of the lumbar dorsal root ganglion in patients with chronic lumbar radicular pain: a randomized, placebo-controlled pilot study. J Pain Res 2014;7:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Prakash B. Patient satisfaction. J Cutan Aesthet Surg 2010;3:151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sluijter ME, Racz G. Technical aspects of radiofrequency. Pain Pract 2002;2:195–200. [DOI] [PubMed] [Google Scholar]