Abstract
To assess the relationship between retinal vein occlusion (RVO) and the incidence of cardiovascular (CV) events.
This was a single-institution, retrospective cohort study. We enrolled 57 patients diagnosed with RVO between January 2012 and December 2019, and 125 non-RVO patients who had undergone cataract surgery by a single surgeon between January and April 2012. We compared the relative risk and incidence rate ratio of CV events between the 2 groups. In addition, survival analysis was performed to calculate the hazard ratio (HR) using the Cox proportional hazards model. RVO, age, sex, blood pressure, body mass index, presence of diabetes, blood sample data, and smoking were considered confounders.
The mean observation period (± standard deviation) for the RVO and non-RVO groups was 2.68 ± 2.04 and 2.81 ± 2.70 years, respectively. Seven CV events were observed in the RVO group and 2 in the non-RVO group. Relative risk and incidence rate ratio were 7.68 (95% confidence interval [CI]: 1.65–35.8) and 8.07 (95% CI: 1.54–79.6), respectively. Multivariate analysis revealed that the RVO group had a high HR for CV events (HR: 16.13 [95% CI: 2.29–113.74]) and older age (HR: 1.26 [95% CI: 1.06–1.49]).
RVO can predict future CV events, especially in the elderly population. Fundus observations should be shared between ophthalmologists and internists to prevent future CV events.
Keywords: cardiovascular events, Japanese elderly, retinal vein occlusion, survival analysis
1. Introduction
Cardiovascular disease (CVD) is one of the most important causes of morbidity and a leading cause of mortality in highly developed countries.[1,2] Atherosclerosis is a significant risk factor for CVD. Progression of atherosclerosis is associated with lifestyle, including an unbalanced diet, lack of exercise, smoking, and alcohol consumption. Such behaviors lead to hypertension, diabetes mellitus, dyslipidemia, and chronic kidney disease.
In ophthalmology, diabetic retinopathy[3] and retinal vein occlusion (RVO)[4,5] are closely correlated with lifestyle and lifestyle-related diseases. We previously reported a high incidence of cardiovascular (CV) events in neovascular glaucoma: 7.5 events per 100 person-years.[6] Neovascular glaucoma is an ischemic eye disease caused by diabetic retinopathy, RVO, and other inflammatory eye diseases. It is refractory to treatment but preventable; therefore, early intervention is recommended. In other words, early treatment with RVO could reduce the incidence of CVD and prevent severe visual impairment or blindness. However, few studies to date have examined the association between RVO and CVD incidence.
In East Asia, epidemiological studies have been conducted in Taiwan[4,7,8] and the Republic of Korea,[9,10,11] but to the best of our knowledge, there have been no reports from Japan on the relationship between symptomatic CV events and RVO. Therefore, we aimed to explore the incidence of CV events and their risk factors in Japanese patients with RVO.
2. Methods
2.1. Study design and participants
This was a single-center, retrospective cohort study based on medical charts. The primary outcome was defined as primary diagnosis, including ischemic heart disease (IHD) requiring hospitalization, such as myocardial infarction, angina, heart failure, and cerebrovascular diseases such as stroke, transient ischemic attack, and death from the CV event itself. Patients in the RVO group were diagnosed between January 2012 and December 2019 and met the following inclusion criteria: RVO diagnosis; existence of fundus photographs; records of height and weight; record of systolic and diastolic blood pressure(s); description of medication and lifestyle (smoking and alcohol consumption); blood sampling data; and description of comorbidities, hypertension, diabetes mellitus, and dyslipidemia. Exclusion criteria for patients with RVO included uncertain or incorrect diagnoses, ocular states for which fundus examination was not possible (e.g., vitreous hemorrhage, retinal detachment, uveitis), a history of vitrectomy, and dialysis. Non-RVO patients were consecutively selected from patients who had undergone cataract surgery at our institution by a single surgeon between January and April 2012.
2.2. Data collection
The following study variables were collected based on the literature[4,7,12]: age, sex, blood pressure, total cholesterol (mg/dL), low-density lipoprotein cholesterol (mg/dL), high-density lipoprotein cholesterol (HDL-C) (mg/dL), non-HDL-C (mg/dL), body mass index (kg/m2), estimated glomerular filtration rate (eGFR; mL/min/1.73 m2), diabetes mellitus (yes/no), alcohol intake (yes/no), history of smoking (yes/no), medication use (anticoagulants, antiplatelets, and prostaglandin analogs), and number of vitreous injections of anti-vascular endothelial growth factor (VEGF). Diabetes was defined as hypoglycemic drug use and diagnosis, as described in the medical charts. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were divided into 4 categories, in accordance with a previous report[13]: SBP < 120 and DBP < 80; 120 ≤ SBP < 140 or 80 ≤ DBP < 90; 140 ≤ SBP < 160 or 90 ≤ DBP < 100; SBP ≥ 160; or DBP ≥ 100.
2.3. Statistical analyses
All data are expressed as the mean and standard deviation (SD) for continuous variables, and categorical variables were expressed as percentages. Continuous data were compared using the t test, and categorical data were compared using the chi-square test or Fisher exact test. Individuals contributed person-years (PYs) from study entry until the first CV event, loss to follow-up, or end of study, whichever occurred first. The cumulative incidence of CV events in each group was then calculated. We also computed relative risk (RR) and incidence rate ratio (IRR) with 95% confidence intervals (CIs).
In addition, we performed survival analysis by drawing a Kaplan–Meier curve, as follows: we defined any CV event as “death”; the date of entry for patients in the RVO group was when the diagnosis was made, and for the non-RVO group it was when cataract surgery was performed; the date of exit was when a CV event occurred or recurred, or when the last observation was made for those without a CV event. The observation period was the difference between the date of entry and the date of exit. A Cox proportional hazards model was used to calculate crude and adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). We excluded alcohol consumption from the statistical model because there were few previous reports that included it as a confounder. Statistical significance was set at P < .05.
All statistical analyses were performed using STATA/SE 15.0, for Mac (Stata Corp., College Station, TX).
2.4. Ethical considerations
This retrospective chart review study involving human participants was conducted in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board of the Juntendo Tokyo Koto Geriatric Medical Center. (Receipt numbers: 86-2 and 107-6. Approval dates February 24, 2016 and July 29, 2020, respectively.)
3. Results
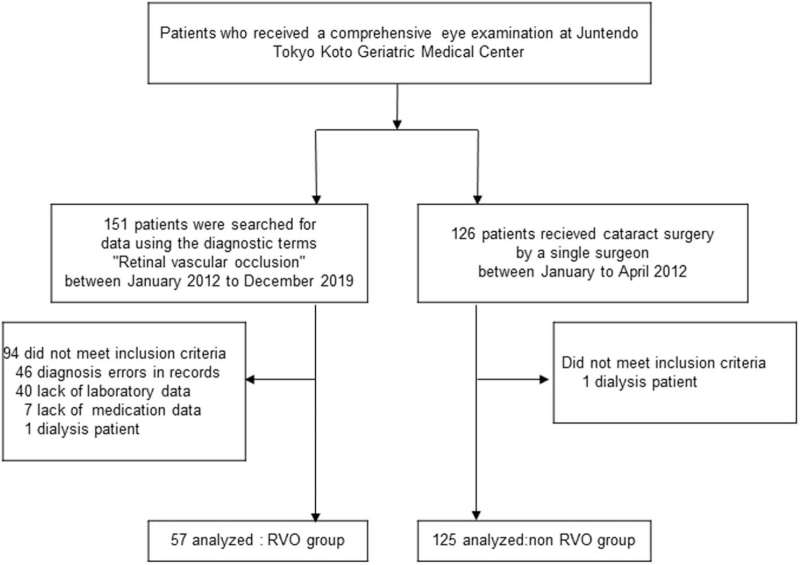
The patient selection flowchart for this study is presented in Fig. 1. Fifty-seven patients were included in the RVO group, and 125 subjects who met the criteria were included in the non-RVO group.
Figure 1.
Flowchart of participants. RVO = retinal vein occlusion.
Detailed information on each group is provided in Table 1. Patients in the RVO group were more likely to have hypertension, lower HDL-C levels, and lower eGFR levels than those in the non-RVO group. The mean ± SD observation period for the RVO and non-RVO patients was 2.68 ± 2.04 and 2.81 ± 2.70 years, respectively.
Table 1.
Demography of patients with and without RVO.
| Variables | RVO (n = 57) | non-RVO (n = 125) | P-value |
| Age, ya | 76.8 (9.4) | 75.6 (8.6) | .783∗ |
| Sex: Maleb | 23 (40.4) | 43 (34.4) | .439∗ |
| HTN, mm Hg | |||
| SBPa | 137.1 (17.7) | 125.9 (13.2) | <.001∗ |
| DBPa | 72.7 (11.8) | 65.6 (8.0) | <.001∗ |
| 120> and 80>b | 8 (14.0) | 38 (30.4) | |
| 120 ≤ SBP < 140 or 80 ≤ DBP < 90b | 25 (43.9) | 62 (49.6) | <.001∗∗ |
| 140 ≤ SBP < 160 or 90 ≤ DBP < 100b | 18 (31.6) | 25 (20.0) | |
| SBP ≥ 160 or DBP ≥ 100b | 6 (10.5) | 0 | |
| TC, mg/dLa | 203.4 (33.5) | 203.8 (35.4) | .468∗ |
| LDL-C, mg/dLa | 119.6 (29.9) | 115.5 (27.3) | .819∗ |
| HDL-C, mg/dLa | 55.0 (15.2) | 59.1 (14.0) | .039∗ |
| Non-HDL-C, mg/dLa | 148.4 (32.4) | 144.7 (31.1) | .135∗ |
| BMI, kg/m2 a | 22.5 (3.5) | 23.0 (3.6) | .180∗ |
| eGFR (mL/min/1.73 m2)a | 67.6 (23.7) | 76.9 (22.8) | .006∗ |
| DMb | 16 (28.1) | 26 (20.8) | .280∗∗ |
| Alcohol intakeb | 12 (21.1) | 41 (32.8) | .106∗∗ |
| Smokingb | 12 (21.1) | 31 (24.8) | .110∗∗ |
| Medication | |||
| Anticoagulantb | 5 (8.8) | 6 (4.8) | .297∗∗ |
| Antiplatelet drugb | 12 (21.1) | 25 (20.0) | .870∗∗ |
| Othersb | 5 (8.8) | 5 (4.0) | .190∗∗ |
| Anti-VEGFc: number of times 0b | 12 (21.1) | 120 (96) | |
| 1b | 12 (21.1) | 0 | <.001∗∗ |
| 2b | 5 (8.8) | 1 (0.8) | |
| ≥3b | 28 (49.1) | 4 (3.2) | |
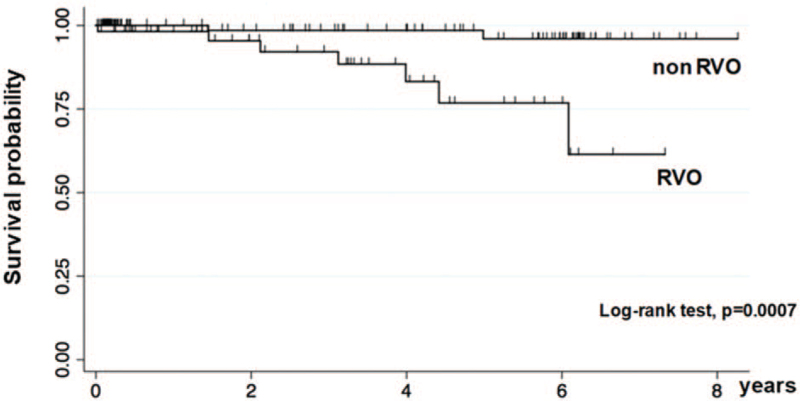
Seven CV events were observed in the RVO group (12.3% [95% CI: 4.0–21.0]; 4.59/100 PYs [95% CI: 3.83–5.73]), while 2 events were observed in the non-RVO group (1.6%, [95% CI: 0.0–3.8]; 0.57/100 PYs [95% CI: 0.49–0.68]) (Table 2). The RVO group also had a higher RR and IRR than the non-RVO group (RR: 7.68 [95% CI: 1.65–35.8] and IRR: 8.07 [95% CI: 1.54–79.6]), respectively. Kaplan–Meier survival curves are shown in Fig. 2. There was a significant difference in survival between the 2 groups (log-rank test, P = .0007). Table 3 shows the crude and adjusted HRs of the CV events in the 2 groups. A significantly higher HR was observed in the RVO group and in older patients in both the simple and multivariate models. The HRs of CV events adjusted only by RVO medication are shown in Table 4. Neither the number of anti-VEGF intravitreal injections nor protective oral medications was associated with CV events.
Table 2.
Incidence of CVD by fundus status.
| RVO | non-RVO | P-value∗ | |
| N | 57 | 125 | |
| Total CV events (MI + HF + TIA + Stroke) | 7 (12.3) | 2 (1.6) | .005∗∗ |
| MI | 0 (0) | 1 (0.8) | 1.000 |
| HF | 1 (1.75) | 1 (0.8) | .529 |
| TIA | 1 (1.75) | 0 (0) | .313 |
| Stroke | 5 (8.77) | 0 (0) | .003 |
Figure 2.
Kaplan–Meier curves of CV event-free survival probability for patients in the RVO group and the non-RVO group. CV = cardiovascular, RVO = retinal vein occlusion.
Table 3.
Major of association between CV incidence and variables selected.
| Simple model | Multivariate model | |||||
| Crude HR | 95% CI | P-value | Adjusted HR | 95% CI | P-value | |
| RVO | 9.61 | 1.97–46.9 | .005∗ | 16.13 | 2.29–113.7 | .005∗ |
| Non-RVO | 1 | – | – | 1 | – | – |
| Age | 1.15 | 1.03–1.27 | .009∗ | 1.26 | 1.06–1.49 | .008∗ |
| Sex | ||||||
| Male | 1 | – | – | 1 | – | – |
| Female | 0.62 | 0.17–2.30 | .473 | 0.37 | 0.03–4.26 | .429 |
| HTN, mm Hg | ||||||
| 120> and 80> | 1 | – | – | 1 | – | – |
| 120 ≤ SBP < 139 or 80 ≤ DBP < 90 | 1.15 | 0.21–6.29 | .873 | 0.91 | 0.11–7.60 | .929 |
| 140 ≤ SBP < 160 or 90 ≤ DBP < 100 | 0.96 | 0.14–6.87 | .971 | 0.69 | 0.05–9.80 | .783 |
| SBP ≥ 160 or DBP ≥ 100 | 8.15 | 0.69–96.4 | .096 | 1.38 | 0.04–46.4 | .856 |
| BMI | 1.03 | 0.86–1.22 | .771 | 1.14 | 0.87–1.48 | .335 |
| HDL-C | 0.96 | 0.92–1.01 | .148 | 1.03 | 0.97–1.09 | .349 |
| DM | ||||||
| Yes | 0.32 | 0.04–2.55 | .279 | 0.25 | 0.02–2.96 | .273 |
| No | 1 | – | – | 1 | – | – |
| Smoking | ||||||
| Yes | 1.8 | 0.45–7.23 | .407 | 2.23 | 0.16–30.7 | .548 |
| No | 1 | – | – | 1 | – | – |
| eGFR | 0.98 | 0.95–1.01 | .109 | 0.97 | 0.92–1.02 | .201 |
Table 4.
Major of association between CV incidence and medical intervention for RVO group.
| Variables | Adjusted HR∗ | 95% CI | P-value |
| Anticoagulant | 2.87 | 0.16–50.61 | .471 |
| Antiplatelet | 1.71 | 0.20–14.49 | .625 |
| Anti-VEGF† | |||
| 0 | 1.00 | ||
| 1 | 0.28 | 0.01–8.36 | .461 |
| 2 | 0.63 | 0.26–15.26 | .776 |
| ≥3 | 0.36 | 0.48–2.63 | .311 |
4. Discussion
This retrospective cohort study found that patients with RVO had a significantly higher incidence of CV events than patients without RVO. This was consistent with the observation of a high HR in a simple and multivariate model. The higher HR for RVO patients supports the notion that RVO could be a sign of cerebral infarction or other systemic atherosclerosis.[14] In our analysis, older age was another predictor of CV events. Along with environmental exposure, age is one of the most important contributors to atherosclerosis.[15,16] Age-associated oxidative stress and inflammation change the structure of arteries,[16] particularly by increasing the diameter of the arterial ducts, leading to arteriosclerosis.[15,16]
The central retinal artery and vein are side by side in the center of the optic nerve and covered with fibrous material. At the arteriovenous intersection within the retina or on the surface of the optic disc, the fibrous tissue and the thickened, hardened retinal arteries compress the underlying veins. These obstructions are complex and multidimensional, and their pathogenesis is not completely understood.[17] Damage to the retinal vessel wall from atherosclerosis alters the compression of the vein, causing a visible narrowing at the crossing site, sometimes with marked upstream dilation of the vein.[18] A previous study[12] showed that retinal vein occlusion was significantly associated with the presence of arteriovenous nicking, which suggests that localized arteriosclerotic processes may contribute to stasis and occlusion in adjacent retinal veins. Thus, these studies support our findings that patients with RVO develop more atherosclerosis than the general healthy population.
Supporting the above hypothesis, some epidemiological studies have revealed a higher risk of CV events in patients with RVO. Martin et al[19] reported that patients with RVO had a significantly higher risk of CVD than those with a standardized risk population (20.6% vs 15.7%, P = .009). In a matched case–control study, Di Capua et al[20] reported that the presence of RVO is an independent risk factor for developing CVD. Our results are consistent with those of previous studies.
We selected a composite primary endpoint of IHD or cerebrovascular disease because the incidence of myocardial infarction or stroke is extremely low.[21,22] Several community-based epidemiologic studies in Japan have described the incidence of IHD and stroke[21,22,23]; however, these data varied widely due to the different age groups investigated in the population. For example, in one study,[21] the median (±SD) age was 61 ± 12 years, which was younger than that of our oldest subjects. Therefore, it was difficult to investigate either IHD or cerebrovascular disease in our single institute, which is why we selected the composite endpoint.
It is well known that lowering blood pressure reduces the risk of major CV events.[24] In theory, the HR should be greater in patients with high systolic and diastolic blood pressures, but there was no dose–response relationship between the hypertension category and CV events. The reason for this is unclear, but may reflect that only a single blood pressure measurement at the first visit was included. Diabetes has also been reported to enhance CV events[25]; however, the present results do not support this hypothesis. This might be because many of our cases were frequently managed and well controlled during the follow-up period, or the diagnosis of diabetes may have been omitted from the medical chart. Although excluded in the present study, if patients on dialysis were included, the results may have differed.
In the RVO group, 20 patients (35%) used antithrombotic drugs; neither anticoagulation nor antiplatelet therapy was associated with the occurrence or recurrence of CVD. Whether antithrombotic drugs are protective against CV events remains a matter of debate.[26] In addition, it remains controversial whether antithrombotic drugs improve visual acuity in RVO.[17,27] Intravitreal injection of a VEGF inhibitor in the treatment of macular edema followed by RVO was found to be safe and effective in randomized controlled trials,[28,29] but experts have warned of the risk of systemic thromboembolism after anti-VEGF injection,[30,31] although the association between thromboembolic events and anti-VEGF therapy has not been confirmed. Further investigation is needed, and frequent follow-up is important.
The strength of our research is the collection of all personal information directly from medical records. In addition, we were able to review personalized medications, clinical records, height and weight, vital signs, and laboratory data, eliminating all incorrect diagnoses. However, this study had several limitations. First, these were censored cases, so it was difficult to follow up the care due to other events, such as walking difficulties due to dementia or degenerative joint disease, nursing care for family members, and moving houses. A survival analysis was performed to avoid selection bias. Second, we were unable to assess variables such as the Suita score[13] or Framingham risk score,[32] which are standard tools used to predict the incidence of IHD. Fasting and non-fasting blood sampling data were mixed in our study; usually, fasting blood samples are needed to assess dyslipidemia, but we had to include non-fasting blood samples in this study because patients who normally come to an ophthalmologist have not always fasted. Recently, HDL-C level has become a biomarker for dyslipidemia; therefore, we used HDL-C level as one of our variables.[33] Third, it may be difficult to generalize the results of this study to a wider population because we excluded patients who were undergoing dialysis. We excluded these patients because eGFR calculation takes into account serum creatinine level and age, but the dialysis patients already had serum creatinine levels that were far from normal.
In conclusion, our study revealed a higher risk of CV events in patients with RVO. Fundus assessment is a meaningful tool for predicting CV events, especially in elderly people; therefore, fundus observations should be shared between ophthalmologists and internists. Further research is required to evaluate the efficacy of early RVO treatment in preventing cardiovascular diseases.
Acknowledgments
This thesis received the Excellent Poster Award at the 74th Annual Congress of Japan Clinical Ophthalmology held in 2020.
Author contributions
All authors contributed to the conception and design of this study. Material creation, data collection, and analysis were performed by all authors. The first draft of the manuscript was written by Reiko Umeya, and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Conceptualization: Reiko Umeya, Yuto Yoshida, Koichi Ono.
Data curation: Reiko Umeya, Yuto Yoshida, Koichi Ono.
Formal analysis: Reiko Umeya, Yuto Yoshida, Koichi Ono.
Funding acquisition: Koichi Ono.
Investigation: Reiko Umeya, Yuto Yoshida, Koichi Ono.
Methodology: Reiko Umeya, Yuto Yoshida, Koichi Ono.
Project administration: Reiko Umeya, Yuto Yoshida, Koichi Ono.
Resources: Reiko Umeya, Yuto Yoshida, Koichi Ono.
Software: Reiko Umeya, Yuto Yoshida, Koichi Ono.
Supervision: Koichi Ono.
Validation: Reiko Umeya, Yuto Yoshida, Koichi Ono.
Visualization: Reiko Umeya, Yuto Yoshida, Koichi Ono.
Writing – original draft: Reiko Umeya.
Writing – review & editing: Reiko Umeya, Yuto Yoshida, Koichi Ono.
Footnotes
Abbreviations: CI = confidence interval, CV = cardiovascular, CVD = cardiovascular disease, DBP = diastolic blood pressure, eGFR = estimated glomerular filtration rate, HDL-C = high-density lipoprotein-cholesterol, HR = hazard ratio, IHD = ischemic heart disease, IRR = incidence rate ratio, PYs = person-years, RR = relative risk, RVO = retinal vein occlusion, SBP = systolic blood pressure, SD = standard deviation, VEGF = vascular endothelial growth factor.
How to cite this article: Umeya R, Yoshida Y, Ono K. Impact of retinal vein occlusion on cardiovascular events in elderly Japanese patients. Medicine. 2021;100:52(e28424).
Competing Interests: The authors have no relevant financial or non-financial interests to disclose.
The authors have no funding and conflicts of interest to disclose. Data Availability: The derived data supporting the findings of this study are available from the corresponding author (KO) on request.
Animal Research (Ethics): This article does not applicable.
Consent to Participate (Ethics): All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board of Juntendo Tokyo Koto Geriatric Medical Center. (Receipt numbers 86-2 and 107-6. Approval dates 24/02/2016 and 29/07/2020, respectively.)
Consent to Publish (Ethics): We have read and understood your journal's policies, and we believe that neither the manuscript nor the study violates any of these. All the authors agree with the submission of this article.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
BMI = body mass index, DBP = diastolic blood pressure, DM = diabetes mellitus, eGFR = estimated glomerular filtration rate, HDL-C = high-density lipoprotein cholesterol, HTN = hypertension, LDL-C = low-density lipoprotein cholesterol, Non-HDL-C = non-high-density lipoprotein cholesterol, RVO = retinal vein occlusion, SBP = systolic blood pressure, TC = total cholesterol, VEGF = vascular endothelial growth factor.
Mean (standard deviation).
n (%).
Vitreous injection therapy.
Student t test.
Chi-square test. P < .05 was considered statistically significant.
Data are presented as the number (%).
CV = cardiovascular, CVD = cardiovascular disease, HF = heart failure, MI = myocardial infarction, RVO = retinal vein occlusion, TIA = transient ischemic attack.
Calculated using the Fisher exact test for variables.
P < .05.
BMI = body mass index, CI = confidence interval, CV = cardiovascular, DBP = diastolic blood pressure, DM = diabetes mellitus, eGFR = estimated glomerular filtration rate, HDL-C = high-density lipoprotein cholesterol, HTN = hypertension, HR = hazard ratio, RVO = retinal vein occlusion, SBP = systolic blood pressure.
P < .05.
CI = confidence interval, HR = hazard ratio, RVO = retinal vein occlusion, VEGF = vascular endothelial growth factor.
Adjusted for age and eGFR use.
Number of vitreous injections.
References
- [1].Causes of death: Mortality and health status. WHO data and statistics. Available at: http://origin.who.int/gho/mortality_burden_disease/en/. Accessed May 6, 2020. [Google Scholar]
- [2].Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442:2011–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kawasaki R, Tanaka S, Tanaka S, et al. Risk of cardiovascular diseases is increased even with mild diabetic retinopathy: the Japan Diabetes Complications Study. Ophthalmology 2013;120:574–82. [DOI] [PubMed] [Google Scholar]
- [4].Ho JD, Liou SW, Lin HC. Retinal vein occlusion and the risk of stroke development: a five-year follow-up study. Am J Ophthalmol 2009;147:283.e2–90.e2. [DOI] [PubMed] [Google Scholar]
- [5].Werther W, Chu L, Holekamp N, Do DV, Rubio RG. Myocardial infarction and cerebrovascular accident in patients with retinal vein occlusion. Arch Ophthalmol 2011;129:326–31. [DOI] [PubMed] [Google Scholar]
- [6].Nishimoto A, Ono K, Umeya R, et al. Cerebrovascular and cardiovascular events and risk factor analysis of patients with neovascular glaucoma and diabetes. Gankarinsyokiyou 2019;12:811–5. (in Japanese). [Google Scholar]
- [7].Hu CC, Ho JD, Lin HC. Retinal vein occlusion and the risk of acute myocardial infarction: a 3-year follow-up study. Br J Ophthalmol 2009;93:717–20. [DOI] [PubMed] [Google Scholar]
- [8].Shih CH, Ou SY, Shih CJ, Chen YT, Ou SM, Lee YJ. Bidirectional association between the risk of comorbidities and the diagnosis of retinal vein occlusion in an elderly population: a nationwide population-based study. Int J Cardiol 2015;178:256–61. [DOI] [PubMed] [Google Scholar]
- [9].Rim TH, Kim DW, Han JS, Chung EJ. Retinal vein occlusion and the risk of stroke development: a 9-year nationwide population-based study. Ophthalmology 2015;122:1187–94. [DOI] [PubMed] [Google Scholar]
- [10].Rim TH, Han JS, Oh J, Kim DW, Kang SM, Chung EJ. Retinal vein occlusion and the risk of acute myocardial infarction development: a 12-year nationwide cohort study. Sci Rep 2016;6:01–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rim TH, Han J, Choi YS, et al. Retinal artery occlusion and the risk of stroke development: twelve-year Nationwide Cohort Study. Stroke 2016;47:376–82. [DOI] [PubMed] [Google Scholar]
- [12].Wong TY, Larsen EK, Klein R, et al. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli: the atherosclerosis risk in communities & cardiovascular health studies. Ophthalmology 2005;112:540–7. [DOI] [PubMed] [Google Scholar]
- [13].Nishimura K, Okamura T, Watanabe M, et al. Predicting Coronary Heart Disease using risk factor categories for a Japanese urban population, and comparison with the framingham risk score: the Suita Study. J Atheroscler Thromb 2014;21:784–98. [DOI] [PubMed] [Google Scholar]
- [14].Ueda Y, Kanazawa S, Ohira A, et al. Retinal vascular obstruction and asymptomatic cerebral infarction. Jpn J Ophthalmol 2002;46:209–14. [DOI] [PubMed] [Google Scholar]
- [15].Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part i: aging arteries: a “Set Up” for vascular disease. Circulation 2003;107:139–46. [DOI] [PubMed] [Google Scholar]
- [16].Thijssen DH, Carter SE, Green DJ. Arterial structure and function in vascular ageing: are you as old as your arteries? J Physiol 2016;594:2275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wong TY, Scott IU. Clinical practice. Retinal-vein occlusion. N Engl J Med 2010;363:2135–44. [DOI] [PubMed] [Google Scholar]
- [18].Christoffersen NL, Larsen M. Pathophysiology and hemodynamics of branch retinal vein occlusion. Ophthalmology 1999;106:2054–62. [DOI] [PubMed] [Google Scholar]
- [19].Martin SC, Butcher A, Martin N, et al. Cardiovascular risk assessment in patients with retinal vein occlusion. Br J Ophthalmol 2002;86:774–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Capua MD, Minno MN, Guida A, et al. Coronary artery disease, cerebral non-fatal ischemic stroke in retinal vein occlusion: an 8-yr follow-up. Nutr Metab Cardiovasc Dis 2012;22:23–7. [DOI] [PubMed] [Google Scholar]
- [21].Hata J, Ninomiya T, Hirakawa Y, et al. Secular trends in cardiovascular disease and its risk factors in Japanese: half-century data from the Hisayama Study (1961–2009). Circulation 2013;128:1198–205. [DOI] [PubMed] [Google Scholar]
- [22].Rumana N, Kita Y, Turin TC, et al. Trend of increase in the incidence of acute myocardial infarction in a Japanese population: Takashima AMI Registry, 1990–2001. Am J Epidemiol 2008;167:1358–64. [DOI] [PubMed] [Google Scholar]
- [23].Kita Y, Turin TC, Ichikawa M, et al. Trend of stroke incidence in a Japanese population: Takashima stroke registry, 1990–2001. Int J Stroke 2009;4:241–9. [DOI] [PubMed] [Google Scholar]
- [24].Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957–67. [DOI] [PubMed] [Google Scholar]
- [25].Fuller JH, Stevens LK, Wang SL. Risk factors for cardiovascular mortality and morbidity: the WHO mutinational study of vascular disease in diabetes. Diabetologia 2001;44: (suppl): S54–64. [DOI] [PubMed] [Google Scholar]
- [26].Di Capua M, Coppola A, Albisinni R, et al. Cardiovascular risk factors and outcome in patients with retinal vein occlusion. J Thromb Thrombolysis 2010;30:16–22. [DOI] [PubMed] [Google Scholar]
- [27].Matei VM, Xia JY, Nguyen C. Poor outcomes despite aspirin or statin use in high-risk patients with retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 2017;255:761–6. [DOI] [PubMed] [Google Scholar]
- [28].Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010;117:1102.e1–12.e1. [DOI] [PubMed] [Google Scholar]
- [29].Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology 2011;118:1594–602. [DOI] [PubMed] [Google Scholar]
- [30].Semeraro F, Morescalchi F, Duse S, Gambicorti E, Romano MR, Costagliola C. Systemic thromboembolic adverse events in patients treated with intravitreal anti-VEGF drugs for neovascular age-related macular degeneration: an overview. Expert Opin Drug Saf 2014;13:785–802. [DOI] [PubMed] [Google Scholar]
- [31].Yashkin AP, Hahn P, Sloan FA. Introducing anti-vascular endothelial growth factor therapies for AMD did not raise risk of myocardial infarction, stroke, and death. Ophthalmology 2016;123:2225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–47. [DOI] [PubMed] [Google Scholar]
- [33].Emerging Risk Factors Collaboration, Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]