Abstract
PURPOSE
Definitive or postoperative chemoradiation (CRT) is curative for human papillomavirus–associated (HPV+) oropharynx cancer (OPC) but induces significant toxicity. As a deintensification strategy, we studied primary transoral surgery (TOS) and reduced postoperative radiation therapy (RT) in intermediate-risk HPV+ OPC.
METHODS
E3311 is a phase II randomized trial of reduced- or standard-dose postoperative RT for resected stage III-IVa (American Joint Committee on Cancer-seventh edition) HPV+ OPC, determined by pathologic parameters. Primary goals were feasibility of prospective multi-institutional study of TOS for HPV+ OPC, and oncologic efficacy (2-year progression-free survival) of TOS and adjuvant therapy in intermediate-risk patients after resection. TOS plus 50 Gy was considered promising if the lower limit of the exact 90% binomial confidence intervals exceeded 85%. Quality of life and swallowing were measured by functional assessment of cancer therapy-head and neck and MD Anderson Dysphagia Index.
RESULTS
Credentialed surgeons performed TOS for 495 patients. Eligible and treated patients were assigned as follows: arm A (low risk, n = 38) enrolled 11%, intermediate risk arms B (50 Gy, n = 100) or C (60 Gy, n = 108) randomly allocated 58%, and arm D (high risk, n = 113) enrolled 31%. With a median 35.2-month follow-up for 359 evaluable (eligible and treated) patients, 2-year progression-free survival Kaplan-Meier estimate is 96.9% (90% CI, 91.9 to 100) for arm A (observation), 94.9% (90% CI, 91.3 to 98.6]) for arm B (50 Gy), 96.0% (90% CI, 92.8 to 99.3) for arm C (60 Gy), and 90.7% (90% CI, 86.2 to 95.4) for arm D (66 Gy plus weekly cisplatin). Treatment arm distribution and oncologic outcome for ineligible or step 2 untreated patients (n = 136) mirrored the 359 evaluable patients. Exploratory comparison of functional assessment of cancer therapy-head and neck total scores between arms B and C is presented.
CONCLUSION
Primary TOS and reduced postoperative RT result in outstanding oncologic outcome and favorable functional outcomes in intermediate-risk HPV+ OPC.
INTRODUCTION
Approximately 15,000 cases of human papillomavirus–associated (HPV+) oropharynx cancer (OPC) are diagnosed annually in the United States and have superior cure rates to tobacco- and alcohol-associated OPC.1-3 HPV+ OPC commonly presents with a small primary tumor and cervical lymphadenopathy amenable to surgical treatment.4,5 Transoral robotic surgery (TORS) or transoral laser microsurgery (TLM) may address this malignancy in a minimally invasive manner6 and, when combined with risk-adjusted postoperative radiation therapy (PORT), may achieve comparable cure rates. Retrospective reports suggest improved functional results with primary transoral surgery (TOS),7,8 yet its role in multidisciplinary management and treatment deintensification remains uncertain.9
CONTEXT
Key Objective
Our primary objectives were to demonstrate the feasibility of a prospective multi-institutional study of transoral surgery (TOS) for human papillomavirus+ oropharynx cancer followed by risk-adjusted adjuvant therapy, and to assess the 2-year progression-free survival of TOS and reduced adjuvant therapy in intermediate-risk patients.
Knowledge Generated
This novel randomized phase II clinical trial reports the first prospective multicenter data for TOS in head and neck cancer. For the 70% of patients who underwent deintensified postoperative adjuvant therapy, outstanding progression-free survival supports the safety and efficacy of treatment deintensification (elimination of chemotherapy, and 10 Gy less radiation). Exploratory comparison suggests a trend toward better quality-of-life scores associated with reduced radiation therapy and chemotherapy.
Relevance
Primary TOS and reduced postoperative radiation therapy result in outstanding oncologic outcome and favorable functional outcomes in intermediate-risk human papillomavirus+ oropharynx cancer.
Treatment with either surgery and adjuvant therapy10,11 or with definitive chemoradiation (CRT) achieves high rates of cure for HPV+ OPC.2 However, as patients are likely to live longer and experience the associated long-term toxicity of definitive CRT,12 there is interest in radiation and/or chemotherapy deintensification for those patients with excellent prognosis. Both surgical and nonsurgical deintensification strategies are actively being pursued in numerous clinical trials.13,14 TOS with de-escalated postoperative management is one potential deintensification strategy.7,10 Retrospective studies of TOS compared with definitive CRT show high oncologic efficacy, and suggest improved functional results in patients undergoing surgery with decreased gastrostomy tube dependency.7,8,15 At the time this study was designed, sufficient normative data for design of a phase III trial comparing TOS and deintensified postoperative therapy were not available, and thus, Eastern Cooperative Oncology Group (ECOG) and the American College of Radiology Imaging Network (ACRIN) Cancer Research Group E3311 was conducted as a randomized phase II trial of primary TOS, with adjuvant therapy based on pathologic risk assessment, for HPV+ OPC. Our primary objectives were to demonstrate the feasibility of a prospective multi-institutional study of TOS for HPV+ OPC followed by risk-adjusted adjuvant therapy, and to assess the oncologic efficacy, reflected in 2-year progression-free survival (PFS), of transoral resection and adjuvant therapy in patients determined to be at intermediate risk after surgical excision.
Study Design and Participants
E3311 (NCT01898494) is a randomized phase II study of transoral surgery (TOS) followed as indicated by postoperative radiation therapy (PORT) with or without chemotherapy for stage III-IVA human papillomavirus+ oropharynx cancer (OPC; using American Joint Committee on Cancer-seventh edition). Patients defined as having intermediate risk were randomly allocated to reduced (50 Gy)- or standard (60 Gy)-dose radiation. Eligible patients had T1-2 p16+ squamous cell carcinoma of the oropharynx amenable to transoral resection, with no matted nodes, and were candidates for radiation and cisplatin. The study Protocol (online only) and eligibility criteria have been previously described in greater detail.16 The institutional review board (IRB) at each site approved the study Protocol, which was approved by the National Cancer Institute (NCI) central IRB. All study subjects provided written informed consent and were enrolled on step 1 (TOS). Step 2 adjuvant treatment arm assignment was determined by pathologic risk parameters (Appendix Table A1, online only), as follows: low-risk patients defined T1-T2 resected with negative (> 3 mm) margins, N0-N1 and no extranodal extension (ENE), were observed (arm A); intermediate-risk patients defined T1-T2 resected to negative or close (< 3 mm) margins, N1-N2 with ≤ 1 mm ENE, or up to four positive nodes were randomly allocated to 50 Gy (arm B) or 60 Gy (arm C) of PORT; higher-risk patients (arm D) were those with positive margins, > 1 mm of ENE, and/or ≥ 5 metastatic lymph nodes, and received 66 Gy of PORT with concurrent weekly cisplatin at 40 mg/m2. The random assignment in the intermediate-risk patient group was stratified by smoking history (≤ 10 v > 10 pack-years). Validated patient-reported outcomes (PRO) measuring quality of life and swallowing were collected.
Statistical Analysis
The study was designed to estimate the 2-year PFS rate for intermediate-risk patients who received TOS followed by reduced-dose PORT (arms B and C). The regimen of TOS plus reduced-dose radiation (arm B and C, analyzed separately) would be considered worthy of further study if, for the given arm, the upper limit of the exact 90% binomial CI exceeded 85%, indicating a result consistent with noninferiority to the outcomes observed in our prior study E2399. It was expected that 48% of the patient population would be evaluable intermediate risk, and therefore a sample size of 377 was targeted to enroll at least 180 intermediate-risk patients.
RESULTS
Of 120 applications, 87 surgeons at 59 sites were credentialed,17 and 68 accrued at least one patient. We separately reported details regarding surgical quality assurance including positive margin rate, postoperative grade III or IV oropharyngeal bleeding, and number of nodes removed.17 One noncredentialed surgeon accrued three patients (see Fig 1 CONSORT diagram). The surgeon underwent a retrospective credentialing procedure, according to ECOG ACRIN and institutional review board guidance, and was found to qualify. After ligation of cervical vessels was made a strong recommendation in a trial amendment (activated January 13, 2016), the only grade V fatal oropharyngeal bleeding occurred (out of 256 enrolled patients after amendment activation) with the omission of recommended vessel ligation. No difference in grade III-V oral bleeding events was observed before and after amendment mandating ligation (6.1% v 6.1%; P = .99).
FIG 1.
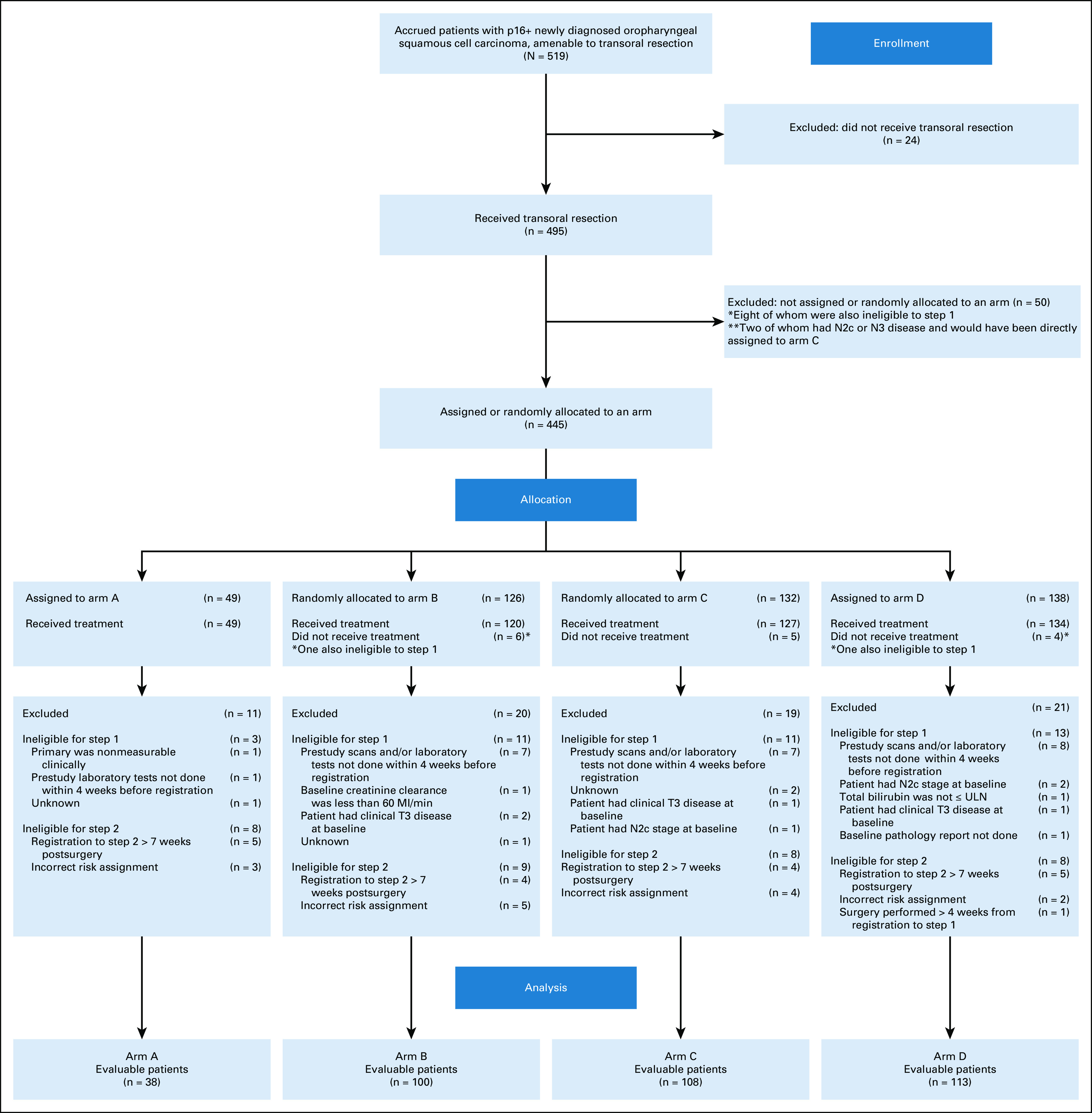
CONSORT diagram.
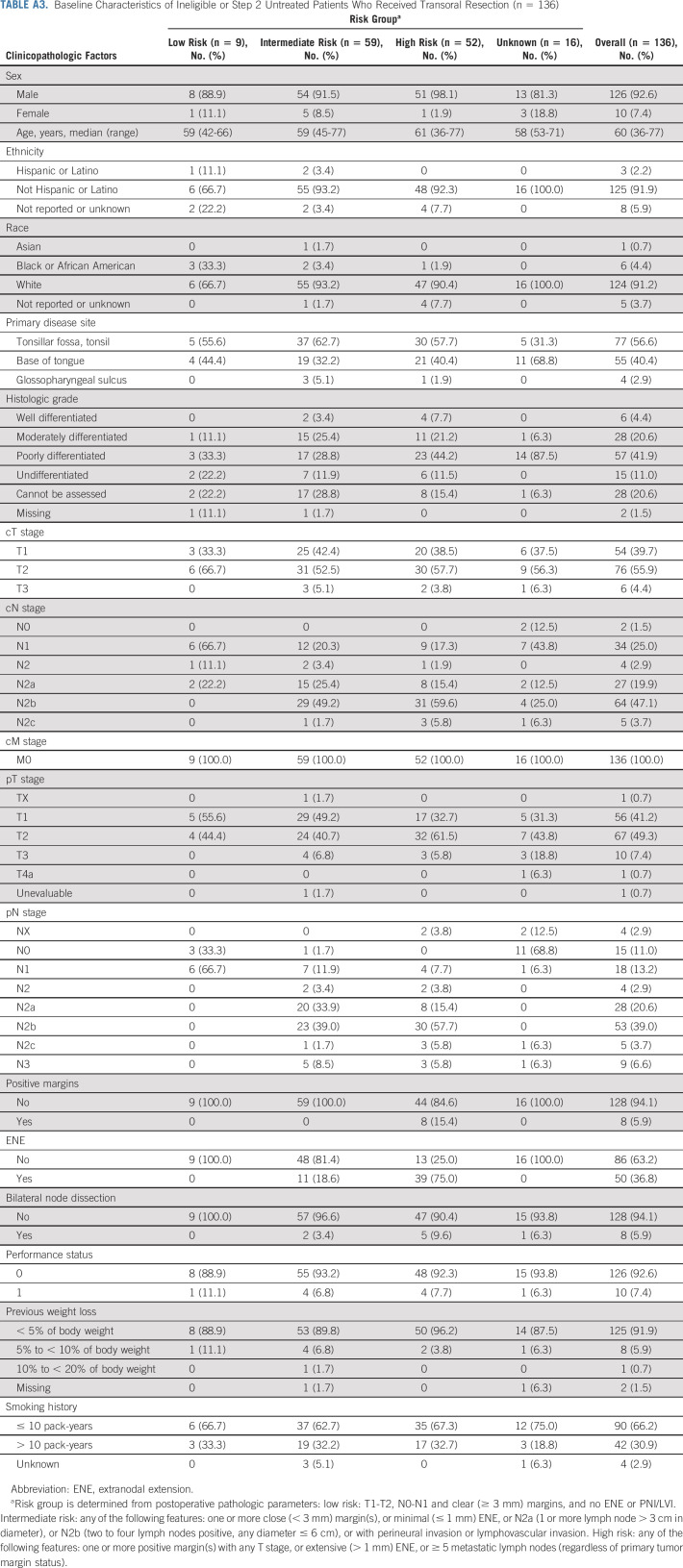
From December 2013 to July 2017, 68 credentialed surgeons enrolled 519 patients and performed TOS for 495 patients with HPV+ OPC. Among the 495 patients who underwent planned surgery, 445 were assigned or randomly allocated to a postoperative treatment arm. Among the 495 patients who underwent planned surgery, 443 (89.5%) underwent TORS, 41 (8.3%) underwent TLM, and 11 (2.2%) underwent surgery using standard equipment. We did not observe differences in assignment to postoperative adjuvant treatment based on surgical technique. Among patients who underwent TORS, 11% were assigned to arm A, 28% and 30% randomly allocated to arms B and C, respectively, and 31% were assigned to arm D. Among patients who underwent TLM, 11% were assigned to arm A, 32% and 24% were randomly allocated to arms B and C, respectively, and 34% were assigned to arm D. Eighty-one patients (71 of whom were eventually treated) were deemed ineligible; 48 patients were ineligible for step 1 (surgery) and 33 patients were ineligible for step 2. The most common reasons for ineligibility at step 1 included prestudy scans and/or laboratory tests completed before the 4-week preregistration window (n = 27) and presence of clinical T3 disease at baseline (n = 4). Ineligibility at step 2 was predominantly because of registration to step 2 at > 7 weeks after surgery (n = 18) and incorrect risk assignment (n = 14) after central review. Although we report evaluable patients (n = 359) for the primary end point and demographics of this group as shown in Table 1, we also present data for all ineligible or untreated (on step 2) patients who underwent planned surgery (n = 136), as well as ineligible and step 2 treated patients (n = 71), because ineligibility determinations were made during data cleaning, only including those patients who had received accurate treatment arm assignments to, and been treated on, step 2 of the study (Appendix Table A2, online only). The proportion of patients assigned to each arm in step 2 was comparable between the total and treated population and the eligible and treated population: 11% to arm A (N = 49); 57% to arms B (N = 120) and C (N = 127); and 31% to arm D (N = 134). We continued follow-up of ineligible or misclassified patients (n = 71) for safety reporting and outcome.
TABLE 1.
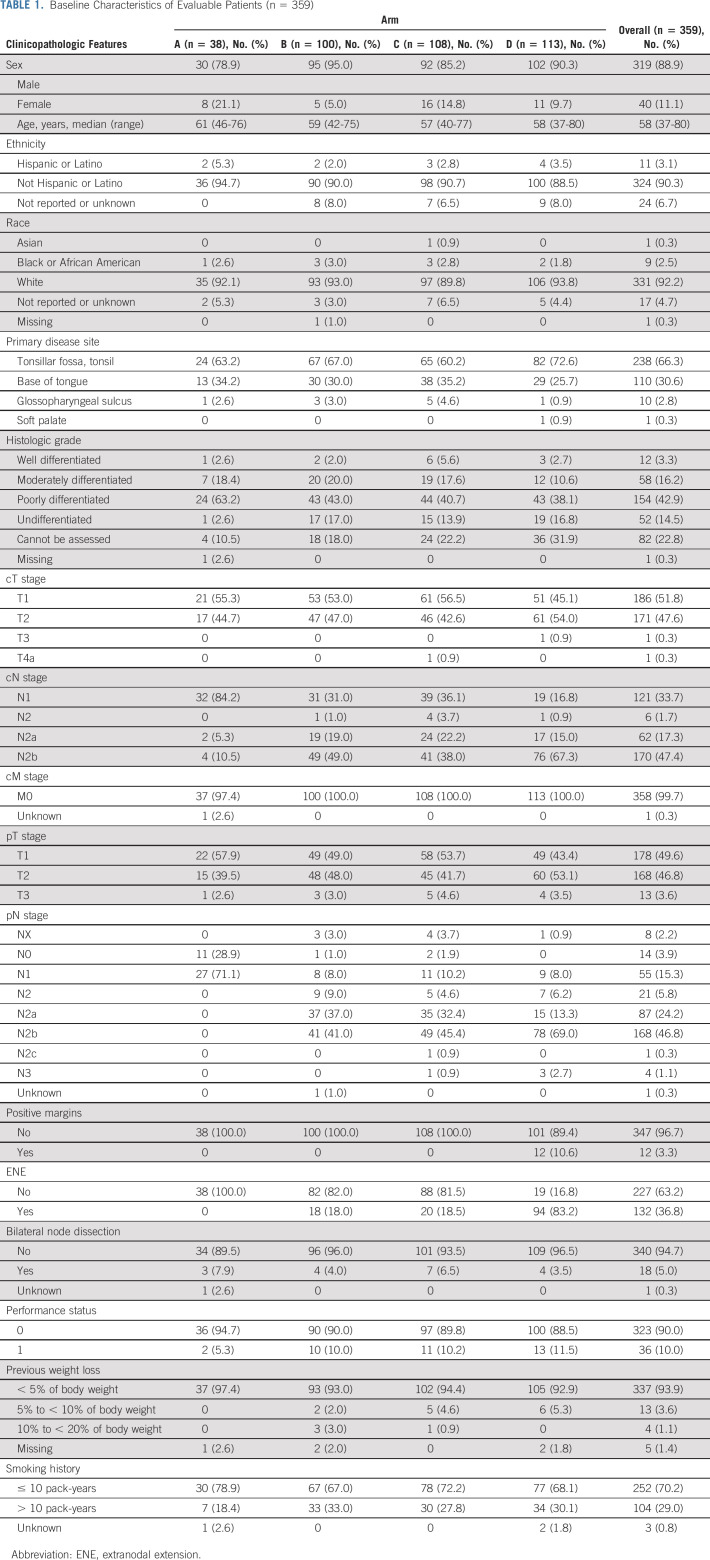
Baseline Characteristics of Evaluable Patients (n = 359)
Patient demographics are shown in Table 1 for those evaluable (n = 359), who were analyzed for the primary end point (Fig 1, CONSORT diagram), as well as in Appendix Table A3 (online only) for ineligible or step 2 untreated patients who received TOS (n = 136). The median age was 58 years (range of 37-80 years), and the majority were male (88.9%), with primary disease site of tonsil (66.3%), and ≤ 10 pack-years of smoking history (70.2%). Among the 359 evaluable, arm A (N = 38) enrolled 11%, arms B (50 Gy, N = 100) or C (60 Gy, N = 108) randomly allocated 58%, and arm D (N = 113) enrolled 31%. Arm D assignment was based on > 1 mm extranodal extension (ENE; 77%), > 4 nodes (27%), and/or positive margins (11%). ENE of any extent was present in 37% of patients on E3311. ENE ≤ 1 mm comprised 34% of the patients with any ENE, with the other 66% scored > 1 mm and leading to arm D assignment. Indeed, 77% of arm D patients had ENE > 1 mm, and some patients had multiple arm D risk factors. The positive margin rate was 3.3% overall. Forty-six patients received (and were eligible for) TOS (step 1) and assigned to adjuvant therapy (step 2) but subsequently did not start treatment or deemed ineligible for step 2 (17% in arm A [N = 8]; 59% in arms B [N = 14] and C [N = 13]; and 24% in arm D [N = 11]).
In September 2015, the data safety monitoring committee reviewed the interim analyses results regarding accrual, risk distribution, and surgical quality. The observed accrual rate was 18.5 patients per month, higher than the assumed eight patients per month. Among the first 59 patients completing transoral resection, 29 were intermediate risk. Among the first 59 eligible patients, five grade 3 bleeding events and two cases with positive margins were reported. Thus, the prespecified stopping rules were not met. The final composite quality assurance end point was a 9.1% rate of combined grade III or IV oropharyngeal bleeding or positive margins (4.0% positive margins, 5.9% grade III or IV oropharyngeal bleeding, 6.1% grade III-V oropharyngeal bleeding).
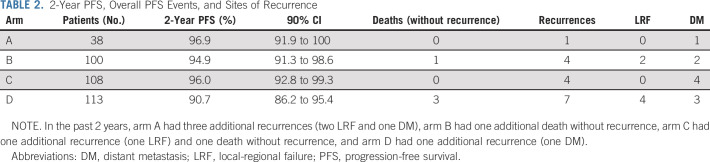
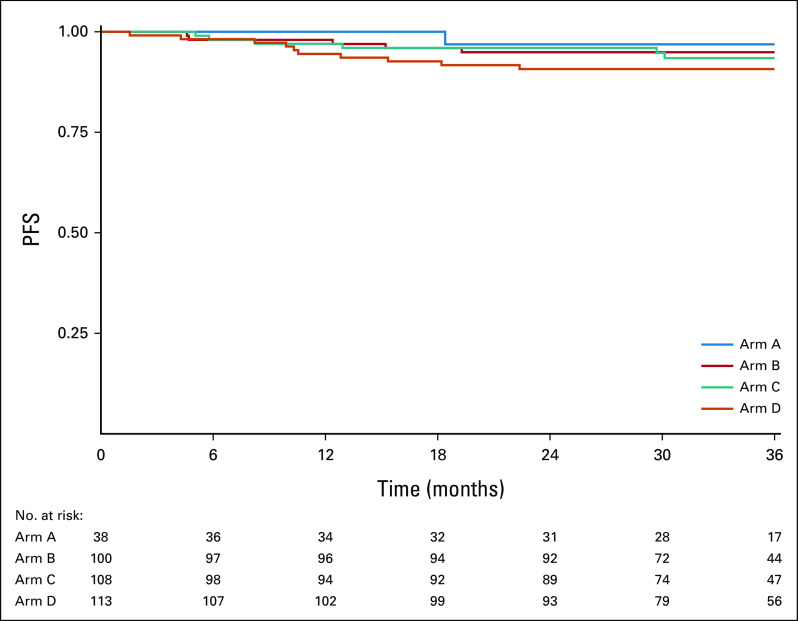
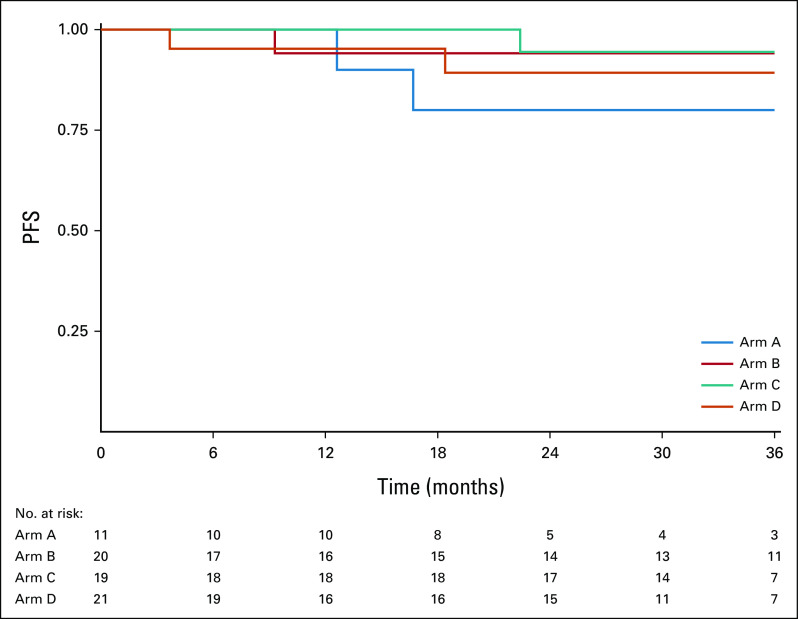
Among the 359 evaluable patients, current median follow-up time among patients who did not progress is 35.2 months, and it is 35.0 months among evaluable intermediate-risk patients who did not progress. Among the 21 recurrences observed at the time of this analysis, 10 were local-regional and 11 were distant (Table 2). Figure 2 displays PFS curves for all evaluable patients, by arm. The 2-year PFS Kaplan-Meier estimate was 96.9% (90% CI, 91.9 to 100) for arm A, 94.9% (90% CI, 91.3 to 98.6) for arm B, 96.0% (90% CI, 92.8 to 99.3) for arm C, and 90.7% (90% CI, 86.2 to 95.4) for arm D. The 3-year PFS estimates were 96.9% (90% CI, 91.9 to 100) for arm A, 94.9% (90% CI, 91.3 to 98.6) for arm B, 93.4% (90% CI, 89.2 to 97.8) for arm C, and 90.7% (90% CI, 86.2 to 95.4) for arm D. The single patient with the unexpected finding of N3 disease without other high-risk features, who was assigned to arm C, experienced a local late recurrence at 30 months; sensitivity analyses excluding this patient showed results consistent with the primary analysis (2-year PFS of 95.9%; 90% CI, 92.7 to 99.3). Exploratory comparisons of PFS between the arms (using the log-rank test) revealed no significant differences (P values = 0.90 for B v C; 0.30 for B v D; 0.30 for C v D), although caution should be taken in the interpretation of these results as the study was not powered for the direct comparison of arms B and C, and differences in both treatment and risk confound the comparisons of arms B or C and D.
TABLE 2.
2-Year PFS, Overall PFS Events, and Sites of Recurrence
FIG 2.
Kaplan-Meier estimates of PFS by arm for 359 evaluable patients. PFS, progression-free survival.
Those with smoking history (> 10 v ≤ 10 pack-years) did not have a detectably worse 3-year PFS in arms B or C (97%; 90% CI, 92.2 to 100 v 93.9%; 90% CI, 89.1 to 98.9 in arm B; 95.2%; 90% CI, 87.9 to 100 v 92.8%; 90% CI, 87.8 to 98.1 in arm C; 93.1% 90% CI, 85.7 to 100% v 89.6%; 90% CI, 84.0 to 95.5% in arm D).
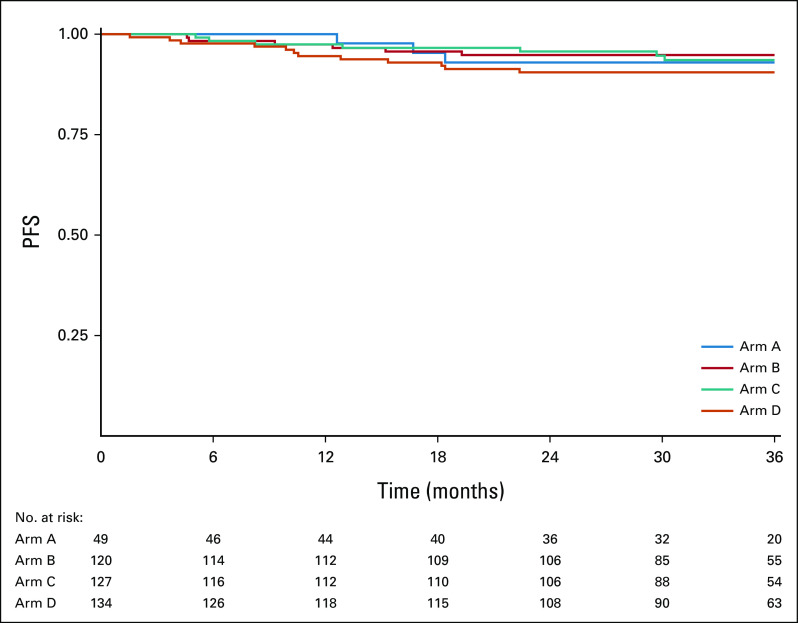
Appendix Figure A1 (online only) displays PFS curves for ineligible and step 2 treated patients (n = 71), by arm. The 2-year PFS Kaplan-Meier estimate was 80.0% (90% CI, 61.7 to 100) for arm A, 94.1% (90% CI, 85.2 to 100) for arm B, 94.4% (90% CI, 86.0 to 100) for arm C, and 89.3% (90% CI, 78.2 to 100) for arm D. Appendix Figure A3 (online only) displays PFS curves for all step 2 treated patients (eligible and ineligible, n = 430), by arm. The 2-year PFS Kaplan-Meier estimate was 93.0% (90% CI, 86.7 to 99.6) for arm A, 94.8% (90% CI, 91.5 to 98.3) for arm B, 95.7% (90% CI, 92.7 to 98.8) for arm C, and 90.5% (90% CI, 86.3 to 94.9) for arm D. The 3-year PFS Kaplan-Meier estimate was 93.0% (90% CI, 86.7 to 99.6) for arm A, 94.8% (90% CI, 91.5 to 98.3) for arm B, 93.6% (90% CI, 89.8 to 97.5) for arm C, and 90.5% (90% CI, 86.3 to 94.9) for arm D.
Among the 359 evaluable patients, 16 patients had died at the time of this analysis (one in arm A, two in arm B, six in arm C, and seven in arm D). The current median follow-up time among patients who are alive is 35.4 months. Among evaluable intermediate-risk patients who are alive, it is 34.8 months.
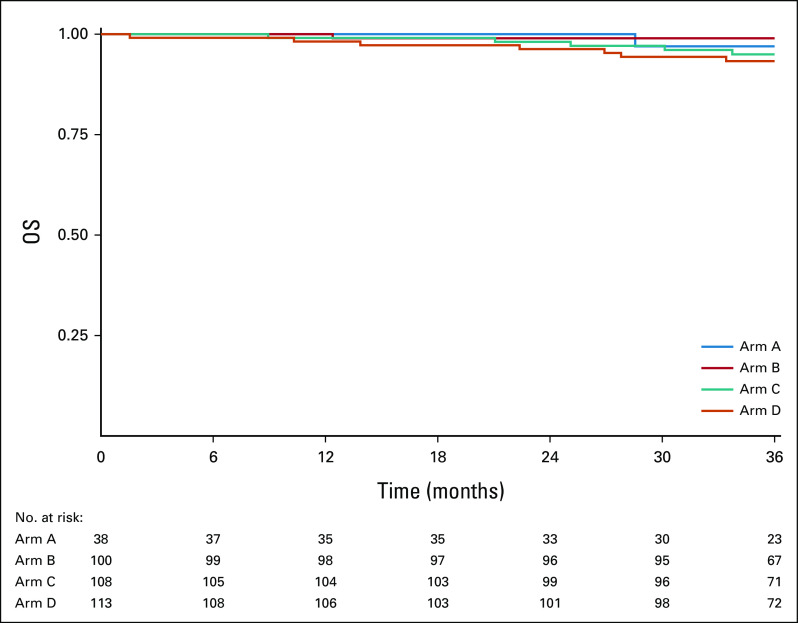
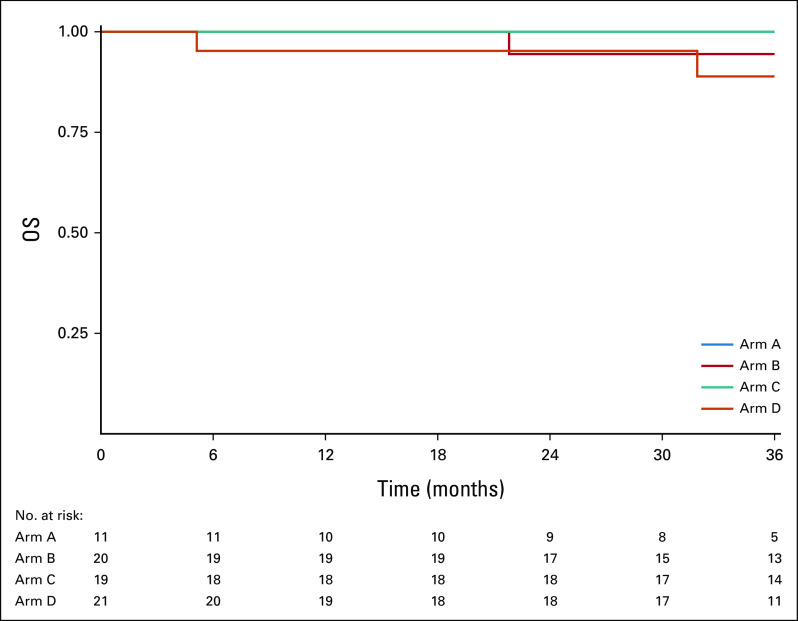
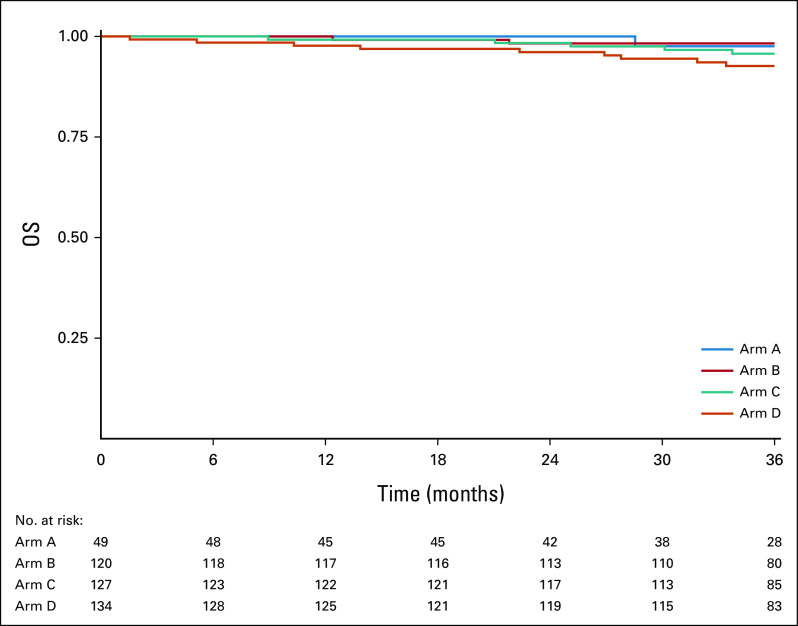
Figure 3 displays OS curves for all evaluable patients, by arm. The 2-year OS Kaplan-Meier estimate was 100% for arm A, 99.0% (90% CI, 9.3 to 100) for arm B, 98.1% (90% CI, 95.9 to 100) for arm C, and 96.3% (90% CI, 93.3 to 99.3) for arm D. Appendix Figure A2 (online only) displays OS curves for ineligible and step 2 treated patients, by arm. The 2-year OS Kaplan-Meier estimate was 100% for arm A, 94.4% (90% CI, 86.0 to 100) for arm B, 100% for arm C, and 95.2% (90% CI, 87.9 to 100) for arm D. Appendix Figure A4 (online only) displays OS curves for all step 2 treated patients (eligible and ineligible, n = 430), by arm. The 2-year OS Kaplan-Meier estimate was 100% for arm A, 98.3% (90% CI, 96.3 to 100) for arm B, 98.4% (90% CI, 96.5 to 100) for arm C, and 96.1% (90% CI, 93.3 to 99.0) for arm D.
FIG 3.
Kaplan-Meier estimates of OS by arm for 359 evaluable patients. OS, overall survival.
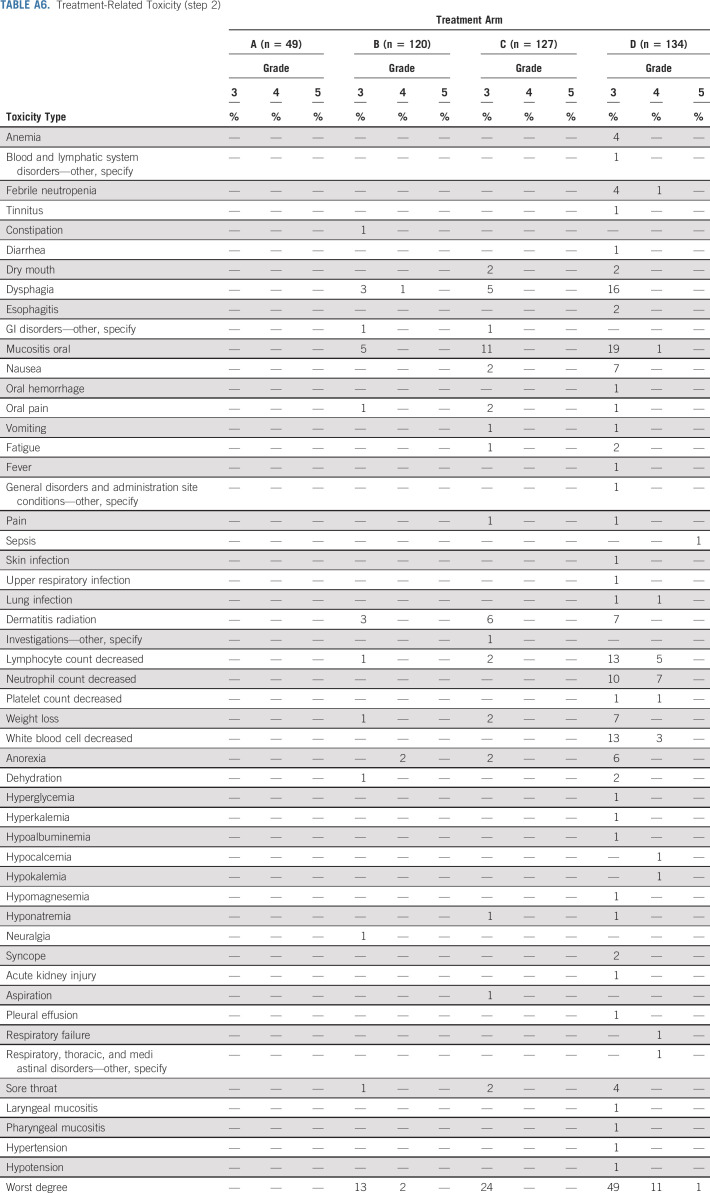
Appendix Table A5 (online only) summarizes treatment-related toxicities (possible, probable, or definite) for step 1. Fifteen percent (n = 74) of patients on step 1 experienced grade 3 treatment-related toxicities, 2% (n = 10) experienced grade 4 treatment-related toxicities, and < 1% (n = 1) patient experienced grade 5 treatment-related toxicities. The most common grade 3 toxicities were dysphagia (6%, n = 31) and oral hemorrhage (3%, n = 13). Appendix Table A6 (online only) summarizes treatment-related toxicities for step 2. Grade 3, 4, and 5 treatment-related toxicity rates were 13%, 2%, and 0% on arm B, 24%, 0%, and 0% on arm C, and 49%, 11%, and 1% on arm D, respectively. The most common grade 3 toxicities were oral mucositis (5% in arm B, 11% in arm C, and 19% in arm D) and dysphagia (3% in arm B, 5% in arm C, and 16% in arm D). There were four treatment-related deaths (one surgical and three on arm D). Significantly different grade III-V toxicity rates were observed between patients on arm B and arm C (14%, 17 of 120 v 24%, 31 of 127, P value = .030), arms B and D (14%, 17 of 120 v 60%, 81 of 134, P value < .0001), and arms C and D (24%, 31 of 127 v 60%, 81 of 134, P value < .0001).
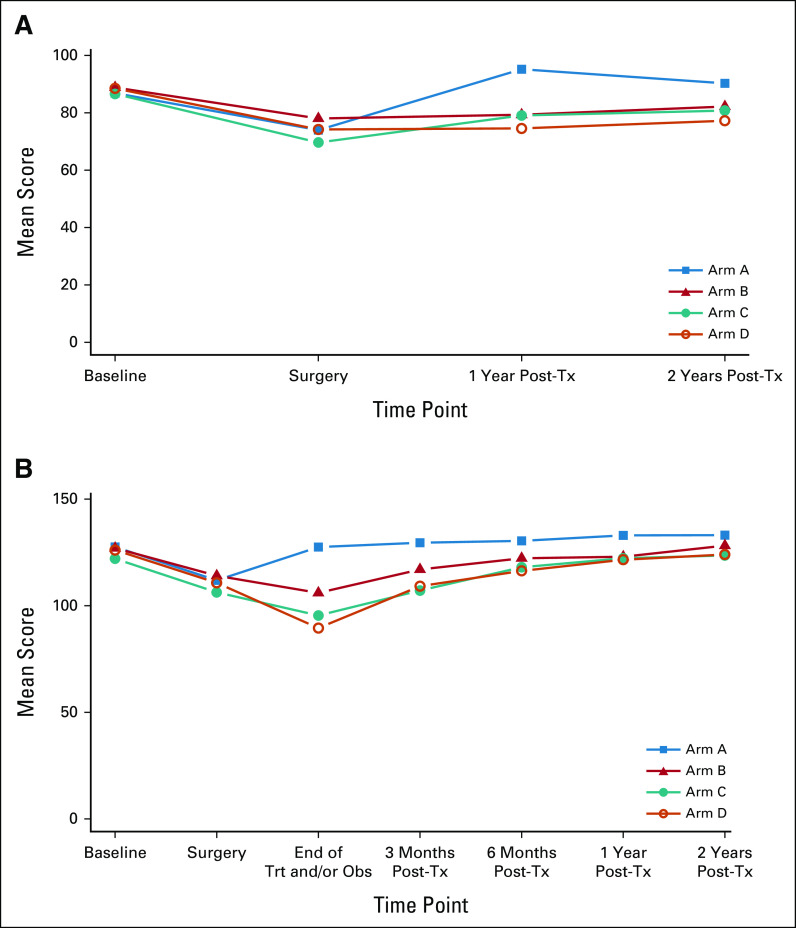
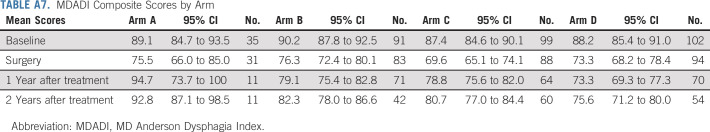
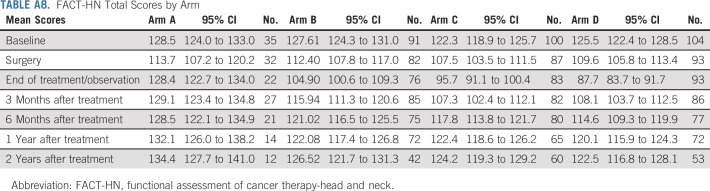
We measured patient-reported outcome (PRO) for quality of life (QOL; functional assessment of cancer therapy-head and neck [FACT-HN]) and swallowing (MD Anderson Dysphagia Index [MDADI]) in arms A-D (Figs 4A and 4B; Appendix Tables A7 and A8, online only), with 70% compliance at 6-month post-treatment for FACT-HN. Although consistent decline in QOL and swallowing scores was observed during treatment, these recovered to baseline in arms A-C with slightly lower scores after adjuvant therapy in arm D. Comparing change in FACT-HN total score from baseline to 6-month post-treatment, between arms B or C against D, 56% of patients in arms B or C had a stable or improved score compared with 38% of patients in arm D (P value = .009, using a one-sided Fisher's exact test). An exploratory comparison between arms B and C revealed a marginally significant difference (63% in arm B and 49% in arm C had a stable or improved score, P value = .066). However, these results should be interpreted with caution as the study design was not powered for a comparison between the randomized arms, and differences in both treatment and risk confound the comparison of arms B or C versus D. With an intent-to-treat analysis of all step 2 treated patients, the FACT-HN score differences remain numerically stable; however, the comparisons gain statistical significance (at 6 months, 57% of patients in arms B or C had stable or improved FACT-HN scores v 39% of arm D, P = .005; 65% of patients in arm B had stable or improved FACT-HN scores v 50% in arm C, P = .033.) Additional PROs and analyses of functional swallowing using objective measurements (eg, modified barium swallow) studies will be reported separately.
FIG 4.
(A) Swallowing and (B) QOL. QOL, quality of life; Trt and/or Obs, treatment and/or observation; Tx, treatment.
DISCUSSION
Treatment deintensification for HPV+ OPC is an active area of investigation.18,19 Transoral, minimally invasive surgery has been practiced widely since 2009 when TORS was FDA-cleared, but its role in permitting reduction in adjuvant therapy as a means to deintensify therapy has not been tested prospectively.4 The ECON-ACRIN (E3311) Trial was designed to collect normative information regarding feasibility and surgical quality in a multi-institutional trial, treatment arm assignment, and PFS of reduced-dose (50 Gy) or standard-dose (60 Gy) radiation therapy (RT) without chemotherapy. We also collected correlative PROs for QOL and swallowing function using MDADI and FACT-HN, as well as functional, modified barium swallow studies at defined time points for comparison between arms.
Deintensification approaches for HPV+ OPC have generally focused on modifying nonsurgical treatment, such as reduced RT or chemotherapy. RTOG 1016 demonstrated that cisplatin chemotherapy was superior to cetuximab when added to standard-dose, definitive RT (70 Gy), confirmed in the DeESCALATE trial.20,21 The NRG HN-002 trial14 suggested inferior oncologic outcomes for patients treated nonsurgically with reduced-dose RT (60 Gy) and without chemotherapy for favorable-risk HPV+ OPC. Single- or multi-institutional case series suggest that primary TOS is feasible and safe.22-26 However, lack of intent-to-treat analyses and heterogeneous postoperative management directed by inconsistent, locally defined criteria complicate interpretation of these studies. Indeed, level I data for using primary TOS and prespecified criteria to deintensify adjuvant therapy for good prognosis of HPV+ OPC have been lacking. The ECON-ACRIN (E3311) Trial now demonstrates the safety, feasibility, and high oncologic efficacy in a large multicenter cooperative group trial. Use of primary TOS and 50 Gy PORT for HPV + OPC with multiple positive nodes or ≤ 1 mm ENE (up to half of those with pathologic ENE) would provide 70% of patients with a deintensified therapeutic option after transoral resection.
Historically, PORT dose prescription has been guided by pathologic risk assessment dictating the RT dose, with many seminal studies27-29 occurring before transoral surgical approaches. These older studies typically provided RT doses approximating 60 Gy to the surgical bed but pathologically negative or positive but with intermediate pathologic risk features. Where pathologic risk features associated with a higher risk of relapse such as ENE or multiple pathologic risk factors, a dose of 63 Gy in 1.8 Gy per fraction was recommended. In E3311, we adopted a similar approach to intermediate-risk pathologic features with our standard dose of 60 Gy to sites of pathology but did not require pathologically negative regions to be irradiated to 60 Gy, and allowed treatment to 50 Gy at the discretion of the treating radiation oncologist. The primary invasive tumor site was prescribed for irradiation to 60 Gy in arm C. By contrast, arm B treated the dissected primary and neck to 50 Gy. Our findings suggest that both approaches are associated with a high 2-year PFS.
We designed this trial to generate normative data regarding TOS and the feasibility of reduced postoperative radiation dose. Our finding of 95% 2-year PFS among intermediate-risk patients who received 50 Gy PORT is among the best results yet described for this population, comparing favorably to comparable-stage patients managed with definitive chemoradiation,20 to favorable risk nonsmokers treated with definitive chemoradiation,14 and favorable-risk nonsmokers treated with postoperative docetaxel and reduced-dose radiation.30 An expanding literature regarding the activity of lower-dose radiation in HPV+ OPC in both the definitive and postoperative setting supports our conclusion that TOS and 50 Gy PORT is oncologically appropriate in intermediate-risk patients. Although median follow-up was over 35 months for the primary end point of 2-year PFS, the natural history of recurrence after definitive treatment of HPV+ OPC may require longer follow-up, particularly for distant metastatic disease, in addition to detecting differences in late toxicity.31 Continued observation of this valuable study population will provide the first large, multi-institutional cohort of HPV+ OPC treated with primary TOS and reduced PORT to document the kinetics of locoregional versus distant recurrence, which may have implications for follow-up schedule using imaging and clinical exam. A planned phase III trial is in development using the arm (50 Gy) regimen as the experimental arm, because of a combination of favorable oncologic and toxicity results from PROs presented here, to be compared with standard-of-care CRT.
TOS with dose-reduced postoperative therapy is one of several deintensification approaches being studied in HPV+ OPC.14,32-34 None of these deintensification approaches has been directly compared with standard of care or an alternate deintensification strategy in a completed phase III trial, although such trials are ongoing (NCT03952585). Optimal deintensification strategies may differ for patients with different clinical risk features and anatomic suitability for TOS, and perhaps by molecular characteristics. The outstanding oncologic and functional results of our study provide compelling rationale for a phase III trial comparing the E3311 arm B regimen with standard CRT using 70 Gy of radiation and concurrent cisplatin across all tobacco risk groups.
Patients with HPV+ OPC experience a profound, acute decrement in general physical functioning and cancer-specific QOL as a result of surgical and nonsurgical treatments, and a significant percentage of patients fail to return to baseline functioning. Here, we provide initial analyses of QOL and swallowing outcomes, measured using validated PROs. Clear decrement of both outcomes during adjuvant treatment was observed, with modest differences seen between arms B and C and the intensified arm D. Whether differences emerge in long-term PRO (> 5 years) because of reduction in RT dose and avoidance of cisplatin chemotherapy will be of interest to compare.
Future enhancement of this approach to deintensification would result from better patient selection, to avoid higher-risk (arm D) patients undergoing TOS followed by chemoradiation. Such preoperative patient selection might be accomplished using radiomics, genomic or transcriptional selection, or other methods to better define those truly at increased risk. For example, the most common indication in our trial for inclusion in arm D was ENE > 1 mm but it remains unknown at what threshold ENE is oncologically meaningful for resected HPV+ OPC. For arm D–like higher-risk patients, alternative therapeutic regimens such as reducing RT and/or chemotherapy dose, or replacing the latter with targeted therapeutic agents or immunotherapy should also be explored for deintensification.35 This group could also be monitored clinically and risk-stratified using surrogates for residual tumor such as salivary or circulating tumor DNA. Others are already testing some of these possibilities,30,36 including lower RT or omitting chemotherapy for arm D–like patients with more extensive ENE. With the very high PFS rates demonstrated here, continued focus on minimizing late consequences of curative therapy remains of great importance for this growing population of patients.
APPENDIX.
Procedures
Briefly, patients with resectable p16+ oropharynx cancer (American Joint Committee on Cancer-seventh edition tumor stage III-IVa without evidence for distant metastasis) and no evidence of matted or fixed pathologic adenopathy provided written informed consent and underwent radiographic staging. Adherence to contraindications to TOS was recommended during investigator communications; this included no gross or radiographic evidence of ENE (Weinstein, GS et al: Eur Arch Otorhinolaryngol 272:1551-1552, 2015). Patients were required to have adequate end-organ function and not to have previously received radiation above the clavicles. Full eligibility criteria are listed in the trial Protocol section 3.1. Primary treatment was TOS and neck dissection, followed by risk-based adjuvant therapy by arm assignment, which was reviewed and confirmed centrally. TOS consisted of transoral robotic surgery, transoral laser microsurgery, or standard cautery equipment, and approximately 90% of the patients were accrued by transoral robotic surgery–credentialed surgeons, as published previously by Ferris et al.17 Patients with N3 disease but no high-risk (arm D) features (N = 1) received 60 Gy PORT (arm C). Sensitivity analyses were conducted with the exclusion of this one patient. Confirmation of eligibility was repeated upon registration to step 2 (Appendix Tables A2 and A3).
Postoperative radiotherapy was based on pathologic risk stratification guiding radiation therapy dose prescription to the region of the pharynx or neck demonstrating the risk features. PORT was administered to the primary site and neck, with the primary site volume defined by the invasive tumor base rather than preoperative imaging that may have included intraluminal tumor (Appendix Table A4). Ipsilateral neck PORT could be elected if the mucosal invasive tumor base was > 1 cm from the midline mucosa as determined at the time of the surgery and if primary tumor adverse pathologic features were not present on the medial tumor margin. The standard PORT dose was a minimum of 60 Gy with the exception of patients assigned to arm B, for whom 50 Gy was prescribed to the primary site and the involved dissected neck.
Adverse events were evaluated by NCI Common Terminology Criteria for Adverse Events Version 4 (CTCAE V4) and assessed at baseline, weekly during radiotherapy, end of treatment, and 1 and 3 months after treatment completion (St Guily JL, et al: J Clin Virol 51:100-104, 2011; Appendix Tables A5 and A6). Criteria for dose reduction or delay were prespecified. Per Protocol, disease assessment (physical examination, including laryngopharyngoscopy and if indicated, computed tomography or magnetic resonance imaging of the head and neck, and late adverse event were required every 3 months for 2 years, every 6 months through year 5, and then annually). Chest x-ray or computed tomography of chest was performed annually. PRO included head and neck–cancer specific quality of life (FACT-HN) and swallowing perception and performance (MDADI) (Appendix Tables A7 and A8, online only). The FACT-H&N is a self-reported instrument, which consists of a 27-item core (functional assessment of cancer therapy-general) and 10 head and neck–specific items. Items are rated on a 5-point Likert-type scale. The score ranges from 0 to 148, with higher scores indicative of higher quality of life. Change in score was categorized as improved (change ≥ 7 points), stable (–6 ≤ change ≤ 6), or worsened (change ≤ –7). The MDADI is also a self-reported instrument, which consists of 20 items that assess an individual's perception of their swallowing ability. Items are rated on a 5-point scale. The composite score ranges from 20 (extremely low functioning) to 100 (high functioning). PROs were assessed at baseline, 4-6 weeks following surgery, end of treatment, and at 3, 6, 12, and 24 months thereafter. Quality assurance review of surgical quality and radiation therapy including stopping rules was performed per the trial Protocol.
Outcomes
Primary end points are feasibility of prospective multi-institutional study of TOS followed by risk-adjusted adjuvant therapy (Appendix Table A9), and 2-year progression-free survival (PFS) of 50 Gy or 60 Gy PORT for patients with intermediate risk. Feasibility was assessed through interim monitoring of the (1) accrual rate during months 13-18 after activation (required to be > 80% of projected rate); (2) risk distribution among the first 59 patients completing transoral resection (requiring at least 22 patients of intermediate risk); and (3) surgical quality as assessed by the rate of grade 3-4 bleeding events during surgery or positive margins after surgery (interim analysis among the first 59 eligible patients requiring < 13 patients with either outcome). Two-year PFS is defined as the proportion of patients alive and progression-free at 24 months, among all evaluable (eligible and treated on step 2) patients. Secondary end points include toxicity, overall survival (OS), swallowing function, and PROs. Comparisons of 2-year PFS rates between arms B and C, arms B and D, and arms C and D were specified as exploratory end points.
Statistical Design and Analyses
Planned interim analyses included 1-year PFS rates for arms A, B and C, as well as surgical quality and risk distribution within the first 59 patients completing surgery. Because this interim analysis demonstrated a higher-than-projected proportion of evaluable patients with high-risk features, the total accrual goal was increased from 377 to 515 (protocol amendment January 2016) to assure an adequate sample of intermediate-risk patients.
Among the 359 evaluable patients, 38 patients had follow-up for < 24 months. Therefore, 2-year PFS (and OS) was estimated using the Kaplan-Meier method, rather than as a binomial proportion. Corresponding 90% CIs were estimated using Greenwood's formula. PFS was measured from registration to step 2 to progression or death because of any cause, and patients without documented progression or death were censored at last disease evaluation date. OS was defined as the time from registration to step 2 to death from any cause or censored at last date known alive. When last disease evaluation date was not available, patients were censored at their date of registration.
Secondary quality-of-life objectives included the comparison of the change in FACT-HN total score from baseline (before TOS) to 6 months after treatment, between arms B and C combined and arm D. A one-sided Fisher's exact test was used to compare the proportion of patients with improved or stable score at 6 months after treatment, between arms B or C and D. A prespecified exploratory comparison between arms B and C was also performed. A one-sided Fisher's exact test was used to compare the toxicity rates of arms B versus C, B versus D, and C versus D.
Our plan was to select arm B or arm C as the experimental arm in a phase III comparison with definitive chemoradiation. Therefore, a prespecified exploratory comparison was made of the 2-year PFS rate between arms B and C. Prespecified exploratory comparisons of PFS between arms B and D, and arms C and D, were also performed, with a divided type I error (0.025 each), for a family-wise error rate of 0.10. A log-rank test was used to compare PFS between groups. Toxicity analyses were performed on all patients treated on step 2, whereas all other prespecified analyses were performed on the cohort of evaluable patients.
Several post hoc analyses were performed, including exploratory analyses of (1) ineligible patients only and (2) all step 2 treated patients, for PFS, OS, and change in FACT-HN total score from baseline to 6-month post-treatment. Additionally, an exploratory comparison of PFS was done, by smoking history (> 10 v ≤ 10 pack-years) for arms B, C, and D (evaluable patients). The final data lock was November 30, 2020.
FIG A1.
Kaplan-Meier estimates of PFS for 71 ineligible and step 2 treated patients. PFS, progression-free survival.
FIG A2.
Kaplan-Meier estimates of OS for 71 ineligible and step 2 treated patients. OS, overall survival.
FIG A3.
Kaplan-Meier estimates of PFS for 430 step 2 treated patients (eligible and ineligible combined). PFS, progression-free survival.
FIG A4.
Kaplan-Meier estimates of OS for 430 step 2 treated patients (eligible and ineligible combined). OS, overall survival.
TABLE A1.
Pathologic Risk Criteria for Treatment Arm Assignment
TABLE A2.
Reasons for Ineligibility
TABLE A3.
Baseline Characteristics of Ineligible or Step 2 Untreated Patients Who Received Transoral Resection (n = 136)
TABLE A4.
Radiation Dose to Primary Site, Arms B and C (evaluable)
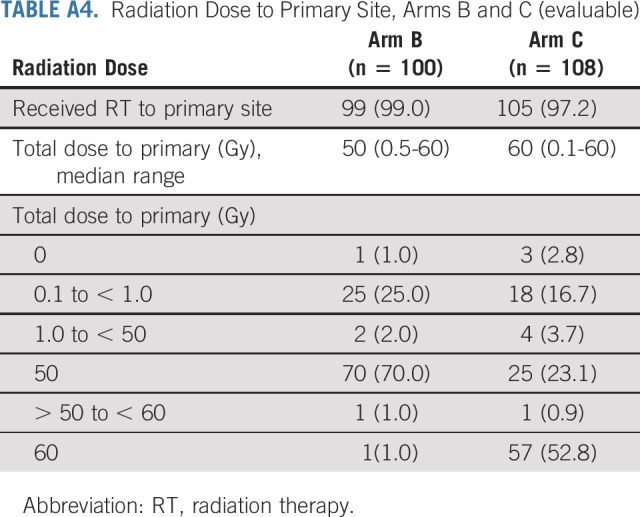
TABLE A5.
Treatment-Related Toxicity (step 1)
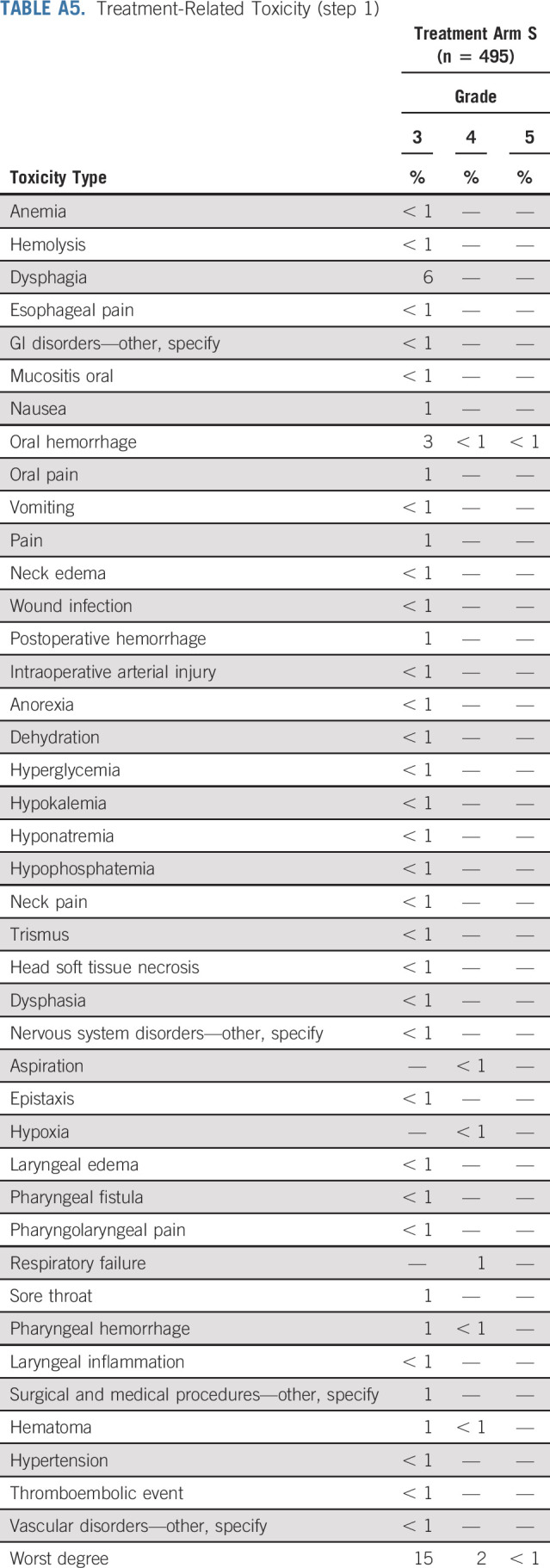
TABLE A6.
Treatment-Related Toxicity (step 2)
TABLE A7.
MDADI Composite Scores by Arm
TABLE A8.
FACT-HN Total Scores by Arm
TABLE A9.
Participating or Accruing Centers
Robert L. Ferris
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Stock and Other Ownership Interests: Novasenta
Consulting or Advisory Role: Merck, Pfizer, EMD Serono, Numab, Macrogenics, Aduro Biotech, Novasenta, Sanofi, Zymeworks, Bristol Myers Squibb
Research Funding: Bristol Myers Squibb, AstraZeneca/MedImmune, Merck, Tesaro, Novasenta
Gregory S. Weinstein
Patents, Royalties, Other Intellectual Property: Olympus
Shuli Li
Employment: Takeda
Stock and Other Ownership Interests: Takeda
Harry Quon
Employment: Johns Hopkins Hospital
Leadership: Pistevo Health
Stock and Other Ownership Interests: Oncospace, Pistevo Health
Honoraria: Sanofi/Regeneron
Consulting or Advisory Role: Pinnacle Biologics, Tactile Medical
Research Funding: Toshiba, Vibrent Health
Patents, Royalties, Other Intellectual Property: Filed under Hopkins, we have several patents related to mobile informatics solutions
Other Relationship: EPIC
Ranee Mehra
Stock and Other Ownership Interests: GlaxoSmithKline (I)
Consulting or Advisory Role: Bayer, Rakuten Medical
Research Funding: AstraZeneca, Merck
Christine H. Chung
Consulting or Advisory Role: Bristol Myers Squibb, CUE Biopharma, Mirati Therapeutics, Sanofi/Regeneron, Exelixis
Research Funding: AstraZeneca, Bristol Myers Squibb, Lilly, Merck, Regeneron, Ignyta, Pfizer, Brooklyn ImmunoTherapeutics, Iovance Biotherapeutics
Travel, Accommodations, Expenses: AstraZeneca, Mirati Therapeutics
Maura L. Gillison
Consulting or Advisory Role: Bristol Myers Squibb, Merck, EMD Serono, Kura Oncology, BioNTech, Shattuck Labs, Bayer, Debiopharm Group¸ Ipsen, Gilead Sciences¸ Bicara Therapeutics, Nektar¸ Istari, LLX Solutions, OncLive, Seagen, Kura Oncology, Mirati Therapeutics, Sensei Biotherapeutics
Research Funding: Bristol Myers Squibb, Genocea Biosciences, Cullinan Oncology, Genentech, Agenus, Kura Oncology
Umamaheswar Duvvuri
Consulting or Advisory Role: ACTIVARTIS Biotech
Research Funding: Kolltan Pharmaceuticals, Medrobotics
Bert W. O'Malley Jr
Patents, Royalties, Other Intellectual Property: Receive royalties from University of Pennsylvania via a licensed technology to Olympus Inc
Neil D. Gross
Honoraria: Intuitive Surgical
Consulting or Advisory Role: PDS Biotechnology, Verb Surgical, Sanofi/Regeneron, Shattuck Labs
Research Funding: Regeneron, MedImmune, Genentech
Patents, Royalties, Other Intellectual Property: UpToDate
R. Bryan Bell
Honoraria: Merck, Regeneron
Consulting or Advisory Role: Macrogenics
Speakers' Bureau: Merck, Regeneron
Research Funding: Bristol Myers Squibb/Celgene
Nabil F. Saba
Honoraria: Merck, CUE Biopharma, BioNTech AG, GlaxoSmithKline
Research Funding: Bristol-Myers Squibb, Exelixis
Travel, Accommodations, Expenses: Merck, Pfizer, Blueprint Medicines
Barbara Burtness
Consulting or Advisory Role: Merck, Debiopharm Group, CUE Biopharma, Maverick Therapeutics, Rakuten Medical, Nanobiotix, Macrogenics, ALX Oncology, IO Biotech, Ipsen, Genentech/Roche, Kura Oncology, Merck KGaA, PPD Global, Exelixis
Research Funding: Merck (Inst), Aduro Biotech (Inst), Formation Biologics (Inst), Bristol Myers (Inst), CUE Biopharma (Inst)
Travel, Accommodations, Expenses: Merck, Debiopharm Group
No other potential conflicts of interest were reported.
See accompanying editorial on page 114
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under awards: U10CA180820, U10CA180794, U10CA180868, UG1CA189953, UG1CA232760, UG1CA233184, UG1CA233196, UG1CA233247, UG1CA233329, UG1CA233328, UG1CA233331, and UG1CA233337.
AUTHOR CONTRIBUTIONS
Conception and design: Robert L. Ferris, Gregory S. Weinstein, Shuli Li, Harry Quon, Ranee Mehra, Christine H. Chung, Maura L. Gillison, Barbara Burtness
Financial support: Robert L. Ferris
Administrative support: Robert L. Ferris
Provision of study materials or patients: Robert L. Ferris, Gregory S. Weinstein, Harry Quon, Ranee Mehra, Umamaheswar Duvvuri, Enver Ozer, Giovana R. Thomas, Wayne M. Koch, R. Bryan Bell, Barbara Burtness
Collection and assembly of data: Robert L. Ferris, Gregory S. Weinstein, Shuli Li, Ranee Mehra, Joaquin J. Garcia, Umamaheswar Duvvuri, Bert W. O'Malley Jr, Enver Ozer, Wayne M. Koch, Neil D. Gross, R. Bryan Bell, Nabil F. Saba, Miriam Lango, Barbara Burtness
Data analysis and interpretation: Robert L. Ferris, Yael Flamand, Gregory S. Weinstein, Shuli Li, Harry Quon, Joaquin J. Garcia, Christine H. Chung, Bert W. O'Malley Jr, Enver Ozer, Giovana R. Thomas, R. Bryan Bell, Nabil F. Saba, Miriam Lango, Barbara Burtness
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Randomized Trial of Transoral Surgery and Low-Dose Intensity Modulated Radiation Therapy in Resectable p16+ Locally Advanced Oropharynx Cancer: An ECOG-ACRIN Cancer Research Group Trial (E3311)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Robert L. Ferris
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Stock and Other Ownership Interests: Novasenta
Consulting or Advisory Role: Merck, Pfizer, EMD Serono, Numab, Macrogenics, Aduro Biotech, Novasenta, Sanofi, Zymeworks, Bristol Myers Squibb
Research Funding: Bristol Myers Squibb, AstraZeneca/MedImmune, Merck, Tesaro, Novasenta
Gregory S. Weinstein
Patents, Royalties, Other Intellectual Property: Olympus
Shuli Li
Employment: Takeda
Stock and Other Ownership Interests: Takeda
Harry Quon
Employment: Johns Hopkins Hospital
Leadership: Pistevo Health
Stock and Other Ownership Interests: Oncospace, Pistevo Health
Honoraria: Sanofi/Regeneron
Consulting or Advisory Role: Pinnacle Biologics, Tactile Medical
Research Funding: Toshiba, Vibrent Health
Patents, Royalties, Other Intellectual Property: Filed under Hopkins, we have several patents related to mobile informatics solutions
Other Relationship: EPIC
Ranee Mehra
Stock and Other Ownership Interests: GlaxoSmithKline (I)
Consulting or Advisory Role: Bayer, Rakuten Medical
Research Funding: AstraZeneca, Merck
Christine H. Chung
Consulting or Advisory Role: Bristol Myers Squibb, CUE Biopharma, Mirati Therapeutics, Sanofi/Regeneron, Exelixis
Research Funding: AstraZeneca, Bristol Myers Squibb, Lilly, Merck, Regeneron, Ignyta, Pfizer, Brooklyn ImmunoTherapeutics, Iovance Biotherapeutics
Travel, Accommodations, Expenses: AstraZeneca, Mirati Therapeutics
Maura L. Gillison
Consulting or Advisory Role: Bristol Myers Squibb, Merck, EMD Serono, Kura Oncology, BioNTech, Shattuck Labs, Bayer, Debiopharm Group¸ Ipsen, Gilead Sciences¸ Bicara Therapeutics, Nektar¸ Istari, LLX Solutions, OncLive, Seagen, Kura Oncology, Mirati Therapeutics, Sensei Biotherapeutics
Research Funding: Bristol Myers Squibb, Genocea Biosciences, Cullinan Oncology, Genentech, Agenus, Kura Oncology
Umamaheswar Duvvuri
Consulting or Advisory Role: ACTIVARTIS Biotech
Research Funding: Kolltan Pharmaceuticals, Medrobotics
Bert W. O'Malley Jr
Patents, Royalties, Other Intellectual Property: Receive royalties from University of Pennsylvania via a licensed technology to Olympus Inc
Neil D. Gross
Honoraria: Intuitive Surgical
Consulting or Advisory Role: PDS Biotechnology, Verb Surgical, Sanofi/Regeneron, Shattuck Labs
Research Funding: Regeneron, MedImmune, Genentech
Patents, Royalties, Other Intellectual Property: UpToDate
R. Bryan Bell
Honoraria: Merck, Regeneron
Consulting or Advisory Role: Macrogenics
Speakers' Bureau: Merck, Regeneron
Research Funding: Bristol Myers Squibb/Celgene
Nabil F. Saba
Honoraria: Merck, CUE Biopharma, BioNTech AG, GlaxoSmithKline
Research Funding: Bristol-Myers Squibb, Exelixis
Travel, Accommodations, Expenses: Merck, Pfizer, Blueprint Medicines
Barbara Burtness
Consulting or Advisory Role: Merck, Debiopharm Group, CUE Biopharma, Maverick Therapeutics, Rakuten Medical, Nanobiotix, Macrogenics, ALX Oncology, IO Biotech, Ipsen, Genentech/Roche, Kura Oncology, Merck KGaA, PPD Global, Exelixis
Research Funding: Merck (Inst), Aduro Biotech (Inst), Formation Biologics (Inst), Bristol Myers (Inst), CUE Biopharma (Inst)
Travel, Accommodations, Expenses: Merck, Debiopharm Group
No other potential conflicts of interest were reported.
REFERENCES
- 1.Adelstein DJ, Ridge JA, Brizel DM, et al. : Transoral resection of pharyngeal cancer: Summary of a National Cancer Institute Head and Neck Cancer Steering Committee Clinical Trials Planning Meeting, November 6-7, 2011, Arlington, Virginia. Head Neck 34: 1681–17032012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ang KK, Harris J, Wheeler R, et al. : Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363: 24–352010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Souza G, Kreimer AR, Viscidi R, et al. : Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356: 1944–19562007 [DOI] [PubMed] [Google Scholar]
- 4.Holsinger FC, Ferris RL: Transoral endoscopic head and neck surgery and its role within the multidisciplinary treatment paradigm of oropharynx cancer: Robotics, lasers, and clinical trials. J Clin Oncol 33: 3285–32922015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Sullivan B, Huang SH, Su J, et al. : Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): A multicentre cohort study. Lancet Oncol 17: 440–4512016 [DOI] [PubMed] [Google Scholar]
- 6.Francissen CM, van la Parra RF, Mulder AH, et al. : Evaluation of the benefit of routine intraoperative frozen section analysis of sentinel lymph nodes in breast cancer. ISRN Oncol 2013: 843793.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstein GS, O'Malley BW, Jr, Magnuson JS, et al. : Transoral robotic surgery: A multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope 122: 1701–17072012 [DOI] [PubMed] [Google Scholar]
- 8.Albergotti WG, Jordan J, Anthony K, et al. : A prospective evaluation of short-term dysphagia after transoral robotic surgery for squamous cell carcinoma of the oropharynx. Cancer 123: 3132–31402017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fakhry C, Westra WH, Li S, et al. : Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100: 261–2692008 [DOI] [PubMed] [Google Scholar]
- 10.Cramer JD, Hicks KE, Rademaker AW, et al. : Validation of the eighth edition American Joint Committee on Cancer staging system for human papillomavirus-associated oropharyngeal cancer. Head Neck 40: 457–4662018 [DOI] [PubMed] [Google Scholar]
- 11.Haughey BH, Hinni ML, Salassa JR, et al. : Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: A United States multicenter study. Head Neck 33: 1683–16942011 [DOI] [PubMed] [Google Scholar]
- 12.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. : Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol 26: 3770–37762008 [DOI] [PubMed] [Google Scholar]
- 13.Quon H, Forastiere AA: Controversies in treatment deintensification of human papillomavirus-associated oropharyngeal carcinomas: Should we, how should we, and for whom? J Clin Oncol 31: 520–5222013 [DOI] [PubMed] [Google Scholar]
- 14.Yom SS, Torres-Saavedra P, Caudell JJ, et al. : NRG-HN002: A randomized phase II trial for patients with p16-positive, non-smoking-associated, locoregionally advanced oropharyngeal cancer. 2019 ASTRO Annual Meeting. Int J Radiat Oncol Biol Phys 105: 384–3852019 [Google Scholar]
- 15.Sharma A, Patel S, Baik FM, et al. : Survival and gastrostomy prevalence in patients with oropharyngeal cancer treated with transoral robotic surgery vs chemoradiotherapy. JAMA Otolaryngol Head Neck Surg 142: 691–6972016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adelstein DJ, Ridge JA, Brizel DM, et al. : Transoral resection of pharyngeal cancer: Summary of a National Cancer Institute head and neck cancer steering committee clinical trials planning meeting, November 6–7, 2011, Arlington, Virginia. Head and Neck 34: 1681–17032012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferris RL, Flamand Y, Holsinger FC, et al. : A novel surgeon credentialing and quality assurance process using transoral surgery for oropharyngeal cancer in ECOG-ACRIN Cancer Research Group Trial E3311. Oral Oncol 110: 104797.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bigelow EO, Seiwert TY, Fakhry C: Deintensification of treatment for human papillomavirus-related oropharyngeal cancer: Current state and future directions. Oral Oncol 105: 104652.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adelstein DJ, Ismaila N, Ku JA, et al. : Role of treatment deintensification in the management of p16+ oropharyngeal cancer: ASCO provisional clinical opinion. J Clin Oncol 37: 1578–15892019 [DOI] [PubMed] [Google Scholar]
- 20.Gillison ML, Trotti AM, Harris J, et al. : Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 393: 40–502019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehanna H, Robinson M, Hartley A, et al. : Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet 393: 51–602019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farooq S, Khandavilli S, Dretzke J, et al. : Transoral tongue base mucosectomy for the identification of the primary site in the work-up of cancers of unknown origin: Systematic review and meta-analysis. Oral Oncol 91: 97–1062019 [DOI] [PubMed] [Google Scholar]
- 23.Chan JYK, Tsang RK, Holsinger FC, et al. : Prospective clinical trial to evaluate safety and feasibility of using a single port flexible robotic system for transoral head and neck surgery. Oral Oncol 94: 101–1052019 [DOI] [PubMed] [Google Scholar]
- 24.Sinha P, Haughey BH, Kallogjeri D, et al. : Long-term analysis of transorally resected p16 + Oropharynx cancer: Outcomes and prognostic factors. Laryngoscope 129: 1141–11492019 [DOI] [PubMed] [Google Scholar]
- 25.Kaczmar JM, Tan KS, Heitjan DF, et al. : HPV-related oropharyngeal cancer: Risk factors for treatment failure in patients managed with primary transoral robotic surgery. Head Neck 38: 59–652016 [DOI] [PubMed] [Google Scholar]
- 26.O'Malley BW, Jr, Weinstein GS, Snyder W, et al. : Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope 116: 1465–14722006 [DOI] [PubMed] [Google Scholar]
- 27.Peters LJ, Goepfert H, Ang KK, et al. : Evaluation of the dose for postoperative radiation therapy of head and neck cancer: First report of a prospective randomized trial. Int J Radiat Oncol Biol Phys 26: 3–111993 [DOI] [PubMed] [Google Scholar]
- 28.Ang KK, Trotti A, Brown BW, et al. : Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 51: 571–5782001 [DOI] [PubMed] [Google Scholar]
- 29.Rosenthal DI, Mohamed ASR, Garden AS, et al. : Final report of a prospective randomized trial to evaluate the dose-response relationship for postoperative radiation therapy and pathologic risk groups in patients with Head and neck cancer. Int J Radiat Oncol Biol Phys 98: 1002–10112017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma DJ, Price KA, Moore EJ, et al. : Phase II evaluation of aggressive dose de-escalation for adjuvant chemoradiotherapy in human papillomavirus-associated oropharynx squamous cell carcinoma. J Clin Oncol 37: 1909–19182019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Machtay M, Moughan J, Trotti A, et al. : Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol 26: 3582–35892008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen AM, Felix C, Wang PC, et al. : Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: A single-arm, phase 2 study. Lancet Oncol 18: 803–8112017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marur S, Li S, Cmelak AJ, et al. : E1308: Phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx- ECOG-ACRIN Cancer Research Group. J Clin Oncol 35: 490–4972017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seiwert TY, Foster CC, Blair EA, et al. : OPTIMA: A phase II dose and volume de-escalation trial for human papillomavirus-positive oropharyngeal cancer. Ann Oncol 30: 1673.2019 [DOI] [PubMed] [Google Scholar]
- 35.Cramer JD, Burtness B, Ferris RL: Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol 99: 104460.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hargreaves S, Beasley M, Hurt C, et al. : Deintensification of adjuvant treatment after transoral surgery in patients with human papillomavirus-positive oropharyngeal cancer: The conception of the PATHOS study and its development. Front Oncol 9: 936.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]