Abstract
Breast cancer patients with liver metastases are associated with high mortality. However, no standardized treatment approach is available for these patients who have undergone chemotherapy and hormonal therapy. We aimed to assess the clinical outcomes of patients with breast cancer liver metastases (BCLM) who underwent drug-eluting beads used for transarterial-chemoembolization (DEB-TACE).
We retrospectively enrolled 14 patients with 39 lesions who underwent DEB-TACE for liver metastases following mastectomy for primary breast cancer. The incidence of complications, overall survival (OS), and local tumor progression-free survival (PFS) were assessed.
A total of 14 patients with 39 liver metastases were treated with DEB-TACE from July 2017 to July 2020. The objective response rates (ORR) and disease control rates (DCR) were 71.4% and 92.8% at the 3-month period and 50% and 71.4% at the 6-month period, respectively. During the follow-up period the local tumor PFS was 8.0 months. The median OS was 20.0 months (range, 8–40 months) and the 1-, 2-year OS rates were 84.4% and 47.4%, respectively. No severe complications caused by this technique were detected.
DEB-TACE for BCLM was characterized as a low trauma technique, with a limited number of complications. The results indicated that this method was safe and effective for patients with BCLM and could be widely adopted as a palliative treatment in clinical practice.
Keywords: drug-eluting beads used for transarterial-chemoembolization, liver metastases, metastatic breast cancer, overall survival
1. Introduction
Breast cancer is the most common malignant tumor that threatens women health worldwide. In 2017, approximately 252,710 women were identified as newly diagnosed breast cancer cases in the United States.[1] Despite the rapid development of medical imaging technology, the early diagnostic rate of breast cancer has greatly increased, whereas approximately 2.4% to 6% of patients still have organ metastases at the time of the first diagnosis.[2,3,4] The common metastatic organs are the bone, lung, liver, and brain. Conventionally, patients with bone metastases may exhibit a better prognosis. The presence of breast cancer liver metastases (BCLM) renders a poor prognosis. This is also caused due to the limited availability of appropriate detection methods and the insensitivity of different types of clinical therapies. Unfortunately, approximately half of breast cancer patients will eventually develop liver metastases and will exhibit a median untreated survival time period as low as 4 months.[5]
In contrast to liver metastases from colorectal cancer, despite being confined to the liver, BCLM are still considered as a systemic disease because of differences in the metastatic routes, biological characteristics. Hence, the mainstay treatments of BCLM are chemotherapy, hormonal therapy, and supportive care. In contrast to the widely accepted local treatment strategy for liver metastasis from colorectal cancer, local treatments for BCLM are rarely performed, and the results vary. In recent years, interventional oncology techniques have gained more attention for the treatment of patients with primary and secondary hepatic malignancies. Although the treatment option for BCLM is palliative, different local treatment modalities such as transarterial-chemoembolization (TACE), radiofrequency ablation (RFA),[6,7] microwave ablation (WMA),[8,9] cryoablation (CA),[10] and laser-induced thermotherapy (LITT)[11] have been applied together with systemic chemotherapeutic agents in order to improve outcomes and also achieved promising results.
The drug-eluting bead transarterial-chemoembolization (DEB-TACE) is a novel drug delivery system, which uses microspheres as embolic agents loaded on chemotherapy drugs. This method has been applied into clinical practice and offers higher intratumoral concentration and lower systemic drug concentrations compared with those noted during conventional TACE (c-TACE). However, the majority of the studies have focused on the evaluation of the efficacy and safety of DEB-TACE applied in patients with primary liver cancer. Few studies have examined DEB-TACE for BCLM to date and the effect of DEB-TACE for BCLM has not been confirmedly established.
Therefore, the present study aimed to retrospectively evaluate the efficacy and safety of CalliSpheres drug-eluting beads used for transarterial-chemoembolization in the treatment of BCLM.
2. Materials and methods
2.1. Patients
A total of 14 patients with BCLM who underwent DEB-TACE at the Shandong Cancer Hospital and Institute from July 2017 to July 2020 were enrolled in the present study. The following inclusion criteria were applied: biopsy-proven liver metastatic lesions from breast cancer, failure of established chemotherapies and/or hormone therapy regimen, or cessation of therapy due to toxic side effects; liver function status at Child-Pugh class A or B; Eastern Cooperative Oncology Group (ECOG) score no >2; the absence of infection; the result of laboratory examinations should meet the following criteria: platelet count >50 × 109/L, hemoglobin >8.0 g/dL, prolongation of the prothrombin time <6 seconds, albumin >2.8 g/dL, bilirubin <51 μmol/L, alanine and aspartate aminotransferase <3 times of the upper limit of the normal range, serum creatinine <1.5 times of the upper limit of the normal range; stable extrahepatic metastases (skeletal metastases, lymph node, or pulmonary metastasis), and expected survival time higher than 6 months.
In addition, patients with liver or renal failure, contraindications for the arterial procedure, presence of serious acute or chronic illness, ascites, liver abscess, and those with allergy to the chemoembolization reagents were excluded from this study.
The present study was conducted according to the basic principles of the Declaration of Helsinki and approved by the Ethical Committee of the Shandong Cancer Hospital and Institute. Written informed consents were collected from all patients.
2.2. Procedures
2.2.1. The process of loading chemotherapy agents
Callispheres (Jiangsu Hengrui Medicine Co. Ltd., Jiangsu, China) beads (100–300 μm or 300–500 μm) were applied as the carrier to load epirubicin or gemcitabine. Epirubicin's loading dose ranged from 60 to 80 mg, whereas the dose of gemcitabine was 400 mg. The loading process was as follows: chemoembolization reagents were dissolved at a concentration of 20 mg/mL; 1 vial of CalliSpheres beads was stirred and the supernatant was extracted. Subsequently, the beads and the chemoembolization solution were mixed by a tee joint; the mixed solution was shaken and allowed to stand for 30 minutes at room temperature. Subsequently, the non-ionic contrast agent was added and the mixed solution was allowed to stand for an additional 5 minutes for further application.
2.2.2. The process of DEB-TACE
Following local disinfection and anesthesia, percutaneous right femoral artery puncture intubation was performed with a modified Seldinger technique and the right femoral artery was punctured. Subsequently, a 5F-RH (Terumo, Japan) catheter was introduced through a 5-F vascular sheath and placed into the common hepatic artery under Digital subtraction angiography guidance to detect the tumor supplying vessels. If the tumor feeding artery was definite, a 2.7-F Progreat microcatheter (Terumo, Japan) was advanced super-selectively into the vessel to perform the embolization. However, if no definite tumor-feeding artery was present, highly selective administration involved embolization of branches leading from the hepatic arteries. The lesion or its feeding branches were preferably selected. The embolization was terminated until the stasis of the contrast agent flow was evident; after approximately 5 minutes, another angiography was performed and the embolization was repeated if the blushed tumor was still evident (Fig. 1).
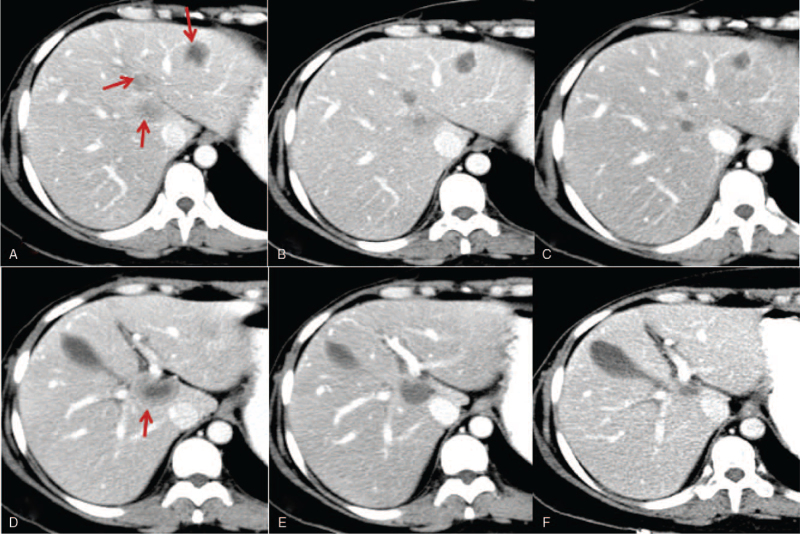
Figure 1.
Image of a 49-year-old woman with BCLM who showed a partial response after DEB-TACE treatment. A, D: Portal phase image of contrast-enhanced CT showed low-attenuated lesions (red arrow): B, E: At 3-months follow-up CT, the metastasis lesions decreased: C, F: At 6-months follow-up CT, the liver metastases decreased significantly. BCLM = breast cancer liver metastases, CT = computed tomography, DEB-TACE = drug-eluting beads used for transarterial-chemoembolization.
2.3. Treatment assessment and follow-up
Individualized computed tomography (CT) or magnetic resonance imaging (MRI) was performed within 1 week prior to initial DEB-TACE in order to evaluate the baseline tumor imaging. Each patient underwent a contrast-enhanced CT scan 1 month following DEB-TACE in order to assess the local tumor response according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST). The objective response (OR) was defined as complete remission (CR) plus partial remission (PR) and the disease control (DC) was defined as CR, PR plus stable disease (SD). The progression of disease (PD) was defined as local recurrence and the presence of new lesions, as well as a combination of both (overall recurrence). The OS was gauged from the date of the initial DEB-TACE until death or the last follow-up visit. The local tumor PFS was calculated as the time between the initial DEB-TACE and the appearance of new lesions in the liver or any increase in the size of the treated lesions (Fig. 2).
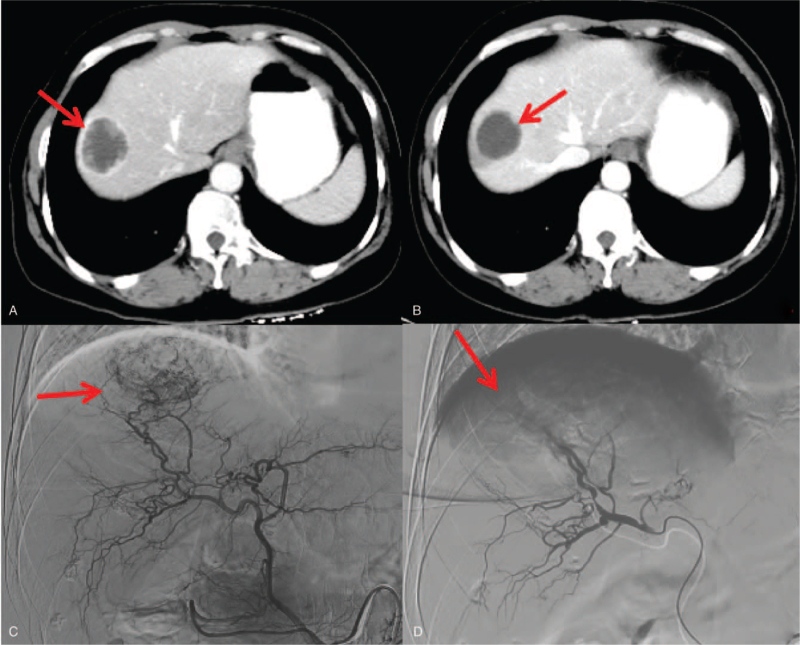
Figure 2.
Hepatic arteriography and post DEB-TACE treatment in a 47-year-old complete responder patient. A. Portal phase image of contrast-enhanced CT showed a low-attenuated lesion (arrow). B. At 3-months follow-up CT, the lesion decreased without contrast enhancement. C. Angiography during the DEB-TACE procedure revealed a comparatively hypervascular lesion. D. Shows the final hepatic angiogram. No remaining tumor blush is visible. CT = computed tomography, DEB-TACE = drug-eluting beads used for transarterial-chemoembolization.
2.4. Safety
Liver function indices including albumin (ALB), total protein (TP), total bilirubin (TBIL), total bile acid (TBA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) were recorded prior to, at the 1-week post-DEB-TACE period and at the 1–3 month post-DEB-TACE treatment period in order to evaluate the influence of DEB-TACE on liver function. In addition, adverse events (AEs) including pain, fever, and nausea/vomiting were recorded during DEB-TACE operation and after 1 month of the DEB-TACE operation.
2.5. Statistical analysis
Statistical analyses were performed using the SPSS 22.0 software (IBM, IL, USA). The local control (LC), overall survival (OS), and local tumor progression-free survival (PFS) rates were calculated using Kaplan–Meier analyses.
3. Results
3.1. Clinical characteristics
The characteristics of the 14 patients are listed in Table 1. A total of 14 patients with 39 liver metastatic lesions were treated with DEB-TACE. All patients were women with a median age of 47 years (range, 36–61 years). The median liver metastasis size was 3.25 (2.4–5.8) cm. A total of 4 patients exhibited 1 metastatic tumor, whereas 1 exhibited 2 tumors, 3 had 3 tumors, and the remaining patient had 4 tumors. A total of 3 exhibited only liver metastasis, while 11 patients presented with >1 metastatic lesion (9, 4, and 2 had bone, lung, and brain metastases, respectively). Liver metastases were observed and were associated with a median of 34.5 months (range, 10–168 months) after the initial diagnosis.
Table 1.
Treatment outcome of individual patients.
| Case | Age | Pathology | Tumor stage | The intervals of liver metastases | No. of metastases | Maximum diameteroflesion, cm | Other metastases | Previous systemic therapy | Previous local therapy in liver | Adjuvant treatment | OS | Alive |
| 1. | 50 | Infiltrating lobular carcinoma | pT2N3M0 | 14 months | 4 | 2.4 | Bone, brain, lung | C, ET | None | None | 15 months | Yes |
| 2 | 42 | Infiltrating ductal carcinoma | pT1aN1M0 | 12 months | 3 | 2.8 | Bone, lung | C, ET | None | C, ET | 24 months | Yes |
| 3 | 49 | Infiltrating ductal carcinoma | pT2N1M0 | 40 months | 4 | 4.4 | Bone | C, ET | None | ET, Palbociclib, Pyrotinib | 12 months | Yes |
| 4 | 49 | Infiltrating ductal carcinoma | pT2N3M0 | 38 months | 4 | 2.4 | Lung | C, ET, H | cTACE | H, C | 17 months | Yes |
| 5 | 43 | Infiltrating ductal carcinoma | pT2N1M0 | 148 months | 3 | 5.8 | Bone, brain, lung, ovary | C, ET | CTACE | Anlotinib | 20 months | No |
| 6 | 61 | Infiltrating ductal carcinoma | pT1N1M0 | 72 months | 2 | 4.5 | Bone | C, ET | None | ET | 12 months | Yes |
| 7 | 53 | Infiltrating lobular carcinoma | pT3N2M0 | 12 months | 4 | 3.7 | Bone | C, ET, H, Pyrotinib, Lapatinib | surgery | Palbociclib | 15 months | No |
| 8 | 60 | Infiltrating ductal carcinoma | pT2N1M0 | 46 months | 1 | 4.4 | Pleura | C, ET, H | None | ET | 35 months | Yes |
| 9 | 56 | Infiltrating ductal carcinoma | pT1N1M0 | 168 months | 1 | 3.5 | None | C, ET | RFA+SBRT | ET | 40 months | Yes |
| 10 | 36 | Infiltrating ductal carcinoma | pT3N3M0 | 12 months | 4 | 3.0 | Bone | C, ET, Palbociclib | none | Palbociclib | 12 months | No |
| 11 | 56 | Infiltrating ductal carcinoma | pT2N3M0 | 10 months | 1 | 4.8 | None | C, ET | None | None | 10 months | Yes |
| 12 | 42 | Infiltrating ductal carcinoma | pT2N2M0 | 46 months | 3 | 3.7 | Bone | C, H, ET, Everolimus | None | ET | 14 months | No |
| 13 | 39 | Infiltrating ductal carcinoma | pT3N1M0 | 31 months | 4 | 4.2 | Bone | C, ET, Palbociclib | None | Olaparib | 8 months | No |
| 14 | 47 | Infiltrating ductal carcinoma | pT1N0M0 | 21 months | 1 | 3.9 | None | C, ET | None | ET | 8 months | Yes |
In all patients, the primary breast tumor histological evaluation was adenocarcinoma, which was divided into invasive ductal carcinoma in 12 (86%) patients and invasive lobular carcinoma in 2 patients (14%). All patients were treated with mastectomy and 10 patients (71%) received postoperative radiotherapy (RT). At diagnosis, 4 patients (28%) were treated with neoadjuvant systemic therapy prior to surgery, while 12 patients (86%) received postoperative systemic chemotherapy. A total of 11 patients (79%) out of the entire cohort were ER positive (+), 8 patients (57%) were PR positive (+), and 3 patients (21%) were cerb-B2 positive (+). A total of 2 patients (14%) had triple negative (–) diseases.
3.2. Treatment prior to DEB-TACE
A total of 13 patients (93%) received chemotherapy during the interval between diagnosis of liver metastases and DEB-TACE. A total of 3 out of 13 patients were treated with the first line of chemotherapy, whereas 7 patients were treated with the second line and 3 patients received the third line of chemotherapy protocols. A total of 13 patients received systemic treatment prior to liver DEB-TACE and the regimens included anthracyclines, taxanes, capecitabine, and gemcitabine. A total of 3 patients with cerb-B2 (+) disease received trastuzumab and 1 patient was treated with combination therapy including trastuzumab and pyrotinib.[12] A total of 11 patients who presented with positive hormone receptor status (+) received hormonal therapy. In addition, with regard to the local therapy, 2 patients performed cTACE, 1 patient received RFA and SBRT, and 1 patient accepted surgery prior to the DEB-TACE.
3.3. Post DEB-TACE treatment
A total of 7 patients from those who received post-DEB-TACE systemic treatment were treated with hormonal therapy. Among these 7 patients, 1 patient was treated with aromatase inhibitors following completion of systemic chemotherapy, 1 patient was combined with palbociclib and pyrotinib, and the remaining 5 patients received aromatase inhibitor treatment only. In addition, 2 patients were treated withpalbociclib only. One patient who had a BRAC mutation received olaparib, whereas 1 patient was treated with herceptin combined with gemcitabine and 1 patient received anlotinib, which is a novel receptor tyrosine kinase inhibitor. A total of 2 patients received no treatment (the details are shown in Table 1).
3.4. Procedure
The chemoembolization technical success rate was 100%. A total of 30 procedures were performed. A total of 4 patients underwent 1 procedure and 5 patients underwent 2 procedures. The remaining patients underwent 3 or 4 procedures. A dose of 60 mg of epirubicin was administered during 20 procedures and a dose of 80 mg was administered in 3 procedures. In addition, a dose of 400 mg gemcitabine was applied in 7 procedures. Among the DEB-TACE procedures, 100 to 300 μm was used in 26 procedures and the remaining used 300 to 500 μm.
3.5. Survival and treatment response of patients
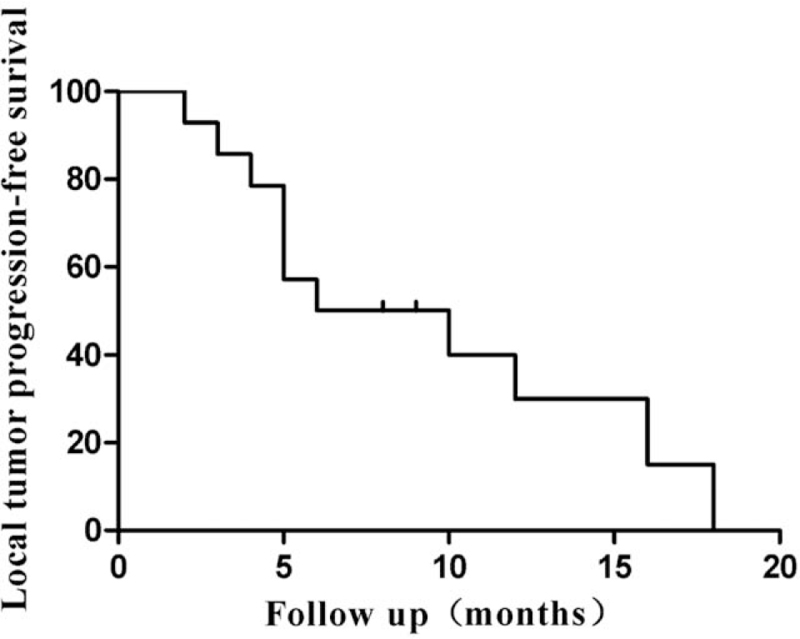
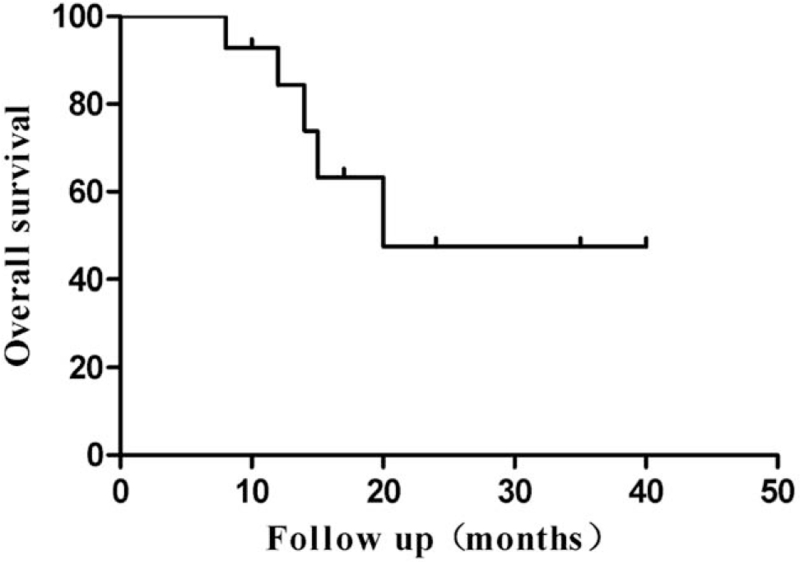
The local tumor response rates are summarized in Table 2. CR, PR, and SD in DEB-TACE were achieved in 5, 5, and 3 patients at the 3-month period and in 3, 4, and 3 patients at the 6-month period, respectively, which resulted in OR and DC rates of 71.4% and 92.8% at 3 months and 50% and 71.4% at 6 months, respectively (Table 2). During the follow-up period, 5 (35.7%) patients did not survive and 9 (64.3%) remained alive till the end of the study. The local tumor PFS was 8.0 months (Fig. 3). The median OS was 20.0 months (range, 8–40 months) in all patients treated with DEB-TACE (Fig. 4), whereas the 1-, 2-year OS rates were 84.4% and 47.4%, respectively.
Table 2.
Local tumor response at 3 and 6 months after DEB-TACE.
| 3 months | 6 months | |
| CR | 5 (35.7%) | 3 (21.4%) |
| PR | 5 (35.7%) | 4 (28.6%) |
| SD | 3 (21.4%) | 3 (21.4%) |
| PD | 1 (7.2%) | 4 (28.6%) |
Figure 3.
Local tumor progression-free survival curve of DEB-TACE for metastatic hepatic tumors from breast cancer. DEB-TACE = drug-eluting beads used for transarterial-chemoembolization.
Figure 4.
Overall survival curve of DEB-TACE for metastatic hepatic tumors from breast cancer. DEB-TACE = drug-eluting beads used for transarterial-chemoembolization.
3.6. Recurrence
During the follow-up, 11 patients presented with a tumor progression in the DEB-TACE lesions or new lesions in the liver or other organs. When these 11 patients were diagnosed with disease progression, 4 patients showed intra- and extrahepatic recurrences and 7 showed only intrahepatic recurrences. Among the 7 patients with intrahepatic recurrences only, 5 showed recurrence only in the remnant liver without recurrences at the lesions treated by DEB-TACE. The single-intrahepatic-recurrence patients mainly underwent another cycle DEB-TACE or SBRT and ablation. The patients with multiple and extrahepatic recurrences underwent chemotherapy, hormonal therapy, supportive treatment. The details of recurrences are present in Table 3.
Table 3.
Treatment in 11 recurred patients.
| Treatment after recurrence | |||||||
| Recurrence pattern | CTx | ET | A | SBRT | DEB-TACE | noTx | Total |
| Intrahepatic recurrences in DEB-TACE lesions | 0 | 0 | 0 | 1 | 1 | 0 | 2 |
| Intrahepatic recurrences in the remnant liver | 5 | ||||||
| Single | 0 | 0 | 1 | 0 | 1 | 0 | 2 |
| Multiple | 0 | 0 | 0 | 0 | 2 | 1 | 3 |
| Intra- and extrahepatic recurrences | 1 | 1 | 0 | 0 | 0 | 2 | 4 |
3.7. Safety profiles of DEB-TACE treatment
With regard to the safety profiles, the most common complication was the post-embolization syndrome. A total of 12 (85.7%), 6 (42.9%), 8 (57.1%), and 4 (28.6%) cases presented with pain, vomiting, fever, and nausea respectively. Serum transaminase levels were elevated in 6 patients. Bone marrow toxicity was detected in 4 patients. They all recovered spontaneously or as a result of timely symptomatic supportive treatment within a week. No other severe complications caused by this technique were detected.
4. Discussion
In contrast to colorectal cancer liver metastases, BCLMs are still considered a systemic disease due to differences in the metastatic routes and distinct biological characteristics. Therefore, systemic therapy such as chemotherapy, hormonal therapy is the mainstay and standard treatment of metastatic breast cancer instead of local therapy.
Although chemotherapy prolongs the survival and delays the progression of patients with BCLM, the survival rates are still quite poor with median survival ranging from 3 to 25 months following systemic treatments.[13] Due to the poor prognosis associated with current treatments, local therapy to oligometastatic lesion has gathered increasing interest by reducing the tumor burden and improving survival rates. Currently, several approaches including surgical resection, RFA, CA, TACE, MWA, or SBRT have been applied in a selected group of patients and the results vary. These outcomes are summarized in Table 4. Theoretically, surgery is an optional choice in BCLM, but its efficacy remains controversial due to relatively high morbidity or mortality derived from their innate invasiveness and consequently is performed only for a selective cohort of patients. Weinrich et al[20] reported that in 29 BCLM patients, the 1-year survival rate of resected patients (n = 21) was 86%, whereas that of non-resected patients (n = 8) was 37.5% following liver resection. The significant prognostic factors were R0 resection, low T- and N-stages as well as a low-grade histopathology of the primary tumor, lower number of liver metastases, and a longer time interval between primary surgery and the occurrence of liver metastasis. In the Lubrano et al[21] study, 16 patients were investigated who underwent partial liver resection for BCLM. Their 1-, 3-, and 5-year OS rates were 94%, 61%, and 33%, respectively. The median survival rate was 42 months. Percutaneous ablations, such as, RFA, MWA, and CA are now considered a good alternative treatment for those high-risk surgical patients and have shown promising results. The literature demonstrated that the BCLM patients who carried out RFA exhibited favorable outcomes, whereas the local control rates were over 90% and the median survival was 10.9 to 60 months. The local tumor progression occurred in a percentage of 13.5% to 58% of treated lesions and the 5-year survival rates ranged from 27% to 30%.[6,15,22,23] WMA indicated a better effectiveness of the method compared with the RFA (100% vs 85–97% coverage of metastatic lesions), a mean survival time of 32 months, and a local progression rate of 9.6%.[8,9,16] Unfortunately, whether RFA or WMA intrahepatic- progression at new sites remain common and long term effect is needed to be proven. In addition, reports of CA and SBRT that have examined the treatment efficacy of BCLM are limited. Onal et al[19] reported the effectiveness and safety of SBRT for the treatment of BCLM. The 1- and 2-year OS rates were 85% and 57%, and the 1- and 2-year PFS rates were 38% and 8%, respectively. In the study by Zhang et al,[10] the CA procedure was used and the 1-year survival from the time of cryoablation was 70.6%, which led to a significant improvement of the quality of life.
Table 4.
Reported series of patients treated with local treatment for breast cancer liver metastases.
| Reference | No. of patients/lesions | Local treatment LM | Mean tumor diameter cm (range) | Local control | PFS (month) | OS (month) |
| Cianni et al,[14] | 52/ | RE | NA | 90% | NA | 11.5 |
| Carrafiello et al,[15] | 13/21 | RFA | 3.5 (0–70) | 66.7% | NA | 10.9 |
| Meloni et al,[6] | 52/87 | RFA | 2.5(0.7–5.0) | 97% | NA | 29.9 |
| Jakobs et al,[7] | 43/111 | RFA | 2.0 (0.5–8.5) | NA | 10.5 | 58.6 |
| Iannitti et al,[16] | 87/224 | WMA | 3.6 (0.5–9) | 97.3% | NA | 47% alive at 19 months |
| Lin et al,[17] | 23/ | TACE | NA | 83% | 8.0 | 17.0 |
| Eichler et al,[18] | 41/ | TACE | NA | 46.3% | 3.3 | 10.2 |
| Onal et al,[19] | 22/29 | SBRT | 2.2 (1.4–6.0) | 1 year 100%2 year 88% | 1 year 38%2 year 8% | 1 year 85%2 year 57% |
| Weinrich et al[20] | 29/56 | S | NA | NA | NA | 1 year 86% |
| Lubrano et al[21] | 16/22 | S | 3.5 (1.0–10) | NA | NA | 42 |
| Zhang et al[10] | 17/39 | CA | 4.0 (2.5–7.5) | 3 month 84% | NA | 1 year 70.6% |
Conventional TACE (c-TACE) offers an alternative noninvasive, and approach for BCLM. The median OS of BCLM who received c-TACE ranged from 10.2 to 21.8 months, whereas the median PFS ranged from 3.0 to 7.9 months, while the tumor response rates ranged from 7% to 13%.[18,24,25,26] The DEB-TACE is currently considered an optimal treatment for PHC patients.[27,28] Currently, it is also widely performed in the treatment of secondary liver malignancies patients. The Response rate (CR+PR) ranges from 10% to 78%. However, the majority of the data regarding the use of DEB-TACE for treating liver metastases are drawn from reports on patients with colorectal cancer,[29,30,31,32] whereas the data regarding DEB-TACE applied in BCLM are limited. Lin et al[17] published a study with Doxorubicin-Loaded 70–150 lm microspheres for liver dominant metastatic breast cancer in 23 patients. They reported a median PFS of 8 months and a median OS of 17 months. The disease control rate and response rate at the 3-month period were 83% and 26% respectively. In the present study, 14 patients who underwent DEB-TACE indicated a median OS of 20 months (range 8–40 months). The local tumor PFS was 8.0 and the 1-, 2-year OS rates were 84.4% and 47.4% respectively. The response rates at the 3- and 6-month periods were 71.4% and 50.0%, respectively. We almost shared the same experience with the study reported by Lin et al, although a better response rate was reported (71.4% vs 26%). In the latter study, in order to avoid missing metastases and unmasking the metastases that were not detected in CT, the authors used a lobar method of chemoembolization administration. However, in the present study, highly selective administration was demanded as far as possible and this may have caused a more complete embolization and reduced liver damage. In addition, different systemic treatments were applied in 86% (12/14) patients. Therefore, micrometastases in the liver can also be controlled despite the selection process. These reasons may be attributed to the relative high response rate.
The most common complication experienced by almost all patients undergoing this type of treatment is the post-embolization syndrome with pain in the right upper quadrant, nausea, vomiting fever, and elevation of liver enzymes. They all recovered spontaneously or as a result of timely symptomatic supportive treatment and no procedure-related death occurred within 3 months. These results may be attributed to the deliberate selection of qualified patients as well as the highly selective administration in the procedure.
To sum up, in the present study we demonstrated the effectiveness and safety of DEB-TACE in the treatment of BCLM patients. The objective response rates and disease control rates were 71.4% and 92.8% at the 3-month period and 50% and 71.4% at the 6-month period, respectively. A satisfied local control rate was observed in BCLM after DEB-TACE. In addition, 81.8% (9/11) of patients had disease progression in other organs or in the remnant liver other than the DEB-TACE treated lesions, thus effective systemic treatment is required to improve treatment outcomes. In our study, 76.9% (10/13) of patients were treated with second or third line chemotherapy prior to DEB-TACE, which may respond poor to any local therapies. So we considered earlier DEB-TACE may result in a better prognosis and further research are necessary to support our thoughts. Moreover, DEB-TACE of liver metastases was well tolerated, and no fatal complications were detected in our study. Currently, TACE has become a regular treatment modality for many malignancies in our institution. More than 4000 TACE procedures were performed every year in our institution and provided the surgeon sufficiently skilled to perform the procedure, fatal side-effects related to the technique are extremely rare. Thus, DEB-TACE seems to be an effective and safe treatment option for BCLM patients.
The present study contains certain limitations. Initially, it contained a retrospective study design, a relatively small cohort, and a short follow-up time. In addition, the majority of the patients of the study had received multiple local or systemic therapies prior to DEB-TACE, which may have led to a relative poor OS and PFS. Also, as different chemotherapeutic drugs were used among the studies (including epirubicin or gemcitabine), it may have a certain impact on the results. Lastly, the systemic treatment varied prior to and following DEB-TACE, which may have potentially influenced the treatment outcomes.
5. Conclusions
DEB-TACE for BCLM is characterized as a low trauma technique, with few complications. The results suggested that it is safe and effective for patients with BCLM. Furthermore, as DEB-TACE gradually develops into a mature technique, this approach could be widely applied as a valid alternative treatment option for patients who are not suitable for surgical resection or develop resistance for systemic therapy. The authors’ initial experience of DEB-TACE seems promising and could be further used and explored for its utility in the systemic treatment of metastasis in further trials using a prospective, controlled design.
Author contributions
Conceptualization: Xu Chang, Jibing Liu, Yinfa Xie.
Data curation: Xu Chang, Peng Sun, Jianxin Zhang, Lin Zhang, Huiyong Wu.
Formal analysis: Xu Chang, Peng Sun, Jianxin Zhang, Lin Zhang, Huiyong Wu, Jibing Liu, Yinfa Xie.
Investigation: Xu Chang, Peng Sun.
Project administration: Jibing Liu, Yinfa Xie.
Writing – original draft: Xu Chang.
Writing – review & editing: Peng Sun, Jianxin Zhang, Lin Zhang, Huiyong Wu, Jibing Liu, Yinfa Xie.
Footnotes
Abbreviations: BCLM = breast cancer liver metastases, DEB-TACE = drug-eluting beads used for transarterial-chemoembolization, OS = overall survival.
How to cite this article: Chang X, Sun P, Zhang J, Zhang L, Wu H, Xie Y, Liu J. CalliSpheres drug-eluting beads transarterial-chemoembolization in the treatment of liver metastases from breast cancer: Initial experience in 14 patients. Medicine. 2021;100:52(e28407).
This work was partly supported by Shandong Province Natural Science ZR2020QH177.
The authors have no conflicts of interest to disclose.
Availability of data and materials: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
CTx = chemotherapy, ET = endocrine hormonal therapy, H = Herceptin, cTACE = conventional transcatheter arterial chemoembolization, RFA = radio frequency ablation, SBRT = stereotactic body radiotherapy.
CR = complete remission, DEB-TACE = drug-eluting beads used for transarterial-chemoembolization, PD = progression of disease, PR = partial remission, SD = stable disease.
A = ablation, CTx = chemotherapy, DEB-TACE = drug-eluting bead transarterial-chemoembolization, ET = endocrine hormonal therapy, SBRT = stereotactic body radiotherapy, Tx = treatment.
CA = cryoablation, RE = 90Yradioembolisation, RFA = radiofrequency ablation, S = surgery, SBRT = stereotactic body radiotherapy, TACE = transcatheter arterial chemoembolization, WMA = microwave ablation.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:07–30. [DOI] [PubMed] [Google Scholar]
- [2].Li J, Zhang BN, Fan JH, et al. A nation-wide multicenter 10-year (1999-2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer 2011;11:364.doi: 10.1186/1471-2407-11-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jung KW, Won YJ, Kong HJ, Oh CM, Shin A, Lee JS. Survival of korean adult cancer patients by stage at diagnosis, 2006-2010: national cancer registry study. Cancer Res Treat 2013;45:162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chutia N, Malakar S, Abdel-Rafee G, et al. Practice of Breast Self-Examination and Knowledge of Breast and Cervical Cancer Screening: A bi-national Survey in Africa. 2020;https://www.researchgate.net/publication/347197786 [Google Scholar]
- [5].Wyld L, Gutteridge E, Pinder SE, et al. Prognostic factors for patients with hepatic metastases from breast cancer. Br J Cancer 2003;89:284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Meloni MF, Andreano A, Laeseke PF, Livraghi T, Sironi S, Lee FT, Jr. Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation--intermediate and long-term survival rates. Radiology 2009;253:861–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jakobs TF, Hoffmann RT, Schrader A, et al. CT-guided radiofrequency ablation in patients with hepatic metastases from breast cancer. Cardiovasc Intervent Radiol 2009;32:38–46. [DOI] [PubMed] [Google Scholar]
- [8].Lorentzen T, Skjoldbye BO, Nolsoe CP. Microwave ablation of liver metastases guided by contrast-enhanced ultrasound: experience with 125 metastases in 39 patients. Ultraschall Med 2011;32:492–6. [DOI] [PubMed] [Google Scholar]
- [9].Abe H, Kurumi Y, Naka S, et al. Open-configuration MR-guided microwave thermocoagulation therapy for metastatic liver tumors from breast cancer. Breast Cancer 2005;12:26–31. [DOI] [PubMed] [Google Scholar]
- [10].Zhang W, Yu H, Guo Z, et al. Percutaneous cryoablation of liver metastases from breast cancer: initial experience in 17 patients. Clin Radiol 2014;69:231–8. [DOI] [PubMed] [Google Scholar]
- [11].Mack MG, Straub R, Eichler K, Sollner O, Lehnert T, Vogl TJ. Breast cancer metastases in liver: laser-induced interstitial thermotherapy--local tumor control rate and survival data. Radiology 2004;233:400–9. [DOI] [PubMed] [Google Scholar]
- [12].Blair HA. Pyrotinib: first global approval. Drugs 2018;78:1751–5. [DOI] [PubMed] [Google Scholar]
- [13].Pivot X, Asmar L, Hortobagyi GN, Theriault R, Pastorini F, Buzdar A. A retrospective study of first indicators of breast cancer recurrence. Oncology 2000;58:185–90. [DOI] [PubMed] [Google Scholar]
- [14].Cianni R, Pelle G, Notarianni E, et al. Radioembolisation with (90)Y-labelled resin microspheres in the treatment of liver metastasis from breast cancer. Eur Radiol 2013;23:182–9. [DOI] [PubMed] [Google Scholar]
- [15].Carrafiello G, Fontana F, Cotta E, et al. Ultrasound-guided thermal radiofrequency ablation (RFA) as an adjunct to systemic chemotherapy for breast cancer liver metastases. Radiol Med 2011;116:1059–66. [DOI] [PubMed] [Google Scholar]
- [16].Iannitti DA, Martin RC, Simon CJ, et al. Hepatic tumor ablation with clustered microwave antennae: the US Phase II trial. HPB 2007;9:120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lin YT, Medioni J, Amouyal G, Dean C, Sapoval M, Pellerin O. Doxorubicin-loaded 70-150 mum microspheres for liver-dominant metastatic breast cancer: results and outcomes of a pilot study. Cardiovasc Intervent Radiol 2017;40:81–9. [DOI] [PubMed] [Google Scholar]
- [18].Eichler K, Jakobi S, Gruber-Rouh T, Hammerstingl R, Vogl TJ, Zangos S. Transarterial chemoembolisation (TACE) with gemcitabine: phase II study in patients with liver metastases of breast cancer. Eur J Radiol 2013;82:e816–22. [DOI] [PubMed] [Google Scholar]
- [19].Onal C, Guler OC, Yildirim BA. Treatment outcomes of breast cancer liver metastasis treated with stereotactic body radiotherapy. Breast 2018;42:150–6. [DOI] [PubMed] [Google Scholar]
- [20].Weinrich M, Weiss C, Schuld J, Rau BM. Liver resections of isolated liver metastasis in breast cancer: results and possible prognostic factors. HPB Surg 2014;2014:893829.doi: 10.1155/2014/893829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lubrano J, Roman H, Tarrab S, Resch B, Marpeau L, Scotte M. Liver resection for breast cancer metastasis: does it improve survival? Surg Today 2008;38:293–9. [DOI] [PubMed] [Google Scholar]
- [22].Gunabushanam G, Sharma S, Thulkar S, et al. Radiofrequency ablation of liver metastases from breast cancer: results in 14 patients. J Vasc Interv Radiol 2007;18(1 pt 1):67–72. [DOI] [PubMed] [Google Scholar]
- [23].Sofocleous CT, Nascimento RG, Gonen M, et al. Radiofrequency ablation in the management of liver metastases from breast cancer. AJR Am J Roentgenol 2007;189:883–9. [DOI] [PubMed] [Google Scholar]
- [24].Giroux MF, Baum RA, Soulen MC. Chemoembolization of liver metastasis from breast carcinoma. J Vasc Interv Radiol 2004;15:289–91. [DOI] [PubMed] [Google Scholar]
- [25].Vogl TJ, Naguib NN, Nour-Eldin NE, Eichler K, Zangos S, Gruber-Rouh T. Transarterial chemoembolization (TACE) with mitomycin C and gemcitabine for liver metastases in breast cancer. Eur Radiol 2010;20:173–80. [DOI] [PubMed] [Google Scholar]
- [26].Wang M, Zhang J, Ji S, et al. Transarterial chemoembolisation for breast cancer with liver metastasis: a systematic review. Breast 2017;36:25–30. [DOI] [PubMed] [Google Scholar]
- [27].Zhou GH, Han J, Sun JH, et al. Efficacy and safety profile of drug-eluting beads transarterial chemoembolization by CalliSpheres(R) beads in Chinese hepatocellular carcinoma patients. BMC cancer 2018;18:644.doi: 10.1186/s12885-018-4566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wu B, Zhou J, Ling G, Zhu D, Long Q. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: a short-term efficacy and safety study. World J Surg Oncol 2018;16:69.doi: 10.1186/s12957-018-1368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ranieri G, Niccoli Asabella A, Altini C, et al. A pilot study employing hepatic intra-arterial irinotecan injection of drug-eluting beads as salvage therapy in liver metastatic colorectal cancer patients without extrahepatic involvement: the first southern Italy experience. OncoTargets Ther 2016;9:7527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Iezzi R, Marsico VA, Guerra A, et al. Trans-arterial chemoembolization with irinotecan-loaded drug-eluting beads (DEBIRI) and capecitabine in refractory liver prevalent colorectal metastases: a phase II single-center study. Cardiovasc Intervent Radiol 2015;38:1523–31. [DOI] [PubMed] [Google Scholar]
- [31].Levy J, Zuckerman J, Garfinkle R, et al. Intra-arterial therapies for unresectable and chemorefractory colorectal cancer liver metastases: a systematic review and meta-analysis. HPB 2018;20:905–15. [DOI] [PubMed] [Google Scholar]
- [32].Bhutiani N, Akinwande O, Martin RC, 2nd. Efficacy and toxicity of hepatic intra-arterial drug-eluting (Irinotecan) bead (DEBIRI) therapy in irinotecan-refractory unresectable colorectal liver metastases. World J Surg 2016;40:1178–90. [DOI] [PubMed] [Google Scholar]