Abstract
Background
People with subjective cognitive decline (SCD) may be at increased risk for Alzheimer’s disease (AD). However, not all studies have observed this increased risk. This project examined whether four common methods of defining SCD yields different patterns of atrophy and future cognitive decline between cognitively normal older adults with (SCD+ ) and without SCD (SCD−).
Methods
Data from 273 Alzheimer’s Disease Neuroimaging Initiative cognitively normal older adults were examined. To operationalize SCD we used four common methods: Cognitive Change Index (CCI), Everyday Cognition Scale (ECog), ECog + Worry, and Worry. Voxel-based logistic regressions were applied to deformation-based morphology results to determine if regional atrophy between SCD− and SCD+ differed by SCD definition. Linear mixed-effects models were used to evaluate differences in future cognitive decline.
Results
Results varied between the four methods of defining SCD. Left hippocampal grading was more similar to AD in SCD+ than SCD− when using the CCI (p = .041) and Worry (p = .021) definitions. The right (p=.008) and left (p=.003) superior temporal regions had smaller volumes in SCD+ than SCD−, but only with the ECog. SCD+ was associated with greater future cognitive decline measured by Alzheimer’s Disease Assessment Scale, but only with the CCI definition. In contrast, only the ECog definition of SCD was associated with future decline on the Montreal Cognitive Assessment.
Conclusion
These findings suggest that the various methods used to differentiate between SCD− and SCD+ influence whether volume differences and findings of cognitive decline are observed between groups in this retrospective analysis.
Keywords: Subjective cognitive decline, Magnetic resonance imaging, Deformation based morphometry, Cognitive decline, Alzheimer’s disease, Regional atrophy
1. Introduction
Alzheimer’s disease (AD) accounts for 50–75% of the 50 million people worldwide living with dementia (International Alzheimer's Disease, 2020). People with AD experience progressive declines in cognitive functioning, including memory and thinking, with symptoms interfering with daily functioning (Alzheimer’s Association, 2021). These symptoms may occur because of brain pathology, including excessive amyloid and tau build-up and neurodegeneration (Serrano-Pozo et al., 2011). This neuropathology may be present for many years before the onset of symptoms (Craig-Schapiro et al., 2009, Sperling et al., 2011). Much of the current AD research has been devoted to finding an early biomarker that can identify individuals with preclinical AD.
Individuals meeting criteria for preclinical AD may also report subjective cognitive decline (SCD), i.e., perceived deficits in memory and/or cognitive functioning in the absence of objective cognitive decline (Jessen et al., 2014), up to 15 years before the onset of AD symptoms (Reisberg et al., 2010). SCD increases the likelihood of progression to clinically probable AD by up to five times (Rabin et al., 2017) and people with SCD show increased neurodegenerative pathology compared to cognitively normal older adults without SCD. Nevertheless, results suggesting atrophy in people with SCD compared to those without SCD are inconsistent. For example, while Jessen et al. observed atrophy in the bilateral entorhinal cortex and not the hippocampus (Jessen et al., 2006), Striepens et al. reported reduced volume in both the bilateral entorhinal cortex and bilateral hippocampus in people with SCD relative to people without SCD (Striepens et al., 2010).
Different methods used to classify SCD may underly the conflicting results. Jessen et al. recruited participants who sought medical help for their self-reported feeling of worsening memory with an onset in the last 5 years (Jessen et al., 2006), while Striepens et al. recruited participants who had both self and informant confirmation of memory impairment within the last 10 years (Striepens et al., 2010). The latter may be closer to clinical decline as informant confirmation is a feature of SCD plus (people with SCD who have features that make them more likely to progress to AD) and might be a better predictor of objective performance as disease severity progresses (Amariglio et al., 2015, Rabin et al., 2017).
Inconsistencies in defining SCD have led to the development of the Subjective Cognitive Decline Initiative (SCD-I) (Jessen et al., 2020, Jessen et al., 2014). This group developed a broad definition of pre-MCI SCD for research, which includes normal performance on cognitive tests but self-experienced persistent decline in cognition compared to a previously normal status not explained by MCI/AD, psychiatric conditions, neurological diseases, medical disorders, medications, or substance abuse. Other important features that may improve SCD identification include the study setting, Apolipoprotein E (APOE) status, memory vs non-memory domain complaints, informant confirmation, and worry. Specific features that increase the likelihood of preclinical AD (SCD plus) include 1) SCD in memory (rather than other domains), 2) onset within the last 5 years, 3) age of onset ≥ 60 years, 4) worry/concern with SCD, and 5) feeling of worse performance than others of the same age.
Despite the SCD-I working groups’ recommendations (Jessen et al., 2020, Jessen et al., 2014), an SCD definition has not been universally implemented, resulting in a concern about the lack of standardization. A recent review shows that since the recommendations by the SCD-I were released, only five out of over forty studies examining structural changes in people with SCD identified SCD using the working group recommendations (see Wang et al., 2020 for review). The review by Wang et al. (2020) reports that some studies use specific questionnaires to define SCD, such as the Cognitive Change Index (CCI; Saykin et al., 2006), Everyday Cognition Scale (ECog; Farias et al., 2008), or Memory Assessment Clinics Questionnaire (MAC-Q; Crook et al., 1992) while others use one or two questions (e.g., do you have memory decline?; are you worried about this decline?), and some use memory clinic consultation to define people with SCD. Only moderate correlations have been found between the ECog and the Blessed Memory scale (van Harten et al., 2018) or between the MAC-Q and the Subjective Memory Complaints (SMC) scale (Vogel et al., 2016), suggesting that the questionnaires may be measuring different cognitive constructs leading to misidentification of people with SCD in both research and clinical settings.
The goal of the current study was to examine whether brain atrophy and future cognitive decline observed between cognitively normal older adults with and without SCD vary between four common methods of differentiating SCD. Many current findings report that cognitive healthy older adults with SCD show a pattern of volumetric atrophy similar to that of people with AD (Peter et al., 2014, Sánchez-Benavides et al., 2018). The most consistent atrophy differences observed between cognitive healthy older adults with and without SCD are observed in the hippocampus (Cantero et al., 2016, Rogne et al., 2016, Yue et al., 2018; see Wang et al., 2020 for review) especially in those who progress to MCI or AD (Verfaillie et al., 2016). Studies examining prediction of MCI and AD observed that the ventricles are sensitive to changes observed between normal aging and MCI (Chou et al., 2009) and the amygdala and superior temporal regions are of importance for progression from SCD to MCI (Yue et al., 2021). Based on these findings we looked specifically at the hippocampus, amygdala, lateral ventricles, and superior temporal regions as several studies have suggested that these regions may be sensitive to progression to MCI and AD in the cognitively healthy older adult population (Chou et al., 2009, Wang et al., 2020, Yue et al., 2021).
2. Methods
2.1. Alzheimer’s disease Neuroimaging Initiative
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public–private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). Participants were selected from the ADNI-2 cohort because ADNI2 was the first cohort to introduce the CCI questionnaire and define participants with significant memory concerns. For consistency with current research standards, we use the term subjective cognitive decline. Participants were between 55 and 90 years old at the time of recruitment. The study received ethical approval from the review boards of all participating institutions. Written informed consent was obtained from participants or their study partner.
2.2. Participants
While there are 420 cognitively normal controls in the ADNI-2 cohort, only 280 participants were included as they had an MRI scan within 6 months of a fully completed SCD questionnaire. The participants were separated into either cognitively normal controls without subjective cognitive decline (i.e., negative for subjective cognitive decline, or SCD−) or cognitively normal controls with subjective cognitive decline (i.e., positive for subjective cognitive decline, or SCD+; not to be confused with SCD plus as defined by Jessen et al. (2014). Neither group had objective evidence of cognitive impairment on cognitive tasks nor the Clinical Dementia Rating or any signs of depression. As four separate definitions of SCD were used to differentiate the participants into either the SCD− or SCD+, participants varied between each analysis as the criteria for SCD differed. Demographic information, by group, for each definition is presented in Table 1. The four SCD+ groups were defined as follows:
-
•
CCI: Participants were considered SCD+ if they had self-reported significant memory concern as quantified by a score of ≥ 16 on the first 12 items (representing memory changes) on the CCI. This score was selected based on previous research by Saykin et al. (2006) and because it is used by ADNI as a criterion to identify participants with significant memory concern (Risacher et al., 2015).
-
•
ECog: Participants were considered SCD+ if they endorsed any item on the ECog with a score ≥ 3. A score of ≥ 3 was used as it represents consistent SCD which has been shown to improve prognostic value of SCD for incident MCI (van Harten et al., 2018).
-
•
ECog + Worry: Participants were considered SCD+ if they had self-reported consistent SCD+ on any item from the ECog (again ≥ 3) as well as indicated worry/concern about their cognitive decline.
-
•
Worry: Participants were considered SCD+ if they indicated worry/concern about their memory/thinking abilities, irrespective of their CCI or ECog scores.
Table 1.
Demographic and clinical characteristics for cognitively normal older adults with and without subjective cognitive decline for the four definitions of SCD.
| Demographic information | CCI | ECog | ECog + Worry | Worry | ||||
|---|---|---|---|---|---|---|---|---|
| SCD− n = 176 |
SCD+ n = 97 |
SCD− n = 130 |
SCD+ n = 143 |
SCD− n = 177 |
SCD+ n = 96 |
SCD− n = 149 |
SCD+ n = 124 |
|
| Age | 73.50 ± 6.27 | 72.44 ± 5.59 | 72.56 ± 6.21 | 73.63 ± 5.86 | 72.94 ± 6.17 | 73.43 ± 5.84 | 72.97 ± 6.17 | 73.29 ± 5.93 |
| Education | 16.55 ± 2.54 | 16.77 ± 2.59 | 16.58 ± 2.65 | 16.67 ± 2.48 | 16.63 ± 2.57 | 16.63 ± 2.56 | 16.64 ± 2.52 | 16.61 ± 2.62 |
| AV-45 | 1.11 ± 0.18 | 1.13 ± 0.19 | 1.10 ± 0.16 | 1.13 ± 0.19 | 1.11 ± 0.18 | 1.14 ± 0.19 | 1.10 ± 0.17 | 1.14 ± 0.19 |
| APOE ε4 + | 51 (29%) | 29 (30%) | 38 (29%) | 42 (29%) | 54 (30%) | 26 (27%) | 44 (30%) | 36 (30%) |
| Amyloid Positivity | 61 (35%) | 42 (43%) | 46 (35%) | 57 (40%) | 59 (33%) | 44 (46%) | 49 (33%) | 54(44%) |
| Male sex | 86(48%) | 61 (41%) | 64 (49%) | 64 (45%) | 88(50%) | 38 (40%) | 83(51%) | 50(40%) |
| ADAS-13 | 9.05 ± 4.44 | 8.79 ± 4.23 | 8.79 ± 4.23 | 9.19 ± 4.19 | 8.93 ± 4.57 | 8.84 ± 3.77 | 8.99 ± 4.77 | 8.78 ± 3.67 |
| MoCA | 25.75 ± 2.37 | 25.65 ± 2.58 | 26.12 ± 2.35 | 25.36 ± 2.47* | 25.83 ± 2.37 | 25.50 ± 2.58 | 25.82 ± 2.36 | 25.58 ± 2.56 |
| MMSE | 29.02 ± 1.23 | 29.01 ± 1.22 | 29.12 ± 1.13 | 28.93 ± 1.30 | 29.06 ± 1.20 | 28.95 ± 1.28 | 29.08 ± 1.17 | 28.95 ± 1.30 |
Values are expressed as mean ± standard deviation, or number (percentage %). APOE ε4+, amyloid positivity, and male sex are represented as total number of sample and percentage of sample. Bold text used to highlight statistically significant differences between SCD− and SCD+ . CCI = Cognitive Change Index. ECog = Everyday Cognition Scale. SCD− = Cognitively normal older adults without subjective cognitive decline. SCD+ = cognitively normal older adults with subjective cognitive decline. ADAS-13 = Assessment Scale–Cognitive Subscale. MoCA = Montreal Cognitive Assessment. MMSE = Mini Mental Status Examination. *Represents the only significant difference between groups, a difference in MoCA score between SCD− and SCD+.
2.3. Structural MRI acquisition and processing
All participants were imaged using a 3T scanner with T1-weighted imaging parameters (see http://adni.loni.usc.edu/methods/mri-tool/mri-analysis/ for the detailed MRI acquisition protocol). Baseline scans were downloaded from the ADNI public website.
T1w scans for each participant were pre-processed through our standard pipeline including noise reduction (Coupé et al., 2008), intensity inhomogeneity correction (Sled et al., 1998), and intensity normalization into range [0–100]. The pre-processed images were then both linearly (9 parameters: 3 translation, 3 rotation, and 3 scaling) (Dadar et al., 2018) and nonlinearly (1 mm3 grid) (Avants et al., 2008) registered to the MNI-ICBM152-2009c average template using the CerbrA atlas (Manera et al., 2020). The quality of the linear and nonlinear registrations was visually verified by an experienced rater. Only seven did not pass this quality control step and were discarded.
2.4. DBM and SNIPE
DBM analysis was performed to measure local anatomical differences in the brain by estimating the Jacobian determinant of the inverse nonlinear deformation field as a proxy of atrophy (Avants et al., 2008). DBM was used because this method has increased sensitivity to subcortical atrophy compared to voxel-based morphometry (Scanlon et al., 2011). Scoring by Nonlocal Image Patch Estimator (SNIPE) was used to measure the extent of AD-related change in the hippocampus using the linearly registered preprocessed T1-weighted images (Coupé et al., 2012b). DBM and SNIPE methods have been are described in detail previously (Dadar et al., 2020b). SNIPE was employed because it has been shown to be sensitive to subtle changes in the brain in normal controls that volumetric measures are unable to detect (Coupé et al., 2012a, Coupé et al., 2015).
2.5. Statistical analysis
Analyses were performed using MATLAB R2019b. Independent sample t-tests were completed on the demographic information presented in Table 1. Volume and grading differences between SCD− and SCD+ participants were analyzed with logistic regressions with ROI volume as the dependent variable and controlled for age, sex, and education. Separate logistic regressions were run for the four separate SCD definitions for each ROI. These analyses were completed with and without APOE ε4 status and amyloid positivity because of their association with increased risk for AD (Rabin et al., 2017).
Linear mixed-effects models were conducted to examine whether SCD diagnosis would influence future cognitive scores. A total of 1421 time points for 273 participants were included for Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS-13) and 820 time points for 273 participants were available for Montreal Cognitive Assessment (MoCA) in the following model:
This model examined the association between CognitiveScore (measured by either ADAS-13 or MoCA) and Diagnosis. The categorical variable of interest was Diagnosis, indicated by SCD− or SCD+ status based on each definition. The models also included Time from baseline, sex, and years of education as covariates. Participant ID was included as a categorical random effect. All continuous variables were z-scored before being entered into the model. To express unit change over time of ADAS and MoCA scores, the estimate from the model was then multiplied by the standard deviation of the scores divided by the standard deviation of the time at baseline.
Linear regressions were also completed to examine whether SCD definition influenced amyloid levels between SCD− and SCD+. Amyloid status was not available for three participants, thus data from 270 participants was used in the model:
This model examined the association between Amyloid and Diagnosis. The categorical variable of interest was Diagnosis, indicated by SCD− or SCD+ status based on each definition. The model also included age and sex as covariates.
Based on previous research, we selected only 4 specific regions apriori (i.e., hippocampus, amygdala, ventricles, and superior temporal region) to be used when examining atrophy changes. For that reason, correction for multiple comparisons were not completed. The threshold of p < .05 was used to determine statistical significance.
3. Results
3.1. Demographics
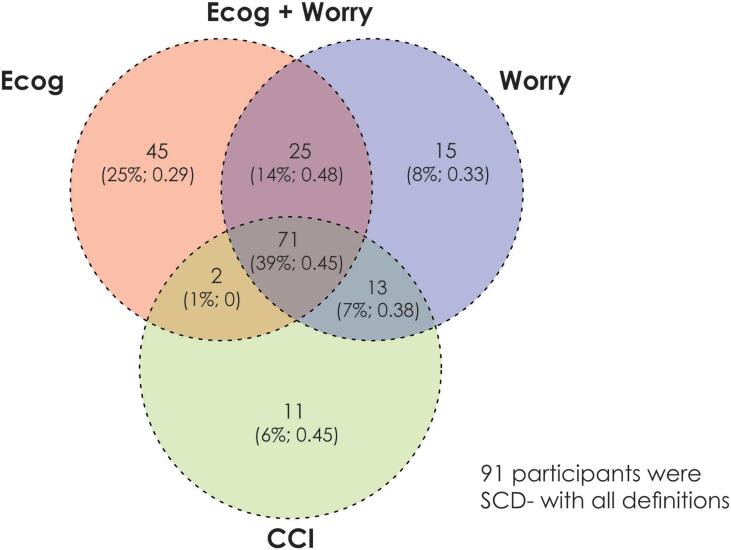
Table 1 shows the demographic information and clinical characteristics for all participants using the four SCD definitions. No statistically significant differences in mean age, education, or male:female ratio between groups were observed for any definition. Only one significant difference in clinical characteristics was observed between the groups. Using the ECog, those defined as SCD+ exhibited lower MoCA scores than SCD− at baseline. Fig. 1 displays a Venn diagram of the participant overlap between the different methods of defining SCD.
Fig. 1.
Venn Diagram representing the overlap of subjective cognitive decline (SCD) diagnosis between the four definitions of SCD. There was a total of 273 participants in the sample, 91/273 (33%) were SCD− with all definitions. The remaining 182 participants are shown in the Venn Diagram with the number of participants, the percentage of the overall SCD+ sample, and the fraction of the sample that was amyloid positive. Overall, there were 72/182 (40%) SCD participants that were amyloid positive. For the four SCD definitions, there were 97 SCD+ subjects defined by CCI, 143 SCD+ defined by ECog, 96 SCD+ defined by ECog &Worry, and 124 defined by Worry only. Finally, only 39% (n = 71) of the 182 SCD+ subjects are common between the four definitions.
Table 2 provides demographic information for the 91 participants who were SCD− with all definitions and the 71 participants that were SCD+ with all definitions. In this comparison the SCD− participants are very specific while the SCD+ participants are very sensitive. Despite this high sensitivity and specificity of groups, the demographic information between the participants did not differ (i.e., no significant differences in mean age, education, or male:female ratio). With regards to clinical characteristics, those who were SCD+ with all definitions had a higher amyloid positivity than those who were SCD− (45% of sample vs 35% of sample).
Table 2.
Demographic and clinical characteristics for older adults who were SCD− and SCD+ with all definitions.
| Demographic information |
All four definitions |
|
|---|---|---|
| SCD− n = 91 |
SCD+ n = 71 |
|
| Age | 72.75 ± 6.29 | 72.90 ± 5.70 |
| Education | 16.58 ± 2.67 | 16.73 ± 2.58 |
| AV-45 | 1.08 ± 0.15 | 1.13 ± 0.19 |
| APOE ε4+ | 24 (26%) | 20 (28%) |
| Amyloid Positivity | 31 (35%) | 42 (45%) |
| Male sex | 48(48%) | 61 (41%) |
| ADAS-13 | 8.77 ± 4.75 | 8.72 ± 3.86 |
| MoCA | 26.16 ± 2.37 | 25.45 ± 2.71 |
| MMSE | 29.14 ± 1.07 | 28.97 ± 1.33 |
Values are expressed as mean ± standard deviation, or number (percentage%). APOE ε4+, amyloid positivity, and male sex are represented as total number of sample and percentage of sample. SCD− = Cognitively normal older adults without subjective cognitive decline. SCD+ = cognitively normal older adults with subjective cognitive decline. ADAS-13 = Assessment Scale–Cognitive Subscale. MoCA = Montreal Cognitive Assessment. MMSE = Mini Mental Status Examination.
3.2. SNIPE analysis
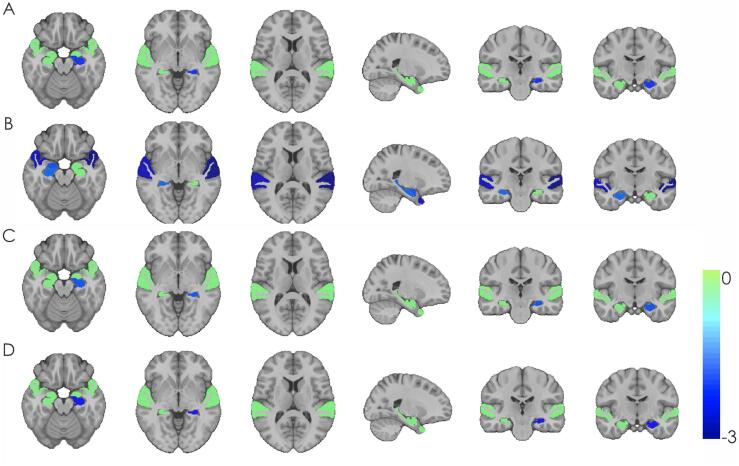
Table 3 summarizes the results of the logistic regression models for both SNIPE and DBM analysis. Fig. 2 shows significant t-statistic values obtained for the categorical diagnosis variable (SCD+ or SCD−) for only the regions tested (left/right hippocampus, amygdala, and superior temporal gyrus). Green regions indicate ROIs examined but not significantly different between the groups. There was an effect of diagnosis on grading in the left hippocampus for both the CCI definition of SCD (OR = 0.74, 95% CI = −0.60 – −0.01, p = .04) and Worry (OR = 0.72, 95% CI = −0.62 – −0.05, p = .02), with an approaching significant effect for ECog + Worry definition (OR = −0.27, 95% CI = −0.56–0.02, p = .065).
Table 3.
Logistic regression model results showing differences between cognitively healthy older adults with and without subjective cognitive decline.
| CCI | ECog | ECog + Worry | Worry Only | |
|---|---|---|---|---|
| SNIPE | ||||
| Grading rHC | ß=−0.03, t = −0.19, p = .85, OR = 0.97 | ß=−0.23 t = −1.69, p = .09, OR = 1.26 | ß=0.09 t = 0.65, p = .51, OR = 1.10 | ß=0.08 t = 0.57, p = .56 OR = 1.08 |
| Grading lHC | ß=−0.30, t = −2.04, p = .041, OR = 0.74 | ß=−0.13, t = −0.94, p = .34, OR = 0.87 | ß=−0.27, t = −1.84, p = .065, OR = 0.76 | ß=−0.33, t =−2.31, p = .021, OR = 0.72 |
| Volume rHC | ß=0.05, t = 0.41, p = .67, OR = 1.10 | ß=−0.01, t = −0.09, p = .93, OR = 1.02 | ß=0.06, t = 0.46, p = .64, OR = 1.06 | ß=−0.02, t = −0.19, p = .85, OR = 0.98 |
| Volume lHC | ß=0.15, t = 1.15, p = .25, OR = 1.16 | ß=−0.02, t = −0.15, p = .88, OR = 0.98 | ß=0.07, t = 0.54, p = .59, OR = 1.07 | ß=0.06, t = 0.47, p = .64, OR = 1.06 |
| DBM- Volume | ||||
| Right amygdala | ß=−0.11, t = −0.88, p = .38, OR = 0.89 | ß=−0.24, t = −1.85, p = .064, OR = 0.79 | ß=0.02, t = 0.16, p = .87, OR = 1.02 | ß=0.11, t = 0.96, p = .34, OR = 1.11 |
| Left amygdala | ß=0.02, t = 0.12, p = .90, OR = 1.02 | ß=−0.18, t = −1.42, p = .15, OR = 0.83 | ß=0.11, t = 0.92, p = .36, OR = 1.12 | ß=0.16, t = 1.43, p = .15, OR = 1.18 |
| Right lateral ventricle | ß=0.05, t = 0.39, p = .70, OR = 1.05 | ß=0.14, t = 1.07, p = .29, OR = 1.15 | ß=0.11, t = 0.95, p = .34, OR = 1.12 | ß=0.04, t = 0.38, p = .70, OR = 1.04 |
| Left lateral ventricle | ß=0.02, t = 0.17, p = .86, OR = 1.02 | ß=0.16, t = 1.20, p = .23, OR = 1.17 | ß=0.18, t = 1.56, p = .11, OR = 1.21 | ß=0.09 t = 0.86, p = .38, OR = 1.10 |
| Right superior temporal | ß=−0.15, t = −1.22, p = .22, OR = 0.86 | ß=−0.33, t =−2.64, p = .008, OR = 0.72 | ß=−0.14, t = −1.18, p = .24, OR = 0.87 | ß=0.03, t = 0.26, p = .79, OR = 1.03 |
| Left superior temporal | ß=−0.14, t = −1.10, p = .27, OR = 0.87 | ß=−0.39, t =−3.00, p = .003, OR = 0.68 | ß=−0.19, t = 1.58, p = .11, OR = 0.83 | ß=0.02, t = 0.15, p = .88, OR = 1.02 |
CCI = Cognitive Change Index. ECog = Everyday Cognition Scale. rHC = right hippocampus. lHC = left hippocampus. OR = Odds ratio. Bolded values represent either significant or approaching significant differences between SCD+ and SCD−.
Fig. 2.
Significant t-statistic values obtained for regions of interest for each definition of subjective cognitive decline. Significant t-statistic values obtained for the categorical diagnosis variable (Subjective Cognitive Decline; SCD+ and SCD−) for the four definitions of SCD for the six regions tested (left/right hippocampus, amygdala, and superior temporal gyrus). Green regions indicate ROIs that were examined but were not significantly different between the groups. Colder blue colors indicate lower DBM values in SCD+ compared to SCD−. A) Cognitive Change Index analysis – smaller left hippocampal grading in SCD+ vs. SCD−. B) Everyday Cognition Scale analysis – smaller right hippocampal grading, right amygdala, and right and left superior temporal regions in SCD+ vs. SCD−. C) Everyday Cognition Scale + Worry analysis – smaller left hippocampal grading in SCD+ vs. SCD−. D) Worry analysis – smaller left hippocampal grading in SCD+ vs. SCD−. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Atlas-based DBM analysis
There were few anatomical differences detected by DBM. Only the right (OR = 0.72, 95% CI = −0.58 – −0.09, p = .008) and left (OR = 0.68, 95% CI = −0.64 – −0.01, p = .003) superior temporal regions were influenced by diagnosis when using the ECog-based definition. A trending effect of diagnosis was also observed in the right amygdala (OR = 0.79, 95% CI = −0.49 – 0.01, p = .064) for the ECog analysis. No other structures were significantly different for SCD+ and SCD− groups using the ECog definition. No structures were found to be different using the other SCD definitions.
3.4. Amyloid & APOE status
As can be observed in Table 4 when amyloid positivity and APOE ε4 status were included in the models, the differences due to diagnosis on volume did not significantly change. Amyloid positivity was significantly associated with grading for all SNIPE analyses, except for the ECog definition. On the other hand, APOE status was not associated with volume change in any of the ROIs or SCD definitions. Levels of amyloid did not differ between SCD− and SCD+ in any of the analyses (see Table 5). APOE ε4+ status did not differ between SCD− and SCD+ populations or between SCD+ groups between the four definitions.
Table 4.
Logistic regression model results including APOE and amyloid status showing the differences between cognitively healthy older adults with and without subjective cognitive decline.
| CCI | ECog | ECog + Worry | Worry Only | |
|---|---|---|---|---|
| SNIPE | ||||
| Grading rHC |
ß=−0.007, t = −0.05, p = .96, OR = 0.99 Amyloid: ß=0.50, t = 1.78, p = .075, OR = 1.65 APOE: ß=−0.23, t = −0.76, p = .44, OR = 0.80 |
ß=0.24, t = 1.76, p = .078, OR = 1.28 Amyloid: ß=0.21, t = 0.79, p = .43, OR = 1.24 APOE: ß=0.01, t = 0.23, p = .98, OR = 1.01 |
ß=0.12, t = 0.81, p = .42, OR = 1.13 Amyloid: ß=0.64, t = 2.28, p = .022, OR = 1.90 APOE: ß=−0.35, t = −1.14, p = .26, OR = 0.70 |
ß=0.10, t = 0.72, p = .47, OR = 1.10 Amyloid: ß=0.50, t = 1.87, p = .061, OR = 1.66 APOE: ß=−0.16, t = −0.54, p = .59, OR = 0.86 |
| Grading lHC |
ß=−0.30, t = −1.98, p = .047, OR = 0.74 Amyloid: ß=0.48, t = 1.72, p = .085, OR = 1.62 APOE: ß=−0.23, t = −0.75, p = .45. OR = 0.80 |
ß=−0.13, t = −0.92, p = .36, OR = 0.88 Amyloid: ß=0.17, t = 0.63, p = .53, OR = 1.18 APOE: ß=−0.01, t = −0.05, p = .96, OR = 0.99 |
ß=−0.27, t = 1.78, p = .075, OR = 0.77 Amyloid: ß=0.61, t = 2.16, p = .030, OR = 1.83 APOE: ß=−0.35, t = −1.15, p = .25, OR = 0.70 |
ß=−0.33, t =−2.26, p = .024, OR = 0.72 Amyloid: ß=0.48, t = 1.76, p = .078, OR = 1.61 APOE: ß=−0.17, t = −0.57, p = .57, OR = 0.85 |
| Volume rHC |
ß=0.04, t = 0.33, p = .74, OR = 1.04 Amyloid: ß=0.50, t = 1.78, p = .075, OR = 1.64 APOE: ß=−0.22, t = −0.73, p = .46, OR = 0.80 |
ß=−0.01, t = −0.10, p = .91, OR = 0.99 Amyloid: ß=0.18, t = 0.66, p = .50, OR = 1.19 APOE: ß=−0.01, t = −0.05, p = .96, 0.99 |
ß=0.05, t = 0.36, p = .72, OR = 1.05 Amyloid: ß=0.62, t = 2.21, p = .027, OR = 1.85 APOE: ß=−0.35, t = −1.13, p = .26, OR = 0.71 |
ß=−0.03, t = −0.26, p = .79, OR = 0.97 Amyloid: ß=0.49, t = 1.83, p = .067, OR = 1.63 APOE: ß=−0.17, t = −0.58, p = .56, OR = 0.85 |
| Volume lHC |
ß=0.14, t = 1.12, p = .26, OR = 1.16 Amyloid: ß=0.49, t = 1.77, p = .076, OR = 1.64 APOE: ß=−0.22, t = −0.74, p = .46, OR = 0.80 |
ß=−0.02, t = −0.16, p = .87, OR = 0.98 Amyloid: ß=0.18, t = 0.66, p = .51, OR = 1.19 APOE: ß=−0.13, t = −0.04, p = .96, OR = 0.99 |
ß=0.06, t = 0.49, p = .62, OR = 1.07 Amyloid: ß=0.62, t = 2.21, p = .027, OR = 1.86 APOE: ß=−0.35, t = −1.14, p = .25, OR = 0.70 |
ß=0.06, t = 0.44, p = .66, OR = 1.06 Amyloid: ß=0.49, t = 1.82, p = .069, OR = 1.63 APOE: ß=−0.16, t = −0.55, p = .58, OR = 0.85 |
| DBM | ||||
| Right amygdala |
ß=−0.11, t = −0.82, p = .41, OR = 0.90 Amyloid: ß=0.41, t = 1.48, p = .13, OR = 1.51 APOE: ß=−0.10, t = −0.35, p = .73, OR = 0.90 |
ß=−0.23, t = −1.83, p = .067, OR = 0.79 Amyloid: ß=0.13, t = 0.47, p = .64, OR = 1.13 APOE: ß=−0.02, t = −0.08, p = .94, OR = 0.98 |
ß=0.02, t = 0.18, p = .86, OR = 1.02 Amyloid: ß=0.13, t = 0.52, p = .60, OR = 1.14 APOE: ß=−0.47, t = −1.72, p = .085, OR = 0.62 |
ß=0.10, t = 0.94, p = .35, OR = 1.11 Amyloid: ß=−0.01, t = −0.06, p = .95, OR = 0.99 APOE: ß=−0.31, t = −1.23, p = .21, OR = 0.74 |
| Left amygdala |
ß=0.03, t=0.23, p=.82, OR=1.03 Amyloid: ß=0.42, t=1.52, p=.13, OR=1.52 APOE: ß=−0.10, t=−0.33, p=.74, OR=0.91 |
ß=−0.17, t=−1.39, p=.16, OR=0.84 Amyloid: ß=0.12, t=0.45, p=.66, OR=1.13 APOE: ß=−0.02, t=−0.06, p=.96, OR=0.98 |
ß=0.12, t=0.97, p=.33, OR=1.13 Amyloid: ß=0.15, t=0.58, p=.56, OR=1.16 APOE: ß=−0.48, t=−1.75, p=.081, OR=0.62 |
ß=0.16, t=1.43, p=.15, OR=1.18 Amyloid: ß=−0.01, t=−0.02, p=.98, OR=1.00 APOE: ß=−0.31, t=−1.26, p=.21, OR=0.73 |
|---|---|---|---|---|
| Right lateral ventricle |
ß=0.04, t=0.30, p=.76, OR=1.04 Amyloid: ß=0.41, t=1.48, p=.14, OR=1.51 APOE: ß=−0.09, t=−0.30, p=.77, OR=0.91 |
ß=0.13, t=1.05, p=.29, OR= 1.15 Amyloid: ß=0.12, t=0.46, p=.64, OR=1.13 APOE: ß=−0.02, t=−0.07, p=.95, OR=1.02 |
ß=0.10, t=0.80, p=.43, OR=1.10 Amyloid: ß=0.12, t=0.49, p=.62, OR=1.13 APOE: ß=−0.45, t=−1.65, p=.10, OR=0.63 |
ß=0.03, t=0.27, p=.79, OR=1.03 Amyloid: ß=−0.03, t=−0.12, p=.90, OR=0.97 APOE: ß=−0.30, t=−1.19, p=.23, OR=0.74 |
| Left lateral ventricle |
ß=0.01, t=0.10, p=.91, OR=1.01 Amyloid: ß=0.42, t=1.50, p=.13, OR=1.51 APOE: ß=−0.10, t=−0.32, p=.75, OR=0.91 |
ß=0.16 t=1.20, p=.23, OR=1.17 Amyloid: ß=0.13, t=0.47, p=.64, OR=1.13 APOE: ß=0.03, t=0.09, p=.93, OR=1.03 |
ß=0.17 t=1.39, p=.16, OR=1.18 Amyloid: ß=0.12, t=0.48, p=.63, OR=1.13 APOE: ß=−0.44, t=−1.58, p=.12, OR=0.65 |
ß=0.08 t=0.74, p=.46, OR=1.09 Amyloid: ß=−0.03, t=−0.13, p=.89, OR=0.97 APOE: ß=−0.28, t=−1.15, p=.25, OR=0.75 |
| Right superior temporal |
ß=−0.15, t=−1.21, p=.23, OR=0.86 Amyloid: ß=0.41, t=1.50, p=.13, OR=1.51 APOE: ß=−0.09, t=−0.30, p=.77, OR=0.92 |
ß=−0.33, t=−2.64, p=.008, OR=0.72 Amyloid: ß=0.14, t=0.53, p=.60, OR=1.15 APOE: ß=0.01, t=0.04, p=.97, OR=1.01 |
ß=−0.13, t=−1.19, p=.23, OR=0.87 Amyloid: ß=0.11, t=0.44, p=.65, OR=1.21 APOE: ß=−0.47, t=−1.72, p=.085, OR=0.62 |
ß=0.02, t=0.23, p=.82, OR=1.03 Amyloid: ß=−0.03, t=−0.11, p=.91, OR=0.97 APOE: ß=−0.30, t=−1.21, p=.22, OR=0.74 |
| Left superior temporal |
ß=−0.14, t=−1.04, p=.30, OR=0.87 Amyloid: ß=0.39, t=1.44, p=.15, OR=1.49 APOE: ß=−0.06, t=−0.19, p=.85, OR=0.94 |
ß=−0.39, t=−3.00, p=.002, OR=0.68 Amyloid: ß=0.10, t=0.36, p=.72, OR=1.10 APOE: ß=0.11, t=0.37, p=.71, OR=1.11 |
ß=−0.18, t=−1.51, p=.13, OR=0.83 Amyloid: ß=0.11, t=0.44, p=.65, OR=1.12 APOE: ß=−0.45, t=−1.66, p=.097, OR=0.63 |
ß=0.02, t=0.19, p=.85, OR=1.02 Amyloid: ß=−0.03, t=−0.11, p=.91, OR=0.97 APOE: ß=−0.30, t=−1.22, p=.22, OR=0.74 |
CCI = Cognitive Change Index. ECog = Everyday Cognition Scale. rHC = right hippocampus. lHC = left hippocampus. Bolded values represent either significant or approaching significant differences between SCD+ and SCD−.
Table 5.
Linear regression model results showing the group differences in AV-45 between SCD− and SCD+.
| Analysis 1–CCI | Analysis 2–ECog | Analysis3–ECog + Worry | Analysis 4–Worry Only | |
|---|---|---|---|---|
| Intercept | ß=0.71, SE = 0.13, t = 5.41, p < .001 | ß=0.71, SE = 0.13, t = 5.56, p < .001 | ß=0.71, SE = 0.13, t = 5.43, p < .001 | ß=0.71, SE = 0.13, t = 5.41, p < .001 |
| Group | ß=0.13, SE = 0.02, t = 0.62, p = .54 | ß=0.02, SE = 0.02, t = 1.13, p = .26 | ß=0.02, SE = 0.02, t = 0.92, p = .35 | ß=0.03, SE = 0.02, t = 1.39, p = .17 |
| Sex- Male | ß=−0.09, SE = 0.02, t =−4.14, p < .001 | ß=−0.09, SE = 0.02, t =−4.14, p < .001 | ß=−0.09, SE = 0.02, t =−4.15, p < .001 | ß=−0.09, SE = 0.02, t =−4.08, p < .001 |
| Age | ß=0.01, SE < 0.01, t = 3.40, p < .001 | ß=0.01, SE < 0.01, t = 3.26, p < .001 | ß=0.01, SE < 0.01, t = 3.40, p < .001 | ß=0.01, SE < 0.01, t = 3.38, p < .001 |
CCI = Cognitive Change Index. ECog = Everyday Cognition Scale. Bolded values represent significant differences between SCD+ and SCD−.
3.5. Cognitive Follow-up
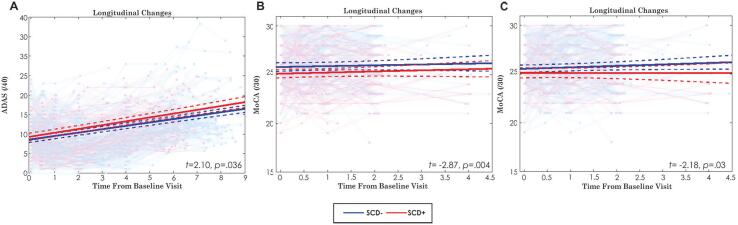
Fig. 3 displays the cognitive scores over time for ADAS13 and MoCA. For the ADAS, the only significant difference was observed when using the CCI definition of SCD (ß = 0.18, SE = 0.09, t = 2.10, p = .036), indicating SCD+ individuals had 0.40 units more of ADAS13 compared SCD−. The model also revealed a significant effect of Age (ß = 0.33, SE = 0.04, t = 7.86, p < .001), Sex (ß = 0.35, SE = 0.09, t = 3.98, p < .001), and Education (ß = −0.09, SE = 0.04, t = −2.23, p = .03). That is, with every year of increased age, ADAS13 increases by 0.73, there was a 0.77-unit difference between males and females, and every year of education decreases ADAS13 scores by 0.20 units. The interaction between Time from Baseline and Diagnosis was not significant (t = 1.31, p = .19). No other definitions of SCD were associated with cognitive change as measured by the ADAS13.
Fig. 3.
Longitudinal cognitive change by group for ADAS and MoCA. This figure shows the only significant differences in cognitive score for each definition. Longitudinal clinical change of each participant group as well as the group change over time is presented in each image. Red lines = SCD+; Blue lines = SCD−. A) Longitudinal change of ADAS scores when defining SCD− and SCD+ based on the CCI. B) Longitudinal change of Montreal Cognitive Assessment (MoCA) scores when defining SCD− and SCD+ based on ECog; C) Longitudinal change of MoCA scores when defining SCD− and SCD+ based on ECog + Worry; (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Longitudinal change in the MoCA was observed for the ECog definition of SCD (ß = −0.27, SE = 0.09, t = −2.87, p = .004). SCD+ individuals had 0.77 units less of MoCA than those who are SCD−. This model also revealed a significant effect of Age (ß = −0.28, SE = 0.05, t = −6.03, p < .001), Sex (ß = −0.31, SE = 0.10, t = −3.32, p = .001), and Education (ß = 0.14, SE = 0.05, t = 2.87, p = .004). The interaction between Time from Baseline and Diagnosis was not significant (|t| < 1, p = .87). Every year of increased age reduces the MoCA by 0.80 units, there was a difference of 0.88 units between males and females, and every year of education increases MoCA by 0.40 units.
The ECog + Worry definition of SCD also revealed a significant group effect (ß = −0.22, SE = 0.10, t = −2.18, p = .03). That is, SCD+ individuals had 0.63 units less of MoCA than those who are SCD−. Similar to the ECog results, this model also revealed a significant effect of Age (ß = −0.29, SE = 0.05, t = −5.94, p < .001), Sex (ß = −0.34, SE = 0.10, t = −3.39, p < .001), and Education (ß = 0.14, SE = 0.05, t = 2.90, p = .004). The interaction between Time from Baseline and Diagnosis was not significant (t = −1.10, p = .27). Every year of increased age reduces MoCA by 0.83 units, there was a difference of 0.97 units between males and females, and every year of education increases MoCA by 0.40 units. The CCI and Worry definition of SCD was not significantly associated with MoCA scores.
4. Discussion
Despite the SCD-I working group report (Jessen et al., 2014), many current studies use widely discrepant methodologies for defining SCD, resulting in inconsistent findings. This inconsistency makes generalizations difficult and may contribute to contradictory results in the current literature examining the associations between SCD, brain changes, and cognitive decline. The current study compares four common methods of defining SCD. Table 1 shows that the four definitions do not separate the participants differently by demographic variables (age, education, sex), biomarker variables (AV-45, APOE ε4+, amyloid positivity) or cognition as measured by ADAS013, MoCA or MMSE at baseline with one exception. The ECog definition separates SCD− from SCD+ for baseline MoCA (p < 0.05, uncorrected), but this difference is no longer significant when the 36 multiple comparisons are corrected for.
This study investigated three main questions: 1) What is the overlap of SCD+ participants using four different SCD definitions? 2) do SCD− and SCD+ atrophy differences vary depending on SCD definition? and 3) are longitudinal cognitive trajectories of SCD− and SCD+ populations different between four definitions of SCD? We observed that these four methods do not categorize older adults similarly, as there was little overlap between the four definitions (see Fig. 1). Both atrophy differences and longitudinal cognitive trajectories between SCD− and SCD+ vary depending on the method used to define SCD.
First, only 39% of the SCD+ participants were common between the four definitions. This finding suggests that there are major inconsistencies in who is identified as having SCD between these methods, which may lead to difficulties when a clinician is attempting to determine which measure to use to predict/screen for AD or future cognitive decline. When early treatment becomes available, clinicians could miss treatment for some individuals at higher risk of AD while providing unnecessary treatment to others. The use of different questionnaires may introduce further inconsistencies across care providers and clinics.
Second, we observed SCD definition dependent SCD−:SCD+ regional volume differences. Hippocampal volume was smaller in SCD+ than SCD− when using both the CCI and Worry and approached significance for the ECog + Worry definition. Such decreased hippocampal volume is consistent with other SCD studies that included worry (Scheef et al., 2012), and memory clinic consultation (Kim et al., 2013, Mollica et al., 2017). However, we did not find hippocampal volume group differences when defining SCD+ with the ECog. This finding is also consistent with several studies that did not report hippocampal atrophy in people with SCD who used multiple questionnaires (Saykin et al., 2006, Shen et al., 2010). The ECog was sensitive to SCD−:SCD+ group differences in the right and left superior temporal regions not observed with the other three definitions. A previous study observed that enlarged white matter at the banks of the superior temporal sulcus was associated with increased progression from SCD to MCI over 7 years (Yue et al., 2021). It is thus possible that ECog may be sensitive to early decline several years in advance of overt symptoms. These differences provide insight into how different definitions of SCD may influence regional atrophy differences observed between SCD+ and SCD−.
Third, we found different cognitive trajectories between the SCD+ groups using the four definitions. While an association between the CCI and future cognitive decline as measured by the ADAS-13 was found, the CCI was not associated with cognitive change on the MoCA. On the other hand, the ECog definition of SCD was associated with a decline on the MoCA, but not on the ADAS-13. The Worry definition was not associated with future cognitive decline as measured by either the ADAS-13 or MoCA. This finding is further supported by a less significant effect observed for the association between ECog + Worry and MoCA scores than for the association between ECog and MoCA; suggesting that the ECog definition is driving the association between SCD and cognitive change for MoCA scores. The differences in cognitive change observed between the definitions may be associated with a combination of the cognitive test sensitivity to detect cognitive decline early in the AD trajectory and the sensitivity of the definitions to classify SCD associated with AD. It is possible that the SCD questionnaires may not all be associated with future AD progression and are sensitive to other forms of decline.
The mixed group volume differences and cognitive trajectories between SCD− and SCD+ observed between the four definitions of SCD suggest these methods may measure different constructs or types of SCD. This hypothesis is further supported with a participant overlap of <40% between the four SCD definitions and the different patterns of atrophy in the four SCD+ groups. The CCI and Worry methods revealed hippocampal volume declines in SCD+ and thus may tap into subjective cognitive declines involved with the left hippocampus such as verbal memory deficits (Ezzati et al., 2016). Hippocampal declines are observed early in AD disease progression. Therefore, the CCI and Worry methods may be sensitive to SCD related to AD. When also taking into consideration the cognitive trajectories of CCI and Worry, only the CCI was associated with future cognitive decline, suggesting this questionnaire may be the most sensitive (of the four measures evaluated here) to preclinical AD.
Reduced volume in the superior temporal region was observed in SCD+ relative to SCD− but only using the ECog definition. Thus, the ECog may be sensitive to cognitive decline related to the superior temporal regions such as episodic memory coding, language comprehension, and speech processing (Wang et al., 2016, Yi et al., 2019), Although atrophy in the superior temporal region has been observed as a sensitive factor for future cognitive decline, this region is not a key component underlying AD-related atrophy. It is possible that the ECog is sensitive to cognitive decline (as measured by MoCA) associated with dementia other than AD. Taken together, the ECog and CCI may be sensitive to different subtypes of SCD. Another recent study also identified multiple SCD subgroups, each characterized by unique patterns of brain atrophy (Diaz-Galvan et al., 2021).
All the methods of defining SCD in this sample had a similar ratio of amyloid positivity, amyloid levels, and APOE ε4+ in the participants. According to the recent National Institute on Aging Alzheimer’s Association (NIA-AA), SCD is part of the transitional “Stage 2” of AD (Jack et al., 2018). In this stage, people should exhibit pathological tau and Aß markers. In the current study, none of the methods used to define SCD resulted in SCD+ groups with higher levels of pathology when compared to the corresponding SCD− groups. This finding suggests that although the CCI appears to be sensitive to AD-related atrophy and cognitive decline, it may not be sensitive or specific in identifying tau and amyloid pathology.
It should also be mentioned that the image processing employed in this study (i.e., DBM and SNIPE) has been developed and extensively validated for use in multi-center and multi-scanner studies. These processing methods have shown patterns of atrophy in cognitively normal, MCI, dementia, and neurodegenerative disease populations, including ADNI (Dadar et al., 2020a, Dadar et al., 2020b, Manera et al., 2019, Zandifar et al., 2020). These techniques thus have the required sensitivity to reveal group differences between SCD− and SCD+ . Thus, the lack of SCD−:SCD+ differences observed in this study is not the result of image processing methods that are not sensitive to observe group differences.
There are a few limitations of the current study. We used cross-sectional MRI data to predict eventual cognitive decline. Future research should use a longitudinal design to determine if the conversion rate to MCI from SCD varies in the assigned groups with all four definitions. This type of study would not only help differentiate between the subtypes of SCD, but would also improve our understanding of how the questionnaires may be associated with regional atrophy in SCD+ vs. SCD−. In the current dataset, the CCI was only administered at screening and thus a follow-up using a longitudinal design with the CCI was not possible. Another limitation of the current study is the use of a population-based cohort. This study design is a limitation because people who seek medical help (i.e., memory clinic consultation) for memory concerns show more hippocampal atrophy than those who do not seek help (Perrotin et al., 2017) and may be more likely to convert to MCI (van Harten et al., 2018). Within our sample, all our participants’ education levels were quite high, thus limiting extrapolation to more representative populations. Atrophy, demographic, and clinical characteristics between the different definitions may have been observable if we were able to examine the participants that were only SCD+ with each definition (i.e., 11 SCD+ with CCI, 15 SCD+ with worry, and 45 SCD+ with ECog). However, because of the limited participant sample size with each definition we were unable to compare the participants from these small groups and the 71 SCD− participants with sufficient power.
5. Conclusion
The current study compared four commonly used methods of defining SCD in the same sample of participants to demonstrate that regional atrophy and future cognitive decline observed in SCD+ populations depend on the SCD definition used. These findings have significant implications for both clinical and research settings where it is crucial to use a definition that has high sensitivity and specificity to identify individuals in the preclinical stages of AD. Misidentifying people who will progress to AD reduces the likelihood of researchers finding an early biomarker for AD. This misidentification also reduces the chances of clinicians providing effective treatments to slow or stop AD progression. Future research needs to determine which questionnaire is sensitive to neurodegeneration, future cognitive decline, and tau and amyloid pathology to be successful at accurately predicting who will progress to AD.
CRediT authorship contribution statement
Cassandra Morrison: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Mahsa Dadar: Conceptualization, Methodology, Formal analysis, Writing – review & editing. Neda Shafiee: Methodology, Formal analysis, Writing – review & editing. Sylvia Villeneuve: Conceptualization, Supervision, Writing – review & editing. D. Louis Collins: Conceptualization, Methodology, Supervision, Funding acquisition, Writing – review & editing.
Acknowledgments
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Funding information
This research was supported by a grant from the Canadian Institutes of Health Research.
Financial disclosures
Dr. Morrison is supported by a postdoctoral fellowship from Canadian Institutes of Health Research, Funding Reference Number: MFE-176608.
Neda Shafiee reports no disclosures.
Dr. Dadar is supported by a scholarship from the Canadian Consortium on Neurodegeneration in Aging as well as an Alzheimer Society Research Program (ASRP) postdoctoral award. The Consortium is supported by a grant from the Canadian Institutes of Health Research with funding from several partners including the Alzheimer Society of Canada, Sanofi, and Women’s Brain Health Initiative.
Neda Shafiee has no conflicts of interest to declare.
Dr. Villeneuve reports work supported by a Canada Research Chair, a Canadian Institutes of Health Research Foundation Grant, a Canada Fund for Innovation Grant, an Alzheimer’s Association Grant, and an Alzheimer’s society of Canada and Fonds de recherche Sante Quebec fellowship.
Dr. Collins reports receiving research funding from Canadian Institutes of Health Research, the Canadian National Science and Engineering Research Council, Brain Canada, the Weston Foundation, and the Famille Louise & André Charron.
References
- Amariglio R.E., Donohue M.C., Marshall G.A., Rentz D.M., Salmon D.P., Ferris S.H., Karantzoulis S., Aisen P.S., Sperling R.A. Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer’s Disease Cooperative Study Cognitive Function Instrument. JAMA Neurol. 2015;72(4):446. doi: 10.1001/jamaneurol.2014.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association, 2021. What is Alzheimer’s disease. Retrieved from https://www.alz.org/alzheimers-dementia/what-is-alzheimers.
- Avants B.B., Epstein C.L., Grossman M., Gee J.C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero J.L., Iglesias J.E., Van Leemput K., Atienza M. Regional hippocampal atrophy and higher levels of plasma amyloid-beta are associated with subjective memory complaints in nondemented elderly subjects. J. Gerontol. Ser. A: Biomed. Sci. Med. Sci. 2016;71(9):1210–1215. doi: 10.1093/gerona/glw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y.Y., Leporé N., Avedissian C., Madsen S.K., Parikshak N., Hua X., Shaw L.M., Trojanowski J.Q., Weiner M.W., Toga A.W., Thompson P.M. Mapping correlations between ventricular expansion and CSF amyloid and tau biomarkers in 240 subjects with Alzheimer’s disease, mild cognitive impairment and elderly controls. Neuroimage. 2009;46(2):394–410. doi: 10.1016/j.neuroimage.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupé P., Fonov V., Bernard C., Zandifar A., Eskildsen S.F., Helmer C., Manjón J.V., Amieva H., Dartigues J.F., Allard M., Catheline G., Collins D.L. Detection of Alzheimer’s disease signature in MR images seven years before conversion to dementia: Toward an early individual prognosis. Hum. Brain Mapp. 2015;36(12):4758–4770. doi: 10.1002/hbm.22926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupé P., Eskildsen S.F., Manjón J.V., Fonov V.S., Collins D.L., Alzheimer's Disease Neuroimaging Initiative Simultaneous segmentation and grading of anatomical structures for patient's classification: application to Alzheimer's disease. NeuroImage. 2012;59(4):3736–3747. doi: 10.1016/j.neuroimage.2011.10.080. [DOI] [PubMed] [Google Scholar]
- Coupé, P., Eskildsen, S. F., Manjón, J. V., Fonov, V. S., Pruessner, J. C., Allard, M., et al., 2012. Scoring by nonlocal image patch estimator for early detection of Alzheimer's disease. NeuroImage: Clin. 1(1), 141–152. https://doi.org/10.1016/j.nicl.2012.10.002. [DOI] [PMC free article] [PubMed]
- Coupé P., Yger P., Prima S., Hellier P., Kervrann C., Barillot C. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans. Med. Imaging. 2008;27(4):425–441. doi: 10.1109/TMI.2007.906087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig-Schapiro R., Fagan A.M., Holtzman D.M. Biomarkers of Alzheimer's disease. Neurobiol. Disease. 2009;35(2):128–140. doi: 10.1016/j.nbd.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook T.H., Feher E.P., Larrabee G.J. Assessment of memory complaint in age-associated memory impairment: the MAC-Q. Int. Psychogeriatr. 1992;4(2):165–176. doi: 10.1017/S1041610292000991. [DOI] [PubMed] [Google Scholar]
- Dadar M., Camicioli R., Duchesne S., Collins D.L., Alzheimer's Disease Neuroimaging Initiative The temporal relationships between white matter hyperintensities, neurodegeneration, amyloid beta, and cognition. Alzheimer's & Dementia: Diagnosis Assessment Disease Monitor. 2020;12(1):e12091. doi: 10.1002/dad2.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar M., Fonov V.S., Collins D.L., Alzheimer’s Disease Neuroimaging Initiative A comparison of publicly available linear MRI stereotaxic registration techniques. Neuroimage. 2018;174:191–200. doi: 10.1016/j.neuroimage.2018.03.025. [DOI] [PubMed] [Google Scholar]
- Dadar Mahsa, Gee Myrlene, Shuaib Ashfaq, Duchesne Simon, Camicioli Richard. Cognitive and motor correlates of grey and white matter pathology in Parkinson’s disease. NeuroImage: Clin. 2020;27:102353. doi: 10.1016/j.nicl.2020.102353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Galvan P., Ferreira D., Cedres N., Falahati F., Hernández-Cabrera J.A., Ames D., Westman E. Comparing different approaches for operationalizing subjective cognitive decline: impact on syndromic and biomarker profiles. Sci. Rep. 2021;11(1):1–15. doi: 10.1002/alz.040982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati A., Katz M.J., Zammit A.R., Lipton M.L., Zimmerman M.E., Sliwinski M.J., Lipton R.B. Differential association of left and right hippocampal volumes with verbal episodic and spatial memory in older adults. Neuropsychologia. 2016;93:380–385. doi: 10.1016/j.neuropsychologia.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias, S.T., Mungas, D., Reed, B.R., Cahn-Weiner, D., Jagust, W., Baynes, K., DeCarli, C., 2008. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology, 22(4), 531. https://dx.doi.org/10.1037%2F0894-4105.22.4.531. [DOI] [PMC free article] [PubMed]
- International Alzheimer's Disease, 2020. Dementia Statistics. Retrieved from https://www.alzint.org/about/dementia-facts-figures/dementia-statistics/.
- Jack C.R., Jr, Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., Silverberg N. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimer's Dementia. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018.NIA-AA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F., Amariglio R.E., Van Boxtel M., Breteler M., Ceccaldi M., Chételat G., Subjective Cognitive Decline Initiative (SCD-I) Working Group A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimer's Dementia. 2014;10(6):844–852. doi: 10.1016/j.jalz.2014.01.001.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F., Amariglio R.E., Buckley R.F., van der Flier W.M., Han Y., Molinuevo J.L., Rabin L., Rentz D.M., Rodriguez-Gomez O., Saykin A.J., Sikkes S., Smart C.M., Wolfsgruber S., Wagner M. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19(3):271–278. doi: 10.1016/S1474-4422(19)30368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F., Feyen L., Freymann K., Tepest R., Maier W., Heun R., Schild HH., Scheef L. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol. Aging. 2006;27(12):1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Kim, M.J., Seo, S.W., Kim, G.H., Kim, S.T., Lee, J.M., Qiu, A., Na, D.L., 2013. Less depressive symptoms are associated with smaller hippocampus in subjective memory impairment. Arch. Gerontol. Geriatr. 57(1), 110–115. https://doi.org/http://dx.doi.org/10.1016/j.archger.2013.01.005. [DOI] [PubMed]
- Manera, A.L., Dadar, M., Collins, D.L., Ducharme, S., Frontotemporal Lobar Degeneration Neuroimaging Initiative, 2019. Deformation based morphometry study of longitudinal MRI changes in behavioral variant frontotemporal dementia. Neuroimage: Clin. 24, 102079. https://doi.org/10.1016/j.nicl.2019.102079. [DOI] [PMC free article] [PubMed]
- Manera A.L., Dadar M., Fonov V., Collins D.L. CerebrA, registration and manual label correction of Mindboggle-101 atlas for MNI-ICBM152 template. Sci. Data. 2020;7(1):1–9. doi: 10.1038/s41597-020-0557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica M.A., Navarra J., Fernández-Prieto I., Olives J., Tort A., Valech N., Coll-Padrós N., Molinuevo J.L., Rami L. Subtle visuomotor difficulties in preclinical Alzheimer’s disease. J. Neuropsychol. 2017;11(1):56–73. doi: 10.1111/jnp.12079. [DOI] [PubMed] [Google Scholar]
- Perrotin A., La Joie R., La Sayette V., Barré L., Mézenge F., Mutlu J., Guilloteau D., Egret S., Eustache F., Chételat G. Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: differential affective and imaging correlates. Alzheimer’s Dementia. 2017;13(5):550–560. doi: 10.1016/j.jalz.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Peter J., Scheef L., Abdulkadir A., Boecker H., Heneka M., Wagner M., Koppara A., Klöppel S., Jessen F. Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimer’s Dementia. 2014;10(1):99–108. doi: 10.1016/j.jalz.2013.05.1764. [DOI] [PubMed] [Google Scholar]
- Rabin L.A., Smart C.M., Amariglio R.E. Subjective cognitive decline in preclinical Alzheimer’s disease. Ann. Rev. Clin. Psychol. 2017;13(1):369–396. doi: 10.1146/clinpsy.2017.13.issue-110.1146/annurev-clinpsy-032816-045136. [DOI] [PubMed] [Google Scholar]
- Reisberg B., Shulman M.B., Torossian C., Leng L., Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimer's Dementia. 2010;6(1):11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risacher S., Kim S., Nho K., Foroud T., Shen L., Petersen R., Jack C., Jr., Beckett L., Aisen P., Koeppe R., Jagust W. APOE effect on Alzheimer’s disease biomarkers in older adults with significant memory concern. Alzheimer’s & Dementia. 2015;11(12):1417–1429. doi: 10.1016/j.jalz.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogne S., Vangberg T., Eldevik P., Wikran G., Mathiesen E.B., Schirmer H. Magnetic resonance volumetry: prediction of subjective memory complaints and mild cognitive impairment, and associations with genetic and cardiovascular risk factors. Dementia Geriatr. Cogn. Disord. Extra. 2016;6(3):529–540. doi: 10.1159/000450885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Benavides G., Grau-Rivera O., Suárez-Calvet M., Minguillon C., Cacciaglia R., Gramunt N., Falcon C., Gispert J.D., Molinuevo J.L. Brain and cognitive correlates of subjective cognitive decline-plus features in a population-based cohort. Alzheimer’s Res. Therapy. 2018;10(1) doi: 10.1186/s13195-018-0449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin A.J., Wishart H.A., Rabin L.A., Santulli R.B., Flashman L.A., West J.D., McHugh T.L., Mamourian A.C. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon C., Mueller S.G., Tosun D., Cheong I., Garcia P., Barakos J., Weiner M.W., Laxer K.D. Impact of methodologic choice for automatic detection of different aspects of brain atrophy by using temporal lobe epilepsy as a model. Am. J. Neuroradiol. 2011;32(9):1669–1676. doi: 10.3174/ajnr.A2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheef L., Spottke A., Daerr M., Joe A., Striepens N., Kolsch H., Popp J., Daamen M., Gorris D., Heneka M.T., Boecker H., Biersack H.J., Maier W., Schild H.H., Wagner M., Jessen F. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79(13):1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo, A., Frosch, M. P., Masliah, E., & Hyman, B. T. (2011). Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspect. Med. 1(1), a006189. [DOI] [PMC free article] [PubMed]
- Shen Li, Saykin Andrew J., Kim Sungeun, Firpi Hiram A., West John D., Risacher Shannon L., McDonald Brenna C., McHugh Tara L., Wishart Heather A., Flashman Laura A. Comparison of manual and automated determination of hippocampal volumes in MCI and early AD. Brain Imaging Behav. 2010;4(1):86–95. doi: 10.1007/s11682-010-9088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sperling Reisa A., Aisen Paul S., Beckett Laurel A., Bennett David A., Craft Suzanne, Fagan Anne M., Iwatsubo Takeshi, Jack Clifford R., Kaye Jeffrey, Montine Thomas J., Park Denise C., Reiman Eric M., Rowe Christopher C., Siemers Eric, Stern Yaakov, Yaffe Kristine, Carrillo Maria C., Thies Bill, Morrison‐Bogorad Marcelle, Wagster Molly V., Phelps Creighton H. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N., Scheef L., Wind A., Popp J., Spottke A., Cooper-Mahkorn D., Suliman H., Wagner M., Schild H.H., Jessen F. Volume loss of the medial temporal lobe structures in subjective memory impairment. Dement. Geriatr. Cogn. Disord. 2010;29(1):75–81. doi: 10.1159/000264630. [DOI] [PubMed] [Google Scholar]
- van Harten Argonde C., Mielke Michelle M., Swenson-Dravis Dana M., Hagen Clinton E., Edwards Kelly K., Roberts Rosebud O., Geda Yonas E., Knopman David S., Petersen Ronald C. Subjective cognitive decline and risk of MCI: the Mayo Clinic Study of Aging. Neurology. 2018;91(4):e300–e312. doi: 10.1212/WNL.0000000000005863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie S.C.J., Tijms B., Versteeg A., Benedictus M.R., Bouwman F.H., Scheltens P., Barkhof F., Vrenken H., Flier W.M. Thinner temporal and parietal cortex is related to incident clinical progression to dementia in patients with subjective cognitive decline. Alzheimer’s Dementia: Diagnosis Assessment Disease Monitor. 2016;5(1):43–52. doi: 10.1016/j.dadm.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A., Salem L.C., Andersen B.B., Waldemar G. Differences in quantitative methods for measuring subjective cognitive decline–results from a prospective memory clinic study. Int. Psychogeriatr. 2016;28(9):1513–1520. doi: 10.1017/S1041610216000272. [DOI] [PubMed] [Google Scholar]
- Wang P., Li J., Li H.J., Huo L., Li R. Mild cognitive impairment is not “mild” at all in altered activation of episodic memory brain networks: evidence from ALE meta-analysis. Front. Aging Neurosci. 2016;8:260. doi: 10.3389/fnagi.2016.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Huang W., Su L., Xing Y., Jessen F., Sun Y., Han Y. Neuroimaging advances regarding subjective cognitive decline in preclinical Alzheimer’s disease. Mol. Neurodegener. 2020;15(1):1–27. doi: 10.1186/s13024-020-00395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H.G., Leonard M.K., Chang E.F. The encoding of speech sounds in the superior temporal gyrus. Neuron. 2019;102(6):1096–1110. doi: 10.1016/j.neuron.2019.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L., Wang T., Wang J., Li G., Wang J., Li X., Li W., Hu M., Xiao S. Asymmetry of hippocampus and amygdala defect in subjective cognitive decline among the community dwelling Chinese. Frontiers in psychiatry. 2018;9:226. doi: 10.3389/fpsyt.2018.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L., Hu D., Zhang H., Wen J., Wu Y., Li W., Sun L., Li X., Wang J., Li G., Wang T., Shen D., Xiao S. Prediction of 7-year’s conversion from subjective cognitive decline to mild cognitive impairment. Hum. Brain Mapp. 2021;42(1):192–203. doi: 10.1002/hbm.v42.110.1002/hbm.25216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandifar, A., Fonov, V. S., Ducharme, S., Belleville, S., Collins, D. L., Alzheimer’s Disease Neuroimaging Initiative, 2020. MRI and cognitive scores complement each other to accurately predict Alzheimer's dementia 2 to 7 years before clinical onset. NeuroImage: Clin. 25, 102121. https://doi.org/10.1016/j.nicl.2019.102121. [DOI] [PMC free article] [PubMed]