Abstract
Purpose
MicroRNAs (miRNAs) are epigenetic post-transcriptional regulators that modulate gene expression and have been identified as biomarkers for several diseases, including cancer. This study aimed to systematically review the relationship between miRNAs and periodontal disease in humans, and to evaluate the potential of miRNAs as diagnostic and prognostic biomarkers of disease.
Methods
The review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (reference number CRD42020180683). The MEDLINE, Scopus, Cochrane Library, Embase, Web of Science, and SciELO databases were searched for clinical studies conducted in humans investigating periodontal diseases and miRNAs. Expression levels of miRNAs across the different groups were analysed using the collected data.
Results
A total of 1,299 references were identified in the initial literature search, and 23 articles were finally included in the review. The study designs were heterogeneous, which prevented a meta-analysis of the data. Most of the studies compared miRNA expression levels between patients with periodontitis and healthy controls. The most widely researched miRNA in periodontal diseases was miR-146a. Most studies reported higher expression levels of miR-146a in patients with periodontitis than in healthy controls. In addition, many studies also focused on identifying target genes of the differentially expressed miRNAs that were significantly related to periodontal inflammation.
Conclusions
The results of the studies that we analysed are promising, but diagnostic tests are needed to confirm the use of miRNAs as biomarkers to monitor and aid in the early diagnosis of periodontitis in clinical practice.
Keywords: Epigenetic biomarker, Humans, miRNA, Periodontal diseases, Periodontitis
Graphical Abstract
INTRODUCTION
Periodontitis is a chronic, multifactorial immunoinflammatory disease, typically caused by anaerobic gram-negative bacteria within dental plaque or biofilm and characterised by the destruction of tooth-supporting tissues; in some cases, this leads to tooth loss [1]. The aetiopathogenesis of this disease is complex. Traditionally, it was considered to be a simple infection caused by different bacterial species that colonised the periodontal pocket. We now know that an inappropriate host immune-inflammatory response against these bacteria and their products drives disease progression in susceptible individuals [2]. Therefore, although bacteria initiate periodontal destruction, disease progression is due to additional factors [3].
For many years, much of the research on periodontics was concerned with the implications of genetic variants or mutations for the aetiopathogenesis of periodontitis, such as the relationship between interleukin (IL)-1 beta gene polymorphism and increased susceptibility to periodontitis [4]. Expanding our knowledge of gene expression modulation by epigenetic regulatory mechanisms is one of the greatest challenges in periodontal research.
Epigenetics is an emerging field of science that investigates changes in gene expression that are not attributed to DNA sequence alterations. Many epigenetic mechanisms, such as those based on microRNAs (miRNAs), are used by cells to activate or inhibit certain genes to produce different proteins [5].
miRNAs constitute a large family of short, non-coding RNA molecules and are ~22 nucleotides in length. These post-transcriptional regulators modulate gene expression either by inducing target messenger RNA (mRNA) degradation or by repressing translation initiation and thus protein synthesis [6]. For this to occur, miRNA must bind to the 3′-untranslated region of target mRNA transcripts, which usually results in gene silencing [6]. However, complete sequence complementarity between a single miRNA and its target mRNA is not required for gene silencing to occur; therefore, a single miRNA has the potential to control the translation of many different genes concurrently [7]. To date, over 2,500 genes encoding miRNAs have been identified in the human genome [8].
Over 2,000 miRNAs have been identified in humans [9]. These single-stranded RNA molecules participate in physiological processes such as cellular development, differentiation, and apoptosis [10]. However, many studies have also reported that miRNA dysregulation may have a substantial impact on the pathophysiology of diseases such as cancer [11], coronary heart disease [12], and diabetes [13], as well as on inflammatory autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus [8].
Furthermore, extracellular miRNAs remain remarkably stable in the bloodstream and other biofluids, such as saliva, urine, and cerebrospinal fluid, making miRNAs ideal candidates as biomarkers for the diagnosis and prognosis of many diseases, including periodontitis [14].
Although studies have shown that miRNAs play important roles in various systemic inflammatory diseases, to date, no systematic review has evaluated the possible association between miRNAs and periodontitis.
This systematic review aimed to analyse the putative relationship between miRNAs and periodontal diseases in humans, and to evaluate their potential as diagnostic and prognostic biomarkers.
MATERIALS AND METHODS
Review question
A systematic review was carried out following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The study was registered in the PRISMA database (PROSPERO), under reference number CRD42020180683. The following population, intervention/exposure, comparison, outcome question was formulated to address the specific aim of the study with reference to humans, miRNAs, healthy subjects without periodontal disease, and periodontitis, respectively: In humans, is there a relationship between miRNA expression levels and periodontal disease?
Inclusion and exclusion criteria
Cross-sectional, case-control, and cohort studies and randomised clinical trials were included in this review. Both prospective and retrospective investigations were included. Clinical case reports, literature reviews, animal studies, in vitro studies, and journal editorials, and studies without a healthy control group were excluded.
Search strategy
Electronic searches of the MEDLINE, Scopus, Cochrane Library, Embase, Web of Science, and SciELO databases were conducted in January 2020 for publications that investigated periodontitis and miRNAs. Detailed search strategies were developed for each database. These were based on a search strategy presented for MEDLINE using the following keywords (MeSH and free terms) combined with the Boolean connectors AND and OR: ((“periodontal” [All Fields] OR “periodontally” [All Fields] OR “periodontically” [All Fields] OR “periodontics” [MeSH Terms] OR “periodontics” [All Fields] OR “periodontic” [All Fields] OR “periodontitis” [MeSH Terms] OR “periodontitis” [All Fields]) AND (“micrornas” [MeSH Terms] OR “micrornas” [All Fields] OR “mirna” [All Fields] OR “mirnas” [All Fields] OR “mirna’s” [All Fields])). No year restrictions were applied for the electronic database search.
In addition, online manual searching of the following key periodontal journals was conducted, for articles published between 2010 and the present date: Journal of Clinical Periodontology, Journal of Periodontology, Journal of Periodontal Research, and Journal of Dental Research. Only articles published in English or Spanish were included.
Two reviewers (PMM and PAP) appraised the titles and abstracts, and reviewed the full texts of the selected articles. The kappa statistic (κ) was calculated to assess inter-rater reliability. In cases of disagreement between the reviewers, a third reviewer was consulted (ALR). Duplicate articles were excluded from the analysis. We also recorded the reasons for rejecting any articles.
Extraction of results and study characteristics
The following information was extracted from each article when available: first author’s surname, publication year, study design, study groups, sample size, mean age of participants, type of sample, type of periodontal disease (chronic or aggressive, following the Armitage classification [15]), analysed miRNAs, and main outcomes. All data were reviewed to consider appropriateness for a meta-analysis.
Qualitative assessment
The Newcastle-Ottawa Quality Assessment Scale (NOS) was used to assess the methodological quality and risk of bias of the non-randomised studies. This checklist consists of 8 detailed quality items divided into 3 categories (selection, comparability, and outcome). Each item could be awarded 1 star, except for comparability, which was awarded 2 stars. Thus, the total maximum score was 9 stars. A score of 7 stars or more indicated a low risk of bias. This assessment was performed independently by 2 reviewers (PMM and PAP), and by a third (ALR) reviewer when there was no consensus.
RESULTS
Selected studies
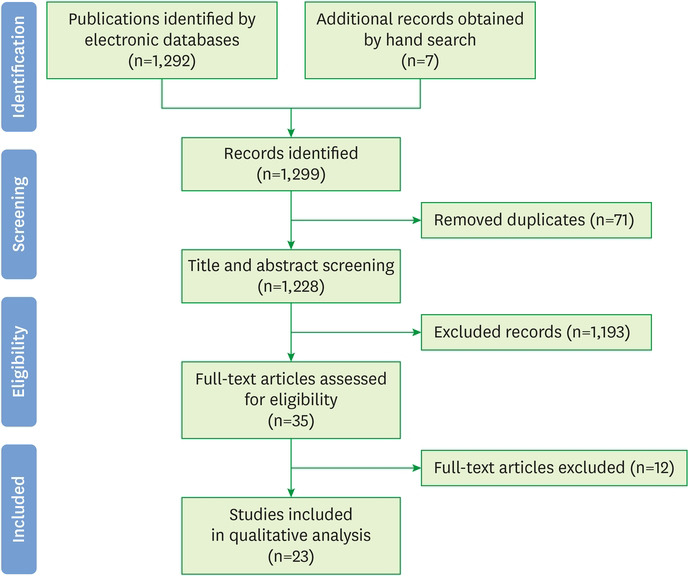
The electronic search generated 1,292 references (287 in PubMed, 178 in Embase, one in the Cochrane Library, 735 in Scopus, 87 in Web of Science, and 4 in SciELO), and 7 additional records were identified by manual searches. A total of 71 duplicates were found using the Mendeley® bibliographic citation management software; these were excluded from the analysis. In addition, after screening 1,228 titles and abstracts, a further 1,193 articles were discarded for the following reasons: 1,060 investigated epigenetic mechanisms and pathologies that were different to miRNAs and periodontitis, respectively, 92 were animal models or in vitro studies, and 41 were literature reviews. A total of 35 publications were obtained as full-text articles; however, 12 of these were later excluded based on our inclusion/exclusion criteria (not written in English or Spanish, n=3; in vitro studies, n=5; literature review, n=1; not relevant to the review objectives, n=1; no control group, n=2). Finally, 23 articles were included in the qualitative analysis. The PRISMA flow diagram in Figure 1 summarizes the study selection criteria.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analysis flow diagram.
Inter-rater reliability
Inter-examiner agreement was high for the full-text screening (κ=0.85).
Characteristics and main outcomes of the included studies
Table 1 summarizes the data extracted from each included article. All 23 studies were cross-sectional studies. Most of the studies performed large-scale sequencing to identify potential diagnostic biomarkers, although some focused on specific miRNAs. Most of the investigations (n=13) obtained biological samples from gingival tissue biopsies, but some analysed blood samples (n=4), saliva (n=2), gingival crevicular fluid (n=3), and subgingival biofilm (n=1). The sample size varied among studies and ranged from 6 [16] to 550 [17] patients. Most of the studies compared miRNA expression levels between patients with periodontitis and healthy controls. However, some authors evaluated how systemic diseases such as obesity [18,19,20], diabetes [21], and coronary heart disease [22,23] influence relative miRNA expression levels. In addition, many studies focused on identifying target genes that were significantly related to periodontal inflammation of the differentially expressed miRNAs.
Table 1. Characteristics and main outcomes of included studies.
| Main outcomes | Analyzed miRNAs | Type of sample | Sample size mean age: years±SD | Study groups | Author, type of study |
|---|---|---|---|---|---|
| - Upregulation of miR-146 in G1, G2, and G3 compared to G4 (OR, 1.43) | miR-146 | Blood sample | 264 | Bagavad Gita et al. [22], cross-sectional | |
| - No significant differences between G1, G2, and G3 | G1: n=66; 52.14±2.5 | G1: CHD without periodontitis | |||
| G2: n=66; 58.24±1.3 | G2: CHD + CP | ||||
| G3: n=66; 43.22±1.9 | G3: Healthy + CP | ||||
| G4: n=66; 48±4.4 | G4: Healthy without CP | ||||
| - Upregulation of miR-142-3p in G1 | miR-142-3p | Gingival biopsy | 46 | Chen et al. [24], cross-sectional | |
| G1: n=26; ND | G1: CP | ||||
| G2: n=20; ND | G2: Healthy | ||||
| - Upregulation of miR-146a in G1 compared to G2 (OR, 17.8) | miR-146a | Gingival biopsy | 28 | Ghotloo et al. [25], cross-sectional | |
| - The higher the PD, the higher the miR-146a expression level | G1: n=18; 27±13 | G1: AP | |||
| - The higher the miR-146a expression level, the lower the PROINF CYT expression level | G2: 4n=10; 32±12 | G2: Healthy | |||
| - Upregulation of both miRNAs in G1 and G2 compared to G3 | miR-146a, miR-499 | Blood sample | 197 | Kadkhodazadeh et al. [26], cross-sectional | |
| G1: n=75; ND | G1: CP | ||||
| G2: n=38; ND | G2: PI | ||||
| G3: n=84; ND | G3: Healthy | ||||
| - miR-200b-5p expression levels were 1.6 times higher in G1 | miR-323a-3p, miR-200b-5p, miR-188-5p, miR-4721, mir-557, miR-196a | Gingival biopsy | 36 | Kalea et al. [18], cross-sectional | |
| G1: ND | G1: Obesity + CP or AP | ||||
| G2: ND | G2: Healthy + CP or AP | ||||
| - Upregulation of these miRNAs in G1: miR-181b (OR, 4.64), miR-19b (OR, 4.79), miR-23a (OR, 4.76), miR-30a (OR, 4.76), let-7a (OR, 9.48), miR-301a (OR, 8.59) | Massive sequencing: 93 miRNAs | Gingival biopsy | ND | Lee et al. [27], cross-sectional | |
| G1: ND | G1: CP | ||||
| G2: ND | G2: Healthy | ||||
| - miR-144-5p expression levels were 4.8 times higher in G1 | 17 miRNAs | Gingival biopsy | 32 | Li et al. [28], cross-sectional | |
| G1: n=16; 41.25±4.89 | G1:CP | ||||
| G2: n=16; 38.00±4.68 | G2: Healthy | ||||
| - miR-1226 expression levels were 15.8 times higher in G1 | miR-671, miR-122, miR-1306, miR-27a, miR-223, miR-1226 | Gingival crevicular fluid | 18 | Micó-Martínez et al. [29], cross-sectional | |
| G1: n=9; 50.44±8.09 | G1: CP | ||||
| G2: n=9; 33.33±12.05 | G2: Healthy | ||||
| - miR-146 expression levels were 32.6 times higher in G1 | miR-146a | Gingival biopsy | 30 | Motedayyen et al. [30], cross-sectional | |
| - The higher the PD, the higher the miR-146a expression level | G1: n=20; 44±8 | G1: CP | |||
| G2: n=10; 32±12 | G2: Healthy | ||||
| - Upregulation of miR-128 (OR >5), miR-34a (OR >5), miR-381 (OR, 10) in G1 | Massive sequencing: 93 miRNAs | Gingival biopsy | ND | Na et al. [31], cross-sectional | |
| - Downregulation of miR-15b (OR, 1), miR-211 (OR, 1), miR-372 (OR >1), miR-656 (OR >1) in G1 | G1: ND | G1: CP | |||
| G2: ND | G2: Healthy | ||||
| - Significant differences in miRNA expression levels in tissue with CP compared to healthy tissue (in both groups); OR ≥1.6 | Massive sequencing | Gingival biopsy | 28 | G1(i): Obesity (tissue with CP) | Naqvi et al. [19], cross-sectional |
| - Significant differences in miRNA expression level between G1 and G2 | G1: n=14; ND | G1(ii): Obesity (healthy tissue) | |||
| G2: n=14; ND | G2(i): Healthy (tissue with CP) | ||||
| G2(ii): Healthy (healthy tissue) | |||||
| - Upregulation of 40, downregulation of 40 miRNAs in G1 | Massive sequencing | Blood sample | 32 | Nisha et al. [32], cross-sectional | |
| - miR-143-3p expression was 5.82 times higher in G1 | G1: n=16; 43.38±9.92 | G1: CP | |||
| G2: n=16; 40.56±8.47 | G2: Healthy | ||||
| - miR-150, miR-223, and miR-200b expression levels were 2.72 times higher in G1 | Massive sequencing | Gingival biopsy | 6 | Ogata et al. [16], cross-sectional | |
| G1: n=3; ND | G1: CP | ||||
| G2: n=3; ND | G2: Healthy | ||||
| - Obesity led to a hyperinflammatory state, increasing the risk for CP | Massive sequencing: 88 miRNAs | Gingival biopsy | 24 | G1: Obesity + CP | Perri et al. [20], cross-sectional |
| - Highlights the overexpression of miR-106b in G1 (OR, 6.4) | 4 participants per group | G2: Obesity without CP | |||
| 45±13.3 (overall mean age of all participants) | G3: Healthy + CP | ||||
| G4: Healthy without CP | |||||
| - Upregulation of both miRNAs in G1 and G3 | miR-146, miR-155 | Gingival crevicular fluid | 48 | Radović et al. [21], cross-sectional | |
| - After NSPT, miRNA expression levels were similar among groups | G1: n=24; 54.9±25.45 | G1: DM2 + CP | |||
| G2: n=24; 33.2±26.93 | G2: DM2 without CP | ||||
| G3: n=24; 54.7±27.31 | G3: Healthy + CP | ||||
| G4: n=24; 33.4±26.37 | G4: Healthy without CP | ||||
| - Upregulation of miR-223-3p, miR-203a, and miR-205-5p in G1 and G2 compared to G3 | Massive sequencing: 752 miRNAs | Gingival crevicular fluid | 20 | Saito et al. [33], cross-sectional | |
| G1: n=7; 67.57±ND | G1: CP | ||||
| G2: n=2; 37.5±ND | G2: AP | ||||
| G3: n=11; 32.45±ND | G3: Healthy | ||||
| - 91 upregulated miRNAs (highlighted: miR-451 [OR, 2.63], miR-223 [OR, 2.53], miR-486-5p [OR, 2.46], miR-3917 [OR, 2.08]) | Massive sequencing: 1,349 miRNAs | Gingival biopsy | 198 | Stoecklin-Wasmer et al. [34], cross-sectional | |
| - 68 downregulated miRNAs (highlighted: miR-1246 [OR, 0.33], miR-1260 [OR, 0.44], miR-141 [OR, 0.46], miR-1260b [OR, 0.46], miR-203 [OR, 0.46], miR-210 [OR, 0.47], miR-205 [OR, 0.49]) | 44.5±ND (overall mean age of all participants) | ||||
| G1: n=158; ND | G1: CP + tissue with CP | ||||
| G2: n=40; ND | G2: CP + healthy tissue | ||||
| - No significant differences in miR-146a expression levels between groups | miR-146a, miR-196a2 | Blood sample | 370 | Venugopal et al. [38], cross-sectional | |
| - Downregulation of miR-196a2 in G1 (OR, 0.23) compared to G2 | G1: n=190; 38.16±8.4 | G1: CP | |||
| G2: n=180; 29.64±5.5 | G2: Healthy | ||||
| - Upregulation of miR-125a (OR, 2.07) and miR-499 (OR, 1.54) in G1 compared to G2 | miR-125a, miR-499a | Blood sample | 550 | Venugopal et al. [17], cross-sectional | |
| G1: n=262; ND | G1: CP | ||||
| G2: n=288; ND | G2: Healthy | ||||
| - Upregulation of miR-21 and let-7a in G1 (OR, 2) | miR-125b, miR-21, miR-100, let-7a | Gingival biopsy | 200 | Venugopal et al. [35], cross-sectional | |
| - Upregulation of miR-100 in G1 (OR, 1.6) | G1: n=100; 48.4±11.6 | G1: CP | |||
| - No significant differences in miR-125b expression levels between groups | G2: n=100; 40.4±8.5 | G2: Healthy | |||
| - Upregulation of 96 miRNAs (highlighted: miR-126, miR-20a, miR-190, miR-32, miR-362-3p; OR, 5–10; OR, 5–10) and downregulation of 34 miRNAs (highlighted: miR-155, miR-205; OR, 2–5) in G1 compared to G2 | Massive sequencing: 1,769 miRNAs | Gingival biopsy | 20 | Xie et al. [36], cross-sectional | |
| - Upregulation of miR-146 in G1 (OR, 2) | G1: n=10; 40.6±ND | G1: CP | |||
| G2: n=10; 36.5±ND | G2: Healthy | ||||
| - Upregulation of miR-146: G1 > G2 > G3 | miR-146a | Subgingival biofilm | 90 | Yagnik et al. [23], cross-sectional | |
| - G1: 2-fold increase compared to G3 | G1: n=30; 53.07±7.72 | G1: CP + CHD | |||
| - Positive correlation with BMI, periodontal and cardiac parameters | G2: n=30; 52.27±7.13 | G2: CP without CHD | |||
| G3: n=30; 51.10±7.90 | G3: Healthy | ||||
| - Upregulation of miR-555 (OR, 1.85), miR-130a-5p (OR, 1.71), miR-664a-3p (OR, 1.54), miR-501-5p (OR, 1.57), miR-6770-5p (OR, 0.65), miR-4717-5p (OR, 0.64), miR-21-3p (OR, 0.63) in G1 compared to G2 | Massive sequencing: 2,565 miRNAs | Blood sample | 60 | Yoneda et al. [37], cross-sectional | |
| G1: n=30; 67.0±11.7 | G1: CP | ||||
| G2: n=30; 65.0±13.2 | G2: Healthy |
OR: odds ratio, miRNA: microRNA, SD: standard deviation, G: group, CHD: coronary heart disease, CP: chronic periodontitis, ND: no data, PD: probing depth, PI: peri-implantitis, PROINF CYT: pro-inflammatory cytokines, NSPT: nonsurgical periodontal therapy, DM2: type 2 diabetes mellitus, AP: aggressive periodontitis, BMI: body mass index.
Based on the included studies, most of the evaluated miRNAs were upregulated in periodontally compromised patients [16-337], and miR-146a was the miRNA most-frequently analysed by microarray and reverse-transcriptase polymerase chain reaction (RT-PCR) [21,22,23,25,26,30,36,38]. There appeared to be a positive correlation between miR-146a levels and disease severity as quantified in terms of probing depth, clinical attachment level, and bleeding on probing [23,25,30]. However, the odds ratios (ORs) differed greatly among studies, ranging from 1.43 [22] to 32.6 [30]. After miR-146 [21,22,23,25,26,30,36,38], the next most-frequently investigated miRNAs were miR-142-3p [20,24,36], miR-223 [16,29,33,34], miR-155 [21,36], miR-205 [33,34,36], miR-21 [35,37], let-7a [27,35], miR-200b [16,18], and miR-499 [17,26]. Several studies reported contradictory results for miRNA expression levels; for example, miR-155 was upregulated in the study of Radović et al. [21], but downregulated in that of Xie et al. [36].
The lack of homogeneity between the reports from the different authors prevented a meta-analysis of the data. In addition, some articles included ORs or fold-changes, whereas others solely stated that there were no significant differences between groups without referencing the use of any measure to assess the miRNA expression profiles. For this reason, it was not possible to harmonize the results of the different studies into a single measure or graph.
Assessment of the quality of the included studies
We performed a qualitative analysis of the included studies (Table 2). Since all the studies were observational, the NOS was used. Overall, most studies received ≥7 stars, indicating a low risk of bias (high-quality studies).
Table 2. Quality of included studies.
| Study | Selection | Comparability | Exposure | Total score/risk of bias | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bagavad Gita et al. [22] | * | * | * | * | * | * | * | 7/Low | ||
| Chen et al. [24] | * | * | * | * | * | * | * | 7/Low | ||
| Ghotloo et al. [25] | * | * | * | * | * | * | * | 7/Low | ||
| Kadkhodazadeh et al. [26] | * | * | * | * | * | * | * | * | 8/Low | |
| Kalea et al. [18] | * | * | * | * | * | * | * | * | * | 9/Low |
| Lee et al. [27] | * | * | * | * | * | * | 6/High | |||
| Li et al. [28] | * | * | * | * | * | * | * | * | * | 9/Low |
| Micó-Martínez et al. [29] | * | * | * | * | * | * | * | * | * | 9/Low |
| Motedayyen et al. [30] | * | * | * | * | * | * | * | * | * | 9/Low |
| Na et al. [31] | * | * | * | * | * | * | * | 7/Low | ||
| Naqvi et al. [19] | * | * | * | * | * | * | * | * | * | 9/Low |
| Nisha et al. [32] | * | * | * | * | * | * | * | * | * | 9/Low |
| Ogata et al. [16] | * | * | * | * | * | * | * | 7/Low | ||
| Perri et al. [20] | * | * | * | * | * | * | * | * | * | 9/Low |
| Radović et al. [21] | * | * | * | * | * | * | * | * | * | 9/Low |
| Saito et al. [33] | * | * | * | * | * | * | * | 7/Low | ||
| Stoecklin-Wasmer et al. [34] | * | * | * | * | * | * | * | * | * | 9/Low |
| Venugopal et al. [35] | * | * | * | * | * | * | * | * | 8/Low | |
| Venugopal et al. [17] | * | * | * | * | * | * | * | * | 8/Low | |
| Venugopal et al. [36] | * | * | * | * | * | * | * | * | 8/Low | |
| Xie et al. [37] | * | * | * | * | * | * | * | * | * | 9/Low |
| Yagnik et al. [23] | * | * | * | * | * | * | * | * | 8/Low | |
| Yoneda et al. [38] | * | * | * | * | * | * | * | * | 8/Low | |
DISCUSSION
miRNAs play significant roles in various immune processes, and affect both the innate and humoral responses of the host against the bacterial challenges associated with periodontal disease. The current qualitative systematic review examined the relationship in humans between differentially expressed miRNAs and periodontal disease, and aimed to determine the potential value of these miRNAs as diagnostic or prognostic periodontal biomarkers.
We were not able to conduct a meta-analysis due to the methodological differences among studies. For example, samples were obtained from different biological sources, sample sizes differed greatly among the studies, and some authors focused on specific miRNAs. In contrast, others performed large-scale sequencing using various technologies (such as microarray hybridization, quantitative RT-PCR, and next-generation sequencing), and analysed up to 2,565 different miRNAs (Yoneda et al. [37]). Furthermore, 6 studies [18,19,20,21,22,23] evaluated the impact of systemic diseases such as obesity, diabetes, and coronary heart disease on miRNA expression levels in periodontal patients.
One of the most-researched miRNAs in periodontal diseases was miR-146a [21,22,23,25,26,30,36,38], which is located in the second exon of the LOC285628 gene on human chromosome 5 [36] and belongs to the miR-146 family, along with miR-146b. Despite significant structural similarities between miR-146a and miR-146b, they do not have comparable biological functions. miR-146a serves as a key negative regulator of the innate immune system. Bacterial components of plaque, particularly lipopolysaccharide, stimulate Toll-like receptors (especially TLR-2 and TLR-4), which leads to upregulation in monocytes of miRNAs such as miR-155, miR-21, and miR-146a [39]. Most of the included studies reported that miR-146a expression levels were higher in patients with periodontitis compared to healthy controls (OR, 1.43 [22] to 32.6 [30]) [21,22,23,25,26,30,36]. There also appeared to be a positive correlation between the miR-146a level and disease severity, as assessed in terms of probing depth, clinical attachment level, and bleeding on probing [23,25,30].
However, 1 study did not find any significant association between miR-146a and chronic periodontitis [38]. Venugopal et al. [38] assessed miR-146a single nucleotide polymorphisms (SNPs), which are genetic variants of miRNA that can alter the biogenesis, binding affinity, and specificity to target mRNAs. Meanwhile, Kadkhodazadeh et al., [26] who also evaluated miR-146a SNPs, reported a positive correlation between miR-146a gene polymorphisms and periodontitis and peri-implantitis. Venugopal et al. [38] believed that this discrepancy might have been due to differences in environmental and participant lifestyle factors.
To understand the beneficial (or detrimental) effects of each miRNA in periodontal inflammation, most of the included studies performed target gene predictions of significantly expressed upregulated or downregulated miRNAs using various bioinformatics tools and databases, such as TargetScan, miRDB, microRNA.org, PicTar, etc. For instance, several studies analysed the role of miR-146a as a negative feedback regulator of inflammation in periodontitis [25]. Elevated miR-146a levels are reported to suppress the expression of the IL-1 receptor-associated kinase 1 and tumour necrosis factor receptor-associated factor 6 target genes, and thus inhibit nuclear factor-kappa B activation, which is the transcription factor most heavily implicated in the production of many pro-inflammatory cytokines, such as tumour necrosis factor-alpha, IL-1β, IL-6, and IL-8, chemokines, adhesion molecules, and prostaglandins [39]. Several studies demonstrated that overexpression of miR-146a was accompanied by a reduction in the levels of these pro-inflammatory cytokines [25,30]. However, these results do not agree with those obtained by Bagavad et al., [22] who observed upregulation of both miR-146a and associated cytokines.
Furthermore, no investigations that screened for multiple candidate periodontitis miRNAs simultaneously (massive sequencing) [16,19,20,27,31,32,33,34,36,37] cited miR-146a as the most highly expressed miRNA. For example, Lee et al. [27] highlighted upregulation of let-7a (OR, 9.48), Xie et al. [36] reported upregulation of miR-126, miR-20a, miR-190, miR-32, and miR-362-3p (OR, 5–10) and downregulation of miR-155 and miR-205 (OR, 2–5), Ogata et al. [16] showed upregulation of miR-150, miR-223, and miR-200b (OR, 2.72), and Nisha et al. [32] reported upregulation of miR-143-3p (OR, 5.82). This variability among studies could be due to differences in the profiling techniques and sample media used, and reflects the complex nature of a multifactorial disease such as periodontitis, in which different regulatory networks intervene between miRNAs and periodontal inflammation-related genes.
Periodontopathogens in intimate contact with an inflamed and ulcerated crevice or pocket epithelium may gain entry to the bloodstream. The resultant bacteraemia and associated endotoxaemia in patients with untreated periodontitis could initiate the overproduction of destructive inflammatory mediators at distant sites. Therefore, periodontitis patients may be at increased risk of developing a number of systemic conditions associated with similar overactive host responses to external stimuli, such as coronary heart disease, obesity, and diabetes [20,21,22,23]. There is substantial evidence of the presence of gram-negative periodontal pathogens in atheromatous plaques [22]. These systemic conditions can also alter the host susceptibility to microbial agents, thus exacerbating periodontal destruction. This was reported by Perri et al., [20] who observed higher levels of miR-106b in chronic periodontitis patients with obesity (OR, 6.4) than in those without obesity (OR, 4.9).
Radović et al. [21] compared the expression levels of miR-146a and miR-155 in gingival crevicular fluid before and after nonsurgical periodontal treatment in periodontitis patients with and without type 2 diabetes. They observed significantly higher levels of these miRNAs before treatment compared to periodontally healthy controls; moreover, nonsurgical periodontal therapy significantly reduced the expression of both of these miRNAs [21].
In a recent review on miRNA expression in periodontal and peri-implant diseases, which included animal and human studies, miR-142-3p, miR-155, and miR-146a were cited as potential diagnostic biomarkers for periodontal disease activity. Furthermore, in peri-implantitis studies, most miRNAs were downregulated, except for miR-145, which was significantly upregulated [40].
Despite their promising indications, stability, and straightforward testability, the use of miRNAs as biomarkers for monitoring and early diagnosis of periodontitis has not yet been incorporated into routine clinical practice. This is mainly due to the heterogeneity of existing studies and the lack of diagnostic tests to evaluate the sensitivity and specificity of these miRNAs. Further investigations using standardised sample collection protocols, miRNA sources (saliva, gingival crevicular fluid, etc.) and detection methods are needed to identify specific miRNAs for periodontal diseases with expression levels varying according to disease progression or the response to treatment.
Footnotes
- Conceptualization: Francisco Alpiste-Illueca.
- Data curation: Pablo Micó-Martínez, Pedro J. Almiñana-Pastor.
- Formal analysis: Pablo Micó-Martínez, Andrés López-Roldán.
- Investigation: Pablo Micó-Martínez, Andrés López-Roldán.
- Methodology: Pedro J. Almiñana-Pastor, Francisco Alpiste-Illueca, Andrés López-Roldán.
- Supervision: Francisco Alpiste-Illueca, Andrés López-Roldán.
- Writing - original draft: Pablo Micó-Martínez.
- Writing - review & editing: Francisco Alpiste-Illueca.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Chapple ILC, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 2.Preshaw PM, Seymour RA, Heasman PA. Current concepts in periodontal pathogenesis. Dent Update. 2004;31:570–572. 574–578. doi: 10.12968/denu.2004.31.10.570. [DOI] [PubMed] [Google Scholar]
- 3.Van Dyke TE. Commentary: periodontitis is characterized by an immuno-inflammatory host-mediated destruction of bone and connective tissues that support the teeth. J Periodontol. 2014;85:509–511. doi: 10.1902/jop.2014.130701. [DOI] [PubMed] [Google Scholar]
- 4.Huang W, He BY, Shao J, Jia XW, Yuan YD. Interleukin-1β rs1143627 polymorphism with susceptibility to periodontal disease. Oncotarget. 2017;8:31406–31414. doi: 10.18632/oncotarget.15612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodenhiser D, Mann M. Epigenetics and human disease: translating basic biology into clinical applications. CMAJ. 2006;174:341–348. doi: 10.1503/cmaj.050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behm-Ansmant I, Rehwinkel J, Izaurralde E. MicroRNAs silence gene expression by repressing protein expression and/or by promoting mRNA decay. Cold Spring Harb Symp Quant Biol. 2006;71:523–530. doi: 10.1101/sqb.2006.71.013. [DOI] [PubMed] [Google Scholar]
- 7.Almiñana-Pastor PJ, Boronat-Catalá M, Micó-Martinez P, Bellot-Arcís C, López-Roldán A, Alpiste-Illueca FM. Epigenetics and periodontics: a systematic review. Med Oral Patol Oral Cir Bucal. 2019;24:e659–72. doi: 10.4317/medoral.23008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai R, Ahmed SA. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl Res. 2011;157:163–179. doi: 10.1016/j.trsl.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. doi: 10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palanisamy V, Jakymiw A, Van Tubergen EA, D'Silva NJ, Kirkwood KL. Control of cytokine mRNA expression by RNA-binding proteins and microRNAs. J Dent Res. 2012;91:651–658. doi: 10.1177/0022034512437372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016;1:15004. doi: 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melak T, Baynes HW. Circulating microRNAs as possible biomarkers for coronary artery disease: a narrative review. EJIFCC. 2019;30:179–194. [PMC free article] [PubMed] [Google Scholar]
- 13.Poy MN, Spranger M, Stoffel M. microRNAs and the regulation of glucose and lipid metabolism. Diabetes Obes Metab. 2007;9(Suppl 2):67–73. doi: 10.1111/j.1463-1326.2007.00775.x. [DOI] [PubMed] [Google Scholar]
- 14.Keller A, Leidinger P, Bauer A, Elsharawy A, Haas J, Backes C, et al. Toward the blood-borne miRNome of human diseases. Nat Methods. 2011;8:841–843. doi: 10.1038/nmeth.1682. [DOI] [PubMed] [Google Scholar]
- 15.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Ogata Y, Matsui S, Kato A, Zhou L, Nakayama Y, Takai H. MicroRNA expression in inflamed and noninflamed gingival tissues from Japanese patients. J Oral Sci. 2014;56:253–260. doi: 10.2334/josnusd.56.253. [DOI] [PubMed] [Google Scholar]
- 17.Venugopal P, Lavu V, Rao SR, Venkatesan V. Association of microRNA-125a and microRNA-499a polymorphisms in chronic periodontitis in a sample south Indian population: a hospital-based genetic association study. Gene. 2017;631:10–15. doi: 10.1016/j.gene.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 18.Kalea AZ, Hoteit R, Suvan J, Lovering RC, Palmen J, Cooper JA, et al. Upregulation of gingival tissue miR-200b in obese periodontitis subjects. J Dent Res. 2015;94:59S–69S. doi: 10.1177/0022034514568197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naqvi AR, Brambila MF, Martínez G, Chapa G, Nares S. Dysregulation of human miRNAs and increased prevalence of HHV miRNAs in obese periodontitis subjects. J Clin Periodontol. 2019;46:51–61. doi: 10.1111/jcpe.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perri R, Nares S, Zhang S, Barros SP, Offenbacher S. MicroRNA modulation in obesity and periodontitis. J Dent Res. 2012;91:33–38. doi: 10.1177/0022034511425045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radović N, Nikolić Jakoba N, Petrović N, Milosavljević A, Brković B, Roganović J. MicroRNA-146a and microRNA-155 as novel crevicular fluid biomarkers for periodontitis in non-diabetic and type 2 diabetic patients. J Clin Periodontol. 2018;45:663–671. doi: 10.1111/jcpe.12888. [DOI] [PubMed] [Google Scholar]
- 22.Bagavad Gita J, George AV, Pavithra N, Chandrasekaran SC, Latchumanadhas K, Gnanamani A. Dysregulation of miR-146a by periodontal pathogens: A risk for acute coronary syndrome. J Periodontol. 2019;90:756–765. doi: 10.1002/JPER.18-0466. [DOI] [PubMed] [Google Scholar]
- 23.Yagnik K, Mahendra J, Kurian VM. The periodontal-cardiovascular alliance: evaluation of miRNA-146a in subgingival plaque samples of chronic periodontitis patients with and without coronary heart disease. J Investig Clin Dent. 2019;10:e12442. doi: 10.1111/jicd.12442. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Lan Z, Li Q, Li Y. Abnormal expression of long noncoding RNA FGD5-AS1 affects the development of periodontitis through regulating miR-142-3p/SOCS6/NF-κB pathway. Artif Cells Nanomed Biotechnol. 2019;47:2098–2106. doi: 10.1080/21691401.2019.1620256. [DOI] [PubMed] [Google Scholar]
- 25.Ghotloo S, Motedayyen H, Amani D, Saffari M, Sattari M. Assessment of microRNA-146a in generalized aggressive periodontitis and its association with disease severity. J Periodontal Res. 2019;54:27–32. doi: 10.1111/jre.12538. [DOI] [PubMed] [Google Scholar]
- 26.Kadkhodazadeh M, Jafari AR, Amid R, Ebadian AR, Alipour MM, Mollaverdi F, et al. MiR146a and MiR499 gene polymorphisms in Iranian periodontitis and peri-implantitis patients. J Long Term Eff Med Implants. 2013;23:9–16. doi: 10.1615/jlongtermeffmedimplants.2013007073. [DOI] [PubMed] [Google Scholar]
- 27.Lee YH, Na HS, Jeong SY, Jeong SH, Park HR, Chung J. Comparison of inflammatory microRNA expression in healthy and periodontitis tissues. Biocell. 2011;35:43–49. [PubMed] [Google Scholar]
- 28.Li J, Wang R, Ge Y, Chen D, Wu B, Fang F. Assessment of microRNA-144-5p and its putative targets in inflamed gingiva from chronic periodontitis patients. J Periodontal Res. 2019;54:266–277. doi: 10.1111/jre.12627. [DOI] [PubMed] [Google Scholar]
- 29.Micó-Martínez P, García-Giménez JL, Seco-Cervera M, López-Roldán A, Almiñana-Pastor PJ, Alpiste-Illueca F, et al. miR-1226 detection in GCF as potential biomarker of chronic periodontitis: a pilot study. Med Oral Patol Oral Cir Bucal. 2018;23:e308–14. doi: 10.4317/medoral.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motedayyen H, Ghotloo S, Saffari M, Sattari M, Amid R. Evaluation of microRNA-146a and its targets in gingival tissues of patients with chronic periodontitis. J Periodontol. 2015;86:1380–1385. doi: 10.1902/jop.2015.150319. [DOI] [PubMed] [Google Scholar]
- 31.Na HS, Park MH, Song YR, Kim S, Kim HJ, Lee JY, et al. Elevated microrna-128 in periodontitis mitigates tumor necrosis factor-α response via p38 signaling pathway in macrophages. J Periodontol. 2016;87:e173–82. doi: 10.1902/jop.2016.160033. [DOI] [PubMed] [Google Scholar]
- 32.Nisha KJ, Janam P, Harshakumar K. Identification of a novel salivary biomarker miR-143-3p for periodontal diagnosis: a proof of concept study. J Periodontol. 2019;90:1149–1159. doi: 10.1002/JPER.18-0729. [DOI] [PubMed] [Google Scholar]
- 33.Saito A, Horie M, Ejiri K, Aoki A, Katagiri S, Maekawa S, et al. MicroRNA profiling in gingival crevicular fluid of periodontitis-a pilot study. FEBS Open Bio. 2017;7:981–994. doi: 10.1002/2211-5463.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoecklin-Wasmer C, Guarnieri P, Celenti R, Demmer RT, Kebschull M, Papapanou PN. MicroRNAs and their target genes in gingival tissues. J Dent Res. 2012;91:934–940. doi: 10.1177/0022034512456551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venugopal P, Koshy T, Lavu V, Ranga Rao S, Ramasamy S, Hariharan S, et al. Differential expression of microRNAs let-7a, miR-125b, miR-100, and miR-21 and interaction with NF-kB pathway genes in periodontitis pathogenesis. J Cell Physiol. 2018;233:5877–5884. doi: 10.1002/jcp.26391. [DOI] [PubMed] [Google Scholar]
- 36.Xie YF, Shu R, Jiang SY, Liu DL, Zhang XL. Comparison of microRNA profiles of human periodontal diseased and healthy gingival tissues. Int J Oral Sci. 2011;3:125–134. doi: 10.4248/IJOS11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoneda T, Tomofuji T, Ekuni D, Azuma T, Maruyama T, Fujimori K, et al. Serum microRNAs and chronic periodontitis: a case-control study. Arch Oral Biol. 2019;101:57–63. doi: 10.1016/j.archoralbio.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Venugopal P, Lavu V, RangaRao S, Venkatesan V. Evaluation of a panel of single-nucleotide polymorphisms in miR-146a and miR-196a2 genomic regions in patients with chronic periodontitis. Genet Test Mol Biomarkers. 2017;21:228–235. doi: 10.1089/gtmb.2016.0358. [DOI] [PubMed] [Google Scholar]
- 39.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asa'ad F, Garaicoa-Pazmiño C, Dahlin C, Larsson L. Expression of microRNAs in periodontal and peri-implant diseases: a systematic review and meta-analysis. Int J Mol Sci. 2020;21:4147. doi: 10.3390/ijms21114147. [DOI] [PMC free article] [PubMed] [Google Scholar]