Summary
Background
Exposure to vitamin D in early life has been associated with improved bone mineralization, but no studies have investigated the combined effect of pregnancy supplementation and childhood 25(OH)D concentrations on bone health.
Methods
We analyzed the effect of serum 25(OH)D concentrations at age 6 months and 6 years and the combined effect with prenatal high-dose vitamin D (2800 vs. 400 IU/day) on bone mineral density (BMD) and content (BMC) assessed by dual-energy X-ray absorptiometry (DXA) scans at age 3 and 6 years and longitudinal risk of fractures in a double-blinded, randomized clinical trial in the Copenhagen Prospective Studies on Asthma in Childhood 2010 (COPSAC2010) mother-child cohort with enrollment from March 4, 2009, to November 17, 2010, and clinical follow-up until January 31, 2019 (NCT00856947). All participants randomized to intervention and with complete data were included in the analyses.
Findings
At age 6 months, serum 25(OH)D concentration was measured in 93% (n = 541) of 584 children. Children with sufficient (≥ 75 nmol/l) vs. insufficient (< 75 nmol/l) concentrations did not have lower risk of fractures: incidence rate ratio (95% CI); 0.64 (0.37;1.11), p = 0.11. However, vitamin D sufficient children from mothers receiving high-dose supplementation during pregnancy had a 60% reduced incidence of fractures compared with vitamin D insufficient children from mothers receiving standard-dose: 0.40 (0.19;0.84), p = 0.02.
At age 6 years, serum 25(OH)D concentration was measured in 83% (n = 318) of 383 children with available DXA data. Whole-body bone mineralization was higher in vitamin D sufficient children at age 6 years; BMD, adjusted mean difference (aMD) (95% CI): 0.011 g/cm2 (0.001;0.021), p = 0.03, and BMC, aMD: 12.3 g (-0.8;25.4), p = 0.07, with the largest effect in vitamin D sufficient children from mothers receiving high-dose vitamin D supplementation; BMD, aMD: 0.016 g/cm2 (0.002;0.030), p = 0.03, and BMC, aMD: 23.5 g (5.5;41.5), p = 0.01.
Interpretation
Childhood vitamin D sufficiency improved bone mineralization and in combination with prenatal high-dose vitamin D supplementation reduced the risk of fractures.
Funding
The study was supported by The Lundbeck Foundation R16-A1694, The Danish Ministry of Health 903,516, The Danish Council for Strategic Research 0603–00280B and The European Research Council 946,228.
Keywords: RCT, Vitamin D, COPSAC, BMC, BMD, 25(OH)D, DXA, Child fractures
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMC, bone mineral content; BMD, bone mineral density; COPSAC, copenhagen prospective studies on asthma in childhood; DXA, dual energy X-ray absorptiometry; RCT, randomized clinical trial; TBLH, total body less head; LC-PUFA, long chained polyunsaturated fatty acids
Research in context.
Evidence before this study
A positive association between 25(OH)D concentrations and bone mineralization in childhood has been suggested in a range of observational studies and a protective effect of pregnancy vitamin D supplementation on offspring bone mineralization has been demonstrated in a randomized controlled trial (RCT) in the COPSAC2010 mother-child cohort (PubMed search using terms “vitamin D”, “25(OH)D”, “bone mineral content”, “bone mineral density” and “childhood fractures” including clinical trials, RCTs and systematic reviews until April 2021). Further, a negative association between bone mineralization and risk of fractures in childhood has been suggested.
Added value of this study
This is the first study to show a combination of high-dose vitamin D supplementation during pregnancy and vitamin D sufficiency (≥ 75 nmol/l) in childhood reduces the risk of childhood fractures and improves bone mineralization outcomes at age 6 years. Childhood vitamin D sufficiency also improve bone mineral outcomes by age 6 years independent of the prenatal high-dose supplementation. Finally, a history of fractures was associated with a lower whole-body bone mineralization status.
Implications of all the available evidence
This trial suggests that sufficient childhood levels improve bone mineralization at age 6 years and in combination with prenatal high-dose vitamin D supplementation reduces fracture risk in childhood by 60%, which may contribute to increased peak bone mass and decreased risk of osteoporosis as early life bone accrual has been suggested by the National Osteoporosis Foundation as the most influential factor for preventing current and future fractures.
Alt-text: Unlabelled box
Introduction
The negative implications of early life severe vitamin D deficiency on bone health are well known; rickets in children1 and possibly osteoporosis later in life.2,3 This relationship is the basis for recommending vitamin D supplementation during pregnancy and in early childhood in most countries.4 In observational studies, findings of a positive association between vitamin D status and bone mineral outcomes assessed by dual-energy X-ray absorptiometry (DXA) scans in childhood5,6 and until time of peak bone mass7 have been demonstrated, while others could not confirm this finding.8 In addition, some studies have demonstrated association between maternal vitamin D deficiency in pregnancy and lower offspring bone mass.9,10 Importantly, an inverse association has been demonstrated between bone mineral content in childhood and risk of childhood fractures11 and osteoporosis later in life 2, which is probably due to the bone tracking phenomenon beginning in utero12 and emphasizes the importance of a preventive strategy initiated in early life. Interestingly, the National Osteoporosis Foundation has suggested early life bone accrual as the most influential factor for preventing current and future fractures.13
Recently, our randomized clinical trial (RCT)14 in the Copenhagen Prospective Studies on Asthma in Childhood 2010 (COPSAC2010) mother-child cohort showed that high-dose compared with standard-dose of vitamin D supplementation during pregnancy improved offspring bone outcomes assessed by DXA scans by age 6 years of life.15 Here, we analyzed serum 25-hydroxyvitamin D (25(OH)D) concentrations in childhood and aimed to investigate the combined effect of supplementation in pregnancy and vitamin D sufficiency during childhood on fracture risk and bone mineralization to guide future vitamin D supplementation strategies.
Methods
The COPSAC2010 vitamin D RCT
Participants were from the Danish mother-child cohort COPSAC2010 with enrollment of mothers during pregnancy and prospective monitoring during childhood with deep clinical phenotyping of the children, i.e. the children were followed longitudinally in the clinic at several time-points throughout childhood with collection of detailed information on asthma symptoms, treatment and diagnoses including several objective measurements to clinically phenotype the children. Baseline characteristics and enrollment procedures are previously detailed.14, 15, 16, 17 Healthy women were randomly assigned (1:1) at the COPSAC research clinic during pregnancy week 24 to a high-dose supplementation of 2400 IU/day of vitamin D or placebo on top of the standard recommended intake of 400 IU/day until 1 week after birth; i.e. a dose comparison study of 2800 IU/day vs. 400 IU/day of vitamin D. The exclusion criteria for the RCT were gestational age above week 26, daily vitamin D intake of more than 600 IU or having any endocrine, heart, or kidney disorders. The primary outcome was asthma/persistent wheeze in the first 3 years of life and the women also participated in a factorial design of n-3 long-chain polyunsaturated fatty acids (n-3 LCPUFA) RCT during pregnancy.16 Offspring bone mineralization was a pre-specified secondary outcome, whereas risk of fractures was added as a post hoc analysis.
The trial was registered on clinicaltrials.gov (NCT00856947) and approved by the Danish Ethics Committee (H-B-2008–093), the Danish Data Protection Agency and the Danish Health and Medicines Authority. Both parents gave written informed consent before enrollment. This study adheres to the STROBE reporting guidelines.
Serum 25(OH)D concentrations were measured at age 6 months and age 6 years (see details in Online Supplement). The method for quantitative determination of 25(OH)D is a chemiluminescence immunoassay (CLIA) using the DiaSorin LIAISON 25(OH)D Vitamin D Total Assay. The laboratory used National Institute of Standards and Technology (NIST) level 1 protocol and for quality control for all 25(OH)D measurements, samples of NIST level 1 standard reference material 972 (SRM 972) for vitamin D in human serum were included in each run.
Vitamin D sufficiency was defined as 25(OH)D ≥ 75 nmol/l, insufficiency as 25(OH)D < 75 nmol/l, and deficiency as 25(OH)D < 50 nmol/l according to recognized guidelines.18
Information on fractures was obtained by parental interviews at the COPSAC clinic and validated in the children's medical records until January 31, 2019, as previously described.15 We included all radiologically verified fractures of larger long bones (i.e., clavicle, radius, ulna, tibia, fibula, femur and humerus) in the analyses and excluded fissures (i.e., minor cracks). The fracture outcome was defined as a binary variable 1 (at least one fracture) or 0 (no fractures).
Whole-body DXA scans were performed at age 3 and 6 years with a Lunar iDXA densitometer (GE Healthcare, United States) with ENCORE software for bone mineral analyses with low radiation dose and short scan time.19 The children were scanned in one movement lasting approximately 3 min. All the scans were quality validated by an experienced specialist and only acceptable quality scans were included in the analyses. Weight and height were measured at the time of the scan. The analyses of bone mineral density (BMD) and bone mineral content (BMC) of the total body, total body less head (TBLH) and head were adjusted for body size, age and sex due to the influence of these growth parameters in previous studies.20, 21, 22, 23
The COPSAC2000 replication cohort
We sought replication in the Danish mother-child cohort the COPSAC2000.24 Pregnant mothers with a history of asthma were enrolled before pregnancy week 36 and monitored prospectively with deep clinical phenotyping from age 1 month through 18 years, including assessment of serum 25(OH)D concentrations at age 4 years and DXA scans at age 7 years with the same equipment as in COPSAC2010.24 The study was approved by the Danish Ethics Committee (KF 01–289/96).
Statistical analysis
The combined effect of the high-dose vitamin D supplementation in pregnancy and vitamin D status in childhood on fracture risk and DXA outcomes was analyzed in a four-group model according to intervention group (high-dose vs. standard-dose) and child vitamin D status (sufficient ≥ 75 nmol/l vs. insufficient < 75 nmol/l) combinations.
The effect of vitamin D status (sufficient vs. insufficient) and the combined effect of high-dose supplementation and vitamin D status (four-group model) on fracture risk was analyzed in a Quasi-Poisson regression model adjusted for observation time estimating the incidence rate ratio (IRR). The association between a history of fractures and DXA outcomes was analyzed using a multivariable linear regression model adjusting for age, sex, height and weight.
The effect of vitamin D status at age 6 months and 6 years on DXA outcomes at age 3 and 6 years was analyzed separately using multivariable linear regression models adjusted for age, sex, height and weight,20, 21, 22, 23 whereas the effect of vitamin D status at age 6 months in relation to bone mineralization outcomes was analyzed in a random intercept mixed-effects model including both DXA time points.
Additionally, sub-analyses were performed in a six-group model according to intervention group (high-dose vs. standard-dose) and vitamin D status (deficiency (< 50 nmol/l), insufficiency (≥ 50 nmol and < 75 nmol) and sufficiency (≥ 75 nmol/l)).
The analyses were further adjusted for the high-dose vitamin D and n-3 LCPUFA interventions and sample season.
Statistical analyses were performed with R (version 4.1.1) with p < 0.05 considered indicative of significance. The trial was powered for persistent wheeze as primary outcome. We did not perform a post hoc power calculation for the secondary outcomes. All participants randomized to the pregnancy vitamin D intervention and with complete data were included in the analyses; i.e. complete case analysis. No imputation was performed for missing data as we considered data to be missing completely at random.
Role of funding sources
The funding agencies did not have any role in design and conduct of the study; collection, management, and interpretation of the data; or preparation, review, or approval of the manuscript.
Results
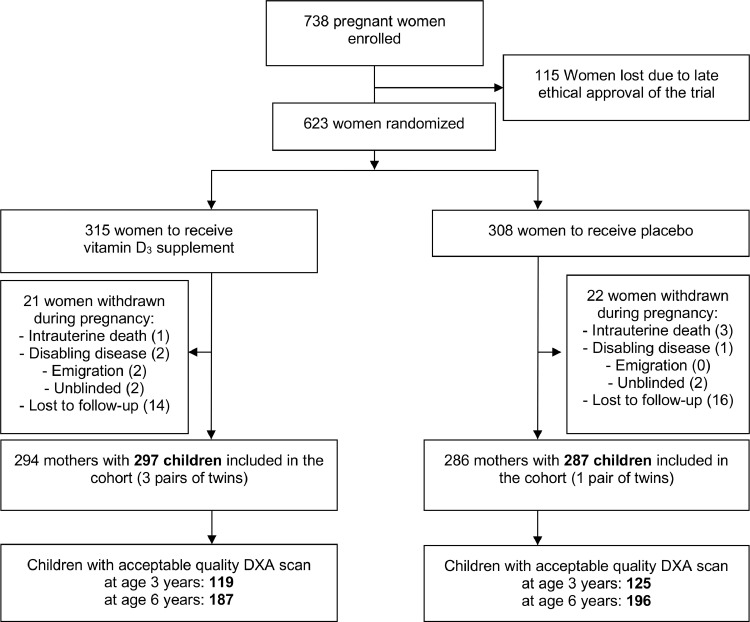
A total of 623 pregnant women were included in the COPSAC2010 vitamin D RCT, where 315 vs. 308 women were successfully randomized to high-dose or standard-dose vitamin D between March 4, 2009, to November 17, 201015 (Figure 1). Of the 584 included children, 541 (93%) and 428 (73%) had serum 25(OH)D concentrations measured at age 6 months and 6 years, respectively. There was no effect of the prenatal high-dose vitamin D supplementation on childhood serum 25(OH)D concentrations at age 6 months, mean difference (95% CI); −0.67 nmol/l (−4.68;3.33) p = 0.74.
Figure 1.
CONSORT Flowchart.
Fracture risk
Among the 541 children with serum 25(OH)D concentrations measured at age 6 months, 65% (n = 351) were vitamin D sufficient and 35% (n = 190) were insufficient. Of these 541 children, 9% (n = 51) had at least one fracture with 55 fractures registered in total (follow-up age, mean (SD): 8.5 (1.3) years). The distribution of fracture types was forearm 53% (n = 29), humerus 24% (n = 13), crus 14% (n = 8) and clavicle 9% (n = 5). A total of 8% (n = 27/351) of the vitamin D sufficient children had a history of fractures in early childhood vs. 13% (n = 24/190) of the vitamin D insufficient children (Table 1).
Table 1.
Risk of fractures in childhood by vitamin D status at age 6 months and in a combination with prenatal intervention group. IRR (incidence rate ratio) was calculated using a Quasi-Poisson regression model.
| 6 months vitamin D status: | Combined: 6 months vitamin D status and prenatal intervention |
|||
|---|---|---|---|---|
| Sufficient (n = 351) vs. insufficient (n = 190) | High-dose and sufficient (n = 179) vs. Standard-dose and insufficient (n = 93) | High-dose and insufficient (n = 97) vs. Standard-dose and insufficient (n = 93) | Standard-dose and sufficient (n = 172) vs. Standard-dose and insufficient (n = 93) | |
| Number of children with fractures,% (n) | 8% (27) vs. 13% (24) | 7% (12) vs. 17% (16) | 8% (8) vs. 17% (16) | 9% (15) vs. 17% (16) |
| Number of fractures, n | 30 vs. 25 | 13 vs. 17 | 8 vs. 17 | 17 vs. 17 |
| IRR | 0.64 (0.37;1.11), p = 0.11 | 0.40 (0.19;0.84), p = 0.02 |
0.47 (0.19;1.07) p = 0.08 | 0.54 (0.27;1.08) p = 0.08 |
| IRR adjusted | *0.61 (0.35;1.07), p = 0.08 | ⁎⁎0.38 (0.18;0.80), p = 0.01 | 0.45 (0.18;1.04) p = 0.07 | 0.50 (0.25;1.02) p = 0.05 |
adjusted for sample season, vitamin D and n-3 LCPUFA interventions.
adjusted for sample season and n-3 LCPUFA intervention.
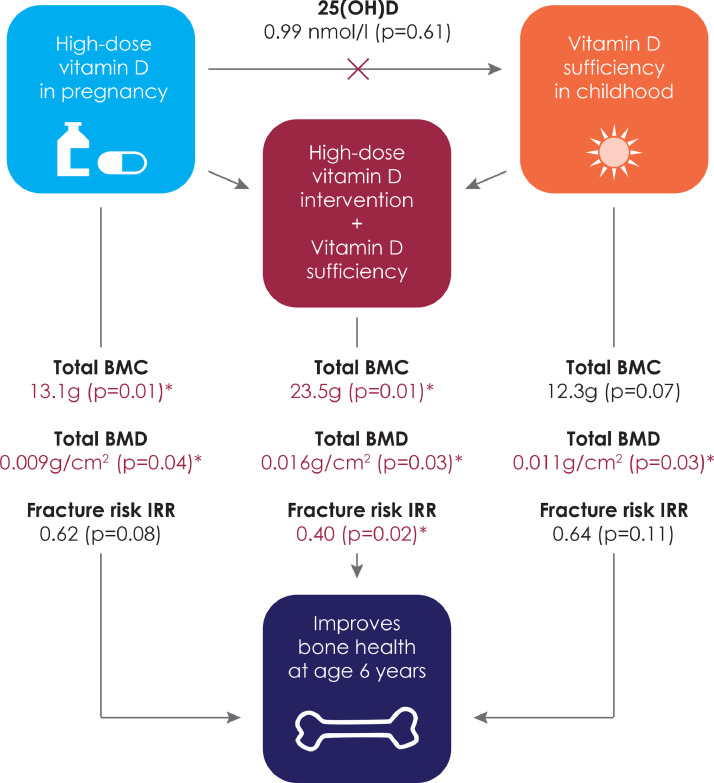
The risk of fractures was not associated with vitamin D status at 6 months: IRR (95% CI), 0.64 (0.37;1.11), p = 0.11. Vitamin D sufficient children at age 6 months born to mothers receiving high-dose vitamin D supplementation in pregnancy (n = 179) had a significantly lower risk of fractures compared with vitamin D insufficient children born to mothers receiving standard-dose (n = 93): 0.40 (0.19;0.84), p = 0.02 (Figure 2 and Table 1). Adjusting the analysis for the vitamin D intervention, n-3 LCPUFA intervention and sample season did not change the results (Table 1). There was no interaction between vitamin D status and the high-dose vitamin D intervention (pinteraction= 0.42).
Figure 2.
An overview of the effects of vitamin D in early life on childhood bone health.
Note: The effect of high-dose vitamin D in pregnancy was reported in Brustad, N. JAMA Pediatr 174, 419–427 (2020).
Children with a history of fractures had lower whole-body bone mineralization at age 6 years compared with children not having a fracture; total BMC: adjusted mean difference (aMD) for age, sex, height and weight (95% CI); −19.6 g (−38.9;−0.30), p = 0.047 (Fig. E1).
Bone mineralization
At age 6 years, 318 (83%) of 383 children with acceptable DXA scans also had assessment of serum 25(OH)D concentrations. Vitamin D sufficiency (n = 88) was significantly associated with higher total BMD: aMD for age, sex, height and weight (95% CI); 0.011 g/cm2 (0.001;0.021), p = 0.03, but not statistically significant higher total BMC; 12.3 g (−0.8;25.4), p = 0.07, TBLH BMD; 0.007 g/cm2 (−0.0004;0.015), p = 0.07, head BMD; 0.026 g/cm2 (−0.002;0.054), p = 0.07, and head BMC 6.5 g (−0.3;13.4), p = 0.06 (Table 2). Adjusting the analyses for the high-dose vitamin D intervention showed similar results (Table 2) and further adjusting for the n-3 LCPUFA intervention and sample season were similar to the main analyses (Table E1). The analyses stratified by intervention group are shown in Table E2.
Table 2.
DXA scan results at age 6 years by vitamin D status.
| COPSAC2010 | 6 years vitamin D status: sufficient (≥ 75 nmol/l) vs. insufficient (< 75 nmol/l) |
|||
|---|---|---|---|---|
| Age 6y DXA | Sufficient Mean (SD) n = 88 |
Insufficient Mean (SD) n = 230 |
aMD (95% CI) n = 318 |
aMD* (95% CI) n = 318 |
| Total BMD, g/cm2 | 0.722 (0.042) | 0.711 (0.040) | 0.011 (0.001;0.021) p = 0.03 | 0.011 (0.001;0.021) p = 0.04 |
| Total BMC, g | 836.0 (54.8) | 823.7 (51.6) | 12.3 (−0.8;25.4) p = 0.07 | 11.7 (−1.38;24.7) p = 0.08 |
| TBLH BMD g/cm2 | 0.563 (0.032) | 0.556 (0.031) | 0.007 (−0.0004;0.015) p = 0.07 | 0.007 (−0.001;0.015) p = 0.07 |
| TBLH BMC g | 533.7 (36.3) | 527.8 (34.9) | 5.8 (−3.1;14.6) p = 0.20 | 5.4 (−3.4;14.2) p = 0.23 |
| Head BMD g/cm2 | 1.434 (0.113) | 1.409 (0.113) | 0.026 (−0.002;0.054) p = 0.07 | 0.025 (−0.003;0.053) p = 0.08 |
| Head BMC g | 302.3 (27.6) | 295.9 (27.6) | 6.5 (−0.3;13.4) p = 0.06 | 6.2 (−0.6;13.1) p = 0.07 |
Vitamin D levels calibrated for age, sex, height and weight. aMD: Adjusted mean difference for age, sex, height and weight.
adjusted for age, sex, height, weight and vitamin D intervention.
The previously reported protective effect of high-dose vitamin D supplementation on bone mineralization at age 6 years was independent of serum 25(OH)D concentrations at age 6 years in the adjusted analyses (Table E3). Finally, there was no interaction between vitamin D status and prenatal high-dose vitamin D supplementation on any of the bone mineral outcomes (all pinteraction>0.05) and the supplementation effect was not mediated by vitamin D status in childhood (all pACME> 0.05) (Table E4).
There was no association between vitamin D status at age 6 months and DXA outcomes at age 3 and 6 years analyzed separately or in a random intercept mixed-effects model in COPSAC2010 (Table E5).
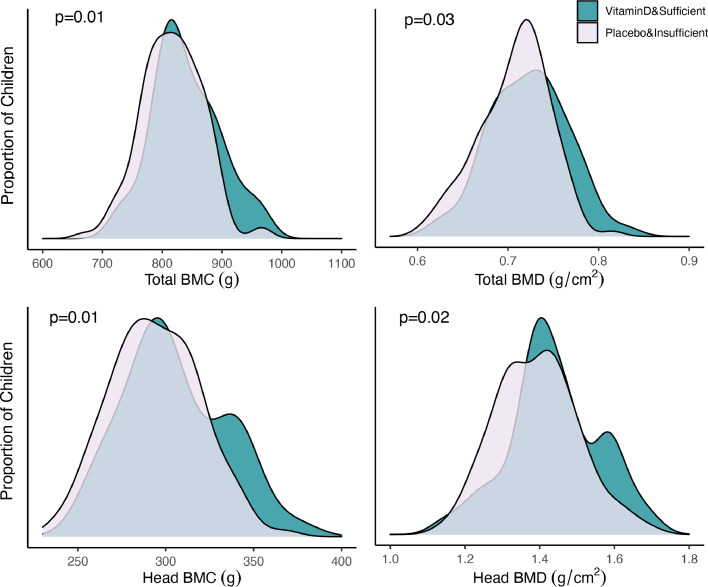
A combined analysis showed that children who were vitamin D sufficient at age 6 years and born to mothers in the high-dose vitamin D supplementation group (n = 47) had the highest bone mineralization, total BMD: aMD (95% CI); 0.016 g/cm2 (0.002;0.030), p = 0.03, total BMC: 23.5 g (5.5;41.5), p = 0.01 head BMD 0.048 (0.009;0.086), p = 0.02 and head BMC: 12.5 g (3.0;22.0) p = 0.01 compared with vitamin D insufficient children born to mothers in the standard-dose group (n = 120) (Table 3, Figure 3).
Table 3.
DXA scan results at age 6 years by combination of vitamin D status and prenatal high-dose vitamin D supplementation.
| COPSAC2010 | Combined: Prenatal supplementation and 6 year vitamin D status |
||
|---|---|---|---|
| Age 6y DXA | High-dose and sufficient (n = 47) vs. Standard-dose and insufficient (n = 120) aMD (95% CI) |
High-dose and insufficient (n = 110) vs. Standard-dose and insufficient (n = 120) aMD (95% CI) |
Standard-dose and sufficient (n = 41) vs. Standard-dose and insufficient (n = 120) aMD (95% CI) |
| Total BMD, g/cm2 | 0.016 (0.002;0.030) p = 0.03 | 0.009 (−0.002;0.019) p = 0.10 | 0.015 (0.0002;0.029) p = 0.047 |
| Total BMC, g | 23.5 (5.5;41.5) p = 0.01 | 13.8 (0.1;27.6) p = 0.049 | 13.9 (−5.0;32.7) p = 0.15 |
| TBLH BMD g/cm2 | 0.008 (−0.002;0.019) p = 0.13 | 0.005 (−0.004;0.013) p = 0.26 | 0.011 (−0.0002;0.022) p = 0.05 |
| TBLH BMC g | 11.0 (−1.1;23.1) p = 0.08 | 7.9 (−1.4;17.2) p = 0.10 | 8.0 (−4.7;20.7) p = 0.22 |
| Head BMD g/cm2 | 0.048 (0.009;0.086) p = 0.02 | 0.035 (0.005;0.064) p = 0.02 | 0.038 (−0.003;0.078) p = 0.07 |
| Head BMC g | 12.5 (3.0;22.0) p = 0.01 | 6.0 (−1.3;13.2) p = 0.11 | 6.0 (−4.0;15.9) p = 0.24 |
aMD: Adjusted mean difference for age, sex, height and weight.
Figure 3.
Density plots of total body and head BMD and BMC at age 6 years by vitamin D status at age 6 years in combination with pregnancy supplementation.
An additional combined analysis utilizing a six-group model dividing vitamin D status at age 6 years into sufficient, insufficient and deficient showed similar improvements in bone outcomes from the combination of high-dose intervention and vitamin D sufficiency (≥ 75 nmol/l) compared with standard-dose and deficiency (< 50 nmol/l) (Table E6). Interestingly, a higher bone mineralization was consistently observed in the vitamin D sufficient vs. insufficient children in all compartments suggesting an optimal bone beneficial threshold of 25(OH)D of at least 75 nmol/l in childhood (Fig. E2). We also analyzed the effect of vitamin D status at age 6 years divided into a threshold of sufficiency (≥ 50 nmol/l) vs. deficiency (< 50 nmol/l) on bone mineralization outcomes (Table E7).
Observational replication analyses in the COPSAC2000 cohort confirmed the positive effect of vitamin D sufficiency in childhood on bone mineralization: total BMC; aMD (95% CI): 18.7 g (1.8;35.6), p = 0.03 (Table E8).
Discussion
This study revealed a significant 60% lower incidence of fractures in children who were vitamin D sufficient at age 6 months and were born to mothers receiving high-dose vitamin D supplementation during pregnancy compared with vitamin D insufficient children born to mothers receiving standard-dose. Furthermore, sufficient vitamin D status at 6 years was associated with improved bone mineralization with independent effects of pregnancy high-dose supplementation and sufficient vitamin D status in childhood, showing a twofold increase in whole-body BMC compared with the individual effects of high-dose supplementation15 and sufficient serum 25(OH)D concentrations. The positive association between childhood vitamin D sufficiency and improved bone mineralization was confirmed in our replication analyses. Finally, we found that children having a fracture had lower whole-body BMC.
The strength of this study is the close longitudinal follow-up of the children with frequent scheduled visits at the COPSAC clinic and high follow-up rate.15 This allowed for thorough registration of fractures. Furthermore, the cohort is population-based allowing for generalization of the findings. The RCT, which is considered to be the most reliable scientific evidence with minimal risk of bias, was designed with persistent wheeze/asthma until age 3 years as the primary outcome, which is the main limitation of this study as it was not powered for fractures or DXA outcomes. Our sample size was limited by the inclusion of complete cases only due to the relatively low amount of DXA scans with acceptable quality. We assumed that our data were missing completely at random with no systematic differences, which was supported by a missing-completely-at-random statistical test for all our observed values (p = 0.175), but this could only be tested among our observed values and our missing data could potentially be related to any unobserved data allowing for differences between observed and missing cases. However, we were able to demonstrate an effect of the high-dose intervention on fractures and DXA outcomes, which was significant and nominally largest in the combined analyses integrating childhood vitamin D status with maternal high-dose supplementation. Another limitation is the lack of information on family history of fractures and detailed information on physical activity and diet of the children as bone health was not the primary outcome. The primary analyses are based on an RCT and confounders should be balanced, however, the observational association analyses between vitamin D status in childhood and bone outcomes could potentially be influenced by these confounders. Further, we found an overall effect on fractures but did not distinguish between types of fractures. We excluded mothers and children with disabling diseases, but we did not screen our study population for connective tissue, myogenic, neurogenic or endocrinologic disorders, which could increase the risk of fractures. In addition, we did not search for family history of sclerosing bone disorders, vascular/neural calcifications or family history of renal failure. However, these disorders are rare and should be evenly distributed given the RCT design of the study.
Low maternal 25(OH)D concentrations in pregnancy has been associated with an increased risk of fractures in the offspring and later osteoporosis,1 suggesting that the intrauterine environment plays an important role in bone health throughout life from prenatal programming.3 In addition, a link between low 25(OH)D concentrations, poor bone mineralization and increased childhood fracture risk has been shown in a pediatric population.25 This association was not confirmed in our study, which did not show a statistically significant lower incidence of fractures in children with vitamin D sufficiency at age 6 months. However, we demonstrated a significant 60% reduced fracture risk and the largest improvement in bone mineralization outcomes in vitamin D sufficient children whose mothers received high-dose vitamin D supplementation during pregnancy compared with vitamin D insufficient children whose mothers received standard-dose, which suggests a combined effect of high in utero exposure and sufficient childhood serum 25(OH)D concentrations. Importantly, there was no association between the pregnancy high-dose intervention and childhood vitamin D status and no evidence of interaction, suggesting that serum 25(OH)D concentrations in pregnancy and childhood have independent effects on bone mineralization outcomes. Further, our causal mediation analyses suggested that the effect of high-dose vitamin D intervention was not mediated through childhood vitamin D status. Finally, we demonstrated that fractures in childhood was associated with lower bone mineralization at age 6 years, which also suggests bone mineral content rather than density as the most sensitive DXA measurement predicting bone health in children.
Both prenatal and early postnatal life seem crucial for optimal bone health in childhood, which tracks into adulthood,26 affects peak bone mass, and most likely reduces future risk of osteoporosis.12 A theoretical analysis has suggested that a 10% increase in peak bone mass would delay the risk of osteoporosis by 13 years and identifies bone mass gain in early life as the single most important factor for preventing osteoporosis compared with age at menopause and non-menopausal bone loss.2 This view is supported by the National Osteoporosis Foundation stating that optimizing bone accrual early in life might be the most influential factor for preventing current and future fractures,13 which aligns with our findings. Osteoporosis is a major global health burden with high economic and individual costs and the number of osteoporotic fractures is expected to rise in the future due to an aging population,27,28 emphasizing the need of implementing preventive strategies. Optimizing vitamin D supplies during pregnancy and continuing to maintain vitamin D sufficiency with 25(OH)D concentrations ≥ 75 nmol/l through childhood could be a safe and cost-effective preventive approach.29
Our finding of association between childhood vitamin D status and bone mineralization is in line with most previous studies5, 6, 7 and is biologically plausible due to the well-known vitamin D effect on calcium and phosphate homeostasis,30 two key components in hydroxyapatite; i.e. bone mineral.31 Our results suggest a 25(OH)D beneficial threshold of minimum 75 nmol/l on bone mineralization and fractures, which is in line with current recommendations of vitamin D sufficiency from the Endocrine Society based on evidence showing up to a 65% increase in calcium absorption when going from 50 nmol/l to 75 nmol/l18 and where the inverse relationship with parathyroid hormone seems to reach a plateau.18,32
The current recommended vitamin D intake of 400 IU/day in infants from the American Academy of Pediatrics,32 the Institute of Medicine33 and the European Food Safety Authority4 is based on a 25(OH)D sufficiency concentration of 50 nmol/l for the prevention of rickets, which may be inadequate to reach our suggested beneficial threshold of 75 nmol/l for improved bone mineralization. The results from a vitamin D dose-response study among infants demonstrated 3.5-times and 9.7-times higher chances of reaching 75 nmol/l after 3 months of supplementation with 800 IU/day and 1200 IU/day, respectively, vs. 400 IU/day.34 The baseline 25(OH)D concentrations in that study were similar to what we observed in our study at age 6 years and are relatively high compared with studies of other ethnicities with more skin pigmentation35 where daily vitamin D requirements may be even larger. The number of intervention studies are limited, but a recent RCT36 in infants reported no differences in bone mineralization from vitamin D supplementation of 400 IU/day vs. 1200 IU/day, but the baseline mean 25(OH)D concentrations of the children was above 80 nmol/l and the findings may reflect that the effect of postnatal supplementation is minimal beyond the 75 nmol/l threshold. However, future large RCTs of infants and preschool children with supplementation around the tolerable upper intake levels of 1000 IU/day (up to 6 months), 1500 IU/day (6–12 months) and 2000 IU/day (from 12 months) are needed to establish the most beneficial vitamin D supplementation regime for optimizing childhood bone health. In our study, there was no evidence of toxicity with no children having 25(OH)D concentrations above the upper threshold of 250 nmol/l defined by the Endocrine Society.18 Further, a meta-analysis including 24 studies has suggested that vitamin D intervention doses during pregnancy up to 5000 IU/day should be considered safe.29
In conclusion, this study suggests an overall 60% reduced risk of fractures in vitamin D sufficient children whose mothers received high-dose vitamin D supplementation in pregnancy compared with vitamin D insufficient children whose mothers received standard-dose. This effect may be limited to certain types of fractures and may not include fractures caused by underlying skeletal diseases. In addition, overall independent effects of vitamin D status in childhood and supplementation in pregnancy on bone mineralization outcomes were demonstrated. These findings suggest vitamin D as a crucial micronutrient in early life for preventing fractures and promoting bone mineralization, which further may contribute to a lower risk of developing osteoporosis in addition to fractures later in life.
Contributors
Conceptualization: NB, BC and HB; Methodology: NB, BC and HB; Formal analysis: NB and JT; Investigation: NB and BC; Resources: BC, MK, JL, SW, JS, KB and HB; Data curation: NB, JT, MK; writing – original draft: NB; writing – review and editing: All authors. NB, BC, MK and HB are responsible for the raw data associated with this study. All authors have read and agreed to the published version of the manuscript and took the decision to submit for publication.
Data sharing statement
Data are available upon request to the corresponding author.
Funding
The study was supported by The Lundbeck Foundation R16-A1694, The Danish Ministry of Health 903,516, The Danish Council for Strategic Research 0603–00280B and The European Research Council 946,228.
Declaration of interests
All authors declare no conflict of interest regarding the content of this manuscript. The funding agencies did not have any role in design and conduct of the study; collection, management, and interpretation of the data; or preparation, review, or approval of the manuscript. JL reports receiving grants from the NIH and the Simons Foundation for Autism and a leadership role in the Metabolomics Society outside of the submitted work. SW reports receiving a grant UH3 OD023268 from the NIH and honoraria from UPTODATE outside of the submitted work. JS reports receiving grants from the NNF and DFF outside of the submitted work. KB reports receiving consulting fees from Sanofi and Astra Zeneca, honoraria from Boehringer Ingelheim and participation in an advisory board from ALK-Abelló Nordic outside of the submitted work.
Acknowledgments
We express our deepest gratitude to the children and families of the COPSAC2000 and COPSAC2010 cohort studies for all their support and commitment. We acknowledge and appreciate the unique efforts of the COPSAC research team.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101254.
Appendix. Supplementary materials
References
- 1.Holick M.F. Resurrection of vitamin D deficiency and rickets. J Clin Investig. 2006;116:2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernandez C.J., Beaupré G.S., Carter D.R. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int. 2003;14:843–847. doi: 10.1007/s00198-003-1454-8. [DOI] [PubMed] [Google Scholar]
- 3.Cooper C., Walker-Bone K., Arden N., Dennison E. Novel insights into the pathogenesis of osteoporosis: the role of intrauterine programming. Rheumatology. 2000;39:1312–1315. doi: 10.1093/rheumatology/39.12.1312. (Oxford) [DOI] [PubMed] [Google Scholar]
- 4.EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2016 Scientific opinion on dietary reference values for vitamin D. EFSA J. 2016;14:4547. doi: 10.2903/j.efsa.2016.4547. [DOI] [Google Scholar]
- 5.Pekkinen M., Viljakainen H., Saarnio E., Lamberg-Allardt C., Mäkitie O. Vitamin D is a major determinant of bone mineral density at school age. PLoS One. 2012;7:e40090. doi: 10.1371/journal.pone.0040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hazell T.J., et al. Vitamin D status is associated with bone mineral density and bone mineral content in preschool-aged children. J Clin Densitom. 2015;18:60–67. doi: 10.1016/j.jocd.2014.04.121. [DOI] [PubMed] [Google Scholar]
- 7.Boot A.M., Krenning E.P., de Muinck Keizer-Schrama S.M.P.F. The relation between 25-hydroxyvitamin D with peak bone mineral density and body composition in healthy young adults. J Pediatr Endocrinol Metab. 2011;24:355–360. doi: 10.1515/jpem.2011.052. [DOI] [PubMed] [Google Scholar]
- 8.Stein E.M., et al. Serum 25-hydroxyvitamin D concentrations in girls aged 4–8 y living in the southeastern United States. Am J Clin Nutr. 2006;83:75–81. doi: 10.1093/ajcn/83.1.75. [DOI] [PubMed] [Google Scholar]
- 9.Zhu K., et al. Maternal vitamin D status during pregnancy and bone mass in offspring at 20 years of age: a prospective cohort study. J Bone Miner Res. 2014;29:1088–1095. doi: 10.1002/jbmr.2138. [DOI] [PubMed] [Google Scholar]
- 10.Javaid M., et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 11.Clark E.M., Tobias J.H., Ness A.R. Association between bone density and fractures in children: a systematic review and meta-analysis. Pediatrics. 2006;117:e291–e297. doi: 10.1542/peds.2005-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heaney R.P., et al. Peak bone mass. Osteoporos Int. 2000;11:985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 13.Weaver C.M., et al. The national osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27:1281–1386. doi: 10.1007/s00198-015-3440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisgaard H., et al. Deep phenotyping of the unselected COPSAC2010 birth cohort study. Clin Exp Allergy. 2013;43:1384–1394. doi: 10.1111/cea.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brustad N., et al. Effect of high-dose vs standard-dose vitamin d supplementation in pregnancy on bone mineralization in offspring until age 6 years: a prespecified secondary analysis of a double-blinded, randomized clinical trial. JAMA Pediatr. 2020;174:419–427. doi: 10.1001/jamapediatrics.2019.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bisgaard H., et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530–2539. doi: 10.1056/NEJMoa1503734. [DOI] [PubMed] [Google Scholar]
- 17.Schoos A.M.M., et al. Environmental and genetic determinants of serum 25(OH)-vitamin D levels during pregnancy and early childhood. Children Basel. 2019;6:116. doi: 10.3390/children6100116. PMID: 31640192; PMCID: PMC6826446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holick M.F., et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 19.Bachrach L.K. Osteoporosis and measurement of bone mass in children and adolescents. Endocrinol Metab Clin N Am. 2005;34:521–535. doi: 10.1016/j.ecl.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Warner J.T., et al. Measured and predicted bone mineral content in healthy boys and girls aged 6-18 years: adjustment for body size and puberty. Acta Paediatr. 1998;87:244–249. doi: 10.1080/08035259850157264. [DOI] [PubMed] [Google Scholar]
- 21.Prentice A., Parsons T., Cole T. Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr. 1994;60:837–842. doi: 10.1093/ajcn/60.6.837. [DOI] [PubMed] [Google Scholar]
- 22.Rupich R.C., Specker B.L., Lieuw-A-Fa M., Ho M. Gender and race differences in bone mass during infancy. Calcif Tissue Int. 1996;58:395–397. doi: 10.1007/BF02509436. [DOI] [PubMed] [Google Scholar]
- 23.Zemel B.S., et al. Height adjustment in assessing dual energy X-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bisgaard H. The copenhagen prospective study on asthma in childhood (COPSAC): design, rationale, and baseline data from a longitudinal birth cohort study. Ann Allergy Asthma Immunol. 2004;93:381–389. doi: 10.1016/S1081-1206(10)61398-1. [DOI] [PubMed] [Google Scholar]
- 25.Ryan L.M., et al. Bone mineral density and vitamin D status among African American children with forearm fractures. Pediatrics. 2012;130:e553–e560. doi: 10.1542/peds.2012-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budek A.Z., Mark T., Michaelsen K.F., Mølgaard C. Tracking of size-adjusted bone mineral content and bone area in boys and girls from 10 to 17 years of age. Osteoporos Int. 2010;21:179–182. doi: 10.1007/s00198-009-0932-z. [DOI] [PubMed] [Google Scholar]
- 27.Si L., Winzenberg T.M., Jiang Q., Chen M., Palmer A.J. Projection of osteoporosis-related fractures and costs in China: 2010-2050. Osteoporos Int. 2015;26:1929–1937. doi: 10.1007/s00198-015-3093-2. [DOI] [PubMed] [Google Scholar]
- 28.Rosengren B.E., Björk J., Cooper C., Abrahamsen B. Recent hip fracture trends in Sweden and Denmark with age-period-cohort effects. Osteoporos Int. 2017;28:139–149. doi: 10.1007/s00198-016-3768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bi W.G., et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality. JAMA Pediatr. 2018;172:635–645. doi: 10.1001/jamapediatrics.2018.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeLuca H.F. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 31.Jeong J., Kim J.H., Shim J.H., Hwang N.S., Heo C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater Res. 2019;23:4. doi: 10.1186/s40824-018-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner C.L., Greer F.R., American Academy of Pediatrics Section on Breastfeeding and Committee on Nutrition Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 33.Institute of Medicine (US) Dietary reference intakes for calcium and vitamin D. National Academies Press; US: 2011. Committee to review dietary reference intakes for vitamin D and calcium. [PubMed] [Google Scholar]
- 34.Gallo S., et al. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA. 2013;309:1785–1792. doi: 10.1001/jama.2013.3404. [DOI] [PubMed] [Google Scholar]
- 35.Mansbach J.M., Ginde A.A., Camargo C.A. Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics. 2009;124:1404–1410. doi: 10.1542/peds.2008-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosendahl J., et al. Effect of higher vs. standard dosage of vitamin D3 supplementation on bone strength and infection in healthy infants: a randomized clinical trial. JAMA Pediatr. 2018;172:646–654. doi: 10.1001/jamapediatrics.2018.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.