Abstract
Stress reduces cognitive flexibility and dopamine D1 receptor-related activity in the prelimbic cortex (PL), effects hypothesized to depend on reduced corticotropic releasing factor receptor type 1 (CRFr1) regulation of dopamine neurons in the ventral tegmental area (VTA). We assessed this hypothesis in rats by examining the effect of chronic unpredictable restraint stress (CUS), mild acute stress, or their combination on cognitive flexibility, CRFr1 expression in the VTA and D1-related activity in PL. In Experiment 1, rats received either CUS or equivalent handling for 14 days before being trained to press two levers to earn distinct food outcomes. Initial learning was assessed using an outcome devaluation test after which cognitive flexibility was assessed by reversing the outcomes earned by the actions. Prior to each reversal training session, half the CUS and controls receiving acute stress with action-outcome updating assessed using a second devaluation test and CRFr1 expression in the VTA assessed using in-situ hybridisation. Although CUS did not itself affect action-outcome learning, its combination with acute stress blocked reversal learning and decreased VTA CRFr1 expression after acute shock. The relationship between these latter two effects was assessed in Experiment 2 by pharmacologically disconnecting the VTA and PL, unilaterally blocking neurons expressing CRFr1 in the VTA and D1 receptors in the contralateral PL during reversal learning after acute stress. Acute stress again blocked reversal learning but only in the group with VTA-PL disconnection, demonstrating that VTA CRFr1-induced facilitation of dopaminergic activity in the PL is necessary for maintaining cognitive flexibility after acute stress. [250].
Keywords: Cognitive flexibility, Stress, CRFr1, Ventral tegmental area, Prelimbic cortex, Dopamine D1 receptor
Highlights
-
•
Acute stress increased CRF receptor1 expression in the VTA.
-
•
Chronic stress attenuated the effect of acute stress on CRFr1 expression.
-
•
Chronic stress plus acute stress produced a loss of cognitive flexibility.
-
•
Blocking VTA CFRr1 and dopamine D1r in PL reduced cognitive flexibility following stress.
1. Introduction
Although the negative influence of stress on cognitive flexibility is well documented, the neural basis of this effect remains unclear. Studies in rodents suggest that exposure to unpredictable stressors remodels cellular processes in the medial prefrontal cortex (mPFC) resulting in a deficit both in cellular activity and in specific cognitive functions (Arnsten, 2009), notably in the capacity for goal-directed action (Dias-Ferreira et al., 2009). Other studies suggest that the functional effects of stress-induced changes in mPFC are related to changes in dopamine (DA) neurotransmission. The acquisition of goal-directed action has been found to rely on the prelimbic prefrontal cortex (PL, area 32) (Balleine, 2019), and within that area, on plasticity involving the interaction of glutamatergic and DAergic processes (Kruse et al., 2009; Tseng and O'Donnell, 2004). Reducing dopamine-related activity using either DA antagonists or by inhibiting the DA input to the PL from its source in the ventral tegmental area (VTA) abolishes such acquisition (Kelly and Fudge, 2018).
Accordingly, DA release in the mPFC is highly sensitive to stress, with mild stress-inducing increases in DA and promoting behavioural flexibility (Sullivan, 2004), whereas more severe stress depletes cortical DA (Matuszewich et al., 2014), produces deficits in behavioral flexibility (Hurtubise and Howland, 2017), in the cognitive and motivational processes required to learn new responses to control stress (Maier and Seligman, 2016); and exacerbates the negative effects of subsequent exposure to even a mildly stressful event (Mor et al., 2017). Corticotropin-releasing factor (CRF) appears to be key to coordinating these neural responses (Orozco-Cabal et al., 2006). Within the VTA the type 1 receptor for CRF (CRFr1) plays a central role in modulating mesocortical DA activity after stress (Kelly and Fudge, 2018; Wanat et al., 2013) and selective disruption of CRFr1 in the VTA reduces both the magnitude and duration of the effects of stress on mPFC DA activity (Refojo et al., 2011; Vranjkovic et al., 2018). Nevertheless, whether CRFr1-mediated changes in VTA activity are responsible for the effects of stress on executive function and cognitive flexibility has yet to be assessed.
To address this question, rats were exposed to chronic unpredictable restraint stress (CUS) before acquiring two specific goal-directed actions involving encoding distinct action-outcome associations on two levers using standard methods; pressing one lever earned food pellets the other a sucrose solution (Balleine and Dickinson, 1998; Bradfield et al., 2013). Half of the rats were then given additional acute stress (AS) before learning that these lever press-outcome contingencies had been reversed. To establish the degree of goal-directed control of lever pressing after exposure to these stressors, we used an outcome devaluation test in which one outcome was selectively devalued using a specific satiety treatment. We then analysed CRFr1 mRNA expression in the VTA to establish whether CUS had altered this expression in response to an AS. In a subsequent study, we assessed the functional effects of disconnecting the pathway between CRFr1 containing neurons in the VTA and D1 receptor-expressing neurons in the PL using the same behavioral assessments. Rats were given asymmetrical infusions of a CRFr1 antagonist into the VTA and a D1 antagonist into the contralateral PL. We predicted that: (i) rats exposed to chronic unpredictable stress will demonstrate an impairment in learning following mild acute stress; (ii) this impaired learning will be associated with an attenuation of CRFr1 mRNA expression in the VTA and (iii) infusions of a CRFr1 antagonist into the VTA will disrupt the DA projection to the PL and impair the flexible updating of goal-directed learning after acute stress.
2. Methods
2.1. Animals
All experimental procedures were approved by the University of New South Wales Animal Ethics Committee (AEC number 19/64B) and were compliant with the ARRIVE guidelines (Kilkenny et al., 2010). Eighty-five male outbred Long Evans rats were housed within a climate-controlled facility on a 12-h light/dark cycle (lights on at 07:00) in groups ranging between two and four rats. Rats had ad-libitum access to water and chow prior to commencement of behavioural training.
2.2. Experiment 1. The effect of CUS on behavioural flexibility
2.2.1. Apparatus
Behavioural training was conducted using 16 operant conditioning chambers (MED Associates) each enclosed within a sound and light attenuating shell. Each chamber contained a pellet dispenser that delivered grain pellets (45 mg, Bioserv Biotechnologies) and a pump that delivered 20% sucrose solution (0.2 ml). Chambers contained two retractable levers and a recessed magazine cantered between them. An infra-red photobeam was positioned at the threshold of the magazine to record entries. Each chamber contained a light (3W, 24V) illuminated for the duration of all behavioural sessions. Sessions were pre-programmed and controlled by microcomputers running MED Associates proprietary software (Med-PC). Lever pressing, magazine entry, reinforcer delivery as well as the presentation time of each lever was captured for each session using this software.
2.2.2. Chronic unpredictable restraint stress
Rats were randomly assigned to Naïve (n = 29) or Chronic Unpredictable Stress (CUS) (n = 33) experimental groups. The CUS treatment involved daily restraint for 2 h inside plexiglass tubes (20 cm length, 6.35 cm diameter, Ibisci, USA) over 14 days. Each day, the restraint was given at a different time between 6 a.m. and 10 p.m. to ensure the stress was unpredictable. Rats allocated to the naïve treatment were handled for a few minutes daily over the 14 days. Percentage body weight change was calculated at two-day intervals during the stressor period.
2.2.3. Instrumental training
Two days prior to commencing training rats were food-restricted with daily intake was restricted to 15g of chow, which was maintained for the remainder of the experiment. Weight was monitored thrice weekly to ensure it remained above 85% of baseline body weight.
Behavioural training started with one session of magazine training. Rats received 20 pellet and 20 sucrose outcomes at 15-s intervals for 15 min. Rats were then trained to press the levers such that, for each rat, one lever was assigned to deliver pellets and the other a sucrose solution, counterbalanced to control for any lever position preference. Each training session was divided into four periods, two on each lever in alternation. During each period, one lever was extended until either 20 outcomes were delivered or 15 min had elapsed after which the lever was retracted and a 2-min break was instituted after which the other lever was inserted, and so on. Two training sessions were conducted each day with the order of lever presentation counterbalanced across sessions.
Outcomes were delivered on a continuous reinforcement schedule for three sessions, then on a random ratio (RR)-5 schedule, i.e, the outcome was delivered after five presses on average, then three sessions on RR10 and two sessions on RR20.
2.2.4. Outcome devaluation by satiety
After this training rats completed two specific satiety-induced outcome devaluation tests on successive days. To induce specific satiety, rats were placed into clean devaluation boxes and given unrestricted access to either sucrose solution or pellets for 45 min. They were then transferred to their operant chamber to compete a 10-min extinction test. Both levers were extended simultaneously, however, no outcomes were delivered during the test. The lever associated with the outcome that was pre-satiated was considered the devalued lever and the other lever valued. The following day, rats received the same protocol only with the alternative outcome presented during the pre-feeding phase.
2.2.5. Mild acute stress and outcome-identity reversal
To assess the rats’ ability to update changes in A-O contingencies they next received three training sessions with the outcome identities reversed; e.g., if, initially, pressing the left lever delivered a pellet and the right lever sucrose solution then pressing the left lever now delivered sucrose solution and the right lever a pellet. In this phase, rats received only one training session per day structured as previously described with the outcomes delivered on an RR-10 schedule.
During this phase each of the previously generated stress groups was subdivided into two sub-groups: one received a mild acute stress (AS), involving 5 min force swim test, prior to each reversal training session, whereas the other received no treatment. This resulted in four groups: naïve (n = 16), naïve with AS (n = 13), CUS (n = 17) and CUS with AS (n = 16). For the forced swim test, rats were placed individually into a white plastic oval bin (100 cm high, 30 cm maximum diameter) filled to a height of 45 cm with clear, fresh water (at 25 ± 1 °C) for 5 min. Rats were then dried with a towel and allowed 10 min for grooming before being placed in the operant conditioning chambers for the reversal training. The sub-groups under each stress condition were matched for performance in the first devaluation test to ensure similar levels of performance during initial training between the sub-groups.
After reversal training, rats completed a second round of outcome devaluation tests conducted as described above except that, after the first test, rats received a fourth refresher RR10 training session with or without the acute stress (as appropriate).
2.2.6. Novel mild stress and euthanasia
To assess the impact of acute stress on VTA CRFr1 expression, all AS rats received a series of foot shocks before euthanasia. Rats were placed into an unfamiliar operant chamber where the stainless-steel rod floor was connected to a shock generator (Med-Associates, USA). In a single session, rats received three foot-shocks (5 mA) at random intervals over the course of 10 min. Rats were then transferred by hand into a novel cage where they were left for 20 min. Euthanasia was completed by rapid decapitation without anaesthesia. Brains were removed and the tissue block containing the midbrain was snap frozen over dry ice for in-situ hybridisation analysis. The remaining rats were similarly euthanised without any additional stress treatment.
2.2.7. Blood collection and corticosterone quantification
Blood was collected at two timepoints; the first via tail-snip the day after the completion of CUS/daily handling treatment, a minimum of 16 h following the last restraint, and the second at decapitation. All samples were collected between 10am and 12pm. For the tail-snip, each rat was briefly restrained using a soft flexible canvas while the tail end was cut to collect blood – and the second after decapitation. At both time points, blood vials were left to coagulate at room temperature for 20 min before being cooled on ice. Vials were then transferred to a centrifuge (5430R, Eppendorf) and spun at 3000×g for 15 min at 4 °C. The supernatant serum was collected and stored at −80 degrees C. Circulating corticosterone levels were assessed using an ELISA kit (R&D Systems) and a microplate reader (SPECTROstar OMEGA, BMG LABTECH, Germany).
2.2.8. In-situ hybridisation
Frozen, unfixed tissue was sliced using a cryostat (Leica Microsystems) and mounted on Superfrost Plus slides. Ten series of 14 μm coronal sections were taken from −4.7 to −5.8 mm from bregma. Slides were stored in slide racks at −20 °C for 1 h and then stored at −80 °C. Identification of CRFr1 mRNA was achieved using the RNAscope® 2.5HD brown reagent kit (Advanced Cell Diagnostics, USA) and CRFr1 probe (Rn-Crhr1-C3, Advanced Cell Diagnostics, USA). Pictures from the center of the left and right parabrachial pigmented nucleus of the VTA were taken using the 40x objectives. Optical densities were calculated using ImageJ.
2.3. Experiment 2: The effect of disconnection of the VTA-PL projection on the effect of AS
Next, we assessed the role of CRFr1 in VTA neurons projecting to the PL in cognitive flexibility in healthy rats following an acute stress. This was achieved by training rats using the same behavioural protocol as in experiment 1, but pharmacologically disconnecting the pathway between CRFr1 containing neurons in the VTA and D1 receptor-expressing neurons in the PL prior to the AS and reversal contingency training.
2.3.1. Surgery
Under isoflurane anaesthesia (5% induction and 2% maintenance in 100% oxygen), rats (n = 25) were placed in a stereotaxic frame (Stoelting) and received a subcutaneous injection of Bupivacaine hydrochloride at the incision site. A midline incision was made to expose the skull, bregma and λ were used to ensure the head was in a horizontal plane, and two small holes were drilled above the target regions. A guide cannula (26 gauge, plastic one) was implanted unilaterally above the VTA (AP: −5.4 mm from bregma, ML ±1.2 from the midline and DV: −8.2 below the skull surface) with a second guide cannula implanted above either the ipsilateral or contralateral prelimbic cortex (AP: 3.7 mm from bregma, ML ±0.6 mm from the midline and DV: 4 mm below the skull surface, at a 10° angle). Three small screws were drilled into the skull and the cannula fixed with dental acrylic. Rats were given seven days recovery.
2.3.2. Behavioural training and testing
After recovery, the rats received a similar instrumental protocol to that described above with a few modifications. Rats received initial instrumental training using the original contingency. Training included magazine training, three continuous reinforcement training sessions, three RR5 and three RR10 training sessions, followed by the first outcome devaluation test. Then all rats received three AS and identity reversal training sessions, but with an infusion of either the CRFr1 antagonist Antalarmin (500 ng in 250 nl over 3 min) or saline into the VTA and dopamine D1 receptor antagonist SCH23390 (200 ng in 250 nl over 3 min) or saline into the prelimbic cortex, prior to the AS. Finally, the reversal learning was assessed by a second devaluation test over two days. The experimental group (n = 8) received the antagonists infused into the VTA and PL in contralateral hemispheres with the aim, therefore, of abolishing VTA CRFr1 modulation of PL dopamine D1r activity bilaterally. Two control groups were used: one given ipsilateral antagonist infusions leaving one hemisphere functionally intact (n = 8), and the second given saline infusions contralaterally, leaving both hemispheres functionally intact (n = 7). At the end of the protocol, rats were decapitated without anaesthesia, separate tissue blocks containing VTA and prelimbic cortex were fixed, sectioned and Nissl stained to confirm cannula placement.
3. Results
3.1. Acute and chronic stressors impair cognitive flexibility
To investigate how cognitive flexibility is affected by exposure to chronic unpredictable stress (CUS), mild acute stress (AS), and the two in combination, we assessed the ability of rats to update action-outcome encoding after outcome-identity reversal (Bradfield and Balleine, 2017). We first assessed the effect of CUS on encoding the initial action-outcome associations and then whether exposure to additional AS prior to reversal training sessions altered updating. As such, there were four experimental groups: Naïve; Naïve with AS; CUS; and CUS with AS.
Two weeks of CUS (2-hr per day; cf. Fig. 1A), resulted in a significant decrease in bodyweight compared Naïve rats, F(1,50) = 44.42, p < .001. There was an effect of time, F(1,50) = 4.20, p = .046, and a significant stress × time interaction, F(1,50) = 60.31, p < .001, indicating the effects of CUS exposure increased over time (Supplementary figure A). Additionally, CUS rats (M = 27.24, SD = 8.60) had significantly higher circulating corticosterone than Naïve rats (M = 14.18, SD = 6.62), t(38) = 0.38, p < .001 (Supplementary figure B).
Fig. 1.
Contingency Learning Following Chronic and Acute Stress Exposure
(A) Experimental design. (B) Group mean lever pressing rates during initial learning and (C) Group mean lever pressing totals over two days of devaluation testing for the initial contingencies. (D) Group mean lever pressing rates during reversal learning and (E) Group mean lever pressing totals over two days of devaluation testing for the reversed contingencies. All error bars represent SEM. *p < .05, *p < .01, **p < .001.
All animals were then food-deprived and trained to press two levers for the pellet and sucrose outcomes on random ratio (RR) schedules of reinforcement. All rats learned to press the levers and increased their performance as the ratio requirement increased. A repeated measures ANOVA showed pressing rates increased over the training, F(11,52) = 30.79, p < .001, but that this increase differed between the CUS and naïve rats yielding a stress × training interaction, F(11,52) = 2.29, p = .023. Pressing rates were significantly lower for CUS than Naïve rats, F(1,62) = 6.75, p = .012, suggesting attenuated rigour after CUS (Fig. 1B). Nevertheless, both groups earned a similar number of outcomes during training (pellet: t(124) = 0.34, p = .929, sucrose t(124) = 0.72, p = .72) (Supplementary figure C).
We assessed the effect of CUS on initial A-O learning using specific-satiety induced outcome devaluation tests. A two-way ANOVA, comparing stress and devaluation treatments, showed a significant devaluation effect, F(1,57) = 73.75, p < .001. The reduced vigour in which the CUS rats pressed lead to a significant interaction between lever pressing and CUS treatment, Λ = 0.90, F(1,57) = 6.10, p = .017. Nevertheless, Bonferroni adjusted pairwise comparisons indicated a significant devaluation effect (devalued < valued) emerged in both Naïve (p < .001) and CUS (p < .001) rats (Fig. 1C). During specific satiety pre-feeding, the CUS rats showed reduced consumption of the outcomes, F(1,56) = 23.29, p < .001. Multiple comparisons revealed that CUS rats consumed less pellets (p < .001) and sucrose (p = .006) than Naïve rats (Supplementary figure D).
Next, we examined whether CUS, AS or CUS with AS affected cognitive flexibility as assayed by the rats’ ability to encode a change in action-outcome contingency. Rats completed additional training with reversed lever-outcome identity contingencies (Fig. 1A). Half of all rats were allocated to an acute stress treatment and completed a 5-min forced swim immediately before each training session. Rats given CUS plus the additional AS showed reduced pressing rates during reversal training (Fig. 1D). A repeated measures ANOVA revealed that pressing rates increased significantly over sessions, F(3,165) = 15.96, p < .001, and there was no interaction was between group and sessions, F(9,165) = 1.81, p = .070. There was, however, evidence of a difference in lever pressing between treatment groups F(3,55) = 6.55, p < .001, with main effects for CUS F(1,55) = 12.38, p < .001 as well as AS F(1,55) = 7.42, p = .008. Bonferroni adjusted pairwise comparisons indicated reduced pressing rates in CUS with AS when compared with naïve rats on training days one (p = .009), two (p = .002) and four (p = .012). These differences in performance resulted in differences in the number of outcomes earned but only on the pellet lever: one-way ANOVA revealed a significant difference in pellets earned between groups F(3,48) = 7.57, p < .001, with post-hoc tests revealing a significance decrease for CUS with AS compared with Naïve, Naïve with AS and CUS groups, t(48) = 4.45, p < .001. There were no differences in the number of sucrose rewards earned during reversal training largest F(3.48) = 2.33, p = .086 (Supplementary Figure E).
A second outcome devaluation test was conducted to assess learning of the new action-outcome contingencies (Fig. 1E). A 2 × 4 Mixed ANOVA revealed a significant overall effect of outcome devaluation, F (1,55) = 27.81, p < .001. However, as is clear from Fig. 1E, this effect interacted with stress treatment, F(3,55) = 3.10, p = .034, and, indeed, post-hoc, Bonferroni adjusted pairwise comparisons revealed the difference between devalued and valued lever was significant for the Naïve (p < .001), Naïve with AS (p < .001) and CUS (p < .003) groups but not for CUS with AS (p = .901). As with the first devaluation assessment, consumption data during outcome devaluation after reversal (Supplementary Figure F) showed a reduction in outcome consumption in the AS, CUS and CUS + AS groups when compared with Naïve group, with a main effect of CUS, F(1,54) = 15.12, p < .001 and AS F(1,54) = 13.12, p < .001, but no CUS × AS interaction F(1,54) = 1.981, p = .165.
3.2. CRFr1 expression in the VTA following chronic and/or acute stress
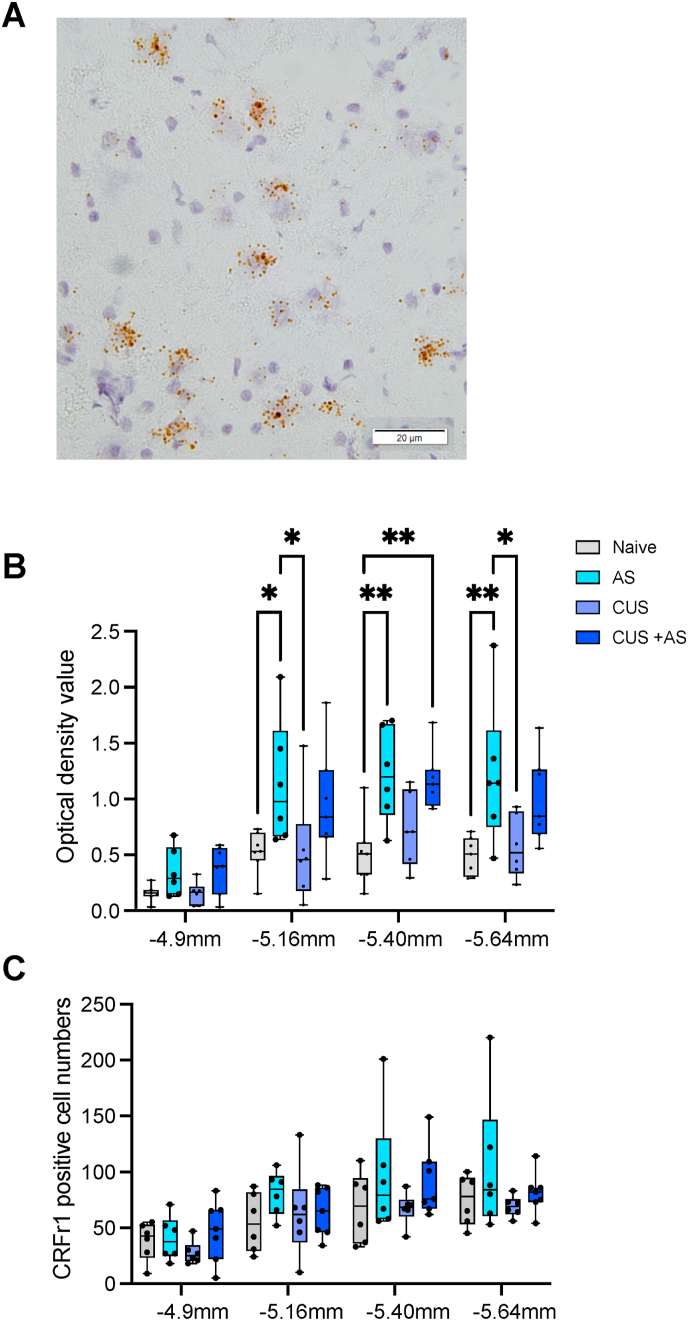
We assessed CRFr1 expression in the VTA at different coronal levels using in situ hybridisation (Fig. 2A) in each of the stress groups. Analysis of staining revealed an increase in total mRNA signal in the posterior part of the VTA when measured as optical density. There was a significant anatomical effect with the more posterior coronal sections showing higher positivity for CRFr1, F(3,75) = 13.79, p < .001, and a significant between-groups effect, F (3,75) = 11.97, p < .001, but no interaction between these variables F(9,75) = 0.58, p = .809. Multiple post hoc comparisons revealed that AS increased total CRFr1 mRNA expression at −5.16 mm (p = .030), −5.40 mm (p = .009) and −5.64 mm (p = .014) posterior to bregma when compared to Naïve rats. However, no differences were observed between Naïve and CUS rats at any coronal levels, p > .05 (Fig. 2B). Furthermore, although it appeared that the increased expression following AS was attenuated in rats with prior exposure to CUS, multiple comparisons analysis revealed no significant difference between CUS and CUS with AS at any coronal level, p > .05. Interestingly, there was no evidence of differences between any of the groups in the number of cells that were positively stained (Fig. 2C) indicating that the increase in the optical density represents increased expression in cells already expressing CRFr1 rather than recruitment of new cells that did not express CRFr1 prior to the AS.
Fig. 2.
CRFr1 mRNA Expression in the VTA
(A) Representative image from the VTA displays CRFr1 mRNA expression in brown staining. (B) Group mean expression of CRFr1 mRNA at each coronal levels by optical density or (C) or cell total. Error bars from SEM. Naïve, AS, and CUS n = 6, CUS and AS n = 7. *p < .05, *p < .01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. VTA-PL disconnection blocks action-outcome updating
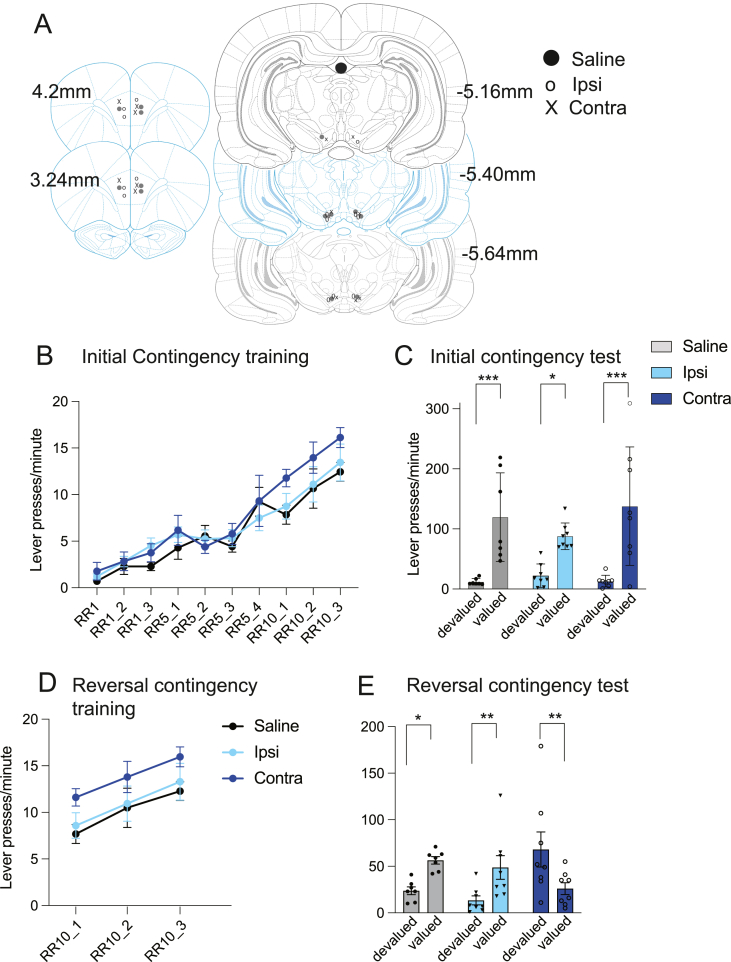
The results of Experiment 1 suggest CUS attenuated the CRFr1 activity in the VTA induced by acute stress and that it was this reduction and its consequent effects on DA activity in the PL that blocked the ability of rats to update the action-outcome associations after outcome identity reversal. To test this claim, Experiment 2 directly assessed the influence of VTA CRFr1-induced DA activity in the PL on cognitive flexibility by disconnecting the VTA input to the PL prior to identity reversal. To achieve this, naïve rats were first implanted with guide cannulae, one above the VTA and a second above either the contralateral or ipsilateral PL. Rats then received a similar instrumental protocol to Experiment 1, consisting of basic training, outcome devaluation, acute stress followed by training with the outcome identities reversed and a second outcome devaluation test (Fig. 1A). Prior to the reversal sessions, rats received an infusion of the CRFr1 antagonist Antalarmin or saline into the VTA and the dopamine D1 receptor antagonist SCH23390 or saline into the prelimbic cortex. They were then given an AS treatment followed by an outcome identity reversal training session. This resulted in three experimental groups: a group given contralateral drug infusions, to block CRF-modulated PL dopamine activity in both hemispheres; a group given ipsilateral drug infusions, to block CRF-modulated PL dopamine activity but only in one hemisphere, and a group given contralateral saline infusions, leaving both hemispheres intact.
Cannulae placements in both the VTA and prelimbic cortex are illustrated in Fig. 3A. All rats have successfully acquired the initial associations, an effect of acquisition p < .001 but not of group or interaction indicated no differences between the three groups in the performance during training (Fig. 3B). The subsequently conducted devaluation test revealed that all groups acquired the initial action-outcome associations (Fig. 3C). A 2 × 3 Mixed ANOVA revealed a significant devaluation effect, F(1,20) = 43.85, p < .001, but effect of group, F(2,20) = 0.60, p = .559, and no evidence of a significant interaction between group and devaluation, F(2,20) = 1.43, p = .263. Post-hoc Bonferroni adjusted pairwise comparisons identified a significant difference in pressing between the devalued and valued levers in the Saline (p < .001), Ipsilateral (p = .018) and Contralateral (p < .001) groups indicating that all groups encoded the initial action-outcome associations.
Fig. 3.
Contingency Learning Following Disconnection
(A) Distributions of guide canula implantation sites within the prelimbic cortex. (B) Group mean lever pressing rates during sessions of initial learning. (C) Group mean lever pressing totals over two days of devaluation testing for the initial contingencies. (D) Group mean lever pressing rates during sessions of reversal learning. (E) Group mean lever pressing totals over two days of devaluation testing for the reversed contingencies. All error bars represent SEM. *p < .05, *p < .01, **p < .001.
The rats then were trained with the outcome identities reversed, receiving the drugs/saline infusions prior to the AS and training sessions (Fig. 3D) Repeated measures ANOVA revealed a main effect of training session, F(1.75,35.02) = 13.46, p < .001, but neither an effect of group, F(2,40) = 2.37, p = .119, nor a group × session interaction, F(4,40) = 0.03, p = .742, indicating no differences in performance during reversal training. Following the second devaluation test (Fig. 3E) a 2 × 3 mixed ANOVA revealed no overall effect of group F(2,20) = 2.372, p > .05 or of devaluation, F(1,20) = 1.99, p = .17; there was, however, a significant interaction between devaluation and group, λ = .37, F(2,20) = 17.41, p < .001. Post-hoc Bonferroni corrected pairwise comparisons revealed significant devaluation effects in the Saline (p < .001), Ipsilateral (p = .003) and Contralateral (p < .008) groups. However, as is clear from Fig. 3E, the difference in group Contralateral was reversed compared to the other two groups. To reveal this, we tested two contrasts: we compared groups Saline and Ipsilateral, which didn't differ from each other F(1,20) = 0.076, p > .05; and then we compared groups Saline + Ipsilateral vs group Contralateral F(1,20) = 20.451, p < .001.
Therefore, these results indicated that both saline infusions and ipsilateral disconnections do not appear to impair the acquisition and updating of lever contingencies. However, the blocking of the CRFr1 receptor in the VTA and D1 receptor in the contralateral prelimbic area appeared completely to abolish the encoding of new action-outcome associations after exposure to AS.
4. Discussion
The capacity to update action-outcome encoding is essential for goal-directed behaviour to remain adaptive in a changing environment, a core component of cognitive flexibility. Here we found that neither exposure to acute nor chronic stress alone impaired this capacity or the ability of rats to incorporate new information for goal-directed control. However, the combined effect of the two stressors led to an impairment in updating action-outcome associations and in goal-directed learning generally. We subsequently found that CRFr1 mRNA expression in the VTA increases following AS and that CRFr1 mediated changes in the dopaminergic output from the VTA to the PL cortex are required for successful updating after exposure to AS. These findings suggest that the impaired learning found in rats receiving both acute and chronic stress was due to an attenuation in the increase in CRFr1 expression in the VTA induced by acute stress.
4.1. The combination of CUS and AS impairs learning
Several measures were taken to ensure the efficacy of the CUS model throughout the duration of the protocol. Elevated circulating corticosterone levels, a hallmark of chronic stress, were found immediately following the CUS period and remained elevated at the completion of behavioural testing. Stress-induced weight loss during the CUS period, even though rats had ad-libitum access to food in the home cage, is also a reliable indicator for the CUS efficacy (Sequeira-Cordero et al., 2019; Willner, 2005). Chronically stressed rats also showed reduced pressing rates when the behavioral requirement to earn reward was increased later in instrumental training, suggesting a reduced level of motivation or engagement with the task when the effort required to achieve the reward was increased but not when the effort required was minimal. As all rats completed the training sessions, the reduced pressing rate did not appear to lead to differences in initial learning during the acquisition of new action-outcome associations and did not translate to changes in performance in the initial devaluation tests. Finally, chronically stressed rats showed some evidence of anhedonia, consuming significantly fewer rewards during the devaluation pre-feeding prior to both devaluation tests. Nevertheless, given that similar consumption levels were sufficient to produce devaluation in the first test, this effect was unlikely to underlie the reduced sensitivity to outcome devaluation in the CUS plus AS group.
The effect of CUS on a range of cognitive flexibility processes changes with the type, duration and severity of stress. Previous studies that demonstrated that CUS impaired cognitive flexibility often used stress paradigms which included longer or more severe stress. Many CUS studies also used a stress paradigm that presents different forms of stress in unpredictable order and times (Hurtubise and Howland, 2017). The current study used one form of stress, a 2-h daily restraint, but was given at a different time each day, to maintain the stress as unpredictable. CUS rats in the current study presented with intact sensitivity to outcome devaluation in the first devaluation test. This indicates that the CUS severity used in the study was not sufficient to disrupt processes such as action-outcome encoding and updating, and producing goal-directed behaviour. This is consistent with a study by Dias-Ferreira and colleagues, that edthat CUS accelerates the transition from goal-directed to habitual control, but demonstrated that this transition required a longer training period than was used in our study (Dias-Ferreira et al., 2009). Furthermore, this study used male rats only; and considering the large literature about difference in response to stress between males and females (Georgiou et al., 2018), it will need to be determined whether this effect is found in females as well.
In the second devaluation test, however, after the contingencies were reversed, Naïve and AS groups showed intact devaluation with comparable performance to the original devaluation, indicating that the amount of reversal training given was sufficient for the rats to acquire the new relationship and that AS on its own does not compromise learning and/or performance. Rats in the CUS groups showed reduced performance in the final devaluation, but still pressed significantly more on the valued lever: therefore, all three groups demonstrated sensitivity both to the change in outcome value and in the contingency (Balleine and Dickinson, 1998). In contrast, rats in the CUS plus AS group did not show sensitivity to devaluation. Failure to show outcome devaluation is typically associated with the transition from goal-directed to habitual control of behaviour (Balleine, 2019), however, given that rats in this group showed intact devaluation after initial training, this effect is more likely to be due to an impaired ability to update the action-outcome contingency after outcome reversal.
Studies on the circuitry mediating reversal learning has focused on the orbitofrontal cortex rather than the prelimbic (Hurtubise and Howland, 2017). However, in these studies, the reversal learning is achieved via negative feedback, where the action delivering the rewards was no longer effective, and the animal needed to learn to use a different action to get the reward. The process of reversal learning we have used does not involve negative feedback, but reversal identities of the two A-O associations. The cognitive task required is updating changes in contingencies, which is a well-known function of the prelimbic cortex (Balleine and Dickinson, 1998; Corbit and Balleine, 2003).
4.2. CUS attenuates the effect of acute stress-induced on VTA CRFr1 expression
CRF is considered to be a neuromodulator; it does not produce fast membrane potential changes as neurotransmitters do, but instead is expressed and released together with other primary neurotransmitters such as glutamate and GABA. Combined with primary neurotransmitters, CRF will influence the general excitatory/inhibitory state of target neurons (Gallagher et al., 2008; Kelly and Fudge, 2018). In the VTA, CRF can be co-released with either glutamate or GABA, onto either dopaminergic neurons or interneurons. The overall effect of CRF release is enhanced dopaminergic output (Wise and Morales, 2010). In Experiment 1 we found an increase of CRFr1 after AS in the mid- and caudal levels of the VTA, but not in the rostral level. Subpopulations of dopaminergic neurons in the VTA influence different physiological processes. Rostromedially, neurons appear more strongly to code reward prediction errors whereas dorsolaterally and caudally, neurons appear more strongly involved in salience coding (Matsumoto and Takada, 2013). This suggests a role for the stress-induced increase in CRFr1 expression in processes such as orienting or preparatory strategies to confront new or uncertain events (Kelly and Fudge, 2018). As VTA CRFr1 activation enhances DA output into the prelimbic cortex, the large increase in CRFr1 expression following AS in naïve rats most likely indicates a significant increase in DA in the prelimbic. Although the pattern of increased CRFr1 expression following AS was also found in CUS rats, the magnitude of the increase was smaller, failing to reach significance. This suggests that the increased DA in the prelimbic would also be attenuated and might not be sufficient to sustain adequate function in the face of acute stress. It is important to note that the link between an increase in CRFr1 mRNA and subsequent changes in DA is based on the assumption that the 30 min timeframe is sufficient for translational and functional effects. This will need to be confirmed in future studies.
While it is known that an acute stress increases activation of dopaminergic neurons projecting specifically to the prelimbic cortex and reducing the expression of CRFr1 in the VTA reduces DA levels in the mPFC, (Refojo et al., 2011; Vranjkovic et al., 2018), it is not clear if the attenuated CRFr1 expression we found reflects reduced activation in these subgroups of neurons specifically. A causative link between the attenuated expression and the learning impairment is yet to be established. It is important to notice that the changes we have found in CRFr1 mRNA expression were in response to a foot shock, and not to an additional forced swim stress. This was done as the study aimed to test the effect of a novel stress on CRFr1 expression, and habituation to the forced swim could have possibly occurred by that stage. However, it is possible that the two stresses produced variable levels of CRFr1 expression, or that the exposure to the forced swim habituated or sensitised the response to the foot shock, and therefore the magnitude of the CRFr1-increased expression, in response to the forced swim prior to reversal training might be varied.
CRF action in the VTA has also been suggested to influence motivational drive via an influence on dopaminergic output to the nucleus accumbens (NAc) (Wanat et al., 2013). It was not clear, however, if this was achieved by activation of CRFr1 VTA neurons projecting to the NAc or indirectly by influencing dopamine release in the NAc by the mPFC (Deutch et al., 1991; Doherty and Gratton, 1996; Lammel et al., 2012); AS reduced motivational drive and dopamine release in NAc in response to a food reward. Blocking CRF activity in the VTA was found to reverse this effect and infusing CRF into the VTA reduced motivational drive without stress. The CRF input to the VTA in vivo was also found to gate afferent inputs to the VTA in a stimulus- and pathway-specific manner and led both to increased and decreased dopamine release in NAc, depending on the source of the CRF (Wanat et al., 2013). These effects are generally consistent with the reduction in pressing rates and increased CRFr1 expression in the VTA we observed after AS. After CUS, on the other hand, although rats still showed a persistent reduction in pressing rates, they did not show increased CRFr1 expression, suggesting that the role of CRF in the VTA in regulating motivational drive may differ in naïve and chronically stressed rats.
There are several processes that may underly attenuated CRFr1 expression after acute stress in CUS rats. A major regulator of the CRFr1 receptor expression is CRF itself (Parham et al., 2004) which is found in a range of structures known to regulate behavioural responses to stress, such as the lateral bed nucleus of stria terminalis, the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus (Rodaros et al., 2007). Attenuation of the CRF input to the VTA from these structures could in turn have led to attenuated CRFr1 expression. Stress-induced epigenetic modifications and expression of CRFr1 promoter regions have also been found in the amygdala, hypothalamus and mPFC (Sotnikov et al., 2014; Viola et al., 2019; Wan et al., 2014) and, therefore, it is possible that a similar effect might alter CRFr1 expression in the VTA following CUS.
4.3. Disconnecting CRF containing neurons in the VTA- D1 containing neurons in the prelimbic cortex impairs learning during stress
The current study also found that CRFr1-related activity in VTA neurons projecting to the PL is essential for cognitive flexibility after stress. Rats in which this pathway was disconnected during reversed contingency training showed similar performance to control rats but did not update changes in the action-outcome contingencies during outcome identity reversal and instead relied on previous learning to choose a course of action. Activation of CRFr1 in the VTA enhances dopamine release in the PL and activates D1 receptors on pyramidal neurons. This process promotes glutamate co-activation, stabilises heightened excitability and brings the membrane potential of pyramidal neurons closer to threshold, thereby enabling a prolonged PL activity (Lewis and O'Donnell, 2000; Thurley et al., 2008). Activation of D1 receptors in the PL has long been implicated in important cognitive functions particularly working memory (Sawaguchi and Goldman-Rakic, 1991; Seamans et al., 1998) and this function of the PL is pivotal for the encoding of new action-outcome associations (Balleine, 2019). The difference in the pattern of deficits induced in Experiments 1 and 2 is important in this regard. Note that, although both CUS plus AS rats in the Experiment 1 and the contralateral group in Experiment 2 could not encode the updated contingencies, in the former, CUS plus AS had a profound effect on choice and animals could not use either the initially learned or the updated contingencies to control performance. This suggests both the stability of initial learning and the ability to update that learning was affected by this treatment. In contrast, after more discrete disconnection of the VTA-PL pathway, only the ability to encode the new contingencies was affected and prior learning was spared. Thus, whereas CUS + AS affects the whole goal-directed circuit, including striatum and its thalamic input, disconnection was specific to the function of the VTA-PL pathway, suggesting the PL is engaged in new learning and not the maintenance of previous learning.
Although dopamine levels in the mPFC are immediately elevated after stress, they peak 20–30 min after stress commencement indicating sustained and enhanced activity in the dopaminergic projection (Pascucci et al., 2007). The current study suggests, therefore, that sustained CRFr1 mRNA expression in the VTA could be a mechanism maintaining dopamine release in the PL, as CRFr1 mRNA was significantly elevated 30 min after stress and blocking this pathway prevented learning for at least 90 min after the stress; i.e., for the duration of the stress and the training session. Therefore, sustained CRFr1 expression is likely to sustain D1 activation of pyramidal neurons, maintaining the NMDA-dependent heightened “up” state and so executive function following stress. In line with this, CUS has been reported to increase D1 receptor excitability in the PL cortex (Anderson et al., 2019) and to cause morphological changes associated with PL impairment in a D1 dependent manner (Lin et al., 2015). As such, although the pathways is important for maintaining behavioural flexibility after AS, it may well form part of the mechanism leading to stress-related pathology in the long term.
5. Conclusions
In summary, this study further develops our understanding of the interaction between the CRF and the VTA DA systems in the brain and how this interaction facilitates cognitive processes following stress. We have shown that exposure to either AS or CUS alone does not impair cognitive flexibility as assessed by the ability to update changes in action-outcome associations for goal-directed action, but the combination of CUS and AS compromises the rats’ ability to maintain behavioural flexibility. We have also shown that exposure to AS increases CRFr1 expression in the VTA, a process that was attenuated in rats previously exposed to CUS. Finally, CRFr1-related activity in VTA neurons projecting to D1 containing neurons in the PL is essential for cognitive flexibility after stress.
CRediT authorship contribution statement
David Mor: Conceptualization, Investigation, Methodology, Validation, Formal analysis, Writing – original draft, Writing – review & editing, Visualization, Funding acquisition. Serena Becchi: Investigation, Formal analysis, Writing – original draft, Visualization. Jeremy Bowring: Investigation, Formal analysis, Writing – original draft. Madeline Tsoukalas: Investigation. Bernard W. Balleine: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by a grant from the Australian Research Council, DP160105070, and a Senior Investigator Award from the NHMRC, GNT1175420, to BWB.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100424.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary figure: Supplementary Descriptors of Behavioural Protocol
Data availability
Data will be made available on request.
References
- Anderson E.M., Gomez D., Caccamise A., McPhail D., Hearing M. Chronic unpredictable stress promotes cell-specific plasticity in prefrontal cortex D1 and D2 pyramidal neurons. Neurobiol. Stress. 2019;10 doi: 10.1016/j.ynstr.2019.100152. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A.F. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10(6):410–422. doi: 10.1038/nrn2648. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B.W. The meaning of behavior: discriminating reflex and volition in the brain. Neuron. 2019;104(1):47–62. doi: 10.1016/j.neuron.2019.09.024. Oct 9. [DOI] [PubMed] [Google Scholar]
- Balleine B.W., Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37(4–5):407–419. doi: 10.1016/s0028-3908(98)00033-1. Apr-May. [DOI] [PubMed] [Google Scholar]
- Bradfield L.A., Balleine B.W. Thalamic control of dorsomedial striatum regulates internal state to guide goal-directed action selection. J. Neurosci. 2017;37(13):3721–3733. doi: 10.1523/jneurosci.3860-16.2017. Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield L.A., Bertran-Gonzalez J., Chieng B., Balleine B.W. The thalamostriatal pathway and cholinergic control of goal-directed action: interlacing new with existing learning in the striatum. Neuron. 2013;79(1):153–166. doi: 10.1016/j.neuron.2013.04.039. Jul 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit L.H., Balleine B.W. The role of prelimbic cortex in instrumental conditioning. Behav. Brain Res. 2003;146(1–2):145–157. doi: 10.1016/j.bbr.2003.09.023. Nov 30. [DOI] [PubMed] [Google Scholar]
- Deutch A.Y., Lee M.C., Gillham M.H., Cameron D.A., Goldstein M., Iadarola M.J. Stress selectively increases fos protein in dopamine neurons innervating the prefrontal cortex. Cerebr. Cortex. 1991;1(4):273–292. doi: 10.1093/cercor/1.4.273. Jul-Aug. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E., Sousa J.C., Melo I., Morgado P., Mesquita A.R., Cerqueira J.J., Costa R.M., Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325(5940):621–625. doi: 10.1126/science.1171203. Jul 31. [DOI] [PubMed] [Google Scholar]
- Doherty M.D., Gratton A. Medial prefrontal cortical D1 receptor modulation of the meso-accumbens dopamine response to stress: an electrochemical study in freely-behaving rats. Brain Res. 1996;715(1–2):86–97. doi: 10.1016/0006-8993(95)01557-4. Apr 9. [DOI] [PubMed] [Google Scholar]
- Gallagher J.P., Orozco-Cabal L.F., Liu J., Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur. J. Pharmacol. 2008;583(2–3):215–225. doi: 10.1016/j.ejphar.2007.11.075. Apr 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou P., Zanos P., Bhat S., Tracy J.K., Merchenthaler I.J., McCarthy M.M., Gould T.D. Dopamine and stress system modulation of sex differences in decision making. Neuropsychopharmacology. 2018;43(2):313–324. doi: 10.1038/npp.2017.161. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtubise J.L., Howland J.G. Effects of stress on behavioral flexibility in rodents. Neuroscience. 2017;345:176–192. doi: 10.1016/j.neuroscience.2016.04.007. Mar 14. [DOI] [PubMed] [Google Scholar]
- Kelly E.A., Fudge J.L. The neuroanatomic complexity of the CRF and DA systems and their interface: what we still don't know. Neurosci. Biobehav. Rev. 2018;90:247–259. doi: 10.1016/j.neubiorev.2018.04.014. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6) doi: 10.1371/journal.pbio.1000412. Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse M.S., Prémont J., Krebs M.O., Jay T.M. Interaction of dopamine D1 with NMDA NR1 receptors in rat prefrontal cortex. Eur. Neuropsychopharmacol. 2009;19(4):296–304. doi: 10.1016/j.euroneuro.2008.12.006. Apr. [DOI] [PubMed] [Google Scholar]
- Lammel S., Lim B.K., Ran C., Huang K.W., Betley M.J., Tye K.M., Deisseroth K., Malenka R.C. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491(7423):212–217. doi: 10.1038/nature11527. Nov 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B.L., O'Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential 'up' states in pyramidal neurons via D(1) dopamine receptors. Cerebr. Cortex. 2000;10(12):1168–1175. doi: 10.1093/cercor/10.12.1168. Dec. [DOI] [PubMed] [Google Scholar]
- Lin G.L., Borders C.B., Lundewall L.J., Wellman C.L. D1 receptors regulate dendritic morphology in normal and stressed prelimbic cortex. Psychoneuroendocrinology. 2015;51:101–111. doi: 10.1016/j.psyneuen.2014.09.020. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S.F., Seligman M.E. Learned helplessness at fifty: insights from neuroscience. Psychol. Rev. 2016;123(4):349–367. doi: 10.1037/rev0000033. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Takada M. Distinct representations of cognitive and motivational signals in midbrain dopamine neurons. Neuron. 2013;79(5):1011–1024. doi: 10.1016/j.neuron.2013.07.002. Sep. 4. [DOI] [PubMed] [Google Scholar]
- Matuszewich L., McFadden L.M., Friedman R.D., Frye C.A. Neurochemical and behavioral effects of chronic unpredictable stress. Behav. Pharmacol. 2014;25(5–6):557–566. doi: 10.1097/fbp.0000000000000061. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor D., Kendig M.D., Kang J.W.M., Gemikonakli G., Austin P.J., Kalman E., Corbit L.H. Peripheral nerve injury impairs the ability to maintain behavioural flexibility following acute stress in the rat. Behav. Brain Res. 2017;328:123–129. doi: 10.1016/j.bbr.2017.04.003. Jun 15. [DOI] [PubMed] [Google Scholar]
- Orozco-Cabal L., Pollandt S., Liu J., Shinnick-Gallagher P., Gallagher J.P. Regulation of synaptic transmission by CRF receptors. Rev. Neurosci. 2006;17(3):279–307. doi: 10.1515/revneuro.2006.17.3.279. [DOI] [PubMed] [Google Scholar]
- Parham K.L., Zervou S., Karteris E., Catalano R.D., Old R.W., Hillhouse E.W. Promoter analysis of human corticotropin-releasing factor (CRF) type 1 receptor and regulation by CRF and urocortin. Endocrinology. 2004;145(8):3971–3983. doi: 10.1210/en.2004-0194. Aug. [DOI] [PubMed] [Google Scholar]
- Pascucci T., Ventura R., Latagliata E.C., Cabib S., Puglisi-Allegra S. The medial prefrontal cortex determines the accumbens dopamine response to stress through the opposing influences of norepinephrine and dopamine. Cerebr. Cortex. 2007;17(12):2796–2804. doi: 10.1093/cercor/bhm008. Dec. [DOI] [PubMed] [Google Scholar]
- Refojo D., Schweizer M., Kuehne C., Ehrenberg S., Thoeringer C., Vogl A.M., Dedic N., Schumacher M., von Wolff G., Avrabos C., Touma C., Engblom D., Schütz G., Nave K.A., Eder M., Wotjak C.T., Sillaber I., Holsboer F., Wurst W., Deussing J.M. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science. 2011;333(6051):1903–1907. doi: 10.1126/science.1202107. Sep. 30. [DOI] [PubMed] [Google Scholar]
- Rodaros D., Caruana D.A., Amir S., Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150(1):8–13. doi: 10.1016/j.neuroscience.2007.09.043. Nov 30. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T., Goldman-Rakic P.S. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251(4996):947–950. doi: 10.1126/science.1825731. Feb 22. [DOI] [PubMed] [Google Scholar]
- Seamans J.K., Floresco S.B., Phillips A.G. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J. Neurosci. 1998;18(4):1613–1621. doi: 10.1523/jneurosci.18-04-01613.1998. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira-Cordero A., Salas-Bastos A., Fornaguera J., Brenes J.C. Behavioural characterisation of chronic unpredictable stress based on ethologically relevant paradigms in rats. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-53624-1. Nov 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotnikov S.V., Markt P.O., Malik V., Chekmareva N.Y., Naik R.R., Sah A., Singewald N., Holsboer F., Czibere L., Landgraf R. Bidirectional rescue of extreme genetic predispositions to anxiety: impact of CRH receptor 1 as epigenetic plasticity gene in the amygdala. Transl. Psychiatry. 2014;4(2) doi: 10.1038/tp.2013.127. Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M. Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Stress. 2004;7(2):131–143. doi: 10.1080/102538900410001679310. Jun. [DOI] [PubMed] [Google Scholar]
- Thurley K., Senn W., Lüscher H.R. Dopamine increases the gain of the input-output response of rat prefrontal pyramidal neurons. J. Neurophysiol. 2008;99(6):2985–2997. doi: 10.1152/jn.01098.2007. Jun. [DOI] [PubMed] [Google Scholar]
- Tseng K.Y., O'Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J. Neurosci. 2004;24(22):5131–5139. doi: 10.1523/jneurosci.1021-04.2004. Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola T.W., Wearick-Silva L.E., Creutzberg K.C., Kestering-Ferreira É., Orso R., Centeno-Silva A., Albrechet-Souza L., Marshall P.R., Li X., Bredy T.W., Riva M.A., Grassi-Oliveira R. Postnatal impoverished housing impairs adolescent risk-assessment and increases risk-taking: a sex-specific effect associated with histone epigenetic regulation of Crfr1 in the medial prefrontal cortex. Psychoneuroendocrinology. 2019;99:8–19. doi: 10.1016/j.psyneuen.2018.08.032. Jan. [DOI] [PubMed] [Google Scholar]
- Vranjkovic O., Van Newenhizen E.C., Nordness M.E., Blacktop J.M., Urbanik L.A., Mathy J.C., McReynolds J.R., Miller A.M., Doncheck E.M., Kloehn T.M., Stinnett G.S., Gerndt C.H., Ketchesin K.D., Baker D.A., Seasholtz A.F., Mantsch J.R. Enhanced CRFR1-dependent regulation of a ventral tegmental area to prelimbic cortex projection establishes susceptibility to stress-induced cocaine seeking. J. Neurosci. 2018;38(50):10657–10671. doi: 10.1523/jneurosci.2080-18.2018. Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Q., Gao K., Rong H., Wu M., Wang H., Wang X., Wang G., Liu Z. Histone modifications of the Crhr1 gene in a rat model of depression following chronic stress. Behav. Brain Res. 2014;271:1–6. doi: 10.1016/j.bbr.2014.05.031. Sep. 1. [DOI] [PubMed] [Google Scholar]
- Wanat M.J., Bonci A., Phillips P.E. CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat. Neurosci. 2013;16(4):383–385. doi: 10.1038/nn.3335. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52(2):90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Wise R.A., Morales M. A ventral tegmental CRF-glutamate-dopamine interaction in addiction. Brain Res. 2010;1314:38–43. doi: 10.1016/j.brainres.2009.09.101. Feb 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure: Supplementary Descriptors of Behavioural Protocol
Data Availability Statement
Data will be made available on request.