Abstract
Cucumber is an important vegetable but highly sensitive to salt stress. The present study was designed to investigate the comparative performance of cucumber genotypes under salt stress (50 mmol L−1) and stress alleviation through an optimized level of triacontanol @ 0.8 mg L−1. Four cucumber genotypes were subjected to foliar application of triacontanol under stress. Different physiological, biochemical, water relations and ionic traits were observed to determine the role of triacontanol in salt stress alleviation. Triacontanol ameliorated the lethal impact of salt stress in all genotypes, but Green long and Marketmore were more responsive than Summer green and 20252 in almost all the attributes that define the genetic potential of genotypes. Triacontanol performs as a good scavenger of ROS by accelerating the activity of antioxidant enzymes (SOD, POD, CAT) and compatible solutes (proline, glycinebetaine, phenolic contents), which lead to improved gas exchange attributes and water relations and in that way enhance the calcium and potassium contents or decline the sodium and chloride contents in cucumber leaves. Furthermore, triacontanol feeding also shows the answer to yield traits of cucumber. It was concluded from the results that the salinity tolerance efficacy of triacontanol is valid in enhancing the productivity of cucumber plants under salt stress. Triacontanol was more pronounced in green long and marketer green than in summer green and 20252. Hence, the findings of this study pave the way towards the usage of triacontanol @ 0.8 mg L−1, and green long and marketer genotypes may be recommended for saline soil.
Subject terms: Ecology, Agroecology
Introduction
Almost 7.0% of the world’s total area is affected by salinization1, representing more than 900 million hectares of land of the world affected by both saline and sodic conditions2, which covers 20% of the cultivated land and half of the irrigated area subjected to high salt levels3. Salt affected soils are a significant problem in Pakistan’s agriculture4,5. Of that total, approximately 6.3 million hectares or 14% of the irrigated land is now affected by salinity6.
Abiotic stresses alter the response of plant growth hormones, resulting in reduced growth and final yield of crop plants7. Once plants are subjected to salinity stress, the balance between the production of reactive oxygen species (ROS) and the quenching action of antioxidant enzymes becomes upset, and the resulting plants experience oxidative stress8. Plants that have higher concentrations of antioxidant enzymes, either constitutive or induced, have healthy resistance to oxidative damage; for example, superoxide dismutase (SOD), guaiacol peroxidase (POD), catalase (CAT), ascorbate peroxidase (APOX) and glutathione reductase (GR) enhance salt tolerance in plants under saline stress9. Additionally, phytohormones have been reported to play a regulatory role in plant growth and development, programmed cell death and survival under changing environmental conditions10. Triacontanol is a saturated primary alcohol and component of plant epicuticular waxes with plant growth promoting properties11,12 and shows growth stimulating properties at low concentrations13, and its foliar application produced significant positive changes in plant photosynthetic pigments, solute accumulation, growth and biomass production under salt stress conditions14,15. Foliar feeding of triacontanol induced stress tolerance16, by regulating the activities of many antioxidant enzymes17, increased the level of osmoprotectants in plant leaf tissues11,18,19, and improved the uptake of essential minerals K+ and Ca2+ instead of Na+14. In addition, triacontanol produced more reducing sugars, soluble proteins and amino acids under stressed environments20–22.
Vegetables are a rich source of phytochemicals and nutrients, which are essential for many metabolic actions in the human body23, and vegetable production is endangered by rising soil salinization, predominantly in irrigated croplands, which produce 40.0% of the world’s food requirements24. Vegetable crops such as onions, cucumbers, eggplants, peppers, and tomatoes are sensitive to salinity25, and cucumber is an important vegetable for human nutrition worldwide26. Salt stress had a significant consequence on the growth rate of cucumber; however, salinity levels higher than 25 mM caused a decline in yield up to 13%27,28. Deleterious effects of salinity on cucumber lead to decreased plant growth and productivity5,29,30. Hence, the current investigation was conducted with the aim of appraising the role of triacontanol in alleviating saline stress in cucumber genotypes and evaluating the ability of triacontanol to stimulate plant growth and productivity. Such information is also key for suggesting a suitable strategy for salt-affected soils. Furthermore, we determined the variations in ionic homeostasis of cucumber genotypes in response to NaCl stress.
Materials and methods
The current study was conducted under the lath house conditions (30–35 °C and relative humidity 40–50%) of the Institute of Horticultural Sciences, University of Agriculture, Faisalabad, Pakistan (31° 25′ 05.10″ N, 73° 04′ 39.27″ E). It has been confirmed that the experimental samples of plants, including the collection of plant material, complied with relevant institutional, national, and international guidelines and legislation with appropriate permissions from Institute authorities of Institute of Horticultural Sciences, University of Agriculture, Faisalabad Pakistan for collection of plant specimens. In advance of the experiment, the cucumber seed surface was sterilized in a 3% solution of sodium hypochlorite for ten minutes and then properly washed with distilled water and air-dried at 25 °C. Four contrasting cucumber genotypes (Green long, Marketmore, Summer green and 20252)31, with varying degrees of salinity tolerance, were used for this experiment. Seeds were sown in plastic 14-L pots in quartz sand with a pH of 6.0–6.5, a field capacity of 7.20% and incipient wilting at a 1.20% volume basis. Four treatments, i.e., T0 = control (nonsaline + deionized water spray), T1 = (saline + deionized water spray), T2 = triacontanol spray (0.80 mg L−1) and T3 = triacontanol + saline (0.80 mg L−1 + NaCl 50 mmol L−1) were applied with four replicates. After ten days of emergence, two plants per pot were maintained by thinning out extra plants. Half strength of Hoagland and Arnon (1950)31 solution was used as a nutrient source. Salt stress was based on NaCl (MERCK, CAS #-7647-14-5), and NaCl was applied after fifty days of seed emergence in the form of salt solution. Moreover, salt stress was developed at intervals to avoid the osmotic shock of sodium chloride by adjusting 10 mmol L−1on Ist day and then gradually increasing after one day intervals until the desired salt level (50 mmol L−1) was achieved31. The levels of salinity were maintained throughout the experiment by recording the EC and pH of the growing media. Foliar spray of triacontanol was performed using a hand sprayer pump with a full cone nozzle; an already optimized dose of triacontanol (0.8 mg L−1) was sprayed after 72 h of stress imposition, while the 2nd spray was applied at the flowering stage and the 3rd spray was applied at the fruit maturing stage. Tween-20 (0.1%) was added to the spray solution as a surfactant to ensure the maximum absorption of the triacontanol solution in the plant tissue. The following data were recorded to evaluate the potential role of triacontanol application.
Stomatal conductance (gs), photosynthetic activity (Pn) and transpiration rate (E) were measured with a portable apparatus termed IRGA (Analytical Development Company, Hoddesdon, England). All readings of the abovementioned physiological attributes were taken at daytime from 11.00 to 12.00 a.m. by describing a method of32,33. SOD activity was examined according to the described procedure of Giannopolitis and Ries34. Peroxidase (POD) and catalase (CAT) activities were calculated by the method of Chance and Maehly35. For the estimation of chlorophyll contents, a meter model (SPAD-502, Konica Minolta Sensing, Inc; Japan) was used to determine the greenness of cucumber plant leaves. Measurements were taken from fully expanded third to fourth youngest leaves from the apex36. Plant cell membrane leakage was calculated using an EC electrical conductivity meter by following the procedure of Lutts37. The proline contents were recorded by the described procedure of Bates et al.38 and Glycine betaine Grieve and Gratan’s39 method. Total phenolic contents were calculated by the described methodology of Julkenen40 by using a spectrophotometer (Model: Hitachi-120, Japan).
Leaf water potential (− Ψw) was measured by a pressure chamber (Model 1000, PMS Instrument Co., Albany, NY, USA) to observe that Ψw data were recorded in the early morning before sunrise (6.00 am). For leaf osmotic potential (− Ψs), the same leaf that was used in a pressure chamber for Ψs was placed in a zipper bag and kept at − 80 °C for one week. Then, the leaf material was thawed at room temperature for 30 min, a disposable syringe was used to extract the cell sap, 10 μL of sap was placed on the sensor of the osmometer (Wescor Model-5500) with a disposable syringe, and readings were noted. Leaf turgor potential (Ψp) is measured by the difference between water (Ψw), and osmotic (Ψπ) potential is known as turgor potential (Ψp). Therefore, Ψp was calculated by the following equation: Ψp = Ψw − Ψπ. Leaf relative water contents (LRWC) recorded by the reported method of Wheatherly and Barrs41. LRWC (%) = [(FW − DW)/(TW − DW)] × 100. Leaf Na+, K+ and Ca+ were determined by a flame photometer (Jenway PFP-7, UK) by the described procedure of Yoshida et al. For chloride (Cl−) determination, leaf material was oven-dried at 65 °C and ground to form a powder. This ground dry leaf material (1 g) was heated in a test tube overnight in 20 ml distilled water at 65 °C in an oven. After obtaining the extract, it was filtered with Whatman-40 filter paper and used for the estimation of Cl− ions with a chloride analyzer (Corning-920; Germany). Total soluble solids (TSS) were estimated by using a digital refractometer (PR-32α, ATAGO, CO; LTD. Tokyo, Japan) that gives the °Brix value, and this method was described by42.
Statistical analysis
The experiment was designed with CRD factorial. Analysis of variance (ANOVA) and multiple comparison test (Tukey test) were computed using Statistix 8.1 computer packages. Differences among treatments were considered significant only when a value was lower than P ≤ 0.05 after statistical analysis.
Results
Stomatal conductance, photosynthesis rate, transpiration rate and water use efficiency
Data for photosynthetic attributes (gs, Pn, E) significantly varied due to NaCl-based salinity and foliar spray of triacontanol (Table 1). A substantial reduction in stomatal conductance was noted in all cucumber genotypes exposed to salt stress. Salt-sensitive genotypes showed a drastic reduction in this gas exchange attribute and resulted in the least decline exhibited by tolerant genotypes; they maintained better conductance of stomata under the saline regime. Foliar application of triacontanol significantly improved stomatal conductance under stressed and nonstressed conditions. Under salinity, the maximum improvement in stomatal conductance was exhibited by Marketmore, followed by Green long in response to foliar-applied triacontanol. On the other hand, transpiration and photosynthetic rate were significantly suppressed in all tested cucumber genotypes due to NaCl stress (Table 1). The summer green genotype showed a better rate of transpiration and photosynthetic rate in the non-saline control but failed to maintain them under salt stress conditions and expressed minimum values for these gas exchange attributes. Foliar application of triacontanol significantly overcomes the lethal effects of salt stress and ameliorates the gas exchange properties of cucumber genotypes. Among the genotypes, Marketmore efficiently responded to exogenously applied triacontanol and resulted in an improved transpiration rate and rate of photosynthesis. Similar to gs, E and Pn, the water use efficiency of cucumber genotypes was also affected by NaCl-based salinity. Summer green and 20252 genotypes showed minimum WUE under stressed conditions, while they showed little improvement with triacontanol, while Marketmore performed well and showed maximum WUE in response to salt stress. Cucumber genotypes subjected to foliar spray of triacontanol significantly improved WUE; however, green long gave higher value for WUE under control and salinity as well and followed by Marketmore.
Table 1.
Effect of triacontanol on leaf gas attributes of four cucumber genotypes under normal and salt stress conditions.
| Treatments | Green long | Marketmore | Summer green | 20252 | Green long | Marketmore | Summer green | 20252 |
|---|---|---|---|---|---|---|---|---|
| Stomatal conductance (μmol m−2 s−1) | Photosynthesis rate (μmol m−2 s−1) | |||||||
| Control | 5.25 ± 0.21cd | 5.49 ± 0.21bc | 5.27 ± 0.19de | 5.56 ± 0.29bc | 3.57 ± 0.03bc | 3.81 ± 0.05bc | 3.27 ± 0.23cd | 3.72 ± 0.07bc |
| Salinity (50 mmol L−1) | 3.87 ± 0.20fg | 4.27 ± 0.13gh | 2.66 ± 0.10j | 2.90 ± 0.34ij | 2.79 ± 0.027def | 2.82 ± 0.13de | 1.90 ± 0.02g | 2.09 ± 0.03ef |
| Triacontanol spray + non-saline | 5.90 ± 0.21ab | 6.07 ± 0.22a | 5.74 ± 0.18bc | 6.13 ± 0.27abc | 4.12 ± 0.11ab | 4.52 ± 0.18a | 3.68 ± 0.10bc | 4.22 ± 0.23ab |
| Triacontanol spray + salinity | 4.56 ± 0.22de | 4.64 ± 0.12ef | 3.65 ± 0.38hi | 3.74 ± 0.45gh | 3.21 ± 0.11cd | 3.28 ± 0.23cd | 2.36 ± 0.04efg | 2.81 ± 0.24de |
| Transpiration rate (mmol H2O m−2 s−1) | Water use efficiency (Pn/E) | |||||||
| Control | 2.64 ± 0.02abc | 2.78 ± 0.03abc | 2.64 ± 0.05abc | 2.86 ± 0.01abc | 1.65 ± 0.04ab | 1.52 ± 0.04ab | 1.31 ± 0.04b | 1.37 ± 0.05b |
| Salinity (50 mmol L−1) | 2.04 ± 0.12cde | 2.04 ± 0.15def | 1.59 ± 0.16ef | 1.67 ± 0.24f | 1.29 ± 0.09b | 1.34 ± 0.11ab | 0.92 ± 0.12b | 0.94 ± 0.20b |
| Triacontanol spray + non-saline | 2.90 ± 0.23abc | 3.1 ± 0.29ab | 2.86 ± 0.14abc | 3.20 ± 0.15a | 1.68 ± 0.07a | 1.47 ± 0.22ab | 1.36 ± 0.05ab | 1.22 ± 0.07b |
| Triacontanol spray + salinity | 2.4 ± 0.06bcd | 2.49 ± 0.13abcd | 2.14 ± 0.12cdef | 2.17 ± 0.20cde | 1.35 ± 0.02ab | 1.29 ± 0.08b | 1.09 ± 0.06b | 1.20 ± 0.11b |
Data represent the means ± SE of four repeats.
Means with different letters are significantly different at P ≤ 0.05 according to Tukey’s HSD test (Salt tolerant, Green long and Marketmore) (Salt sensitive Summer green and 20252).
Antioxidant enzyme activity
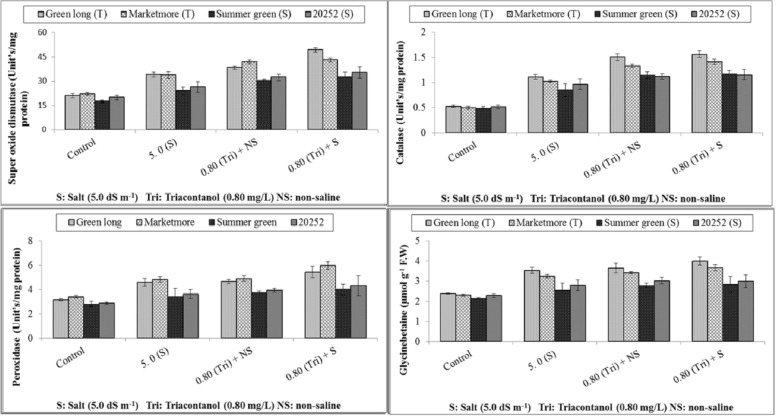
Figure 1 reveals the effects of foliar spray of triacontanol and salinity treatment on the activities of the studied antioxidants in cucumber genotypes. SOD activity considerably increased with salt stress, especially in green long and marketer, whereas summer green and 20252 showed little increase in SOD activity under stressed and nonstressed environments. Exogenously applied triacontanol caused a further increase in SOD activity in green long followed by marketmore, while summer green showed the minimum value for SOD in response to triacontanol application. Similar to SOD, the POD and CAT activities were also significantly enhanced in all cucumber genotypes exposed to salt stress. However, Green long presented maximum activities of CAT in response to salt stress and foliar applied triacontanol, followed by Marketmore. Likewise, the higher scavenging activity of POD was also noted for Marketmore, while Summer green expressed the lowest values of POD in their leaves under all experimental conditions.
Figure 1.
Effect of triacontanol on leaf antioxidants and osmoprotectants of four cucumber genotypes under normal and salt stress conditions.
Estimation of organic solutes
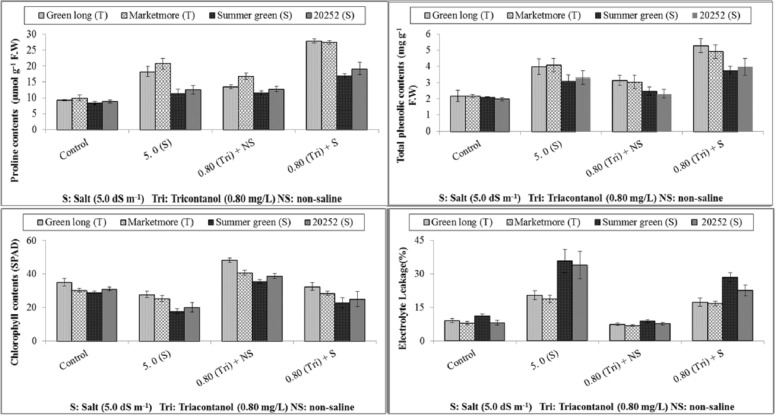
The addition of salinity stress increased the accumulation of osmoprotectants (glycine betaine (GB) and proline (Pro) and antioxidant phenols in all cucumber genotypes. Green long revealed high values of GB and Pro contents by significantly enhancing the endogenous levels under saline regime. All the cucumber genotypes countered the foliar spray and improved the contents of GB and pro; however, long-term green treatment with exogenously applied triacontanol significantly increased the GB and Pro contents and showed maximum values for these attributes. On the other hand, the poor response of Summer green and 20252 to foliar spray of triacontanol was observed and resulted in lower values of GB and Pro, respectively, under salt stress (Table 2 & Fig. 2). In the case of total phenols (TP), a similar trend was found, where green long significantly accumulated the highest total phenolics, followed by marketmore supplied with foliar triacontanol under all growing environments. However, minimum accumulation of TP content was noted in summer green under saline stress and triacontanol treatment.
Table 2.
Effect of triacontanol on leaf water relations of four cucumber genotypes under normal and salt stress conditions.
| Treatments | Green long | Marketmore | Summer green | 20252 | Green long | Marketmore | Summer green | 20252 |
|---|---|---|---|---|---|---|---|---|
| Water potential (Ψw) (− MPa) | Osmotic potential (Ψπ) (− MPa) | |||||||
| Control | 0.44 ± 0.04def | 0.44 ± 0.03ef | 0.54 ± 0.04cde | 0.46 ± 0.05def | 0.72 ± 0.04efg | 0.62 ± 0.04fg | 0.84 ± 0.05cde | 0.77 ± 0.05efg |
| Salinity (50 mmol L−1) | 0.73 ± 0.05bcd | 0.64 ± 0.04bcd | 1.03 ± 0.10a | 0.85 ± 0.10ab | 1.08 ± 0.04cde | 0.99 ± 0.06cdef | 1.67 ± 0.10a | 1.50 ± 0.14ab |
| Triacontanol spray + non-saline | 0.38 ± 0.05ef | 0.39 ± 0.05ef | 0.57 ± 0.03bcd | 0.35 ± 0.03f | 0.64 ± 0.05fg | 0.54 ± 0.03g | 0.80 ± 0.05cdef | 0.69 ± 0.04efg |
| Triacontanol spray + salinity | 0.63 ± 0.05bcd | 0.54 ± 0.04cde | 0.84 ± 0.05ab | 0.75 ± 0.08abc | 0.95 ± 0.10cdef | 0.88 ± 0.05cdef | 1.24 ± 0.13bc | 1.18 ± 0.16bcd |
| Turgor potential (Ψp) (MPa) | Leaf relative water contents (%) | |||||||
| Control | 0.39 ± 0.02bcd | 0.35 ± 0.01cde | 0.32 ± 0.01def | 0.33 ± 0.01def | 77.5 ± 3.93ab | 81.25 ± 2.21a | 73.25 ± 5.11abc | 75 ± 3.87abc |
| Salinity (50 mmol L−1) | 0.29 ± 0.01fg | 0.20 ± 0.02ghi | 0.16 ± 0.01i | 0.18 ± 0.00hi | 59.5 ± 4.11bcd | 58 ± 2.48cde | 46 ± 5.85e | 43.75 ± 5.07e |
| Triacontanol spray + non-saline | 0.51 ± 0.02a | 0.45 ± 0.01ab | 0.41 ± 0.01bcd | 0.43 ± 0.01abc | 81 ± 1.47a | 83.75 ± 1.89a | 75.25 ± 0.85abc | 77.25 ± 1.11ab |
| Triacontanol spray + salinity | 0.39 ± 0.019bcd | 0.30 ± 0.023ef | 0.26 ± 0.019 fgh | 0.28 ± 0.012fg | 67.25 ± 3.64abc | 70.75 ± 2.46abcd | 58.5 ± 5.11cde | 55.5 ± 7.27de |
Data represent the means ± SE of four repeats.
Means with different letters are significantly different at P ≤ 0.05 according to Tukey’s HSD test (Salt tolerant, Green long and Marketmore) (Salt sensitive Summer green and 20252).
Figure 2.
Effect of triacontanol on leaf osmoprotectants and physiological attributes of four cucumber genotypes under normal and salt stress conditions.
Photosynthetic pigments and electrolyte leakage
The results showed that the chlorophyll content of cucumber genotypes was considerably influenced by salt stress. The greenness of cucumber genotypes decreased under salt stress, and a maximum reduction was observed in summer green. In contrast, long green fruits maintained better chlorophyll contents upon exposure to salinity (Fig. 2). All cucumber genotypes effectively enhanced chlorophyll contents upon treatment with foliar-applied triacontanol. Among the hybrids, green long produced maximum chlorophyll contents, while the lowest value was recorded for salt-sensitive summer green, which was revealed by foliar spray of triacontanol in saline environments. Salt stress remarkably affects membrane stability in cucumber genotypes (Fig. 2). Maximum electrolyte leakages were observed in hybrid 20252 followed by Marketmore, whereas Green long explored least electrolyte leakage, which indicates its higher membrane integrity under non-saline controlled conditions. Upon exposure to salinity, Marketmore treated with foliar triacontanol showed maximum membrane stability with low electrolyte leakage compared to other genotypes (Fig. 2).
Water relations
Exogenously applied triacontanol significantly affected the water relations of the four cucumber genotypes under the NaCl stress regime (Table 2). The maximum reduction in osmotic and water potentials was recorded in the summer green genotype; however, Marketmore secured the best water potential when salt stress was imposed. Likewise, a pattern was observed when these genotypes were supplied with foliar sprays of triacontanol compared to untreated plants. The addition of salt stress significantly reduced the turgor potential in the leaves of all the genotypes. However, foliar feeding of triacontanol improved turgor potential in salt-stressed plants compared to their respective controls. The cucumber genotype Green maintained the highest turgor pressure, followed by Marketmore influence by salt stress and triacontanol. However, summer green results in lower values of turgor potential when subjected to triacontanol under all the experimental conditions. In the case of RWC, higher values were noted for Marketmore and Green long in response to foliar application of triacontanol under stressed and nonstressed environments (Table 2). Under salinity, genotype 20252 leaves showed a maximum reduction in RWC and maintained a minimum RWC when sprayed with triacontanol under controlled and NaCl-stressed conditions.
Inorganic osmolytes
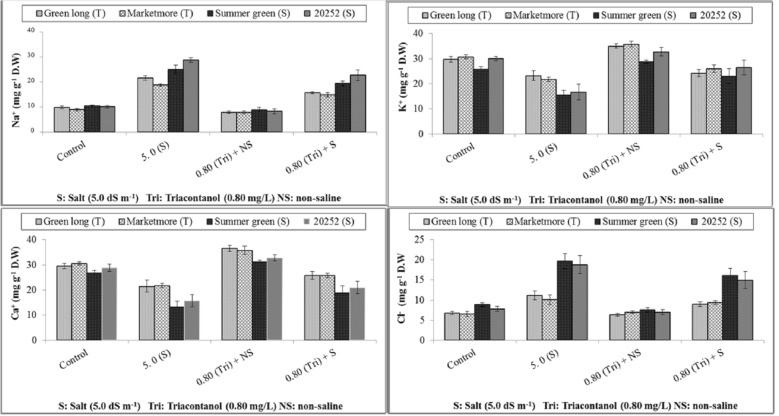
Of many different physiological attributes, leaf ionic contents are a very important feature that reveals the health status of plants and is linked to plant water availability. Salt stress resulted in decreased K+ and Ca+ contents accompanied by a significant increase in Na+ and Cl− contents in the leaves of all cucumber genotypes compared to the non-saline control (Fig. 3). Among the genotypes, lower levels of leaf Na+ and Cl− associated with improved K+ and Ca+ contents were observed in stressed and nonstressed Marketmore and Green plants long sprayed with triacontanol. Summer green accumulated the lowest Ca+ and K+ contents, while the highest Na+ content was observed in genotype 20252 under saline stress; however, foliar spray of triacontanol improved the ionic contents of these genotypes compared to the nonsprayed control.
Figure 3.
Effect of triacontanol on leaf inorganic osmolytes of four cucumber genotypes under normal and salt stress conditions.
Quality and yield related attributes
A marked reduction in the total number of fruits and average fruit weight was observed when cucumber genotypes were sown under salt stress (Fig. 4). These reductions were more prominent in the summer green and 20252 genotypes, whereas Marketmore and green long maintained the highest fruit number and weight upon introduction to salinity. Foliar application of triacontanol decreased the adversities of salt stress and improved the no. of fruits and their weight in all evaluated genotypes. However, Marketmore treated with triacontanol showed the maximum number of fruits having high average weight compared to untreated plants under stressed and controlled conditions. Summer green and 20252 showed maximum soluble solids in cucumber fruit and gave poor performance compared to genotypes Marketmore and Green long, which exhibited the lowest concentrations of total soluble solids.
Figure 4.
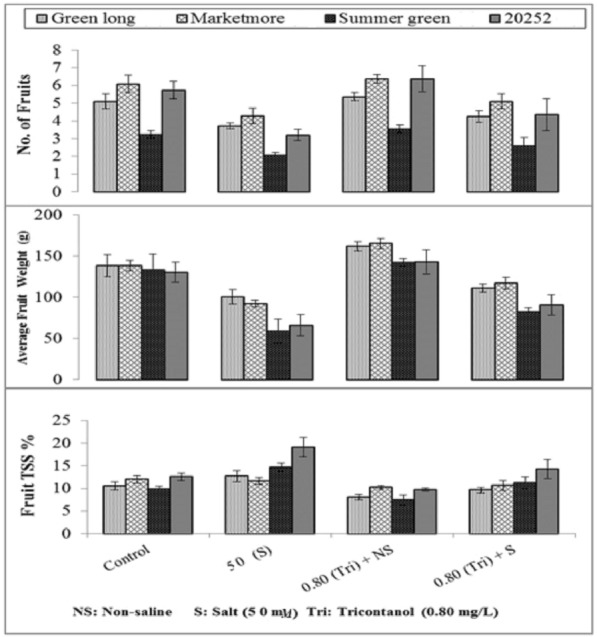
Effect of triacontanol on the yield attributes of four cucumber genotypes under normal and salt stress conditions.
Discussion
This study was conducted to assess the impacts of an optimized dose of triacontanol (0.80 mg L−1) on salt tolerance induction in cucumber genotypes by exploring detailed physiological, biochemical, ionic and yield parameters. Salt stress significantly inhibited the gas exchange mechanisms, while triacontanol (0.80 mg L−1) successfully alleviated the salt stress effect. Furthermore, triacontanol improved gas exchange attributes in both salinized and nonsalinized plants of the investigated cucumber genotypes. The ameliorating effect of triacontanol may be attributed to its role in regulating growth hormones (ABA, IAA and cytokinin) because IAA and cytokinin maintain cell division in the apical root meristem, while ABA helps in the biosynthesis of various antistress proteins, strengthens the antioxidant enzyme system and reduces ROS production, maintains membrane integrity and regulates stomatal conductance, which in turn ensures more CO2 availability to leaf mesophyll cells, thus improving photosynthetic activity, transpiration rate and water use efficiency. Similar results were reported by many investigators who have explored the effects of triacontanol on several basic metabolic processes, including photosynthesis, nutrient uptake and enzymatic activities17. Photosynthetic capacity depends on photosynthetic green pigments such as chlorophyll, and salinity induces a decline in photosynthesis that can be attributed to a reduction in chlorophyll content43. Here, it was reported that an accumulation of chlorophyll contents was significantly induced after triacontanol application. These findings are similar to the results of14. Triacontanol spraying on leaves significantly enhanced the growth, stomatal conductance, photosynthetic activity, transpiration rate and chlorophyll contents, while under salt stress conditions the membrane permeability reduction has been reported. Triacontanol increases the antenna pigment levels and in PS-II the efficiency of energy has been found to be increased. It has been found that the application of triacontanol showed deep effects on photosynthesis, which leads to increase the plant growth through improvement in overall photosynthesis along with the higher accumulation of organic compounds produced through photosynthesis44. It has also been found that the transcription of rbcS gene showed linkage with improved photosynthetesis in plants treated with triacontanol. The triacontanol has been found to be involved in improving the activity of ribulose-1,5-bisphosphate carboxylase oxygenase (RuBisCO) which is an important photosynthetic enzyme due to which the photosystems showed enhanced performace45. The application of triacontanol even in very low concentrations enhanced the CO2 uptake by plants46. Our current study, revelaed that the application of triacontanol was found to be involved in increasing stomata conductance and photosynthesis in cucumber genotypes. The stomata conductance is actually the factor which regualtes photosynthetic rate through regulating the concentration of CO2 uptake by leaf mesophyll tissue which is positively and significantly correlated with photosynthetic activity47. It has also been reported that triacontanol has positive and significant effects for photosynthetic rate, stomata conductance, and the CO2 concentration in leaves of crop plants. Similar, findings were stated by48,49. However, salt-tolerant genotypes gave a more efficient response to triacontanol (0.80 mg L−1) application than susceptible genotypes under saline (50.0 mm L−1) and nonsaline environments. All the water relation attributes (water potential, osmotic potential, turgor potential and leaf relative water contents) were drastically affected by salt stress. In the current experiment, salt stress increased total free proline and glycinebetaine in all genotypes (Green long, Marketmore), and Summer green, 20252) accumulated higher proline. As a positive correlation was found between leaf proline and glycinbetaine contents and leaf water potential (Ψw), which became more negative due to decreased osmotic potential, it could be suggested that these two osmotica play a central role in osmotic adjustment under saline stress50,51. Salinity has been known to disturb the water relations of plants as a consequence of decreased leaf osmotic potential in external soil solutions52. In our study, leaf Ψw and Ψs decreased under the saline regime. Early responses to salinity included a decrease in water potential and LRWC53, and it was observed from the results that plants sprayed with triacontanol @ 0.80 mg L−1 showed an improvement in the activities of enzymes such as SOD, CAT and POD under a saline environment (50 mm L−1). However, tolerant genotypes exhibited an excellent response in terms of higher antioxidant activities, i.e., SOD, CAT and POD, than sensitive genotypes54.
Triacontanol significantly eliminated the drastic effect of salt stress by minimizing ROS with the help and rise of antioxidant enzymes. through the activation and strengthening of the antioxidant system, i.e., SOD and CAT, during osmotic stress because of the accumulation of toxic ions Na+ and Cl− and help sustain growth. Thus, the relationship between triacontanol and antioxidants, such as SOD, was reported in the present study54–56. Membrane stability under saline stress is an indication of salt stress tolerance. However, excessive accumulation of salt (Na+) in the cytosol directly affects membrane stability through enhanced leakage of electrolytes, thereby disturbing cytosolic metabolic activities. It affects physiological and biochemical aspects together with the overproduction of ROS57, which leads to premature senescence and a decline in carbon assimilation and, consequently, plant productivity. However, triacontanol foliar spray reduces oxidative stress by enhancing the production of antioxidants, which in turn scavenges ROS and improves carbon assimilation by reducing electrolyte leakage, as observed in the present study, in which triacontanol spray reduced electrolyte leakage. Nevertheless, (Green long and Marketmore) presented better results than (Summer green and 20252) genotypes. Plants may accumulate compatible solutes such as glycinebetaine and proline under salt stress to enhance their salt stress tolerance58,59. In this experiment, salinity enhanced glycinebetaine and proline. Phenolics are potential antioxidant compounds that play an important role in scavenging singlet oxygen (O2−)60; under salt stress, the concentrations of phenolic compounds are frequently altered61. In this study, saline stress increased the amount of total phenolics in all genotypes. However, foliar-applied triacontanol improved the total phenolic content in tolerant genotypes by presenting a greater degree of improvement. Similar results were reported by62.
Foliar application of triacontanol significantly increased the K+ and Ca+ contents of the leaves. The findings of the present study demonstrated the significant accumulation of mineral contents of K+ and Ca+ in cucumber. Similar findings were also reported: triacontanol application stimulates K+, Ca+, and accumulation by eliciting a secondary messenger, L(+) adenosine, and such raised mineral contents may stimulate plant growth. Increased K+ content of the leaves may play a role in stomatal function. Triacontanol-induced increased accumulation of nutrients might result in enhanced crop yield63,64. However, exogenous application of triacontanol ameliorated the drastic effects of salt stress by reducing Na+ contents and increasing K+ contents. Moreover, triacontanol proved to be more effective for salt tolerance because it reported a high percentage decrease in leaf Na+ contents and a higher percentage increase in K+ contents than nontolerant genotypes, which gave a minimum percentage decrease under nonsaline and saline treatments.
Salt tolerance achieved by triacontanol application may be attributed to its ability to maintain enhanced uptake of K+ and restricting loading of Na+ salts into xylem while maintaining a high K+/Na+ ratio in plant tissues. This is probably due to the overexpression of SOS1 (Na+/H+ antiporter), which moves excess Na+ out of the cytosol and helps maintain low cytosolic Na+ concentrations in almost all tissues, especially in root epidermal cells, particularly root tips in cells bordering vascular tissues. Mainly, SOS1 confers salt stress tolerance by facilitating Na+ efflux from the cytosol to the rhizosphere by (1), increasing Na+ storage time in vacuoles and reducing Na+ buildup in the cytoplasm (2) and controlling long-distance sodium transport through Na+ retrieval among roots and shoots. Triacontanol increased yield per plant, enhanced uptake of nutrients, improved photosynthetic rate and nitrogen fixation, and enhanced the translocation of photosynthates and other metabolites12,65,66. Salt stress significantly decreased crop quality and yield67. Similar findings were reported during our studies; however, triacontanol foliar feeding improved the quality and crop yield11,64 because triacontanol application enhances water uptake, cell division, cell elongation and the permeability of plant cell membranes67, increased the growth, number of inflorescences, and quality of fruits and increased the yield of the majority of annual vegetables and agronomic crops20,66,68–71.
Foliar application of Triacontanol induces the second messenger (TRIM) formation which triggers the infux of ions such as Mg2+, K+ and Ca2+, through opening of their channels in the plasma membrane. Particularly, the level of Ca2+ may control for calmodulin and of Mg2+ and K+ ions which may improve the metabolism of cell by activation of enzymes that may be responsible for plant growth. Modulation of transcription factors such as CAMTA3, GTL and MYB2, that may regulate many anabolic processes which are related to genes. So, better growth of Triacontanol treated plants occurs. Triacontanol reduced the lethal impact of salinity by regulating the stress mitigating genes. Foliar application of Triacontanol over-seed treatment technique could be attributed to its prompt availability to plants. Triacontanol improved the salinity tolerance due to the increased antioxidant enzyme activity, accumulation of osmolytes and maintaining membrane integrity, osmotic adjustment and limiting the lipid peroxidation and ROS production. The lower level of triacontanol mainly showed the useful in improving the salinity tolerance in plants.
Conclusion
An optimized level of triacontanol@0.8 mg L−1 alleviated the lethal effect of salt stress on the studied genotypes under salt-stressed and nonstressed environments. However, the effect of triacontanol was more noticeable on Green long and Marketmore. In view of the salt tolerance potential of these genotypes and the efficacy of triacontanol to mitigate the salt stress impact, cultivation of these genotypes along with foliar application of triacontanol may be endorsed in salty soil.
Author contributions
M.S. conducted research under the supervision of M.S.H. M.S.H. gave the concept of research. S.A. carried out statistical analysis of data. Q.A., M.W.A., and W.M. carried out the final editing of the manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mubeen Sarwar, Email: mubeen.iags@pu.edu.pk.
Qurban Ali, Email: saim1692@gmail.com.
References
- 1.Fahmi AI, Nagaty HH, Eissa RA, Hassan MM. Effects of salt stress on some nitrogen fixation parameters in faba bean. Pak. J. Biol. Sci. 2011;14:385–391. doi: 10.3923/pjbs.2011.385.391. [DOI] [PubMed] [Google Scholar]
- 2.Munns R, Tester M. Mechanism of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 3.Chinnusamy V, Jagendorf A, Zhu J. Understanding and improving salt tolerance in plants. Crop Sci. 2005;45:437–448. [Google Scholar]
- 4.Chaum S, Pokasombat Y, Kirdmanee C. Remediation of salt-affected soil by gypsum and farm yard manure—Importance for the production of Jasmine rice. Austr. J. Crop Sci. 2011;5(4):458–465. [Google Scholar]
- 5.Sarwar M, Amjad M, Ayyub CM. Alleviation of salt stress in cucumber (Cucumis sativus L.) through seed priming with triacontanol. Int. J. Agric. Biol. 2017;19:771–778. [Google Scholar]
- 6.Afzal I, Basra SMA, Ahmad N, Farooq M. Optimization of hormonal priming techniques for alleviation of salinity stress in wheat (Triticum aestivum L.) Caderno de Pesquisa Série Biologia. 2005;17(1):95–109. [Google Scholar]
- 7.Javid MG, Sorooshzadeh A, Moradi F, Sanavy Seyed AMM, Allahdadi I. The role of phytohormones in alleviating salt stress in crop plants. AJCS. 2011;5(6):726–734. [Google Scholar]
- 8.Ahmad P, Abdel Latef AA, Abd-Allah EF, Hashem A, Sarwat M, Anjum NA, Gucel S. Calcium and potassium supplementation enhanced growth, osmolyte secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed chickpea (Cicer arietinum L.) Front. Plant Sci. 2016;7:513. doi: 10.3389/fpls.2016.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittova V, Guy M, Tal M, Volokita M. Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J. Exp. Bot. 2004;55(399):1105–1113. doi: 10.1093/jxb/erh113. [DOI] [PubMed] [Google Scholar]
- 10.Liu P, Yin L, Wang S, Zhang M, Deng X, Zhang S, Tanaka K. Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environ. Exp. Bot. 2015;111:42–51. [Google Scholar]
- 11.Kumaravelu G, Livingstone MD, Ramanujam MP. Triacontanol- induced changes in the growth, photosynthetic pigments, cell metabolites, flowering and yield of green gram. Biol. Plant. 2000;43:287–290. [Google Scholar]
- 12.Khan MMA, Mujibur-Rahman M, Naeem M, Mohammad F, Siddiqui MH, Khan MN. Triacontanol-induced changes in the growth, yield and quality of tomato (Lycopersicon esculentum Mill) Electron. J. Environ. Agric. Food Chem. 2006;5:1492–1499. [Google Scholar]
- 13.Ries SK, Wert VF, Sweeley CC, Leavitt RA. Triacontanol: A new naturally occurring plant growth regulator. Science. 1977;195:1339–1341. doi: 10.1126/science.195.4284.1339. [DOI] [PubMed] [Google Scholar]
- 14.Muthuchelian K, Murugan C, Harigovindan R, Nedunchezhian N, Kulandaivelu G. Ameliorating effect of triacontanol on salt stressed Erythrina variegate seedlings. Changes in growth, biomass, pigments and solute accumulation. Biol. Plant. 1996;38:133–136. [Google Scholar]
- 15.Verma A, Malik CP, Gupta VK, Bajaj BK. Effects of in vitro triacontanol on growth, antioxidant enzymes, and photosynthetic characteristics in Arachis hypogaea hypogea L. Braz. J. Plant Physiol. 2011;23:271–277. [Google Scholar]
- 16.Kilic NK, Duygu E, Donmez G. Triacontanol hormone stimulates population, growth and Brilliant Blue R dye removal by common duckweed from culture media. J. Hazard. Mater. 2010;182:525–530. doi: 10.1016/j.jhazmat.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 17.Naeem M, Khan MMA, Moinuddin M, Idrees K, Aftab T. Triacontanol-mediated regulation of growth and other physiological attributes active constituents and yield of Mentha arvensis L. Plant Growth Regul. 2011;11:9588–9598. [Google Scholar]
- 18.Chen X, Yuan H, Chen R, Zhu L, Du B, Weng Q, He G. Isolation and characterization of triacontanol regulated genes in rice (Oryza sativa L.): Possible role of triacontanol as plant growth stimulator. Plant Cell Physiol. 2002;43(8):869–876. doi: 10.1093/pcp/pcf100. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Yuan H, Chen R, Zhu L, He G. Biochemical and photochemical changes in response to triacontanol in rice (Oryza sativa L.) Plant Growth Regul. 2003;40:249–256. [Google Scholar]
- 20.Reddy BO, Giridhar P, Ravishankar GA. The effect of triacontanol on micropropagation of Capsicum frutescens and Decalepis hamiltonii W&A. Plant Cell Tissue Organ Cult. 2002;71:253–258. [Google Scholar]
- 21.Tantos A, Meszaros A, Farkas T, Szalai J, Horvath G. Triacontanol supported the micropropagation of woody plants. Plant Cell Rep. 2001;20:16–21. doi: 10.1007/s002990000282. [DOI] [PubMed] [Google Scholar]
- 22.Cavusoglu K, Kilic S, Kabar K. Effects of triacontanol pretreatment on seed germination, seedling growth and leaf anatomy under saline (NaCl) conditions. Sdu. Fen. Edebiyat Fakultesi Fen Dergisi (E-Dergi) 2007;2(2):136–145. [Google Scholar]
- 23.Noreen Z, Ashraf M. Assessment of variation in antioxidative defense system in salt- treated pea (Pisum sativum) cultivars and its putative use as salinity tolerance markers. J. Plant Physiol. 2009;166:1764–1774. doi: 10.1016/j.jplph.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 24.FAO . The State of the World’s Land and Water Resources for Food and Agriculture (SOLAW) Managing Systems at Risk. Food and Agriculture Organization of the United Nations; 2012. [Google Scholar]
- 25.Yamaguchi T, Blumwald E. Developing salt-tolerant crop plants: Challenges and opportunities. Trends Plant Sci. 2005;10(12):616–619. doi: 10.1016/j.tplants.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Stepien P, Klobus G. Water relations and photosynthesis in Cucumis sativus L. leaves under salt stress. Biol. Plant. 2006;50:610–616. [Google Scholar]
- 27.Ayers RS, Westcot DW. Water quality for agriculture FAO irrigation and drainage. UN Rome. 1985;29:1. [Google Scholar]
- 28.Dorota Z. Irrigating with High Salinity Water Bulletin 322 Agricultural and Biological Engineering Dep. Florida Cooperative Extension service Institute of Food and Agriculture Sciences University of Florida; 1997. [Google Scholar]
- 29.Wang XJ. Analysis of secondary salination in protected soils. North. Hortic. 1998;3(4):12–13. [Google Scholar]
- 30.Haghighi M, Pessarakli M. Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Hortic. 2013;161:111–117. [Google Scholar]
- 31.Sarwar M, Amjad M, Ayyub CM, Ashraf A, Tehseen S, Manan A, Butt M, Hussain T, Nawaz MA. Evaluation of cucumber germplasm for salinity tolerance based on early growth attributes and leaf inorganic osmolytes. Transylv. Rev. 2016;24(11):1077–1086. [Google Scholar]
- 32.Zekri M. Effects of NaCl on growth and physiology of sour orange and Cleopatra mandarin seedlings. Sci. Hortic. 1991;47:305–315. [Google Scholar]
- 33.Moya JL, Gomez-Cademas A, Primo-Millo E, Talon M. Chloride absorption in salt-sensitive Carrizo citrange and salt tolerant Cleapatra mandarian citrus rootstocks is linked to water use. J. Experi. Bot. 2003;54:825–833. doi: 10.1093/jxb/erg064. [DOI] [PubMed] [Google Scholar]
- 34.Giannopolitis CN, Ries SK. Superoxide dismutase I. Occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chance B, Maehly AC. Assay of catalase and peroxidase. Methods Enzymol. 1955;2:764–775. [Google Scholar]
- 36.Khan W, Prithiviraj B, Smith P. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 2003;160(5):485–492. doi: 10.1078/0176-1617-00865. [DOI] [PubMed] [Google Scholar]
- 37.Lutts S, Kinet JM, Bouharmont J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996;78:389–398. [Google Scholar]
- 38.Bates LS, Waldron RP, Teaxe IW. Rapid determination of free proline for water stress studies. Plant Soil. 1972;39:205–207. [Google Scholar]
- 39.Grieve CM, Gratan SR. Rapid assay for the determination of water soluble quaternary ammonium compounds. Plant Soil. 1983;70:303–307. [Google Scholar]
- 40.Julkenen-Titto R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. Agric. Food Chem. 1985;33(2):213–217. [Google Scholar]
- 41.Wheatherly PE, Barrs C. A reexamination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962;15:413–428. [Google Scholar]
- 42.Dadzie BK, Orchard JE. Routine Postharvest Screening of Banana/Plantain Hybrids: Criteria and Methods. INIBAP Technical Guidelines 2. International Plant Genetic Resources Institute; 1997. pp. 9–11. [Google Scholar]
- 43.Delfine S, Alvino A, Villani MC, Loreto F. Restrictions to carbon dioxide conductance and photosynthesis in spinach leave recovering from salt stress. Plant Physiol. 1999;119:101–106. doi: 10.1104/pp.119.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen SF, Zhu YL, Liu YL, Hu CM, Zhang GW. Effects of NaCl stress on ABA and polyamine contents in leaves of grafted tomato seedlings. Acta Hortic. Sin. 2006;33(1):58–62. [Google Scholar]
- 45.Eriksen AB, Haugstad MK, Nilsen S. Yield of tomato and maize in response to foliar and root applications of triacontanol. Plant Growth Regul. 1982;1:11–14. [Google Scholar]
- 46.Misra A, Srivastava NK. Effects of the triacontanol formulations ‘‘Miraculan’’ on photosynthesis, growth, nutrient uptake, and essential oil yield of lemongrass (Cymbopogon flexuosus) Steud, Watts. Plant Growth Regul. 1991;10:57–63. [Google Scholar]
- 47.Ivanov AG, Angelov MN. Photosynthesis response to triacontanol correlates with increased dynamics of mesophyll protoplast and chloroplast membranes. Plant Growth Regul. 1997;21:145–152. [Google Scholar]
- 48.Shakirova FM, Sakhabutdinova AR, Bezrukova MV, Fatkhutdinova RA, Fatkhutdinova DR. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003;164:317–322. [Google Scholar]
- 49.Aziz R, Shahbaz M, Ashraf M. Influence of foliar application of triacontanol on growth attributes, gas exchange and chlorophyll fluorescence in sunflower (Helianthus annuus L.) under saline stress. Pak. J. Bot. 2013;45(6):1913–1918. [Google Scholar]
- 50.Shao HB, Chen XY, Chu IY, Zhao XN, Ww G, Yuan YB, Zhao CX. Phenol by Synechocystis sp. in media including triacontanol hormone. Water Environ. J. 2006;26:1747–6585. [Google Scholar]
- 51.Moghaieb REA, Saneoka H, Fujita K. Effect of salinity on osmotic adjustment, glycinebetaine accumulation and betaine aldehyde dehydrogenase gene expression in two halophytic plants, Salicornia europaea and Suaeda maritime. Plant Sci. 2004;166(5):1345–1349. [Google Scholar]
- 52.Munns R. Gene and salt tolerance: Bringing them together. New Phytol. 2005;167(3):645–663. doi: 10.1111/j.1469-8137.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 53.Gucci R, Lombardini L, Tattini M. Analysis of leaf water relations in two olive (Olea europaea L.) cultivars differing in tolerance to salinity. Tree Physiol. 1997;17:13–21. doi: 10.1093/treephys/17.1.13. [DOI] [PubMed] [Google Scholar]
- 54.Khandaker MM, Faruq G, Motior RM, Sofian-Azirun M, Nasrulhaq BA. The influence of 1-triacontanol on the growth, flowering, and quality of potted bougainvillea plants (Bougainvillea glabra var. ‘‘Elizabeth Angus’’) under natural conditions. Sci. World J. 2013;10:1–12. doi: 10.1155/2013/308651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gatica AM, Arrieta G, Espinosa AM. Direct somatic embryogenesis in Coffea arabica L cvs catura and catuai: Effect of triacontanol, light condition, and medium consistence. Agron. Costarric. 2008;32(1):139–147. [Google Scholar]
- 56.Naeem M, Khan MMA, Moinuddin M, Siddiqui MH. Triacontanol stimulates nitrogen-fixation, enzyme activities, photosynthesis, crop productivity and quality of hyacinth bean (Lablab purpureus L.) Sci. Hortic. 2009;121:389–396. [Google Scholar]
- 57.Zhu JK. Overexpression of a delta-pyrroline-5-carboxylate synthetase gene and analysis of tolerance to water and salt stress in transgenic rice. Trends Plant Sci. 2001;6:66–72. [Google Scholar]
- 58.Dos-Reis SP, Lima AM, De-Souza CRB. Recent molecular advances on down stream plant responses to abiotic stress. Int. J. Mol. Sci. 2012;13(7):8628–8647. doi: 10.3390/ijms13078628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shahbaz M, Ashraf M, Al-Qurainy F, Harris PJC. Salt tolerance in selected vegetable crops. Crit. Rev. Plant Sci. 2012;31:303–320. [Google Scholar]
- 60.Mahboob W, Rehman H, Basra SMA, Afzal I, Abbas MA, Naeem MA, Sarwar M. Seed priming improves the performance of late sown spring maiz (Zea mays) through better crop stand and physiological attributes. Int. J. Agric. Biol. 2015;17(3):491–498. [Google Scholar]
- 61.Sarwar M, Amjad M, Anjum S, Alam MW, Ahmad S, Ayyub CM, Ashraf A, Hussain R, Mannan A, Ali A, Shahid A, Hussain T. Improving the salt stress tolerance in cucumber (Cucumis sativus L.) using by triacontanol. J. Hortic. Sci. Technol. 2019;2(1):20–26. [Google Scholar]
- 62.Ertani A, Schiavon M, Muscolo A, Nardi S. Alfalfa plant derived bio stimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil. 2012;364:145–158. [Google Scholar]
- 63.Miniraj N, Shanmugavelu KG. Studies on the effect of triacontanol on growth, flowering, yield, quality and nutrient uptake in chillies. South Indian Hortic. 1987;35:362–366. [Google Scholar]
- 64.Aftab T, Khan MMA, Idrees M, Naeem M, Singh M, Ram M. Stimulation of crop productivity, photosynthesis and artemisinin production in Artemisia annua L. by triacontanol and gibberellic acid application. J. Plant Interact. 2010;4:273–481. [Google Scholar]
- 65.Borowski E, Blamowski ZK. The effect of triacontanol ‘TRIA’ and Asahi-SL on the development and metabolic activity of sweet basil (Ocimum basilicum L.) plants treated with chilling. Folia Hortic. 2009;21(1):39–48. [Google Scholar]
- 66.Chaudhary BR, Sharma MD, Shakya SM, Gautam DM. Effect of plant growth regulators on growth, yield and quality of chilli (Capsicum annum L.) at Rampur Chitwan. J. Inst. Agric. Anim. Sci. 2006;27:65–68. [Google Scholar]
- 67.Ashraf M, Akram NA, Arteca RN, Foolad MR. The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Crit. Rev. Plant Sci. 2010;29(3):162–190. [Google Scholar]
- 68.Hangarter R, Ries SK, Carlson P. Effect of triacontanol on plant cell cultures in vitro. Plant Physiol. 1978;61:855–857. doi: 10.1104/pp.61.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kapitsimadi C, Vioryl SA. Effect of a long chain aliphatic alcohol (triacontanol) on growth and yield of different horticultural crops. Acta Hortic. 1995;379:237–243. [Google Scholar]
- 70.Muthuchelian K, Velayutham M, Nedunchezhian N. Ameliorating effect of triacontanol on acidic mist-treated Erythrina variegata seedlings. Changes in growth and photosynthetic activities. Plant Sci. 2003;165:1253–1257. [Google Scholar]
- 71.Khan N, Nazar R, Anjum N. Growth, photosynthesis and antioxidant metabolism in mustard (Brassica juncea L.) cultivars differing in ATP-sulfurylase activity under salinity stress. Sci. Hortic. 2009;122:455–460. [Google Scholar]