Abstract
Outbreaks of neurological disease associated with Sarcocystis calchasi have been observed in captive and free-ranging rock pigeons (Columba livia) in Europe and the United States as well as in wild Brandt's cormorants (Phalacrocorax penicillatus) and captive psittacines in California, USA. Experimental and field studies have identified northern goshawks (Accipiter gentilis) and European sparrowhawks (A. nisus) as definitive hosts in Europe while the definitive hosts elsewhere remain unknown. In this study, we aimed to identify the potential definitive host(s) of S. calchasi through molecular analysis of intestinal samples from seven predatory (n = 85) and one omnivorous (n = 11) bird species in California. In total, apicomplexan-generic 28S rRNA PCR products were obtained and sequenced for 42 raptors. Three of 16 (18.8%) Cooper's hawks (A. cooperii) and two of 26 (5.6%) red-tailed hawks (Buteo jamaicensis) also tested positive for the S. calchasi-specific ITS1 PCR and sequencing of the 28S rRNA PCR product was 100% homologous to S. calchasi. In addition to S. calchasi (5.9%; 5/85), other Sarcocystis spp. detected in raptors included: S. jamaicensis (21.2%; 18/85), S. columbae (8.2%; 7/85), S. turdusi (7.1%; 6/85), and S. halieti (4.7; 4/85%). Infections with closely related S. jamaicensis and S. (Frenkelia) microti (9.4%; 8/85) could not be distinguished for eight raptors. Eumonospora henryae (1.2%; 1/85) was detected in one raptor. Our results indicate for the first time that S. calchasi may have a definitive host range in North America that includes at least two raptors, Cooper's hawks and red-tailed hawks, within the family Accipitridae.
Keywords: Accipitridae, Apicomplexa, Definitive host, Protozoa, Raptor, Sarcocystis calchasi
Graphical abstract
Highlights
-
•
Cooper's hawks and red-tailed hawks likely definitive hosts for Sarcocystis calchasi.
-
•
Raptors may be infected with a diversity of closely related Sarcocystis spp.
-
•
More research needed to clarify life cycles for bird-infecting Sarcocystis spp.
1. Introduction
Sarcocystis spp., which belong to the family Sarcocystidae, are parasites with an obligate two-host life cycle involving a definitive host and an intermediate host. Disease may occur in the intermediate host following the ingestion of sporocysts shed in the feces of the definitive host, typically a predator that consumes the tissue cysts (sarcocysts) present in the muscle of the intermediate host (Dubey et al., 2016). For many Sarcocystis spp., the complete life cycle with the known definitive and intermediate hosts remains undetermined. Historically, Sarcocystis spp. have been identified by the morphology of the sarcocysts found in the muscle or other tissues of the intermediate host while the morphology of the oocysts and sporocysts had little taxonomic value, making it challenging to link these different life stages to a single parasite (Dubey et al., 2016). Laboratory studies involving dosing either a potential intermediate or definitive host with parasites was commonly utilized to demonstrate the complete life cycle (Box and Smith, 1982; Luznar et al., 2001; Olias et al., 2010b). Recently, advances in molecular techniques have enabled more expansive research on potential hosts using PCR to genetically identify Sarcocystis spp. found in the tissues of the intermediate host or in the intestines and feces of the definitive host (Gjerde et al., 2018; Olias et al., 2010c, 2011; Parmentier et al., 2018; Prakas et al., 2011). This methodology continues to expand the host range of many Sarcocystis spp., and consequently, it has been observed that bird-infecting species typically have multiple hosts (Olias et al., 2014).
While raptors can act as intermediate hosts for some Sarcocystis spp., such as S. falcatula and S. neurona (Olson et al., 2007; Wunshmann et al., 2009, 2010), raptors are the recognized definitive host for many Sarcocystis spp. (Dubey et al., 2016). Sporocysts have been recovered from the intestines and/or feces of raptors in the United States and elsewhere. A survey of Sarcocystis spp. from four species of hawks in Georgia, USA between 2001 and 2004 reported infections in 67% of raptors including Cooper's hawks (Accipiter cooperii), sharp-shinned hawks (A. striatus), red-tailed hawks (Buteo jamaicensis), and red-shouldered hawks (B. lineatus) (Yabsley et al., 2009). However, genetic analysis of the 18S rRNA gene segment in this study failed to separate the closely related parasites. More recently, analysis of the 28S rRNA gene segment and the internal transcribed spacer 1 (ITS1) region has been used to differentiate closely related bird-infecting Sarcocystis spp. (Olias et al., 2010c, 2014; Prakas et al., 2018). Although several Sarcocystis spp. are nearly identical at the partial 28S rRNA gene, these species may be differentiated using the ITS1 region.
Sarcocystis calchasi was identified in 2008 as the causative agent of pigeon protozoal encephalitis following an outbreak of neurological disease in racing pigeons (Columba livia f. domestica) in Germany (Olias et al., 2009). Sarcocysts were observed on histology in the skeletal muscles and rare parasites were detected in the severe brain lesions of infected pigeons (Maier et al., 2015; Olias et al., 2010a). Molecular evaluation of brain and muscle tissues from infected pigeons identified S. calchasi (Olias et al., 2009). The northern goshawk (Accipiter gentilis), a common avian predator that frequently preys on rock pigeons, was confirmed as a definitive host of S. calchasi in a laboratory study in Germany (Olias et al., 2010b). Subsequent molecular research in Germany has identified S. calchasi in intestinal samples from European sparrowhawks (A. nisus), another likely definitive host for this parasite in Europe (Olias et al., 2011). Since its initial discovery, S. calchasi has been identified in the United States in captive and free-ranging pigeons (C. livia) (Mete et al., 2019; Olias et al., 2014; Trupkiewicz et al., 2016; Wunshmann et al., 2011), a free-ranging Eurasian collared dove (Streptopelia decaocto) (Hodo et al., 2016), captive psittacines (Rimoldi et al., 2013), and wild Brandt's cormorants (Phalacrocorax penicillatus) (Bamac et al., 2020), and in Japan in a free-ranging rock pigeon (Ushio et al., 2015). The definitive hosts for S. calchasi remain unknown outside of Europe, although raptor species within the genus Accipiter have been suggested.
In the United States, S. calchasi was first detected in captive and free-ranging rock pigeons in Minnesota and Missouri in 2011 and 2012 (Olias et al., 2014; Wunshmann et al., 2011). An outbreak in California during late winter and spring 2017 marked the first widespread mortality event involving free-ranging rock pigeons (Mete et al., 2019). Infection was identified in 21 rock pigeons exhibiting neurologic disease from 10 different counties (Mete et al., 2019). Between March and May 2019, an outbreak along the southern California coast was identified in a seabird, the Brandt's cormorant (Phalacrocorax penicillatus) (Bamac et al., 2020). Wildlife rehabilitation centers had reported increased admissions of cormorants with neurological signs. Sarcocystis calchasi was confirmed in brain and muscle tissues from 7 of 8 cormorants examined, with one cormorant positive for S. falcatula (Bamac et al., 2020). This outbreak of S. calchasi-encephalitis marked the first time this parasite was detected in a seabird with no regular avian predators. The authors hypothesized that sporocysts shed in the feces of terrestrial definitive hosts were washed into the ocean following increased rainfall in late winter where they were ingested, either directly or indirectly, by near-shore Brandt's cormorants (Bamac et al., 2020). The land to sea transmission of other apicomplexan parasites such as S. neurona and Toxoplasma gondii have been documented in marine mammal infections (Miller et al., 2002, 2010). However, unlike S. neurona and T. gondii whose definitive hosts include the Virginia opossum (Didelphis virginiana) and domestic cat (Felis catus), respectively, the definitive hosts of S. calchasi contributing to the fecal contamination of land and sea environments remain unknown.
In the present study, we surveyed eight different species of potential avian predators and scavengers of rock pigeons for Sarcocystis spp. to identify the likely definitive host(s) of S. calchasi. Additionally, we provide a summary of all the Sarcocystis spp. found in the intestines of the evaluated raptor species, expanding the putative definitive host range for some of the parasites we identified through molecular analysis.
2. Materials and methods
2.1. Postmortem examination
Ninety-six birds were evaluated for this study including 85 raptors and 11 American crows (Corvus brachyrhynchos). These carcasses were submitted to the California Department of Fish and Wildlife's (CDFW) Wildlife Health Laboratory (WHL; Rancho Cordova, CA, USA) between 2016 and 2020 by members of the public, wildlife rehabilitation centers, CDFW staff, or other government agencies for postmortem examination and cause of death determination. Carcasses were received at WHL and stored in a freezer (−20 °C) until postmortem examination. Prior to examination, the carcasses were thawed at 4 °C for 3–5 days. A gross necropsy was performed at WHL, and findings were recorded including age, sex, adipose deposition, appearance/condition of organs, and abnormalities (e.g., injuries). Tissue samples of pectoral muscle, lung, heart, liver, spleen, kidneys, pancreas, intestines, and brain were collected into individual whirl-pak® bags (Nasco Sampling, LLC, Fort Atkinson, WI) and stored at −80 °C.
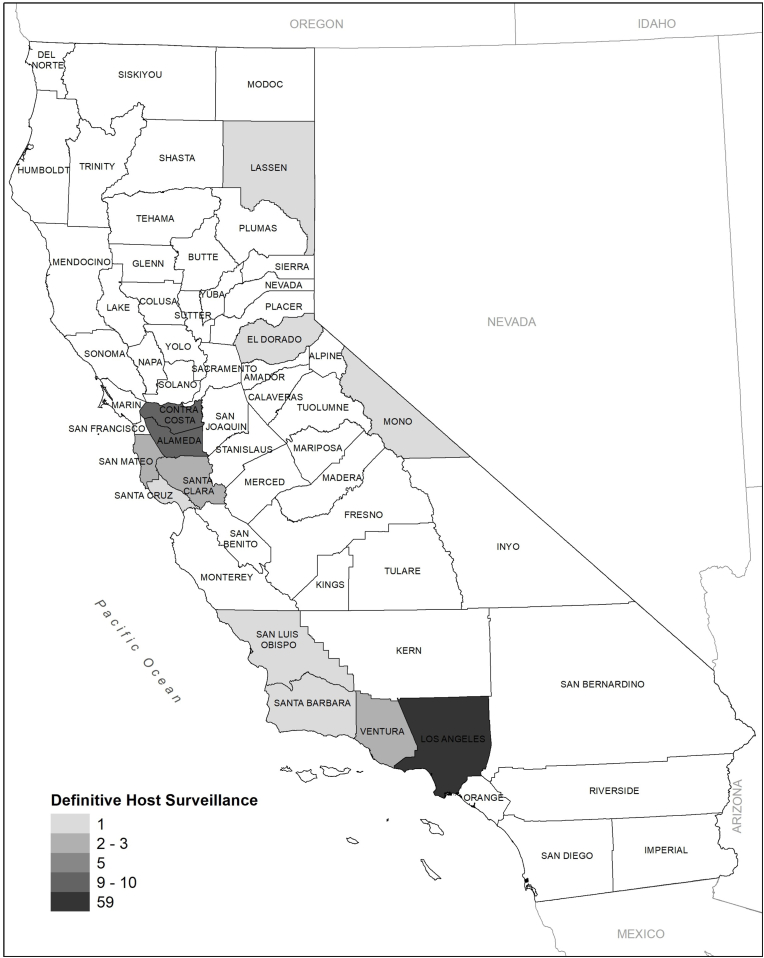
Individuals evaluated for Sarcocystis spp. were preferentially selected based on a collection location and date corresponding with previous S. calchasi outbreaks in rock pigeons (Mete et al., 2019) and Brandt's cormorants (Bamac et al., 2020) and included species that are known to prey or scavenge on rock pigeons (Fig. 1). The species evaluated included American crow (n = 11), Cooper's hawk (n = 16), great horned owl (Bubo virginianus) (n = 12), northern goshawk (n = 2), peregrine falcon (Falco peregrinus) (n = 3), red-shouldered hawk (n = 12), red-tailed hawk (n = 36), and sharp-shinned hawk (n = 4).
Fig. 1.
Number of avian carcasses evaluated for Sarcocystis spp. by county in California, USA between 2016 and 2020. Species include American crow (Corvus brachyrhynchos; n = 11), Cooper's hawk (Accipiter cooperii; n = 16), great horned owl (Bubo virginianus; n = 12), northern goshawk (A. gentilis; n = 2), peregrine falcon (Falco peregrinus; n = 3), red-shouldered hawk (Buteo lineatus; n = 12), red-tailed hawk (B. jamaicensis; n = 36), and sharp-shinned hawk (A. striatus; n = 4).
2.2. Molecular characterization
For the Sarcocystis spp. analysis, the intestines were thawed, and immediately a section of the small intestine was excised, macerated with a sterile blade, collected into a cryovial, and stored at −80 °C until molecular analysis. Archived intestinal tissue was limited for some birds, in which case a section was collected from the available portion. Frozen intestinal samples from all 96 birds included in the study were tested for Sarcocystis spp. by polymerase chain reaction (PCR). Approximately 50–100 mg of tissue from each bird was used to isolate DNA, and 100–250 ng of DNA was subsequently used for PCR as previously described (Bamac et al., 2020). Briefly, the amplification of a ∼350 bp fragment from the 28S rRNA region was achieved by using the Sarcocystis spp. (and other apicomplexan parasites) conserved primers SAD2F and SAD2R. Samples that tested positive were further analyzed by the S. calchasi-specific PCR (ITS1) with the primer pair SCa1 and SCa2, with a predicted amplicon size of 225 bp (Bamac et al., 2020). For sequence analysis, PCR products from 28S rRNA-positive samples were purified and analyzed by Sanger sequencing using the SAD2R primer. Sarcocystis calchasi and S. neurona positive DNA samples were used as positive controls, while nuclease-free water used as a template served as a negative control to ensure no cross-contamination occurred (Bamac et al., 2020). Sequences were analyzed as previously described (Bamac et al., 2020) using BioEdit sequence alignment editor v7.0.5.3 (Hall 1999), comparing sequences available from the National Center for Biotechnology Information (NCBI) GenBank (https://www.ncbi.nlm.nih.gov/genbank/) by BLASTn (Altschul et al., 1990). Generated sequences were deposited in GenBank under the accession numbers OK576416 - OK576465.
2.3. Phylogenetic analysis
Phylogenetic comparisons were made between the apicomplexan parasites found in the intestinal samples from the raptors and the respective closest reference sequence in GenBank. Briefly, a Maximum Likelihood phylogeny test was performed using MEGA-X version 10.2.6 (Kumar et al., 2018) applying the Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Tamura-Nei model (Tamura and Nei, 1993).
2.4. Histopathological examination
Histopathology was performed on intestinal tissue of selected birds to confirm the presence of sporocysts. The remaining archived intestinal tissue was thawed, and sections were immersed in 10% neutral buffered formalin, paraffin-embedded, sectioned at 4 μm, and stained with hematoxylin and eosin for histologic examination by light microscopy at the California Animal Health and Food Safety Laboratory System (CAHFS; Davis, CA, USA).
2.5. Statistical analysis
We analyzed differences in infection prevalence using the chi-square (χ2) test of independence. Values reported are mean ± SE. Statistical analyses were performed using NCSS (Hintze, 2007) and P ≤ 0.05 were considered statistically significant. Maps were prepared using ArcMap (ESRI, Inc., Redlands, CA, USA).
3. Results
3.1. Postmortem examination
The 85 raptor carcasses were collected from 12 counties (Fig. 1) including Alameda (n = 8), Contra Costa (n = 10), El Dorado (n = 1), Lassen (n = 1), Los Angeles (n = 51), Mono (n = 1), San Francisco (n = 5), San Luis Obispo (n = 1), San Mateo (n = 2), Santa Clara (n = 3), Santa Cruz (n = 1), and Ventura (n = 1). The raptors were collected in 2016 (n = 6), 2017 (n = 14), 2018 (n = 22), 2019 (n = 19), and 2020 (n = 24). Of the 85 raptors, 51 were female and 34 were male while 44 were juveniles and 41 were adults. Trauma was the most common cause of death for the raptors followed by anticoagulant rodenticide toxicosis, starvation, and diseases (e.g., West Nile virus, avian trichomonosis, etc.).
The 11 American crow carcasses were collected from four counties (Fig. 1) including Alameda (n = 1), Los Angeles (n = 8), Santa Barbara (n = 1), and Ventura (n = 1) in 2018 (n = 1), 2019 (n = 1), and 2020 (n = 9). Seven of the 11 crows were female and four were male while six were juveniles and five were adults. Starvation and diseases (e.g., West Nile virus) were the most common causes of death for the crows followed by trauma.
3.2. Molecular characterization
In total, the intestinal samples of 46 of 85 (54.1%) raptors tested positive for the apicomplexan-generic 28S rRNA PCR (Table 1). Twenty-eight (44.7%) raptors and 10 American crows tested negative. Samples from the remaining 11 raptors and one crow yielded poor quality DNA (i.e., either too low concentration, 1–10 ng/ul, or an A260/A280 < 1) in repeated extractions and tested negative for the 28S rRNA PCR. These samples were excluded from the analysis because it could not be ruled out that the negative results were due to the poor-quality DNA rather than an actual absence of parasite DNA. Additionally, the PCR products from four of the 46 positive raptors were deemed unreadable despite several sequencing attempts, as the chromatogram showed high background noise and mixed peaks that rendered it impossible to discern between nucleotides. The 28S rRNA PCR products were sequenced for the remaining 42 raptors.
Table 1.
Number and prevalence (%) of Sarcocystis spp. and other Sarcocystidae identified by BLASTn analysis of 28S PCR-positive intestinal samples from dead predatory and omnivorous birds collected in California, USA between 2016 and 2020. The parasite species was matched by BLASTn to the closest reference sequence available in GenBank. Bird species include American crow (Corvus brachyrhynchos), Cooper's hawk (Accipiter cooperii), great horned owl (Bubo virginianus), northern goshawk (A. gentilis), peregrine falcon (Falco peregrinus), red-shouldered hawk (Buteo lineatus), red-tailed hawk (B. jamaicensis), and sharp-shinned hawk (A. striatus).
| Number positive (Prevalence %) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Predatory species | n | Sarcocystis spp. (28S PCR) | S. calchasi | S. columbae | S. halieti | S. turdusi | S. jamaicensis | S. jamaicensis/S. (Frenkelia) microti | Eumonospora henryae |
| Cooper's hawk | 16 | 8 (50.0) | 3 (18.8) | 4 (25.0) | 1 (6.3) | 2 (12.5) | – | – | – |
| Great horned owl | 12 | 1 (8.3) | – | – | – | – | – | – | – |
| Northern goshawk | 2 | 2 (100) | – | 2 (100) | – | 1 (50.0) | – | – | – |
| Peregrine falcon | 3 | – | – | – | – | – | – | – | – |
| Red-shouldered hawk | 12 | 8 (66.7) | – | – | 1 (8.3) | – | 6 (50.0) | 1 (8.3) | – |
| Red-tailed hawk | 36 | 25 (69.4) | 2 (5.6) | 1 (2.8) | 2 (5.6) | 1 (50.0) | 12 (33.3) | 7 (19.4) | 1 (2.8) |
| Sharp-shinned hawk | 4 | 2 (50.0) | – | – | – | 2 (5.6) | – | – | – |
| Total | 85 | 46 (54.1) | 5 (5.9) | 7 (8.2) | 4 (4.7) | 6 (7.1) | 18 (21.2) | 8 (9.4) | 1 (1.2) |
| Omnivorous species |
n |
Sarcocystis spp. (28S PCR) |
S. calchasi |
S. columbae |
S. halieti |
S. turdusi |
S. jamaicensis |
S. jamaicensis/S. (Frenkelia) microti |
Eumonospora henryae |
| American crow | 11 | – | – | – | – | – | – | – | – |
Five of the 42 raptors also tested positive for the S. calchasi-specific ITS1 PCR (Table 1) and all five were further confirmed to be 100% identical to S. calchasi by sequencing of the 28S rRNA PCR product (Table 2). Infected raptors included 3 of 16 (18.8%) Cooper's hawks and 2 of 26 (5.6%) red-tailed hawks (Table 1), all collected from Los Angeles County. The S. calchasi-positive Cooper's hawks were collected in November 2016, May 2017, and June 2017 while the red-tailed hawks were collected in March and June 2020. One positive Cooper's hawk was an adult female and two were juvenile males. Both positive red-tailed hawks were males, one was a juvenile and one was an adult.
Table 2.
Sarcocystis spp. and other Sarcocystidae identified by BLASTn analysis of 28S PCR-positive intestinal samples from dead raptors (n = 85) collected in California, USA between 2016 and 2020. The parasite species was matched by BLASTn to the closest reference sequence available in GenBank. Raptor species include Cooper's hawk (Accipiter cooperii), great horned owl (Bubo virginianus), northern goshawk (A. gentilis), peregrine falcon (Falco peregrinus), red-shouldered hawk (Buteo lineatus), red-tailed hawk (B. jamaicensis), and sharp-shinned hawk (A. striatus).
| Primary infectiona | % homology to GenBank | Secondary infectiona | % homology to GenBank | n (%) |
|---|---|---|---|---|
| S. jamaicensis | 97.0–99.7 | 13 (15.8) | ||
| S. jamaicensis | 97.1 | S. jamaicensis | 96.7 | 1 (1.2) |
| S. jamaicensis | 97.7 | S. jamaicensis or S. (Frenkelia) microtib | 98.7 | 1 (1.2) |
| S. jamaicensis or S. (Frenkelia) microtib | 99.7 | S. jamaicensis or S. (Frenkelia) microtib | 99.3 | 1 (1.2) |
| S. jamaicensis or S. (Frenkelia) microtib | 99.3 | 4 (4.7) | ||
| S. jamaicensis or S. (Frenkelia) microtib | 99.3–99.7 | S. jamaicensis | 97.7 | 2 (2.4) |
| S. calchasi | 100 | 5 (5.9) | ||
| S. turdusi | 98.7–99.7 | 4 (4.7) | ||
| S. turdusi | 98.4 | S. columbae | 99.0 | 1 (1.2) |
| S. columbae | 99.7 | 3 (3.5) | ||
| S. columbae | 99.7 | S. turdusi | 98.7 | 1 (1.2) |
| S. columbae | 99.7 | S. halieti | 100.0 | 1 (1.2) |
| S. columbae | 99.7 | S. jamaicensis | 99.0 | 1 (1.2) |
| S. halieti | 100 | 3 (3.5) | ||
| Eumonospora henryae | 95.7 | 1 (1.2) | ||
| Negative | 28 (32.9) | |||
| Bad quality DNA | 11 (12.9) | |||
| Unreadable sequencesc | 4 (4.7) |
Nine of 85 raptors presented mixed infections, i.e., chromatograms showed clear double peaks in only a few nucleotides. In these cases, two sequences were analyzed separately by combining only the high and low peak nucleotides. The closest BLASTn match for each sequence was considered the primary and secondary infection, respectively.
Eight of 85 raptors had sequences with identical homology percentage to two different sequences by BLASTn for S. jamaicensis and S. (Frenkelia) microti. In these cases, either of these parasites could be considered as the cause of infection.
Sequence considered unreadable when its respective chromatograph presented high background noise or mixed peaks that rendered it impossible to discern between nucleotides.
Screening the sequences of the remaining 28S rRNA-positive raptors through BLASTn, a high percentage of homology (96–100%) to a number of different Sarcocystis spp. and other apicomplexan parasites were found (Table 1, Table 2). Of note, when the chromatograms of the positive raptors were analyzed, nine showed nucleotides with double, overlapping peaks in an otherwise clean sequence with no, or minimal, noise (see examples in Fig. 2). Because these were detected in only a few nucleotides (1–11) in each sequence, and there was always one predominant nucleotide over the other in each double peak, two different sequences were analyzed separately by combining only the high and low peak nucleotides. After BLASTn analysis, the closest match from each sequence was considered as the primary and secondary infection for each raptor, respectively. In addition to S. calchasi (5.9%; 5/85), the following parasites also were detected in raptors: S. jamaicensis (21.2%; 18/85), S. columbae (8.2%; 7/85), S. turdusi (7.1%; 6/85), S. halieti (4.7; 4/85%), and Eumonospora henryae (1.2%; 1/85) (Table 2). Further, eight raptors (9.4%; 8/85) had sequences with the same homology percentage (∼99%) to two different sequences by BLASTn, namely S. jamaicensis and S. (Frenkelia) microti. Therefore, either of these parasites could be considered as the cause of infection (Table 2). Detailed information for each bird and sequence is presented in Supplementary Table 1.
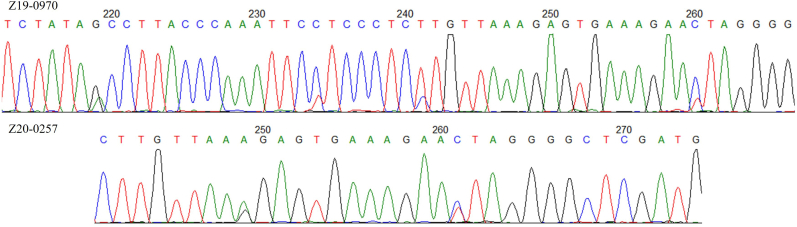
Fig. 2.
Examples of two 28S rRNA chromatograms showing double, overlapping peaks. The chromatogram for northern goshawk (Accipiter gentilis) Z19-0970 (top) from Lassen County, California, USA shows the predominate nucleotide for Sarcocystis columbae (99.7% homology) and secondary nucleotide for S. turdusi (98.7% homology); clear double peaks are patent at positions 219, 234, 241 and 260. The chromatogram for Cooper's hawk (A. cooperii) Z20-0257 (bottom) from Los Angeles County, California, USA shows the predominate nucleotide for S. columbae (99.7% homology) and the secondary nucleotide for S. halieti (100% homology); clear double peaks are patent at positions 249 and 261. Note the marginal presence of noise in the remainder of the sequences.
Overall, significantly more red-tailed hawks were infected compared with the other six raptor species (X2 = 5.9, P = 0.0151). Red-tailed hawks also had the highest diversity of parasites with seven, followed by Cooper's hawks with four and red-shouldered hawks with three. Significantly more juvenile, red-shouldered hawks (X2 = 1.3, P = 0.038) and great horned owls (X2 = 5.5, P = 0.020) were infected compared to adults. No other age or sex differences were observed.
3.3. Phylogenetic analysis
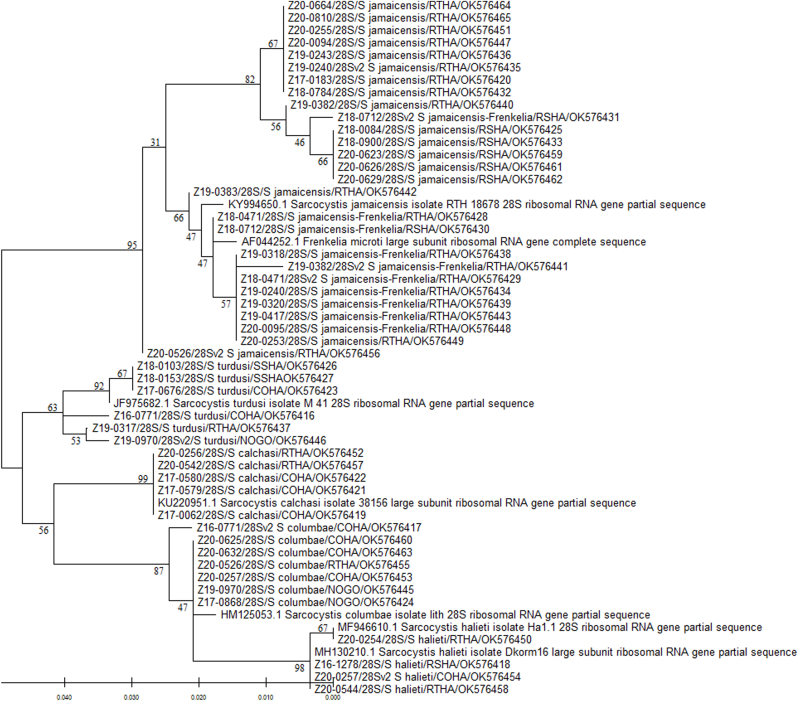
All 28S rRNA sequences from the raptor intestinal samples grouped within the same branch or sub-branch as their respective reference sequence in the phylogenetic tree (Fig. 3). However, for the S. jamaicensis and S. (Frenkelia) microti sequences, two distinct branches were observed (Fig. 3). The first branch included the S. jamaicensis sequences with two separate sub-branches comprised of sequences obtained from red-tailed hawks and red-shouldered hawks, respectively. The second branch included the S. jamaicensis/S. (Frenkelia) microti sequences that had the same homology percentage, including the GenBank reference sequences. All but one of these sequences were obtained from red-tailed hawks, with one sequence from a red-shouldered hawk. The remaining Sarcocystis spp. found in our study formed the lower main branch of the tree, being distributed in sub-branches, one for each Sarcocystis spp. (S. turdusi, S. calchasi, S. columbae, and S. halieti) together with their respective reference sequence (Fig. 3). Finally, the E. henryae sequence from one red-tailed hawk formed a distant branch in the tree (not shown), accounting for the higher number of dissimilar nucleotides compared to the other sequences.
Fig. 3.
Phylogenetic tree based on 28S rRNA sequences of Sarcocystis spp. obtained from raptor intestinal samples and reference sequences available in GenBank. The phylogenetic relationships were determined by the Maximum Likelihood method using MEGA-X version 10.2.6 (Kumar et al., 2018). Numbers above or below nodes represent bootstrap confidence values from 500 replicates. The distances were computed using the Tamura-Nei model (Tamura and Nei, 1993). Branch lengths are proportional to sequence divergence and relate to the scale bar (bottom left). GenBank reference sequences are labeled by their accession number and associated information. Raptor sequences are labeled by animal identification number, region amplified (28S), Sarcocystis spp. with the highest homology found by BLASTn, 4-letter alpha-code for the raptor species common name, and NCBI accession number. COHA: Cooper's hawk (Accipiter cooperii), NOGO: northern goshawk (A. gentilis), RSHA: red-shouldered hawk (Buteo lineatus), RTHA: red-tailed hawk (B. jamaicensis), SSHA: sharp-shinned hawk (A. striatus). Note the Eumonospora henryae-like sequence was not included in the analysis. Detailed information for each bird and sequence is presented in Supplementary Table 1.
3.4. Histopathological examination
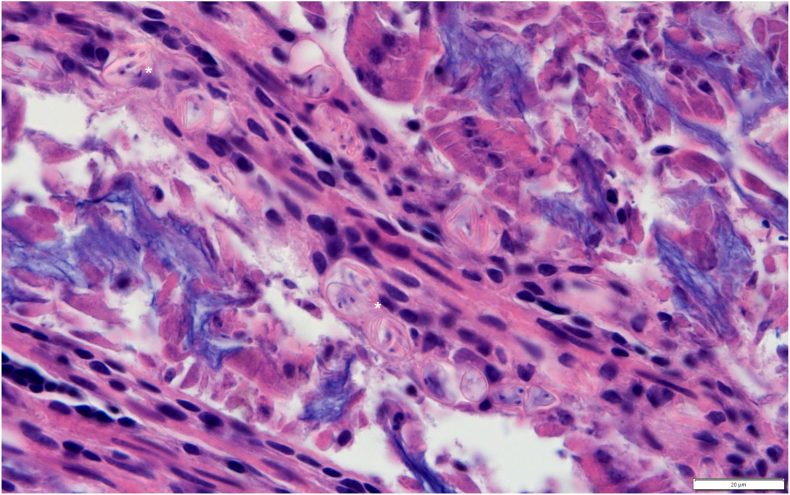
Histopathological evaluation of the intestine was performed on the three Cooper's hawk's and two red-tailed hawk's positive for S. calchasi. Structures consistent with sporocysts/oocysts were observed in the mucosa of the intestine in 2 of 3 Cooper's hawks and both red-tailed hawks (Fig. 4); the intestine of one Cooper's hawk was too autolyzed, limiting evaluation.
Fig. 4.
Photomicrograph of intestine from Sarcocystis calchasi-positive Cooper's hawk (Accipiter cooperii) Z17-0062 collected from Los Angeles County, California, USA, showing sporocysts/oocysts (*) in the mucosa. Hematoxylin and eosin stain; scale bar 20 μm.
4. Discussion
In this study, Cooper's hawks and red-tailed hawks were identified for the first time as likely definitive hosts for S. calchasi in California, the causative agent of protozoal encephalitis in free-ranging rock pigeons and wild Brandt's cormorants. Populations of Cooper's hawks and red-tailed hawks are wide-ranging and may be the definitive hosts of S. calchasi elsewhere in North America. Both species are relatively adaptable to living in urban environments where their density is often higher than in more rural and undisturbed areas (Stout and Rosenfield, 2010; Stout et al., 2006). Similarly, large numbers of non-native rock pigeons, Eurasian collared doves, and psittacines, all competent intermediate hosts for S. calchasi, also reside in urban environments including that of the San Francisco Bay and Los Angeles Metropolitan Areas (Garrett 1998). This combination of factors may help explain the epizootic nature of S. calchasi in these locations with multiple intermediate hosts becoming infected during an overlapping timeframe, which is unique among Sarcocystis spp. with a bird-to-bird life cycle. Higher densities of definitive hosts in urban areas, could then contribute to the land-to-sea transmission of S. calchasi to a near-shore seabird, the Brandt's cormorant, along the southern California coast during periods of high precipitation.
Northern goshawks were identified as a definitive host of S. calchasi following a laboratory study in Germany in which two goshawks were fed pectoral muscle from an infected rock pigeon and began shedding sporocysts on day 6 post-infection (Olias et al., 2010b). Oocysts, each containing two sporocysts, were observed in the mucosa of the small intestine of the goshawk by microscopy. Later, field studies examining the intestinal samples and/or feces of northern goshawks and European sparrowhawks, identified infection with S. calchasi through molecular analysis of the ITS1 and 28S rRNA gene segments (Olias et al., 2011; Parmentier et al., 2018), and oocysts were observed in the lamina propria of some birds (Olias et al., 2011). This expanded the definitive host range for S. calchasi in Germany, and presumably elsewhere in Europe, to northern goshawks and European sparrowhawks, which both belong to the genus Accipiter. In the present study, intestinal samples of seven raptor species and American crows, considered to be possible predators and/or scavengers of rock pigeons, were examined. Three Cooper's hawks and two red-tailed hawks tested positive for S. calchasi with a 100% homology to the GenBank reference sequence. Further, histopathology confirmed the presence of sporocysts in the mucosa of the intestine in four of the five raptors indicating sporogony was occurring, rather than the parasite simply passing though the raptor's digestive tract following a meal.
In the United States, the range of northern goshawks is more restricted, and overlaps only rarely with rock pigeons (Squires et al., 1997). However, the range of a related Accipiter, the Cooper's hawk, is widespread and commonly overlaps with rock pigeons in diverse habitats including urban areas (Rosenfield et al., 2019). Cooper's hawks are primarily an avian predator that will readily consume pigeons. A previous study in Georgia identified a Sarcocystis spp. in a Cooper's hawk compatible with S. calchasi (Yabsley et al., 2009). However, only the 18S rRNA gene segment was evaluated, and therefore, the parasite could not be conclusively identified as S. calchasi. Interestingly in the present study, S. calchasi also was detected in red-tailed hawks. Red-tailed hawks belong to the genus Buteo but are wide-ranging and have a diverse diet feeding on mammals as well as birds, reptiles, and amphibians (Preston and Beane, 2009). While Cooper's hawks and red-tailed hawks belong to different genera, they are within the family Accipitridae, suggesting other Buteos may be definitive hosts for S. calchasi. The study in Georgia also identified closely related Sarcocystis spp. based on the 18S rRNA gene segment in red-tailed and red-shouldered hawks (Yabsley et al., 2009). Additional molecular analysis is needed to evaluate the possibility of other definitive hosts for S. calchasi.
In addition to S. calchasi, multiple closely related Sarcocystis spp. and other apicomplexan protozoa were detected in the intestinal samples from the raptors evaluated in the present study, including S. columbae, S. halieti, S. turdusi, S. jamaicensis, S. jamaicensis/S. (Frenkelia) microti, and E. henryae. Co-infection with multiple Sarcocystis spp. were found in nine raptors. While the 28S and ITS1 PCRs successfully identified the DNA of apicomplexans in the intestine of 54% (46/85) of the raptors in our study, this methodology may overlook the presence of other protozoa that are not detectable with these primers (Gjerde et al., 2018). Furthermore, some sequences had only 96–99% homology to reference sequences in GenBank; thus, comparing sequences from other conserved regions (e.g., ITS1) and employing additional techniques that allow screening for multiple genera and/or species, such as restriction fragment length polymorphism, are needed to confirm the identity of these parasites.
In the present study, new potential definitive hosts were identified for several previously recognized Sarcocystis spp. Northern goshawks and European sparrowhawks had been identified as definitive hosts for S. columbae in Europe (Olias et al., 2011) whereas in the United States, a species related to S. columbae had been detected in the intestinal tract of a single Cooper's hawk from North Carolina (Lindsay et al., 2017). Northern goshawks and red-tailed hawks also were identified as possible definitive hosts for S. columbae in the present study. Muscle cysts of S. columbae were previously observed in native wood pigeons (Columba palumbus) from Germany and Lithuania (Olias et al., 2010c; Prakas et al., 2011). A comparable intermediate host for S. columbae in the western United States is the native band-tailed pigeon (Patagioenas fasciata) which inhabit conifer and deciduous forests (Keppie and Braun, 2000). However, since band-tailed pigeons do not occur in the eastern United States, other intermediate hosts are likely. Sarcocysts of S. columbae also were found in one herring gull (Larus argentatus) in Lithuania with sequences 99.9–100% identical to those from wood pigeons (Prakas et al., 2020), which supports an expansive host range.
Definitive hosts of S. turdusi had not been previously established although muscle cysts of S. turdusi were found in common blackbirds (Turdus merula) in Lithuania (Kutkiene et al., 2012). In the present study, three Accipiter spp. and red-tailed hawks were identified as possible definitive hosts of S. turdusi suggesting that North American blackbirds also may harbor this parasite. For S. halieti, the white-tailed sea eagle (Haliaeetus albicilla) and the much smaller European sparrowhawk have been recognized as definitive hosts in Europe (Gjerde et al., 2018; Mayr et al., 2016). This suggests that in addition to great cormorants (Phalacrocorax carbo) and herring gulls (Prakas et al., 2018, 2020), other intermediate hosts such as songbirds are likely for S. halieti, which is supported by the diversity of potential definitive hosts identified in the present study. Interestingly, two raptor species were recently identified as intermediate hosts of S. halieti although the morphology of the sarcocysts in the muscle varied from those described in non-raptorial intermediate hosts (Prakas et al., 2021). Encephalitis in a little owl (Athene noctua) also was associated with S. halieti infection (Maier-Sam et al., 2021). Studies evaluating the transmission of Sarcocystis spp., including S. halieti, are needed to clarify the life cycles of these parasites.
The taxonomy of some parasites remains unclear and requires further study, for example, S. jamaicensis and S. (Frenkelia) microti. Red-tailed hawks have been identified as a definitive host for both parasites with various rodents being the likely intermediate host for S. jamaicensis and known intermediate host for S. (Frenkelia) microti (Upton and McKown, 1992; Verma et al., 2017). Our analysis has identified red-shouldered hawks as another possible definitive host for these parasites. Early researchers established Frenkelia as a separate genus from Sarcocystis based on the sarcocysts identified in the brain of rodents infected with Frenkelia spp.; however, later it became clear that some Sarcocystis spp. also may form cysts in the brain in addition to the muscle tissue (Dubey et al., 2016; Votypka et al., 1998). Recent molecular analyses indicate that Frenkelia is closely related to Sarcocystis, and that Frenkelia should be a junior synonym to Sarcocystis (Verma et al., 2017). Our analysis supports this finding and suggests that S. jamaicensis and S. microti are different species as they have two single nucleotide polymorphisms (SNP), although these SNPs occur at different locations. The S. jamaicensis and S. microti sequences also comprised two separate branches in the phylogenetic tree. Interestingly, within the S. jamaicensis branch, there were two distinct sub-branches for sequences from red-tailed hawks and red-shouldered hawks, respectively. This suggests these S. jamaicensis-like parasites could be new variants or subspecies of S. jamaicensis and that a particular variant may be more common in different raptor species. Given the small number of SNPs and less than 100% homology to the GenBank reference sequence, further studies are needed to evaluate these variants.
Another genus with unclear taxonomy is Eumonospora. The Eumonospora-like parasite identified in the present study was distantly related to the Sarcocystis spp. found in the raptor intestinal samples. Recently, Chou et al. (2020) suggested Eumonospora should be synonymized with Caryospora and Avispora. In Japan, E. henryae was identified in fecal samples from five captive owls, including four different species (Chou et al., 2020), and a captive merlin (F. columbarius) (Chou et al., 2021). The merlin was exhibiting severe watery diarrhea, and infections with Caryospora spp. reportedly have been associated with diarrhea in captive-bred raptors in Europe and the Middle East (Forbes et al., 1997; Mateuta and Samour, 2017). The E. henryae-like sequence detected in the single red-tailed hawk in the present study was only 95.7% homologous to the sequence from the merlin (Chou et al., 2021), which may indicate a different, yet-to-be defined but closely related, parasite. The red-tailed hawk was admitted to a wildlife rehabilitation center after it was found grounded and presented with emaciation and copious green diarrhea; coccidia oocysts were noted on fecal floatation. Additional research is needed to determine if red-tailed hawks are a true definitive host for E. henryae.
5. Conclusions
In this study, we identify for the first time Cooper's hawks and red-tailed hawks as likely definitive hosts for S. calchasi in California, as well as identify new possible definitive hosts for other related Sarcocystis spp. by using ITS1 and 28S sequence analysis. Further research is needed to determine the competency of S. calchasi-definitive hosts for producing and shedding sporocysts into the environment to better understand infection dynamics. Specifically for S. calchasi, the management of non-native populations of columbids and psittacines in urban areas may be warranted to help protect the health of sensitive marine species susceptible to infection following periods of high precipitation. Additionally, further molecular surveillance for Sarcocystis spp. in both definitive and intermediate hosts is needed to elucidate the complete life cycle for closely related bird-infecting Sarcocystis spp. Experimental infection studies also are necessary to confirm the definitive and intermediate hosts for these parasites.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
We thank the staff and volunteers at the California Wildlife Center (Calabasas, CA), Defiance Canyon Raptor Rescue (Bieber, CA), East Bay Regional Park District (Oakland, CA), Golden Gate Raptor Observatory (San Rafael, CA), Greater Los Angeles County Vector Control District (Sylmar, CA), Lindsay Wildlife Experience (Walnut Creek, CA), Midpeninsula Regional Open Space District (Los Altos, CA), Ohlone Humane Society Wildlife Rehabilitation Center (Fremont, CA), Pasadena Humane (Pasadena, CA), Peninsula Humane Society and SPCA (Burlingame, CA), Presidio Trust (San Francisco, CA), Santa Barbara Wildlife Care Network (Goleta, CA), Wildcare Eastern Sierra (Bishop, CA), Wildlife Center of Silicon Valley (San Jose, CA) for submitting carcasses. We thank T. Giudici, T. Kasteen, S. McMillin, B. Munk, K. Rush, N. Shirkey, and T. Tognazzini with CDFW for assisting with the mortality investigations. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2021.12.008.
Contributor Information
Krysta H. Rogers, Email: krysta.rogers@wildlife.ca.gov.
David Arranz-Solís, Email: darranz@ucdavis.edu.
Jeroen P.J. Saeij, Email: jsaeij@ucdavis.edu.
Stephany Lewis, Email: stephany@ojairaptorcenter.org.
Aslı Mete, Email: amete@ucdavis.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bamac O.E., Rogers K.H., Arranz-Solís D., Saeij J.P., Lewis S., Duerr R., Skoglund J., Peronne L., Mete A. Protozoal encephalitis associated with Sarcocystis calchasi and S. falcatula during an epizootic involving Brandt's cormorants (Phalacrocorax penicillatus) in coastal Southern California, USA. Int. J. Parasitol. Parasites Wildl. 2020;12:185–191. doi: 10.1016/j.ijppaw.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box E.D., Smith J.H. The intermediate host spectrum in a Sarcocystis species of birds. J. Parasitol. 1982;68:668–673. [PubMed] [Google Scholar]
- Chou S., Izawa N., Ike K., Tokiwa T. Detection of Eumonospora henryae (Apicomplexa: Sarcocystidae) from Falco columbarius (Falconiformes: Aves): comparison of host–parasite phylogram and comments on the family Sarcocystidae Poche, 1913. Int. J. Parasitol. Parasites Wildl. 2021;14:75–83. doi: 10.1016/j.ijppaw.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S., Tokiwa T., Hadano S., Izawa N., Ueda M., Kojima A., Ike K. Resurrection of the genus Eumonospora (Apicomplexa: Sarcocystidae) for Caryospora species without stieda body. Parasitol. Int. 2020;77:102101. doi: 10.1016/j.parint.2020.102101. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Calero-Bernal R., Rosenthal B.M., Speer C.A., Fayer R. second ed. CEC Press, Taylor and Francis Group; Boca Raton, Florida: 2016. Sarcocystosis of Animals and Humans. [Google Scholar]
- Forbes N.A., Simpson G.N. Caryospora neofalconis: an emerging threat to captive-bred raptors in the United Kingdom. J. Avian Med. Surg. 1997;11:110–114. [Google Scholar]
- Garrett K.L. In: Proc. 18th Verteb. Pest Conf., Univ. of California, Davis. Baker R.O., Crabb A.C., editors. 1998. Population trends and ecological attributes of introduced parrots, doves, and finches in California; pp. 46–54. [Google Scholar]
- Gjerde B., Vikøren T., Hamnes I.S. Molecular identification of Sarcocystis halieti n. sp., Sarcocystis lari and Sarcocystis truncata in the intestine of a white-tailed sea eagle (Haliaeetus albicilla) in Norway. Int. J. Parasitol. Parasites Wildl. 2018;7:1–11. doi: 10.1016/j.ijppaw.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Hintze J. NCSS, LLC; Kaysville, Utah: 2007. NCSS. Statistical Software. Version 07.1.21. [Google Scholar]
- Hodo C.L., Whitley D.B., Hamer S.A., Corapi W.V., Snowden K., Heatley J.J., Hoffmann A.R. Histopathologic and molecular characterization of Sarcocystis calchasi encephalitis in white-winged doves (Zenaida asiatica) and Eurasian collared doves (Streptopelia decaocto), East-central Texas, USA, 2010-13. J. Wildl. Dis. 2016;52:395–399. doi: 10.7589/2015-10-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppie D.M., Braun C.E. In: Birds of the World. Poole A.F., Gill F.B., editors. Cornell Lab of Ornithology; Ithaca, New York, USA: 2000. Band-tailed pigeon (Patagioenas fasciata), version 1.0. Available online: [DOI] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutkienė L., Prakas P., Butkauskas D., Sruoga A. Description of Sarcocystis turdusi sp. nov. from the common blackbird (Turdus merula) Parasitology. 2012;139:1438–1443. doi: 10.1017/S0031182012000819. [DOI] [PubMed] [Google Scholar]
- Lindsay D.S., Verma S.K., Scott D., Dubey J.P., von Dohlen A.R. Isolation, molecular characterization, and in vitro schizogonic development of Sarcocystis sp. ex Accipiter cooperii from a naturally infected Cooper's hawk (Accipiter cooperii) Parasitol. Int. 2017;66:106–111. doi: 10.1016/j.parint.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Luznar S.L., Avery M.L., Dame J.B., MacKay R.J., Greiner E.C. Development of Sarcocystis falcatula in its intermediate host, the brown-headed cowbird (Molothrus ater) Vet. Parasitol. 2001;95:327–334. doi: 10.1016/s0304-4017(00)00399-x. [DOI] [PubMed] [Google Scholar]
- Maier K., Olias P., Enderlein D., Klopfleisch R., Mayr S.L., Gruber A.D., Lierz M. Parasite distribution and early-stage encephalitis in Sarcocystis calchasi infections in domestic pigeons (Columba livia f. domestica) Avian Pathol. 2015;44:5–12. doi: 10.1080/03079457.2014.978263. [DOI] [PubMed] [Google Scholar]
- Maier-Sam K., Kaiponen T., Schmitz A., Schulze C., Bock S., Hlinak A., Olias P. Encephalitis associated with Sarcocystis halieti infection in a free-ranging little owl (Athene noctua) J. Wildl. Dis. 2021;57:712–714. doi: 10.7589/JWD-D-20-00184. [DOI] [PubMed] [Google Scholar]
- Mateuta V.D., Samour J.H. Prevalence of Caryospora species (Apicomplexa: Eimeriidae) in falcons in the United Arab Emirates. J. Avian Med. Surg. 2017;31:327–334. doi: 10.1647/2016-221. [DOI] [PubMed] [Google Scholar]
- Mayr S.L., Maier K., Müller J., Enderlein D., Gruber A.D., Lierz M. Accipiter hawks (Accipitridae) confirmed as definitive hosts of Sarcocystis turdusi, Sarcocystis cornixi and Sarcocystis sp. ex Phalacrocorax carbo. Parasitol. Res. 2016;115:3041–3047. doi: 10.1007/s00436-016-5059-5. [DOI] [PubMed] [Google Scholar]
- Mete A., Rogers K.H., Wolking R., Bradway D.S., Kelly T., Piazza M., Crossley B. Sarcocystis calchasi outbreak in feral rock pigeons (Columba livia) in California. Vet. Pathol. 2019;56:317–321. doi: 10.1177/0300985818794262. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Conrad P.A., Harris M., Hatfield B., Langlois G., Jessup D.A., Magargal S.L., Packham A.E., Toy-Choutka S., Melli A.C. A protozoal-associated epizootic impacting marine wildlife: mass-mortality of southern sea otters (Enhydra lutris nereis) due to Sarcocystis neurona infection. Vet. Parasitol. 2010;172:183–194. doi: 10.1016/j.vetpar.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.A., Gardner I.A., Kreuder C., Paradies D.M., Worcester K.R., Jessup D.A., Dodd E., Harris M., Ames J.A., Packham A.E., Conrad P.A. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis) Int. J. Parasitol. 2002;32:997–1006. doi: 10.1016/s0020-7519(02)00069-3. [DOI] [PubMed] [Google Scholar]
- Olias P., Olias L., Krücken J., Lierz M., Gruber A.D. High prevalence of Sarcocystis calchasi sporocysts in European Accipiter hawks. Vet. Parasitol. 2011;175:230–236. doi: 10.1016/j.vetpar.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Olias P., Gruber A.D., Heydorn A.O., Kohls A., Hafez H.M., Lierz M. Unusual biphasic disease in domestic pigeons (Columba livia f. domestica) following experimental infection with Sarcocystis calchasi. Avian Dis. 2010;54:1032–1037. doi: 10.1637/9303-031110-Reg.1. [DOI] [PubMed] [Google Scholar]
- Olias P., Gruber A.D., Heydorn A.O., Kohls A., Mehlhorn H., Hafez H.M., Lierz M. A novel Sarcocystis-associated encephalitis and myositis in racing pigeons. Avian Pathol. 2009;38:121–128. doi: 10.1080/03079450902737847. [DOI] [PubMed] [Google Scholar]
- Olias P., Gruber A.D., Kohls A., Hafez H.M., Heydorn A.O., Mehlhorn H., Lierz M. Sarcocystis species lethal for domestic pigeons. Emerg. Infect. Dis. 2010;16:497. doi: 10.3201/eid1603.090860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olias P., Maier K., Wuenschmann A., Reed L., Armién A.G., Shaw D.P., Gruber A.D., Lierz M. Sarcocystis calchasi has an expanded host range and induces neurological disease in cockatiels (Nymphicus hollandicus) and North American rock pigeons (Columbia livia f. dom.) Vet. Parasitol. 2014;200:59–65. doi: 10.1016/j.vetpar.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Olias P., Olias L., Lierz M., Mehlhorn H., Gruber A.D. Sarcocystis calchasi is distinct to Sarcocystis columbae sp. nov. from the wood pigeon (Columba palumbus) and Sarcocystis sp. from the sparrowhawk (Accipiter nisus) Vet. Parasitol. 2010;171:7–14. doi: 10.1016/j.vetpar.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Olson E.J., Wünschmann A., Dubey J.P. Sarcocystis sp.-associated meningoencephalitis in a bald eagle (Haliaeetus leucocephalus) J. Vet. Diagn. Invest. 2007;19:564–568. doi: 10.1177/104063870701900519. [DOI] [PubMed] [Google Scholar]
- Parmentier S.L., Maier-Sam K., Failing K., Enderlein D., Gruber A.D., Lierz M. Prevalence of Sarcocystis calchasi in free-ranging host species: Accipiter hawks and common wood pigeon in Germany. Sci. Rep. 2018;8:1–8. doi: 10.1038/s41598-018-35862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakas P., Bea A., Juozaitytė-Ngugu E., Olano I., Villanúa D., Švažas S., Butkauskas D. Molecular identification of Sarcocystis halieti in the muscles of two species of birds of prey from Spain. Parasites Vectors. 2021;14:1–7. doi: 10.1186/s13071-021-04921-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakas P., Butkauskas D., Juozaitytė-Ngugu E. Molecular identification of four Sarcocystis species in the herring gull, Larus argentatus, from Lithuania. Parasites Vectors. 2020;13:1–6. doi: 10.1186/s13071-019-3869-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakas P., Butkauskas D., Sruoga A., Švažas S., Kutkienė L. Identification of Sarcocystis columbae in wood pigeons (Columba palumbus) in Lithuania. Vet. Med. Zoot. 2011;55:33–39. [Google Scholar]
- Prakas P., Butkauskas D., Švažas S., Stanevičius V. Morphological and genetic characterization of Sarcocystis halieti from the great cormorant (Phalacrocorax carbo) Parasitol. Res. 2018;117:3663–3667. doi: 10.1007/s00436-018-6083-4. [DOI] [PubMed] [Google Scholar]
- Preston C.R., Beane R.D. In: Birds of the World. Poole A.F., editor. Cornell Lab of Ornithology; Ithaca, New York, USA: 2009. Red-tailed hawk (Buteo jamaicensis), version 1.0. Available online: [DOI] [Google Scholar]
- Rimoldi G., Speer B., Wellehan J.F., Jr., Bradway D.S., Wright L., Reavill D., Barr B.C., Childress A., Shivaprasad H.L., Chin R.P. An outbreak of Sarcocystis calchasi encephalitis in multiple psittacine species within an enclosed zoological aviary. J. Vet. Diagn. Invest. 2013;25:775–781. doi: 10.1177/1040638713502981. [DOI] [PubMed] [Google Scholar]
- Rosenfield R.N., Madden K.K., Bielefeldt J., Curtis O.E. In: Birds of the World. Rodewald P.G., editor. Cornell Lab of Ornithology; Ithaca, NY, USA: 2019. Cooper's hawk (Accipiter cooperii), version 1.0. Available online: [DOI] [Google Scholar]
- Squires J.R., Reynolds R.T., Orta J., Marks J.S. In: Birds of the World. Billerman S.M., editor. Cornell Lab of Ornithology; Ithaca, NY, USA: 1997. Northern goshawk (Accipiter gentilis), version 1.0. Available online: [DOI] [Google Scholar]
- Stout W.E., Rosenfield R.N. Colonization, growth, and density of a pioneer Cooper's hawk population in a large metropolitan environment. J. Raptor Res. 2010;44:255–267. [Google Scholar]
- Stout W.E., Temple S.A., Cary J.R. Landscape feathers of red-tailed hawk nesting habitat in an urban/suburban environment. J. Raptor Res. 2006;40:181–192. [Google Scholar]
- Tamura K., Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Trupkiewicz J.G., Calero-Bernal R., Verma S.K., Mowery J., Davison S., Habecker P., Georoff T.A., Ialeggio D.M., Dubey J.P. Acute, fatal Sarcocystis calchasi-associated hepatitis in Roller pigeons (Columba livia f. dom.) at Philadelphia Zoo. Vet. Parasitol. 2016;216:52–58. doi: 10.1016/j.vetpar.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Upton S.J., McKown R.D. The red-tailed hawk, Buteo jamaicensis, a native definitive host of Frenkelia microti (Apicomplexa) in North America. J. Wildl. Dis. 1992;28:85–90. doi: 10.7589/0090-3558-28.1.85. [DOI] [PubMed] [Google Scholar]
- Ushio N., Watanabe K.I., Chambers J.K., Shibato T., Nakayama H., Uchida K. Sarcocystis calchasi encephalitis in a rock pigeon. J. Vet. Med. Sci. 2015;77:1523–1526. doi: 10.1292/jvms.15-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S.K., Von Dohlen A.R., Mowery J.D., Scott D., Rosenthal B.M., Dubey J.P., Lindsay D.S. Sarcocystis jamaicensis n. sp., from red-tailed hawks (Buteo jamaicensis) definitive host and IFN-γ gene knockout mice as experimental intermediate host. J. Parasitol. 2017;103:555–564. doi: 10.1645/17-10. [DOI] [PubMed] [Google Scholar]
- Votypka J., Hypša V., Jirku M., Flegr J., Vavra J., Lukes J. Molecular phylogenetic relatedness of Frenkelia spp. (Protozoa, Apicomplexa) to Sarcocystis falcatula Stiles 1893: is the genus Sarcocystis paraphyletic? J. Eukaryot. Microbiol. 1998;45:137–141. doi: 10.1111/j.1550-7408.1998.tb05081.x. [DOI] [PubMed] [Google Scholar]
- Wünschmann A., Armien A.G., Reed L., Gruber A.D., Olias P. Sarcocystis calchasi‐associated neurologic disease in a domestic pigeon in North America. Transbound. Emerg. Dis. 2011;58:526–530. doi: 10.1111/j.1865-1682.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- Wünschmann A., Rejmanek D., Conrad P.A., Hall N., Cruz-Martinez L., Vaughn S.B., Barr B.C. Natural fatal Sarcocystis falcatula infections in free-ranging eagles in North America. J. Vet. Diagn. Invest. 2010;22:282–289. doi: 10.1177/104063871002200222. [DOI] [PubMed] [Google Scholar]
- Wünschmann A., Rejmanek D., Cruz-Martinez L., Barr B.C. Sarcocystis falcatula-associated encephalitis in a free-ranging great horned owl (Bubo virginianus) J. Vet. Diagn. Invest. 2009;21:283–287. doi: 10.1177/104063870902100223. [DOI] [PubMed] [Google Scholar]
- Yabsley M.J., Ellis A.E., Stallknecht D.E., Howerth E.W. Characterization of Sarcocystis from four species of hawks from Georgia, USA. J. Parasitol. 2009;95:256–259. doi: 10.1645/GE-1567.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.