Abstract
Background
Influenza-like Illness (ILI) refers to a wide range of viral infections with an important cause of morbidity and mortality worldwide. The global incidence of ILI is estimated at 5–10% in adults and 20–30% in children. In India influenza accounts for 20–42% of monthly acute medical illness hospitalizations during the peak rainy season. AYUSH-64, a poly-herbal drug, is in practice for 40 years for various clinical conditions like fevers, microfilaremia, and inflammatory conditions.
Objective
A pilot study was conducted to evaluate the safety and efficacy of Ayurvedic formulation, AYUSH-64 in clinically diagnosed ILI for accelerating the recovery.
Material and Methods
A prospective, open-label, nonrandomized, single group, single-center pilot clinical study with pre-test and post-test design was conducted at Raja Ramdeo Anandilal Podar Central Ayurveda Research Institute for Cancer, Mumbai, an institute of Central Council for Research in Ayurvedic Sciences (CCRAS) between June 2018 and July 2019. A total of 38 participants of clinically diagnosed ILI (18–65 years) were studied with an one-week intervention of ‘AYUSH 64’ in a dose of 3 gm/day and three weeks post-treatment observation period. Assessment of parameters viz. improvement in the symptoms of ILI, frequency of usage of acetaminophen, antihistaminic and cough syrup, hematology, liver function and kidney function tests along with incidence of secondary complications, and time to return to a normal routine was done.
Results
One-week intervention of AYUSH 64 helped to recover from ILI symptoms with reduced frequency of usage of acetaminophen and antihistaminic. The intervention was safe on hematology and biochemical parameters. No serious adverse effects were observed during the study.
Conclusion
AYUSH 64 along-with standard care in ILI is safe and efficacious and this may be used in other viral infections with pyrexia as add-on to standard care for early recovery and better outcome.
Keywords: Influenza-like illness (ILI), Vata-Kaphaja Jvara, AYUSH 64, Safety, Efficacy, Ayurveda intervention
1. Introduction
Influenza is an important cause of morbidity and mortality worldwide including low- and middle-income countries (LMICs) in tropical regions like India where highest point estimates rates of Influenza-like illness (ILI) and influenza-associated ILI among young and older adults [1]. ILI refers to a wide range of viral infections with clinical manifestation ranging from fever, malaise and other constitutional symptoms mostly depending upon tissue, organ affected, host defense, age of the patient, and the affecting strain of the virus and bacterial load. Management of uncomplicated ILI remains symptom-based rather than antiviral therapy where in antipyretic, antihistaminic or cough syrup is used for symptomatic relief which again has its own side effects. Patients are advised to take rest and maintain proper hydration during illness and asked to return to full activity after gradual recovery from illness. Viruses constantly change their antigenicity as a strategy for evasion from immune-mediated elimination [2]. The classical example is of influenza virus where this strategy leads to the emergence of new strains and the failure of previously generated immunity protects against fresh infections. This has led to the difficulties in generating vaccines against these infections. The unavailability of optimal medication which would effectively hinder the disease pathogenesis while easing the recovery process and simultaneously boosting the immune mechanism has probed the exploration of traditional and such other medicines to promote natural healing in ILI.
The clinical presentation of ILI is similar with Vata-Kapha Jvara (fever with predominance of Vata and Kapha dosha) as per the description in Ayurveda classics [3]. The treatment of Jvara aims at digestion of ama (partially digested metabolic waste) through langhana (fasting/restricted calorie intake) and formulations (single herb/polyherbal) containing ingredients having tikta rasa, ushna virya, katu vipaka and amapachaka (anti-inflammatory) properties [4]. Many botanicals which are documented in Ayurveda Pharmacopoeia viz. Swertia chirata (Kiratatikta), Alstonia scholaris (Saptaparna), Pircrorria kurroa (Kutaki) etc. are traditionally used for fevers like malaria [5]. AYUSH 64, a polyherbal formulation is in practice since 1980s against various conditions like fevers, inflammation and joint pains across CCRAS peripheral institutes. Its efficacy has been reported in conditions like Vishamjvara (malarial fever), Shleepada (microfilaremia) and chikungunya. During the malaria epidemic in Rajasthan and Assam in 1994 and 1996 respectively, AYUSH 64 was used in 3600 fever cases in Rajasthan and 2294 fever cases in Assam. A decline in infectivity rate was observed when AYUSH 64 was given to all fever cases along with anti-malarial, and a good response was reported in villages where AYUSH 64 administration was done on prophylaxis basis for fever cases [[6], [7], [8], [9], [10]]. Vishamjvara and Vata-kapha jvara vis-à-vis ILI are the types of Jvara and share common pathology, however Vata and Kapha doshas are predominant in ILI and manifest symptoms as Shitaka (chills), Gaurava (heaviness), Parvaruka (pain in small joints), Pratishyaya (cold/catarrh), and Kasa (cough), etc. The ingredients in AYUSH 64 are Jwarahara (useful in fevers), Shwasa-Kaasaghna (useful in asthma, cough), Shothahara (relieves inflammation). Details of pharamalogical profile of plant ingredients present in AYUSH 64 is provided in Table 1. On this background, this pilot study was aimed to evaluate safety and efficacy of AYUSH64 in the management of clinically diagnosed ILI.
Table 1.
Details of pharmacological profile of plant ingredients present in AYUSH 64.
| Name of the plant | Rasa | Guna | Virya | Vipaaka | Karma |
|---|---|---|---|---|---|
| Saptaparna [[37], [38], [39], [40], [41]] (Alstonia scholaris R. Br.) | Tikta, Kashaya | Sara, Snigdha, Deepan, Laghu | Ushna | Katu | Shoolahara (relieves pain), Gulmahara (relieves bloating), Krimihara (wormicidal), Hrudya (cardiac tonic), Shwashara (userful in asthma), Vranahara (wound healer), Asradoshahara (useful in blood related diseases), Jantuhara (antihelminthic), Tridoshaghna (pacifies kapha, pitta and vata), Jwarahara (useful in fevers) |
| Katuki [42] (Picrorhiza kurroa Royle ex. Benth) | Tikta | Laghu, Ruksha | Shita | Katu | Bhedana (causes purgation), Deepana (improves digestion), Hrudya (cardiac tonic), Jwarahara (useful in fevers), Vishamjvaranashini ( useful in recurrent fevers) Kaphapittahara (pacifies Kapha and pitta), Pramehaghna (useful in urinary disorders/ diabetes), Shwasa-Kaasaghna (useful in asthma, cough), Dahaghna (relieves burning sensation), Kushthaghna (userful in skin disorders) |
| Kiratatikta [43] (Swertia Chirata Pexbex. Karst) | Tikta | Laghu, Ruksha | Shita | Katu | Sannipatajwarahara (useful in chronic and recurrent fevers), Shwasahara (userful in asthma), Kaphapittahara (pacifies Kapha and pitta), Asradoshahara (useful in blood related diseases), Dahashamana (relieves burning sensation), Kasahara (useful in cough), Shothahara (relieves inflammation), Trishnashamaka (relieves thirst), Kushthahara (useful in skin disorders), Vranahara (wound healer), Krimihara (wormicidal) |
| Kuberaksha [44,45] (Caesalpinia crista L.) | Katu, Tikta, Kashaya | Laghu Ruksha | Ushna | Katu |
Kaphavatahara (pacifies Kapha and vata), Deepana (improves digestion), Shoolaghna (relieves pain), Gulmanaashaka (relieves bloating), Kriminashaka (wormicidal), Kushthanaashaka (useful in skin disorders), Pramehajit (useful in urinary disorders/ diabetes), Pittarshanaashaka (useful in haemorrhoids), Vamihara (antiemetic) Vishama jvarahara (useful in recurrent fevers), Kasahara (useful in cough) |
2. Methods
2.1. Study design and trail site
As 4-week single-center pilot study was conducted in a prospective, open-label, non-randomized, single group, pre and post-test design at Raja Ramdeo Anandilal Podar Central Ayurveda Research Institute for Cancer, an institute of CCRAS, Ministry of AYUSH, Government of India.
2.2. Patient enrolment and inclusion criteria
Patients who were clinically diagnosed cases with ILI having axillary temperature ≥38 °C and with at least two constitutional symptoms (headache, chills, myalgia or fatigue) and one respiratory symptom (cough, sore throat or coryza) with the onset of illness since not more than 36 h, of either sex, age between 18 and 65 years and who voluntarily signed the informed consent to participate in the study were included in the study.
2.3. Exclusion criteria
Exclusion criteria were the following: Cases of bronchitis, pneumonia, pleural effusion, interstitial lesions, patients having white blood cells (WBC) count greater than the upper limit of normal (WBC>11.0 × 109 L) and lesser than normal (WBC<4.0 × 109/L) or neutrophil count ≥75%, patients coughing purulent sputum or with suppurative tonsillitis, patients with uncontrolled diabetes, COPD, hepatic insufficiency (ALT or AST 2 times above normal or higher); renal insufficiency (serum creatinine more than the upper lab value); chronic congestive heart failure, psychiatric diseases, patients who already have taken antiviral drugs or related traditional medicine after the onset/before the screening, women in pregnancy or lactation period, women of childbearing age with a plan of a pregnancy, immune-compromised patients or taking immunosuppressant in last 3 months, with dubious or confirmed alcohol and drug abuse history, suffering from an acute respiratory infection, otitis media or sinusitis 2 weeks before, with history of vaccination for seasonal or new influenza A (H1N1) vaccine 6 months before.
2.4. Consent and ethical approval
The study was approved by the Institutional Ethics Committee of this institute and registered prospectively at CTRI (CTRI/2017/10/010145 Registered on: 23/10/2017). Recruitment of participants was done as per the inclusion and exclusion criteria after taking informed written consent from the eligible patients.
2.5. Intervention
The study drug AYUSH-64 was procured from Indian Medicines Pharmaceutical Corporation Limited (IMPCL) Almora, Uttarakhand, India. Each capsule of AYUSH 64 consists of A. scholaris R. Br (Saptaparna) bark aqueous extract 100 mg, P. kurroa Royle ex. Benth (Katuki) root aqueous extract 100 mg, S. chirata Pexbex. Karst (Kiratatikta) whole-plant aqueous extract 100 mg, C. crista L. (Kuberaksha) seed powder 200 mg. Quality control and safety parameters of the ingredients and the formulation complied with API limits/Inhouse limits as appropriate (Quality Control Analysis enclosed as supplementary files).
The drug was given in a dose of 2 capsules (500 mg) thrice daily i.e. 3 gm/day orally after food along-with water for 7 days from baseline. One of the authors, a specialist in modern medicine prescribed acetaminophen, antihistaminic and cough syrup as per the standard guidelines and monitored the patients along with Ayurveda physicians.
2.6. Outcome measure/efficacy evaluation
The efficacy evaluation of the drug was done by assessing the ILI symptoms viz. fever, headache, myalgia, running nose, nasal obstruction/congestion, cough, sore throat, fatigue, chills/aversion to cold and sweating. The symptoms were assessed on a scale of 0–100 cm using VAS, where 0 represents no symptom and 100 represents the maximum severity of the symptom [11]. These symptoms were assessed at baseline and on 3rd, 7th, 14th, 21st and 28th day. Improvement in fever was measured as time to defervescence, i.e. the time from the first dose of study medication to the time when the body temperature declined to 37.4 °C or below and was sustained for ≥24 h. Severity of the illness was assessed by computing the area under the curve (AUC) for all the symptoms together. Average score of all the ten symptoms for each patient was computed for calculating the AUC. The area under the curve was evaluated between two consecutive time points. Integration approximating with the trapezoidal rule was used to calculate the areas [12]. The frequency of usage of acetaminophen, antihistaminic and cough syrup and incidence of secondary complications of ILI e.g. bronchitis, otitis media, pneumonitis etc. and adverse reactions related to the intervention were noted at all visits. The time to return to normal state of health and activity was defined as the time (in hours) from study drug initiation to the first 24h period in which participants returned to their normal state and remained so for 24h. The participants were assessed for Ayurvedic variables with the interest to study diagnostic classifications according to Ayurveda (Table 2).
Table 2.
Timeline procedure of the study.
| Time Points | Schedule of Activity |
|---|---|
| Prior to selection (Screening Visit) |
|
| During Selection (Baseline Visit, 0 day) |
|
| During Intervention (3rd Day) |
|
| End of Intervention (7th Day) |
|
| Post Intervention- Follow-up (14th and 21st Day) |
|
| Post Intervention Follow-up (28thDay) |
|
2.7. Sample size and statistical methods
As a pilot study, sample size of 30 + 25% drop out amounting to 38 was considered for study. The data on discrete variables has been represented as n (%). The data on continuous variables has been represented as mean (SD). The continuous data has been analyzed by using One-way Repeated Measure Anova with Bonferroni correction in post-hoc analysis. A p-value of <0.05 has been considered significant. The data was analyzed using SPSS Version 15.0.
3. Results
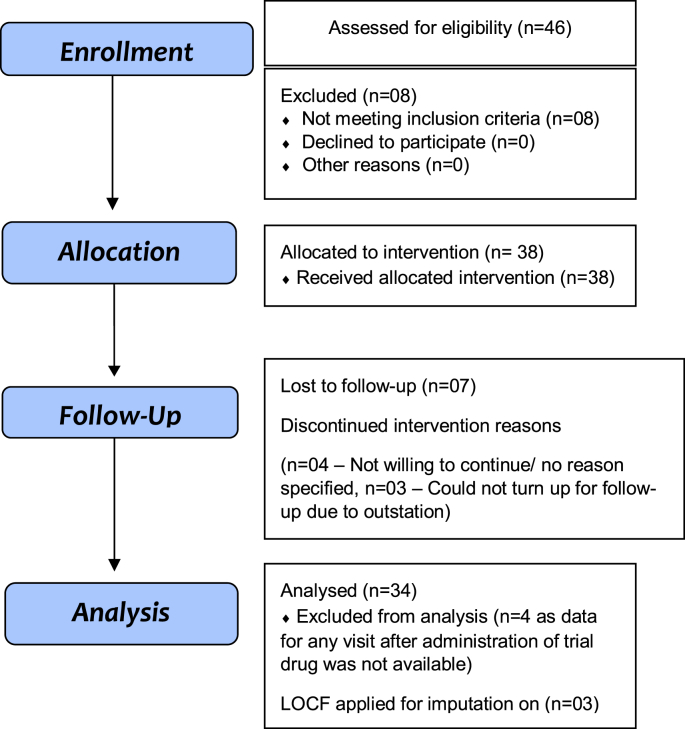
Between June 2018 and July 2019, a total of 46 participants were screened, out of which 38 were enrolled in the study (Fig. 1). The patients were given AYUSH 64 for 7 days only and later observed for 21 days. Response assessment was done on day 3, 7, 14, 21 and 28.
Fig. 1.
CONSORT 2010 Flow Diagram.
3.1. Baseline data
Out of a total 34 participants, 14 patients were male and 20 were females. All the participants were residents of Mumbai. No participant was having diabetes mellitus; however one participant was a known case of hypertension with ongoing medicine. Acute onset of disease was observed in 32 participants and 2 participants had insidious onset of symptoms of ILI. The demographic details of the participants is provided in Table 3.
Table 3.
Demographic Profile of the participants.
| Variables | n (%) |
|---|---|
| Gender | |
| Male | 14 (41.2) |
| Female | 20 (58.8) |
| Age Group | |
| 23 - 32 | 14 (41.2) |
| 33 - 42 | 7 (20.6) |
| 43 - 52 | 7 (20.6) |
| 53 - 62 | 5 (14.7) |
| 63- 64 | 1 (2.9) |
| Marital Status | |
| Married | 23 (67.6) |
| Unmarried | 8 (23.5) |
| Widow(er) | 2 (5.9) |
| Divorcee | 1 (2.9) |
| Educational status | |
| Illiterate | 1 (2.9) |
| Read and write | 33 (97.1) |
| Occupation | |
| Desk work | 6 (17.6) |
| Field work with physical labour | 9 (26.5) |
| Field work | 8 (23.5) |
| House wife | 11 (32.4) |
| Socio-economic status | |
| Above poverty line | 28 (82.6) |
| Below poverty line | 6 (17.4) |
| Religion | |
| Hindu | 32 (94.1) |
| Muslim | 1 (2.9) |
| Others | 1(2.9) |
3.2. Outcomes and estimation
Mean Visual Analogue Scale (VAS) scores for all the ILI symptoms at different time points have been enlisted in (Table 4) Statistically significant improvement was seen in all the ILI symptoms from day 3 onwards. Fever was completely relieved in 18 participants on 3rd day and in 10 participants on 7th day of intervention. At day 3, 16 patients took acetaminophen, 8 participants till 7th day. Later 6 patients took acetaminophen out of which only one participant needed it for fever, others had taken for headache or body ache. Antihistaminic and cough syrup were taken by 6 participants till day 7. Normal health was achieved by 6 participants on day 3 while 17 participants achieved it on day 7 and 3 participants on day 14. (Table 5).
Table 4.
Effect of trial drug on chief complaints.
| Chief Complaints | Assessment Stages |
|||||
|---|---|---|---|---|---|---|
| Baseline | 3rd day | 7th day | 14th day | 21st day | 28th day | |
| Headache | 57.65 ± 32.127 | 29.12 ± 24.385** | 10.88 ± 14.846** | 10.44 ± 18.273** | 4.85 ± 8.302** | 6.91 ± 14.356** |
| Myalgia | 67.35 ± 26.975 | 31.32 ± 28.744** | 11.03 ± 15.801** | 11.03 ± 18.537** | 4.85 ± 8.918** | 7.21 ± 14.934** |
| Running Nose | 33.68 ± 32.921 | 15.29 ± 25.254** | 8.09 ± 20.114** | 5.00 ± 15.076** | 5.15 ± 13.953** | 4.12 ± 10.185** |
| Nasal Obstruction / Congestion | 36.62 ± 32.769 | 18.97 ± 21.943* | 6.47 ± 14.063** | 5.29 ± 11.930** | 6.62 ± 15.750** | 2.65 ± 6.989** |
| Cough | 36.91 ± 29.362 | 21.91 ± 27.192* | 17.50 ± 23.940** | 9.71 ± 19.304** | 11.76 ± 24.676** | 5.15 ± 14.061** |
| Sore Throat | 45.88 ± 31.754 | 17.79 ± 22.737** | 12.65 ± 20.197** | 8.24 ± 16.091** | 7.94 ± 17.927** | 3.97 ± 12.540** |
| Fatigue | 73.38 ± 17.655 | 42.35 ± 26.834** | 23.68 ± 24.812** | 14.56 ± 19.124** | 9.85 ± 13.953** | 9.26 ± 13.714** |
| Chills | 39.12 ± 31.273 | 8.97 ± 17.046** | 2.50 ± 6.770** | 1.47 ± 4.357** | 1.03 ± 4.569** | 0.59 ± 2.388** |
| Sweating | 33.53 ± 29.427 | 11.76 ± 18.459** | 2.94 ± 7.084** | 4.03 ± 10.656** | 1.53 ± 4.907** | 1.32 ± 4.816** |
Values have been expressed as Mean ±SD. *p-value <0.01, **p-value<0.001.
Compared using Repeated Measure ANOVA with bonferroni correction.
Table 5.
Effect on other parameters- Frequency of using other medicines etc.
| Assessment Criteria: n | Baseline | Day 3 | Day 7 | Day14 | Day 21 | Day 28 |
|---|---|---|---|---|---|---|
| Frequency of use of Acetaminophen | 1 | 16 | 8 | 1 | 3 | 2 |
| Frequency of use of Antihistaminic | 2 | 5 | 3 | 0 | 1 | 0 |
| Frequency of use of Cough syrups | - | 1 | 3 | 0 | 0 | 0 |
| Time to return to normal health | - | 5 | 14 | 9 | 5 | 1 |
| Complete defervescence | - | 16 | 13 | 4 | 1 | 0 |
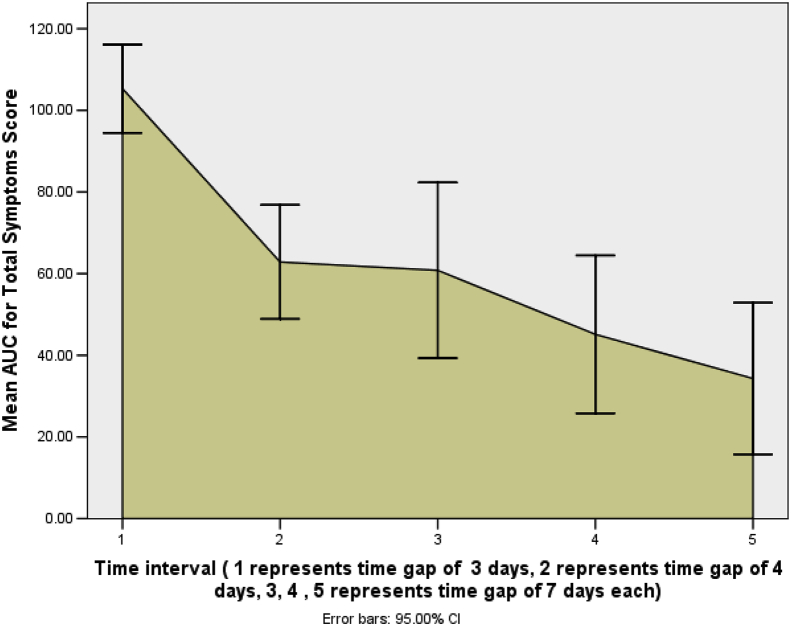
Severity of illness as assessed by mean AUC for total symptoms score at 1st time interval of 3 days was 105.30 (95% CI: 94.49 to 116.12), at 2nd time interval of 4 days (day 7) was 62.85 (95% CI: 48.89 to 76.81), at 3rd time interval of 7 days (day 14) it was 60.81 (95% CI: 39.30 to 82.32), at 4th time interval of another 7 days (day 21) it was 45.12 (95% CI: 25.80 to 64.45), and at the 5th time interval of 7 days (day 28) it was 34.30 (95% CI: 15.77 to 52.82). This shows a continuous decrease in the severity of illness with the passage of time (Fig. 2).
Fig. 2.
Severity of illness as assessed by mean AUC for Total Symptoms Score.
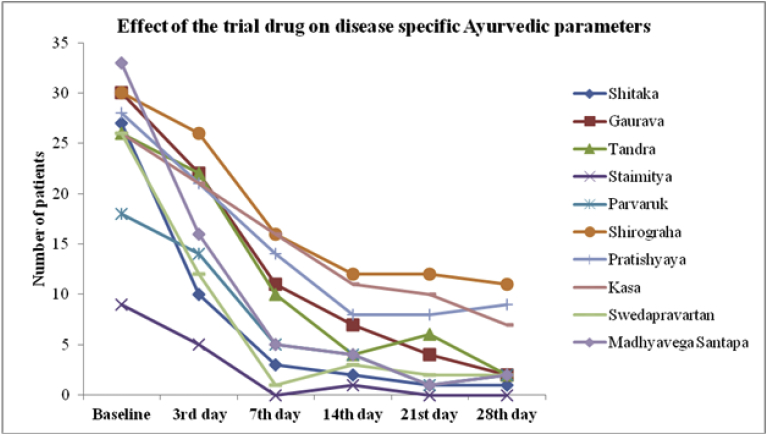
Ayurveda variables viz. Shitaka (chills), Gaurava (generalized heaviness), Tandra (lethargy), Staimitya (feeling as if wrapped up/numb), Parvaruk (pain in joints), Shirograha (headache), Pratishyaya (cold), Kasa (cough), Swedapravartan (absence of sweating), Madhyavega Santapa (mild fever) seen to have sharp decline by the 7th day of intervention and slow decline in the post treatment phase (Fig. 3).
Fig. 3.
Effect of the trial drug on disease specific Ayurvedic parameters.
Hematological parameters including liver function test and kidney function tests were within the normal limits during the treatment period and no significant change was observed in either of them. Secondary complications of ILI were not observed during the study period in any participant. No serious adverse reactions related to the intervention were reported in any participant.
4. Discussion
ILI refers to a wide range of viral infections, quite common in children and old people due to lowered immunity. The conventional management of ILI depends on severity, mild to moderate cases of category A and B are managed by acetaminophen, antihistaminic and severe cases of category C are treated with antiviral, antibiotics, intravenous fluids and other supportive care [13]. In an internet-based cohort study, it was found that the quality-adjusted life-day (QALD) loss was found to be over three times higher for ILI than for acute respiratory infections [14]. Early recovery is needed for returning to jobs/works along-with arrest to spread of infection. The innate immune response plays a crucial role in the viral elimination and recovery from disease.
It is reported that the symptom scores in influenza infections are directly correlated with IL-6 and IFN-α [15]. Significant production of IL-6, TNF- α, IFN- α, IFN-γ and IL-10 occurs in response to community acquired influenza A illness [16]. Bian et al. has noted that the levels of interleukin (IL)- 6, IL-33 and tumor necrosis factor (TNF)- α were significantly higher in Influenza A patients while IL-6, IL-17 A, IL-29, interferon (IFN)- γ and interferon gamma-induced protein (IP)-10 were significantly higher in Influenza B patients [17].
Multiple host-based intervention strategies against influenza are under development and these offer advantages over conventional antivirals. New therapeutic options in the treatment of Influenza infection will be targeting virus-induced metabolic changes to restore host normal metabolism, and research in the immunometabolism field along with studies on modulating immune response [18]. A class of herbal medicines, known as immunomodulators vis-à-vis Rasayana, modulate the secretion of multiple cytokines [[19], [20], [21]].
The botanicals in AYUSH-64, reported to have anti-inflammatory and immunomodulatory activity. The aqueous bark extract of A. scholaris in BALB/c mouse induced the cellular immune response at 50 mg/kg body weight once a day for 7 consecutive days while at 100 mg/kg body weight inhibited the delayed type of hypersentivity reaction [22]. Yun-Li Zhao et al. studied the effects of indole alkaloids and total alkaloids of A. scholaris on post infectious cough in mice and airway inflammation in rats respectively. Indole alkaloids showed down-regulation of inflammatory cells, cytokines (IL-6) and the balance of antioxidants. Total alkaloids inhibited the production of inflammatory cytokines TNF-α and IL-8 in bronchoalveolar lavage fluid and lung [23,24].
The biopolymeric fraction RLJ-NE-205 from the rhizomes of P. kurroa improved the immune system of mouse through increased proliferation of lymphocytes and cytokine levels (IL-4 and IFN-gamma) in serum, phagocytic index and CD4/CD8 population [25]. Pre-treatment with P. kurroa rhizome extract exhibited anti-inflammatory activity through the suppression of macrophage-derived cytokines (TNF-α, IL-1β, IL-6, IL-10) and mediators via suppression of NF-κB signaling [26]. Picroside II, active ingredient from P. scrophulariiflora showed the promising effects of anti-inflammation in cells and animals through decrease in concentrations of TNF-α, IL-1β, and IL-6. It suppressed the activation of p65 NF-κB signaling pathway compared with lipopolysaccharide (LPS) stimulation. The pathologic changes of lung tissues had been alleviated and lung wet/dry weight ratio was decreased after Picroside II treatment [27].
Swertia chirata is found to inhibit NF-kB/DNA interactions and also reduced pro-inflammatory IL- 8 expression in cystic fibrosis cells at IC50 concentrations [28]. Bellidifolin and Swerchirin, the two main xanthones from S. chirata inhibit the production of the proinflammatory cytokines IL-6 and TNF- α. Bellidifolin potently inhibited the prostaglandin Es (PGE2) by suppressing the protein expression of cyclooxygenase-2 (COX-2) [29]. The CHCl3 soluble, crude extract of the whole S. chirata inhibited the expression of Viral protein R (Vpr) in HeLa cells harboring the TREx plasmid encoding full-length Vpr (TREx-HeLa-Vpr cells) [30]. Crude extract of swertia plant showed antiviral properties against Herpes simplex virus type-1 [31].
Administration of methanolic extracts of C. crista reduced TNF- α, IL-1β and IL-6 mRNA expression in hippocampus and the frontal cortex brain areas in rats [32]. Caesalpinia bonducella seed extract produced dose dependent increase in both the parameters, i.e. antibody production and delayed type hypersensitivity in rats indicating promising immunostimulant properties [33]. A water-soluble gluco-arabinan isolated from the alkaline extract of the endosperm of seeds of C. bonducella showed immunostimulant activity through splenocytes and thymocytes activations [34]. Whole ethanolic seed extract of C. bonducella seeds in experimental albino rats displayed anti-inflammatory, antipyretic and analgesic activities [35,36].
As per the Ayurvedic understanding, the weakened Agni in Jvara leads to formation of Ama (proinflammatory undigested substance) leading to amavastha that results into weak immune status. The Ayurvedic management aims at correction of Agni status through Agnideepana and Amapachana resulting into niramavastha (devoid of ama) i.e. reversing the pathology and improving the immune status. The ingredients in AYUSH 64 are having Tikta rasa which is Agnideepak and Amapachak (anti-inflammatory). AYUSH 64 acts as amapachaka and converts the status from sama to nirama. Niramavastha results into restoring the normal metabolism and strengthening immune status.
In this study, the declining trend in symptoms of ILI is seen from the 3rd day of intervention to the 7th day. The combination effect of botanicals is Jwarahara (relieves fever), Kasahara (useful in cough), and Shwasahara (useful in asthma). This effect is seen from the reduction in the requirement of acetaminophen from 3rd day onwards after the intervention and minimal use of antihistaminic and cough syrup during the study period. The recovery time and returning to normal life was 3rd day in 6 patients, 7th day for 17 patients and 14th day for 3 patients. The seven-day treatment of AYUSH 64 along with standard care has shown reduction in symptoms of majority patients. Symptoms like fever, svedapravartana, shitaka, staimitya, tandra, parvaruk, kasa have shown sharp decline by 7th day of intervention. The post-treatment follow-up period has seen mild symptoms and requirement of antihistaminic and acetaminophen in 7 patients. Declining trend was seen in all the symptoms of ILI with fairly early recovery, however a longer duration of AYUSH 64 may have shown better results in terms of the restoration of metabolism and further strengthening the immune status. As far herb–drug interaction is concerned, AYUSH 64 was used along-with standard antimalarial treatment in earlier malaria epidemic. In this study the seven-day administration of AYUSH 64 along with standard care was tolerated well and no adverse events were recorded.
4.1. Limitations
This is single-group pre and post test design study with a small sample size and was focused on clinically diagnosed ILI condition with only clinical safety related biochemical parameters. However, for substantial evidence, a larger multicentric study with a robust design with standard diagnostic investigations and cytokine response will be helpful to validate the efficacy of this drug.
5. Conclusion
AYUSH 64 along-with standard care in ILI is safe and efficacious and it may be used in other viral infections with pyrexia as an add-on to standard care for early recovery and better outcome.
Clinical Trial Registry
Clinical Trial Registry of India (CTRI), number: CTRI/2017/10/010145.
Source of support
CCRAS, Ministry of AYUSH, GoI.
Declaration of Competing Interest
Nil.
Acknowledgment
The authors are thankful to CCRAS, Ministry of AYUSH, Government of India for grants for this study under CCRAS Research policy.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2020.05.010.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Saha S., Gupta V., Dawood F.S., Broor S., Lafond K.E., Chadha M.S., et al. Estimation of community-level influenza-associated illness in a low resource rural setting in India. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0196495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.https://www.cdc.gov/flu/about/viruses/change.htm 11.51pm.
- 3.Vaidya Jadavaji T.A., editor. Charaka Samhita of Acharya Charaka, Chikitsa Sthana, Jvara chikitsa, 3rd Chapter, Verse 86-87. 5th ed. Chaukambha Sanskrit Sansthan; Varanasi: 2001. p. 406. [Google Scholar]
- 4.Vaidya Jadavaji T.A., editor. Charaka Samhita of Acharya Charaka, Chikitsa Sthana, Jvara chikitsa, 3rd Chapter, Verse 142-143. 5th ed. Chaukambha Sanskrit Sansthan; Varanasi: 2001. p. 409. [Google Scholar]
- 5.Shankar R., Deb S., Sharma B.K. Antimalarial plants of northeast India: An overview. J Ayurveda Integr Med. 2012 Jan;3(1):10–16. doi: 10.4103/0975-9476.93940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anonymous . CCRAS, MoHFW, Govt of India; 1987. Monograph on AYUSH 64- A New Antimalarial Herbal Compound. [Google Scholar]
- 7.Rao T.S., Rao P.V., Kusuma K.K., Netaji B., Nisteswar K., Sharma C.R.R. Clinical Trial of Ayush-64 in Sleepada. J Res Ay Sid. 1982;3(1 & 2):9–12. [Google Scholar]
- 8.Pandey P.N., Kishore P. Effect of Ayush-64 and Saptaparnaghana Vati on Microfilaraemia. J Res Ay Sid Vol XII. 1989;3-4:145–150. [Google Scholar]
- 9.Anonymous . CCRAS, Dept of AYUSH, MoHFW, Govt of India; 2009. Management of Chikungunya through Ayurveda and Siddha, A Technical Report. [Google Scholar]
- 10.Bhatia D. Role of AYUSH 64 in malaria epidemic. J Res Ay Sid Vol XVIII. 1997;1-2:71–76. [Google Scholar]
- 11.Zakay- Rones Z., Thom E., Wollan T., Wadstein J. Randomized study of the efficacy and safety of oral elderberry extract in the treatment of influenza A and B virus infections. J Int Med Res. 2004 Mar-Apr;32(2):132. doi: 10.1177/147323000403200205. [DOI] [PubMed] [Google Scholar]
- 12.Sowunmi A., Gbotosho G.O., Happi C.T., Folarin O., Okuboyejo T., Michael O., et al. Use of area under the curve to evaluate the effects of antimalarial drugs on malaria associated anemia after treatment. Am J Ther. 2011 May;18(3):190–197. doi: 10.1097/MJT.0b013e3181d169c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical Management Protocol for Seasonal Influenza (Revised on 25.02.2019) https://ncdc.gov.in/showfile.php?lid=364 available at:
- 14.Camacho A., Eames K., Adler A., Funk S., Edmunds J. Estimation of the quality of life effect of seasonal influenza infection in the UK with the internet-based Flu survey cohort: an observational cohort study. Lancet. 2013;382:S8. doi: 10.1016/S0140-6736(13)62433-2. [DOI] [Google Scholar]
- 15.Hayden F.G., Fritz R., Lobo M.C., Alvord W., Strober W., Straus S.E. Local and systemic cytokine responses during experimental human influenza A virus infection relation to symptom formation and host defense. J Clin Invest. 1998 Feb 1;101(3):643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser L., Fritz R.S., Straus S.E., Gubareva L., Hayden F.G. Symptom pathogenesis during acute influenza: Interleukin-6 and other cytokine responses. J Med Virol. 2001 Jul;64(3):262–268. doi: 10.1002/jmv.1045. [DOI] [PubMed] [Google Scholar]
- 17.Bian J.R., Nie W., Zang Y.S., Fang Z., Xiu Q.Y., Xu X.X. Clinical aspects and cytokine response in adults with seasonal influenza infection. Int J Clin Exp Med. 2014 Dec 15;7(12):5593–5602. [PMC free article] [PubMed] [Google Scholar]
- 18.Yip T.F., Selim A.S.M., Lian I., Lee S.M. Advancements in host-based interventions for influenza treatment. Front Immunol. 2018 Jul 10;9:1547. doi: 10.3389/fimmu.2018.01547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spelman K., Burns J., Nichols D., Winters N., Ottersberg S., Tensborg M. Modulation of cytokine expression by traditional medicines: a review of herbal immunomodulators. Altern Med Rev. 2006 Jun;11(2):128–150. [PubMed] [Google Scholar]
- 20.Burns J.J., Zhao L., Taylor E.W., Spelman K. The influence of traditional herbal formulas on cytokine activity. Toxicology. 2010 Nov 28;278(1):140–159. doi: 10.1016/j.tox.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Balasubramani S.P., Venkatasubramanian P., Kukkupuni S.K., Patwardhan B. Plant-based rasayana drugs from ayurveda. Chin J Integr Med. 2011 Feb;17(2):88–94. doi: 10.1007/s11655-011-0659-5. [DOI] [PubMed] [Google Scholar]
- 22.Iwo M.I., Soemardji A.A., Retnoningrum D.S., Sukrasno U.U.M. Immunostimulating effect of pule (Alstonia scholaris L. R.Br., Apocynaceae) bark extracts. Clin Hemorheol Microcirc. 2000;23(2-4):177–183. [PubMed] [Google Scholar]
- 23.Zhaoa Y.L., Yang Z.F., Shang J.H., Huang W.Y., Wang B., Wei X., et al. Effects of indole alkaloids from leaf of Alstonia scholaris on post-infectious cough in mice. J Ethnopharmacol. 2018 May 23;218:69–75. doi: 10.1016/j.jep.2018.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y.L., Shang J.H., Pu S.B., Wang H.S., Wang B., Liu L., et al. Effect of total alkaloids from Alstonia scholaris on airway inflammation in rats. J Ethnopharmacol. 2016 Feb 3;178:258–265. doi: 10.1016/j.jep.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 25.Gupta A., Khajuria A., Singh J., Bedi K.L., Satti N.K., Dutt P., et al. Immunomodulatory activity of biopolymeric fraction RLJ-NE-205 from Picrorhiza kurroa. Int Immunopharmacol. 2006 Oct;6(10):1543–1549. doi: 10.1016/j.intimp.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R., Gupta Y.K., Singh S., Raj A. Anti-inflammatory effect of Picrorhiza kurroa in experimental models of inflammation. Planta Med. 2016 Nov;82(16):1403–1409. doi: 10.1055/s-0042-106304. [DOI] [PubMed] [Google Scholar]
- 27.Shen B., Zhao C., Chen C., Li Z., Li Y., Tian Y., et al. Picroside II protects rat lung and A549 cell against LPS-induced inflammation by the NF-κB pathway. Inflammation. 2017 Jun;40(3):752–761. doi: 10.1007/s10753-017-0519-3. [DOI] [PubMed] [Google Scholar]
- 28.Guerrini A., Mancini I., Maietti S., Rossi D., Poli F., Sacchetti G., et al. Expression of pro-inflammatory interleukin-8 is reduced by ayurvedic decoctions. Phytother Res. 2014 Aug;28(8):1173–1181. doi: 10.1002/ptr.5109. [DOI] [PubMed] [Google Scholar]
- 29.Hu T.Y., Ju J.M., Mo L.H., Ma L., Hu W.H., You R.R., et al. Anti-inflammation action of xanthones from Swertia chirayita by Regulating COX-2/NF-kB/MAPKs/Akt signaling pathways in RAW 264.7 Macrophage cells. Phytomedicine. 2019 Mar 1;55:214–221. doi: 10.1016/j.phymed.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Woo S.Y., Win N.N., Noe Oo W.M., Ngwe H., Ito T., Abe I., et al. Viral protein R inhibitors from Swertia chirata of Myanmar. J Biosci Bioeng. 2019 Oct;128(4):445–449. doi: 10.1016/j.jbiosc.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Verma H., Patil P.R., Kolhapure R.M., Gopalkrishna V. Antiviral activity of the Indian medicinal plant extract Swertia chirata against herpes simplex viruses: a study by in-vitro and molecular approach. Indian J Med Microbiol. 2008 Oct-Dec;26(4):322–326. [PubMed] [Google Scholar]
- 32.Ravi S.K., Ramesh B.N., Mundugaru R., Vincent B. Multiple pharmacological activities of Caesalpinia crista against aluminium-induced neurodegeneration in rats: Relevance for Alzheimer's disease. Environ Toxicol Pharmacol. 2018 Mar;58:202–211. doi: 10.1016/j.etap.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Shukla S., Mehta A., Mehta P., Vyas S.P., Shivaprasad H.N. In vivo immunomodulatory activities of the aqueous extract of bonduc nut Caesalpinia bonducella seeds. Pharm Biol. 2010 Feb;48(2):227–230. doi: 10.3109/13880200903085474. [DOI] [PubMed] [Google Scholar]
- 34.Mandal E.K., Mandal S., Maity S., Behera B., Maiti Tk, Islam S.S. Structural studies of an immunostimulating gluco-arabinan from seeds of Caesalpinia bonduc. Carbohydr Polym. 2013 Jan 30;92(1):704–711. doi: 10.1016/j.carbpol.2012.08.093. [DOI] [PubMed] [Google Scholar]
- 35.Shukla S., Mehta A. In vivo anti-inflammatory, analgesic and antipyretic activities of a medicinal plant, Caesalpinia bonducella F. Pak J Pharm Sci. 2015 Jul;28(4 Suppl):1517–1521. [PubMed] [Google Scholar]
- 36.Shukla S., Mehta A., Mehta P., Vyas S.P., Shukla S., Bajpai V.K. Studies on anti-inflammatory, antipyretic and analgesic properties of Caesalpinia bonducella F. seed oil in experimental animal models. Food Chem Toxicol. 2010 Jan;48(1):61–64. doi: 10.1016/j.fct.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Sharma P.V., editor. Dhanvantari Nighantu. 3rd ed. Chaukhambha Orientalia; Varanasi: 2002. p. 105. [Google Scholar]
- 38.Madanpala . First ed. Chaukhambha Orientalia; Varanasi: 2010. Madanpala Nighantu. (Illustrated by Shastry J. L. N) pp. 541–542. [Google Scholar]
- 39.Kaiyadeva . In: Kaiyadeva Nighantu. Sharma P.V., Sharma G.P., editors. Chaukhambha Orientalia; Varanasi: 2009. p. 176. Reprint. [Google Scholar]
- 40.Bhavamishra . In: Bhavprakash Nighantu. Pandey G.S., editor. Chaukhambha Bharati Academy; Varanasi: 2004. p. 534. (Commentary by Chunekar K.C) Reprint: 2015. [Google Scholar]
- 41.Anonymous . Department of AYUSH, Ministry of Health and Family Welfare, Government of India; 1986. pp. 129–131. (The Ayurvedic Pharmacopoeia of India, Part- I, Vol– I). [Google Scholar]
- 42.Bhavamishra . In: Bhavprakash Nighantu. Pandey G.S., editor. Chaukhambha Bharati Academy; Varanasi: 2004. p. 67. (Commentary by Chunekar K.C) Reprint:2015. [Google Scholar]
- 43.Bhavamishra . In: Bhavprakash Nighantu. Pandey G.S., editor. Chaukhambha Bharati Academy; Varanasi: 2004. p. 70. (Commentary by Chunekar K.C) Reprint:2015. [Google Scholar]
- 44.Narhari Pandit. 2nd Ed. Krishnadas Academy; Varanasi: 1998. Rajnighantu; p. 244. (Illustrated by Tripathi Indradev) [Google Scholar]
- 45.Anonymous . Department of AYUSH, Ministry of Health and Family Welfare, Government of India; 2006. pp. 112–114. (The Ayurvedic Pharmacopoeia of India, Part- I, Vol– V). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.