Abstract
PURPOSE
Most patients with mantle cell lymphoma (MCL) are older. In this study, we investigated the efficacy and safety of a chemotherapy-free combination with ibrutinib and rituximab (IR) in previously untreated older patients with MCL (age ≥ 65 years).
METHODS
We enrolled 50 patients with MCL in this single-institution, single-arm, phase II clinical trial (NCT01880567). Patients with Ki-67% ≥ 50% and blastoid morphology were excluded. Ibrutinib was administered with rituximab up to 2 years with continuation of ibrutinib alone. The primary objective was to assess the overall response rate and safety of IR. In evaluable samples, whole-exome sequencing and bulk RNA sequencing from baseline tissue samples were performed.
RESULTS
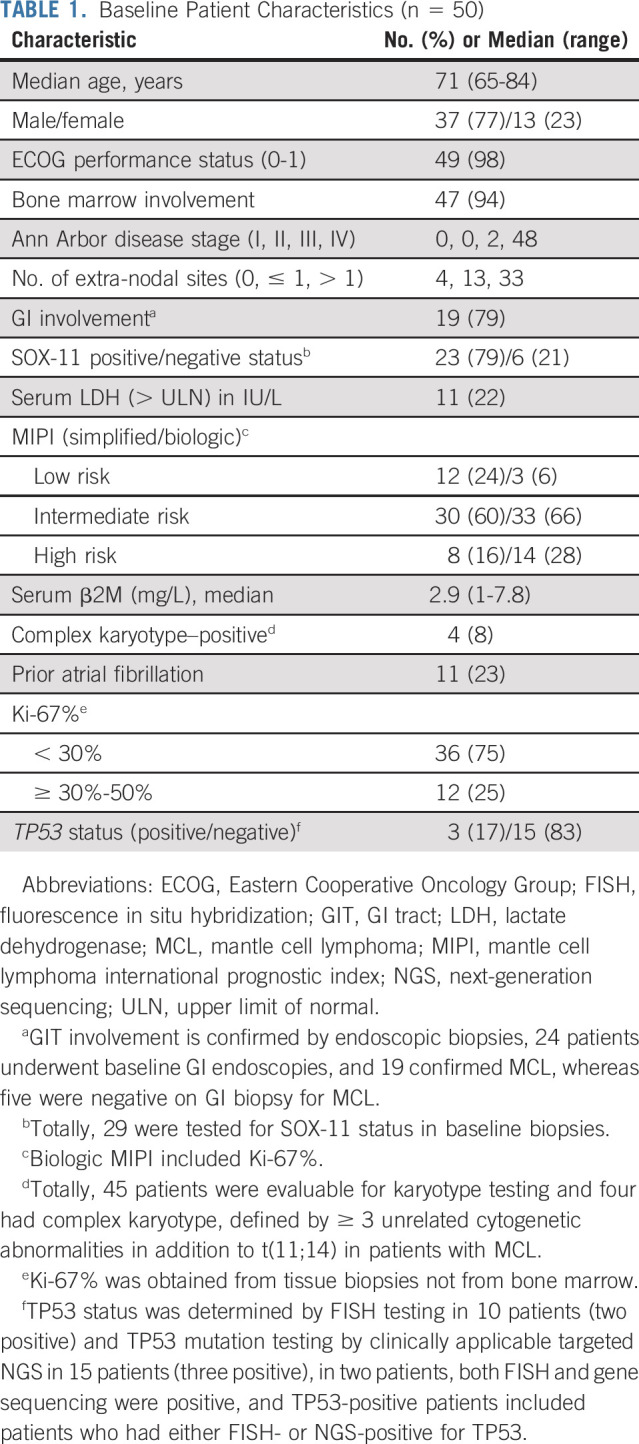
The median age was 71 years (interquartile range 69-76 years). Sixteen percent of patients had high-risk simplified MCL international prognostic index. The Ki-67% was low (< 30%) in 38 (76%) and moderately high (≥ 30%-50%) in 12 (24%) patients. The best overall response rate was 96% (71% complete response). After a median follow-up of 45 months (interquartile range 24-56 months), 28 (56%) patients came off study for various reasons (including four progression, 21 toxicities, and three miscellaneous reasons). The median progression-free survival and overall survival were not reached, and 3-year survival was 87% and 94%, respectively. None of the patients died on study therapy. Notably, 11 (22%) patients had grade 3 atrial fibrillation. Grade 3-4 myelosuppression was seen in < 5% of patients. Differential overexpression of CCND1, BIRC3, BANK1, SETBP1, AXIN2, and IL2RA was noted in partial responders compared with patients with complete response.
CONCLUSION
IR combination is effective in older patients with MCL. Baseline evaluation for cardiovascular risks is highly recommended. Randomized trial is needed for definitive conclusions.
INTRODUCTION
Most patients with mantle cell lymphoma (MCL) are elderly with a median age of 69 or 71 years.1,2 Apart from high-risk disease characteristics,3 advanced age, poor performance status, and number of comorbidities exhibit an adverse prognostic impact in patients with MCL.4,5 Patients treated with systemic chemotherapy and advancing age have an inferior outcome compared with young patients with MCL (age ≤ 65 years).2 The standard-of-care treatment for elderly patients with MCL is rituximab-based chemoimmunotherapy followed by rituximab maintenance (in some studies),6-9 providing a median progression-free survival (PFS) of 3-5 years and an overall response rate (ORR) of about 90% with 40%-50% complete response (CR; except the combination of bendamustine, rituximab, and cytarabine, which induced a CR of 91%).6-9 High incidence of grade 3-4 myelosuppression, hospitalization rates, infectious complications, and second cancers (including therapy-related myelodysplasia) after chemoimmunotherapy are significant complications in elderly patients with MCL.
CONTEXT
Key Objective
Ibrutinib is an approved Bruton's tyrosine kinase inhibitor in relapsed mantle cell lymphoma (MCL). To our knowledge, this single-arm phase II study is the first chemotherapy-free combination of ibrutinib-rituximab (IR) in newly diagnosed elderly patients with MCL.
Knowledge Generated
IR combination demonstrated high response rates and durable survival in elderly patients with MCL. After a nearly 4-year median follow-up, 56% of patients discontinued treatment. Treatment-related intolerance, especially atrial fibrillation, was noticeable in comparison with other therapies in elderly patients with MCL. Furthermore, an integrated genomic and transcriptomic analysis from pretreatment biopsies demonstrated the significance of B-cell receptor pathway aberrations as a predictor of response to ibrutinib.
Relevance (J. W. Friedberg)
-
These early results of the IR combination in older patients with MCL support planned and ongoing randomized trials comparing Bruton's tyrosine kinase inhibitor–based regimens with chemoimmunotherapy in this setting. High rates of cardiac toxicity suggest that patients should be carefully evaluated for cardiac risk factors before receiving the IR combination.*
*Relevance section written by JCO Editor-in-Chief Jonathan W. Friedberg, MD.
With the advent of orally administered Bruton's tyrosine kinase (BTK) inhibitors such as ibrutinib, acalabrutinib, zanubrutinib, and pirtobrutinib10-14 alone or ibrutinib with rituximab15,16 and/or ibrutinib with Bcl2 antagonist venetoclax in treating patients with relapsed MCL,17,18 many investigators are exploring earlier use of chemotherapy-free novel targeted therapeutic approaches for patients with MCL. On the basis of favorable efficacy and safety data (particularly lack of myelosuppression), ease of administration, our own clinical experience using the ibrutinib-rituximab combination in patients16 with relapsed MCL (88% ORR, 58% CR after a 4-year follow-up15 with 16 of 50 [32%] patients' age > 70 years), and the desire to develop chemotherapy-free treatment modalities, we investigated the ibrutinib-rituximab combination (IR) in the frontline therapy for elderly patients with MCL in this single-center, phase II study.
METHODS
Study Design and Patient Population
This is an investigator-initiated, institutional review board–approved, open-label, single-institution, single-arm, phase II clinical trial. The study was originally designed to investigate the efficacy and safety of the IR combination in relapsed MCL (reported previously16) and was modified in 2015 to include a cohort of newly diagnosed elderly patients with MCL. In this study, we report the results of the 50 previously untreated elderly patients with MCL who participated in this clinical trial after obtaining informed consent as per the Declaration of Helsinki. The key eligibility criteria included the following: previously untreated elderly patients with MCL, age ≥ 65 years with nonblastoid or pleomorphic histology and/or with a Ki-67% < 50% (chosen to avoid inadequate therapy for very aggressive MCL), and an Eastern Cooperative Oncology Group performance status of ≤ 2. Patients with a history of controlled atrial fibrillation were included (Protocol, online only).
Treatment
Ibrutinib was administered orally at 560 mg once daily in 28-day cycles with rituximab. Rituximab was administered as intravenous infusion at a fixed dose of 375 mg/m2 once weekly for 4 weeks in cycle 1 followed by day 1 of every cycle starting in cycles 3-8. After cycle 8, rituximab was given on day 1 of every 2 months for up to 2 years, and after 2 years, ibrutinib was administered in continuous cycles until disease progression or unacceptable toxicity or any other reason of discontinuation. None of the patients received stem-cell transplantation.
Response Assessment
ORR included partial response (PR) and CR according to the Lugano 2014 criteria.19 Response assessments were performed using computed tomography scanning every two cycles until cycle 8, followed by assessments every 4 months. After achievement of CR and for patients receiving ibrutinib treatment after 2 years, response assessments were performed every 6-12 months. A 18F-fluorodeoxyglucose–positron-emission tomography (PET) scan was performed at baseline and to confirm the CR at best response. Best response is the best response that the patient had achieved while on therapy. Deauville scoring, with scores ranging from 1 to 5, with a score of 1 to 3 indicating a complete metabolic response, was used. Bone marrow examination using flow cytometry–based assay with a minimum sensitivity of 0.01%-0.1% was performed at best response, among evaluable patients who had initial evidence of bone marrow involvement by MCL. Adverse events were graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE, version 4.0).20
Genomic Studies
Among evaluable patients, DNA and RNA extraction was performed from archived formalin-fixed paraffin-embedded tissues baseline tissue samples (node, extra-nodal tissue, gastrointestinal tract, or bone marrow biopsies) with > 30% cellularity with MCL and germline samples (when available). Whole-exome sequencing (WES) and bulk RNA sequencing were performed to assess the somatic mutation profile, copy number abnormalities, and differential gene expression (DEG), and gene set enrichment analysis was performed among various response categories to identify genomic predictors of response and resistance to IR. All WES sequencing was performed with a NovaSeq6000 SP-XP flow cell using the 150bp paired end format, whereas for RNA sequencing, Illumina HiSeq4000 using the 76bp paired end configuration was used (detailed methods are given in the Data Supplement, online only).
Statistical Analysis
The primary objective was to estimate ORR. The secondary objectives included PFS and overall survival (OS). PFS was measured from the treatment start date until disease progression or death whichever occurs earlier. OS was measured from the treatment start date to the date of death or last follow-up. Safety assessment was performed during every patient visit. Time to event outcomes were estimated using the Kaplan-Meier method. All statistical tests were based on a two-sided alpha level of .05. Statistical analyses were performed using Stata/SE version 16.0 statistical software (Stata Corp LP, College Station, TX).
RESULTS
Patient Characteristics and Disposition
Fifty previously untreated elderly patients with MCL were enrolled in the study between October 2015 and November 2019. Baseline patient characteristics are summarized in Table 1. The median age was 71 years (interquartile range [IQR] 69-76 years), and 77% were men. Bone marrow involvement and GI tract involvement were observed in 94% (47 of 50) and 79% (19 of 24) of evaluable patients, respectively. High mantle cell lymphoma international prognostic index (MIPI) risk score was observed in 16%, and high biologic MIPI score including Ki-67% was observed in 28% of patients. TP53 aberrations were detected 3 of 18 (17%) patients in the bone marrow by either targeted next-generation sequencing (NGS) or fluorescence in situ hybridization, whereas 4 of 45 (8%) evaluable patients had complex karyotype. Eleven patients (23%) had a history of atrial fibrillation.
TABLE 1.
Baseline Patient Characteristics (n = 50)
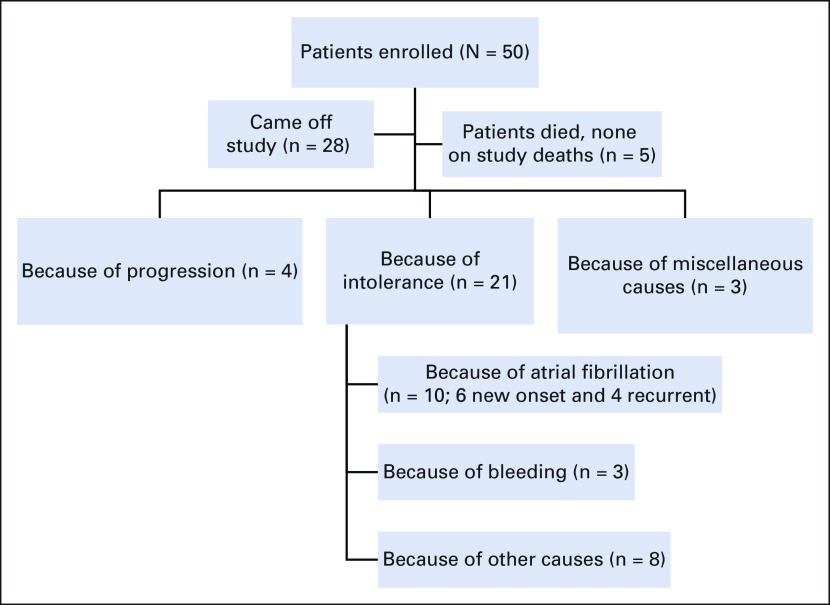
After a median follow-up of 45 months (IQR 24-56 months), five patients had died and 45 patients were alive. Overall, four patients developed disease progression (including three patients who transformed from classic to blastoid MCL in two and from classic to pleomorphic in one). Among these four patients with progression, the Ki-67% at baseline was 15%, 20%, 30%, and 35% and one patient had a TP53 mutation. Figure 1 shows the flowchart of patients.
FIG 1.
Flowchart of patient treatment and disposition. The induction treatment consisted of ibrutinib administered at 560 mg once daily on days 1-28 of a 28-day cycle and rituximab weekly for 4 weeks during cycle 1 and then day 1 of every cycle starting in cycles 3-8. After cycle 8, rituximab was given on day 1 of every 2 months for up to 2 years, and after 2 years, ibrutinib was administered in continuous cycles until disease progression or unacceptable toxicity or any other reason of discontinuation. Of the 50 patients enrolled, 28 came off study for various reasons.
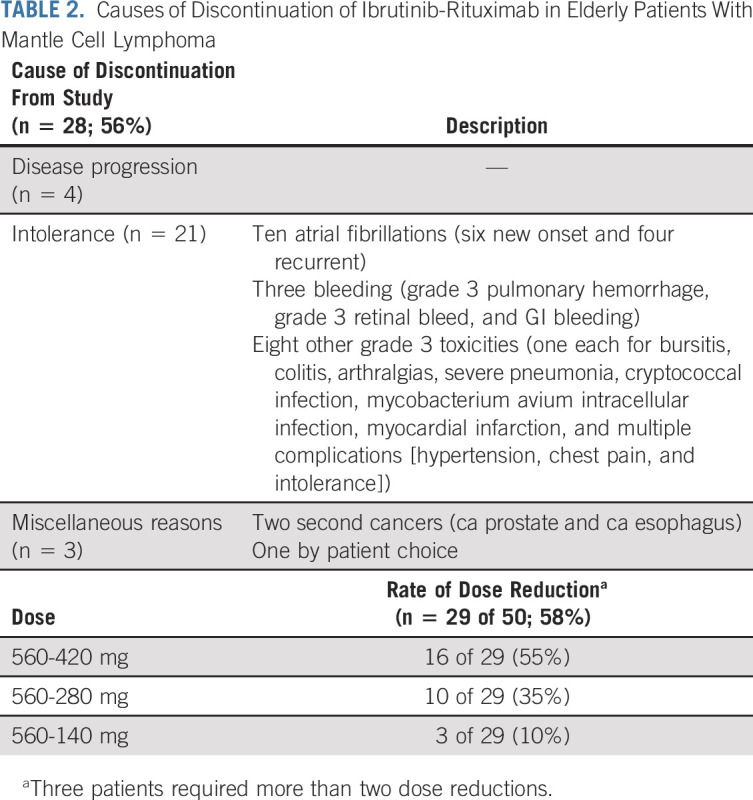
As of January 2021, 28 of 50 (56%) patients discontinued IR therapy and came off study for various reasons (4 of 50 [8%] disease progression, 21 of 50 [42%] because of toxicities [including 10 because of grade 3 atrial fibrillation], and 3 of 50 [6%] for miscellaneous reasons). At the time of study discontinuation, 16 of 28 (57%) patients were in CR. Among the 10 patients with atrial fibrillation, six patients were new onset and four patients had a history of atrial fibrillation. Among the 14 patients who discontinued IR therapy for miscellaneous reasons, two were due to other cancers, three were due to bleeding, three were due to infections, five were due to intolerance, and one was by patient choice. The causes of discontinuation (n = 28) and causes of death (n = 5) are summarized in Table 2.
TABLE 2.
Causes of Discontinuation of Ibrutinib-Rituximab in Elderly Patients With Mantle Cell Lymphoma
Efficacy
The investigator-assessed best ORR was 96% (46 of 48), CR 71% (34 of 48), PR 25% (12 of 48), and stable disease 4% (2 of 48). Two patients were not evaluable for response assessment since they came off study within 1 month after therapy initiation (one because of GI bleed and the other because of grade 3 atrial fibrillation).With an intent to treat, the best ORR was 92% (46 of 50) and CR was 68% (34 of 50). Forty patients had baseline PET-computed tomography scans performed, and 35 were positive. Among these 35 patients, 26 achieved complete metabolic response at best response (74%), eight did not have PET scan at best response, and one patient had residual disease. Of the 26 patients with CMR, 21 (81%) had bone marrow–negative for MCL, one had residual disease, and four patients did not have bone marrow evaluation. The median number of IR cycles to reach CR was seven (range 2-51).
We further evaluated responses in patients with Ki-67% (< 30%) and Ki-67% (≥ 30%-50%), and the n of N, ORR (n of N; CR) were 37 of 38, 97% (28 of 38; 74%) and 9 of 12, 75% (6 of 12; 50%), respectively (P < .001). Within the 18 evaluable patients for TP53 aberrations (three positive and 15 negative for TP53 aberrations), the n of N, ORR (CR) were 2 of 3, 66% (1 of 3, 33%) and 14 of 15, 94% (10 of 15, 67%), respectively, P < .001. Bone marrow flow cytometry assessment was performed at their best response in 32 patients, and 27 of 32 patients (84%) were negative for MCL cells.
Time to Event Outcomes
With a median follow-up of 45 months (IQR 24-56 months), overall, five patients had died and seven patients progressed or died (whichever occurred earlier). None of the deaths were on study. The median PFS and OS were not reached, and the 3-year PFS and OS were 87% (95% CI, 0.73 to 0.94) and 94% (95% CI, 0.82 to 0.98), respectively (Figs 2A and 2D). Patients with high Ki-67% (≥ 30%-50%) had a trend of higher risk of progression and/or death compared with those with low Ki-67% (< 30%), P = not significant (Figs 1E and 2B). Patients who achieved CR as their best response had significantly longer PFS and OS compared with those who did not achieve CR, Figures 1F and 2C.
FIG 2.
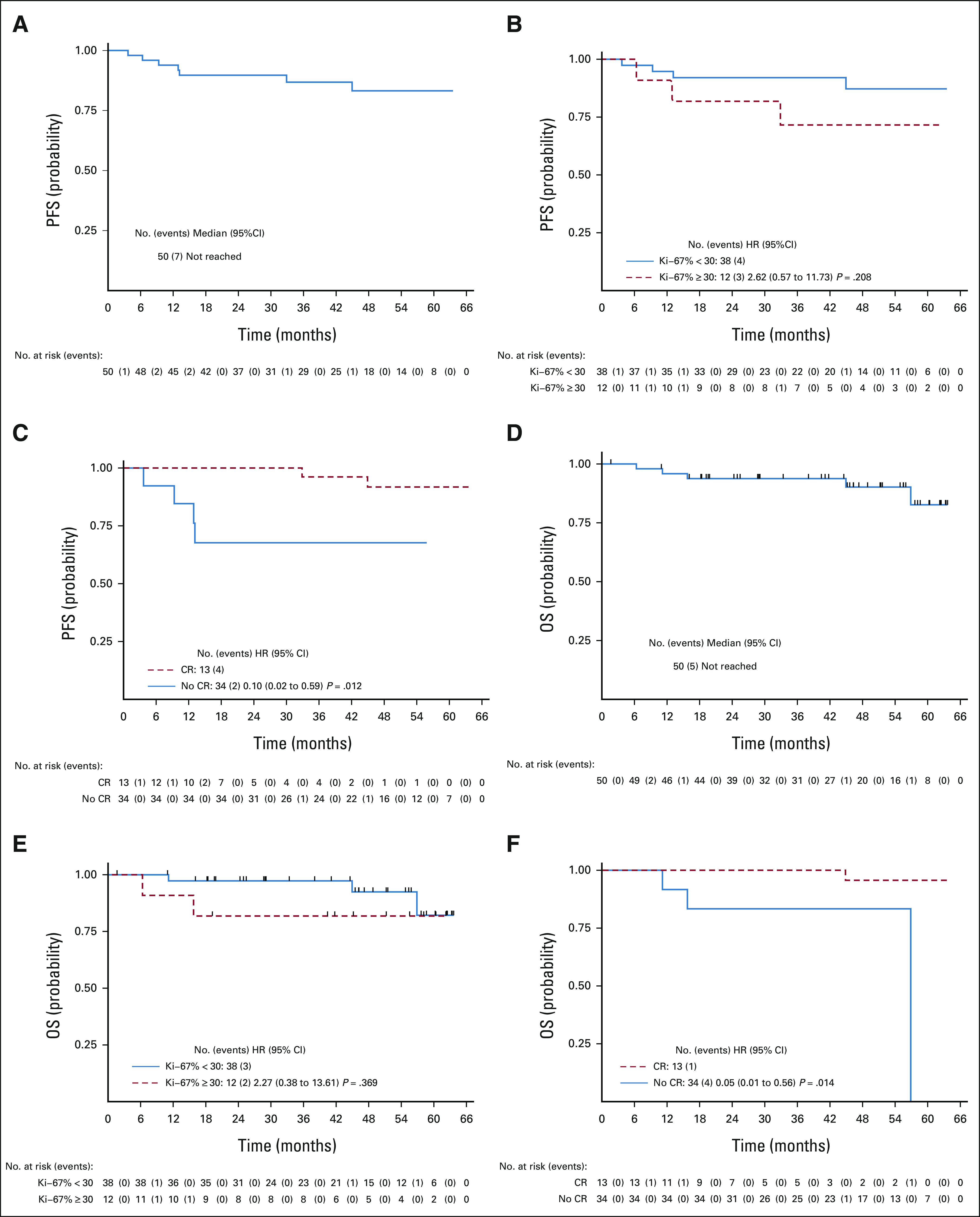
Survival outcomes after a median follow-up of 45 months. (A) The median PFS in all patients was not reached. (B) PFS by Ki-67% was not significantly different in high (Ki-67% ≥ 30%) versus low Ki-67% (< 30%) although higher hazard of progression was noted in those patients with high Ki-67%, HR of 2.62, P = .190. (C) PFS by CR status. Patients who achieved CR as the best response to IR therapy had a significantly better PFS compared with those patients without CR as the best response, P = .003. (D) The median OS in all patients was not reached. (E) OS by Ki-67% was not significantly different between low and high Ki-67% categories (P = .356). (F) OS by CR status. Patients who achieved CR as the best response to IR therapy had a significantly better OS compared with those patients without CR as the best response, P = .002. CR, complete response; HR, hazard ratio; IR, ibrutinib-rituximab; OS, overall survival; PFS, progression-free survival.
Furthermore, significantly higher risk of progression was noted in patients with high-risk simplified and modified MIPI score (Data Supplement). No difference in PFS was observed in patients with or without TP53 aberrations and those with or without complex karyotype (Data Supplement); however, OS was inferior in high-risk patients (Data Supplement).
Adverse Events
The adverse event profile on IR therapy is summarized in Table 3. Most adverse events were grade 1 or 2 (≥ 50% frequency—fatigue, neuropathy, diarrhea, myalgia, and oral mucositis were common). The most frequent grade 3-4 toxicities were 22% atrial fibrillation (n = 11), 18% fatigue (n = 9), 14% diarrhea (n = 6), and 14% myalgias (n = 7). Grade 3-4 hematologic toxicities were 4% anemia (n = 2), 8% neutropenia (n = 4), and 4% thrombocytopenia (n = 2). Four patients developed grade 3-4 bleeding while on ibrutinib (included hematuria, bronchopulmonary hemorrhage, GI bleeding, and retinal bleeding), 3 of 4 patients were on aspirin and/or enoxaparin (two on both and one only on aspirin), and one patient with retinal bleeding had recent glaucoma surgery and had history of vitreous surgery. One patient had grade 3 hematuria and Gram-negative urinary tract infection, which resolved after antibiotics and therefore resumed on study and maintained remission. None had grade 5 toxicities.
TABLE 3.
Summary of Adverse Events (AE) During Ibrutinib-Rituximab Treatment
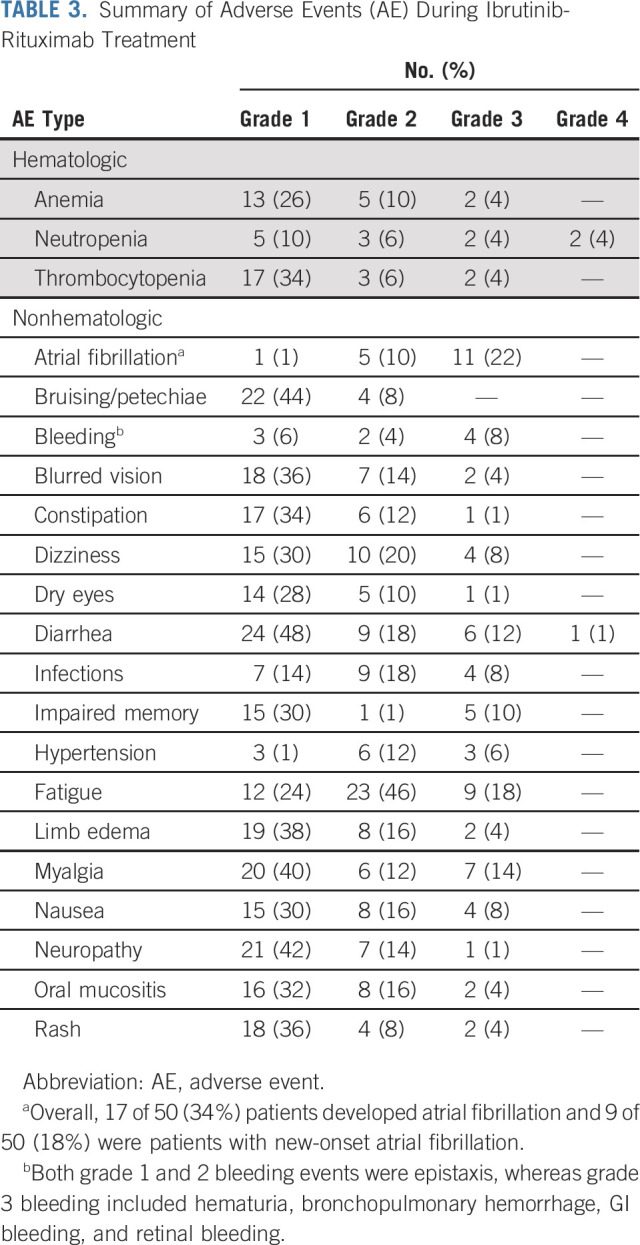
Overall, 17 of 50 (34%) patients developed atrial fibrillation and 10 of 17 had discontinued ibrutinib because of grade 3 atrial fibrillation. Of these 17 patients, nine patients (53%) were without a history of atrial fibrillation and six of them had baseline ECG abnormalities (including first-degree atrioventricular block in one, right bundle branch block in one, sinus bradycardia in three, and one with miscellaneous abnormalities). Eight patients had a history of atrial fibrillation. Fourteen patients were receiving 560 mg ibrutinib once daily at the onset of atrial fibrillation, whereas one patient each was taking ibrutinib 420, 280, and 140 mg once daily, respectively. The median age of patients who developed atrial fibrillation was 71 years (range 66-82 years), the median ejection fraction in pretreatment echocardiogram was 60% (range 44-75%), and the median time to onset of atrial fibrillation from the start of ibrutinib treatment was 9.5 months (range 1-48 months). In the Data Supplement, we have described the characteristics of patients who developed atrial fibrillation and those patients who did not develop atrial fibrillation. Of particular note, patients with atrial fibrillation had a higher median number of baseline cardiovascular risk factors, four (range 1-8), and by contrast, the median number of baseline cardiovascular risk factors in those patients who did not develop atrial fibrillation was two (range 1-4). Overall, dose reduction of ibrutinib was performed in 29 (58%) patients for various reasons (seven atrial fibrillation [two improved, whereas five patients had persistent atrial fibrillation or had recurrence], five infections, five bleeding, five myalgias, and seven miscellaneous).
Genomic Profiles in Patients according to the Response to IR Combination
We then performed WES in 25 pretreatment tissue biopsies. We divided the patients according to best response on IR therapy (CR and PR). Figure 3A depicts the pattern of somatic mutations and distribution according to CR versus PR. Patients with PR were enriched in KMT2D, FAT4, ROS1, CARD11, ATM, NOTCH1, CCND1, and FAT1 mutations. In Figure 3B, we evaluated the copy number variation and identified deletions in FAT1, FAT4, ROS1, and KMT2C were predominantly observed in patients with PR. Gain of MALT1 in the CR group and gain of SMARCA4 in the PR group were observed. Intratumoral heterogeneity and domains of specific proteins (KMT2D, CCND1, and NCOR2 mutants) are shown in the Data Supplement.
FIG 3.
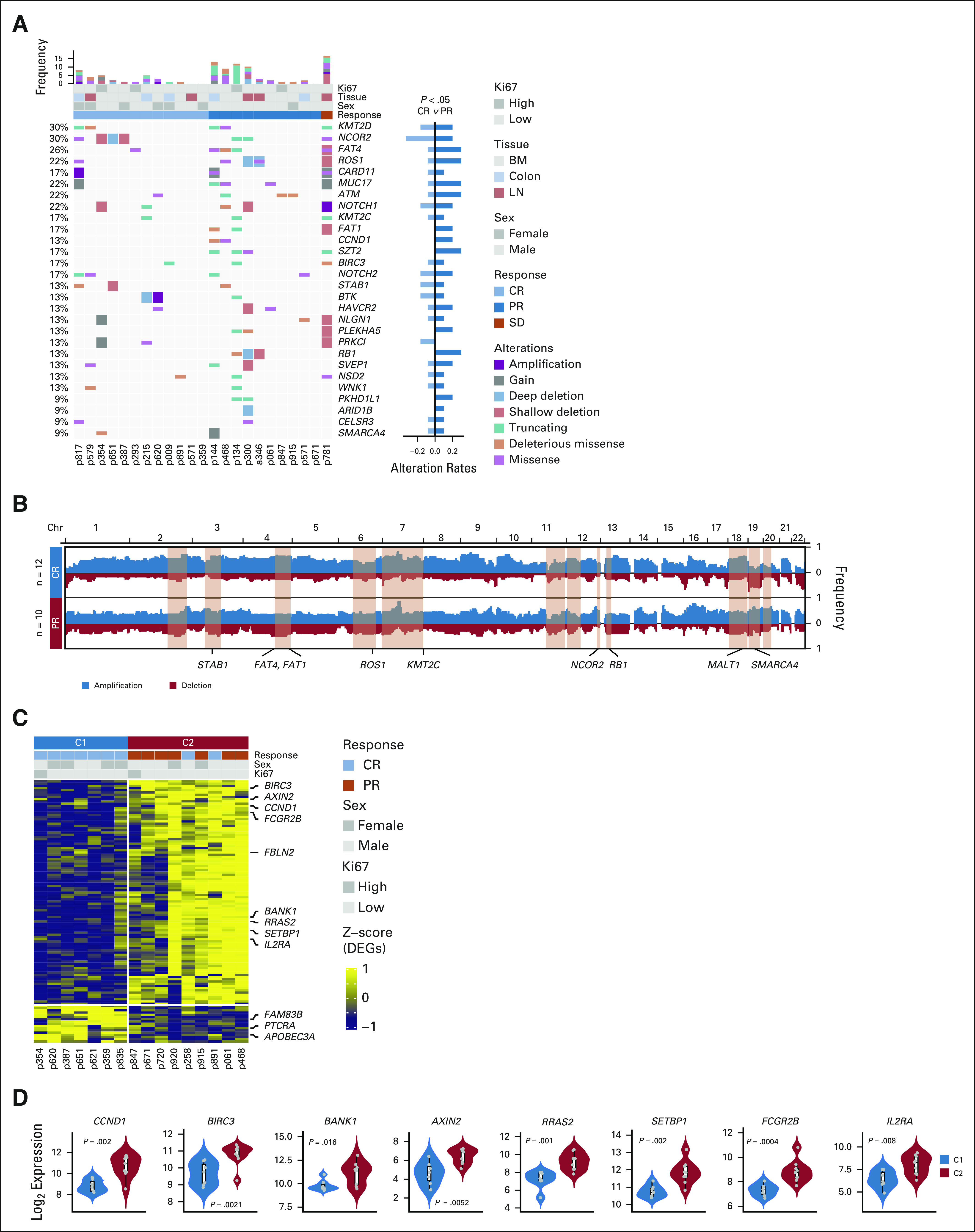
Somatic mutation profile by WES and transcriptomic profile by bulk RNA sequencing and differential gene expression in patients, on the basis of achieving CR (C1) or PR (C2) after treatment with ibrutinib-rituximab. (A) The landscape of somatic mutations from pretreatment MCL samples (n = 25). The bottom panel shows somatic mutations and gene-level copy number alterations by sample (column) and by gene (row). The middle tracks display the clinical characteristics. The histogram on the top shows the number of alterations accumulated on 28 listed genes in each individual patient. The right bar plots show the composite of all mutations between CR (C1) and PR (C2) groups. Fisher's exact test, P < .05. (B) Composite of copy number profiles between CR and PR groups, with gains in red and losses in blue. The regions that showed a difference in the frequency of copy number alterations between two subtypes are shaded in light red rectangles, and within which the names of cancer-related or biologically important genes are labeled (STAB1, FAT1, FAT4, KMT2C, MALT1, SMARCA4, ROS1, NCOR2, and RB1). (C) Transcriptomic profile of baseline tumor specimens from 16 patients with MCL is shown, CR (C1) and PR (C2). Unsupervised hierarchical clustering of DEGs on RNA-seq analysis is shown. Genes with log2(fold change) > 1 and a FDR q < 0.05 were applied to filter DEGs. Biologically important genes are labeled on the right of the plot. The top tracks show clinical characteristics among the samples. A Fisher's exact test is used to identify significant clinical factors; response was significantly correlated with the DEGs (P = .003). (D) Violin plot indicates biologically important DEGs among the three clusters. BM, bone marrow; CR, complete response; DEG, differentially expressed gene; FDR, false discovery rate; MCL, mantle cell lymphoma; PR, partial response; WES, whole-exome sequencing.
Bulk RNA sequencing and DEG according to the response to IR combination.
Sixteen baseline samples were evaluable for bulk RNA sequencing. A distinct pattern of differentially expressed genes including CCND1, BIRC3, BANK1, SETBP1, RRAS2, AXIN2, and IL2RA was observed in patients who achieved a PR (n = 7) compared with patients who achieved CR (n = 9; Figs 3C and 3D). In the Data Supplement, pathways that are differentially enriched in PR versus CR are depicted. Gene set enrichment pathway analysis demonstrated that B-cell receptor (BCR) pathways were predominantly upregulated in PR patients (Data Supplement), whereas mitogen-activated protein kinase, TP53, calcium signaling, and hypoxia-associated pathways were upregulated in those with CR.
DISCUSSION
Treatment of previously untreated older patients with MCL is challenging because of generally coexisting comorbidities and age-related complications. Traditionally, older patients with MCL are treated with chemoimmunotherapy. In general, adverse effects from chemoimmunotherapies can limit the quality of life in older patients. The adverse impact of conventional chemoimmunotherapies2,6-9 on patient's performance status, worsening of comorbidities, risk of severe infections, second cancers, and the need for hospitalization are the major factors to consider in treating older patients with MCL. Therefore, development of safe and efficacious therapies for older patients with MCL is a major unmet need. The advent of orally administered and well-tolerated nonchemotherapeutic targeted agents, such as ibrutinib, was significant for older patients with MCL. After the approval of BTK inhibitors in relapsed MCL,10-14 it was natural to investigate ibrutinib with rituximab combination in the frontline setting. Therefore, we designed this completely chemotherapy-free approach with IR combination for previously untreated older patients with MCL.
There has been a continuous natural evolution of frontline therapies for the elderly patients with MCL. In the Data Supplement, we have summarized the results from previous studies in elderly patients with MCL after other standard therapies. Chemoimmunotherapy, such as rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine and prednisone–based studies, induced an ORR (CR) of 86% (34%) in the MCL elderly study,6 and bortezomib–rituximab, cyclophosphamide, doxorubicin, and prednisone,7 92% (53%), whereas the other commonly practiced bendamustine-rituximab–based studies induced an ORR (CR) of 93% (40%) in the StiL study.21 Other bendamustine-rituximab–based studies with rituximab, bendamustine, and cytarabine induced an ORR (CR) of 91% (91%),8 and in the rituximab, bendamustine, bortezomib and dexamethasone22 study, 84% (75%). Of note, the rituximab, bendamustine, and cytarabine therapy8 was also myelosuppressive (50%) in older patients with MCL. With chemoimmunotherapies, the grade 3-4 myelosuppression was observed in 40%-50% of patients and with a longer follow-up, about 10% of patients developed second cancers. Furthermore, lenalidomide with rituximab23 combination was investigated in the frontline setting (excluding blastoid/pleomorphic histology) in a phase II multicenter study (n = 38; 24 patients age > 60 years), but 16% of patients developed second cancers and 42% developed grade 3 neutropenia, similar to myelosuppression observed with chemoimmunotherapies. The combination of lenalidomide with bendamustine rituximab24 was also investigated in elderly patients with MCL and was associated with 38% grade 3 neutropenia, 42% severe infections, and 16% risk of second primary cancers.
It is in such a historical context that we designed the current trial. Our study excluded patients with Ki-67% > 50% and/or blastoid/pleomorphic histology because we were not confident that this combination would be effective in patients with high-risk MCL. Our data demonstrated a lower rate of grade 3-4 myelosuppression and a lower risk of hospitalization for infections (< 10%) than previously published chemoimmunotherapy results.6-8 These were the major advantages of IR combination in elderly patients with MCL, compared with other treatment modalities with chemoimmunotherapy or lenalidomide-rituximab.
Of note, after a median follow-up of 45 months, 28 (56%) patients had discontinued IR therapy for various reasons, mainly because of intolerance in 21 of 28 patients. The median time to IR discontinuation was 32.6 months (Data Supplement). Although the rate of discontinuation because of disease progression (8%) was lower than some other therapies (40%-60%),6,23 the rate of study discontinuation because of intolerance (42%) was higher in this study compared with previous studies with other therapies (10%-25%).6,8,23 Furthermore, with a nearly similar median follow-up, the rate of study discontinuation because of intolerance was higher in our study compared with the IR combination in relapsed MCL15 (56% v 18%, respectively). Possible reasons for these differences could be elderly patient population with comorbidities since 6 of 9 patients who discontinued IR combination in relapsed MCL were age ≥ 65 years.
Moreover, IR combination in relapsed MCL demonstrated that the incidence of atrial fibrillation was < 12%.15,25 In this study, 17 patients developed atrial fibrillation. The numbers of baseline cardiovascular risk factors and baseline asymptomatic ECG abnormalities were higher in patients who developed atrial fibrillation compared with patients who did not develop atrial fibrillation, with median of 4 (range 1-8) vs median of 2 (range 1-4) and 13 of 17 (76%) versus 17 of 33 (51%) patients, respectively (described in the Data Supplement). These differences could potentially explain the increased incidence of atrial fibrillation observed in this study. At the time of initial enrollment in 2015 for this study, our screening process did not include a comprehensive cardiology evaluation in any patients before treatment with ibrutinib. Our results demonstrate that appropriate patient selection from cardiology standpoint before IR therapy is important. Recently, an echocardiogram-based baseline parameter such as left atrial volume index ≥ 40 mL/m2 has been reported26 by cardiologists to identify patients who are prone to develop ibrutinib-associated atrial fibrillation.27 To mitigate the risk of discontinuation because of intolerance, a frontline phase II study with acalabrutinib-rituximab in elderly patients with MCL is conducted at our center (NCT04765111).
With respect to survival outcomes, the median PFS and OS were not reached after almost a 4-year follow-up, but significantly higher risk of events was noted in patients with high-risk MIPI and those who did not attain CR. On comparing patients with a high (≥ 30%) and low (< 30%) Ki-67 index, the PFS and OS were not significantly different, but higher risk of progression event was noticeable in those patients with Ki-67 higher than 30%. Only three patients among the 18 evaluable patients had TP53 aberrations, where one patient progressed while other two discontinued therapy because of intolerance. The response rates in this limited number of patients with TP53 aberration were lower compared with patients without TP53 aberrations. These data further suggest that for elderly patients with high-risk3 MCL, IR combination alone may not be sufficient and we will need to develop newer treatment modalities for these elderly patients with high-risk MCL. Furthermore, this is a single-arm study, which excluded high-risk (blastoid or Ki-67% > 50%) patients and may induce a selection bias, and therefore, the true efficacy of this regimen in MCL across various risk categories should be further evaluated in a randomized study and should be compared with standard treatments.
We further investigated for genomic predictors of response, using WES and bulk RNA sequencing, among the evaluable baseline tumor tissues. Our WES data demonstrate that partial responders to IR were enriched in KMT2D, FAT1, FAT4 (cell adhesion factor), ROS1, CARD11, NOTCH1, NSD2, and CCND1 mutations. These findings are consistent with other previous studies28-32 showing disease resistance to BTK inhibitors with these mutations; however, STAB1 gene (lymphocyte homing and angiogenesis) deletions and ROS1 mutations were not previously reported in MCL. NCOR2 mutations were clustered in those with CR, and this could mediate the downregulation of targeted genes, which are unknown at this time in MCL.
Furthermore, the DEG data are interesting and demonstrate that BCR signaling pathway aberrations are predominant in patients with PR compared with those in CR. A cluster of differentially overexpressed genes—CCND1, BIRC3, BANK1, SETBP1, RRAS2, AXIN2, and IL2RA, were observed in patients who achieved a PR (n = 7) compared with patients who achieved CR (n = 9). Of note, BANK1 gene33 expression is associated with sustained BCR signaling in lupus patients. These genomic data provide us a cleaner evidence to evaluate the baseline transcriptomic signature and understand the complexity of the BCR signaling pathway,34 adapter proteins, and their relationship with the tumor microenvironment in MCL.
To conclude, IR combination is an effective, easily administered, and chemotherapy-free option in elderly patients with nonblastoid (and/or Ki-67 < 50%) MCL. New onset atrial fibrillation was observed in nine patients (18%). We recommend that pretreatment assessment of cardiovascular risk factors is beneficial before IR therapy. Long-term follow-up and randomized studies with standard treatments are needed to further evaluate the efficacy, safety, and pattern of relapse with IR combination.
ACKNOWLEDGMENT
The authors thank the patients who participated in this trial and their families, the study investigators and coordinators at MD Anderson for sample and data collection, Core grant CA016672 (ATGC) for conducting genomic sequencing, and NIH 1S10OD024977-01 grant for using NovaSeq6000 data.
Preetesh Jain
Honoraria: Kite, a Gilead company, Lilly
Consulting or Advisory Role: Lilly
Hun Ju Lee
Honoraria: Aptitude Health, Cancer Experts Now, Curio Science, Century Therapeutics
Consulting or Advisory Role: BMS, Guidepoint Global,
Research Funding: Seattle Genetics, BMS, Takeda, Oncternal Therapeutics, Celgene,
Chi Young Ok
Research Funding: Seattle Genetics
Fredrick B. Hagemeister
Consulting or Advisory Role: Genentech
Nathan Fowler
Employment: BostonGene
Consulting or Advisory Role: Roche/Genentech, TG Therapeutics, Verastem, Bayer, Celgene, Novartis,
Research Funding: Roche, Celgene, Gilead Sciences, TG Therapeutics, Novartis, Abbvie, BeiGene
Luis Fayad
Consulting or Advisory Role: EUSA Pharma
Francisco Vega
Honoraria: i3Health
Research Funding: CRISPR Therapeutics, Geron
Christopher R. Flowers
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Bayer, Gilead Sciences¸ Spectrum Pharmaceuticals, Abbvie, Celgene, Denovo Biopharma, BeiGene, Karyopharm Therapeutics, Pharmacyclics/Janssen, Genentech/Roche, Epizyme
Research Funding: Acerta Pharma (Inst), Janssen Oncology (Inst), Gilead Sciences (Inst), Celgene (Inst), TG Therapeutics (Inst), Genentech/Roche (Inst), Pharmacyclics (Inst), Abbvie (Inst), Millennium (Inst), Alimera Sciences (Inst), Xencor (Inst)
Michael L. Wang
Honoraria: Janssen Research & Development, DAVA Oncology, OM Pharmaceutical Industries, AstraZeneca, CAHON, Hebei Cancer Prevention Federation, Mumbai Hematology Group, Acerta Pharma, Anticancer Association, BeiGene, Chinese Medical Association, Clinical Care Options, Epizyme, Imbruvica, Imedex, Kite, a Gilead company, Miltenyi Biomedicine GmbH, Moffit Cancer Center, Newbridge Pharmaceuticals, Physicians Education Resources (PER), Scripps, The First Afflicted Hospital of Zhejiang University, BGICS,
Consulting or Advisory Role: AstraZeneca, Janssen Research & Development, Juno Therapeutics, Bioinvent, Pharmacyclics/Janssen, Pulse Biosciences, Guidepoint Global, Loxo, Kite, a Gilead company, InnoCare, Oncternal Therapeutics, CStone Pharmaceuticals, Genentech, Bayer, BeiGene, DTRM Biopharma (Cayman) Limited, Epizyme, Miltenyi Biomedicine GmbH, VelosBio
Research Funding: AstraZeneca, Janssen Research & Development, Pharmacyclics, Kite, a Gilead company¸ Juno Therapeutics, BeiGene, Acerta Pharma, Oncternal Therapeutics, Bioinvent, Loxo, Celgene, VelosBio, Molecular Templates, Lilly, InnoCare
Travel, Accommodations, Expenses: Janssen Research & Development, AstraZeneca, Celgene, DAVA Oncology, OM Pharmaceutical Industries
No other potential conflicts of interest were reported.
SUPPORT
Supported by the Pharmacyclics LLC and Janssen trial registration: ClinicalTrials.gov Identifier: NCT02427620.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Once published, clinical outcome data (deidentified) will be provided. In addition, study protocol, statistical analysis plan, informed consent form, and amendments of the study protocol will be provided. The data will be made available on demand, and corresponding author e-mail address will be provided to request any data. The use of available data can be made after discussion with the corresponding author, and possibilities of future collaborations will be explored.
AUTHOR CONTRIBUTIONS
Conception and design: Michael L. Wang
Administrative support: Preetesh Jain, Maria Badillo, Michael L. Wang
Provision of study materials or patients: Preetesh Jain, Hun Ju Lee, Chi Young Ok, Rashmi Kanagal-Shamanna, Fredrick B. Hagemeister, Nathan Fowler, Luis Fayad, Selvi Thirumurthi, David Santos, Michael L. Wang
Collection and assembly of data: Preetesh Jain, Holly A. Hill, Yixin Yao, Yang Liu, Omar B. Moghrabi, Lucy Navsaria, Guofan Xu, L. Jeffrey Medeiros, Francisco Vega, Michelle Avellaneda, Michael L. Wang
Data analysis and interpretation: Preetesh Jain, Shuangtao Zhao, Holly A. Hill, Lei Feng, Graciela M. Nogueras Gonzalez, Cezar Iliescu, Guilin Tang, Christopher R. Flowers, Linghua Wang, Michael L. Wang
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Ibrutinib with Rituximab in First-Line Treatment of Older Patients With Mantle Cell Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Preetesh Jain
Honoraria: Kite, a Gilead company, Lilly
Consulting or Advisory Role: Lilly
Hun Ju Lee
Honoraria: Aptitude Health, Cancer Experts Now, Curio Science, Century Therapeutics
Consulting or Advisory Role: BMS, Guidepoint Global,
Research Funding: Seattle Genetics, BMS, Takeda, Oncternal Therapeutics, Celgene,
Chi Young Ok
Research Funding: Seattle Genetics
Fredrick B. Hagemeister
Consulting or Advisory Role: Genentech
Nathan Fowler
Employment: BostonGene
Consulting or Advisory Role: Roche/Genentech, TG Therapeutics, Verastem, Bayer, Celgene, Novartis,
Research Funding: Roche, Celgene, Gilead Sciences, TG Therapeutics, Novartis, Abbvie, BeiGene
Luis Fayad
Consulting or Advisory Role: EUSA Pharma
Francisco Vega
Honoraria: i3Health
Research Funding: CRISPR Therapeutics, Geron
Christopher R. Flowers
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Bayer, Gilead Sciences¸ Spectrum Pharmaceuticals, Abbvie, Celgene, Denovo Biopharma, BeiGene, Karyopharm Therapeutics, Pharmacyclics/Janssen, Genentech/Roche, Epizyme
Research Funding: Acerta Pharma (Inst), Janssen Oncology (Inst), Gilead Sciences (Inst), Celgene (Inst), TG Therapeutics (Inst), Genentech/Roche (Inst), Pharmacyclics (Inst), Abbvie (Inst), Millennium (Inst), Alimera Sciences (Inst), Xencor (Inst)
Michael L. Wang
Honoraria: Janssen Research & Development, DAVA Oncology, OM Pharmaceutical Industries, AstraZeneca, CAHON, Hebei Cancer Prevention Federation, Mumbai Hematology Group, Acerta Pharma, Anticancer Association, BeiGene, Chinese Medical Association, Clinical Care Options, Epizyme, Imbruvica, Imedex, Kite, a Gilead company, Miltenyi Biomedicine GmbH, Moffit Cancer Center, Newbridge Pharmaceuticals, Physicians Education Resources (PER), Scripps, The First Afflicted Hospital of Zhejiang University, BGICS,
Consulting or Advisory Role: AstraZeneca, Janssen Research & Development, Juno Therapeutics, Bioinvent, Pharmacyclics/Janssen, Pulse Biosciences, Guidepoint Global, Loxo, Kite, a Gilead company, InnoCare, Oncternal Therapeutics, CStone Pharmaceuticals, Genentech, Bayer, BeiGene, DTRM Biopharma (Cayman) Limited, Epizyme, Miltenyi Biomedicine GmbH, VelosBio
Research Funding: AstraZeneca, Janssen Research & Development, Pharmacyclics, Kite, a Gilead company¸ Juno Therapeutics, BeiGene, Acerta Pharma, Oncternal Therapeutics, Bioinvent, Loxo, Celgene, VelosBio, Molecular Templates, Lilly, InnoCare
Travel, Accommodations, Expenses: Janssen Research & Development, AstraZeneca, Celgene, DAVA Oncology, OM Pharmaceutical Industries
No other potential conflicts of interest were reported.
REFERENCES
- 1.Fu S, Wang M, Lairson DR, et al. : Trends and variations in mantle cell lymphoma incidence from 1995 to 2013: A comparative study between Texas and National SEER areas. Oncotarget 8: 112516–1125292017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrahamsson A, Albertsson-Lindblad A, Brown PN, et al. : Real world data on primary treatment for mantle cell lymphoma: A Nordic Lymphoma Group observational study. Blood 124: 1288–12952014 [DOI] [PubMed] [Google Scholar]
- 3.Jain P, Dreyling M, Seymour JF, et al. : High-risk mantle cell lymphoma: Definition, current challenges, and management. J Clin Oncol 38: 4302–43162020 [DOI] [PubMed] [Google Scholar]
- 4.Weaver JA, Peng Y, Ji Y, et al. : A medicare database analysis of practice patterns in patients with mantle cell lymphoma. J Geriatr Oncol 11: eaau1167.2021 [DOI] [PubMed] [Google Scholar]
- 5.Glimelius I, Smedby KE, Eloranta S, et al. : Comorbidities and sex differences in causes of death among mantle cell lymphoma patients—A nationwide population-based cohort study. Br J Haematol 189: 106–1162020 [DOI] [PubMed] [Google Scholar]
- 6.Kluin-Nelemans HC, Hoster E, Hermine O, et al. : Treatment of older patients with mantle cell lymphoma (MCL): Long-term follow-up of the randomized European MCL elderly trial. J Clin Oncol 38: 248–2562020 [DOI] [PubMed] [Google Scholar]
- 7.Robak T, Jin J, Pylypenko H, et al. : Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: Final overall survival results of a randomised, open-label, phase 3 study. Lancet Oncol 19: 1449–14582018 [DOI] [PubMed] [Google Scholar]
- 8.Visco C, Chiappella A, Nassi L, et al. : Rituximab, bendamustine, and low-dose cytarabine as induction therapy in elderly patients with mantle cell lymphoma: A multicentre, phase 2 trial from Fondazione Italiana Linfomi. Lancet Haematol 4: e15–e232017 [DOI] [PubMed] [Google Scholar]
- 9.Flinn IW, van der Jagt R, Kahl B, et al. : First-line treatment of patients with indolent non-Hodgkin lymphoma or mantle-cell lymphoma with bendamustine plus rituximab versus R-CHOP or R-CVP: Results of the BRIGHT 5-year follow-up study. J Clin Oncol 37: 984–9912019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang ML, Rule S, Martin P, et al. : Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 369: 507–5162013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Rule S, Zinzani PL, et al. : Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): A single-arm, multicentre, phase 2 trial. Lancet 391: 659–6672018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Shah NN, Alencar AJ, et al. : LOXO-305, A next generation, highly selective, non-covalent BTK inhibitor in previously treated mantle cell lymphoma, Waldenström's macroglobulinemia, and other non-Hodgkin lymphomas: Results from the phase 1/2 BRUIN study. Blood 136: 8–102020. 32614959 [Google Scholar]
- 13.Song Y, Zhou KS, Zou D, et al. : Treatment of patients with relapsed or refractory mantle cell lymphoma with zanubrutinib, a selective inhibitor of Bruton's tyrosine kinase. Clin Cancer Res 26: 4216–42242020 [DOI] [PubMed] [Google Scholar]
- 14.Mato AR, Shah NN, Jurczak W, et al. : Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): A phase 1/2 study. Lancet 397: 892–9012021 [DOI] [PubMed] [Google Scholar]
- 15.Jain P, Romaguera J, Srour SA, et al. : Four-year follow-up of a single arm, phase II clinical trial of ibrutinib with rituximab (IR) in patients with relapsed/refractory mantle cell lymphoma (MCL). Br J Haematol 182: 404–4112018 [DOI] [PubMed] [Google Scholar]
- 16.Wang ML, Lee H, Chuang H, et al. : Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: A single-centre, open-label, phase 2 trial. Lancet Oncol 17: 48–562016 [DOI] [PubMed] [Google Scholar]
- 17.Tam CS, Anderson MA, Pott C, et al. : Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med 378: 1211–12232018 [DOI] [PubMed] [Google Scholar]
- 18.Zhao S, Kanagal-Shamanna R, Navsaria L, et al. : Efficacy of venetoclax in high risk relapsed mantle cell lymphoma (MCL) - outcomes and mutation profile from venetoclax resistant MCL patients. Am J Hematol 95: 623–6292020 [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Fisher RI, Barrington SF, et al. : Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 32: 3059–30682014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(CTEP) NCICTEP : Common Toxicity Criteria for Adverse Events (v4.03). Bethesda, MD, US Department of Health and Human Services, 2009
- 21.Rummel MJ, Niederle N, Maschmeyer G, et al. : Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 381: 1203–12102013 [DOI] [PubMed] [Google Scholar]
- 22.Gressin R, Daguindau N, Tempescul A, et al. : A phase 2 study of rituximab, bendamustine, bortezomib and dexamethasone for first-line treatment of older patients with mantle cell lymphoma. Haematologica 104: 138–1462019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruan J, Martin P, Christos P, et al. : Five-year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Blood 132: 2016–20252018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albertsson-Lindblad A, Kolstad A, Laurell A, et al. : Lenalidomide-bendamustine-rituximab in patients older than 65 years with untreated mantle cell lymphoma. Blood 128: 1814–18202016 [DOI] [PubMed] [Google Scholar]
- 25.Rule S, Dreyling M, Goy A, et al. : Outcomes in 370 patients with mantle cell lymphoma treated with ibrutinib: A pooled analysis from three open-label studies. Br J Haematol 179: 430–4382017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baptiste F, Cautela J, Ancedy Y, et al. : High incidence of atrial fibrillation in patients treated with ibrutinib. Open Heart 6: e001049.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fradley MG, Gliksman M, Emole J, et al. : Rates and risk of atrial arrhythmias in patients treated with ibrutinib compared with cytotoxic chemotherapy. Am J Cardiol 124: 539–5442019 [DOI] [PubMed] [Google Scholar]
- 28.Ferrero S, Rossi D, Rinaldi A, et al. : KMT2D mutations and TP53 disruptions are poor prognostic biomarkers in mantle cell lymphoma receiving high-dose therapy: A FIL study. Haematologica 105: 1604–16122020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu C, de Miranda NF, Chen L, et al. : Genetic heterogeneity in primary and relapsed mantle cell lymphomas: Impact of recurrent CARD11 mutations. Oncotarget 7: 38180–381902016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain P, Kanagal-Shamanna R, Zhang S, et al. : Long-term outcomes and mutation profiling of patients with mantle cell lymphoma (MCL) who discontinued ibrutinib. Br J Haematol 183: 578–5872018 [DOI] [PubMed] [Google Scholar]
- 31.Bea S, Valdes-Mas R, Navarro A, et al. : Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc Natl Acad Sci USA 110: 18250–182552013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Jima D, Moffitt AB, et al. : The genomic landscape of mantle cell lymphoma is related to the epigenetically determined chromatin state of normal B cells. Blood 123: 2988–29962014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozyrev SV, Abelson AK, Wojcik J, et al. : Functional variants in the B-cell gene BANK1 are associated with systemic lupus erythematosus. Nat Genet 40: 211–2162008 [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Yao Y, Zhang S, et al. : Metabolic reprogramming toward oxidative phosphorylation identifies a therapeutic target for mantle cell lymphoma. Sci Transl Med 11: eaau1167.2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Once published, clinical outcome data (deidentified) will be provided. In addition, study protocol, statistical analysis plan, informed consent form, and amendments of the study protocol will be provided. The data will be made available on demand, and corresponding author e-mail address will be provided to request any data. The use of available data can be made after discussion with the corresponding author, and possibilities of future collaborations will be explored.