Abstract
Mosquito vectors in the genera Anopheles, Aedes, and Culex transmit a variety of medically important pathogens. Current vector control tools are reaching the limits of their effectiveness, necessitating the introduction of innovative vector control technologies. RNAi, which facilitates functional characterization of mosquito genes in the laboratory, could one day be applied as a new method of vector control. Recent advances in the oral administration of microbial-based systems for delivery of species-specific interfering RNA pesticides to mosquitoes may facilitate translation of this technology to the field. Oral RNAi-based pesticides represent a new class of biorational pesticides that could combat increased global incidence of insecticide resistance and which could one day become critical components of integrated human disease vector mosquito control programs.
Introduction:
The mosquito genome projects facilitated research in new facets of mosquito biology, including functional genetic studies in medically important Aedes (dengue, Zika, chikungunya, and yellow fever vector), Culex (West Nile and lymphatic filariasis vector), and Anopheles (malaria vector) disease vector mosquitoes. Genetic advancements in mosquitoes have made the potential for using gene-centered vector control strategies a reality, challenging researchers to identify potential gene targets for vector control, as well as reliable methods for manipulating mosquito gene function in the laboratory that could one day be extended to the field [1]. RNAi, initially employed in Caenorhabditis elegans, in which microbial systems for oral delivery of interfering RNA were pioneered, is a cellular mechanism that prevents expression of mRNA transcripts when triggered by interfering RNAs such as double-stranded RNA (dsRNA), small interfering RNA (siRNA), and short hairpin RNA (shRNA) [2, 3]. RNAi has revolutionized the field of functional genomics by permitting the interrogation and characterization of candidate genes in a wide variety of insects, including mosquitoes [4]. Silencing efficiency, however, is variable and dependent on a number of parameters including the: (i) interfering RNA species (dsRNA, siRNA or shRNA) administered and its molecular stability, (ii) delivery system, which impacts upon biochemical processing downstream of the anatomical site of entry [5], and (iii) cellular physiology of the arthropod [6]. These factors, either singularly or combined, have historically hampered development of RNAi as a mosquito control technology.
This review focuses on recent advancements in oral delivery of interfering RNA to medically important mosquitoes in the context of its value to fundamental biological research, potential as a biorational vector control intervention, and the commercial and regulatory challenges that must be resolved while developing such a product for the global market.
RNAi-mediated analysis of mosquito gene function in the post-CRISPR-Cas9 era:
RNAi has played a key role in functional genomics, a cross-disciplinary field that has expanded our knowledge of insect evolutionary, developmental, physiological, and molecular biology at a remarkable pace. Although RNAi does not generate heritable germline mutations, an advantage of CRISPR-Cas9 gene editing strategies that have been successfully adapted in mosquitoes [7-10], it offers several advantages that may be of utility. First, RNAi is a conditional method of gene silencing that requires no long-term maintenance of genetically modified mosquito strains. The conditional nature of RNAi also allows researchers to control the stage at which gene silencing initiates, circumventing challenges such as developmental lethality or sterility which can hinder the production and maintenance of strains bearing heritable mutations. Moreover, even with significant CRISPR-Cas9 advancements, genetic engineering of non-model insects is still a relatively labor-intensive and expensive process. Thus, despite concerns for off-site targeting and variations in the level of silencing obtained, which can be dependent on the gene targeted and the tissue in which it is silenced, RNAi is often employed for functional genetics studies in mosquitoes and other insects [1]. Although a majority of laboratories use long dsRNA (~300-400 bp) molecules in their studies, the use of siRNAs and shRNAs enables confirmation of phenotypes using multiple interfering RNAs that target different 21-25 bp sites in a single gene [11], which can alleviate concerns that the phenotype under analysis has arisen from off-site targeting. As discussed below, the use of siRNAs/shRNAs also permits the targeting of short target nucleotide sequences that are conserved in multiple insect species, facilitating comparative studies of gene function [12, 13].
Orally-mediated delivery of interfering RNA:
Microinjection is widely used for delivery of interfering RNA to mosquito embryos, larvae, pupae, and adults [14, 15]. Despite the widespread use of microinjection, this technique is labor-intensive and requires both technical skill and a microinjection setup [16]. The stress of microinjection to the organism can complicate phenotype characterization, particularly for analysis of behaviors, and this technique cannot be extended to the field [16]. To address these concerns, a variety of oral delivery strategies, the subject of this review, have been successfully employed in mosquitoes [4]. These include: direct ingestion [17, 18], larval soaking (that most likely occurs through ingestion) [19-23], nanoparticle-mediated uptake with chitosan or other nanoparticles [15, 16, 24-26], and microbially-expressed vector systems such as bacteria and yeast [1, 12, 13, 20-22, 27]. Recent studies have focused on oral RNAi-mediated inhibition of developmental transcripts that cause defects in the development of mosquito tissues of vector importance or that interfere with reproductive traits. For example, targeting semaphorin-1a results in severe olfactory [28] and visual system [29] defects. Silencing of fasciculation and elongation protein zeta 2, leukocyte receptor cluster, suppressor of actin, offtrack, synaptotagmin, and semaphorin-1a in the larval brain correlate with high levels of mosquito larval mortality [12, 13, 21, 22]. Oral RNAi-mediated silencing of doublesex disrupts fitness and lifespan of Aedes aegypti [15] and Anopheles gambiae [27] female mosquitoes. By functionally characterizing these and other genes that are essential for mosquito survival and reproduction, researchers are identifying potential genetic targets for vector control, as well as potential means of sex-sorting mosquitoes for large-scale male release programs.
In addition to discovery of target genes that can be manipulated for vector control, the identification of effective oral interfering RNA delivery systems that function well in the laboratory and which might translate to the field is critical. Incongruencies exist in the level of gene silencing that is generated by oral RNAi techniques, with variation of RNAi inefficiency likely resulting from the molecular conformation of RNA, its stability during and after cellular uptake, and transportation to the target site [5, 6, 18, 30-32]. Several recent efforts have been directed toward improvement of chitosan-based strategies and exploration of other nanoparticle interfering RNA delivery systems in mosquitoes and other insects [33-36]. Further work in this area will permit identification of the most efficient nanoparticle-based systems for RNA delivery to mosquitoes. However, the present costs of RNA synthesis are a significant factor for soaking and nanoparticle-based delivery systems, particularly when considering large-scale laboratory and field applications.
The use of microbial interfering RNA expression and delivery systems, which facilitate cost-effective RNA propagation, has attracted attention in recent years. Kumar et al. [37] explored the use of transgenic Chlamydomonas reinhardtii for oral delivery of larvicidal dsRNA to Anopheles stephensi. Although this technique shows promise, the pursuit of regulatory permits for the release of live genetic model organisms, and in some geographical contexts, invasive species, could impact translation of algal delivery systems to the field. Comparisons of the relative efficacy of orally-based delivery mechanisms in A. aegypti and A. gambiae larvae suggest that bacterial and yeast-based strategies for delivery of larvicidal interfering RNA to mosquitoes generate higher degrees of target gene silencing and larval mortality than do other delivery systems [21, 22]. Furthermore, these microbes can be heat-killed with no resulting loss of larvicide activity [21, 38], a likely advantage for field applications, as no live genetically modified organisms would be released into the environment.
Escherichia coli strains that were engineered to produce both long and short dsRNA molecules targeting mosquito genes of interest have facilitated gene silencing in A. aegypti [20, 21, 39] and A. gambiae [22, 27] larvae. Pichia pastoris (yeast) expressing long hairpin RNA (lhRNA) was used to target juvenile hormone acid methyl transferase in A. aegypti larvae [40]. More recently, shRNAs targeting mosquito genes that are required for larval viability were expressed in Saccharomyces cerevisiae (baker’s yeast), in which shRNA was either expressed from plasmids or expression cassettes that had been stably integrated into the yeast genome [1, 11-13, 21, 22]. Following culturing, the S. cerevisiae strains were prepared as dried tablets that were fed to larvae, resulting in silencing of developmental transcripts and high mortality rates (>90%) in multiple mosquito larval species.
The success of microbial-based interfering RNA delivery strategies in mosquitoes suggests that microbe-interfering RNA complexes survive the mosquito gastrointestinal environment. The flour beetle Tribolium castaneum is classed as RNAi-efficient because it processes ingested dsRNA into siRNA via clathrin-dependent endocytosis [41], thus avoiding degradation by gut lumen dsRNases and trapping by cytoplasmic endosomes. StaufenC was recently identified as an obligatory dsRNA binding protein for RNAi initiation in Coleoptera, as well as a potential target for development of RNAi resistance [42]. Although endocytosis is likely the mechanism for microbial-based interfering RNA uptake in mosquitoes, this has not yet been confirmed, and StaufenC has not been identified in mosquitoes, in which RNAi resistance has not yet been reported, but is nevertheless a concern. The mosquito RNAi research agenda should therefore focus on elucidation of the cellular machinery that permits interfering RNA and microbe-interfering RNA transport, how these species are processed and degraded, whether the mechanisms differ between delivery methods, and assessment of the potential for developing resistance to RNAi when utilizing different delivery strategies.
Orally-induced RNAi in vector control applications:
Insecticide resistance is a primary obstacle to global vector control operations, and the development of new classes of environmentally safe pesticides with no cross-resistance to current insecticides is critical [43]. RNAi-based pesticides, promising candidates that satisfy these requirements, have an overwhelmingly good safety profile, particularly with respect to existing chemical pesticides [44]. While recent studies demonstrated a reversal of insecticide resistance by soaking larvae in dsRNAs that targeted genes known to contribute to resistance pathways in A. aegypti [45] and An. stephensi [23], most RNAi-based larvicides are designed to directly kill mosquito larvae. High-throughput screens in A. aegypti and A. gambiae have identified hundreds of genes required for larval viability, several of which have been characterized in detail [12, 13, 21, 22]. An arsenal of interfering RNA pesticides could be developed as a new class of species-specific biorational mosquito larvicides. This would permit rotated use of interfering RNA pesticides, combating the potential for development of point mutations in interfering RNA target sites.
Larvicide screens also led to the discovery of a number of interfering RNAs with target sites that are conserved in multiple mosquito species, but which are not found in humans or other non-target organisms [12, 13]. Yeast-mediated delivery of shRNAs targeting conserved sites in the synaptotagmin (syt) [12] and semaphorin-1a [13] genes effectively silenced target gene expression and induced high levels of larval mortality in A. aegypti, Aedes albopictus, A. gambiae, and Culex quinquefasciatus, but was not toxic to non-target arthropods. Yeast, which can be used directly for RNA production, as a delivery system, and as larval bait, is a strong odorant attractant for larvae [28] and gravid A. aegypti females, which are attracted to deposit eggs in treated containers [21]. These advantages, as well as the potential for greater acceptance of yeast vs. bacteria among consumers, make it an attractive delivery system for potential development as a mosquito control mechanism [11].
Novel RNAi-based control strategies can also be directed toward adult mosquitoes, a strategy that requires the identification of effective delivery methods to adults. Although reliable methods for topical applications of interfering RNAs to mosquitoes have not yet been identified, sugar-baited oral delivery systems are of increasing interest. Attractant Toxic Sugar Baits (ATSBs) are a simple concept designed to attract an insect to a baited-sugar source that induces mortality upon ingestion [46]. Current ATSB technologies, which often employ insecticides that are not specific to mosquitoes [46], could potentially be enhanced through the use of species-specific interfering RNA pesticides. Studies with A. aegypti have shown potential for incorporating interfering RNA into sugar solutions [17], and sugar-baited delivery of siRNAs targeting neural genes have induced 100% A. aegypti morbidity in laboratory trials (MDS, unpublished results). In addition to ATSBs, viral RNA expression systems present another potential method of lethal RNAi pesticide delivery (see discussion in [4]). The use of symbiotic microorganisms as a means of delivering lethal interfering RNA is also gaining traction [47], but may face greater regulatory hurdles than the use dead microbial interfering RNA delivery systems. Finally, in addition to killing adult mosquitoes, it may also be possible to use RNAi to induce pathogen resistance in mosquitoes, a concept investigated in two recent studies [48, 49], or to impact behaviors that contribute to the spread of disease, for example human host location and blood feeding behavior (see [4] for detailed discussions).
Conclusions and future directions:
In the two decades since it was identified, RNAi has advanced as a critical research tool with the potential to translate innovative bench technology towards operational vector control strategies (Fig. 1). In addition to proof of concept outdoor semi-field trials, which were successfully conducted for yeast interfering RNA larvicides [12, 13], the development and evaluation of commercial formulations, followed by analysis of the potential for scaled production of these formulations, are critical. Careful consideration must be given to the impacts of scaled production on interfering RNA stability. The stability of the commercial product over time is also crucial, particularly given that vector control operations often require long-term storage and operational use at high temperatures [11]. The cost of production, which can be challenging to estimate for novel technologies, will ultimately drive commercialization decisions, and input from specialists who can accurately estimate costs associated with development and manufacture of RNAi-based products is crucial. Evaluation of stakeholder acceptance, a critical component of the successful development of new vector control strategies, is also essential.
Fig. 1. Oral RNAi-based vector control product commercialization process.
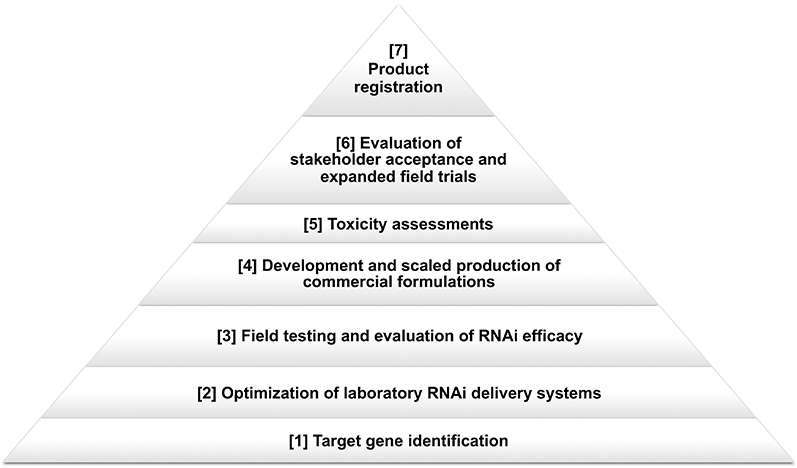
The pathway for commercialization, initiating with target gene discovery and concluding with product registry, is summarized. See text for further discussion.
Following the development and scaled production of commercially-ready pesticide formulations, further toxicology and expanded field testing of these formulations are requisite for registry applications through the United States Environmental Protection Agency (EPA) and comparable entities in other countries. The use of heat-inactivated microbial delivery systems, which retain interfering RNA activity [21, 38], may support these applications. Registry of mosquito interfering RNA insecticides by the EPA, which following extensive satisfactory risk assessment [50] recently approved an RNAi-based pesticide targeting an insect agricultural pest, could increase the likelihood of gaining approval for these technologies at additional sites across the globe. However, some nations lack a regulatory body equivalent to the EPA, which will complicate global deployment of RNAi-based interventions. Although further research and regulatory hurdles remain, RNAi-based pesticides represent a new class of biorational insecticides that could combat increased global incidence of pesticide resistance and which could one day become critical components of integrated mosquito control programs.
Highlights:
Oral RNA interference (RNAi) permits functional characterization of mosquito genes.
Microbial-based oral RNAi methods are highly effective in mosquitoes.
Interfering RNAs are a new class of pesticides that can combat resistance.
Oral RNAi may facilitate species-specific biorational mosquito control.
Further development and evaluation of RNAi control strategies is critical.
Acknowledgments:
We thank members of the Scheel lab for useful discussions. Our RNAi pesticide research has been supported by National Institutes of Health/National Institute of Allergy and Infectious Disease [1 R21 AI128116-01 and 1R21 AI128116-01]; the United States Agency for International Development [AID-OAA-F-16-00097]; the U.S. Department of Defense [W81XWH-17-1-0294]; the Innovative Vector Control Consortium; and the U.S. Department of Defense Deployed Warfighter Protection Program [W911QY-17-1-0002]. The sponsors of this research did not play a role in study design, in the collection, analysis and interpretation of data discussed herein, in the writing of the review, nor the decision to submit the article for publication.
Footnotes
Conflict of interest statement:
RW: Nothing declared. MDS is an inventor on pending Patent Applications PCT/US2017/041919 and PCT/US2019/058232. These applications did not impact her interpretation of the data described in this review.
CRediT author statement:
RW: Conceptualization, Writing-Original draft preparation, reviewing and editing, Figure preparation. MDS: Conceptualization, Writing-Original draft preparation, reviewing and editing, Figure preparation.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- •[1]. Mysore K, Hapairai LK, Wei N, Realey JS, Scheel ND, Severson DW, Duman-Scheel M: Preparation and use of a yeast shRNA delivery system for gene silencing in mosquito larvae. Methods Mol Biol 2019, 1858:213–231. This methods protocol describes a detailed procedure for generation of S. cerevisiae strains that express interfering RNA, yeast culturing, larvicide tablet preparation, mosquito larval feeding assays, and methods for confirming gene silencing. The protocol, which was prepared in an effort to help other laboratories use the yeast interfering RNA delivery procedure to study genes of interest, includes an introduction which describes the benefits and challenges of using RNAi and a methodology troubleshooting section that will assist researchers.
- [2].Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC: Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391:806–811. [DOI] [PubMed] [Google Scholar]
- [3].Timmons L, Court DL, Fire A: Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 2001, 263:103–112. [DOI] [PubMed] [Google Scholar]
- ••[4]. Airs PM, Bartholomay LC: RNA interference for mosquito and mosquito-borne disease control. Insects 2017, 8,4. Readers are referred to this comprehensive review for further discussion of the history, present status, and challenges for development of RNAi strategies for mosquito control, including a more comprehensive discussion of environmental considerations, gene targets, and interfering RNA delivery systems.
- [5].Whitten MM: Novel RNAi delivery systems in the control of medical and veterinary pests. Curr Opin Insect Sci 2019, 34:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kunte N, McGraw E, Bell S, Held D, Avila LA: Prospects, challenges and current status of RNAi through insect feeding. Pest Manag Sci 2020, 76: 26–41. [DOI] [PubMed] [Google Scholar]
- [7].Kistler KE, Vosshall LB, Matthews BJ: Genome engineering with CRISPR-Cas9 in the mosquito Aedes aegypti. Cell Rep 2015, 11:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gantz VM, Jasinskiene N, Tatarenkova O, Fazekas A, Macias VM, Bier E, James AA: Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proceedings of the National Academy of Sciences of the United States of America 2015, 112:E6736–6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hammond A, Galizi R, Kyrou K, Simoni A, Siniscalchi C, Katsanos D, Gribble M, Baker D, Marois E, Russell S et al. : A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nature biotechnology 2016, 34:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••[10]. Li M, Li T, Liu N, Raban R, Wang X, Akbari OS: Methods for the generation of heritable germline mutations in the disease vector Culex quinquefasciatus using CRISPR/Cas9. Insect Mol Biol 2019, doi: 10.1111/imb.12626. This publication, as well as several others cited in this review, describes methods for generating stable, heritable, mutations in mosquitoes through use of CRISPR/Cas9 technology. Although this technology is not the focus of this review, CRISPR/Cas has revolutionized genome editing in mosquitoes and other non-model insects and may be the gene knockdown method of choice depending on the application desired. As discussed in this review, this will depend on available resources and expertise, as well as the gene, biological process, or life cycle stage that is under investigation.
- •[11]. Duman-Scheel M: Saccharomyces cerevisiae (baker's yeast) as an interfering RNA expression and delivery system. Curr Drug Targets 2019, 20:942–952. This review details the benefits and potential applications of S. cerevisiae as an interfering RNA expression and delivery system, together with a more detailed overview of prospects and challenges for its commercialization, including environmental implications, as well as considerations for scaling production and formulation development.
- ••[12]. Mysore K, Li P, Wang CW, Hapairai LK, Scheel ND, Realey JS, Sun L, Roethele JB, Severson DW, Wei N et al. : Characterization of a yeast interfering RNA larvicide with a target site conserved in the synaptotagmin gene of multiple disease vector mosquitoes. PLoS Negl Trop Dis 2019, 13:e0007422. This study presents a biorational mosquito larvicide that could be implemented as a novel control intervention. S. cerevisiae were engineered to express shRNA targeting a conserved site in mosquito synaptotagmin genes that is not found in humans or other non-target organisms. Larval consumption of the yeast resulted in severe neural defects and high rates of mortality in A. aegypti, A. albopictus, A. gambiae, and C. quinquefasciatus larvae, but was not toxic to non-target arthropods. Successful outdoor semi-field trials were conducted on A. aegypti larvae.
- [13].Mysore K, Li P, Wang CW, Hapairai LK, Scheel ND, Realey JS, Sun L, Severson DW, Wei N, Duman-Scheel M: Characterization of a broad-based mosquito yeast interfering RNA larvicide with a conserved target site in mosquito semaphorin-1a genes. Parasit Vectors 2019, 12:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Clemons A, Haugen M, Le C, Mori A, Tomchaney M, Severson DW, Duman-Scheel M: siRNA-mediated gene targeting in Aedes aegypti embryos reveals that frazzled regulates vector mosquito CNS development. PLoS One 2011, 6:e16730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mysore K, Sun L, Tomchaney M, Sullivan G, Adams H, Piscoya AS, Severson DW, Syed Z, Duman-Scheel M: siRNA-mediated silencing of doublesex during female development of the dengue vector mosquito Aedes aegypti. PLoS Negl Trop Dis 2015, 9:e0004213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang X, Mysore K, Flannery E, Michel K, Severson DW, Zhu KY, Duman-Scheel M: Chitosan/interfering RNA nanoparticle mediated gene silencing in disease vector mosquito larvae. J Vis Exp 2015, 97: e52523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Coy MR, Sanscrainte ND, Chalaire KC, Inberg A, Maayan I, Glick E, Paldi N, Becnel JJ: Gene silencing in adult Aedes aegypti mosquitoes through oral delivery of double-stranded RNA. J. Appl. Entomol 2012, 2012:741–748. [Google Scholar]
- •[18]. Singh IK, Singh S, Mogilicherla K, Shukla JN, Palli SR: Comparative analysis of double-stranded RNA degradation and processing in insects. Sci Rep 2017, 7:17059. Microinjection and oral-mediated delivery of dsRNA in 37 insect species from five insect orders (Coleoptera, Diptera, Hemiptera, Lepidoptera, Orthoptera) were compared to assess variance in RNAi efficiency. The data presented suggest that dsRNAse activity and dsRNA processing to siRNAs are significant factors that contribute to differential RNAi efficiency observed among insects.
- [19].Singh AD, Wong S, Ryan CP, Whyard S: Oral delivery of double-stranded RNA in larvae of the yellow fever mosquito, Aedes aegypti: implications for pest mosquito control. J Insect Sci 2013, 13:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Whyard S, Erdelyan CN, Partridge AL, Singh AD, Beebe NW, Capina R: Silencing the buzz: a new approach to population suppression of mosquitoes by feeding larvae double-stranded RNAs. Parasit Vectors 2015, 8:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hapairai LK, Mysore K, Chen Y, Harper EI, Scheel MP, Lesnik AM, Sun L, Severson DW, Wei N, Duman-Scheel M: Lure-and-kill yeast interfering RNA larvicides targeting neural genes in the human disease vector mosquito Aedes aegypti. Sci Rep 2017, 7:13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mysore K, Hapairai LK, Sun L, Harper EI, Chen Y, Eggleson KK, Realey JS, Scheel ND, Severson DW, Wei N et al. : Yeast interfering RNA larvicides targeting neural genes induce high rates of Anopheles larval mortality. Malar J 2017, 16:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Negri A, Ferrari M, Nodari R, Coppa E, Mastrantonio V, Zanzani S, Porretta D, Bandi C, Urbanelli S, Epis S: Gene silencing through RNAi and antisense vivo-morpholino increases the efficacy of pyrethroids on larvae of Anopheles stephensi. Malar J 2019, 18:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ramesh Kumar D, Saravana Kumar P, Gandhi MR, Al-Dhabi NA, Paulraj MG, Ignacimuthu S: Delivery of chitosan/dsRNA nanoparticles for silencing of wing development vestigial (vg) gene in Aedes aegypti mosquitoes. Int J Biol Macromol 2016, 86:89–95. [DOI] [PubMed] [Google Scholar]
- [25].Chen J, Lu HR, Zhang L, Liao CH, Han Q: RNA interference-mediated knockdown of 3, 4-dihydroxyphenylacetaldehyde synthase affects larval development and adult survival in the mosquito Aedes aegypti. Parasit Vectors 2019, 12:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •[26]. Dhandapani RK, Gurusamy D, Howell JL, Palli SR: Development of CS-TPP-dsRNA nanoparticles to enhance RNAi efficiency in the yellow fever mosquito, Aedes aegypti. Sci Rep 2019, 9:8775. Chitosan and sodium tripolyphosphate (CS-TPP) were cross-linked in a dsRNA nanoparticle complex to enhance RNAi efficiency in A. aegypti. Knockdown of expression and improved larval mortality demonstrate that these nanoparticles can effectively deliver dsRNA to their target cells. Although levels of mortality achieved were less than what has been described for microbial delivery systems, this article highlights an important area of research that centers on the improvement of nanoparticle delivery systems for mosquito control.
- •[27]. Taracena ML, Hunt CM, Benedict MQ, Pennington PM, Dotson EM: Downregulation of female doublesex expression by oral-mediated RNA interference reduces number and fitness of Anopheles gambiae adult females. Parasit Vectors 2019, 12:170. Larval expression of the female specific doublesex (dsxF) variant was silenced by oral dsRNA delivery using an E. coli delivery system. The effects were then observed in adults in which a sex-specific knockdown resulted in fewer females that had less progeny; no impacts on males were observed. This technology could be integrated into established control strategies requiring efficient sex separation, and male-only releases.
- [28].Mysore K, Flannery EM, Tomchaney M, Severson DW, Duman-Scheel M: Disruption of Aedes aegypti olfactory system development through chitosan/siRNA nanoparticle targeting of semaphorin-1a. PLoS Negl Trop Dis 2013, 7:e2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mysore K, Flannery E, Leming MT, Tomchaney M, Shi L, Sun L, O'Tousa JE, Severson DW, Duman-Scheel M: Role of semaphorin-1a in the developing visual system of the disease vector mosquito Aedes aegypti. Dev Dyn 2014, 243:1457–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wynant N, Santos D, Vanden Broeck J: Biological mechanisms determining the success of RNA interference in insects. Int Rev Cell Mol Biol 2014, 312:139–167. [DOI] [PubMed] [Google Scholar]
- [31].Joga MR, Zotti MJ, Smagghe G, Christiaens O: RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: what we know so far. Front Physiol 2016, 7:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shukla JN, Kalsi M, Sethi A, Narva KE, Fishilevich E, Singh S, Mogilicherla K, Palli SR: Reduced stability and intracellular transport of dsRNA contribute to poor RNAi response in lepidopteran insects. RNA Biol 2016, 13:656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Das S, Debnath N, Cui Y, Unrine J, Palli SR: Chitosan, carbon quantum dot, and silica nanoparticle mediated dsRNA delivery for gene silencing in Aedes aegypti: A Comparative Analysis. ACS Appl Mater Interfaces 2015, 7:19530–19535. [DOI] [PubMed] [Google Scholar]
- [34].Gillet FX, Garcia RA, Macedo LLP, Albuquerque EVS, Silva MCM, Grossi-de-Sa MF: Investigating engineered ribonucleoprotein particles to improve oral RNAi delivery in crop insect pests. Front Physiol 2017, 8:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lin YH, Huang JH, Liu Y, Belles X, Lee HJ: Oral delivery of dsRNA lipoplexes to German cockroach protects dsRNA from degradation and induces RNAi response. Pest Manag Sci 2017, 73:960–966. [DOI] [PubMed] [Google Scholar]
- [36].Castellanos NL, Smagghe G, Sharma R, Oliveira EE, Christiaens O: Liposome encapsulation and EDTA formulation of dsRNA targeting essential genes increase oral RNAi-caused mortality in the neotropical stink bug Euschistus heros. Pest Manag Sci 2019, 75:537–548. [DOI] [PubMed] [Google Scholar]
- [37].Kumar A WS, Ou R, Samrakandi M, Beerntsen B, Sayre R: Development of an RNAi based microalgal larvicide to control mosquitoes. Malar World J 2013, 4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Whyard S, Singh AD, Wong S: Ingested double-stranded RNAs can act as species-specific insecticides. Insect biochemistry and molecular biology 2009, 39:824–832. [DOI] [PubMed] [Google Scholar]
- [39].Lopez SBG, Guimaraes-Ribeiro V, Rodriguez JVG, Dorand F, Salles TS, Sa-Guimaraes TE, Alvarenga ESL, Melo ACA, Almeida RV, Moreira MF: RNAi-based bioinsecticide for Aedes mosquito control. Sci Rep 2019, 9:4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Van Ekert E, Powell CA, Shatters RG Jr., Borovsky D: Control of larval and egg development in Aedes aegypti with RNA interference against juvenile hormone acid methyl transferase. J Insect Physiol 2014, 70:143–150. [DOI] [PubMed] [Google Scholar]
- [41].Xiao D, Gao X, Xu J, Liang X, Li Q, Yao J, Zhu KY: Clathrin-dependent endocytosis plays a predominant role in cellular uptake of double-stranded RNA in the red flour beetle. Insect biochemistry and molecular biology 2015, 60:68–77. [DOI] [PubMed] [Google Scholar]
- ••[42]. Yoon JS, Mogilicherla K, Gurusamy D, Chen X, Chereddy S, Palli SR: Double-stranded RNA binding protein, Staufen, is required for the initiation of RNAi in coleopteran insects. Proceedings of the National Academy of Sciences of the United States of America 2018, 115:8334–8339. This study reports the discovery of StaufenC, an RNA-binding protein that, to date, has yet to be located in any other insect order. The presence of StaufenC, which facilitates intracellular dsRNA transportation, may contribute to the high levels of RNAi efficiency in coleopterans, and could facilitate development of methodology that might improve RNAi efficiency in mosquitoes and other insects. The study also demonstrates that StaufenC is a potential target for RNAi resistance, a topic that was not highlighted in this review due to space limitations, but which is nevertheless an important area of research and a phenomenon that could impact the ultimate success of RNAi-based mosquito control interventions.
- [43].Hemingway J: The role of vector control in stopping the transmission of malaria: threats and opportunities. Philos Trans R Soc Lond B Biol Sci 2014, 369:20130431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].U.S. Environmental Protection Agency. RNAi technology as a pesticide: problem formulation for human health and ecological risk assessment. 2014; Docket ID: EPA-HQ-OPP-2013-0485. [Google Scholar]
- [45].Bona AC, Chitolina RF, Fermino ML, de Castro Poncio L, Weiss A, Lima JB, Paldi N, Bernardes ES, Henen J, Maori E: Larval application of sodium channel homologous dsRNA restores pyrethroid insecticide susceptibility in a resistant adult mosquito population. Parasit Vectors 2016, 9:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fiorenzano JM, Koehler PG, Xue RD: Attractive toxic sugar bait (ATSB) for control of mosquitoes and its impact on non-target organisms: a review. Int J Environ Res Public Health 2017, 14:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Abrieux A, Chiu JC: Oral delivery of dsRNA by microbes: beyond pest control. Commun Integr Biol 2016, 9:e1236163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kang S, Shields AR, Jupatanakul N, Dimopoulos G: Suppressing dengue-2 infection by chemical inhibition of Aedes aegypti host factors. PLoS Negl Trop Dis 2014, 8:e3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •[49]. Magalhaes T, Bergren NA, Bennett SL, Borland EM, Hartman DA, Lymperopoulos K, Sayre R, Borlee BR, Campbell CL, Foy BD et al. : Induction of RNA interference to block Zika virus replication and transmission in the mosquito Aedes aegypti. Insect biochemistry and molecular biology 2019, 111:103169. This recent study highlights the potential for using RNAi to block Zika viral transmission, an alternative to killing mosquitoes. This investigation lays the requisite groundwork for pursuit of siRNA-mediated arboviral suppression in natural A. aegypti populations.
- [50].Bachman PM, Huizinga KM, Jensen PD, Mueller G, Tan J, Uffman J, Levine SL: Ecological risk assessment for DvSNf7RNA: a plant-incorporated protectant with targeted activity against western corn rootworm. Regul Toxicol Pharmacol 2016, 81:77–88. [DOI] [PubMed] [Google Scholar]


