Abstract
Sour is one of the fundamental taste modalities that enable taste perception in animals. Chemoreceptors embedded in taste organs are pivotal to discriminate between different chemicals to ensure survival. Animals generally prefer slightly acidic food and avoid highly acidic alternatives. We recently proposed that all acids are aversive at high concentrations, a response that is mediated by low pH as well as specific anions in Drosophila melanogaster. Particularly, some carboxylic acids such as glycolic acid, citric acid, and lactic acid are highly attractive to Drosophila compared with acetic acid. The present study determined that attractive carboxylic acids were mediated by broadly expressed Ir25a and Ir76b, as demonstrated by a candidate mutant library screen. The mutant deficits were completely recovered via wild-type cDNA expression in sweet-sensing gustatory receptor neurons. Furthermore, sweet gustatory receptors such as Gr5a, Gr61a, and Gr64a-f modulate attractive responses. These genetic defects were confirmed using binary food choice assays as well as electrophysiology in the labellum. Taken together, our findings demonstrate that at least two different kinds of receptors are required to discriminate attractive carboxylic acids from other acids.
Keywords: citric acid, glycolic acid, gustatory receptor, ionotropic receptor, lactic acid
INTRODUCTION
The sense of taste is crucial for the identification of nutritious foods and avoiding potentially toxic foods. Taste perception is mediated by receptors housed in the taste organs and enables animals to localize and discriminate between potential food items in the environment. Chemical signals from foods are distinguished by specialized receptors, which possess a discrete ligand response profile (Rimal and Lee, 2018). Sour is one of the five basic taste modalities of the gustatory system. Unlike sweet, bitter, and umami, salty and sour perception is bidirectional. In other words, animals prefer low concentrations of salt and sour compounds but also actively avoid high concentrations (Chen and Dahanukar, 2020).
In the fruit fly (Drosophila melanogaster), taste is primarily detected through ionotropic receptors (ion permeable receptors), including gustatory receptors (GRs), ionotropic receptors (IRs), and transient receptor potential (TRP) channels (Rimal and Lee, 2018). Fly GRs are generally required for sensing sweet and bitter compounds (Dahanukar et al., 2007; Weiss et al., 2011). Further, except for nine sweet-sensing GRs, most GRs are considered bitter-sensing GRs (Shrestha and Lee, 2021). These nine sweet-sensing GRs are categorized by the expression of reporters and recently verified functions. Gr5a is expressed in a large number of sweet-sensing gustatory receptor neurons (GRNs) (Marella et al., 2006) and is required for sensing trehalose (Dahanukar et al., 2001). Gr43a has a crucial role in internal fructose sensing in the brain (Miyamoto and Amrein, 2014). In fact, the Gr64a cluster (Gr64a-f) is the most important cluster for the perception of most sugars such as sucrose, maltose, trehalose, and glucose given the role of Gr64f as a sugar co-receptor (Jiao et al., 2008). Furthermore, Gr64e is required for sensing glycerol (Wisotsky et al., 2011) and fatty acids (Kim et al., 2018). However, Gr61a is only a putative sweet-sensing GR based on the reporter expression in sweet-sensing GRNs (Dahanukar et al., 2007; Miyamoto et al., 2013). Fly IRs are required for sensing saltiness and sourness in foods (Chen and Amrein, 2017; Zhang et al., 2013). Additionally, flies use IRs for tasting Ca2+ (Lee et al., 2018). Fly TRPs taste aversive and pungent chemicals such as wasabi and aristolochic acid (Al-Anzi et al., 2006; Kim et al., 2010).
The labellum, the major teste organ in Drosophila, harbors many taste sensilla that are classified based on length as short (S-type), intermediate (I-type), and long (L-type) (Hiroi et al., 2002; Shanbhag et al., 2001). Each taste sensillum comprises an apical pore system, which facilitates the recording of neural activity (Wieczorek and Wolff, 1989). The taste sensillum encompasses 2-4 GRNs. Generally, each L- and S-type sensillum harbors 4 GRNs, whereas I-type sensillum has two GRNs (Dethier, 1976; Falk et al., 1976; Fujishiro et al., 1984; Hiroi et al., 2004; Meunier et al., 2003; Nayak and Singh, 1983). Individual neurons for each taste modality are embodied by a discrete repertoire of receptors, which drive the transduction of stimuli elicited by a particular sensor to a higher brain center. After decoding these messages, the brain determines whether or not the food is safe to eat (Roper and Chaudhari, 2017).
Acid has a sour taste and a pungent smell. Odor sensation related to acids has been identified in Drosophila melanogaster and this process is known to be mediated by IRs. Electrophysiological analyses and calcium imaging have demonstrated that IR31a, IR64a, IR84a, IR75a, IR75b, and IR75c are specifically tuned to acidic molecules, in addition to the broadly tuned co-receptor IR8a (Abuin et al., 2011; Prieto-Godino et al., 2016; 2017; Silbering et al., 2011). However, acid perception in Drosophila remains largely uncharacterized. We recently proposed that IR7a mediates repulsive responses to acetic acid in D. melanogaster (Rimal et al., 2019). Furthermore, many carboxylic acids such as glycolic acid (GA), citric acid (CA), and lactic acid (LA) are very attractive even at extremely low pH (~2.5) (Rimal et al., 2019). In another study, starved flies exhibited appetitive responses to acetic acid (Devineni et al., 2019). Additionally, two broadly expressed IRs, IR25a and IR76b, detect acids during oviposition (Chen and Amrein, 2017).
Here, we conducted screening experiments using loss of function mutants to elucidate the transduction mechanism of attractive acid perception. Further, feeding behavior assays and electrophysiological analyses were conducted to identify candidate taste receptors for carboxylic acids. Our findings demonstrated that two broadly required IRs, IR25a and IR76b, and most sweet-sensing GRs are essential to discriminate attractive sour tastes.
MATERIALS AND METHODS
Fly stocks
Strain w1118 was used as a control strain in this study. All flies were maintained at 25°C under a 12-h light/12-h dark cycle. Both male and female flies were used randomly for the experiments. The following lines were obtained from the Bloomington Drosophila Stock Center (https://bdsc.indiana.edu/): Ir7g1 (BL42420), Ir8a1 (BL41744), Ir10a1 (BL23842), Ir21a1 (BL10975), Ir48a1 (BL26453), Ir48b1 (BL23473), Ir51b1 (BL10046), Ir52b1 (BL25212), Ir56b1 (BL27818), Ir62a1 (BL32713), Ir67a1 (BL56583), Ir75d1 (BL24205), Ir85a1 (BL24590), Ir92a1 (BL23638), Ir94b1 (BL23424), Ir94d1 (BL33132), Ir94f1 (BL33095), Ir94g1 (BL25551), Ir100a1 (BL31853), and UAS-Kir2.1 (BL6596). We previously described the source of the following lines in our previous study: Ir7a1, Ir47a1, Ir52a1, Ir56a1, Ir60b3, Ir94a1, Ir94c1, Ir94h1 (Rimal et al., 2019), UAS-Ir25a (Lee et al., 2018). C. Montell kindly provided strains Ir76b1, Ir76b-GAL4, and UAS-Ir76b. Additionally, J.R. Carlson generously provided strains Gr5a∆5, Gr5a∆5/FM7;UAS-Gr5a/CyO, Gr5a∆5/FM7;Gr5a-GAL4/CyO, Gr61a1, Gr61a-GAL4, and Gr61a1;UAS-Gr61a. K. Scott provided strains ppk23-GAL4 and ppk28-GAL4. Gr43aGAL4, Gr66a-GAL4, Gr5a-GAL4, and ∆Gr64a-f were provided by H. Amrein. Ir20a1 and Gr64f-GAL4 were obtained from A. Dahanukar and L.B. Vosshall provided strains Ir25a-GAL4 and Ir25a2.
Chemical sources
GA (CAS No. 79-14-1), CA (CAS No. 77-92-9), LA (CAS No. 50-21-5), sucrose (CAS No. 57-50-1), tricholine citrate (TCC) (CAS No. 546-63-4), and sulforhodamine B (CAS No. 3520-42-1) were purchased from Sigma-Aldrich (USA). Brilliant blue FCF (CAS No. 3844-45-9, Cat No. 027-12842) was purchased from Wako Pure Chemical Industry (Japan).
Electrophysiology
Electrophysiology (i.e., tip recording assay) was performed as described in a previous study (Lee et al., 2009). First, 4- to 7-day old flies were anesthetized on ice. A reference glass electrode filled with Ringer’s solution was inserted into the thorax of the flies. The electrode was then slowly extended towards the proboscis of the fly. To avoid experimental biases, 5 to 6 live insects were prepared per set-up and the same procedure was repeated for several rounds on different days. For the recordings, the sensilla were stimulated for 5 s with a mixture of chemical stimulant in a 30 mM TCC solution (i.e., electrolyte solution) in recording pipettes (10-20 μm tip diameter) connected to a preamplifier. The recorded signals were collected and amplified 10× using a signal connection interface box (Syntech, Netherlands) in conjunction with a 100-3,000 Hz band-pass filter. Recordings of action potentials were acquired using a 12-kHz sampling rate and analyzed using the Autospike 3.1 software (Syntech). To obtain proper signals, all recordings were conducted at 1 min intervals. The number of action potentials was counted from 50-550 ms after application of the stimulus. Therefore, the numbers of spikes/s in Figures mean that the numbers of spikes during 50-550 ms are divided by 1/2. The dots in each figure indicate the number of insects tested.
Binary food choice assay
Binary food choice assays were conducted as described in a previous study (Lee et al., 2010). Approximately 50-70 flies (3-6 days old; mixed sexes) were starved for 18 h in a humidified chamber. Two different food sources containing 1% agarose were then prepared. One food source contained 2 mM sucrose, whereas the other contained 2 mM sucrose mixed with different concentrations of acid. These food sources were mixed with either blue food coloring (brilliant blue FCF, 0.125 mg/ml) or red food coloring (sulforhodamine B, 0.1 mg/ml). The two mixtures were distributed in alternating wells of a 72-well microtiter dish (Cat. No. 438733; Thermo Fisher Scientific, USA). Approximately 50-70 starved flies were then transferred to the dish within 30 min of food preparation. The dishes were incubated in a dark, humidified chamber, and the flies were allowed to feed for 90 min at room temperature. After the feeding process, the flies were sacrificed at –20°C. The color of their abdomens was analyzed under a stereomicroscope. Upon visual inspection, blue (NB), red (NR), or purple (NP) flies were tabulated. The preference index (PI) was calculated according to the following equation: (NB – NR)/(NR + NB + NP) or (NR – NB)/(NR + NB + NP), based on the dye/tastant combinations. PI values of 1.0 or –1.0 indicated that the flies exhibited a complete preference for one food choice or the other, whereas a PI of 0.0 indicated that the flies had no preference between the two food choices.
Quantification and statistical analyses
All the experiments were repeated on different days and the data were analyzed using GraphPad Prism 8 (GraphPad Software, USA) (RRID: SCR_002798). The dots in the graph represent the number of trials for the experiment. All error bars represent SEM. Multiple sets of data were compared using single-factor ANOVA coupled with Scheffe’s post hoc analysis. All statistical analyses were conducted using the origin program (OriginLab [USA]) (RRID: SCR_002815). The asterisks in the figures indicate statistical significance (*P < 0.05, **P < 0.01).
RESULTS
Low concentrations of carboxylic acids are attractive to Drosophila
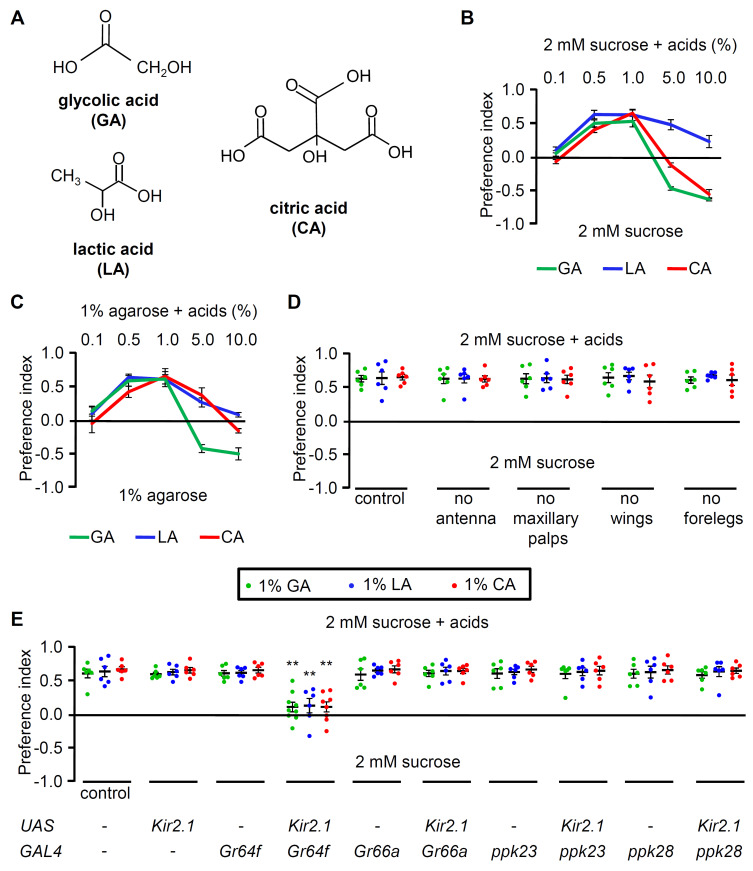
Many organic acids such as GA, LA, and CA are edible and can be used as energy sources (Fig. 1A). GA is the smallest α-hydroxyl acid, which is produced during photorespiration in sugar-crop plants. LA is produced from simple carbohydrates by LA bacteria. Furthermore, CA is abundant in most fruits and vegetables. These carboxylic acids elicit appetitive responses in animals at environmentally relevant concentrations (Rimal et al., 2019). Binary food choice assays were conducted to characterize sour feeding behavior in flies (see the Materials and Methods section for more details). Control flies exhibited almost no bias to 0.1% carboxylic acids (pH ~3) (Fig. 1B). According to our observations, the flies preferred a 0.5%-1% carboxylic acid range (pH 2.4-2.7) with 2 mM of sucrose (Fig. 1B) or without sucrose (Fig. 1C). However, this attraction was reversed by increasing the concentrations of carboxylic acids (Figs. 1B and 1C). Sucrose did not affect the discrimination of these acids except in the 5%-10% CA treatment. Specifically, the preference for relatively high concentrations of CA was affected by the presence of sucrose (Figs. 1B and 1C). Therefore, our findings demonstrated that sour taste is biphasic and that the flies preferred ecologically relevant concentrations of GA, LA, and CA.
Fig. 1. Low concentrations of carboxylic acids are attractive to Drosophila.
(A) Chemical structures of GA, LA, and CA. (B) Binary food choice assays using control flies (w1118). The food choices were prepared with either 2 mM sucrose only or 2 mM sucrose mixed with different concentrations of GA, LA, and CA (n = 6). (C) Binary food choice assays using control flies (w1118) in the absence of sucrose with different concentrations of GA, LA, and CA (n = 6). (D) Binary food choice assays with organ-ablated control flies (2 mM sucrose or 2 mM sucrose with 1% GA, 1% LA, or 1% CA) (n = 6). (E) Binary food choice assays after inactivating different GRNs using UAS-Kir2.1 under the control of the indicated GAL4s. 1% GA, 1% LA, and 1% CA were tested (n = 6). Multiple sets of data were compared using single-factor ANOVA coupled with Scheffe’s post hoc test. Asterisks indicate statistical significance compared with the control (**P < 0.01). All error bars represent the SEM.
Sweet-sensing GRNs are involved in the attractive response to carboxylic acids
Next, surgical dissections were conducted to test whether other chemosensory organs besides the labellum were required for carboxylic acid attraction. Flies have two main olfactory organs: the antennae and maxillary palp. When we removed each organ, flies generally preferred ingesting 1% GA, LA, and CA (Fig. 1D). In addition to the labellum, the legs and wing margin also contain taste sensilla. Dissecting the wings or forelegs did not affect carboxylic acid preference (Fig. 1D). However, these observations cannot completely rule out the possible contribution of these chemosensory organs to carboxylic acid perception, as removing the remaining midlegs and hindlegs was impossible without affecting the behavioral assays. Furthermore, many taste receptors in flies are also expressed in the legs. Next, we sought to determine which GRNs were required to detect 1% GA, LA, and CA (Fig. 1E). Flies have four types of GRNs. Specifically, sweet-sensing and water-sensing GRNs are required for attraction, whereas bitter-sensing and calcium-sensing GRNs are necessary for aversion. When the sweet-sensing GRNs were inactivated by expressing inwardly rectifying Kir2.1 (Paradis et al., 2001) under the control of a Gr64f-GAL4 driver (Dahanukar et al., 2007), GA-, LA-, and CA-mediated attraction was attenuated (Fig. 1E). Conversely, inactivating the water-sensing GRNs using the ppk28-GAL4 driver line (Cameron et al., 2010) had no effect on carboxylic acid feeding preference (Fig. 1E). Furthermore, attraction remained unchanged in flies with inactivated bitter-sensing (Gr66a-GAL4) and calcium-sensing (ppk23-GAL4) GRNs (Dunipace et al., 2001; Lee et al., 2018; Moon et al., 2006) (Fig. 1E). These results suggest that sweet-sensing GRNs mediate the preference for appetitive concentrations of carboxylic acids.
Some L-type sensilla mediate robust responses to physiologically relevant concentrations of carboxylic acids
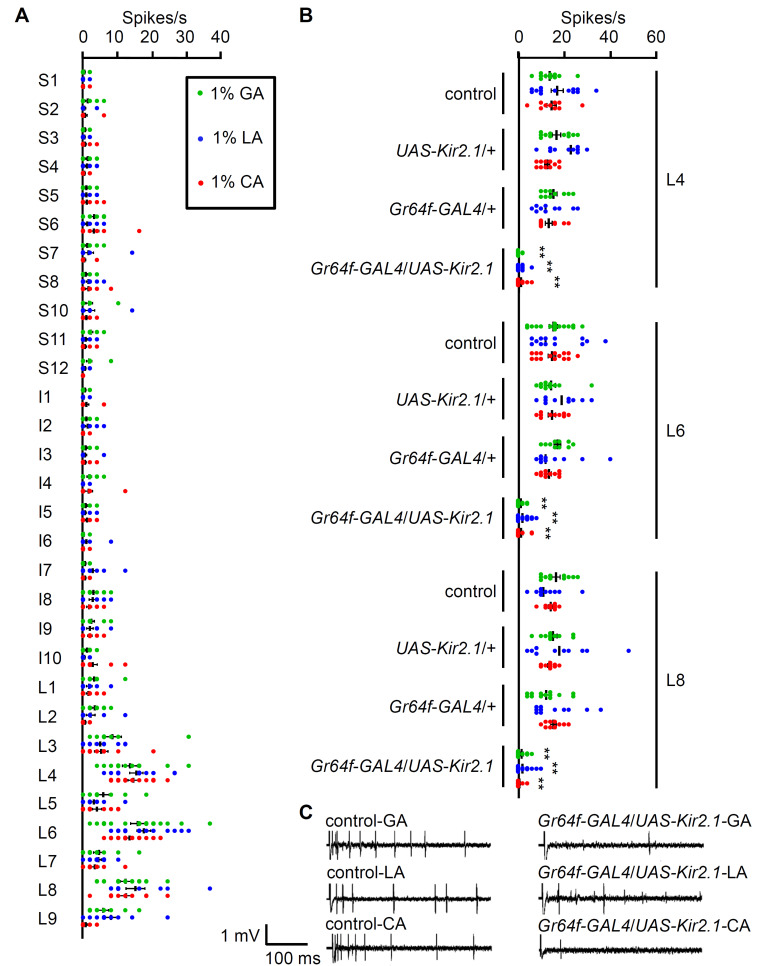
Each sensillum possesses one sweet-sensing GRN, which was labeled using the Gr64f-GAL4 driver. To identify relevant sensilla in the labellum that were sensitive to 1% GA, LA, and CA, we investigated the peripheral nerve responses to each carboxylic acid by performing tip recordings with control flies. The L4, L6, and L8 sensilla robustly responded to all three acids (Fig. 2A). Four additional L-type sensilla (L3, L5, L7, and L9) showed moderate responses to the same stimuli (Fig. 2A). However, none of the S-type and I-type sensilla or the L1 and L2 sensilla were significantly activated by the acids (Fig. 2A). Next, the behavioral abnormalities of the flies with inactivated sweet-sensing GRNs (Gr64f-GAL4/UAS-Kir2.1) were confirmed by conducting electrophysiology tests from the robust L4, L6, and L8 sensilla (Figs. 2B and 2C). As expected, we found that high-frequency action potentials in the L4, L6, and L8 sensilla of the control flies were significantly suppressed, although the nerve activity of both parent strains was similar to that of the control (Fig. 2B). These data suggest that molecular sensors for carboxylic acids are present in L-type sensilla.
Fig. 2. 1% carboxylic acid-induced neuronal responses in the labellum.
(A) 1% GA, 1% LA, and 1% CA were tested from all the labellar sensilla for electrophysiology mapping (n = 10-18). All error bars represent the SEM. (B) Tip recordings from L4, L6, and L8 sensilla after inactivating sweet-sensing GRNs using UAS-Kir2.1 under the control of Gr64f-GAL4 (n = 10-15). All error bars represent the SEM. Multiple sets of data were compared using single-factor ANOVA coupled with Scheffe’s post hoc test. Asterisks indicate statistical significance compared with the controls (**P < 0.01). (C) Representative sample traces of (B).
IR25a and IR76b are indispensable mediators of carboxylic acid-induced nerve activity in L-type sensilla
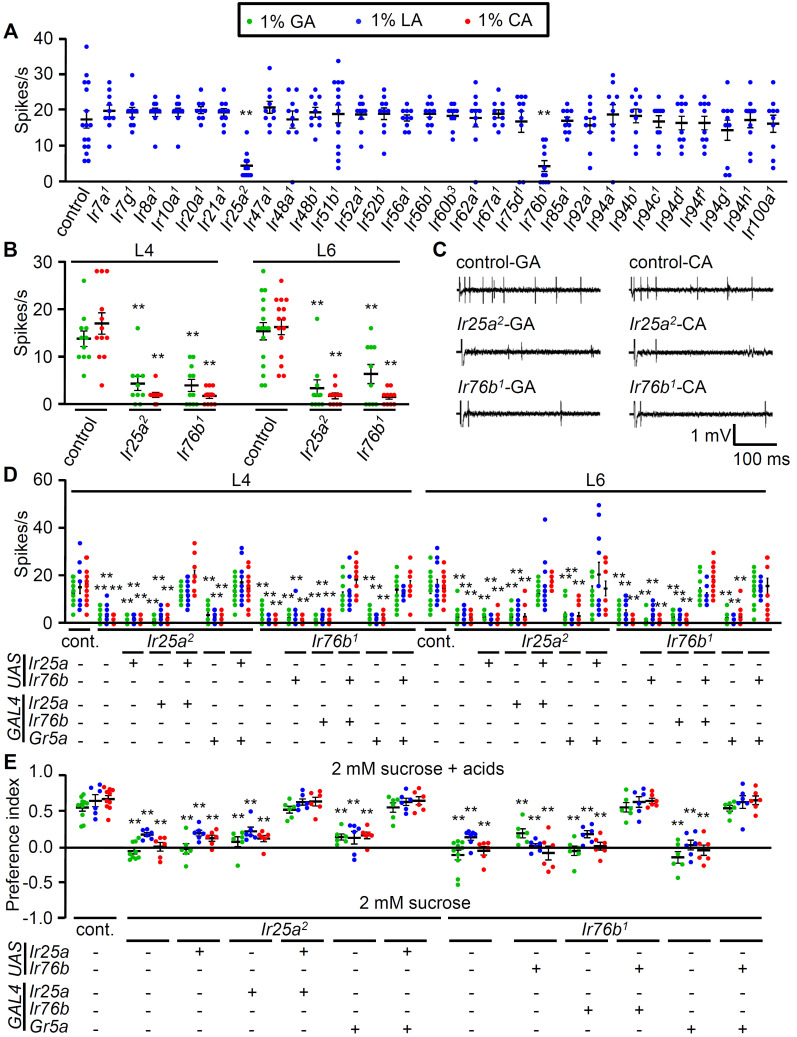
To identify which molecules are involved in carboxylic acid detection, 30 candidate Ir loss-of-function mutants were tested via electrophysiology assays (Benton et al., 2009; Lee et al., 2018; Rimal et al., 2019) (Fig. 3A). The library included the Ir7a and Ir62a mutants, which are essential for the repulsion of acetic acid and Ca2+, respectively (Lee et al., 2018; Rimal et al., 2019). Ir76b is required for sensing Na+ and Ca2+ minerals and amino acids (Ganguly et al., 2017; Lee et al., 2017; 2018; Zhang et al., 2013). Ir25a is also broadly expressed and required for sensing minerals (Chen and Amrein, 2017; Lee et al., 2018; Sánchez-Alcañiz et al., 2018). We thus focused on the L4, L6, and L8 sensilla, which exhibit relatively high action potential frequencies in response to carboxylic acids (Fig. 2A). Our screening results indicated that the Ir25a2 and Ir76b1 mutations significantly impaired LA-induced action potentials (Fig. 3A). In contrast, the other 28 mutations elicited normal LA responses (Fig. 3A). To address whether IR25a and IR76b are necessary for neuronal firing in response to other acids, we further evaluated the response of L4 and L6 sensilla to 1% GA and 1% CA (Figs. 3B and 3C). Our findings indicated that the strong responses to the carboxylic acids in the sweet-sensing GRNs were mediated by IR25a and IR76b.
Fig. 3. IR25a and IR76b are required for sensing attractive carboxylic acids in sweet-sensing GRNs.
(A) Screening candidate Ir mutants from L6 sensilla stimulated with 1% LA (n = 10-14). (B) Neuronal firing responses of control, Ir25a2, and Ir76b1 from L4 and L6 sensilla stimulated with 1% GA and 1% CA (n = 10-16). (C) Representative sample traces of (A) and (B). (D) Electrophysiological rescue of Ir25a2 and Ir76b1 mutant defects elicited by the indicated acids from L4 and L6 sensilla using broadly expressed specific GAL4s (Ir25a-GAL4 and Ir76b-GAL4). Gr5a-GAL4 (sweet-sensing GRNs marker) was also used to drive both UAS lines. +/- indicate the presence or absence of the transgene, respectively (n = 10-14). cont., control. (E) Behavioral rescue of Ir25a2 and Ir76b1 mutant deficits to acid-attraction with the indicated acids in the binary food choice assay. UAS-Ir25a was driven by Ir25a-GAL4 or Gr5a-GAL4 in Ir25a2 mutant while UAS-Ir76b was driven by Ir76b-GAL4 or Gr5a-GAL4 in Ir76b1 mutant. +/- indicate the presence or absence of the transgene, respectively (n = 6-8). All error bars represent the SEM. Multiple sets of data were compared using single-factor ANOVA coupled with Scheffe’s post hoc test. Asterisks indicate statistical significance compared to the control (**P < 0.01).
To test whether Ir25a and Ir76b are required in these GRNs, recovery experiments were conducted using their specific enhancer GAL4s, which are very broadly expressed in the labellum (Lee et al., 2018). Ir25a-GAL4 and Ir76b-GAL4 are expressed not only in sweet-sensing GRNs but also in bitter- and calcium-sensing GRNs (Lee et al., 2018). Each wild-type cDNA was expressed in the mutant background under the control of its specific GAL4 (Figs. 3D and 3E). We found that each GAL4 in combination with its specific UAS-Ir25a or UAS-Ir76b fully rescued the GA-, CA-, and LA-induced action potentials in the neurons (Fig. 3D) and restored the attraction to 1% carboxylic acids (Fig. 3E). Furthermore, we also expressed UAS-Ir25a or UAS-Ir76b under the control of Gr5a-GAL4, which is exclusively expressed in the sweet-sensing GRNs (Thorne et al., 2004) for cell-type-specific rescue (Figs. 3D and 3E). Again, the expression of IR25a and IR76b in the sweet-sensing GRNs was enough to recover the neuronal activity as well as attractive behavior (Figs. 3D and 3E). However, UAS-only or GAL4-only parent strains in the mutant background had deficits that were similar to the mutants (Figs. 3D and 3E). These genetic experiments clearly confirmed that IR25a and IR76b are necessary for carboxylic acid-induced nerve activity.
Sweet Grs are required for carboxylic acid-induced attraction and neuronal activity
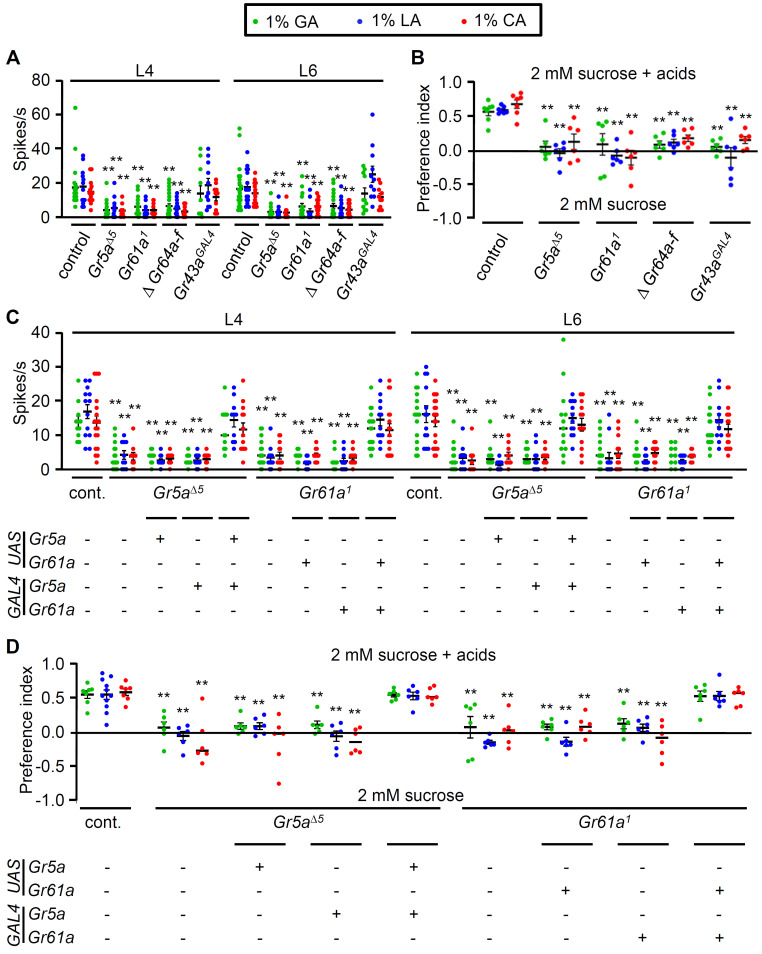
Given that sweet-sensing GRNs are required for acid sensation (Figs. 1E and 2B), sweet Gr mutants were examined in more detail. Fruit flies possess nine sweet Grs including Gr5a (trehalose), Gr43a (fructose), Gr61a (unknown), and Gr64a-f cluster (sucrose, maltose, trehalose, glucose, glycerol, and fatty acids) (Dahanukar et al., 2001; Jiao et al., 2007; 2008; Kim et al., 2018; Miyamoto and Amrein, 2014; Miyamoto et al., 2012). Surprisingly, we found that all the sweet Grs except for Gr43a had very suppressed neuronal responses to all the carboxylic acids (Fig. 4A). Furthermore, all mutants including the Gr43aGAL4 mutant exhibited severe deficits in the binary food choice assays (Fig. 4B), indicating that the physiology and behavior results for Gr43a were inconsistent. However, Gr43a is a known fructose sensor in the brain, suggesting that the ingestion of carboxylic acids as an energy source is controlled both by internal sensors as well as peripheral sensation. These results suggest that sweet-sensing GRs are involved in the signal transduction of acid sensation rather than direct sensors.
Fig. 4. Sweet GRs are required for carboxylic acid sensation.
(A) Neuronal firing responses of the control, Gr5a∆5, Gr61a1, ∆Gr64a-f, and Gr43aGAL4 mutants from L4 and L6 sensilla stimulated by the indicated acids (n = 10-14). (B) Binary food choice assays of control and sweet Gr mutants: Gr5a∆5, Gr61a1, ∆Gr64a-f, and Gr43aGAL4 (n = 6-8). (C) Electrophysiological rescue of Gr5a∆5 and Gr61a1 mutant defects on L4 and L6 sensilla elicited by the indicated acids by expressing UAS-Gr5a or UAS-Gr61a under the control of its specific GAL4s (n = 10-16). (D) Behavioral rescue of Gr5a∆5 and Gr61a1 deficits in the indicated acid attraction by expressing UAS-Gr5a or UAS-Gr61a under the control of their specific GAL4s (n = 6-8). All error bars represent the SEM. Multiple sets of data were compared using single-factor ANOVA coupled with Scheffe’s post hoc test. Asterisks indicate statistical significance compared with the control (**P < 0.01).
The GAL4/UAS system in the mutant background was used to genetically recover the deficits of Gr5a and Gr61a. Using its specific GAL4 drivers, the mutant deficits of Gr5a and Gr61a were significantly recovered to levels similar to those of the wild-type control upon exposure to GA, LA, and CA, as demonstrated by our electrophysiology analyses (Fig. 4C) and binary food choice assays (Fig. 4D). However, we did not further verify six genes of the Gr64 cluster. Nevertheless, these results strongly suggest that sweet GRs are involved in the tuning of acid responses in the labellum. Furthermore, our study is the first to demonstrate the role of Gr61a deficits in carboxylic acid attraction. Gr61a is known to be expressed in sweet-sensing GRNs and is phylogenetically close to sweet GRs (Dahanukar et al., 2007; Robertson et al., 2003). Therefore, Gr61a was functionally verified as a sweet Gr in this study.
DISCUSSION
Carboxylic acids such as glycolic acid, lactic acid, and citric acid are especially attractive to flies at ecologically relevant concentrations
A major unresolved question in the field of taste perception is whether animals distinguish different acid levels based only on relative acidity. Based on the outcomes of this study as well as our previous findings, flies clearly prefer GA, LA, and CA at a pH level of approximately 2.5. However, we previously demonstrated that wild-type flies clearly avoided acetic acid and propionic acid at pH 2.5 (Rimal et al., 2019). These observations made us speculate whether these preferences were driven by the carbon backbone of the carboxylic acid compounds. Specific anions and their concentrations are also important factors that determine food preference in flies, which is very similar to how other animals distinguish different carbohydrates. In mice, for example, the G-protein coupled receptors T1R2 and T1R3 are required for carbohydrate sensing (Puri and Lee, 2021; Zhao et al., 2003). GR64f is a co-receptor in flies (Jiao et al., 2008), but the mechanisms by which different sugars can be identified by different combinations of sweet GRs remain uncertain. Heterologous expression of a minimum number of sweet GRs should be conducted to address these questions. However, previous attempts to conduct these experiments based on many trials of several groups have not been successful.
Acid sensing is thought to prevent the consumption of unripe foods or foods containing harmful bacteria. However, we found that ecologically relevant concentrations (range, 10-150 mM) of carboxylic acids may provide a good energy source to flies. This attractive behavior is mediated by the expression of two IRs (IR25a and IR76b) and sweet GRs (except GR43a) in the sweet-sensing GRNs.
Molecular sensors for sour taste
Recent studies (including this study) have proposed that sour sensation is quite complex in flies compared with sugar or bitter sensation (Ganguly et al., 2021; Mi et al., 2021; Rimal et al., 2019; Stanley et al., 2021). Wild-type females prefer acid-laced food to lay eggs, which is mediated by the expression of IR25a and IR76b in the GRNs of the tarsal segment (Chen and Amrein, 2017). Here, we demonstrated that flies with excised forelegs fed normally with acid-laced food, whereas the inactivation of sweet-sensing GRNs suppressed the attractive behavior. IR25a and IR76b are very broadly expressed in the labellum as well as the tarsal segments (Chen and Amrein, 2017; Lee et al., 2018). These observations suggest that IR25a and IR76b in the labellum and legs are important for the detection of acid-laced foods. Furthermore, the role of IR25a in LA sensation was also evaluated (Stanley et al., 2021). However, the authors found that IR76b did not contribute to LA attraction, which was controversial among different groups. Our study provides not only feeding behavior data, but also single sensillum recordings in mutant flies and genetic recovery with specific GAL4 drivers, as well as with the sweet-sensing GRN marker Gr5a-GAL4. Furthermore, our study evaluated the response of flies to three different carboxylic acids. Therefore, we believe that our findings provide solid evidence for the involvement of IR76b and IR25a in acid attraction.
Our study also demonstrated that most sweet GRs are required for acid attraction and neuronal firing in sweet-sensing GRNs. While we were preparing for the present study, the involvement of sweet GRs in LA attraction was also evaluated by another group (Stanley et al., 2021). The authors confirmed that the ΔGr64a-f mutant could not normally sense LA using calcium imaging (Stanley et al., 2021). Together with our present and previous findings, the aforementioned study demonstrated that sweet GRs mediate acid-induced neuronal activation in sweet-sensing GRNs. Further, as demonstrated by our behavioral experiments and tip recordings, we genetically restored the ability of the Gr5a and Gr61a mutants to exhibit attractive responses to three carboxylic acids. We finally sought to identify the anatomical structures where Gr43a is required for the sensing of carboxylic acids. Our findings indicated that GR43a did not mediate acid sensation in the labellum, as normal neuronal activities to the acids were identified in the Gr43a mutant. However, the Gr43a mutant exhibited defects that were similar to those of other sweet Gr mutants.
Previous studies on IRs and GRs have not been able to conclusively demonstrate their role as molecular sensors for sour taste because none of these studies have demonstrated the direct activation of the IRs by acid. Recently, however, several studies have proposed otopetrin (i.e., a proton-selective channel protein) as a molecular sensor of sour taste in mammals and flies (Ganguly et al., 2021; Mi et al., 2021; Tu et al., 2018). These studies have demonstrated the direct activation of the otopetrin channel coupled with deficits in acid-mediated behavior, suggesting that this evolutionarily conserved channel protein has a role in proton sensing. Again, sour taste appears to be mediated by not only protons but also anions and their concentrations. Moreover, the integration of peripheral acid-sensing information in the brain appears to be much more complex than we anticipated. Nevertheless, we propose that IRs and GRs are equally important to discriminate acid attraction.
ACKNOWLEDGMENTS
This work was supported by grants to Y.L. from the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1A2C1007628) and the Korea Environmental Industry and Technology Institute (KEITI) grant funded by the Ministry of Environment of Korea. B.S. was supported by the Global Scholarship Program for Foreign Graduate Students at Kookmin University in Korea. We would like to thank S. Dhakal for the help of tip recordings.
Footnotes
AUTHOR CONTRIBUTIONS
B.S. and Y.L. conceived and designed the experiments. B.S. performed the experiments. B.S. and Y.L. wrote the manuscript. Y.L. supervised the study.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Abuin L., Bargeton B., Ulbrich M.H., Isacoff E.Y., Kellenberger S., Benton R. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 2011;69:44–60. doi: 10.1016/j.neuron.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Anzi B., Tracey W.D., Jr, Benzer S., Jr Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr. Biol. 2006;16:1034–1040. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Benton R., Vannice K.S., Gomez-Diaz C., Vosshall L.B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P., Hiroi M., Ngai J., Scott K. The molecular basis for water taste in Drosophila. Nature. 2010;465:91–95. doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Amrein H. Ionotropic receptors mediate Drosophila oviposition preference through sour gustatory receptor neurons. Curr. Biol. 2017;27:2741–2750.e4. doi: 10.1016/j.cub.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C.D., Dahanukar A. Recent advances in the genetic basis of taste detection in Drosophila. Cell. Mol. Life Sci. 2020;77:1087–1101. doi: 10.1007/s00018-019-03320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahanukar A., Foster K., van der Goes, van Naters W.M., Carlson J.R. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat. Neurosci. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- Dahanukar A., Lei Y.T., Kwon J.Y., Carlson J.R. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethier V.G. The Hungry Fly: A Physiological Study of the Behavior Associated with Feeding. Harvard University Press; Cambridge: 1976. [Google Scholar]
- Devineni A.V., Sun B., Zhukovskaya A., Axel R. Acetic acid activates distinct taste pathways in Drosophila to elicit opposing, state-dependent feeding responses. Elife. 2019;8:e47677. doi: 10.7554/eLife.47677.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunipace L., Meister S., McNealy C., Amrein H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr. Biol. 2001;11:822–835. doi: 10.1016/S0960-9822(01)00258-5. [DOI] [PubMed] [Google Scholar]
- Falk R., Bleiser-Avivi N., Atidia J. Labellar taste organs of Drosophila melanogaster. J. Morphol. 1976;150:327–341. doi: 10.1002/jmor.1051500206. [DOI] [PubMed] [Google Scholar]
- Fujishiro N., Kijima H., Morita H. Impulse frequency and action potential amplitude in labellar chemosensory neurones of Drosophila melanogaster. J. Insect Physiol. 1984;30:317–325. doi: 10.1016/0022-1910(84)90133-1. [DOI] [Google Scholar]
- Ganguly A., Chandel A., Turner H., Wang S., Liman E.R., Montell C. Requirement for an Otopetrin-Like protein for acid taste in Drosophila. BioRxiv. 2021 doi: 10.1101/2021.06.18.449071. https://doi.org/10.1101/2021.06.18.449071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A., Pang L., Duong V.K., Lee A., Schoniger H., Varady E., Dahanukar A. A molecular and cellular context-dependent role for Ir76b in detection of amino acid taste. Cell Rep. 2017;18:737–750. doi: 10.1016/j.celrep.2016.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi M., Marion-Poll F., Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoolog. Sci. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- Hiroi M., Meunier N., Marion-Poll F., Tanimura T. Two antagonistic gustatory receptor neurons responding to sweet‐salty and bitter taste in Drosophila. J. Neurobiol. 2004;61:333–342. doi: 10.1002/neu.20063. [DOI] [PubMed] [Google Scholar]
- Jiao Y., Moon S.J., Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Moon S.J., Wang X., Ren Q., Montell C. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr. Biol. 2008;18:1797–1801. doi: 10.1016/j.cub.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Kim H., Kwon J.Y., Seo J.T., Shin D.M., Moon S.J. Drosophila Gr64e mediates fatty acid sensing via the phospholipase C pathway. PLoS Genet. 2018;14:e1007229. doi: 10.1371/journal.pgen.1007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Lee Y., Akitake B., Woodward O.M., Guggino W.B., Montell C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc. Natl. Acad. Sci. U. S. A. 2010;107:8440–8445. doi: 10.1073/pnas.1001425107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.J., Sung H.Y., Jo H., Kim H.W., Choi M.S., Kwon J.Y., Kang K. Ionotropic receptor 76b is required for gustatory aversion to excessive Na+ in Drosophila. Mol. Cells. 2017;40:787–795. doi: 10.14348/molcells.2017.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Kim S.H., Montell C. Avoiding DEET through insect gustatory receptors. Neuron. 2010;67:555–561. doi: 10.1016/j.neuron.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Moon S.J., Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Poudel S., Kim Y., Thakur D., Montell C. Calcium taste avoidance in Drosophila. Neuron. 2018;97:67–74.e4. doi: 10.1016/j.neuron.2017.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella S., Fischler W., Kong P., Asgarian S., Rueckert E., Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Meunier N., Marion-Poll F., Rospars J.P., Tanimura T. Peripheral coding of bitter taste in Drosophila. J. Neurobiol. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
- Mi T., Mack J.O., Lee C.M., Zhang Y.V. Molecular and cellular basis of acid taste sensation in Drosophila. Nat. Commun. 2021;12:3730. doi: 10.1038/s41467-021-23490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T., Amrein H. Diverse roles for the Drosophila fructose sensor Gr43a. Fly (Austin) 2014;8:19–25. doi: 10.4161/fly.27241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T., Chen Y., Slone J., Amrein H. Identification of a Drosophila glucose receptor using Ca2+ imaging of single chemosensory neurons. PLoS One. 2013;8:e56304. doi: 10.1371/journal.pone.0056304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T., Slone J., Song X., Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–1125. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S.J., Köttgen M., Jiao Y., Xu H., Montell C. A taste receptor required for the caffeine response in vivo. Curr. Biol. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Nayak S.V., Singh R.N. Sensilla on the tarsal segments and mouthparts of adult Drosophila melanogaster Meigen (Diptera: Drosophilidae) Int. J. Insect Morphol. Embryol. 1983;12:273–291. doi: 10.1016/0020-7322(83)90023-5. [DOI] [Google Scholar]
- Paradis S., Sweeney S.T., Davis G.W. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001;30:737–749. doi: 10.1016/S0896-6273(01)00326-9. [DOI] [PubMed] [Google Scholar]
- Prieto-Godino L.L., Rytz R., Bargeton B., Abuin L., Arguello J.R., Dal Peraro M., Benton R. Olfactory receptor pseudo-pseudogenes. Nature. 2016;539:93–97. doi: 10.1038/nature19824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Godino L.L., Rytz R., Cruchet S., Bargeton B., Abuin L., Silbering A.F., Ruta V., Dal Peraro M., Benton R. Evolution of acid-sensing olfactory circuits in drosophilids. Neuron. 2017;93:661–676.e6. doi: 10.1016/j.neuron.2016.12.024. [DOI] [PubMed] [Google Scholar]
- Puri S., Lee Y. Salt sensation and regulation. Metabolites. 2021;11:175. doi: 10.3390/metabo11030175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimal S., Lee Y. The multidimensional ionotropic receptors of Drosophila melanogaster. Insect Mol. Biol. 2018;27:1–7. doi: 10.1111/imb.12347. [DOI] [PubMed] [Google Scholar]
- Rimal S., Sang J., Poudel S., Thakur D., Montell C., Lee Y. Mechanism of acetic acid gustatory repulsion in Drosophila. Cell Rep. 2019;26:1432–1442.e4. doi: 10.1016/j.celrep.2019.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H.M., Warr C.G., Carlson J.R. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A. 2003;100(Suppl 2):14537–14542. doi: 10.1073/pnas.2335847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper S.D., Chaudhari N. Taste buds: cells, signals and synapses. Nat. Rev. Neurosci. 2017;18:485–497. doi: 10.1038/nrn.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Alcañiz J.A., Silbering A.F., Croset V., Zappia G., Sivasubramaniam A.K., Abuin L., Sahai S.Y., Münch D., Steck K., Auer T.O., et al. An expression atlas of variant ionotropic glutamate receptors identifies a molecular basis of carbonation sensing. Nat. Commun. 2018;9:4252. doi: 10.1038/s41467-018-06453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag S., Park S.K., Pikielny C., Steinbrecht R.A. Gustatory organs of Drosophila melanogaster: fine structure and expression of the putative odorant-binding protein PBPRP2. Cell Tissue Res. 2001;304:423–437. doi: 10.1007/s004410100388. [DOI] [PubMed] [Google Scholar]
- Shrestha B., Lee Y. Mechanisms of DEET gustation in Drosophila. Insect Biochem. Mol. Biol. 2021;131:103550. doi: 10.1016/j.ibmb.2021.103550. [DOI] [PubMed] [Google Scholar]
- Silbering A.F., Rytz R., Grosjean Y., Abuin L., Ramdya P., Jefferis G.S., Benton R. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J. Neurosci. 2011;31:13357–13375. doi: 10.1523/JNEUROSCI.2360-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley M., Ghosh B., Weiss Z.F., Christiaanse J., Gordon M.D. Mechanisms of lactic acid gustatory attraction in Drosophila. BioRxiv. 2021 doi: 10.1101/2021.01.22.427705. https://doi.org/10.1101/2021.01.22.427705. [DOI] [PubMed] [Google Scholar]
- Thorne N., Chromey C., Bray S., Amrein H. Taste perception and coding in Drosophila. Curr. Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Tu Y.H., Cooper A.J., Teng B., Chang R.B., Artiga D.J., Turner H.N., Mulhall E.M., Ye W., Smith A.D., Liman E.R. An evolutionarily conserved gene family encodes proton-selective ion channels. Science. 2018;359:1047–1050. doi: 10.1126/science.aao3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L.A., Dahanukar A., Kwon J.Y., Banerjee D., Carlson J.R. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek H., Wolff G. The labellar sugar receptor of Drosophila. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 1989;164:825–834. doi: 10.1007/BF00616754. [DOI] [Google Scholar]
- Wisotsky Z., Medina A., Freeman E., Dahanukar A. Evolutionary differences in food preference rely on Gr64e, a receptor for glycerol. Nat. Neurosci. 2011;14:1534–1541. doi: 10.1038/nn.2944. [DOI] [PubMed] [Google Scholar]
- Zhang Y.V., Ni J., Montell C. The molecular basis for attractive salt-taste coding in Drosophila. Science. 2013;340:1334–1338. doi: 10.1126/science.1234133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G.Q., Zhang Y., Hoon M.A., Chandrashekar J., Erlenbach I., Ryba N.J., Zuker C.S. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/S0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]