Abstract
Endophytic fungi (EF) are a group of fascinating host-associated fungal communities that colonize the intercellular or intracellular spaces of host tissues, providing beneficial effects to their hosts while gaining advantages. In recent decades, accumulated research on endophytic fungi has revealed their biodiversity, wide-ranging ecological distribution, and multidimensional interactions with host plants and other microbiomes in the symbiotic continuum. In this review, we highlight the role of secondary metabolites (SMs) as effectors in these multidimensional interactions, and the biosynthesis of SMs in symbiosis via complex gene expression regulation mechanisms in the symbiotic continuum and via the mimicry or alteration of phytochemical production in host plants. Alternative biological applications of SMs in modern medicine, agriculture, and industry and their major classes are also discussed. This review recapitulates an introduction to the research background, progress, and prospects of endophytic biology, and discusses problems and substantive challenges that need further study.
Keywords: symbiosis, endophytic fungi, endophytic fungi-host interaction, genetic regulation, secondary metabolites
Introduction
Plants, especially perennials, are colonized by many types of endophytic microorganisms (Stone et al., 2004; Demain, 2014), which live inside plant tissues either throughout their lives or during a certain period of their life cycles without causing visible damage or morphological changes in their hosts. These microorganisms include both fungi and bacteria (Zhang et al., 2006; Porras-Alfaro and Bayman, 2011; Gouda et al., 2016), and usually coexist with pathogens. According to their colonizing behaviors, endophytic microflora can be sorted into facultative and obligate categories. Facultative endophytes colonize plants at certain stages of their life cycles, but they may also reside outside the plant at other stages to form an association with the immediate rhizosphere soil of host plants (Abreu-Tarazi et al., 2010). In contrast, obligate strains live in plants throughout their entire life cycles. They usually proliferate across plant generations through vertical transmission and use or alter the metabolic machinery and products of plants for their own survival (Hardoim et al., 2008; Gouda et al., 2016).
Among these endophytic microorganisms, endophytic fungi (EFs) have attracted much research interest because they have provided not only novel sources of cytotoxic compounds, such as anticarcinogenic molecules (Uzma et al., 2018) and antibacterial substances (Radic and Strukelj, 2012), but also biostimulants for essential oil biosynthesis (El Enshasy et al., 2019). They may enhance nutrient solubilization in the plant rhizosphere (Mehta et al., 2019), promote plant growth (Poveda et al., 2021), act as biological control agents (Poveda and Baptista, 2021), or activate plant systemic resistances to biotic (Poveda et al., 2020a) or abiotic (Cui et al., 2021) stresses. In this review, we provide a comprehensive overview of the biological aspects of EFs that are related to their diversity, their distribution, and their multidimensional interactions with multiple players, including host plants, epiphytes, and pathogens, in their communities. We also examine the role of secondary metabolites (SMs) in these multidimensional interactions and how SMs are biosynthesized through gene expression regulation and through the mimicry or alteration of phytochemical production in host plants. Finally, we identify the principal categories of SMs, characterize their attributes, and review their alternative biological potential. This review provides readers with a profound understanding of EFs and SMs.
Biodiversity and Distribution of Endophytic Fungi in Nature
The geographical distribution of biota is characterized as a continuous gradient distribution of traits. Biodiversities at various levels, including species, function, and phylogenesis, are the basis of this continuous distribution (Violle et al., 2014). Although little is known about the mechanisms of biodiversity formation in a particular geographical habitat, fungal strains mediate many processes and may play a crucial role in their habitats (Vandenkoornhuyse et al., 2002). Plant tissue is arranged in multi-layers, forming a spatial and temporal supportive refuge, like a natural habitat, for various endophytic microorganisms. Based on an accepted estimation of a 1:4 or 1:5 ratio of vascular plants to fungal strains, there could be more than one million strains of EFs remaining to be discovered (Sun and Guo, 2012). However, our limited recognition of EF diversity renders the ratio a biased estimation, because EFs thrive ubiquitously in species diversity, while rare species and those that are characterized as non-sporulating, non-culturable, or asceptic cannot be examined properly in current laboratory isolation and fermentation attempts (Stone et al., 2004; Zhang et al., 2006; Ding et al., 2017; Alvear-Daza et al., 2021).
According to the reproductive pattern and host occurrence, EF communities can be sorted into two categories: the Clavicipitaceous/Balansiaceous group (C-group) and the non-Clavicipitaceous/non-Balansiaceous group (NC-group). C-group EFs infect the ovules of host plants and transmit vertically from parents to progenies through host seeds. The target tissues for their colonization are living rhizomes and shoots of host plants, but the host range is restricted to grass species (Poaceae). C-group species are typical obligate endophytes (Carroll, 1988), which protect their hosts from herbivore attacks or enable the hosts to survive under drought conditions by secreting defensive or supportive bioactive metabolites, respectively (Roberts and Lindow, 2014; Poveda, 2021). NC-group EFs, which are non-grass-host related (Ascomycota, Basidiomycota), have a wide biodiversity and distribution from tropical to polar areas, with their hosts including nonvascular, vascular, and woody plant communities. They transmit sexually or asexually by producing spores or conidia, which contribute to horizontal propagation, i.e., the induction of symbiosis. These EFs are not closely associated the host plants because they can exist in a quiescent state until they sense the chemical changes from host plants suffering injuries, wounds, or other environmental stresses (Carroll, 1988; Rodriguez et al., 2008; Mishra et al., 2021). The colonization of the NC-group in aerial organs is usually local, restricted, limited, and mainly intercellular, but the colonization of this group in roots or the rhizosphere is extensive, organized, systematic, intercellular, and intracellular. Some illustrative endophytic mycobiomes of the NC-group include Fusarium spp., Piriformospora indica, and dark septate mycobiota (Varma et al., 2000; Schulz et al., 2002).
Recent progress in molecular techniques, such as metagenomic sequencing, DNA fingerprinting, and phylogenetic analysis, has been successfully employed to detect and identify the species, community composition, and diversity of EFs. These technologies provide more precise methods of fungal identification and accommodation of the asceptic strains in situ than conventional isolation attempts (Arnold and Lutzoni, 2007; Tao et al., 2008; Tejesvi et al., 2009; Bahram et al., 2018; Jiang et al., 2018; Huang et al., 2019; Zhang J. et al., 2019). Studies have demonstrated that the host range of EFs includes algae (Tan and Zou, 2001), liverworts, mosses, hornworts (Davey and Currah, 2006; Aly et al., 2010; Sun and Guo, 2012), grasses (Muller and Krauss, 2005; Su et al., 2010), lycophytes, ferns, equisetopsida (Aly et al., 2010), shrubs, deciduous and coniferous trees (Gao et al., 2008; Albrectsen et al., 2010; Mohamed et al., 2010; Sun et al., 2011), gymnosperms, angiosperms, and annual/perennial herbaceous and broad-leaved plants. EFs are also distributed in a broad range of geographic habitats, such as tropical, temperate, arctic tundra, alpine, aquatic, and xerophytic ecosystems (Tan and Zou, 2001; Stone et al., 2004; Zhang et al., 2006) for more than 400 million years (Sun and Guo, 2012).
Endophytic Fungi and Their Multidimensional Interactions
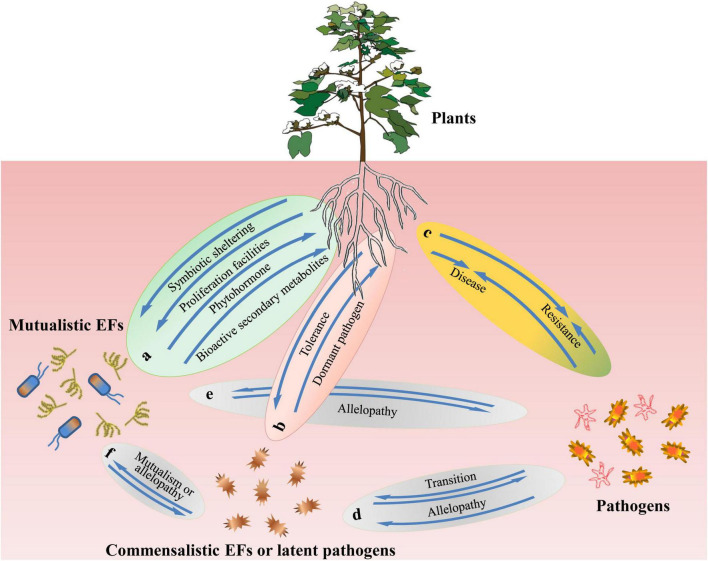
During their biogenesis and the establishment of symbiosis, EFs encounter specific host groups (Stone et al., 2004; Sun et al., 2008; Chadha et al., 2014), non-host plant communities, epiphytes, and pathogens. These multiple encounters prompt EFs to develop multidimensional interactions with the organisms they encounter (Figure 1). Major evolutionary and ecological novelties may direct or correlate to these interactions.
FIGURE 1.
A schematic model of plant and microbiome interactions. The interactions include: (a) interactions between plants and mutualistic endophytic fungi (EFs), (b) interactions between plants and commensalistic EFs or latent pathogens, (c) interactions between Plants and pathogens, and (d–f) interactions between microbiomes.  Mutualistic endophytic fungus (EF),
Mutualistic endophytic fungus (EF), commensalistic
commensalistic  EF,
EF,  pathogens.
pathogens.
Endophytic Fungi Interactions With Host Plants
Fungi can colonize the intercellular or intracellular spaces of plants, but systematic and extensive colonization is most likely to occur in the roots rather than in the aerial leaves or stems. Colonization in aerial organs primarily depends on the host’s apoplastic fluid as the nutrient source to support the normal reproduction of EFs in aerial organs (Schulz et al., 2002). During infection, fungi form three corresponding types of interactions with hosts: mutualistic (beneficial endophytes), commensalistic (non-beneficial/virulent endophytes), and pathogenic (virulent pathogens) (Figure 1), depending on the physiological status or specific circumstances that host plants experience. According to these three modes of action, fungal strains can increase, have no palpable effects on, or decrease host fitness (Kogel et al., 2006).
Mutualistic Symbiosis
In mutualistic symbiosis, both partners of EFs and host plants enjoy this beneficial symbiotic continuum (Jia et al., 2016) and eventually achieve evolutionary and ecological success. EFs alter the metabolic mechanism of host plants, improve metal and drought tolerance, enhance growth, and promote nutrient acquisition (Hunt and Newman, 2005; Rodriguez and Redman, 2008; Mejia et al., 2014; Poveda et al., 2021). They also enhance the defense efficiency of host plants against herbivorous animals and pests as well as against infections of pathogenic microorganisms (Cui et al., 2021). In response to these benefits, host plants provide symbiotic shelters and other proliferation facilities, such as an adequate nutrient supply and protection, to enable fungi to safely complete their life cycles during colonization (Figure 1a). The exact mechanism of mutual interaction between EFs and plants remains to be elucidated (Haas and Défago, 2005), but EFs offer these potential advantages to host plants in several possible ways. One of these ways is to enhance the plant’s immune system by producing a plethora of bioactive SMs as protective agents. It is speculated that increasing the number of SMs causes physiological changes in the infected host plant that further stimulate the plant immune system (Poveda et al., 2020a). Various experiments on endophytic and pathogenic fungi and monitoring their comparative effects on host plants suggest that EFs promote the defense mechanisms of host plants by synthesizing bioactive SMs and herbicidal metabolites in hosts (Figueiredo et al., 2008). In an in vitro tripartite interaction assay, it was observed that Fusarium oxysporum rapidly killed A. thaliana plants, whereas the presence of Paraconiothyrium variabile reduced plant death by up to 85% (Bärenstrauch et al., 2020). This hypothesis was confirmed in the following experiments. When mosquito larva were assayed with ethanol extracts from Poa ampla Merr. (big bluegrass), the results showed that only the extracts from the plants that were inoculated with Neotyphodium typhnium were effective against the insect, whereas the extracts from the plants that were not inoculated with the fungus were inactive (Ju et al., 1998). Another way that an EF bestows host plant benefits is that EFs promote plant growth by producing and providing phytohormones, including auxins, cytokines, and gibberellins. This has been confirmed by the discovery of a common gibberellin biosynthesis pathway in fungi and higher plants (Tan and Zou, 2001; Kumar et al., 2013). Studies have demonstrated that endophytes can improve the growth and proliferation of plants by enhancing their defensive systems, like ethylene and jasmonic acid do (Kunkel and Brooks, 2002; Van der Ent et al., 2009; Di et al., 2016; Verbon and Liberman, 2016; Forni et al., 2017; Yang et al., 2019), or by interacting with ethylene-targeted transcription factors (Camehl et al., 2010). Neotyphodium, an EF, colonizes in tall fescue ryegrass and confers protection and stability on host plants in hostile conditions; in return, ryegrass provides facilities to promote fungal proliferation through ryegrass seeds infected with fungal hyphae (Tan and Zou, 2001). The resulting competitive advantage provides both hosts and symbionts with greater potential for growth and survival than the non-symbiotic plants and fungi.
Commensalistic to Latent Pathogenic Relationships
In a commensalistic or latent pathogenic relationship, EFs sporulate rapidly and interact with host plants through a relationship with or without any significant beneficial effects on plant physiology (Stone et al., 2004; Hiruma et al., 2016). Studies have reported that these endophytes exist as latent pathogens in hosts under normal conditions (Brown et al., 1998; Photita et al., 2004; Rodriguez and Redman, 2008; Gorzynska et al., 2018; Figure 1b), while some studies have described various species and genera of EFs from host plants as active pathogens under unusual physiological stresses (Photita et al., 2004; Figure 1c). Fungi that have been identified as endophytes that are also possible pathogens include Cladosporium, Fusarium, Colletotrichum, Cordana, Deightoniella, Periconiella, Verticillium, Curvularia, Nigrospora, Guignardia, and Phoma (Photita et al., 2004; Cui et al., 2021). These EFs stay in latent or dormant state in the tissue of their host plants long before the outbreak of disease symptoms. In such cases, the dormancy phase is essential because it determines the time when the fungus is harmless as an endophyte and when it is virulent as a pathogen. In the virulent phase, EFs cause obvious symptoms and change the morphology and physiology of host plants under adverse conditions (Figure 1d). It is precisely these hostile conditions, including malnutrition, disruption of ontogenetic state (Sieber, 2007; Rodriguez and Redman, 2008), biotic stresses, drastic climate changes (such as elevated temperature and excessive humidity), and senescence, that break the balance between EFs and their hosts and lead to the transition of EFs from latent mode to active virulent pathogens, although there are no obvious disease symptoms before transition (Romero et al., 2001; Photita et al., 2004; Poveda et al., 2020b). There are also endemic fungal species, which typically include the majority of F. oxysporum strains, that live in host tissues without causing disease symptoms. Some strains even confer beneficial effects (Imazaki and Kadota, 2015; Di et al., 2016), such as C. tofieldiae, which promotes plant growth and fertility as an endophyte under phosphorus-deficient conditions (Hiruma et al., 2016). It is assumed that a combination of effectors, enzymes, and secondary metabolites determines the outcome of an interaction; that is, whether it is endophytic or pathogenic (Di et al., 2016; Poveda et al., 2020b). Nutrient status may have facilitated the transition of C. tofieldiae from pathogenicity to symbiosis (Hiruma et al., 2016).
Endophytic Fungi Interactions With Other Plant Microbiomes
As one of the numerous microbial players in the endophyte–host continuum, EFs inevitably have dynamic and complex interactions with other plant microbial communities, including endosphere-associated fungal and bacterial strains, regardless of whether they are pathogenic or symbiotic under natural conditions (Strobel, 2018; Figures 1d–f). Studies have observed that fungal endophytic metabolomic profiles can be affected by pathogen infection (Combes et al., 2012), indicating that antagonistic effects, or chemical communications, exist between the two microorganisms (Combes et al., 2012; Saikkonen et al., 2013). Endophytic fungi may also harbor a variety of bacterial species (endohyphal bacteria) in their hyphae (Hoffman and Arnold, 2010). When a foliar endophyte hosts the endohyphal bacterium Luteibacter sp., its indole acetic acid (IAA) production is significantly enhanced. However, the axenic culture of the bacterium does not show IAA production (Hoffman et al., 2013). Host plants may provide direct interfaces facilitating interactions between EFs and bacterial microflora. It is observed that two mutualistic EFs, Neotyphodium sp. and Epichloë sp., protect the leaves of fescue grasses from herbivores by producing loline alkaloids. On the leaf surface of fescue grass that is not infected with such endophytes or on the leaf surface of other plants that are infected with endophytes incapable of producing loline alkaloids, there is no accumulation of loline-consuming bacteria (Roberts and Lindow, 2014). In some cases, indirect interactions between EFs and other microorganisms may involve the participation of a third organism. A typical example is the interaction between the EF N. coenophialum and grass yellow dwarf virus (Hunt and Newman, 2005). N. coenophialum protects its host plants from aphids, and aphids are primary vectors of viruses (Grafton et al., 1982; Malmstrom et al., 2005; Yi and Gray, 2020). The N. coenophialum strains that provide tall fescue with better prevention against bird-cherry-oat aphids also inhibit the spread of the virus (Hunt and Newman, 2005).
Why Do Endophytic Fungi Produce Secondary Metabolites?
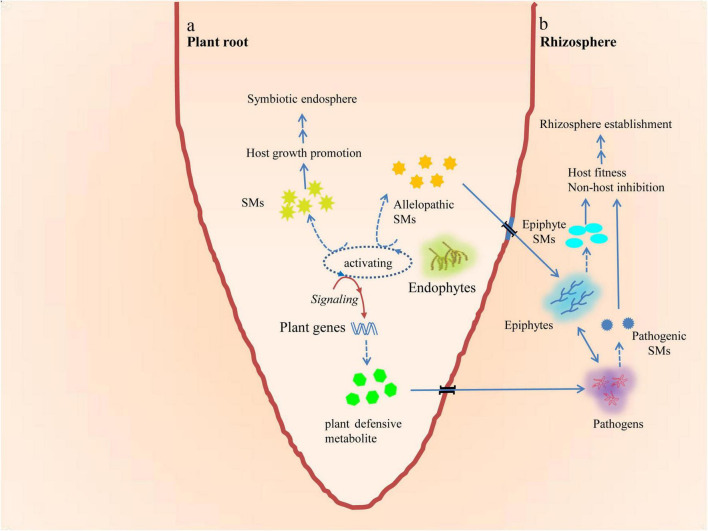
When different microorganisms occupy the same habitat, they must compete for the resources of that habitat for nutrition, living space, reproduction, and other needs throughout their life cycles. Compared to microorganisms that have poor adaptability, adaptable microorganisms are more likely to obtain adequate resources and increase their abundance when the resources are insufficient to meet the needs of the community. As a result, the former may not be able to survive in adverse conditions. In order to survive, organisms have developed two effective strategies to compete. One is to produce allelochemicals that inhibit the growth of their competitors and eliminate toxic effects produced by their competitors in the vicinity (Macías-Rubalcava et al., 2008; Konarzewska et al., 2020; Poveda, 2021). The other is to produce allelochemicals that help their producers form alliances through symbiotic relationships with symbionts or hosts. These symbiotic relationships enable both parties to survive and reproduce safely, even in extremely adverse environments (Macías-Rubalcava et al., 2008). According to the hypothesis of long-term coevolution within biological communities (Ji et al., 2009), this mutual orientation of EFs and their hosts leads to each EF having developed a specific range of host species, enabling them to accumulate in a specific eukaryotic host group (Figure 2a).
FIGURE 2.
Mutual orientation of microbiome and plant (a) and multidirectional crosstalks or chemical communications among plants, endophytes, epiphytes, and pathogens under natural ecological conditions (b).  Endophytic fungus (EF),
Endophytic fungus (EF),  EF secondary metabolite (SM),
EF secondary metabolite (SM),  allelopathic SMs,
allelopathic SMs,  pathogens, pathogenic SM,
pathogens, pathogenic SM,  epiphytes,
epiphytes,  epiphytic SM,
epiphytic SM,  plant defensive metabolite.
plant defensive metabolite.
Most of these allelochemicals are SMs, a variety of usually low-molecular-weight and amazingly heterogeneous chemicals that were previously thought to have no direct functional effect on the growth, development, and reproduction of the organisms that produce them (Keller et al., 2005; Yu and Keller, 2005; Fox and Howlett, 2008; Shwab and Keller, 2008). Volatile organic compounds (VOCs) are a large group of such chemicals that allow their producers (including plants and microorganisms) to defend themselves against attacks by pests or pathogens, or to convey warnings intra- or inter-specifically during such attacks (for details of VOCs, please refer to Poveda, 2021). Historically, the term “secondary” used for such natural metabolites has been associated with their “inessentiality,” but they have been demonstrated to play important roles in the growth and development of organisms in indirect ways (Pichersky and Gang, 2000; Deepika et al., 2016).
Secondary Metabolites Serve as Agents to Help Endophytic Fungi Compete and Survive
By preventing competition from other organisms, SMs help the organisms that produce them survive. They may also cause harm to other individuals of the same species. Examples of these SMs include those that help organisms access limited resources and survive in a specific adverse niche and those that inhibit competitors (Williams et al., 1989; Vaishnav and Demain, 2010; Xie et al., 2019; Figure 2b). In an in vitro competition experiment, naphthoquinone spiroketals, isolated from a newly identified EF strain, exhibited an allelochemical inhibitory effect against other EFs, such as Colletotrichum sp., Phomopsis sp. (Wang et al., 2016), and G. manguifera (Macías-Rubalcava et al., 2008; Macías-Rubalcava et al., 2014). In another in vitro dual culture experiment, it was observed that P. variabile, an EF, exhibited direct antagonism against F. oxysporum, a phytopathogen, by secreting the induced metabolite hydroperoxin oxylipin, which decreased the concentration of pathogenic mycotoxins, whereas none of the pure axenic cultures showed an increase or decrease of this metabolite (Combes et al., 2012). Further study has revealed that the biosynthesis of hydroperoxy oxylipins is catalyzed by lipoxygenases and the two lipoxygenase genes (pvlox1 and pvlox2) in P. variabile, and that only pvlox2 is specifically up-regulated during the interaction (Bärenstrauch et al., 2020).
Secondary Metabolites Form the Phylogenesis of Symbiosis of Endophytic Fungi and Their Host Plants
The concept of coevolution has been applied to characterize the biochemical interactions between EFs and hosts. It is thought that the coevolution of endophytes and their host plants shapes the production of SMs, which play important roles in endophyte-host communication for mutual adaptation and their orientation to different environments (Debbab et al., 2011; Lind et al., 2017). There are three main schools of thought to interpret the relationship between the biosynthesis pathway of common SMs and the evolution of symbiosis between endophytes and their hosts. According to the hypothesis of the first school, this may occur in the era of coevolution when a responsive relationship has been established between EFs and host plants. EFs and host plants were adapted to each other, leading them to share common biosynthetic pathways of natural active SMs. In this case, the endophyte–host association is imperative because it may be the critical factor affecting the secretion of bioactive metabolites (Vaishnav and Demain, 2010). The environmental factors that affect symbiosis formation will also affect SM biosynthesis. The second school of thought suggests that endophytes gradually acquired adaptations to the internal microenvironment of hosts by horizontal gene transfer (HGT) between endophytes and plants during the primeval period (Slot and Rokas, 2011; Soanes and Richards, 2014). In such genetic variations, fungal strains insert some fragments of their genetic materials into plant genomes, or uptake some plant genetic fragments into their own genomes (see section “Horizontal Gene Transfer”). The third school of thought has argued that both endophytes and plants synthesize these common metabolites and transfer them to their corresponding symbiotic systems (Zhang et al., 2006).
Some investigations support the hypothesis that phytoendophytes originate from phytopathogens. Phylogenetic analysis has revealed the interconnections between phytoendophytes and phytopathogens in various environments (Arnold et al., 2009). SMs might also be involved in such interconnections (Di et al., 2016). Recent studies reported that the transient evolutionary roots of two fungal endophytic communities, Clavicipitaceous sp. N. coenophialum, an endophyte of tall fescue, and Harpophora oryzae, a beneficial endosymbiont of wild rice, originated from insect parasitoid and phytopathogenic ancestors, respectively (Spatafora et al., 2007; Xu et al., 2014). The exact phylogenetic mechanism of endophytes from apparent pathogens still remains unclear. It has been observed that the transfer of one specific F. oxysporum f. sp. lycopersici chromosome, which contains most of its effector genes and a secondary metabolite coding gene cluster, confers pathogenicity to an endophytic strain (Ma et al., 2010; Di et al., 2016). Studies suggest that environmental stresses, sudden drastic climate changes, or senescence may facilitate the conversion of endophytes into pathogens in the host to adapt to these ecological changes (Romero et al., 2001; Photita et al., 2004). They may also play a role in phylogenesis from pathogens to endophytes (Cui et al., 2021). Given such a prolonged virulent contradiction between pathogens and plants, the loss-of-function mutations of the virulence genes in pathogenic strains and the alteration of the SM biosynthetic pathway and accumulation of SMs eventually convert such pathogen–host interactions into mutual symbiotic relationships that are beneficial to both parties (Tan and Zou, 2001; Kemen et al., 2015). It has been observed that plants infected with EFs have more bioactive chemical compounds than those infected with pathogenic fungi (Schulz et al., 2002). Generally, when facultative EFs begin to colonize host plants, they face three kinds of competitors: epiphytes, pathogens, and the host defense systems. This may explain why plants infected with EFs produce more defensive chemicals than plants infected with pathogens (Schulz et al., 2002; Hunt and Newman, 2005; Figure 2b). These examples illustrate the significance of metabolic communications for the construction of multiple interactions among EFs, host plants, and other plant microbiomes, and for the identification of diverse metabolic chemicals secreted by plants or EFs during their interaction (Friesen et al., 2011; Quambusch et al., 2014; Hardoim et al., 2015).
Diverse Secondary Metabolites Help Form Endophytic Fungi Diversity and Thus Enhance Plant Diversity
In competitive and unfavorable ecological environments, plant species modify their biological systems by producing various defense reactions, which are mainly manifested in the synthesis of defensive SMs. It is estimated that plant genomes may contain more than 70,000 genes (Wang et al., 2018), of which 15–25% are involved in encoding enzymes that participate in secondary metabolism (Bevan et al., 1998; Somerville and Somerville, 1999). This indicates that defensive SMs are often highly diverse within and across populations. EFs also take part in the establishment of host defense mechanisms through SMs to enhance the host capacity to adapt to a wide range of biogeographical ecosystems (Violle et al., 2014). One reason for the diversity of the SMs of an EF species is the diverse arrangement of coding genes in the SM synthetic pathway. As the coding genes of SM synthetic pathways in EFs are usually in clusters (Andersen et al., 2013), the divergent rearrangements of the clusters within an EF species or across EF species may also contribute to the diversity of SMs. Recently, at least five divergent types of variation in SM gene clusters in the fungal species Aspergillus fumigatus were identified, revealing its diverse secondary metabolism (Lind et al., 2017). Although their mechanism is still poorly understood, SMs may cause the morphological or physiological alteration of host plants (Braga et al., 2018). These alterations are believed to be linked to plant diversity, which means that the colonization of EFs enhances the host adaptability and thus its viability under various environmental conditions (Hyde and Soytong, 2008); in return, the survival of EFs may also be enhanced through their host diversifications.
How Do Endophytic Fungi in Symbiosis Produce Diverse Secondary Metabolites?
Gene Expression Regulation of Secondary Metabolite Biosynthesis in Endophytic Fungi
Analyzing the gene expression regulation mechanism of EFs, their hosts, and their symbiosis contributes greatly to further understanding the interactions between the two symbiotic partners (Deepika et al., 2016). The expression of SM coding genes in a symbiotic continuum may be regulated by factors including gene clustering, transcription factors, the presence of EFs, and HGT.
Gene Clustering
The diversity of EF genomes in eukaryotic systems and fungal genetic studies has revealed that genes coding specific SM biosynthesis pathways are clustered. In some cases, such as when the pathway function requires specific transcription factors or transporters, the genes encoding these factors (enzymes) are also located in these clusters (Nützmann et al., 2018). Two findings suggest that the architecture or structure of fungal secondary metabolic clusters varies with the situation. First, some highly complex SMs are synthesized through collaboration between different clusters (Chiang et al., 2016; Henke et al., 2016). Second, in some cases clusters of different pathways are adjacent to each other in the genome (Nützmann et al., 2018). These clusters are usually found in the dynamic regions of chromosomes or near telomeres. Subtelomeric regions are well-known hotspots in chromosomal recombinations and segmental duplications (Field et al., 2011; Andersen et al., 2013; Lind et al., 2017). The mechanism of SM-coding gene clusters in unstable DNA regions is still unclear, but an acceptable explanation is that it may be related to gene expression regulation (Osbourn, 2010a). The proximity of clustered genes may be a necessary condition for the synthesis of bioactive products related to a pathway, because it keeps the pathway genes closer during genomic rearrangements (Deepika et al., 2016). This clustering genetic format may facilitate the co-inheritance of favorable combinations of alleles at these multigene loci (Chu et al., 2011; Field et al., 2011). It may also monitor the synchronization of the clustered gene expression by altering the arrangement of chromatins (Hurst et al., 2004; Sproul et al., 2005; Field and Osbourn, 2008; Osbourn and Field, 2009; Osbourn, 2010b; Field et al., 2011) and by exchanging the non-contiguous regulatory elements, which are harbored in the clusters (Hurst et al., 2004; Sproul et al., 2005; Osbourn and Field, 2009; Osbourn, 2010b). In the genomes of higher plants, the functionally correlated genes interspersed in the genome may form clusters at the transcriptional level through helix-loop-helix domains (Nützmann et al., 2018). The formation of DNA loops causes cis-elements to be located adjacent to each other and creates high local concentrations of transcription factors that are close to the transcription initiation sites of the genes, thereby initiating transcription (Mendes et al., 2013). Any interference with the transcription of clustered genes will not only cause the loss of the coding products of these genes, but may also cause toxic intermediates to accumulate in the biochemical pathways (Hurst et al., 2004; Sproul et al., 2005). Evidence indicates that gene regulation at chromatin levels is important for the expression of secondary metabolic gene clusters (Osbourn, 2010b).
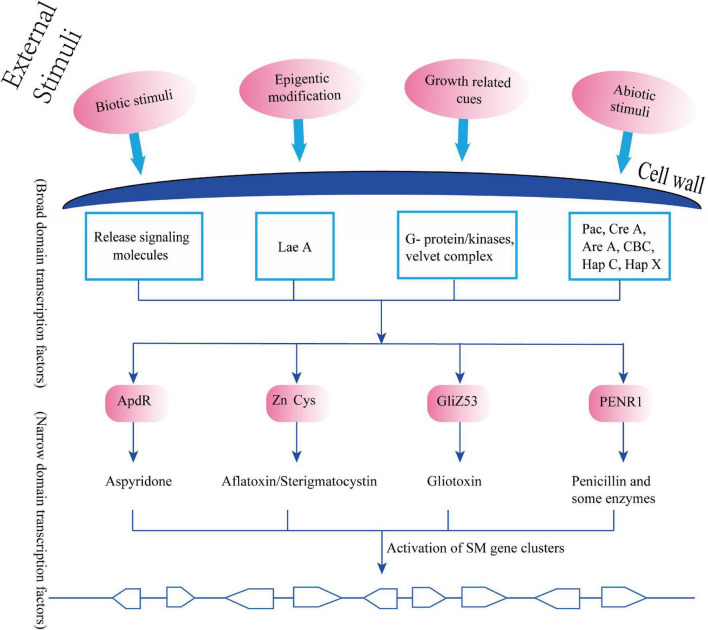
Transcription Factors
The activities of these clustered genes in the secondary metabolism are further regulated by two main groups of transcription factors: narrow domain transcription factors (NDTFs) (Table 1) and broad domain transcription factors (BDTFs) (Table 2; Keller et al., 2005). NDTFs act on the genes in the cluster, and may also act on the clustered genes at different genomic locations from NDTFs themselves. This can be illustrated by the typical NDTF AflR, which is a well-characterized Zn2Cys6 transcription factor that regulates the aflatoxin and sterigmatocystin gene clusters through binding to the palindromic sequence 5′-TCG(N5)CGA-3′, an 11-bp motif in the promoter regions of coding genes in a few Aspergillus species (Slot and Rokas, 2011). It also regulates three more genes outside the aflatoxin metabolite gene cluster (Woloshuk et al., 1994; Yu et al., 1996; Cánovas et al., 2017). Some typical NDTFs are shown in Table 1. BDTFs, or global transcription factors, are upper hierarchical-level control systems that respond to external stimuli that are not directly related to secondary biochemical gene clusters (Keller et al., 2005; Yu and Keller, 2005; Young et al., 2006; Hoffmeister and Keller, 2007; Fox and Howlett, 2008; Shwab and Keller, 2008; Deepika et al., 2016). Studies have revealed that EF signals interfere preferentially with ethylene-targeted transcription factors (Camehl et al., 2010). It is well-accepted that the biosynthesis of SMs depends on a combination of developmental competence and the stimulation of environmental factors, such as nutrient availability, illumination, pH, injury, infection, and developmental changes during the host life cycle (Bayram and Braus, 2012; Xie et al., 2019). BDTFs play an essential role in the transmission of environmental stimuli to the genome. They create and regulate the signaling transduction from environmental cues to cellular responses in the formation of specific SMs (Keller et al., 2005). Some typical BDTFs are shown in Table 2.
TABLE 1.
Narrow domain transcription factors (NDTFs).
| Transcriptional factors (regulatory proteins) | Class of regulatory transcriptional proteins | Microfungal organisms | Functional metabolites | References |
| AflR | Zinc binuclear cluster protein of Zn2Cys6 type | A. flavus and A. parasiticus | Aflatoxin and sterigmato cystin | Woloshuk et al., 1994; Chang et al., 1995; Yu et al., 1996; Fernandes et al., 1998; Slot and Rokas, 2011 |
| AflJ | Zn2Cys6 DNA-binding protein | A. parasiticus | Aflatoxin and sterigmato cystin | Meyers et al., 1998; Chang et al., 2000 |
| MlcR | Zinc binuclear cluster protein of Zn2Cys6 type | P. citrinum | Compactin | Abe et al., 2002; Deepika et al., 2016 |
| ApdR | GAL4-type Zn2Cys6 need to check | A. nidulans, A. flavus | Aspyridone A and B | Bergmann et al., 2007 |
| GliZ53 | Zinc finger transcription factor GliZ53 | A. nidulans | Gliotoxin | Cramer et al., 2006 |
| PENR1 | HAP-like transcription factor | A. nidulans | Penicillin and some enzymes like cellobiohydrolase, xylanase, and taka-amylase | Brakhage et al., 1999 |
| ToxE | Ankyrin repeat protein | Cochliobolus sp. | HC-Toxin | Pedley and Walton, 2001; Keller et al., 2005 |
| AcFKH1 | 2-Peptide forkhead protein | A. chrysogenum | Cephalosporin C | Schmitt et al., 2004 |
| CPCR1 | 2-Peptide forkhead protein | A. chrysogenum | Cephalosporin C | Schmitt et al., 2004 |
| Tri4, Tri5, Tri6 | Cys2His2 zinc finger proteins | F. sporotrichioides | Trichothecene | Proctor et al., 1995 |
| MRTR14, MRTR15, MRTR16 | Cys2His2 zinc finger proteins | F. sporotrichioides, Myrothecium roridum | Trichothecene | Trapp et al., 1998; Keller et al., 2005; Liu et al., 2016 |
TABLE 2.
Broad domain transcription factors (BDTFs)/global transcription factors.
| Transcriptional factors (regulatory proteins) | Class of regulatory transcriptional proteins | External cues | Microfungal strains | Functional metabolites | References |
| CreA | Zinc finger protein of Cys2His2 type | Carbon signaling | A. nidulans | Penicillium | Shwab and Keller, 2008; Deepika et al., 2016 |
| PacC/CBC | Zinc finger protein of Cys2His2 type | Alkaline pH signaling | A. nidulans | Penicillium/ β-lactam | Shwab and Keller, 2008; Deepika et al., 2016 |
| AreA | Zinc finger protein of Cys2His2 type | Nitrogen signaling | F. fujikusori | Gibberellins | Deepika et al., 2016 |
| AreA | Zinc finger protein of Cys2His2 type | Nitrogen signaling | F. verticillioides | Fumonisin B1 | Kim and Woloshuk, 2008 |
| FadA/homologous of FadA | G-protein signaling regulator | Growth related hormone like extracellular ligands |
A. nidulans/ F. sporotrichioides |
Penicillium/ trichothecene |
Tag et al., 2000 |
| PkaA | Protein kinases | Growth related cues jointly work with G-proteins | A. nidulans | Penicillium | Hicks et al., 1997; Shimizu and Keller, 2001 |
| FlbA | G-protein signaling regulator | Asexual sporulation cues | A. nidulans | Aflatoxin and sterigmatocystin synthesis | Deepika et al., 2016 |
| HapB, HapC, HapE, HapX | CCAAT-binding complex | pH, Iron-depriving, and redox status signaling | A. nidulans | Penicillium, iron-scavenging siderophores | |
| LaeA, VeA, VelB | Velvet complex | Light dependent regulatory developmental cues | A. nidulans, A. chryroseum, P. chryroseum, A. flavus | β-lactam, aflatoxin | Kato et al., 2003; Dreyer et al., 2007; Bayram et al., 2008; Kosalkova et al., 2009; Bayram and Braus, 2012 |
| LaeA | Protein methyltransferase | Light dependent signaling | A. nidulans, A. fumigatus, A. terreus | Sterigmatocystin (ST) biosynthesis, chromatin modification | Bok and Keller, 2004; Lee et al., 2005 |
The close relationship between NDTFs and BDTFs has been extensively reviewed by various reports, which have explained how BDTFs or global transcription factors perceive the environmental and developmental cues and transduct these external messages to NDTFs through chromatin and histone modification or through specific biochemical cascade reactions, including methylation, phosphorylation, and acetylation. These reactions are essential for activating the silent clustered genes associated with specific SMs that are required in particular cellular metabolisms, growth stages, or environmental conditions (Figure 3; Foss et al., 1993; Kouzminova and Selker, 2001; Tamaru and Selker, 2001; Calvo et al., 2002; Yu and Keller, 2005; Shwab and Keller, 2008).
FIGURE 3.
Synchronized regulatory model of secondary metabolites (SMs) biosynthesis and external/morphological indicators.
Alterations in Plant Genetic Makeup in the Presence of Endophytic Fungi
Plant phenotypes depend not only on the genetic makeup of the plant, but also on the activity of microbiome and environmental factors (Jia et al., 2016). It has been observed that the presence of EFs enhances the resistance potential of host plants to biotic and abiotic stresses (Rodriguez et al., 2008; Shoresh et al., 2010; Busby et al., 2017; Yan et al., 2019) or improves nutrient uptake (Xie et al., 2019). Although the exact mechanism still remains to be elucidated, evidence has shown that plant genetic expression profiles are altered in the presence of EFs (Mejia et al., 2014; Cui et al., 2017; Liao et al., 2019; Xie et al., 2019). Endophytes reprogram the host genomic expression through epigenetic interactions with the host. In epigenetic events, DNA methylation and demethylation induced by endophytes enhance the host’s defensive processes (Kouzminova and Selker, 2001; Strobel, 2018; Geng et al., 2019). EFs stimulate the immune systems of plants and increase the quantity of SMs (Cui et al., 2017; Xie et al., 2019), which may induce some physiological changes in infected host plants. Bailey et al. (2006) regarded these altered or differentiated gene expressions in the presence of EFs as a complex system of genetic crosstalk between EFs and hosts. During the crosstalk, the genomic expressions of both endophytes and hosts are altered. Additional examples are given in Table 3.
TABLE 3.
Selected examples of plant gene alteration in the presence of endophytic fungi (EFs) and their resulted beneficial expression.
| Host Plant | Endophytic fungal community | Gene ontology (up regulation/down regulation) | Phenotypic expression/gene function | References |
| Taxus (young/old plantlets) | Paraconiothyrium SSM001 | Up regulation of TS, DXR, HMGR genes (related to taxol synthesis) | Increase the concentration of host plant taxol | Soliman et al., 2013 |
| Soybean plant (Glycine max) | P. indica | 61 genes are up- regulation and 238 genes are down-regulated | Enhancing the iron transport, lignin biosynthesis, hormone signaling, nutrient acquisition, biosynthesis of phenylpropanoids, flavonols, siderophores, and flavonoids (61 genes) 238 genes involved in encoding the heat shock protein, and several other abiotic stress related defense responses | Bajaj et al., 2018 |
| Rhodiola crenulata | Trimmatostroma sp. ZPRs-R11 | Up regulatory genes are TYDC, MAOA, C4H, TAT, PAL, PCD | Induced the accumulation of tyrosol and salidroside | Cui et al., 2017 |
| Lolium perenne (perennial ryegrass) | N. lolii | Up regulation of MRP/PDR like ABC and GST transporter systems related genes and downregulation of carbohydrate metabolism and photosynthesis related genes | Activates the cellular transport, transporter system, protein synthesis. | Khan et al., 2010 |
| Hordeum vulgare L. (barley) | P. indica | PR-5 gene | Induced tolerance in salt stress and the systemic disease resistance via elevating the conc. of antioxidant metabolites (ascorbate-glutathione pathway). | Waller et al., 2005 |
Horizontal Gene Transfer
Horizontal gene transfer refers to the exchange of genetic materials between isolated lineages through asexual processes. A growing body of evidence has suggested that frequent HGT occurs between independent organisms and between EFs and their hosts (Richards et al., 2011; Bapteste et al., 2012). Most HGTs identified in microbiota are associated with invading, degrading, and manipulating hosts (Soanes and Richards, 2014), which supports the hypothesis that HGT may be a robust source of novel adaptive traits of EFs and that HGT is a strong driving force for fungal adaptive evolution (Feurtey and Stukenbrock, 2018). As the coding genes of a metabolic pathway are always located in a cluster, HGT of an intact metabolic cluster may enable the recipient organism to obtain a new and complete metabolic pathway (Slot and Rokas, 2011). Bapteste et al. (2012) identified a particular evolutionary unit of genetic materials that introgress into different host structures and propagate within these structures. These evolutionary units may leave recognizable patterns in resemblance networks. This finding suggests that HGT also plays an important role in the emergence of new fungal strains (Feurtey and Stukenbrock, 2018).
The expression of horizontally transferred genes in recipient organisms is the first step for HGT to play a role, and determine the colonization mode and capacity of EFs in host plants (Soanes and Richards, 2014). The mechanism of how horizontally transferred genes initiate their expression in recipient organisms remains to be discussed. Slot and Rokas (2011) demonstrated that when the gene cluster encoding the biosynthesis of sterigmatocystin (a toxic SM) was transferred from an Aspergillus species to Podospora anserine, the transferred cluster was functional because it was expressed in the latter. Many studies have demonstrated that EFs can produce host-mediated compounds, whereas the latter can also produce some EF-originated metabolites. For instance, djalonensone has been detected as a fungal metabolite in various Alternaria fungi, but it was first identified in extracts from cauliflower plants. Similarly, the EF metabolite aureonitol produced by Chaetomium sp. can also be detected in the extract of Helichrysum aureonitens. Many reported EF-originated compounds, such as alternariol, alternariol monomethyl ether, altenusin, macrosporin, and methylalaternin, were further detected in their host plants, Polygonum senegalense and Urospermum picroides. These results provide evidence for HGT or genetic recombination during the coevolution between hosts and endophytes, thus forming the genetic regulation mechanism of SM biosynthesis (Aly et al., 2010; Kozyrovska, 2013).
When HGT occurs between prokaryotes and eukaryotes, the integrated genes are more likely to be expressed in a modified manner in the recipient organism due to the fundamentally distinct gene expression mechanisms between the two genomes. The failure of HGT expression may lead to the appearance of pseudogenes in recipient organisms (Andersson, 2009).
Endophytic Fungi Mimic or Alter Phytochemical Production in Host Plants
The wide range of biotic and abiotic stresses that plants constantly face in natural or agricultural environments lead to specific transcriptional variations at the individual gene level, with a high degree of variability and stress specificity (Zhang and Sonnewald, 2017). This was supported by a study of plant responses to combined heat and drought stress, in which the genes involved in secondary metabolism were significantly up-regulated (Prasch and Sonnewald, 2013). In symbiosis, EFs mimic the production of SMs in hosts using primary phytocompounds as precursors. In contrast, proteins secreted by EFs may potentially act as effectors altering host metabolism (Spanu et al., 2010; Soanes and Richards, 2014; Kemen et al., 2015), with the specificity of each effector targeting a distinct hormone signaling pathway (Di et al., 2016).
Endophytic Fungi Sense the Same Stimuli as Hosts
A “xenohormesis” hypothesis proposed by Howitz and Sinclair (2008) suggests that heterotrophic organisms (animals and microbes) may sense plant signals or molecules induced under stress. By doing so, the heterotrophic organisms may take advantage of the defensive responses of autotrophs to increase their own survival chances. EFs may also have the ability to sense chemical cues in plants, and begin to produce similar SMs (Kusari et al., 2012). As ecological adaptions and survival elements, SMs are not synthesized throughout the whole life cycle, but only when necessary, especially in the early differentiation or late senescence stages of an organism (Deepika et al., 2016; Stahl et al., 2018; Siddhardha and Meena, 2020). In general, their synthesis is minimal when the organism grows rapidly and is maximal when the growth of the organism ceases. For an organism, the core indicator point of its synthesis of SMs is that it is in a state of rapid response rather than in a state of rapid growth (Deepika et al., 2016). In such circumstances, the genetic materials of both EFs and their hosts are cross-activated under hostile conditions (Stierle et al., 1993; Zhou et al., 2010). The best example, which well supports the previous hypothesis, is the pioneering identification of the anti-carcinogenic drug Taxol (paclitaxel), which is a worldwide selling cancer drug, with annual sales of million USD, from an EF, Taxomyces andreanae. This EF was originally identified from Taxus brevifolia, the host plant of T. andreanae, indicating that both the EF and its host produce the same SM in response to environmental stimuli. Since its initial discovery in the past few decades, Taxol has been proven to be more efficiently produced in the EF than in the host. Studies also show that Taxol can be effectively isolated from several other EF strains and Pacific yew plants (Stierle et al., 1993; Zhou et al., 2010; Chandra, 2012; Zaiyou et al., 2017; Uzma et al., 2018).
Endophytic Fungi Share Common Precursors With Hosts
Primary metabolites are the end products of primary metabolic pathways, including carbohydrates, amino acids, proteins, and lipids. They play a primary metabolic role in the construction and development of an organism. Without them, the growth and development of the organism are at extreme risk for defects. An important role of primary metabolism is that the products of some key steps provide precursors for the synthesis of SMs. Endophytes and their host plants share these precursors in their respective SM biosynthesis pathways. The biosynthetic pathway of SMs in EFs may be the result of their mimicking of the host pathways (Kirby and Keasling, 2009; Deepika et al., 2016). The synthetic pathways of some phytochemicals, including ergot alkaloids, aflatoxin, and lovastatin, have been studied by blocking mutant and radio labeling techniques (Keller et al., 2005). It has been revealed that although diverse, SMs are produced by a few common biosynthetic pathways and the metabolomic pathways of endophytic fungal communities and their host plants are similar. At this stage, the question still remains of whether these low molecular weight phytochemicals are synthesized by plants or as a result of symbiosis with microorganisms living inside their tissues. The combination of some possible inducers has promoted the accumulation of bioactive metabolites in EFs and plants, indicating that EFs play a significant role in the biosynthesis of SMs. These inducers include nutrient deficiencies, morphological development, and growth rates (Kirby and Keasling, 2009; Deepika et al., 2016).
Classes of Fungal Secondary Metabolites and Their Biological Potential
EFs are considered to be rich sources of diverse bioactive SMs and phytohormones to support plant growth and enable plants to survive under biotic or abiotic stresses (Tan and Zou, 2001; Chadha et al., 2014). Some of the most important commercially exploited SMs, including antibiotics, anticarcinogenics, cytotoxics, insecticides, and allelopathic compounds, can also be biosynthesized by EFs (Schneider et al., 2008). They have broad, promising commercial prospects in the pharmaceutical, medical, agricultural, nutraceutical, cosmetic, flavor, and fragrance industries, making EFs an attractive topic in the field of endophytism research (Hyde and Soytong, 2008; Table 4). Therefore, exploring the benign symbiotic relationship that synthesizes these bioactive SMs between EFs and plants and its impact on the genetic materials of EFs and plants will provide a promising framework for the discovery and development of new bioactive SMs through metabolomic and genetic engineering in the future (Kirby and Keasling, 2009; Deepika et al., 2016). Of the numerous SMs biosynthesized by EFs and hosts, most can be sorted into the following classes: alkaloids, terpenoids, polyketides, phenylpropanoids and lignins, flavonoids, saponins, phenols and phenolic acids, aliphatic, and chlorinated metabolites, peptides, and steroids. To facilitate the comparison of these SMs, they are presented in tabular form in Table 5 and Figure 4 after a brief description of their EF sources, chemical structures, and biological application potential.
TABLE 4.
Commercial applications of bioactive natural products with endophytic fungi (EFs)-based biogenesis.
| Application fields | Reported products | Endophytic fungal sources | References |
| Pharmaceuticals | Taxol (anticarcinogenic agent) | Paraconiothyrium SSM001 | Soliman et al., 2013 |
| Cycloepoxytriol B (antibiotic agent) | Phomopsis sp. | Hussain et al., 2009 | |
| Flavor and fragrance | Methyl eugenol [1,2-dimethoxy 4-(2-propenyl) benzene] | Alternaria sp. | Kaul et al., 2008 |
| Cosmetics (cream, shampoos, lotions, toothpaste, etc.) | Fatty acids (e.g., oleic, stearic, linoleic, and palmitic acid) | Bionectria ochroleuca, C. truncatum, Chaetomium sp. | George et al., 2011; Kumar and Kaushik, 2013; Yang Y. et al., 2015 |
| Chitosan | A. flavus, C. cladosporioides, Phoma sp. | George et al., 2011 | |
| Food industry | Chitosan (as food additive) | A. flavus, C. cladosporioides, Phoma sp. | Liu et al., 2008; George et al., 2011 |
| 7-amino-4-methylcoumarin (food preservative agent) | Xylaria sp. | Liu et al., 2008 | |
| Bioinsecticides | Loline alkaloids | N. uncinatum | Aly et al., 2010 |
| Bioherbicides | Ascotoxin (growth inhibitory effect) | Paraconiothyrium sp. | Khan et al., 2012 |
| Nutraceuticals | Saponins | Aspergillus, Bulgaria, Penicillium, Phomopsis sp. | Nicoletti and Fiorentino, 2015 |
TABLE 5.
Classes of endophytic fungal secondary metabolites (SMs) with biological potential activities.
| Classes of SMs | Sub classes of SMs | Compounds with references | Endophytic fungal sources | Chemical structures* | Potential biological properties |
| a. Alkaloids | Indole derivative alkaloids | Vinblastine, vincristine (Keglevich et al., 2012; Kumar et al., 2013) | F. oxysporum | 1, 2 | Antitumor drugs |
| Chaetoglobosin (Zhang Y. et al., 2012; Huang et al., 2016) | C. elatum | 3 | Antitumor activity against breast tumor and cholangiocarcinoma cell lines | ||
| Pyridines and pyrrolizidines | Asperfumoid (Zhang Y. et al., 2012; Li et al., 2015) | Penicillium sp. | 4 | Potent cytotoxic | |
| 7,8-dimethyl-isoalloxazine (Owen and West, 1971; Zhang Y. et al., 2012; Li et al., 2015) | Penicillium sp. | 5 | Cytotoxic agent | ||
| Lolines (Bush et al., 1997; Tan and Zou, 2001) | 6 | Allelopathic and insecticidal properties | |||
| Amines and amides | Peramine (Schardl and Phillips, 1997; Tan and Zou, 2001) | Neotyphodium sp., Epichloë sp. | 7 | Insecticidal- pyrrolopyrazine alkaloid | |
| Phomoenamide (Rukachaisirikul et al., 2008) | Phomopsis sp. | 8 | Antibacterial properties | ||
| Ergovaline (Flieger et al., 1997; Duringer et al., 2007; Rukachaisirikul et al., 2008; Zhang Y. et al., 2012) | Neotyphodium sp., Claviceps sp. | 9 | Neurotoxicity in livestock (feeding repellent) | ||
| Quinoline and isoquinoline | Camptothecin (Sriram et al., 2005; Shao et al., 2010; Zhang Y. et al., 2012; Wu et al., 2015) | Nothapodytes fortida | 10 | Potent cytotoxic drug, antiprotozoal, and anti-HIV properties | |
| Penicinoline and its derivatives (Shao et al., 2010; Zhang Y. et al., 2012; Bladt et al., 2013; Naveen et al., 2017) | Penicillium sp., Auxarthron reticulatum, and mangroves associated endophytic fungal species | 11 | Cytotoxic compound | ||
| b. Terpenoids | Sesquiterpenes | Chokols and its derivatives (A, C, D, F) (Hiroyuki et al., 1989) | E. typhina | 12, 13, 14, 15 | Fungicidal properties against C. phlei pathogen |
| Heptelidic acid and hydroheptelidic acid (Calhoun et al., 1992; Tan and Zou, 2001) | Phyllosticta sp. | 16, 17 | Toxic against C. fumiferana larvae | ||
| Diterpenes | Taxol (paclitaxel) (Nicolaou et al., 1994; Tan and Zou, 2001; Lin et al., 2014) | T. andreanae | 18 | Anticarcinogenic drug | |
| Subglutinol A and B (Nicolaou et al., 1994; Tan and Zou, 2001; Lin et al., 2014) | F. subglutinans | 19, 20 | Immunosuppressive property | ||
| c. Polyketides | 6-O-Methylalaternin (Mousa and Raizada, 2013) | Ampelomyces sp. | 21 | Biocontrol agent against parasitic fungi | |
| Altersolanol A (Mousa and Raizada, 2013) | A. solani | 22 | Antibiotic (antibacterial) properties | ||
| Palmarumycin CP17 (Martínez-Luis et al., 2008) | Edenia sp. (Pleosporaceae) | 23 | Antiparasitic compound especially against protozoans, antineoplastic effects via G2/M stage in mammalian cell cycle | ||
| Rugulosin (Mousa and Raizada, 2013) | Hormonema dematioides | 24 | Act as a mycotoxins due to having cell necrosis, fatty acids degeneration effects makes it a natural cytotoxic compound | ||
| Pestalachloride B (Li et al., 2008) | P. adusta | 25 | Antibiotic (antifungal) activities | ||
| CR377 (Brady and Clardy, 2000; Mousa and Raizada, 2013) | Fusarium sp. | 26 | Antibiotic (antifungal) activities | ||
| Pestalotheol C (Mousa and Raizada, 2013) | Pestalotiopsis theae | 27 | Inhibitory effect | ||
| Chaetomugilin A (Qin et al., 2009) | C. globosum | 28 | Cytotoxic effect against brine shrimp larvae | ||
| d. Phenylpropanoids and lignans | Coniferin (Falshaw et al., 1969; Chapela et al., 1991; Daubresse et al., 1997) | Xylariaceae sp. | 29 | Reduced the biosynthesis of lignins via inhibition of oxidases | |
| Syringin (Eleutheroside B) (Falshaw et al., 1969; Chapela et al., 1991; Cho et al., 2001; Li et al., 2017) | Xylariaceae sp. | 30 | Antioxidant effects, anti-inflammatory, immunomodulatory, and most remarkably used in cardiac disease (cardiac hypertrophy) | ||
| Phillyrin (Zhang Q. et al., 2012; Chen et al., 2016) | C. gloeosporioides | 31 | Antioxidant, anti-inflammatory, and antipyretic activities | ||
| Sesamin (Lee et al., 2011; Nicoletti and Fiorentino, 2015) | A. ilanense | 32 | Antitumor, antioxidantive, antihypertensive properties | ||
| Syringaresinol (Cheng et al., 2013; Nicoletti and Fiorentino, 2015; Kim et al., 2016) | A. ilanense | 33 | Activating the SIRT1 gene expression, leading to slow the cellular senescence, and enhanced the function of endothelial cells | ||
| 4-Ketopinoresinol (Chen et al., 2012; Cheng et al., 2013; Nicoletti and Fiorentino, 2015) | A. ilanense | 34 | Nrf2/ARE-mediated transcription activator and eliminate the oxidative stress effects | ||
| e. Flavonoids | Cajanol (Liang et al., 2013; Zhao et al., 2013; Nicoletti and Fiorentino, 2015) | Hypocrea lixii | 35 | Anticarcinogenic and antimalarial properties | |
| Kaempferol (Vellosa et al., 2011; Nicoletti and Fiorentino, 2015) | F. chlamydosporum | 36 | Cytotoxic and antioxidant properties | ||
| Quercetin (Materska, 2008; Huang et al., 2013; Nicoletti and Fiorentino, 2015) | A. ilanense | 37 | Reduce degenerative disease, apoptotic activity against liver cancer, antioxidant drug | ||
| Silymarin (AbouZid, 2012; Nicoletti and Fiorentino, 2015) | A. iizukae | 38a–38g including 7 flavonolignans (silybin A, B, isosilybin A, B, silychristin A, B, and silydianin) | Anti-inflammatory, anticarcinogenic, anti-asthma, hyperprolactinemia, hepatoprotective, immunostimulant | ||
| Tricin (Tan and Zou, 2001; Mousa and Raizada, 2013) | N. typhnium infected bluegrass | 39 | Toxic effect against mosquito larvae and acted as antimalarial agent | ||
| Flavones glycosides (Tan and Zou, 2001; Mousa and Raizada, 2013) | N. typhnium infected bluegrass | 40 | Antimalarial agent | ||
| f. Saponins | Diosgenin (Nicoletti and Fiorentino, 2015) | Fusarium sp., Cephalosporium sp., Paecilomyces sp. | 41 | Pharmaceutically effective drug and important precursor of progesterone, corticosteroids, and other several steroidal drugs | |
| Gymnemagenin (Nicoletti and Fiorentino, 2015) | P. oxalicum | 42 | Antidiabetic properties | ||
| g. Phenols and phenolic acids | 2-Hydroxy-6-methyl benzoic acid (Yang et al., 1994; Zou et al., 2000) | Phoma sp. | 43 | Antibiotic activity | |
| Tyrosol (Zou et al., 2000; Rodríguez-Morató et al., 2015) | E. typhina | 44 | Antifungal | ||
| cis- and trans- p-coumaric acids (Zou et al., 2000; Sigurdson et al., 2018) | E. typhina | 45, 46 | Antimicrobial activities | ||
| Colletotric acid (Zou et al., 2000) | C. gloeosporioides | 47 | Antimicrobial compound | ||
| h. Aliphatic and chlorinated metabolites | Phomodiol (Calhoun et al., 1992; Horn et al., 1996) | Phomopsis sp. | 48 | Antimicrobial, insecticidal, algicidal properties | |
| Phomopsolide B (aliphatic ester related compounds) (Tan and Zou, 2001) | Phomopsis sp. | 49 | Antimicrobial activities | ||
| Mycorrhizin A (Tan and Zou, 2001) | Phyllosticta sp. strain 76 | 50 | Antibiotic drug | ||
| Cryptosporiopsin (chlorinated compounds) (Tan and Zou, 2001) | Pezicula sp., P. livida | 51 | Algicidal drug | ||
| i. Peptides | Leucinostatin A (Tan and Zou, 2001) | Acremonium sp. | 52 | Fungicidal, antitumor, phytotoxic properties | |
| Echinocandins A, B, D, H (Tan and Zou, 2001) | A. rugulosus, Cryptosporiopsis sp., Pezicula sp. | 53 | Antibiotic activities | ||
| Cryptocandin (Tan and Zou, 2001) | Cryptosporiopsis cf. quercina | 54 | Antifungal properties | ||
| j. Steroids | 3β,5α-dihydroxy-6β-acetoxyergosta-7,22-diene and 3β,5α-dihydroxy-6β-phenylacetoxyergosta-7,22-diene (Lu et al., 2000) | Colletotricum sp. | 55, 56 | Fungicidal activity | |
| 3β-hydroxyergosta-5-ene and 3-oxoergosta-4,6,8(14),22-tetraene (Lu et al., 2000) | Colletotricum sp. | 57, 58 | Fungicidal activity | ||
| Ergosterol (Yu et al., 2010; Yang H. et al., 2015; Nowak et al., 2016) | Nodulisporium sp. | 59 | Antimicrobial activity | ||
| 5a, 8a-epidioxy ergosterol (Yu et al., 2010; Nowak et al., 2016) | Nodulisporium sp. | 60 | Antimicrobial activity |
*The chemical structure of the secondary metabolite represented by each number is shown in Figure 4.
FIGURE 4.
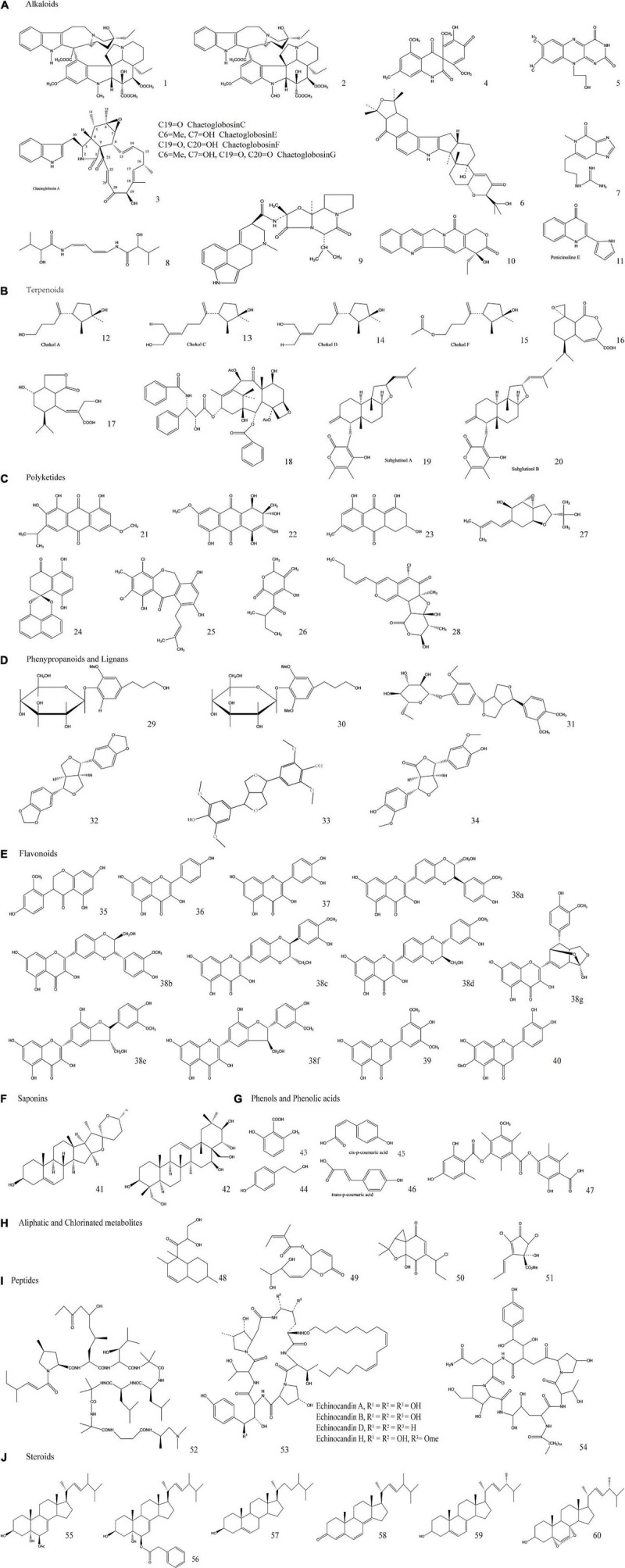
Molecular structures of some typical secondary metabolites (SMs). The classes, subclasses, compounds with references, endophytic fungal sources, together with their potential biological properties of the SMs are briefed in Table 5.
a. Alkaloids
Alkaloids include indole derivatives, pyrimidine and pyrrolizidine, quinoline and isoquinoline, amines, and amides (Zhang Y. et al., 2012). They are isolated from EFs that colonize in grass (Clavicipitaceous genera: Epichloë sp.) (Guerre, 2015), and because of their toxicity, they enable hosts to inhibit insect and herbivore attacks. In addition, their concentration levels are mainly dependent on EF strains, species, or genotypes and less dependent on environmental factors and host genotypes (Zhang et al., 2006; Gundel et al., 2018). Alkaloids are composed of bioactive compounds. In addition to complex chemical properties, alkaloids have bioactive properties, such as fungicidal, antibiotic, and antitumor activities, making them the main focus of numerous drug research and development projects (Zhang et al., 2006; Song et al., 2020).
b. Terpenoids
Terpenoids are the second major group of plant SMs. They also are obtained from endophytic mycobiota. Their primary characteristics include defensive agents, pollinator attractors (mainly odoriferous or color components), and allelochemicals in competitive environments. Among terpenoids, sesquiterpenoids, and diterpenoids can be easily isolated from endophytic cultures without degradation (Jakubczyk and Dussart, 2020).
c. Polyketides
Polyketides are the most copious and well-characterized fungal metabolites, and are exclusively detected in mycospecies. Polyketide synthases play the most important role in their biosynthesis. These synthases are similar to those of eukaryotic fatty acid, but their products are different due to the selective reduction of the β-carbon in polyketides rather than the compulsive reduction of that position in fatty acids (Keller et al., 2005; Jakubczyk and Dussart, 2020).
d. Phenylpropanoids and lignins
These types of SMs are produced in wounds and lesion conditions to protect the host from pathogenic attacks. Lignin compounds play a role in initiating wound healing (Yadav et al., 2020). EFs also produce these phytochemicals to improve the adaptability of host plants to adversity. These compounds can be isolated from both infected plants and separately cultured EFs (Dixon and Paiva, 1995; Sampangi-Ramaiah et al., 2020).
e. Flavonoids
Flavonoids are essential plant natural products that have polyphenolic moieties, but they are also EF derived. Due to their antioxidant, anti-inflammatory, antimicrobial, antimutagenic, and anticarcinogenic effects, they have significant miscellaneous biochemical, cosmetic, nutritional, and pharmaceutical applications for the treatment of various ailments, such as Alzheimer’s disease, cancer, and atherosclerosis. In addition, flavonoids are basic ingredients in the field of cosmetics (Panche et al., 2016).
f. Saponins
Saponins are a kind of glycoside compound in which the sugar moiety attaches to sapogenin through a glycosidic bond. Because of their antimicrobial properties, saponins play a defensive role in their symbiosis with host plants. Other applications are related to their anticancer, antinutritive, and anticholesterol properties (Nicoletti and Fiorentino, 2015).
g. Phenols and phenolic acids
Phenols and phenolic acids are a diversified class of SMs that are principally synthesized by plants and have also been isolated and identified from EF sources. Their major function is to act as signal factors in plant–microbial associations. They also act as defensive agents, promoting plant growth in nutrient-limited soil during symbiosis (Singh et al., 2011). This may be due to the higher concentrations of phenolic acids, phenols, and their derivatives in host plants inoculated with EFs (Mandal et al., 2010).
h. Aliphatic and chlorinated metabolites
Aliphatic and chlorinated metabolites have simple chemical structures but are considered xenobiotic compounds. These metabolites are widely biosynthesized by several forest and wood litter-degrading fungal species and endophytes. They have antibiotic activities against pathogenic microorganisms, insects, and algae, but they are also carcinogenic and genotoxic to animals and humans (Lin et al., 2011).
i. Peptides
Endophytic fungal peptides are another class of SMs that act as defense agents. They are protein forms with a molecular weight of less than 10 kDa (Ng, 2004). Significant scientific efforts have been directed toward identifying and isolating peptides as candidate drugs due to their high degree of interactions with their specific targets. Studies have reported endophyte isolates as potential sources of peptide-based drugs for the treatment of a variety of illnesses (Abdalla and Matasyoh, 2014). The most important group of anticarcinogenic and antifungal peptides, Leucinostatin, has been extracted from the fungal endophyte Acremonium sp., which is isolated from T. baccata (European yew plant) (Abdalla and Matasyoh, 2014).
j. Steroids
Steroids are natural chemical substances that are abundantly produced not only in plants and animals, but also in microbial communities. Steroids are bio-lipid-based terpenoids that have a structure of four fused carbon skeleton-based rings, which is characterized as the steroid nucleus or sterane. Steroids vary in structure and function because of the different oxidation states of functional groups attached to the rings (Theodorakidou et al., 2018).
It has already been demonstrated that endophytic steroids such as ergosterol exhibit extra pharmaceutical activities and natural roles in their producers. The 5a- and 8a-epidioxy ergosterols that have been isolated from Nodulisporium sp. have potent antimicrobial activities against a series of pathogenic microbial strains (Yu et al., 2010; Yang H. et al., 2015; Nowak et al., 2016).
Substantial Challenges and Future Perspectives in Endophytic Fungi Studies and Conclusion
At present, endogenous biology is receiving increasing attention due to the great application potential of the chemicals secreted by EF–host symbiotic associations in sustainable agriculture and biomedicine. Scientists are interested in understanding the underlying mechanisms of endophytism and its biological and ecological roles. Its importance is highlighted by the large number of studies in the field of endophytic biological research (Hyde and Soytong, 2008; Deepika et al., 2016; Nützmann et al., 2018; Fang et al., 2019; Huang et al., 2019). Although EF research has attracted great attention, this field still faces substantial challenges to be addressed in the coming decades, including:
-
•
The selection of suitable host plants and their healthy organs or tissues to identify and isolate new EFs and to dissect their related mutualistic or antagonistic signaling mechanisms during symbiosis (Strobel, 2018).
-
•
The complexities in the process of artificial culture due to the aseptic or non-culturable characteristics of some fungal strains. It is important to introduce de novo bioengineering systems or to modify conventional isolation techniques to address this challenge (Stone et al., 2004; Zhang et al., 2006; Zhang P. et al., 2019).
-
•
The biosynthesis of natural products of EFs, especially SMs, requires inducing stimuli from host plants or from symbiosis. In artificial axenic culture, EFs may not be able to synthesize the same chemicals as they do in a symbiotic continuum due to the absence of these plant-mediated stimuli or signals. For the EFs that have been successfully isolated and cultured, their SM production decreases with successive subcultures under axenic monoculture conditions (Cánovas et al., 2017). Therefore, it is also a challenge to monitor the in vivo stimuli of successfully isolated EFs under quasi-natural conditions and maintain their “competency” in culture.
-
•
Fungal SMs play critical roles in understanding the endosymbiotic mechanisms between EFs and hosts. This endosymbiosis can be indirectly monitored by the transformation biogenesis of SMs under artificial culture conditions. Considering the significant impact of different types and concentrations of nutrients in artificial media in EF culture (Ruiz et al., 2010), this is not an easy job.
-
•
The degradability of SMs extracted from target EFs needs to be addressed. Compounds isolated from a symbiotic continuum may be highly unstable in vitro or in axenic culture. Thus, it is difficult to obtain these required novel compounds in an artificial medium.
-
•
The number of EFs that have been explored is limited. It is estimated that only 1-2% of about 300,000 plant species have been investigated, and at present, little information has been obtained from hydro-ecosystems, which means that the vast majority of EF symbiotic relationships remain elusive (Strobel, 2018).
In the current “omics” era, tools including genomics, epigenomics, transcriptomics, proteomics, and their related meta-omics (metagenomics, metatranscriptomics, and metaproteomics) (Zhang J. et al., 2019), will be extraordinarily supportive in illuminating the gray areas of myco-endophytisms and in tackling the aforementioned challenges to reveal complementary information on these symbionts and their interactions inside the internal niches of host plants (Peršoh, 2015; Nowack and Weber, 2018; Zhang J. et al., 2019). Furthermore, collaborative research between omics tools and other disciplines, such as combinatorial chemistry, will be more effective and fruitful in constructing novel molecular models of these EF–host interactions (Nowack and Weber, 2018).
Conclusion
Secondary metabolites play a pivotal role in mediating biochemical communications between EFs and host plants. These biochemical communications guide the multidimensional interactions among EFs, host plants, and pathogens in their community and determine the host range of an EF and endophyte populations in a plant host. The biosynthesis of SMs during symbiosis is precisely regulated by several genetic mechanisms, including gene clustering, transcription factors, the altering of the genetic makeup of the host in the presence of EFs, and HGT. These regulation mechanisms may have coevolved with the initiation of EF–host symbiosis. Recently, SMs have attracted widespread research efforts due to their great biological potential in the discovery of modern medicines, sustainable agriculture, and industry. Further investigations at the molecular level may still be needed for gaining a better understanding the endophyte–host relationship in natural ecosystems at the genomic level, and for efficiently identifying the hidden genes involved in the biosynthesis of SMs and new compounds in axenic culture.
Author Contributions
BA and WG: conceive the idea and write the initial draft of the manuscript. JL and QG: help organizing and editing the manuscript. QG and WG: perform the network construction and figure presentation. MK, JG, and SM: proofreading the manuscript. YY and WG: contribute to the final editing of the manuscript. All authors contribute in the interpretation of the manuscript and approve it.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge SciLang for its linguistic assistance during the preparation of this manuscript and Baocai Zhang, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, for his critical review and valuable suggestions to the manuscript.
Funding
This work was funded by the Natural Science Foundation of China (31621005), the National Key R&D Program of China (2017YFD0101603-11, 2016YFD0100500, and 2016YFD0101401), the Agricultural Science and Technology Innovation Program for CAAS (CAAS-ASTIP-ICRCAAS), the National High Technology Research and Development Program of China (2012AA101108 and 2009AA101104), and the Central Level of the Scientific Research Institutes for Basic R&D Special Fund Business (1610162014008).
References
- Abdalla M. A., Matasyoh J. C. (2014). Endophytes as producers of peptides: an overview about the recently discovered peptides from endophytic microbes. Nat. Prod. Bioprospect. 4 257–270. 10.1007/s13659-014-0038-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe Y., Ono C., Hosobuchi M., Yoshikawa H. (2002). Functional analysis of mlcR, a regulatory gene for ML-236B (compactin) biosynthesis in Penicillium citrinum. Mol. Genet. Genomics 268 352–361. 10.1007/s00438-002-0755-5 [DOI] [PubMed] [Google Scholar]
- AbouZid S. (2012). “Silymarin, natural flavonolignans from milk thistle,” in Phytochemicals-A Global Perspective of Their Role in Nutrition and Health, ed. Rao V. (London: IntechOpen; ). [Google Scholar]
- Abreu-Tarazi M. F., Navarrete A. A., Andreote F. D., Almeida C. V., Tsai S. M., Almeida M. (2010). Endophytic bacteria in long-term in vitro cultivated “axenic” pineapple microplants revealed by PCR–DGGE. World J. Microbiol. Biotechnol. 26 555–560. 10.1007/s11274-009-0191-3 [DOI] [Google Scholar]
- Albrectsen B. R., Witzell J., Robinson K. M., Wulff S., Luquez V. M. C., Ågren R., et al. (2010). Large scale geographic clines of parasite damage to Populus tremula L. Ecography 33 483–493. 10.1111/j.1600-0587.2009.05982.x [DOI] [Google Scholar]
- Alvear-Daza J. J., García-Barco A., Osorio-Vargas P., Gutiérrez-Zapata H. M., Sanabria J., Rengifo-Herrera J. A. (2021). Resistance and induction of viable but non culturable states (VBNC) during inactivation of E. coli and Klebsiella pneumoniae by addition of H2O2 to natural well water under simulated solar irradiation. Water Res. 188:116499. 10.1016/j.watres.2020.116499 [DOI] [PubMed] [Google Scholar]
- Aly A. H., Debbab A., Kjer J., Proksch P. (2010). Fungal endophytes from higher plants: a prolific source of phytochemicals and other bioactive natural products. Fungal Divers. 41 1–16. [Google Scholar]
- Andersen M. R., Nielsen J. B., Klitgaard A., Petersen L. M., Zachariasen M., Hansen T. J., et al. (2013). Accurate prediction of secondary metabolite gene clusters in filamentous fungi. Proc. Natl. Acad. Sci. U.S.A. 110 E99–E107. 10.1073/pnas.1205532110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J. O. (2009). Gene transfer and diversification of microbial eukaryotes. Annu. Rev. Microbiol. 63 177–193. 10.1146/annurev.micro.091208.073203 [DOI] [PubMed] [Google Scholar]
- Arnold A. E., Lutzoni F. (2007). Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology 88 541–549. [DOI] [PubMed] [Google Scholar]
- Arnold A. E., Miadlikowska J., Higgins K. L., Sarvate S. D., Gugger P., Way A., et al. (2009). A phylogenetic estimation of trophic transition networks for ascomycetous fungi: are lichens cradles of symbiotrophic fungal diversification? Syst. Biol. 58 283–297. 10.1093/sysbio/syp001 [DOI] [PubMed] [Google Scholar]
- Bahram M., Hildebrand F., Forslund S. K., Anderson J. L., Soudzilovskaia N. A., Bodegom P. M., et al. (2018). Structure and function of the global topsoil microbiome. Nature 560 233–237. 10.1038/s41586-018-0386-6 [DOI] [PubMed] [Google Scholar]
- Bailey B. A., Bae H., Strem M. D., Roberts D. P., Thomas S. E., Crozier J., et al. (2006). Fungal and plant gene expression during the colonization of cacao seedlings by endophytic isolates of four Trichoderma species. Planta 224 1449–1464. 10.1007/s00425-006-0314-0 [DOI] [PubMed] [Google Scholar]
- Bajaj R., Huang Y., Gebrechristos S., Mikolajczyk B., Brown H., Prasad R., et al. (2018). Transcriptional responses of soybean roots to colonization with the root endophytic fungus Piriformospora indica reveals altered phenylpropanoid and secondary metabolism. Sci. Rep. 8:10227. 10.1038/s41598-018-26809-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapteste E., Lopez P., Bouchard F., Baquero F., McInerney J. O., Burian R. M. (2012). Evolutionary analyses of non-genealogical bonds produced by introgressive descent. Proc. Natl. Acad. Sci. U.S.A. 109 18266–18272. 10.1073/pnas.1206541109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bärenstrauch M., Mann S., Jacquemin C., Bibi S., Sylla O. K., Baudouin E., et al. (2020). Molecular crosstalk between the endophyte Paraconiothyrium variabile and the phytopathogen Fusarium oxysporum – modulation of lipoxygenase activity and beauvericin production during the interaction. Fungal Genet. Biol. 139:103383. 10.1016/j.fgb.2020.103383 [DOI] [PubMed] [Google Scholar]
- Bayram Ö, Braus G. H. (2012). Coordination of secondary metabolism and development in fungi: the velvet family of regulatory proteins. FEMS Microbiol. Rev. 36 1–24. 10.1111/j.1574-6976.2011.00285.x [DOI] [PubMed] [Google Scholar]
- Bayram O., Krappmann S., Ni M., Bok J. W., Helmstaedt K., Valerius O., et al. (2008). VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 320 1504–1506. 10.1126/science.1155888 [DOI] [PubMed] [Google Scholar]
- Bergmann S., Schumann J., Scherlach K., Lange C., Brakhage A. A., Hertweck C. (2007). Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat. Chem. Biol. 3 213–217. 10.1038/nchembio869 [DOI] [PubMed] [Google Scholar]
- Bevan M., Bancroft I., Bent E., Love K., Goodman H., Dean C., et al. (1998). Analysis of 1.9 Mb of contiguous sequence from chromosome 4 of Arabidopsis thaliana. Nature 391 485–488. 10.1038/35140 [DOI] [PubMed] [Google Scholar]
- Bladt T., Frisvad J., Knudsen P., Larsen T. (2013). Anticancer and antifungal compounds from Aspergillus, Penicillium and other filamentous fungi. Molecules 18 11338–11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok J. W., Keller N. P. (2004). LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot. Cell 3 527–535. 10.1128/ec.3.2.527-535.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady S. F., Clardy J. (2000). CR377, a new pentaketide antifungal agent isolated from an endophytic fungus. J. Nat. Prod. 63 1447–1448. [DOI] [PubMed] [Google Scholar]
- Braga R. M., Padilla G., Araújo W. L. (2018). The biotechnological potential of Epicoccum spp.: diversity of secondary metabolites. Crit. Rev. Microbiol. 44 759–778. 10.1080/1040841x.2018.1514364 [DOI] [PubMed] [Google Scholar]
- Brakhage A. A., Andrianopoulos A., Kato M., Steidl S., Davis M. A., Tsukagoshi N., et al. (1999). HAP-Like CCAAT-binding complexes in filamentous fungi: implications for biotechnology. Fungal Genet. Biol. 27 243–252. 10.1006/fgbi.1999.1136 [DOI] [PubMed] [Google Scholar]
- Brown K. B., Hyde K. D., Guets D. I. (1998). Preliminary studies on endophytic fungal communities of Musa acuminata species comples in Hong Kong and Australia. Fungal Divers. 1 27–51. [Google Scholar]
- Busby P. E., Soman C., Wagner M. R., Friesen M. L., Kremer J., Bennett A., et al. (2017). Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 15:e2001793. 10.1371/journal.pbio.2001793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush L. P., Wilkinson H. H., Schardl C. L. (1997). Bioprotective alkaloids of grass-fungal endophyte symbioses. Plant Physiol. 114 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun L. A., Findlay J. A., Miller J. D., Whitney N. J. (1992). Metabolites toxic to spruce budworm from balsam fir needle endophytes. Mycol. Res. 96 281–286. [Google Scholar]
- Calvo A. M., Wilson R. A., Woo B. J., Keller N. P. (2002). Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 66 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camehl I., Sherameti I., Venus Y., Bethke G., Varma A., Lee J., et al. (2010). Ethylene signalling and ethylene-targeted transcription factors are required to balance beneficial and nonbeneficial traits in the symbiosis between the endophytic fungus Piriformospora indica and Arabidopsis thaliana. New Phytol. 185 1062–1073. 10.1111/j.1469-8137.2009.03149.x [DOI] [PubMed] [Google Scholar]
- Cánovas D., Studt L., Marcos A. T., Strauss J. (2017). High-throughput format for the phenotyping of fungi on solid substrates. Sci. Rep. 7:4289. 10.1038/s41598-017-03598-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll G. (1988). Fungal endophytes in stems and leaves: from latent pathogen to mutualistic symbiont. Ecology 69 2–9. [Google Scholar]
- Chadha N., Mishra M., Prasad R., Varma A. (2014). Root endophytic fungi: research update. J. Biol. Life Sci. 5 135–158. 10.5296/jbls.v5i2.5960 [DOI] [Google Scholar]
- Chandra S. (2012). Endophytic fungi: novel sources of anticancer lead molecules. Appl. Microbiol. Biotechnol. 95 47–59. 10.1007/s00253-012-4128-7 [DOI] [PubMed] [Google Scholar]
- Chang P. K., Ehrlich K. C., Yu J., Bhatnagar D., Cleveland T. E. (1995). Increased expression of Aspergillus parasiticus aflR, encoding a sequence-specific DNA-binding protein, relieves nitrate inhibition of aflatoxin biosynthesis. Appl. Environ. Microbiol. 61 2372–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P. K., Yu J., Bhatnagar D., Cleveland T. E. (2000). Characterization of the Aspergillus parasiticus major nitrogen regulatory gene, area. Biochim. Biophys. Acta 1491 263–266. 10.1016/s0167-4781(00)00004-x [DOI] [PubMed] [Google Scholar]
- Chapela I., Petrini O., Hagmann L. (1991). Monolignol glucosides as specific recognition messengers in fungus-plant symbioses. Physiol. Mol. Plant Pathol. 39 289–298. [Google Scholar]
- Chen H.-H., Chen Y.-T., Huang Y.-W., Tsai H.-J., Kuo C.-C. (2012). 4-Ketopinoresinol, a novel naturally occurring ARE activator, induces the Nrf2/HO-1 axis and protects against oxidative stress-induced cell injury via activation of PI3K/AKT signaling. Free Radic. Biol. Med. 52 1054–1066. [DOI] [PubMed] [Google Scholar]
- Chen J., Chen Q., Yu F., Huang H., Li P., Zhu J., et al. (2016). Comprehensive characterization and quantification of phillyrin in the fruits of Forsythia suspensa and its medicinal preparations by liquid chromatography-ion trap mass spectrometry. Acta Chromatogr. 28 145–157. [Google Scholar]
- Cheng M.-J., Wu M.-D., Chen J.-J., Hsieh S.-Y., Yuan G.-F., Chen I.-S., et al. (2013). Secondary metabolites from the endophytic fungus of Annulohypoxylon ilanense. Chem. Nat. Compd. 49 523–525. [Google Scholar]
- Chiang Y. M., Ahuja M., Oakley C. E., Entwistle R., Asokan A., Zutz C., et al. (2016). Development of genetic dereplication strains in Aspergillus nidulans results in the discovery of aspercryptin. Angew. Chem. 55 1662–1665. 10.1002/anie.201507097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J. Y., Nam K. H., Kim A. R., Park J., Yoo E. S., Baik K. U., et al. (2001). In-vitro and in-vivo immunomodulatory effects of syringin. J. Pharm. Pharmacol. 53 1287–1294. [DOI] [PubMed] [Google Scholar]
- Chu H. Y., Wegel E., Osbourn A. (2011). From hormones to secondary metabolism: the emergence of metabolic gene clusters in plants. Plant J. 66 66–79. 10.1111/j.1365-313X.2011.04503.x [DOI] [PubMed] [Google Scholar]
- Combes A., Ndoye I., Bance C., Bruzaud J., Djediat C., Dupont J., et al. (2012). Chemical communication between the endophytic fungus Paraconiothyrium variabile and the phytopathogen Fusarium oxysporum. PLoS One 7:e47313. 10.1371/journal.pone.0047313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer R. A., Jr., Gamcsik M. P., Brooking R. M., Najvar L. K., Kirkpatrick W. R., Patterson T. F., et al. (2006). Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot. Cell 5 972–980. 10.1128/EC.00049-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J. L., Wang Y. N., Jiao J., Gong Y., Wang J. H., Wang M. L. (2017). Fungal endophyte-induced salidroside and tyrosol biosynthesis combined with signal cross-talk and the mechanism of enzyme gene expression in Rhodiola crenulata. Sci. Rep. 7:12540. 10.1038/s41598-017-12895-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui R., Lu X., Chen X., Malik W. A., Wang D., Wang J., et al. (2021). A novel raffinose biological pathway is observed by symbionts of cotton=Verticillium dahliae to improve salt tolerance genetically on cotton. J. Agron. Crop Sci. 207 956–969. 10.1111/jac.12556 [DOI] [Google Scholar]
- Daubresse N., Chupeau Y., Francesch C., Lapierre C., Pollet B., Rolando C. (1997). Rational design, synthesis and biological evaluation of the first inhibitor of lignin polymerization. Chem. Commun. 1489–1490. [Google Scholar]
- Davey M. L., Currah R. S. (2006). Interactions between mosses (Bryophyta) and fungi. Can. J. Bot. 84 1509–1519. [Google Scholar]
- Debbab A., Aly A. H., Proksch P. (2011). Bioactive secondary metabolites from endophytes and associated marine derived fungi. Fungal Divers. 49 1–12. 10.1007/s13225-011-0114-0 [DOI] [Google Scholar]
- Deepika V. B., Murali T. S., Satyamoorthy K. (2016). Modulation of genetic clusters for synthesis of bioactive molecules in fungal endophytes: a review. Microbiol. Res. 182 125–140. 10.1016/j.micres.2015.10.009 [DOI] [PubMed] [Google Scholar]
- Demain A. L. (2014). Importance of microbial natural products and the need to revitalize their discovery. J. Ind. Microbiol. Biotechnol. 41 185–201. [DOI] [PubMed] [Google Scholar]
- Di X., Takken F. L., Tintor N. (2016). How phytohormones shape interactions between plants and the soil-borne fungus Fusarium oxysporum. Front. Plant Sci. 7:170. 10.3389/fpls.2016.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding T., Suo Y., Xiang Q., Zhao X., Chen S., Ye X., et al. (2017). Significance of viable but nonculturable Escherichia coli: induction, detection, and control. J. Microbiol. Biotechnol. 27 417–428. 10.4014/jmb.1609.09063 [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Paiva N. L. (1995). Stress-induced phenylpropanoid metabolism. Plant Cell 7 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer J., Eichhorn H., Friedlin E., Kurnsteiner H., Kuck U. (2007). A homologue of the Aspergillus velvet gene regulates both cephalosporin C biosynthesis and hyphal fragmentation in Acremonium chrysogenum. Appl. Environ. Microbiol. 73 3412–3422. 10.1128/AEM.00129-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duringer J., DeLorme M., Lehner A., Craig A. (2007). “A review of the ergot alkaloids found in endophyte-infected tall fescue and perennial ryegrass and their metabolism after ingestion by livestock,” in Proceedings of the 6th International Symposium on Fungal Endophytes of Grasses, (Christchurch: ), 377–382. [Google Scholar]
- El Enshasy H. A., Hanapi S. Z., Malek R. A., Abdelgalil S. A., Leng O. M. (2019). “Endophytic fungi: the desired biostimulants for essential oil production,” in Advances in Endophytic Fungal Research: Present Status and Future Challenges, ed. Singh B. P. (Cham: Springer International Publishing; ), 211–232. [Google Scholar]
- Falshaw C. P., Ollis W. D., Ormand K. L., Mongkolsuk S., Podimuang V. (1969). The spectroscopic identification of coniferin. Phytochemistry 8 913–915. 10.1016/s0031-9422(00)85883-5 [DOI] [Google Scholar]
- Fang C., Fernie A. R., Luo J. (2019). Exploring the diversity of plant metabolism. Trends Plant Sci. 24 83–98. 10.1016/j.tplants.2018.09.006 [DOI] [PubMed] [Google Scholar]
- Fernandes M., Keller N. P., Adams T. H. (1998). Sequence-specific binding by Aspergillus nidulans AflR, a C6 zinc cluster protein regulating mycotoxin biosynthesis. Mol. Microbiol. 28 1355–1365. [DOI] [PubMed] [Google Scholar]
- Feurtey A., Stukenbrock E. H. (2018). Interspecific gene exchange as a driver of adaptive evolution in fungi. Annu. Rev. Microbiol. 72 377–398. 10.1146/annurev-micro-090817-062753 [DOI] [PubMed] [Google Scholar]
- Field B., Osbourn A. E. (2008). Metabolic diversification–independent assembly of operon-like gene clusters in different plants. Science 320 543–547. 10.1126/science.1154990 [DOI] [PubMed] [Google Scholar]
- Field B., Fiston-Lavier A. S., Kemen A., Geisler K., Quesneville H., Osbourn A. E. (2011). Formation of plant metabolic gene clusters within dynamic chromosomal regions. Proc. Natl. Acad. Sci. U.S.A. 108 16116–16121. 10.1073/pnas.1109273108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo A. C., Barroso J. G., Pedro L. G., Scheffer J. J. C. (2008). Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Fragr. J. 23 213–226. 10.1002/ffj.1875 [DOI] [Google Scholar]
- Flieger M., Wurst M., Shelby R. (1997). Ergot alkaloids—sources, structures and analytical methods. Folia Microbiol. 42 3–30. [DOI] [PubMed] [Google Scholar]
- Forni C., Duca D., Glick B. R. (2017). Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 410 335–356. [Google Scholar]
- Foss H. M., Roberts C. J., Claeys K. M., Selker E. U. (1993). Abnormal chromosome behavior in Neurospora mutants defective in DNA methylation. Science 262 1737–1741. 10.1126/science.7505062 [DOI] [PubMed] [Google Scholar]
- Fox E. M., Howlett B. J. (2008). Secondary metabolism: regulation and role in fungal biology. Curr. Opin. Microbiol. 11 481–487. 10.1016/j.mib.2008.10.007 [DOI] [PubMed] [Google Scholar]
- Friesen M. L., Porter S. S., Stark S. C., von Wettberg E. J., Sachs J. L., Martinez-Romero E. (2011). Microbially mediated plant functional traits. Annu. Rev. Ecol. Evol. Syst. 42 23–46. 10.1146/annurev-ecolsys-102710-145039 [DOI] [Google Scholar]
- Gao L. D., Huang G. R., Wang Y. (2008). Seasonal and tissue age influences on endophytic fungi of Pinus tabulaeformis (Pinaceae) in the Dongling Mountains, Beijing. J. Integr. Plant Biol. 50 997–1003. 10.1111/j.1744-7909.2008.00394x [DOI] [PubMed] [Google Scholar]
- Geng S., Kong X., Song G., Jia M., Guan J., Wang F., et al. (2019). DNA methylation dynamics during the interaction of wheat progenitor Aegilops tauschii with the obligate biotrophic fungus Blumeria graminis f. sp. tritici. New Phytol. 221 1023–1035. 10.1111/nph.15432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George T. S., Guru K. S. S., Vasanthi N. S., Kannan K. P. (2011). Extraction, purification and characterization of chitosan from endophytic fungi isolated from medicinal plants. World J. Sci. Technol. 1 43–48. [Google Scholar]
- Gorzynska K., Slachetka M., Ryszka P., Turnau K., Plachno B. J., Lembicz M. (2018). Incidence, identification, and mycoparasitic activity of clonostachys Epichloë, a hyperparasite of the fungal endophyte Epichloë typhina. Plant Dis. 102 1973–1980. 10.1094/PDIS-02-18-0320-RE [DOI] [PubMed] [Google Scholar]
- Gouda S., Das G., Sen S. K., Shin H. S., Patra J. K. (2016). Endophytes: a treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 7:1538. 10.3389/fmicb.2016.01538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton K. F., Poehlman J. M., Sehgal O. P., Sechler D. T. (1982). Tall fescue as a natural host and aphid vectors of barley yellow dwarf virus in Missouri. Plant Dis. 66 318–320. [Google Scholar]
- Guerre P. (2015). Ergot alkaloids produced by endophytic fungi of the genus Epichloë. Toxins 7 773–790. 10.3390/toxins7030773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundel P. E., Seal C. E., Biganzoli F., Martínez-Ghersa M. A., Molina-Montenegro M. A., Vázquez-de-Aldana B. R., et al. (2018). Occurrence of alkaloids in grass seeds symbiotic with vertically-transmitted Epichloë fungal endophytes and its relationship with antioxidants. Front. Ecol. Evol. 6:211. 10.3389/fevo.2018.00211 [DOI] [Google Scholar]
- Haas D., Défago G. (2005). Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 3 307–319. 10.1038/nrmicro1129 [DOI] [PubMed] [Google Scholar]
- Hardoim P. R., van Overbeek L. S., van Elsas J. D. (2008). Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 16 463–471. 10.1016/j.tim.2008.07.008 [DOI] [PubMed] [Google Scholar]
- Hardoim P. R., van Overbeek L. S., Berg G., Pirttila A. M., Compant S., Campisano A., et al. (2015). The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 79 293–320. 10.1128/MMBR.00050-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke M. T., Soukup A. A., Goering A. W., McClure R. A., Thomson R. J., Keller N. P., et al. (2016). New aspercryptins, lipopeptide natural products, revealed by HDAC inhibition in Aspergillus nidulans. ACS Chem. Biol. 11 2117–2123. 10.1021/acschembio.6b00398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J. K., Yu J. H., Keller N. P., Adams T. H. (1997). Aspergillus sporulation and mycotoxin production both require inactivation of the FadA G alpha protein-dependent signaling pathway. EMBO J. 16 4916–4923. 10.1093/emboj/16.16.4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroyuki K., Satoshi T., Shun-ichi T., Yoshihara T., Sakamura S., Shimanuki T., et al. (1989). New fungitoxic sesquiterpenoids, chokols AG, from stromata of Epichloë typhina and the absolute configuration of chokol E. Agric. Biol. Chem. 53 789–796. [Google Scholar]
- Hiruma K., Gerlach N., Sacristan S., Nakano R. T., Hacquard S., Kracher B., et al. (2016). Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell 165 464–474. 10.1016/j.cell.2016.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman M. T., Arnold A. E. (2010). Diverse bacteria inhabit living hyphae of phylogenetically diverse fungal endophytes. Appl. Environ. Microbiol. 76 4063–4075. 10.1128/AEM.02928-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman M. T., Gunatilaka M. K., Wijeratne K., Gunatilaka L., Arnold A. E. (2013). Endohyphal bacterium enhances production of indole-3-acetic acid by a foliar fungal endophyte. PLoS One 8:e73132. 10.1371/journal.pone.0073132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister D., Keller N. P. (2007). Natural products of filamentous fungi: enzymes, genes, and their regulation. Nat. Prod. Rep. 24 393–416. 10.1039/b603084j [DOI] [PubMed] [Google Scholar]
- Horn W. S., Simmonds M. S., Schwartz R. E., Blaney W. M. (1996). Variation in production of phomodiol and phomopsolide B by Phomopsis spp. Mycologia 88 588–595. [Google Scholar]
- Howitz K. T., Sinclair D. A. (2008). Xenohormesis: sensing the chemical cues of other species. Cell 133 387–391. 10.1016/j.cell.2008.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. C., Jiang T., Liu Y. X., Bai Y. C., Reed J., Qu B., et al. (2019). A specialized metabolic network selectively modulates Arabidopsis root microbiota. Science 364:eaau6389. 10.1126/science.aau6389 [DOI] [PubMed] [Google Scholar]
- Huang S., Chen H., Li W., Zhu X., Ding W., Li C. (2016). Bioactive chaetoglobosins from the mangrove endophytic fungus Penicillium chrysogenum. Mar. Drugs 14:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Wan C., Zhou S. (2013). Quercetin-A flavonoid compound from Sarcopyramis bodinieri var delicate with potential apoptotic activity in HepG2 liver cancer cells. Trop. J. Pharm. Res. 12 529–533. [Google Scholar]
- Hunt M. G., Newman J. A. (2005). Reduced herbivore resistance from a novel grass-endophyte association. J. Appl. Ecol. 42 762–769. 10.1111/j.1365-2664.2005.01061.x [DOI] [Google Scholar]
- Hurst L. D., Pal C., Lercher M. J. (2004). The evolutionary dynamics of eukaryotic gene order. Nat. Rev. Genet. 5 299–310. 10.1038/nrg1319 [DOI] [PubMed] [Google Scholar]
- Hussain H., Akhtar N., Draeger S., Schulz B., Pescitelli G., Salvadori P., et al. (2009). New bioactive 2,3-epoxycyclohexenes and isocoumarins from the endophytic fungus Phomopsis sp. from Laurus azorica. Eur. J. Organ. Chem. 2009 749–756. 10.1002/ejoc.200801052 [DOI] [Google Scholar]
- Hyde K. D., Soytong K. (2008). The fungal endophyte dilemma. Fungal Divers. 33 163–173. [Google Scholar]
- Imazaki I., Kadota I. (2015). Molecular phylogeny and diversity of Fusarium endophytes isolated from tomato stems. FEMS Microbiol. Ecol. 91:fiv098. 10.1093/femsec/fiv098 [DOI] [PubMed] [Google Scholar]
- Jakubczyk D., Dussart F. (2020). Selected fungal natural products with antimicrobial properties. Molecules 25:911. 10.3390/molecules25040911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H. F., Li X. J., Zhang H. Y. (2009). Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 10 194–200. 10.1038/embor.2009.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia M., Chen L., Xin H. L., Zheng C. J., Rahman K., Han T., et al. (2016). A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front. Microbiol. 7:906. 10.3389/fmicb.2016.00906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Zerfass C., Feng S., Eichmann R., Asally M., Schafer P., et al. (2018). Impact of spatial organization on a novel auxotrophic interaction among soil microbes. ISME J. 12 1443–1456. 10.1038/s41396-018-0095-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y., Sacalis J. N., Still C. C. (1998). Bioactive flavonoids from endophyte-infected blue grass (Poa ampla). J. Agric. Food Chem. 46 3785–3788. [Google Scholar]
- Kato N., Brooks W., Calvo A. M. (2003). The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot. Cell 2 1178–1186. 10.1128/ec.2.6.1178-1186.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul S., Wani M., Dhar K. L., Dhar M. K. (2008). Production and GC-MS trace analysis of methyl eugenol from endophytic isolate of Alternaria from rose. Ann. Microbiol. 58:443. [Google Scholar]
- Keglevich P., Hazai L., Kalaus G., Szántay C. (2012). Modifications on the basic skeletons of vinblastine and vincristine. Molecules 17 5893–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller N. P., Turner G., Bennett J. W. (2005). Fungal secondary metabolism – from biochemistry to genomics. Nat. Rev. Microbiol. 3 937–947. 10.1038/nrmicro1286 [DOI] [PubMed] [Google Scholar]
- Kemen A. C., Agler M. T., Kemen E. (2015). Host-microbe and microbe-microbe interactions in the evolution of obligate plant parasitism. New Phytol. 206 1207–1228. 10.1111/nph.13284 [DOI] [PubMed] [Google Scholar]
- Khan A. L., Hamayun M., Hussain J., Kang S.-M., Lee I.-J. (2012). The newly isolated endophytic fungus Paraconiothyrium sp. LK1 produces ascotoxin. Molecules 17 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Bassett S., Voisey C., Gaborit C., Johnson L., Christensen M., et al. (2010). Gene expression profiling of the endophytic fungus Neotyphodium lolii in association with its host plant perennial ryegrass. Aust. Plant Pathol. 39 467–476. [Google Scholar]
- Kim H., Woloshuk C. P. (2008). Role of AREA, a regulator of nitrogen metabolism, during colonization of maize kernels and fumonisin biosynthesis in Fusarium verticillioides. Fungal Genet. Biol. 45 947–953. 10.1016/j.fgb.2008.03.007 [DOI] [PubMed] [Google Scholar]
- Kim J., Cho S. Y., Kim S. H., Kim S., Park C.-W., Cho D., et al. (2016). Ginseng berry and its biological effects as a natural phytochemical. Nat. Prod. Chem. Res. 4:2. [Google Scholar]
- Kirby J., Keasling J. D. (2009). Biosynthesis of plant isoprenoids: perspectives for microbial engineering. Annu. Rev. Plant Biol. 60 335–355. 10.1146/annurev.arplant.043008.091955 [DOI] [PubMed] [Google Scholar]
- Kogel K. H., Franken P., Heückelhoven R. (2006). Endophyte or parasite—–what decides? Curr. Opin. Plant Biol. 9 358–363. 10.1016/j.pbi.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Konarzewska Z., Śliwińska-Wilczewska S., Felpeto A. B., Vasconcelos V., Latala A. (2020). Assessment of the allelochemical activity and biochemical profile of different phenotypes of picocyanobacteria from the genus Synechococcus. Mar. Drugs 18:179. 10.3390/md18040179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosalkova K., Garcia-Estrada C., Ullan R. V., Godio R. P., Feltrer R., Teijeira F., et al. (2009). The global regulator LaeA controls penicillin biosynthesis, pigmentation and sporulation, but not roquefortine C synthesis in Penicillium chrysogenum. Biochimie 91 214–225. 10.1016/j.biochi.2008.09.004 [DOI] [PubMed] [Google Scholar]
- Kouzminova E., Selker E. U. (2001). dim-2 encodes a DNA methyltransferase responsible for all known cytosine methylation in Neurospora. EMBO J. 20 4309–4323. 10.1093/emboj/20.15.4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozyrovska N. O. (2013). Crosstalk between endophytes and a plant host within information-processing networks. Biopolym. Cell 29 234–243. 10.7124/bc.00081D [DOI] [Google Scholar]
- Kumar A., Patil D., Rajamohanan P. R., Ahmad A. (2013). Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS One 8:e71805. 10.1371/journal.pone.0071805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Kaushik N. (2013). Endophytic fungi isolated from oil-seed crop Jatropha curcas produces oil and exhibit antifungal activity. PLoS One 8:e56202. 10.1371/journal.pone.0056202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel B. N., Brooks D. M. (2002). Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Biol. 5 325–331. [DOI] [PubMed] [Google Scholar]
- Kusari S., Hertweck C., Spiteller M. (2012). Chemical ecology of endophytic fungi: origins of secondary metabolites. Chem. Biol. 19 792–798. 10.1016/j.chembiol.2012.06.004 [DOI] [PubMed] [Google Scholar]
- Lee C.-C., Liu K.-J., Wu Y.-C., Lin S.-J., Chang C.-C., Huang T.-S. (2011). Sesamin inhibits macrophage-induced vascular endothelial growth factor and matrix metalloproteinase-9 expression and proangiogenic activity in breast cancer cells. Inflammation 34 209–221. [DOI] [PubMed] [Google Scholar]
- Lee D. Y., Teyssier C., Strahl B. D., Stallcup M. R. (2005). Role of protein methylation in regulation of transcription. Endocr. Rev. 26 147–170. 10.1210/er.2004-0008 [DOI] [PubMed] [Google Scholar]
- Li E., Jiang L., Guo L., Zhang H., Che Y. (2008). Pestalachlorides A–C, antifungal metabolites from the plant endophytic fungus Pestalotiopsis adusta. Bioorg. Med. Chem. 16 7894–7899. [DOI] [PubMed] [Google Scholar]
- Li F., Zhang N., Wu Q., Yuan Y., Yang Z., Zhou M., et al. (2017). Syringin prevents cardiac hypertrophy induced by pressure overload through the attenuation of autophagy. Int. J. Mol. Med. 39 199–207. [DOI] [PubMed] [Google Scholar]
- Li S., Li Z., Qian L., Xiao-Wen L., Ju-Qun X., Gui-Mei K., et al. (2015). Fumigaclavine I, a new alkaloid isolated from endophyte Aspergillus terreus. Chin. J. Nat. Med. 13 937–941. [DOI] [PubMed] [Google Scholar]
- Liang L., Gao C., Luo M., Zhao C., Wang W., Gu C., et al. (2013). The phytoestrogenic compound Cajanol from Pigeonpea roots is associated with the activation of estrogen receptor α−dependent signaling pathway in human prostate cancer cells. Phytother. Res. 27 1834–1841. [DOI] [PubMed] [Google Scholar]
- Liao H. L., Bonito G., Rojas J. A., Hameed K., Wu S., Schadt C. W., et al. (2019). Fungal endophytes of Populus trichocarpa alter host phenotype, gene expression, and rhizobiome composition. Mol. Plant Microbe Interact. 32 853–864. [DOI] [PubMed] [Google Scholar]
- Lin C., Yang L., Xu G., Wu J. (2011). Biodegradation and metabolic pathway of β-chlorinated aliphatic acid in Bacillus sp. CGMCC no. 4196. Appl. Microbiol. Biotechnol. 90 689–696. 10.1007/s00253-010-3081-6 [DOI] [PubMed] [Google Scholar]
- Lin R., Kim H., Hong J., Li Q.-J. (2014). Biological evaluation of subglutinol A as a novel immunosuppressive agent for inflammation intervention. ACS Med. Chem. Lett. 5 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind A. L., Wisecaver J. H., Lameiras C., Wiemann P., Palmer J. M., Keller N. P., et al. (2017). Drivers of genetic diversity in secondary metabolic gene clusters within a fungal species. PLoS Biol. 15:e2003583. 10.1371/journal.pbio.2003583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. X., Liu W. Z., Chen Y. C., Sun Z. H., Tan Y. Z., Li H. H., et al. (2016). Cytotoxic trichothecene macrolides from the endophyte fungus Myrothecium roridum. J. Asian Nat. Prod. Res. 18 684–689. 10.1080/10286020.2015.1134505 [DOI] [PubMed] [Google Scholar]
- Liu X., Dong M., Chen X., Jiang M., Lv X., Zhou J. (2008). Antimicrobial activity of an endophytic Xylaria sp.YX-28 and identification of its antimicrobial compound 7-amino-4-methylcoumarin. Appl. Microbiol. Biotechnol. 78 241–247. 10.1007/s00253-007-1305-1 [DOI] [PubMed] [Google Scholar]
- Lu H., Zou W. X., Meng J. C., Hu J., Tan R. X. (2000). New bioactive metabolites produced by Colletotrichum sp., an endophytic fungus in Artemisia annua. Plant Sci. 151 67–73. [Google Scholar]
- Ma L.-J., van der Does H. C., Borkovich K. A., Coleman J. J., Daboussi M.-J., Di Pietro A., et al. (2010). Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 464 367–373. 10.1038/nature08850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macías-Rubalcava M. L., Hernández-Bautista B. E., Jiménez-Estrada M., González M. C., Glenn A. E., Hanlin R. T., et al. (2008). Naphthoquinone spiroketal with allelochemical activity from the newly discovered endophytic fungus Edenia gomezpompae. Phytochemistry 69 1185–1196. [DOI] [PubMed] [Google Scholar]
- Macías-Rubalcava M. L., Sobrino M. E. R.-V., Meléndez-González C., Hernández-Ortega S. (2014). Naphthoquinone spiroketals and organic extracts from the endophytic fungus Edenia gomezpompae as potential herbicides. J. Agric. Food Chem. 62 3553–3562. [DOI] [PubMed] [Google Scholar]
- Malmstrom C. M., McCullough A. J., Johnson H. A., Newton L. A., Borer E. T. (2005). Invasive annual grasses indirectly increase virus incidence in California native perennial bunchgrasses. Oecologia 145 153–164. 10.1007/s00442-005-0099-z [DOI] [PubMed] [Google Scholar]
- Mandal S. M., Chakraborty D., Dey S. (2010). Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 5 359–368. 10.4161/psb.5.4.10871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Luis S., Della-Togna G., Coley P. D., Kursar T. A., Gerwick W. H., Cubilla-Rios L. (2008). Antileishmanial constituents of the Panamanian endophytic fungus Edenia sp. J. Nat. Prod. 71 2011–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materska M. (2008). Quercetin and its derivatives: chemical structure and bioactivity-a review. Pol. J. Food Nutr. Sci. 58 407–413. [Google Scholar]
- Mehta P., Sharma R., Putatunda C., Walia A. (2019). “Endophytic fungi: role in phosphate solubilization,” in Advances in Endophytic Fungal Research: Present Status and Future Challenges, ed. Singh B. P. (Cham: Springer International Publishing; ), 183–209. [Google Scholar]
- Mejia L. C., Herre E. A., Sparks J. P., Winter K., Garcia M. N., Van Bael S. A., et al. (2014). Pervasive effects of a dominant foliar endophytic fungus on host genetic and phenotypic expression in a tropical tree. Front. Microbiol. 5:479. 10.3389/fmicb.2014.00479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes M. A., Guerra R. F., Berns M. C., Manzo C., Masiero S., Finzi L., et al. (2013). MADS domain transcription factors mediate short-range DNA looping that is essential for target gene expression in Arabidopsis. Plant Cell 25 2560–2572. 10.1105/tpc.112.108688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers D. M., Obrian G., Du W. L., Bhatnagar D., Payne G. A. (1998). Characterization of aflJ, a gene required for conversion of pathway intermediates to aflatoxin. Appl. Environ. Microbiol. 64 3713–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra Y., Sharma L., Dhiman M., Sharma M. M. (2021). “Endophytic fungal diversity of selected medicinal plants and their bio-potential applications,” in Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology, Chap. 10, eds Sharma V. K., Shah M. P., Parmar S., Kumar A. (Cambridge, MA: Academic Press; ), 227–283. [Google Scholar]
- Mohamed R., Jong P. L., Zali M. S. (2010). Fungal diversity in wounded stems of Aquilaria malaccensis. Fungal Divers. 43 67–74. [Google Scholar]
- Mousa W. K., Raizada M. N. (2013). The diversity of anti-microbial secondary metabolites produced by fungal endophytes: an interdisciplinary perspective. Front. Microbiol. 4:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C. B., Krauss J. (2005). Symbiosis between grasses and asexual fungal endophytes. Curr. Opin. Plant Biol. 8 450–456. 10.1016/j.pbi.2005.05.007 [DOI] [PubMed] [Google Scholar]
- Naveen B., Ommi N. B., Mudiraj A., Mallikarjuna T., Babu P. P., Nagarajan R. (2017). Total synthesis of penicinoline E, marinamide, methyl marinamide and their antimalarial activity. ChemistrySelect 2 3256–3261. [Google Scholar]
- Ng T. B. (2004). Peptides and proteins from fungi. Peptides 25 1055–1073. 10.1016/j.peptides.2004.03.013 [DOI] [PubMed] [Google Scholar]
- Nicolaou K., Yang Z., Liu J., Ueno H., Nantermet P., Guy R., et al. (1994). Total synthesis of taxol. Nature 367:630. [DOI] [PubMed] [Google Scholar]
- Nicoletti R., Fiorentino A. (2015). Plant bioactive metabolites and drugs produced by endophytic fungi of Spermatophyta. Agriculture 5 918–970. [Google Scholar]
- Nowack E. C. M., Weber A. P. M. (2018). Genomics-informed insights into endosymbiotic organelle evolution in photosynthetic eukaryotes. Annu. Rev. Plant Biol. 69 51–84. 10.1146/annurev-arplant-042817-040209 [DOI] [PubMed] [Google Scholar]
- Nowak R., Drozd M., Mendyk E., Lemieszek M., Krakowiak O., Kisiel W., et al. (2016). A new method for the isolation of ergosterol and peroxyergosterol as active compounds of Hygrophoropsis aurantiaca and in vitro antiproliferative activity of isolated ergosterol peroxide. Molecules 21:946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nützmann H. W., Scazzocchio C., Osbourn A. (2018). Metabolic gene clusters in eukaryotes. Annu. Rev. Genet. 52 159–183. 10.1146/annurev-genet-120417-031237 [DOI] [PubMed] [Google Scholar]
- Osbourn A. (2010a). Gene clusters for secondary metabolic pathways: an emerging theme in plant biology. Plant Physiol. 154 531–535. 10.1104/pp.110.161315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osbourn A. (2010b). Secondary metabolic gene clusters: evolutionary toolkits for chemical innovation. Trends Genet. 26 449–457. 10.1016/j.tig.2010.07.001 [DOI] [PubMed] [Google Scholar]
- Osbourn A. E., Field B. (2009). Operons. Cell. Mol. Life Sci. 66 3755–3775. 10.1007/s00018-009-0114-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen E., West D. (1971). “Isolation and identification of 7, 8-dimethyl-10-(2’-hydroxyethyl) isoalloxazine from natural sources,” in Methods in Enzymology, eds McCormick D. B., Wright L. D. (Amsterdam: Elsevier; ), 574–579. [Google Scholar]
- Panche A. N., Diwan A. D., Chandra S. R. (2016). Flavonoids: an overview. J. Nutr. Sci. 5:e47. 10.1017/jns.2016.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley K. F., Walton J. D. (2001). Regulation of cyclic peptide biosynthesis in a plant pathogenic fungus by a novel transcription factor. Proc. Natl. Acad. Sci. U.S.A. 98 14174–14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peršoh D. (2015). Plant-associated fungal communities in the light of meta’omics. Fungal Divers. 75 1–25. 10.1007/s13225-015-0334-9 [DOI] [Google Scholar]
- Photita W., Lumyong S., Lumyong P., McKenzie E. H. C., Hyde K. D. (2004). Are some fungi isolated as endophytes of Musa acuminata latent pathogens? Fungal Divers. 16 131–140. [Google Scholar]
- Pichersky E., Gang D. R. (2000). Genetics and biochemistry of secondary metabolites in plants: an evolutionary perspective. Trends Plant Sci. 5 439–445. [DOI] [PubMed] [Google Scholar]
- Porras-Alfaro A., Bayman P. (2011). Hidden fungi, emergent properties: endophytes and microbiomes. Annu. Rev. Phytopathol. 49 291–315. 10.1146/annurev-phyto-080508-081831 [DOI] [PubMed] [Google Scholar]
- Poveda J. (2021). Beneficial effects of microbial volatile organic compounds (MVOCs) in plants. Appl. Soil Ecol. 168:104118. 10.1016/j.apsoil.2021.104118 [DOI] [Google Scholar]
- Poveda J., Abril-Urias P., Escobar C. (2020a). Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, Mycorrhizal and endophytic fungi. Front. Microbiol. 11:992. 10.3389/fmicb.2020.00992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poveda J., Zabalgogeazcoa I., Soengas P., Rodríguez V. M., Cartea M. E., Abilleira R., et al. (2020b). Brassica oleracea var. acephala (kale) improvement by biological activity of root endophytic fungi. Sci. Rep. 10:20224. 10.1038/s41598-020-77215-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poveda J., Baptista P. (2021). Filamentous fungi as biocontrol agents in olive (Olea europaea L.) diseases: Mycorrhizal and endophytic fungi. Crop Prot. 146:105672. 10.1016/j.cropro.2021.105672 [DOI] [Google Scholar]
- Poveda J., Eugui D., Abril-Urías P., Velasco P. (2021). Endophytic fungi as direct plant growth promoters for sustainable agricultural production. Symbiosis 85 1–19. 10.1007/s13199-021-00789-x [DOI] [Google Scholar]
- Prasch C. M., Sonnewald U. (2013). Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiol. 162 1849–1866. 10.1104/pp.113.221044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. H., Hohn T. M., McCormick S. P., Desjardins A. E. (1995). Tri6 encodes an unusual zinc finger protein involved in regulation of trichothecene biosynthesis in Fusarium sporotrichioides. Appl. Environ. Microbiol. 61 1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J.-C., Zhang Y.-M., Gao J.-M., Bai M.-S., Yang S.-X., Laatsch H., et al. (2009). Bioactive metabolites produced by Chaetomium globosum, an endophytic fungus isolated from Ginkgo biloba. Bioorg. Med. Chem. Lett. 19 1572–1574. [DOI] [PubMed] [Google Scholar]
- Quambusch M., Pirttilä A. M., Tejesvi M. V., Winkelmann T., Bartsch M. (2014). Endophytic bacteria in plant tissue culture: differences between easy-and difficult-to-propagate Prunus avium genotypes. Tree Physiol. 34 524–533. [DOI] [PubMed] [Google Scholar]
- Radic N., Strukelj B. (2012). Endophytic fungi: the treasure chest of antibacterial substances. Phytomedicine 19 1270–1284. 10.1016/j.phymed.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Richards T. A., Leonard G., Soanes D. M., Talbot N. J. (2011). Gene transfer into the fungi. Fungal Biol. Rev. 25 98–110. 10.1016/j.fbr.2011.04.003 [DOI] [Google Scholar]
- Roberts E., Lindow S. (2014). Loline alkaloid production by fungal endophytes of Fescue species select for particular epiphytic bacterial microflora. ISME J. 8 359–368. 10.1038/ismej.2013.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R. J., Henson J., Van Volkenburgh E., Hoy M., Wright L., Beckwith F., et al. (2008). Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2 404–416. [DOI] [PubMed] [Google Scholar]
- Rodriguez R., Redman R. (2008). More than 400 million years of evolution and some plants still can’t make it on their own: plant stress tolerance via fungal symbiosis. J. Exp. Bot. 59 1109–1114. 10.1093/jxb/erm342 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Morató J., Xicota L., Fitó M., Farré M., Dierssen M., de la Torre R. (2015). Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules 20 4655–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero A., Carrion G., Rico-Gray V. (2001). Fungal latent pathogens and endophytes from leaves of Parthenium hysterophorus (Asteraceae). Fungal Divers. 7 81–87. [Google Scholar]
- Ruiz B., Chavez A., Forero A., Garcia-Huante Y., Romero A., Sanchez M., et al. (2010). Production of microbial secondary metabolites: regulation by the carbon source. Crit. Rev. Microbiol. 36 146–167. 10.3109/10408410903489576 [DOI] [PubMed] [Google Scholar]
- Rukachaisirikul V., Sommart U., Phongpaichit S., Sakayaroj J., Kirtikara K. (2008). Metabolites from the endophytic fungus Phomopsis sp. PSU-D15. Phytochemistry 69 783–787. [DOI] [PubMed] [Google Scholar]
- Saikkonen K., Gundel P. E., Helander M. (2013). Chemical ecology mediated by fungal endophytes in grasses. J. Chem. Ecol. 39 962–968. 10.1007/s10886-013-0310-3 [DOI] [PubMed] [Google Scholar]
- Sampangi-Ramaiah M. H., Dey P., Jambagi S., Kumari M. V., Oelmüller R., Nataraja K. N., et al. (2020). An endophyte from salt-adapted Pokkali rice confers salt-tolerance to a salt-sensitive rice variety and targets a unique pattern of genes in its new host. Sci. Rep. 10:3237. 10.1038/s41598-020-59998-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardl C. L., Phillips T. D. (1997). Protective grass endophytes: where are they from and where are they going? Plant Dis. 81 430–438. [DOI] [PubMed] [Google Scholar]
- Schmitt E. K., Hoff B., Kück U. (2004). AcFKH1, a novel member of the forkhead family, associates with the RFX transcription factor CPCR1 in the cephalosporin C-producing fungus Acremonium chrysogenum. Gene 342 269–281. [DOI] [PubMed] [Google Scholar]
- Schneider P., Misiek M., Hoffmeister D. (2008). In vivo and in vitro production options for fungal secondary metabolites. Mol. Pharm. 5 234–242. 10.1021/mp7001544 [DOI] [PubMed] [Google Scholar]
- Schulz B., Boyle C., Draeger S., Römmert A. K., Krohn K. (2002). Endophytic fungi: a source of novel biologically active secondary metabolites. Mycol. Res. 106 996–1004. [Google Scholar]
- Shao C.-L., Wang C.-Y., Gu Y.-C., Wei M.-Y., Pan J.-H., Deng D.-S., et al. (2010). Penicinoline, a new pyrrolyl 4-quinolinone alkaloid with an unprecedented ring system from an endophytic fungus Penicillium sp. Bioorg. Med. Chem. Lett. 20 3284–3286. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Keller N. P. (2001). Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoresh M., Harman G. E., Mastouri F. (2010). Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 48 21–43. 10.1146/annurev-phyto-073009-114450 [DOI] [PubMed] [Google Scholar]
- Shwab E. K., Keller N. P. (2008). Regulation of secondary metabolite production in filamentous ascomycetes. Mycol. Res. 112(Pt 2) 225–230. 10.1016/j.mycres.2007.08.021 [DOI] [PubMed] [Google Scholar]
- Siddhardha B., Meena H. (2020). “Microbial secondary metabolites: natural benediction elements for plants during abiotic and biotic stress conditions,” in Microbial Services in Restoration Ecology, eds Singh J. S., Vimal S. R. (Elsevier), 99–107. [Google Scholar]
- Sieber T. N. (2007). Endophytic fungi in forest trees: are they mutualists? Fungal Biol. Rev. 21 75–89. [Google Scholar]
- Sigurdson G., Tang P., Giusti M. (2018). Cis–trans configuration of coumaric acid acylation affects the spectral and colorimetric properties of anthocyanins. Molecules 23:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh L. P., Gill S. S., Tuteja N. (2011). Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signal. Behav. 6 175–191. 10.4161/psb.6.2.14146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. C., Rokas A. (2011). Horizontal transfer of a large and highly toxic secondary metabolic gene cluster between fungi. Curr. Biol. 21 134–139. 10.1016/j.cub.2010.12.020 [DOI] [PubMed] [Google Scholar]
- Soanes D., Richards T. A. (2014). Horizontal gene transfer in eukaryotic plant pathogens. Annu. Rev. Phytopathol. 52 583–614. 10.1146/annurev-phyto-102313-050127 [DOI] [PubMed] [Google Scholar]
- Soliman S. S., Trobacher C. P., Tsao R., Greenwood J. S., Raizada M. N. (2013). A fungal endophyte induces transcription of genes encoding a redundant fungicide pathway in its host plant. BMC Plant Biol. 13:93. 10.1186/1471-2229-13-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C., Somerville S. (1999). Plant functional genomics. Science 285 380–383. 10.1126/science.285.5426.380 [DOI] [PubMed] [Google Scholar]
- Song Q., Li F., Nan Z., Coulter J. A., Wei W. (2020). Do Epichloë endophytes and their grass symbiosis only producetoxic alkaloids to insects and livestock? J. Agric. Food Chem. 68 1169–1185. 10.1021/acs.jafc.9b06614 [DOI] [PubMed] [Google Scholar]
- Spanu P. D., Abbott J. C., Amselem J., Burgis T. A., Soanes D. M., Stüber K., et al. (2010). Genome expansion and gene loss in powdery mildew fungi reveal tradeoffs in extreme parasitism. Science 330 1543–1546. 10.1126/science.1194573 [DOI] [PubMed] [Google Scholar]
- Spatafora J. W., Sung G. H., Sung J. M., Hywel-Jones N. L., White J. F. J. (2007). Phylogenetic evidence for an animal pathogen origin of ergot and the grass endophytes. Mol. Ecol. 16 1701–1711. 10.1111/j.1365-294X.2007.03225.x [DOI] [PubMed] [Google Scholar]
- Sproul D., Gilbert N., Bickmore W. A. (2005). The role of chromatin structure in regulating the expression of clustered genes. Nat. Rev. Genet. 6 775–781. 10.1038/nrg1688 [DOI] [PubMed] [Google Scholar]
- Sriram D., Yogeeswari P., Thirumurugan R., Ratan Bal T. (2005). Camptothecin and its analogues: a review on their chemotherapeutic potential. Nat. Prod. Res. 19 393–412. [DOI] [PubMed] [Google Scholar]
- Stahl E., Hilfiker O., Reymond P. (2018). Plant-arthropod interactions: who is the winner? Plant J. 93 703–728. 10.1111/tpj.13773 [DOI] [PubMed] [Google Scholar]
- Stierle A., Strobel G., Stierle D. (1993). Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 260 214–216. 10.1126/science.8097061 [DOI] [PubMed] [Google Scholar]
- Stone J., Polishook J. D., White J. F. (2004). “Endophytic fungi,” in Biodiversity of Fungi, eds Mueller G. M., Bills G. F., White J. F. (Amsterdam: Elsevier; ), 241–270. [Google Scholar]
- Strobel G. (2018). The emergence of endophytic microbes and their biological promise. J. Fungi 4:57. 10.3390/jof4020057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y. Y., Guo L. D., Hyde K. D. (2010). Response of endophytic fungi of Stipa grandis to experimental plant function group removal in Inner Mongolia steppe, China. Fungal Divers. 43 93–101. [Google Scholar]
- Sun J., Guo L., Zang W., Ping W., Chi D. (2008). Diversity and ecological distribution of endophytic fungi associated with medicinal plants. Sci. China C Life Sci. 51 751–759. 10.1007/s11427-008-0091-z [DOI] [PubMed] [Google Scholar]
- Sun X., Guo L.-D. (2012). Endophytic fungal diversity: review of traditional and molecular techniques. Mycology 3 65–76. 10.1080/21501203.2012.656724 [DOI] [Google Scholar]
- Sun X., Guo L. D., Hyde K. D. (2011). Comunity composition of endophytic fungi in Acer truncatum and their role in decomposition. Fungal Divers. 47 85–95. [Google Scholar]
- Tag A., Hicks J., Garifullina G., Ake C., Jr., Phillips T. D., Beremand M., et al. (2000). G-protein signalling mediates differential production of toxic secondary metabolites. Mol. Microbiol. 38 658–665. [DOI] [PubMed] [Google Scholar]
- Tamaru H., Selker E. U. (2001). A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature 414 277–283. 10.1038/35104508 [DOI] [PubMed] [Google Scholar]
- Tan R. X., Zou W. X. (2001). Endophytes: a rich source of functional metabolites. Nat. Prod. Rep. 18 448–459. [DOI] [PubMed] [Google Scholar]
- Tao G., Liu Z. Y., Hyde K. D., Liu X. Z., Yu Z. N. (2008). Whole rDNA analysis reveals novel and endophytic fungi in Bletilla ochracea (Orchidaceae). Fungal Divers. 33 101–112. [Google Scholar]
- Tejesvi M. V., Tamhankar S. A., Kini K. R., Rao V. S., Prakash H. S. (2009). Phylogenetic analysis of endophytic Pestalotiopsis species from ethnopharmaceutically important medicinal trees. Fungal Divers. 38 167–183. [Google Scholar]
- Theodorakidou M., Nikola O.-A., Lambrou G. I. (2018). The multiple roles of steroids and anabolic steroids and its relations to cardiovasular and musculoskeletal pathology: a brief review. J. Res. Pract. Musculoskelet. Syst. 2 22–30. 10.22540/jrpms-02-022 [DOI] [Google Scholar]
- Trapp S. C., Hohn T. M., McCormick S., Jarvis B. B. (1998). Characterization of the gene cluster for biosynthesis of macrocyclic trichothecenes in Myrothecium roridum. Mol. Gen. Genet. 257 421–432. [DOI] [PubMed] [Google Scholar]
- Uzma F., Mohan C. D., Hashem A., Konappa N. M., Rangappa S., Kamath P. V., et al. (2018). Endophytic fungi-alternative sources of cytotoxic compounds: a review. Front. Pharmacol. 9:309. 10.3389/fphar.2018.00309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnav P., Demain A. L. (2010). Unexpected applications of secondary metabolites. Biotechnol. Adv. 29 223–229. 10.1016/j.biotechadv.2010.11.006 [DOI] [PubMed] [Google Scholar]
- Van der Ent S., Van Wees S. C., Pieterse C. M. (2009). Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochemistry 70 1581–1588. [DOI] [PubMed] [Google Scholar]
- Vandenkoornhuyse P., Baldauf S. L., Leyval C., Straczek J., Young J. P. (2002). Extensive fungal diversity in plant roots. Science 295:2051. 10.1126/science.295.5562.2051 [DOI] [PubMed] [Google Scholar]
- Varma A., Rai M., Sahay S., Sahay N. S. (2000). “Microbial-biotechnology: new-paradigms and role in sustainable agriculture,” in Microbial Biotechnology for Sustainable Development and Productivity, ed. Rajak R. C. (India: Scientific Publishers; ), 22–37. [Google Scholar]
- Vellosa J. C. R., Regasini L. O., Khalil N. M., Bolzani V. D. S., Khalil O. A., Manente F. A., et al. (2011). Antioxidant and cytotoxic studies for kaempferol, quercetin and isoquercitrin. Eclética Quim. 36 7–20. [Google Scholar]
- Verbon E. H., Liberman L. M. (2016). Beneficial microbes affect endogenous mechanisms controlling root development. Trends Plant Sci. 21 218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violle C., Reich P. B., Pacala S. W., Enquist B. J., Kattge J. (2014). The emergence and promise of functional biogeography. Proc. Natl. Acad. Sci. U.S.A. 111 13690–13696. 10.1073/pnas.1415442111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller F., Achatz B., Baltruschat H., Fodor J., Becker K., Fischer M., et al. (2005). The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc. Natl. Acad. Sci. U.S.A. 102 13386–13391. 10.1073/pnas.0504423102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Tu L., Yuan D., Zhu D., Shen C., Li J., et al. (2018). Reference genome sequences of two cultivated allotetraploid cottons, Gossypium hirsutum and Gossypium barbadense. Nat. Genet. 51 224–229. 10.1038/s41588-018-0282-x [DOI] [PubMed] [Google Scholar]
- Wang R., Liu G., Yang M., Wang M., Zhou L. (2016). Total synthesis and antifungal activity of palmarumycin CP17 and its methoxy analogues. Molecules 21:600. 10.3390/molecules21050600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. H., Stone M. J., Hauck P. R., Rahman S. K. (1989). Why are secondary metabolites (natural products) biosynthesized? J. Nat. Prod. 52 1189–1208. [DOI] [PubMed] [Google Scholar]
- Woloshuk C. P., Foutz K. R., Brewer J. F., Bhatnagar D., Cleveland T. E., Payne G. A. (1994). Molecular characterization of aflR, a regulatory locus for aflatoxin biosynthesis. Appl. Environ. Microbiol. 60 2408–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Zhang S.-Y., Liu Y.-Q., Wu X.-B., Zhu G.-X., Zhang Y., et al. (2015). Synthesis, biological activities, and quantitative structure–activity relationship (QSAR) study of novel camptothecin analogues. Molecules 20 8634–8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Hao Z., Yu M., Wu Z., Zhao A., Li J., et al. (2019). Improved phosphorus nutrition by arbuscular mycorrhizal symbiosis as a key factor facilitating glycyrrhizin and liquiritin accumulation in Glycyrrhiza uralensis. Plant Soil 439 243–257. 10.1007/s11104-018-3861-9 [DOI] [Google Scholar]
- Xu X. H., Su Z. Z., Wang C., Kubicek C. P., Feng X. X., Mao L. J., et al. (2014). The rice endophyte Harpophora oryzae genome reveals evolution from a pathogen to a mutualistic endophyte. Sci. Rep. 4:5783. 10.1038/srep05783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav V., Wang Z., Wei C., Amo A., Ahmed B., Yang X., et al. (2020). Phenylpropanoid pathway engineering: an emerging approach towards plant defense. Pathogens 9:312. 10.3390/pathogens9040312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Zhu J., Zhao X., Shi J., Jiang C., Shao D. (2019). Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 103 3327–3340. 10.1007/s00253-019-09713-2 [DOI] [PubMed] [Google Scholar]
- Yang H., Tong J., Lee C. W., Ha S., Eom S. H., Im Y. J. (2015). Structural mechanism of ergosterol regulation by fungal sterol transcription factor Upc2. Nat. Commun. 6:6129. [DOI] [PubMed] [Google Scholar]
- Yang H.-R., Yuan J., Liu L.-H., Zhang W., Chen F., Dai C.-C. (2019). Endophytic Pseudomonas fluorescens induced sesquiterpenoid accumulation mediated by gibberellic acid and jasmonic acid in Atractylodes macrocephala Koidz plantlets. Plant Cell Tissue Organ Cult. 138 445–457. [Google Scholar]
- Yang X., Strobel G., Stierle A., Hess W., Lee J., Clardy J. (1994). A fungal endophyte-tree relationship: Phoma sp. in Taxus wallachiana. Plant Sci. 102 1–9. [Google Scholar]
- Yang Y., Jin Z., Jin Q., Dong M. (2015). Isolation and fatty acid analysis of lipid-producing endophytic fungi from wild Chinese Torreya Grandis. Microbiology 84 710–716. [Google Scholar]
- Yi X. U., Gray S. M. (2020). Aphids and their transmitted potato viruses: a continuous challenges in potato crops. J. Integr. Agric. 19 367–375. [Google Scholar]
- Young C. A., Felitti S., Shields K., Spangenberg G., Johnson R. D., Bryan G. T., et al. (2006). A complex gene cluster for indole-diterpene biosynthesis in the grass endophyte Neotyphodium lolii. Fungal Genet. Biol. 43 679–693. 10.1016/j.fgb.2006.04.004 [DOI] [PubMed] [Google Scholar]
- Yu H., Zhang L., Li L., Zheng C., Guo L., Li W., et al. (2010). Recent developments and future prospects of antimicrobial metabolites produced by endophytes. Microbiol. Res. 165 437–449. [DOI] [PubMed] [Google Scholar]
- Yu J. H., Keller N. (2005). Regulation of secondary metabolism in filamentous fungi. Annu. Rev. Phytopathol. 43 437–458. 10.1146/annurev.phyto.43.040204.140214 [DOI] [PubMed] [Google Scholar]
- Yu J. H., Butchko R. A., Fernandes M., Keller N. P., Leonard T. J., Adams T. H. (1996). Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus. Curr. Genet. 29 549–555. [DOI] [PubMed] [Google Scholar]
- Zaiyou J., Li M., Xiqiao H. (2017). An endophytic fungus efficiently producing paclitaxel isolated from Taxus wallichiana var. mairei. Medicine 96:e7406. 10.1097/MD.0000000000007406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. W., Song Y. C., Tan R. X. (2006). Biology and chemistry of endophytes. Nat. Prod. Rep. 23 753–771. 10.1039/b609472b [DOI] [PubMed] [Google Scholar]
- Zhang H., Sonnewald U. (2017). Differences and commonalities of plant responses to single and combined stresses. Plant J. 90 839–855. [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu Y. X., Zhang N., Hu B., Jin T., Xu H., et al. (2019). NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 37 676–684. 10.1038/s41587-019-0104-4 [DOI] [PubMed] [Google Scholar]
- Zhang P., Yuan X.-L., Du Y.-M., Zhang H.-B., Shen G.-M., Zhang Z.-F., et al. (2019). Angularly prenylated indole alkaloids with antimicrobial and insecticidal activities from an endophytic fungus Fusarium sambucinum TE-6L. J. Agric. Food Chem. 67 11994–12001. 10.1021/acs.jafc.9b05827 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Wei X., Wang J. (2012). Phillyrin produced by Colletotrichum gloeosporioides, an endophytic fungus isolated from Forsythia suspensa. Fitoterapia 83 1500–1505. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Han T., Ming Q., Wu L., Rahman K., Qin L. (2012). Alkaloids produced by endophytic fungi: a review. Nat. Prod. Commun. 7 963–968. [PubMed] [Google Scholar]
- Zhao J., Li C., Wang W., Zhao C., Luo M., Mu F., et al. (2013). Hypocrea lixii, novel endophytic fungi producing anticancer agent cajanol, isolated from pigeon pea (C ajanus cajan [L.] M illsp.). J. Appl. Microbiol. 115 102–113. [DOI] [PubMed] [Google Scholar]
- Zhou X., Zhu H., Liu L., Lin J., Tang K. (2010). A review: recent advances and future prospects of taxol-producing endophytic fungi. Appl. Microbiol. Biotechnol. 86 1707–1717. 10.1007/s00253-010-2546-y [DOI] [PubMed] [Google Scholar]
- Zou W., Meng J., Lu H., Chen G., Shi G., Zhang T., et al. (2000). Metabolites of Colletotrichum gloeosporioides, an endophytic fungus in Artemisia mongolica. J. Nat. Prod. 63 1529–1530. [DOI] [PubMed] [Google Scholar]