Abstract
Using an immobilized template assay, we observed two steps in assembly of the yeast RNA polymerase I (Pol I) preinitiation complex: stable binding of upstream activating factor (UAF) followed by recruitment of Pol I-Rrn3p and core factor (CF). Pol I is required for stable association of CF with the promoter and can be recruited in the absence of Rrn3p. Upon transcription initiation, Pol I-Rrn3p and CF dissociate from the promoter while UAF remains behind. These findings support a novel model in which the Pol I basal machinery cycles on and off the promoter with each round of transcription. This model accounts for previous observations that rRNA synthesis may be controlled by regulating both promoter accessibility and polymerase activity.
Transcription of the rRNA genes in the yeast Saccharomyces cerevisiae requires the TATA binding protein (TBP), Rrn3p, and three multiprotein complexes: upstream activating factor (UAF), core factor (CF), and RNA polymerase I (Pol I). Their arrangement within the ribosomal gene preinitiation complex (PIC) is shown schematically in Fig. 1. CF contains three polypeptides, Rrn6p, Rrn7p, and Rrn11p (16, 17, 18), binds to the core promoter element, and is able to direct a basal level of Pol I transcription. UAF interacts with the upstream promoter element and is a complex of six polypeptides including Rrn5p, Rrn9p, Rrn10p, the two histones H3 and H4, and an uncharacterized p30 (13, 15). UAF is not absolutely required for specific initiation but stimulates CF-directed transcription both in vivo and in vitro. Although TBP is required for Pol I transcription (6, 32), its function remains unclear. An in vitro system reconstituted from purified components was shown to direct specific initiation by Pol I in the apparent absence of either UAF or TBP (14). On the other hand, TBP interacts with both UAF and CF in vitro (18, 34) and is essential for transcription activation in vivo (35).
FIG. 1.
Immobilized templates and experimental design. The WT template contains yeast rDNA sequence from −200 to +41 relative to the site of transcription initiation (indicated by an arrow). The upstream promoter element (upe) and core promoter element (core) are shown by open boxes; vector DNA is shown as a thick line. Template Δ−42 has a deletion extending from −200 to −42, while template Δ−2 has a further deletion extending to −2. All templates are shown attached to a Dynal magnetic bead. In control experiments (data not shown), the Δ−42 template had the same transcription activity whether or not it was beaded. Thus, at this distance the bead does not interfere with factor binding.
Pol I, the largest component of the system, consists of 14 polypeptides (5, 11) and has been shown to exist in two forms (19): one population which contains an additional polypeptide, Rrn3p (37), and a larger fraction which lacks Rrn3p. Only the Rrn3p-containing fraction is capable of promoter-specific transcription. Rrn3p dissociates from Pol I during transcription and is not associated with Pol I in extracts from cells grown to stationary phase (19). These results suggest that Rrn3p regulates rRNA production by reversible association with the polymerase.
A homolog of yeast Rrn3p has been identified and cloned from human cells (21). Remarkably, human Rrn3p functions at nearly wild-type (WT) levels when expressed in yeast, indicating that its function has been strongly conserved in evolution. By several criteria, human Rrn3p was proposed to be the same as transcription initiation factor IA (TIF-IA) (3, 21), a Pol I regulatory factor previously purified from mouse and human cells (30, 31). Recently it has been shown that Rrn3p interacts both genetically and biochemically with the Rpa43p subunit of Pol I as well as with the Rrn6p subunit of CF (25). These interactions suggest that Rrn3p may act as a bridge between Pol I and CF to promote productive integration of Pol I into the PIC.
In this paper we have used in vitro transcription on immobilized templates to explore the roles of UAF, CF, Pol I, and Rrn3p in PIC formation and to examine the fate of these factors upon initiation of transcription. Contrary to our expectation, we found that Pol I can be recruited to the promoter in the absence of Rrn3p; however, PICs formed by this route are transcriptionally inactive. We also found that CF is not recruited to the PIC in the absence of Pol I, suggesting that Pol I is required for its stable association. Most surprising, we found that CF and TBP are released from the PIC upon transcription initiation, along with Pol I and Rrn3p. Based on these results we propose a model in which Pol I-Rrn3p, CF, and TBP cycle on and off the promoter with each round of transcription.
MATERIALS AND METHODS
Yeast strains.
Except as noted, strain W303-1a (with the rad5 mutation repaired [40]) and its derivatives were the source of all transcription extracts. For immunodetection of CF (Rrn7p, Rrn11p), UAF (Rrn5p, Rrn9p), Pol I (Rpa43p, Rpa34p, or Rpa135p) or Rrn3p, a separate W303-1a derivative was created in which the relevant open reading frame was tagged at the C terminus with a triple FLAG epitope tag by using homologous recombination (9). The WT (RRN3) strain used was RLY302 (rrn3::HIS3) carrying a 2μm plasmid expressing polyomavirus and FLAG epitope-tagged Rrn3p from its own promoter (plasmid yPyWT) (see Fig. 3). The RRN3ts strain is the same except that the plasmid-expressed Rrn3p carries a temperature-sensitive mutation (L143P). The strains RLY302 (RRN3), RRN3ts, and RLY303 (ΔRRN3) have been described previously (21). RPA190 was deleted by replacement of the entire open reading frame with LEU2 in strains carrying FLAG epitope-tagged versions of either RRN3, RRN7, or RRN9. Strains with RRN7 or RRN5 deleted as well as a strain carrying a temperature-sensitive mutation in RPA190 have been previously described (2).
FIG. 3.
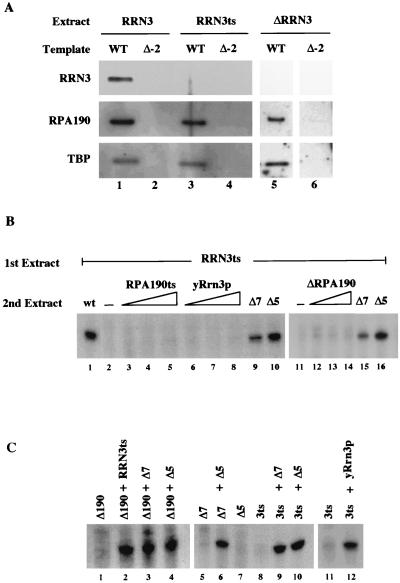
Pol I is recruited in the absence of Rrn3p. (A) PIC formation in the absence of Rrn3p. PICs were formed on either WT or Δ−2 templates in extract containing WT Rrn3p (RRN3, lanes 1 and 2), temperature-sensitive Rrn3p (RRN3ts, lanes 3 and 4), or in an extract lacking Rrn3p (ΔRRN3, lanes 5 and 6). After washing, bound proteins were detected by Western blotting. (B) Rescue of transcription activity on PICs lacking Rrn3p. PICs were formed on WT template by incubation with 2 μl of the RRN3ts extract for 45 min. After washing, PICs were incubated either with buffer alone (lanes 2 and 11), a WT extract (2 μl; lane 1), an RPA190ts extract (1, 2, and 4 μl; lanes 3 to 5), affinity-purified yeast Rrn3p (1, 2, and 4 μl; lanes 6 to 8), a ΔRPA190 extract (0.5, 1, and 2 μl; lanes 12 to 14), a ΔRRN7 extract (2 μl; lanes 9 and 15) or a ΔRRN5 extract (5 μl; lanes 10 and 16). Second incubations were also for 45 min followed by washing and resuspension in YTB with NTPs. After 15 min of incubation, transcripts were assayed by primer extension. Partial rescue was obtained with a second extract containing both Pol I and Rrn3p (ΔRRN7; lanes 9 and 15), while an extract containing Pol I, Rrn3p, and CF (ΔRRN5; lanes 10 and 16) rescued fully. (C) Complementation activity of mutant extracts. Extracts were premixed and incubated for 10 min before addition of template. The WT template was added and incubation continued for 45 min followed by NTP addition. Transcription was stopped after 30 min and assayed by primer extension. Volumes of extracts tested were as follows: ΔRPA190, 0.5 μl; RRN3ts, 2 μl; ΔRRN7, 2 μl; ΔRRN5, 5 μl; yRrn3p, 2 μl.
Note that strains deleted for essential Pol I transcription factors all contain a multicopy plasmid, pNOY103, which produces rRNA under control of a galactose-dependent RNA polymerase II promoter (GAL7 promoter) (23).
Immobilized template preparation and purification of Rrn3p.
Templates were prepared by PCR using a 5′-biotinylated primer, PCRITA5B (5′CGCCAGGGTTTTCCCAGTCAC3′) and a nonbiotinylated primer, PCRITA3 (5′CTTTACACTTTATGCTTCCGGCTC3′). Templates for PCR, pUCWT, pUCΔ-42, and pUCΔ-2 have been described elsewhere (2). Biotinylated templates were purified with an S-300 spin column (Pharmacia) and attached to Dynal magnetic beads essentially as described elsewhere (26). Immobilized templates were stored in transcription buffer at a concentration of 30 ng of DNA/μl.
Yeast Rrn3p was purified from a ΔRPA190 strain expressing FLAG-tagged Rrn3p (see Fig. 3). An S-100 whole-cell extract was loaded onto a column of DEAE-Sepharose CL6B (Pharmacia), washed with two bed volumes of CB100 (18), and eluted with CB400. Fractions showing maximum transcription activity when mixed with a ΔRRN3 extract were further purified by binding to anti-FLAG affinity resin and elution with FLAG peptide.
In vitro transcription using an immobilized template.
Yeast whole-cell transcription extracts were prepared as described previously (2). Extracts from strains with temperature-sensitive transcription factors were not heat treated, since experience has shown that most temperature-sensitive factors are inactive in extracts (for example, see reference 32). Extracts were titrated to determine the amount of extract yielding the maximum level of specific Pol I transcription activity. In a typical reaction mixture, 30 to 45 μg of extract was incubated in 50 μl of YTB (20 mM HEPES [pH 7.9], 5 mM MgCl2, 100 mM KCl, 5 mM EGTA, 0.05 mM EDTA, 2.5 mM dithiothreitol, 10% glycerol, 0.5% Tween 20, 10 μg of α-amanitin/ml, and 10 μg of plasmid pUC19/ml as nonspecific competitor). After 10 min at 25°C, 0.5 μl of immobilized template (15 ng of DNA) was added and incubated for a further 45 min with gentle agitation to prevent settling of the beads. Transcription was initiated by addition of nucleoside triphosphates (NTPs) to concentrations of 0.25 mM each. Alternatively, templates were washed three times with 300 μl of YTB by magnetic concentration and resuspension followed by suspension in 50 μl of YTB with NTPs. Transcription was stopped after 15 to 20 min by addition of 250 μl of stop mix (0.1 M sodium acetate, 10 mM EDTA, 10 μg of tRNA/ml), extraction with phenol-chloroform, and ethanol precipitation. RNA products were analyzed by primer extension as described at the Hahn Laboratory website (http://www.fhcrc.org/labs/hahn/), using oligonucleotide PR75 (5′ATGACCATGATTACGCCAAG3′).
Western blotting.
To analyze proteins bound to immobilized templates or released during transcription, standard transcription reaction mixtures were scaled up twofold. After incubation for 45 min, immobilized templates were washed four times with 300 μl of YTB, suspended in 20 μl of sodium dodecyl sulfate (SDS) loading buffer, boiled 5 min, and resolved by electrophoresis on SDS–4 to 12% polyacrylamide gels (Novex). For proteins released during transcription, PICs were formed and washed by the standard preincubation protocol. Transcription was then initiated by suspension of templates in 15 μl of YTB supplemented with NTPs. After incubation for 15 min at 25°C, the factors released to the supernatant were separated from the factors that remained on the template by using a magnetic concentrator. Released and bound fractions were resuspended in the same volume of the SDS loading buffer and resolved by gel electrophoresis. Resolved proteins were transferred to nitrocellulose and detected by luminescence (Pierce ECL kit). Monoclonal antibody M2 (Sigma) used to detect the FLAG epitope, and polyclonal antibodies raised in rabbits were used to detect either TBP (18) or the Rpa190p.
RESULTS
Formation of the Pol I PIC is promoter dependent.
To study the role of Pol I transcription factors in PIC formation, we have developed an in vitro transcription system using immobilized templates coupled to magnetic beads. The basic protocol outlined in Fig. 1 is similar to one previously used to study initiation by RNA polymerase II (26, 38, 39). Also shown in Fig. 1 are the three DNA templates used in this study. The WT template contains the yeast ribosomal DNA (rDNA) promoter region from −200 to +41 relative to the site of transcription initiation that is flanked by vector DNA. These sequences include both the upstream and core promoter elements. Also shown in Fig. 1 are two mutant templates used as specificity controls to monitor PIC formation and transcription initiation. The Δ−42 template is similar to the WT template except that the upstream promoter element has been removed by deletion of the −200 to −42 region, eliminating the binding site for UAF. The Δ−2 template has been further deleted to position −2, removing both the upsteam and core promoter elements.
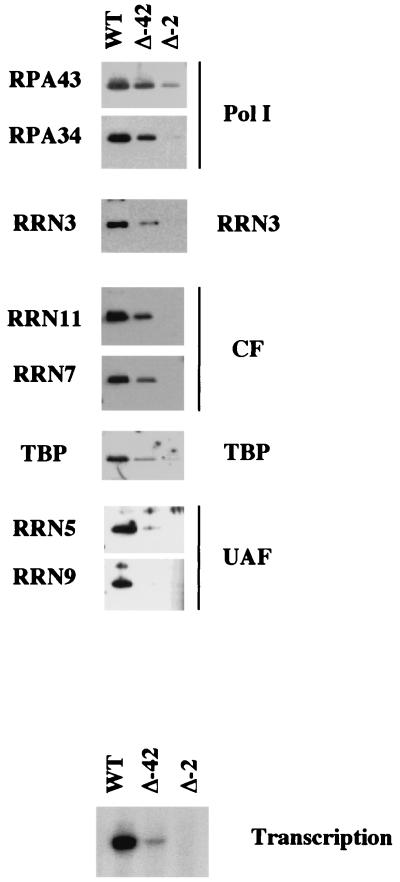
We monitored PIC formation and compared the transcription activity of all three templates (Fig. 2). Immobilized templates were incubated with WT whole-cell extract for 45 min, the time required for maximal complex assembly (data not shown). Immobilized PICs were then washed and either subjected to Western blot analysis to determine their protein composition or were resuspended in buffer with NTPs to measure their ability to direct Pol I initiation. As shown in Fig. 2, PICs formed on the WT template support high levels of transcription and contain the full complement of transcription factors, including UAF (Rrn5p and Rrn9p), CF (Rrn7p and Rrn11p), TBP, Pol I (Rpa43p and Rpa34p), and Rrn3p. PICs formed on the Δ−42 template contain all of the Pol I machinery except UAF and support a much-reduced level of transcription. PICs formed on the Δ−2 template are transcriptionally inactive and contain none of the Pol I factors except for trace amounts of Pol I and TBP. This residual binding most likely reflects the ability of both factors to bind DNA nonspecifically.
FIG. 2.
PIC formation is promoter dependent. PICs were formed by incubation of WT whole-cell extracts with immobilized templates (either WT, Δ−42, or Δ−2) for 45 min. PICs were then washed and analyzed either for specific Pol I transcription (by addition of NTPs followed by primer extension) or for bound proteins (by Western blotting).
We also asked if the system supports single- or multiple-round transcription. PICs were formed on the WT template by preincubation in extract without NTPs for 45 min and then split into two portions. Triphosphates were added to one portion to initiate transcription, while the other portion was washed before triphosphate addition. The same level of transcription was obtained from either washed or unwashed complexes (data not shown), and in both cases maximal transcription was obtained within 5 min of triphosphate addition. These data indicate that our system supports one or at most a few rounds of transcription and establish that PIC formation under these conditions is promoter dependent.
Role of Rrn3 in PIC formation and transcription.
Rrn3p is essential for specific initiation by Pol I (37), and it has been shown to interact with both the Pol I subunit Rpa43p and CF subunit Rrn6p (25). Because these data suggest that Rrn3p may facilitate Pol I recruitment by mediating its interactions with promoter-bound CF, we wished to examine the role of Rrn3p in PIC formation. PICs were formed on either WT or Δ−2 templates by using extracts made from either a WT strain (RRN3), a strain carrying a temperature-sensitive mutant of RRN3 (RRN3ts), or a strain in which the RRN3 gene is deleted (ΔRRN3) (21). After the PICs were washed, they were examined for the presence of Rrn3p, Pol I (Rpa190p), and TBP by Western blotting (Fig. 3A). As expected, all three factors were present in PICs formed using WT extract on a WT template (lane 1) but were not observed on the Δ−2 control template (lane 2). To our surprise, Pol I was recruited in the absence of Rrn3p when either the RRN3ts extract (lane 3) or the ΔRRN3 extract (lane 5) was assayed. The RRN3ts extract is transcriptionally inactive (Fig. 3C, lanes 8 and 11) even though the inactive Rrn3p is still present, as revealed by Western blotting (reference 21 and data not shown). However, activity can be rescued by adding either purified yeast Rrn3p (lane 12) or by adding extracts specifically deficient for either Pol I (lane 2), Rrn7p (lane 9), or Rrn5p (lane 10) activities (these extracts rescue activity because they all presumably contain active Rrn3p). Similar transcription complementation results were also observed when using the ΔRRN3 extract (data not shown).
The data in Fig. 3A show that Rrn3p is not required for recruitment of Pol I to the PIC, even though it is required for initiation of transcription by Pol I. To address its requirement for PIC activity, we attempted to restore transcription activity of PICs formed in the RRN3ts extract by addition of purified Rrn3p. As shown in Fig. 3B, PICs were formed by incubating the WT template in RRN3ts extract for 45 min and subsequently washing and incubating it with either purified Rrn3p or various mutant extracts. After the second incubation, PICs were again washed and resuspended in buffer with triphosphates, and transcription activity was monitored by primer extension. As expected, the PICs formed by the RRN3ts extract were unable to support transcription in the absence of further addition (lanes 2 and 11). To our surprise, the transcriptional activity of the PICs formed in the absence of Rrn3p was not restored upon addition of either the RPA190ts extract, the ΔRPA190 extract, or purified yeast Rrn3p (lanes 2 to 8 and 12 to 14). In contrast, addition of a CF knockout extract (ΔRRN7) partially restored transcription activity (lanes 9 and 15), while addition of a UAF knockout extract (ΔRRN5) complemented the Rrn3ts extract to the same level as that observed with a WT extract (lane 1). The difference in the abilities of the ΔRRN7 and ΔRRN5 extracts to restore transcription in these assays is not due to differences in the specific transcription activity of the extracts, as both knockout extracts restored the RRN3ts extract to full activity if they were added before PIC formation (Fig. 3C).
Overall, the data presented in Fig. 3 show that Pol I can be recruited to the PIC in the absence of Rrn3p but that the resulting complex is transcriptionally inactive. Rrn3p alone is not sufficient to reactivate a Rrn3p-deficient PIC, but it is capable of a modest level of reactivation when complexed with Pol I, presumably by displacing Pol I lacking Rrn3p from the complex. When Rrn3p, Pol I, and CF are all present, transcription can be restored to WT levels. This raises the possibility that Rrn3p, Pol I, and CF may enter the PIC in a concerted fashion.
PIC formation in the absence of Pol I.
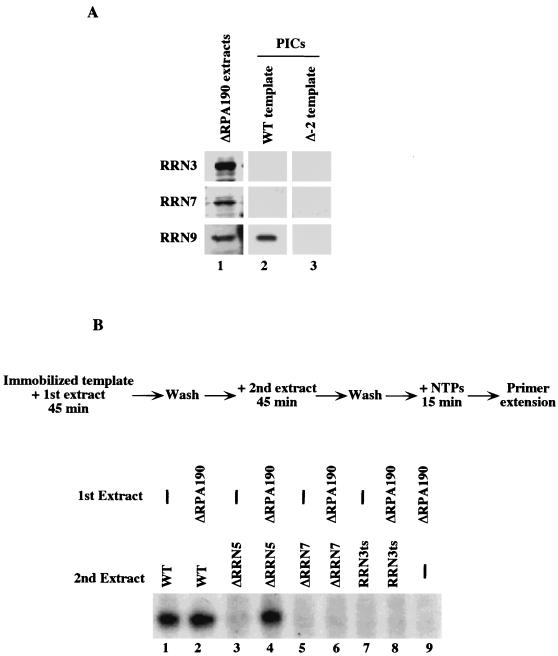
It has been proposed that Pol I PIC assembly in yeast proceeds in a stepwise manner in which UAF recruits CF to form a stable complex that in turn recruits Pol I-Rrn3p (34). To test this model we have examined PIC assembly on a WT template by using extracts from cells lacking Pol I (ΔRPA190) and carrying an epitope-tagged genomic copy of either RRN3, RRN7 (CF), or RRN9 (UAF). Western analysis revealed that the PICs formed by each of these extracts contained UAF (Rrn9p) but lacked Rrn3p (Fig. 4A). Unexpectedly, the PICs also lacked CF (Rrn7p), despite the facts that mixing the ΔRPA190 extract with a ΔRRN7 extract restored transcription (Fig. 3C, lane 3), demonstrating that active CF was present in the ΔRPA190 extract, and that CF was readily detected by Western blotting (Fig. 4A, lane 1). This result shows that UAF cannot stably recruit CF in the absence of Pol I.
FIG. 4.
CF is not recruited in the absence of Pol I. (A) PIC formation in the absence of Pol I. PICs were formed on either the WT or Δ−2 template (lanes 2 and 3) using ΔRPA190 extracts where either RRN9, RRN7, or RRN3 were epitope tagged. After washing, proteins were detected by Western blotting. The presence of intact proteins in each extract is shown in lane 1. In the absence of Pol I, UAF binds to the promoter but not to CF or Rrn3p (lane 2). (B) Complementation of Pol I-free PICs with mutant extracts. WT template was first incubated either with buffer (lanes 1, 3, 5, and 7) or ΔRPA190 extract (lanes 2, 4, 6, 8, and 9) for 45 min and washed. Templates were then incubated for 45 min with a second extract, either WT (lanes 1 and 2), ΔRRN5 (lanes 3 and 4), ΔRRN7 (lanes 5 and 6), RRN3ts (lanes 7 and 8), or buffer alone (lane 9), washed, and incubated in YTB with NTPs. After a 15-min incubation, transcripts were assayed by primer extension. PICs formed in the absence of Pol I were only rescued by a second extract lacking UAF (ΔRRN5; lane 4) or the WT extract (lane 2).
To further investigate this result, we attempted to rescue PICs formed in the ΔRPA190 extract by incubating them with extracts deficient in either UAF (ΔRRN5), CF (ΔRRN7), or RRN3 (RRN3ts). As shown in Fig. 4B, PICs formed in the absence of Pol I are not rescued by incubation with extracts lacking either Rrn3p (lane 8) or CF (lane 6). However, they are rescued to WT levels by incubation in a UAF-deficient extract (lane 4). This result further supports the observation that CF is not associated at the promoter when PICs are formed in the absence of Pol I. Taken together, the results shown in Fig. 4 indicate that Pol I is absolutely required for stable association of the CF with the promoter during PIC formation, while UAF can bind stably to the promoter in the absence of both Pol I and CF.
Fate of PIC components after transcription initiation.
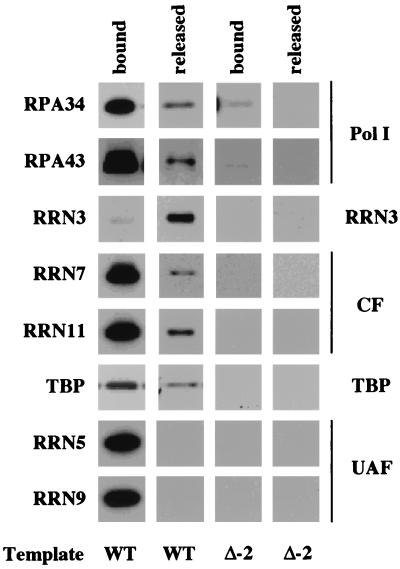
The current model of Pol I transcription predicts that polymerase and Rrn3p exit the promoter upon initiation, leaving behind UAF and CF as a scaffold for the next round of transcription (19, 34). To test this model we examined the fate of Pol I transcription factors during transcription initiation. PICs were formed on the WT template using WT extracts containing epitope-tagged components, and transcription was initiated by resuspending the washed PICs in transcription buffer containing NTPs. Factors which either remained bound to the template or were released to the supernatant during transcription were separated and analyzed by Western blotting. Figure 5 shows a comparison of the factors released during transcription with factors that remained associated with the templates. As expected, Pol I (RPA34, RPA43) and Rrn3p were found in the released fraction, which additionally contained not only the CF subunits (RRN7, RRN11) but also TBP. In contrast, the UAF complex (RRN5, RRN9) remained tightly associated with promoter template throughout the transcription cycle. Moreover, Fig. 5 shows that nearly all of the Rrn3p dissociates from the promoter template during transcription, while only a small fraction of Pol I, CF, and TBP are released upon NTP addition.
FIG. 5.
Release of Pol I factors during transcription. PICs were formed for 45 min on either the WT or Δ−2 template using WT extract. After washing, PICs were resuspended in YTB with NTPs for 15 min. Factors that remain bound to the immobilized template were separated from released factors with a magnetic separator, resolved on an SDS-polyacrylamide gel, and identified by using Western blotting. Note that essentially all of Rrn3p releases from the WT template, a small fraction of Pol I (RPA34, RPA43), CF (RRN7, RRN11) and TBP release, and no UAF releases during transcription.
These results show that both active and inactive PICs are assembled on the WT promoter template in our extracts and that the presence of Rrn3p is the distinguishing feature of the transcriptionally active class, since it is quantitatively released from the promoter complex when transcription is initiated by addition of NTPs. Furthermore, the factor composition of inactive PICs is identical to that of PICs formed in the absence of Rrn3p (Fig. 3). UAF is not released upon triphosphate addition, in agreement with previous demonstrations that UAF is the primary stabilizing element of the PIC (34, 37).
Nucleotide requirements for factor release.
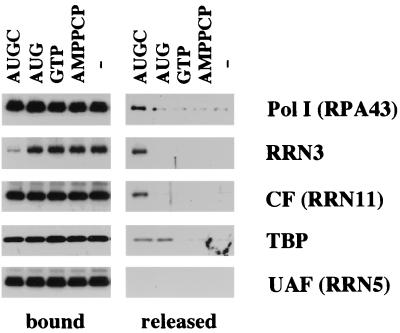
The factor release shown in Fig. 5 was obtained in the presence of all four NTPs. To confirm that the observed factor release was the result of transcription initiation, we tried adding various subsets of triphosphates to the assembled PICs (Fig. 6). With the exception of a small amount of Pol I which dissociated in the absence of any triphosphate addition, Pol I, Rrn3p, and CF were released only in the presence of all four triphosphates. Since Rrn3 is not released in the absence of NTPs, the minor Pol I fraction released under these conditions probably corresponds to the fraction of Pol I which is nonspecifically associated with the immobilized template. In contrast, the amount of TBP released upon addition of all four NTPs is identical to the amount released when only the first three nucleotides of the transcript are added (the sequence of the Pol I transcript begins 5′-AUGCGAAAGCAGUUGAAGAC). Further work is needed to understand the role of TBP and to define exactly when CF and Rrn3p leave the template.
FIG. 6.
Nucleotide requirements for factor release. PICs were formed on the WT template, washed, and incubated for 15 min in YTB with either all four NTPs (AUGC), triphosphates corresponding to the first three nucleotides of the transcript (AUG), GTP alone, a nonhydrolyzable ATP analog (AMPPCP), or buffer alone. After separation into bound and released fractions, factors were identified by Western blotting. As expected from Fig. 5, UAF does not release under any conditions. Pol I, Rrn3p, and CF release only in the presence of all four NTPs (AUGC) except for a small nonspecific release of Pol I that occurs in buffer alone. TBP releases in the presence of only AUG as well as in the presence of AUGC.
DISCUSSION
A model for the Pol I transcription cycle.
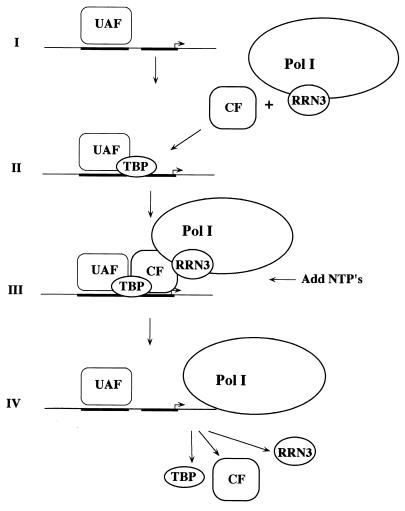
The data reported in this paper suggest a new model for the Pol I transcription cycle; this model illustrated in Fig. 7. The cycle begins with formation of the Pol I PIC in two discernible steps: sequence-specific localization of the promoter by the UAF complex, followed by the recruitment of CF, Pol I-Rrn3, and possibly TBP. Upon addition of NTPs, CF, Pol I-Rrn3, and TBP dissociate from the template while UAF remains stably bound to the promoter, presumably serving as a scaffold for reinitiation.
FIG. 7.
Model of the Pol I transcription cycle. We define two steps in formation of the Pol I PIC. In the first step, UAF locates the promoter and binds stably in a sequence-specific manner. In the second step, Pol I-Rrn3p and CF are recruited by UAF. TBP is required for this recruitment, but whether it enters with CF and Pol I-Rrn3p or is separately recruited by UAF is not yet determined. Upon initiation of transcription, CF, Pol I/Rrn3p, and TBP all leave the promoter. UAF remains bound as a scaffold for further rounds of recruitment and transcription.
It has been previously suggested that UAF is the factor that first binds the promoter to initiate the process of PIC assembly (34). This is supported both by in vitro studies showing that UAF alone can bind stably to the rDNA promoter in the absence of CF (15) as well as by in vivo footprint analyses of the rRNA genes, which revealed that UAF remains stably associated with the upstream promoter element (36). The results presented in Fig. 4, showing that UAF can bind stably to the rDNA promoter in the absence of both CF and Pol I, agree with these studies. We therefore favor the notion that UAF binding initiates transcription complex formation at rRNA genes.
The next step in PIC formation is the recruitment of CF and Pol I. The data presented in Fig. 4 demonstrate that CF does not remain stably associated with the promoter in the absence of polymerase. Western blotting revealed that CF is not associated with the PIC in extracts lacking Pol I (Fig. 4A), even though it contains transcriptionally competent CF, and in vitro transcription assays demonstrated that extracts lacking CF cannot direct transcription from a complex formed in the absence of active Pol I (Fig. 4B). Thus, Pol I is essential for stable association of CF with the rDNA promoter.
Although Pol I appears to be required for formation of a stable PIC, it can be specifically recruited to the promoter in the absence of Rrn3p (Fig. 3A). In vitro, this results in the formation of a transcriptionally inactive complex. These inactive complexes cannot be rendered transcriptionally competent by adding purified Rrn3p (Fig. 3B). In fact, the data shown in Fig. 3B suggest that an Rrn3p-Pol I complex is required to direct transcription from PICs assembled in the absence of Rrn3p, as only those extracts containing both Rrn3p and Pol I were capable of restoring transcription activity. This result was unexpected, since it has previously been suggested that Rrn3p is required for recruitment of Pol I, based on its ability to interact with both Pol I (Rpa43p) and the core factor subunit Rrn6p (25). In addition, recent experiments in human cell systems have been interpreted to mean that human Rrn3 (hRrn3) is essential for Pol I recruitment (20).
The present finding that Rrn3p can be dispensable for Pol I recruitment suggests that it is required, in combination with Pol I-CF interactions, to induce an active conformation of the polymerase within the PIC. This may be similar to what has been reported for Pol III transcription, where TFIIIB appears to participate actively in initiation by possibly changing the conformation of the polymerase (12).
Upon transcription initiation, Pol I-Rrn3p, CF, and TBP all escape the promoter while UAF remains bound (Fig. 5). The fractions of Pol I, CF, and TBP that escape are relatively small while almost all of Rrn3p is released. This suggests that two types of PICs are formed in vitro: transcriptionally competent PICs containing Rrn3p and transcriptionally inactive PICs lacking Rrn3p. At present we do not understand what limits the fraction of PICs that contain Rrn3p. Overexpression of Rrn3p leads to extracts with greatly increased in vitro transcription activity but does not materially increase the fraction of Rrn3p-containing PICs (B. Moorefield and P. Aprikian, unpublished results). Thus, some other as-yet-unidentified factor is probably limiting.
The role of TBP in PIC formation is currently unclear. TBP has been shown to bind to both CF and UAF in vitro (18, 34, 35) and is required for transcriptional activation by UAF in vivo (34). Using the immobilized template assay, we have observed that TBP can be recruited in the absence of UAF, presumably by binding to CF (Fig. 2). Conversely, TBP can be recruited to the promoter in the absence of CF, presumably by binding to UAF subunits (P. Aprikian, unpublished results). However, we do not know if TBP recruited to these partial complexes can participate in the formation of transcriptionally active PICs. Since TBP as well as CF and Pol I-Rrn3p dissociate from promoter following transcription initiation, we favor the notion that all these components enter the PIC in the same step. Further experiments are needed to test this possibility.
Comparison with previous models of PIC formation.
Stepwise models of Pol I PIC formation have been proposed for both yeast (34) and mammals (30). In these earlier models an activator (yeast UAF, mammalian upstream binding factor) recruits a basal complex (yeast CF, mammalian SL1-TIF-IB) which in turn recruits Pol I plus Rrn3p (yeast) or Pol I plus hRrn3-TIF-IA (mammals). These stepwise models are now being reconsidered in view of multiple reports of Pol I “holoenzymes” in plants and mammals (1, 10, 20, 29, 33). While the composition of these various complexes is not yet well defined, they all share the property of containing Pol I along with enough other factors to support specific initiation in vitro. Although a Pol I holoenzyme has yet to be identified in yeast, the model shown in Fig. 7 could readily accommodate the existence of a complex containing Pol I-Rrn3 and CF.
However, all of the models of Pol I PIC formation proposed so far envision either SL1 or CF remaining at the promoter upon transcription initiation (20, 30, 34). A major novelty of our results is the demonstration that this is not so, at least in yeast. Transcription initiation involves the release of both CF and TBP in addition to Pol I-Rrn3.
There is precedent for assembly pathways that have been worked out with purified factors to differ from those observed with crude extracts. For example, a stepwise pathway for Pol II PIC formation has been proposed on the basis of order-of-addition experiments with purified factors (see review in reference 24). In this stepwise model, TBP binding to the TATA box is a prerequisite for TFIIB recruitment. Pol II and TFIIF are subsequently recruited, followed by TFIIE and TFIIH, and TFIIA can enter the PIC at any point after TBP binding. This stepwise model has been challenged by discovery of a Pol II holoenzyme which is stably associated with a subset of general transcription factors (reviewed in reference 22). In agreement with the holoenzyme model, studies of Pol II PIC assembly using immobilized templates and unfractionated nuclear extracts suggest that the assembly pathway consists of only two major steps (26). In the first step, TFIIA and the TBP-containing factor TFIID are recruited in a sequence-specific manner that is stimulated by activators. The second step is concerted recruitment of TFIIB plus Pol II holoenzyme and is also stimulated by activators. Subsequent studies indicate that initiation of transcription results in Pol II, TFIIB, and TFIIF leaving the PIC (39). This leaves behind a scaffold consisting of TFIID, TFIIA, TFIIH, TFIIE, and a subcomplex of the holoenzyme called mediator which apparently serves as a platform for subsequent rounds of reinitiation.
Our model for Pol I resembles the Pol II holoenzyme recruitment model in that polymerase plus a subset of factors dissociates from the template upon transcription initiation while activator(s) remain behind. Our model appears to differ from the Pol II situation in that the factors dissociating from the promoter include the entire basal machinery.
Relevance of our model to Pol I transcription in vivo.
In vivo footprinting has been utilized to examine the binding of Pol I transcription factors to the ribosomal gene promoter in living yeast (4) and has been able to detect binding of both UAF and CF to the promoters of WT cells. UAF binding at WT levels was also detected in cells lacking either CF (ΔRRN7) or Pol I (ΔRPA43), while CF binding was not detected in cells lacking UAF (ΔRRN5) or Pol I (ΔRPA43). The authors interpreted these results to mean that UAF binding did not depend upon either CF or Pol I and that CF binding was dependent upon both UAF and Pol I. These in vivo data are in complete agreement with the model in Fig. 7 which was derived from our in vitro experiments.
Bordi et al. further found that UAF binding was present in a strain lacking Rrn3p (ΔRRN3), but CF binding was absent (4). This indicates that the inactive, Rrn3p-lacking complexes which formed in our experiments are due to some deficiency in the in vitro system and do not form in vivo. Despite some optimistic calculations in the literature, it is our impression that this is a feature of most eukaryotic in vitro systems, in which only a fraction of the complexes that form are transcriptionally active (for example, see reference 26). In our case we were able to measure the active fraction and distinguish it from the inactive fraction.
Implications of the Pol I transcription cycle for regulation of rRNA synthesis.
We have previously proposed that eukaryotic rRNA transcription has the potential of being regulated on two levels that are mechanistically distinct (27, 28). One level of regulation appears to control whether or not a given promoter is open and capable of directing transcription. Evidence for regulation of promoter opening in yeast comes from psoralen cross-linking experiments which show that only a fraction of the ribosomal genes are active during exponential cell growth (7). Electron micrographs of ribosomal genes from rapidly growing cells typically show genes that are tightly packed with elongating polymerase. This suggests that under some circumstances the factor(s) required to open genes are rate limiting while the components needed for transcription initiation are in excess. Regulation at the level of promoter opening is further supported by the observation that the number of open, transcriptionally active ribosomal genes varies in proportion to yeast cellular growth rate (7).
Based on work presented in this and in previous papers, the UAF complex has the characteristics expected of a factor that might regulate Pol I promoter opening. UAF is capable of binding to the promoter in the absence of other factors (15) (Fig. 4A), does not readily exchange once it is bound, and remains behind after transcription initiation (Fig. 5 and 6). In addition, UAF is tightly associated with histones H3 and H4 (13), suggesting that it may be particularly suited for altering histone interactions in the process of alleviating repressive chromatin structures.
Each repeating unit of yeast rDNA also contains an enhancer element located just downstream of the Pol I terminator site. When a Pol I promoter on an extrachromosomal plasmid is placed in competition with the Pol I promoters in the chromosome, the presence of an adjacent enhancer element is essential for transcriptional activity of the plasmid-borne promoter (8). Thus, it is possible that the enhancer elements also influence Pol I promoter opening. However, the mechanism of enhancer-dependent activation is unknown at present.
Once a Pol I promoter is open, the rate of Pol I loading is controlled by a secondary mechanism. Regulation at this level is likely to involve Rrn3p or its mammalian homolog, TIF-IA. Rrn3p appears to determine the fraction of active Pol I (19) and is necessary to form a transcriptionally competent PIC (Fig. 3 and 5). Regulation at the level of promoter loading has been monitored during the rapid down regulation of rRNA gene transcription which occurs when yeast undergo the transition from log-phase growth to stationary phase. This transcriptional shutoff is accompanied by a marked decline in the levels of Rrn3p-associated Pol I (19), while the number of psoralen-accessible, presumably open genes decreases very little (7). Thus, rRNA gene transcription may be regulated at the level of promoter opening or at the level of polymerase loading in response to different physiological conditions.
At present much attention is focused on the possibility that Rrn3p activity is controlled by a regulatory cycle in which it dissociates from Pol I during initiation, is inactivated, and must be reactivated before it can direct initiation by a second polymerase. The observation that CF and TBP also leave the PIC upon initiation raises the possibility that they too may undergo a similar cycle of inactivation and reactivation during successive rounds of transcription.
ACKNOWLEDGMENTS
We thank J. Roan and K. Johnson for excellent technical assistance and T. Tsukiyama for review of the manuscript. S. Hahn and members of his laboratory helped us adapt the immobilized template assay for Pol I transcription and also offered critical comments on the manuscript.
This work was partially supported by a grant to R.H.R. (GM26624).
REFERENCES
- 1.Albert A C, Denton M, Kermekchiev M, Pikaard C S. Histone acetyltransferase and protein kinase activities copurify with a putative Xenopus RNA polymerase I holoenzyme self-sufficient for promoter-dependent transcription. Mol Cell Biol. 1999;19:796–806. doi: 10.1128/mcb.19.1.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aprikian P, Moorefield B, Lin C-W, Reeder R H. TATA binding protein can stimulate core-directed transcription by yeast RNA polymerase I. Mol Cell Biol. 2000;20:5269–5275. doi: 10.1128/mcb.20.14.5269-5275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodem J, Dobreva G, Hoffmann-Rohrer U, Iben S, Zentgraf H, Delius H, Vingron M, Grummt I. TIF-IA, the factor mediating growth-dependent control of ribosomal RNA synthesis, is the mammalian homolog of yeast Rrn3p. EMBO Rep. 2000;1:171–175. doi: 10.1093/embo-reports/kvd032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bordi L, Cioci F, Camilloni G. In vivo binding and hierarchy of assembly of the yeast RNA polymerase I transcription factors. Mol Biol Cell. 2001;12:753–760. doi: 10.1091/mbc.12.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carles C, Riva M. Yeast RNA polymerase I subunits and genes. In: Paule I, editor. Transcription of ribosomal RNA genes by eukaryotic RNA polymerase. Berlin, Germany: Springer Verlag; 1998. [Google Scholar]
- 6.Cormack B P, Struhl K. The TATA-binding protein is required for transcription by all three nuclear RNA polymerases in yeast cells. Cell. 1992;69:685–696. doi: 10.1016/0092-8674(92)90232-2. [DOI] [PubMed] [Google Scholar]
- 7.Dammann R, Lucchini R, Koller T, Sogo J M. Chromatin structures and transcription of rDNA in yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:2331–2338. doi: 10.1093/nar/21.10.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elion E, Warner J R. An RNA polymerase I enhancer in Saccharomyces cerevisiae. Mol Cell Biol. 1986;6:2089–2097. doi: 10.1128/mcb.6.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldmark J P, Fazzio T G, Estep P W, Church G M, Tsukiyama T. The Isw2 chromatin remodeling complex represses early meiotic genes upon recruitment by Ume6p. Cell. 2000;103:423–433. doi: 10.1016/s0092-8674(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 10.Hannan R D, Cavanaugh A, Hempel W M, Moss T, Rothblum L. Identification of a mammalian RNA polymerase I holoenzyme containing components of the DNA repair/replication system. Nucleic Acids Res. 1999;27:3720–3727. doi: 10.1093/nar/27.18.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishihama A, Kimura M, Mitsuzawa H. Subunits of yeast RNA polymerases: structure and function. Curr Opin Microbiol. 1998;1:190–196. doi: 10.1016/s1369-5274(98)80010-6. [DOI] [PubMed] [Google Scholar]
- 12.Kassavetis G A, Kumar A, Letts G A, Geiduschek E P. A post-recruitment function for the RNA polymerase III transcription-initiation factor IIIB. Proc Natl Acad Sci USA. 1998;95:9196–9201. doi: 10.1073/pnas.95.16.9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keener J, Dodd J A, Lalo D, Nomura M. Histones H3 and H4 are components of upstream activation factor required for the high-level transcription of yeast rDNA by RNA polymerase I. Proc Natl Acad Sci USA. 1997;94:13458–13462. doi: 10.1073/pnas.94.25.13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keener J, Josaitis C A, Dodd J A, Nomura M. Reconstitution of yeast RNA polymerase I transcription in vitro from purified components. J Biol Chem. 1998;173:33795–33802. doi: 10.1074/jbc.273.50.33795. [DOI] [PubMed] [Google Scholar]
- 15.Keys D A, Lee B-S, Dodd J A, Nguyen T T, Vu L, Fantino E, Burson L M, Nogi Y, Nomura M. Multiprotein transcription factor UAF interacts with the upstream element of the yeast RNA polymerase I promoter and forms a stable preinitiation complex. Genes Dev. 1996;10:887–903. doi: 10.1101/gad.10.7.887. [DOI] [PubMed] [Google Scholar]
- 16.Keys D A, Steffan J S, Dodd J A, Yamamoto R T, Nogi Y, Nomura M. RRN6 and RRN7 encode subunits of a multiprotein complex essential for the initiation of rDNA transcription by RNA polymerase I in Saccharomyces cerevisiae. Genes Dev. 1994;8:2349–2362. doi: 10.1101/gad.8.19.2349. [DOI] [PubMed] [Google Scholar]
- 17.Lalo D, Steffan J S, Dodd J A, Nomura M. RRN11 encodes the third subunit of the complex containing Rrn6p and Rrn7p that is essential for the initiation of rDNA transcription by yeast RNA polymerase I. J Biol Chem. 1996;271:21062–21067. doi: 10.1074/jbc.271.35.21062. [DOI] [PubMed] [Google Scholar]
- 18.Lin C-W, Moorefield B, Payne J, Aprikian P, Mitomo K, Reeder R H. A novel 66-kilodalton protein complexes with Rrn6, Rrn7, and TATA-binding protein to promote polymerase I transcription initiation in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:6436–6443. doi: 10.1128/mcb.16.11.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milkereit P, Tschochner H. A specialized form of RNA polymerase I, essential for initiation and growth-dependent regulation of rRNA synthesis, is disrupted during transcription. EMBO J. 1998;17:3692–3703. doi: 10.1093/emboj/17.13.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller G, Panov K I, Friedrich J K, Trinkle-Mulcahy L, Zomerdijk J C B M. hRRN3 is essential in the SL1-mediated recruitment of RNA polymerase I to rRNA gene promoters. EMBO J. 2001;20:1373–1382. doi: 10.1093/emboj/20.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moorefield B, Greene E A, Reeder R H. RNA polymerase I transcription factor Rrn3 is functionally conserved between yeast and human. Proc Natl Acad Sci USA. 2000;97:4724–4729. doi: 10.1073/pnas.080063997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myer V E, Young R A. RNA polymerase II holoenzymes and subcomplexes. J Biol Chem. 1998;273:27757–27760. doi: 10.1074/jbc.273.43.27757. [DOI] [PubMed] [Google Scholar]
- 23.Nogi Y, Yano R, Nomura M. Synthesis of large rRNAs by RNA polymerase II in mutants of Saccharomyces cerevisiae defective in RNA polymerase I. Proc Natl Acad Sci USA. 1991;88:3962–3966. doi: 10.1073/pnas.88.9.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orphanides G, Lagrange T, Reinberg D. General initiation factors of RNA polymerase II. Genes Dev. 1996;10:2657–2683. doi: 10.1101/gad.10.21.2657. [DOI] [PubMed] [Google Scholar]
- 25.Peyroche G, Milkereit P, Bischler N, Tschochner H, Schultz P, Sentenac A, Carles C, Riva M. The recruitment of RNA polymerase I on rDNA is mediated by the interaction of the A43 subunit with Rrn3. EMBO J. 2000;19:5473–5482. doi: 10.1093/emboj/19.20.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranish J A, Yudkovsky N, Hahn S. Intermediates in formation and activity of the RNA polymerase II preinitiation complex: holoenzyme recruitment and a postrecruitment role for the TATA box and TFIIB. Genes Dev. 1999;13:49–63. doi: 10.1101/gad.13.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reeder R H. Regulatory elements of the generic ribosomal gene. Curr Opin Cell Biol. 1989;1:466–474. doi: 10.1016/0955-0674(89)90007-0. [DOI] [PubMed] [Google Scholar]
- 28.Reeder R H. The regulation of transcription by RNA polymerase I. In: Yamamoto K, McKnight S, editors. Transcriptional regulation. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 29.SaezVasquez J, Pikaard C S. Extensive purification of a putative RNA polymerase I holoenzyme from plants that accurately initiates rRNA gene transcription in vitro. Proc Natl Acad Sci USA. 1997;94:11869–11874. doi: 10.1073/pnas.94.22.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnapp A, Grummt I. Transcription complex formation at the mouse rDNA promoter involves the stepwise association of four transcription factors and RNA polymerase I. J Biol Chem. 1991;266:24588–24595. [PubMed] [Google Scholar]
- 31.Schnapp A, Schnapp G, Erny B, Grummt I. Function of the growth-regulated transcription initiation factor TIF-IA in initiation complex formation at the murine ribosomal gene promoter. Mol Cell Biol. 1993;13:6723–6732. doi: 10.1128/mcb.13.11.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultz M C, Reeder R H, Hahn S. Variants of the TATA binding protein can distinguish subsets of RNA polymerase I, II, and III promoters. Cell. 1992;69:697–702. doi: 10.1016/0092-8674(92)90233-3. [DOI] [PubMed] [Google Scholar]
- 33.Seither P, Iben S, Grummt I. Mammalian RNA polymerase I exists as a holoenzyme with associated basal transcription factors. J Mol Biol. 1998;275:43–53. doi: 10.1006/jmbi.1997.1434. [DOI] [PubMed] [Google Scholar]
- 34.Steffan J S, Keys D A, Dodd J A, Nomura M. The role of TBP in rDNA transcription by RNA polymerase I in Saccharomyces cerevisiae: TBP is required for upstream activation factor-dependent recruitment of core factor. Genes Dev. 1996;10:2551–2563. doi: 10.1101/gad.10.20.2551. [DOI] [PubMed] [Google Scholar]
- 35.Steffan J S, Keys D A, Vu L, Nomura M. Interaction of TATA-binding protein with upstream activation factor is required for activated transcription of ribosomal DNA by RNA polymerase I in Saccharomyces cerevisiae in vivo. Mol Cell Biol. 1998;18:3752–3761. doi: 10.1128/mcb.18.7.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogelauer M, Cioci F, Camilloni G. DNA protein-interactions at the Saccharomyces cerevisiae 35S rRNA promoter and in its surrounding region. J Mol Biol. 1998;275:197–209. doi: 10.1006/jmbi.1997.1451. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto R T, Nogi Y, Dodd J A, Nomura M. RRN3 gene of Saccharomyces cerevisiae encodes an essential RNA polymerase I transcription factor which interacts with the polymerase independently of DNA template. EMBO J. 1996;15:3964–3973. [PMC free article] [PubMed] [Google Scholar]
- 38.Yudkovsky N, Logie C, Hahn S, Peterson C L. Recruitment of the SWI/SNF chromatin remodeling complex by transcriptional activators. Genes Dev. 1999;13:2369–2374. doi: 10.1101/gad.13.18.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yudkovsky N, Ranish J A, Hahn S. A transcription reinitiation intermediate that is stabilized by activator. Nature. 2000;408:225–229. doi: 10.1038/35041603. [DOI] [PubMed] [Google Scholar]
- 40.Zhao X, Muller E G, Rothstein R. A suppressor of two essential checkpoint genes identified a novel protein that negatively affects dNTP pools. Mol Cell. 1998;2:329–340. doi: 10.1016/s1097-2765(00)80277-4. [DOI] [PubMed] [Google Scholar]