Summary
Preeclampsia is pregnancy-specific, and significantly contributes to maternal, and perinatal morbidity and mortality worldwide. An effective predictive test for preeclampsia would facilitate early diagnosis, targeted surveillance and timely delivery; however limited options currently exist. A first-trimester screening algorithm has been developed and validated to predict preterm preeclampsia, with poor utility for term disease, where the greatest burden lies. Biomarkers such as sFlt-1 and placental growth factor are also now being used clinically in cases of suspected preterm preeclampsia; their high negative predictive value enables confident exclusion of disease in women with normal results, but sensitivity is modest. There has been a concerted effort to identify potential novel biomarkers that might improve prediction. These largely originate from organs involved in preeclampsia's pathogenesis, including placental, cardiovascular and urinary biomarkers. This review outlines the clinical imperative for an effective test and those already in use and summarises current preeclampsia biomarker research.
Keywords: Preeclampsia, Prediction, Biomarkers, Placenta
Introduction; Preeclampsia and the need for improved prediction
Preeclampsia is a pregnancy-specific disease, affecting 3-5% of all pregnancies. Its hallmark features are high blood pressure (hypertension) and endothelial dysfunction, leading to widespread end-organ injury. This includes the liver, blood, kidneys, brain and placenta. Preeclampsia is a significant contributor to maternal morbidity (including outcomes as severe as liver rupture, renal failure, seizures (eclampsia) and stroke) and mortality worldwide. With delivery the only current cure, preeclampsia also contributes significantly to prematurity, neonatal morbidity and perinatal mortality.1,2
Recently, there has been increased interest in predictive biomarkers for preeclampsia. An effective predictive test would facilitate early diagnosis, targeted surveillance and timely delivery. A biomarker able to predict high risk women in early pregnancy (less than 16 weeks3) has clinical utility in preventing preterm preeclampsia (and associated preterm birth and perinatal morbidity) through administration of low-dose prophylaxis with aspirin to reduce preterm disease.4, 5, 6, 7, 8 The benefit of identifying patients at higher risk of preeclampsia in late pregnancy (enabling increased surveillance and well-timed delivery) is evidenced by the PHOENIX trial.9 This randomised study provided strong evidence that planned delivery for preeclamptic patients reduces maternal morbidity compared to expectant management.9 In this review, we outline the clinical imperative for an effective test, review those already in use and summarise current preeclampsia biomarker research.
Screening tests – what's needed in the clinic?
To develop new, clinically relevant tests, clarity is needed around the objective. The greatest clinical value is likely to be realised with new screening tests to identify women at high risk of developing – or of already having – established disease, enabling risk stratification for ongoing care. In preeclampsia, such tests could identify women who may benefit from increased clinical surveillance and carefully timed birth. Conversely, they may identify low risk patients who could safely reduce their antenatal visits. Like predicting the weather, screening tests for preeclampsia generally perform better closer to disease development10 ie. prospective biomarkers to predict term preeclampsia perform better when sampled at later, compared to earlier, gestations.
Measuring blood pressure is a screening test for preeclampsia that has been used for over a century. However, hypertension is often only useful when preeclampsia has already started to develop, performing only modestly in predicting later preeclampsia. Another screening test currently applied in early pregnancy is identification of clinical risk factors for preeclampsia11,12 (Table 1), yet this too has very limited predictive ability.
Table 1.
Advantages and disadvantages of current tests used to screen for preeclampsia
| Test | Specifications | Advantages | Disadvantages |
|---|---|---|---|
| Clinical guidelines to apply a preeclampsia risk score. Examples include NICE and ACOG guidelines. | Maternal and pregnancy characteristics and existing medical conditions are classified as high or moderate risk factors | (I) Only requires an assessment of clinical factors that are readily attainable. (ii) Is applicable to all women at the first visit (to determine who might benefit from low-dose aspirin for preterm preeclampsia prophyaxis) (iii) Doesn't require access to specific blood-testing or Doppler ultrasonography (iv) No additional cost |
(I) Modest test performance. Its sensitivity is ∼41% for pre-term preeclampsia and it performs worse for all preeclampsia. (ii) When applied in the real world setting, it has been found not to achieve high compliance with prophylactic low-dose aspirin for women at risk |
| First trimester combined algorithm for preeclampsia | Combines maternal characteristics with mean arterial blood pressure, mean uterine artery resistance, and circulating PlGF to stratify risk | (I) Achieves a higher sensitivity for preterm preeclampsia (∼82%). (ii) Shown to achieve a high compliance with prophylactic aspirin. |
(I) Additional cost for blood testing for PlGF and ultrasonography to determine maternal uterine artery resistance (ii) Does not predict term preeclampsia with high sensitivity. Overall it detects only 42.5% of all preeclampsia. (iii) Its use (combined with aspirin prophylaxis) cannot reduce rates of preeclampsia occurring at >37 weeks, which is the majority of the disease. |
| sFlt1:PlGF ratio | >38 represents screen positive | (I) If sFlt1:PlGF is 38 or less there is 99.3% negative predictive value for preeclampsia within a week – a strong “rule out” test (ii) Reduces admissions for women with suspected preeclampsia |
(I) Limited to suspected preeclampsia at <37 weeks – not applicable to the general pregnant population (ii) Unable to accurately predict (“rule in”) who will develop preeclampsia, with low sensitivity and positive predictive values |
| PlGF alone | <100pg /ml represents screen positive | (I) Screen positive in women with suspected preeclampsia at <35 weeks achieves 96% sensitivity and 98% negative predictive value for preeclampsia developing within 2 weeks (ii) Its use has been shown to reduce the time to diagnosis, adverse maternal outcomes, outpatient attendances and costs to healthcare service |
(I) Limited to suspected preeclampsia at <35 weeks – not applicable to the general pregnant population (ii) Unable to accurately predict (“rule in”) who will develop preeclampsia at term |
Over the last decade, two screening tests have been developed that have been integrated into clinical care in some settings. The first is a first trimester screening test that identifies those at risk of developing preterm preeclampsia. The second is designed for later pregnancy where there is clinical uncertainty whether preeclampsia is established or likely to evolve. This latter test is highly accurate in ruling out development of preeclampsia within the next week (high negative predictive value) and modestly accurate in predicting whether preeclampsia will develop (positive predictive value). We will discuss these established tests below, before turning to new biomarkers in the second half of this review.
First-trimester screening for preeclampsia risk
The advantages and disadvantages of existing tests to identify women at risk of developing preeclampsia are summarised in Table 1. Several guidelines exist to stratify risk using pregnancy factors and maternal characteristics. The National Institute for Health and Care Excellence (NICE) and American College of Obstetricians and Gynecologists (ACOG) guidelines are frequently used examples. Both list previous preeclampsia, chronic renal disease, chronic hypertension, pre-existing diabetes mellitus and autoimmune disease as “high” risk factors for preeclampsia.11,12 The ACOG guideline also includes multifetal gestation11 (a “moderate” risk factor in the NICE guidelines12). Other “moderate” risk factors (where women with ≥ two moderate risk factors are considered at high risk of preeclampsia) include nulliparity, advanced age and high body mass index (albeit at differing cut-offs), inter-pregnancy interval of >10 years and family history of preeclampsia.11,12 These guidelines are easy to apply to all pregnant women without cost, or additional testing, however, perform with poor sensitivity. For example, only 41% of women destined to deliver preterm preeclampsia will be screen-positive using the NICE guidelines.13,14 Predictive performance for late gestation preeclampsia is even poorer.13
To address this gap, a new first-trimester screening algorithm has been developed and validated to predict preterm preeclampsia. It combines mean arterial blood pressure, Doppler ultrasound measured maternal uterine artery resistance, and levels of circulating Placental Growth Factor (PlGF). This test is superior in predicting preterm preeclampsia compared to clinical risk factors alone. It correctly detects 82% of cases – doubling the detection rate achieved by application of clinical factors using the NICE guidelines (table 1).13 When used to identify who should be offered aspirin to prevent preeclampsia, it significantly reduces preterm preeclampsia. In turn, this reduces hospital costs and reduced financial and long-term human costs associated with preterm birth.15, 16, 17, 18 Several international societies now recommend first trimester combination screening for preterm preeclampsia.19,20 However, the costs of implementing the test (given the ultrasound expertise and assays to measure PlGF required) have meant implementation has not been universal.
Moreover, while individual costs of preterm preeclampsia are high, preterm preeclampsia is rare, with most disease burden associated with late pregnancy and term disease. Early-onset preeclampsia occurring at <34 weeks complicates just 0.38% of pregnancies. Late-onset preeclampsia occurs at more than seven times that rate.21 Importantly, late-onset preeclampsia can be severe, contributing significantly to global maternal and perinatal mortality and morbidity.21, 22, 23 While first trimester preeclampsia screening and intervention with aspirin is an exciting development, this regimen does not predict or prevent most cases of preeclampsia. Therefore, there remains an unmet need to find predictive, diagnostic and therapeutic approaches to reduce the burden of preeclampsia occurring at later gestations.
Because screening does not predict most cases of preeclampsia, clinical triage remains the mainstay of care. At each antenatal visit, pregnant women have blood pressure measured and may have an assessment of urinary protein. Given the increasing prevalence of disease as term approaches, the frequency of visits become progressively more intensive: for example, every 2-3 weeks between 28 and 36 weeks, and then weekly thereafter. This time-honoured clinical approach is designed to identify early, women where the disease has already established, but a predictive test for late-onset preeclampsia could enable personalised stratification into high- or low-intensity surveillance.
Biomarker testing to triage care in suspected preterm preeclampsia
Besides the first trimester combined screening algorithm, another biomarker innovation for preeclampsia has entered the clinic. This screening test (usually applied during the third trimester of pregnancy) is selectively offered when clinicians are uncertain whether a pregnant woman is on the cusp of developing preeclampsia or not; where the clinical picture is ambiguous. Examples include those with borderline hypertension, or non-specific symptoms (such as headache) or for women with persistently high blood pressure, who have not met the diagnostic criteria for preeclampsia (no strong evidence of other organ involvement).
Soluble fms-like tyrosine kinase 1 (sFlt-1) and PlGF are anti- and pro-angiogenic factors (respectively) significantly deranged in preeclampsia. The PROGNOSIS study showed that a sFlt-1:PlGF ratio of 38 or lower can accurately rule out the likelihood of developing preeclampsia over the next week, with 99.3%, negative predictive value, among women at less than 37 weeks, who have the test for suspected preeclampsia.10 This test has the potential to reduce admissions for blood pressure monitoring, as it can confidently exclude the likelihood of having the condition. As such, it has been translated to clinical practice in some centres. Conversely an sFlt-1:PlGF ratio >38 is only modestly accurate in predicting who will develop preeclampsia, with positive predictive value of 36.7% for preeclampsia within four weeks, and sensitivity of 66.2%.10
PlGF alone (without expressing it in a ratio with sFlt-1) has also been evaluated to triage care in women with suspected preterm preeclampsia. Besides having a very high negative predictive value it has also been shown to predict preeclampsia requiring delivery within two weeks with greater accuracy than other commonly used clinical tests (blood pressure, urate, alanine transaminase (a liver function test), and proteinuria24). Specifically, PlGF <100pg/ml in women presenting with suspected preeclampsia at <35 weeks’ gestation, performed with 96% sensitivity and 98% negative predictive value in predicting whether preeclampsia will occur over the next two weeks.24 Although recent data demonstrates that PlGF alters with gestational age, and thus a blanket definition of screen positive with PlGF <100pg/ml may pick up numerous false positives.25 As such, generation of gestation-specific references ranges for diverse populations may further enhance the clinical utility of PlGF. We also note that PlGF is not good at predicting preeclampsia if applied to a low risk population – such as the general pregnant population without clinical suspicion of disease. Adding PlGF to the work up of those with suspected preeclampsia, compared to usual care, shortened time to diagnosis and reduced severe maternal adverse outcomes for women with an intermediately low PlGF of 12-100pg/ml – potentially otherwise triaged as lower risk.26 These improved maternal outcomes could also be achieved with reduced cost and reduced inconvenience of outpatient attendances,27 highlighting the clinical and financial value of ‘point of care’ biomarker testing in cases of suspected preeclampsia. When compared, the sFlt-1:PlGF ratio and PlGF alone performed similarly in predicting delivery within two weeks in cases of suspected preterm preeclampsia.28 The high negative predictive value enables confident exclusion of disease in women with normal results.28
A ‘rule in’ test for term preeclampsia remains an unmet clinical need
While exciting advances have been made over the past decade with the introduction of these new biomarker tests into the clinic, there remains a significant unmet clinical need. There is still no test that can be applied universally with sufficiently strong performance characteristics to reliably identify those destined to develop preeclampsia at term. If such a test were available, this could dramatically reconfigure antenatal clinical care. The number of visits could be safely reduced for those who screen negative, saving pregnant women valuable time and vastly reducing medical costs. A positive test would allow careful surveillance, earlier diagnosis, and an opportunity to perhaps offer preventative drugs (candidate treatments that may prove useful in future clinical trials29) to improve outcomes. Until one is discovered, costly weekly antenatal visits with blood pressure checks will remain the status quo.
Identifying new biomarkers for preeclampsia
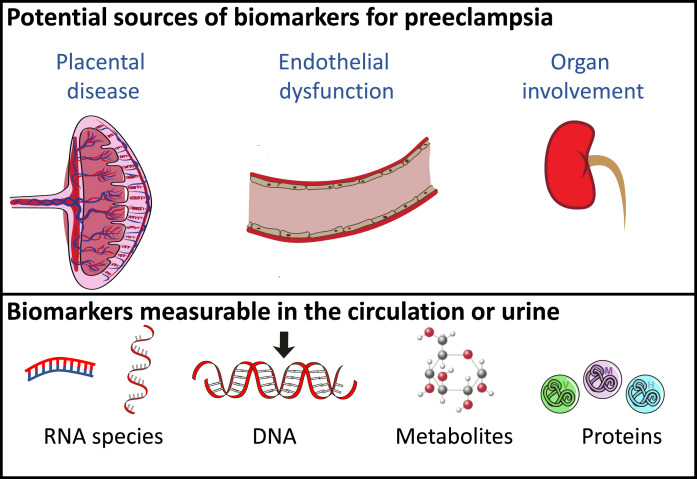
In this second half of the review, we highlight new discovery research: novel biomarkers for preeclampsia. This field offers a rich literature, with teams from around the world taking diverse approaches to identify preeclampsia biomarkers of interest. For this review, we have focused on potential biomarkers originating from organs involved in the pathogenesis of the condition (Figure 1). In this update we specifically focus our discussions around placental and cardiovascular biomarkers. However, many have undertaken broader screening approaches, utilising omics to screen for biomarkers including epigenetics, transcriptomics, GWAS, proteomics and metabolomics.30 For example, two recent independent studies have utilised mass spectrometry platforms to identify 4-hyroxyglutamate as a novel predictor of preeclampsia, before validating in an external cohort.31,32 We also note that urinary biomarkers arising as a result of kidney compromise is another avenue of important work in this field.
Fig. 1.
Schematic outlining the potential sources of biomarkers for preeclampsia and types that may be measured. Preeclampsia is associated with placental insufficiency that leads to significant endothelial dysfunction and organ involvement. As such, the placenta and endothelial cells represent potential sources of potential biomarkers that may be in the form of RNAs, DNA, metabolites proteins.
Before discussing current research efforts targeting placental and cardiovascular biomarkers, it is worth reflecting on the importance of the appropriate methodology to mine for prospective biomarkers. To accurately identify predictive biomarkers for a low-risk population where diagnostic performance (sensitivity, specificity) can be calculated, large prospective cohort collections are required. In such cohort studies, samples are collected at specific gestations and candidate biomarkers’ levels compared among those who developed the condition and those who did not. Ideally the disease will be represented at population incidence (e.g. 3-5% for preeclampsia). This means such studies in low risk populations require collection of hundreds, ideally thousands, of samples. Collecting and curating such cohorts is expensive and thus to mine for candidate biomarkers, a case cohort can be selected from the samples to contain costs of laboratory discovery.32
Because of the expense of collecting large cohorts, most preeclampsia biomarker studies have instead used convenience samples collected from women already diagnosed with preeclampsia at sampling, compared to those without. This is pragmatic and can provide excellent insights into the condition and can identify candidate biomarkers. However, it cannot be assumed that biomarkers differentially expressed in such cohorts can predict the condition. Indeed, it is possible that circulating biomarkers differentially expressed in women who have preeclampsia (versus those without) will fail (or perform very modestly) when tested in large cohorts for their predictive potential weeks before the condition develops. Even sFlt-1 and PIGF perform modestly at predicting the onset of preeclampsia when applied to a low risk cohort.33
Finally, besides the limitations of using samples from cohorts of established disease, another significant limitation in the field of preeclampsia biomarker research is that validation studies of prospective biomarkers (in a fresh, independent cohort) are rarely performed. Another option to overcome this is via national or international collaboration and exchange of samples for this purpose.31, 32, 33
Placental biomarkers
Given the two-stage theory of preeclampsia34,35 – proposing placental disease precedes maternal endothelial dysfunction – placenta-released molecules offer a rational starting point. Our team has specifically studied placenta-enriched molecules (those highly expressed in the placenta), including messenger RNAs (mRNAs),36, 37, 38, 39 microRNAs (miRNAs)37,40 and proteins,33,41,42 as potential biomarkers.
Placental RNAs
The placenta releases a host of nucleic acids directly into the maternal circulation which are detectable during pregnancy, and quickly cleared post delivery.43, 44, 45 Numerous recent studies demonstrate alterations in placental mRNA, miRNA, long-non coding RNA and circular RNA between healthy and preeclamptic pregnancies.46, 47, 48 However, changes in the placenta are not always reflected within the maternal circulation, necessitating direct measurement of potential biomarkers within maternal blood, serum or plasma.
Tarca et al49 studied 20 circulating mRNAs previously identified as deranged in patients with established preeclampsia and deemed as “extravillous cytotrophoblast” (EVT) specific by single-cell RNA sequencing across gestation. They demonstrated that this EVT signature was increased as early as 11-17 weeks’ gestation and further increased at 32-34 weeks once diagnosis was established. Our team has assessed mRNA expression of placenta-enriched RNAs, including adrenomedullin (Adm).50 In a nested case control study, and confirmed in an entire cohort of 1004 samples, Adm was significantly reduced at both 28 and 36 weeks in those destined to develop preeclampsia at term gestations.39 While the reduction was subtle, resulting in modest predictive ability, Adm may have clinical utility if combined with other molecules. Evidence that mRNA biomarkers could improve prediction if combined with proteins was demonstrated by Zhou et al.51 A combination of Hoxb3 with sFlt-1 resulted in greater area under the Receiver Operator Curve (ROC) than either biomarker alone, but a major limitation is that samples were collected following diagnosis. Thus, the true predictive capacity of Hoxb3 with sFlt-1 requires validation. Differential expression in circulating mRNAs NR4A2, EMP1, PGM5, SKIL, and UGT2B1, in pregnancies with severe placental insufficiency (FGR, preeclampsia or both) at imminent risk of stillbirth has also been identified.52 We are currently investigating whether these are of placental origin and could be used as clinically useful predictors of placental insufficiency. miRNAs are endogenous short non-coding RNA molecules that can have post-transcriptional effects on mRNAs by repressing their translation to protein or promoting their degradation. They either circulate freely or can be encapsulated in extracellular vesicles, including exosomes, following release from the cells of origin. Presently there is no ‘gold standard’ method for miRNA measurement; studies utilise a mix of whole blood, plasma, serum or isolated exosomes from one of these, making comparisons between studies challenging.
The primate specific chromosome 19 miRNA cluster (C19MC), consisting of 56 mature miRNAs, is exclusively expressed in placenta, embryonic cells and certain cancers.53 Therefore, C19MC miRNAs have been the focus of numerous biomarker studies. In first trimester, C19MC miRNA expression has been assessed in both exosomes isolated from plasma and in whole maternal plasma.54 Exosomal expression produced higher predictive performance for preeclampsia. Combined miR-517-5p, miR520a-5p and miR-525-5p demonstrated 44.2% sensitivity at 90% specificity for prediction of late-preterm preeclampsia.54 Our work screening C19MC cluster miRNAs in whole blood at 36 weeks preceding term preeclampsia only identified modest changes in one of the cluster members studied, miR-128340.
A recent systematic review55 assessing circulating nucleic acids in maternal plasma and serum concluded that of 83 eligible studies, variation in populations and limitations in adjustments for clinically relevant variables, made it difficult to draw firm conclusions around the value of mRNAs, miRNA and other RNA species in preeclampsia prediction. Further work is needed to determine whether these molecules hold promise as future biomarkers.
Placental proteins
Compared to RNA species, measurement of circulating proteins may be more consistent between studies, although variation remains, depending on whether plasma or serum is utilised. Systematic reviews56 consistently identify PlGF and placental protein 13 (PP13) as the best predictive candidates. PlGF demonstrated 65% sensitivity at 89% specificity, and PP13 just 27% sensitivity, with a specificity of 88%. As detailed earlier, PlGF alone, and a ratio of sFlt-1/PlGF are being used clinically as rule-out tests in some centres.10,24 In this section we highlight proteins that might have potential if tested in combination with established biomarkers.
The Pregnancy Outcome Prediction study assessed combined first trimester Pregnancy-associated plasma protein A (PAPP-A) and Alpha fetoprotein (AFP) in a large (n=4057) unselected cohort of nulliparous women.57 PAPP-A is measured in the first trimester as part of aneuploidy screening and has been associated with pregnancy complications, including preeclampsia. Second trimester AFP, produced primarily by the fetal liver, has also been robustly linked to adverse pregnancy outcomes including preeclampsia,58 with a large retrospective study (n=3325) reporting first trimester elevations, but poor predictive performance.59 When combined, a ratio of AFP/PAPP-A above 10 resulted in a relative risk for severe preeclampsia of 2.12, with slightly improved (although not significant) area under the ROC curve (of 0.60 – reflecting only modest predictive potential) compared to either biomarker alone.57
We have also assessed the potential of combining placental biomarkers to improve prediction. Growth Differentiation Factor 15 (GDF-15), a member of the transforming growth factor β superfamily, is most highly expressed in placenta under physiological conditions.60,61 It is stress-induced, responding to cellular injury and inflammation, and deranged in cardiovascular disease.60,62,63 Plasma GDF15 levels are increased in the circulation of patients with preterm preeclampsia, and in our large prospective cohort collection (the Fetal Longitudinal Assessment of Growth (FLAG) study), we showed an association with term preeclampsia when measured at 36 weeks. A combination of GDF15 x sFlt1/PlGF yielded 68.3% sensitivity and 83.2% specificity 33; higher than each analyte's performance alone. However, other recent works found increased serum GDF15 at 30-34 weeks in those destined to develop early onset preeclampsia, but no significant change at 35-37 weeks prior to preeclampsia at term.64 The most important difference between these studies was the use of plasma in one, and serum in the other. Caution must be exercised when using different sample types for comparison or validation of biomarkers.
In both early and late pregnancy, combining placental biomarkers may hold promise for enhanced sensitivity. Future studies should focus upon identifying biomarkers that could be added to enhance the predictive performance of sFlt-1, PlGF and PP13.
Endothelial/cardiovascular biomarkers
Given the significant maternal endothelial dysfunction characteristic of preeclampsia, there has been considerable interest in measuring circulating biomarkers that originate from the maternal vasculature or that are associated with endothelial dysfunction.
Endothelial RNAs
Like the placenta, endothelial cells release miRNAs that likely play an important role in regulating endothelial and possibly cardiovascular function. mir-574-5p, mir-1972, and mir-4793 have been identified as elevated in preeclampsia relative to healthy patients with the greatest fold change and lowest false discovery rate.65 All three have been linked to endothelial dysfunction including impaired wound healing and decreasing proliferation.
We studied endothelial miRNAs to predict term preeclampsia using RNA from whole blood. miR363 regulates endothelial cell properties by post transcriptional regulation of tissue inhibitor of metalloproteinases-1 and thrombospondin 3. We found significantly reduced miR363 at 28 and 36 weeks’ gestation preceding term preeclampsia, and significantly reduced miR149, miR424 and miR18a at 36 weeks. These miRs are all implicated in endothelial dysfunction, or have anti-angiogenic properties.40 Despite this, they demonstrated only modest predictive potential. The best combination, miR149 and miR363, demonstrated 45% sensitivity at 90% specificity. Further, GATA2 is a transcription factor expressed in endothelium. It regulates vascular homeostasis by controlling transcription of genes and miRNAs, including miR126. Whole blood Gata2 and miR126 were both reduced in the circulation of patients with early onset preeclampsia (<34wks)38 and prior to preeclampsia diagnosis at term. Gata2 was reduced up to 12 weeks prior. These findings suggest potential to combine endothelial related RNAs in a multi-marker test with clinical utility.
Endothelial proteins
Nitric oxide (NO) is an important signalling messenger in the cardiovascular system where it maintains endothelial integrity by regulating vasodilation, adhesion of leukocytes and platelet aggregation.66,67 NO is synthesised by Nitric oxide synthase (NOS), using L-arginine as a precursor. Multiple groups have assessed the biomarker potential of Asymmetric dimethylarginine (ADMA) for preeclampsia. It is a methylated product of L-arginine that endogenously inhibits NOS to reduce NO production. Two meta-analyses have recently assessed ADMA showing it is deranged in those destined to develop early onset preeclampsia if samples are taken after 20 weeks, although with modest predictive efficacy.68,69
Endothelin-1 (ET-1) is a potent vasoconstrictive peptide, predominantly secreted by endothelial and vascular smooth muscle cells. Early studies, and more recent works70 have demonstrated 2-3 fold increases in circulating ET-1 in preeclamptic compared with normal pregnancies, however, there have been few studies examining the predictive potential of ET-1 preceding diagnosis. Malte and others71 assessed its stable circulating precursor protein, C-terminal proendothelin-1 (CT-pro-ET1) in healthy patients versus those with suspected preeclampsia at the time of enrolment. A combination of CT-pro-ET1, sFlt-1 and systolic blood pressure produced sensitivity of 80% at 90% specificity for development of severe preeclampsia within 1 week in women with either subclinical preeclampsia, gestational hypertension, essential hypertension or moderate preeclampsia. Thus ET-1, or its precursor protein CT-pro-ET1 may hold potential in prediction, or risk stratification of disease.
A host of other ‘endothelium-related’ biomarkers have also been assessed for their potential to identify preeclampsia. Vascular cell adhesion molecule-1 (VCAM-1) is upregulated in response to endothelial inflammation. Soluble VCAM-1 was significantly increased in a small cohort of early onset preeclamptics relative to normal pregnancies.72 While the combination of VCAM-1 with hyularon improved prediction further,73 this combination was out-performed by PlGF alone.74 Endocan, also involved in inflammation and endothelial dysfunction, has modest biomarker potential, with meta-analyses finding higher levels in women with early onset preeclampsia.75
In summary, many studies have reported that endothelial/cardiovascular biomarkers are deranged in established preeclampsia, but few have shown predictive potential. Nevertheless, there is likely untapped potential in combining biomarkers, and future research combining markers of placental and endothelial origin should be pursued with vigour.
Conclusions
Preeclampsia remains one of the most severe pregnancy complications with a significant legacy of both maternal and perinatal morbidity. Early detection improves outcomes, yet at present there is no reliable screening test to predict its development – especially at term gestations where the greatest burden of disease exists. Many potential biomarkers have been identified through exploratory studies utilising samples from established disease. These studies have generated hypotheses for potential biomarkers, with less focus on prediction. It is possible that combining biomarkers derived from multiple organ and cellular sources may yield the best predictive performance. Utilising large prospective cohort collections in unselected populations provides the best avenue for discovering novel biomarkers, but these markers – or combinations – must be rigorously validated in external cohorts to ensure they achieve their potential to improve outcomes for pregnant people and their babies.
Outstanding questions
While progress has been made toward developing useful screening options for preterm preeclampsia, there is still much work to do before a screening test could be applied to predict term preeclampsia, where the greatest burden of disease lies. Some outstanding questions that need answering include:
-
•
Is there a time point in pregnancy that offers a pragmatic time for screening, but is close enough to disease onset that a test might perform with high sensitivity and positive predictive value? We believe 28 weeks’ gestation might offer an ideal time, given it is around this time that glucose tolerance tests might be offered, but closer in proximity to the time at which late preterm or term preeclampsia might develop. It is also possible that a late gestation test, at perhaps 36 weeks’ gestation could offer clinical value.
-
•
Can novel biomarkers improve the sensitivity and positive predictive value of current biomarkers such as sFlt-1 and PlGF? The PROGNOSIS study has shown that a ratio of sFlt-1/PlGF performs well for ruling out disease development, but consideration should be given as to whether novel markers can either be combined to this ratio to enhance sensitivity, or indeed out-perform these markers. To achieve this, teams that are examining novel biomarkers must also measure sFlt-1 and PlGF.
-
•
Can biomarkers discovered in samples collected from patients with established disease be validated in the general pregnancy population preceding diagnosis, and can they also be validated in external cohorts? As detailed above, this is a limitation of many studies within the field and the true clinical utility of a test relies on these questions being answered properly.
Contributors
TMM, ST and TKL contributed to the conception and design of the review. The first draft was written by TMM and TKL. SPW, NJH and ST critically revised the manuscript. All authors contributed to the approved and submitted version.
Declaration of Competing Interest
TKL, TM, SW and ST hold a provisional patent (PCT/AU2019/050516) relating to the use of SPINT1 and syndecan-1 as diagnostic markers for placental insufficiency. The remaining authors have no conflicts of interest to declare.
Acknowledgments
Acknowledgements
TKL (#1159261) NJH (#1146128) and ST (#1136418) are funded by fellowships from the National Health and Medical Research Council of Australia. The funders played no role in design, interpretation or writing of the manuscript.
Search strategy and selection criteria
For the section covering novel biomarkers, we searched PubMed from Jan 1 2016 to Sept 30 2021 with the following search terms: “preeclampsia” cross-referenced with “biomarker”, “prediction”, “prognosis” and “diagnosis”. We also searched specifically for “mRNA preeclampsia blood prediction”, “C19MC cluster preeclampsia”, “microRNA miRNA blood preeclampsia prediction” and “preeclampsia endothelial dysfunction biomarkers”. Our search was performed between Aug 24 and Sept 30th, 2021. We prioritised biomarkers studied by numerous groups with consistent findings, or for which validation data exists. Only articles published in English were included.
References
- 1.Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. 2021 doi: 10.1016/S0140-6736(20)32335-7. London, England. [DOI] [PubMed] [Google Scholar]
- 2.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323–e333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 3.Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018;218(3):287–293. doi: 10.1016/j.ajog.2017.11.561. e1. [DOI] [PubMed] [Google Scholar]
- 4.Rolnik DL, Wright D, Poon LC, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. 2017;377(7):613–622. doi: 10.1056/NEJMoa1704559. [DOI] [PubMed] [Google Scholar]
- 5.Beaufils M, Uzan S, Donsimoni R, Colau JC. Prevention of pre-eclampsia by early antiplatelet therapy. Lancet. 1985;1(8433):840–842. doi: 10.1016/s0140-6736(85)92207-x. [DOI] [PubMed] [Google Scholar]
- 6.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791–1798. doi: 10.1016/S0140-6736(07)60712-0. [DOI] [PubMed] [Google Scholar]
- 7.Duley L, Meher S, Hunter KE, Seidler AL, Askie LM. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2019;2019(10) doi: 10.1002/14651858.CD004659.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright D, Rolnik DL, Syngelaki A, et al. Aspirin for Evidence-Based Preeclampsia Prevention trial: effect of aspirin on length of stay in the neonatal intensive care unit. Am J Obstet Gynecol. 2018;218(6):612. doi: 10.1016/j.ajog.2018.02.014. e1-e6. [DOI] [PubMed] [Google Scholar]
- 9.Chappell LC, Brocklehurst P, Green ME, et al. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet. 2019;394(10204):1181–1190. doi: 10.1016/S0140-6736(19)31963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeisler H, Llurba E, Chantraine F, et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N Engl J Med. 2016;374(1):13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 11.ACOG Committee Opinion No. 743 Low-Dose Aspirin Use During Pregnancy. Obstetrics and gynecology. 2018;132(1):e44–e52. doi: 10.1097/AOG.0000000000002708. [DOI] [PubMed] [Google Scholar]
- 12.Visintin C, Mugglestone MA, Almerie MQ, Nherera LM, James D, Walkinshaw S. Management of hypertensive disorders during pregnancy: summary of NICE guidance. Bmj. 2010;341:c2207. doi: 10.1136/bmj.c2207. [DOI] [PubMed] [Google Scholar]
- 13.Tan MY, Wright D, Syngelaki A, et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2018;51(6):743–750. doi: 10.1002/uog.19039. [DOI] [PubMed] [Google Scholar]
- 14.Helou A, Walker S, Stewart K, George J. Management of pregnancies complicated by hypertensive disorders of pregnancy: Could we do better? The Australian & New Zealand journal of obstetrics & gynaecology. 2017;57(3):253–259. doi: 10.1111/ajo.12499. [DOI] [PubMed] [Google Scholar]
- 15.Park F, Russo K, Williams P, et al. Prediction and prevention of early-onset pre-eclampsia: impact of aspirin after first-trimester screening. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2015;46(4):419–423. doi: 10.1002/uog.14819. [DOI] [PubMed] [Google Scholar]
- 16.Park F, Deeming S, Bennett N, Hyett J. Cost effectiveness analysis of a model of first trimester prediction and prevention for preterm preeclampsia against usual care. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2020 doi: 10.1002/uog.22193. [DOI] [PubMed] [Google Scholar]
- 17.Guy GP, Leslie K, Diaz Gomez D, et al. Implementation of routine first trimester combined screening for pre-eclampsia: a clinical effectiveness study. BJOG: an international journal of obstetrics and gynaecology. 2021;128(2):149–156. doi: 10.1111/1471-0528.16361. [DOI] [PubMed] [Google Scholar]
- 18.Ortved D, Hawkins TL, Johnson JA, Hyett J, Metcalfe A. Cost-effectiveness of first-trimester screening with early preventative use of aspirin in women at high risk of early-onset pre-eclampsia. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2019;53(2):239–244. doi: 10.1002/uog.19076. [DOI] [PubMed] [Google Scholar]
- 19.Brown MA, Magee LA, Kenny LC, et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension. 1979;72(1):24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. Dallas, Tex2018. [DOI] [PubMed] [Google Scholar]
- 20.Sotiriadis A, Hernandez-Andrade E, da Silva Costa F, et al. ISUOG Practice Guidelines: role of ultrasound in screening for and follow-up of pre-eclampsia. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2019;53(1):7–22. doi: 10.1002/uog.20105. [DOI] [PubMed] [Google Scholar]
- 21.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. American journal of obstetrics and gynecology. 2013;209(6):e1–e12. doi: 10.1016/j.ajog.2013.08.019. 544. [DOI] [PubMed] [Google Scholar]
- 22.Tuffnell DJ, Jankowicz D, Lindow SW, et al. Outcomes of severe pre-eclampsia/eclampsia in Yorkshire 1999/2003. BJOG: an international journal of obstetrics and gynaecology. 2005;112(7):875–880. doi: 10.1111/j.1471-0528.2005.00565.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Joseph KS, Liston RM, et al. Incidence, risk factors, and associated complications of eclampsia. Obstetrics and gynecology. 2011;118(5):987–994. doi: 10.1097/AOG.0b013e31823311c1. [DOI] [PubMed] [Google Scholar]
- 24.Chappell LC, Duckworth S, Seed PT, et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation. 2013;128(19):2121–2131. doi: 10.1161/CIRCULATIONAHA.113.003215. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin K, Hobson SR, Chandran AR, et al. Circulating maternal placental growth factor responses to low-molecular-weight heparin in pregnant patients at risk of placental dysfunction. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Duhig KE, Myers JE, Gale C, et al. Placental growth factor measurements in the assessment of women with suspected Preeclampsia: A stratified analysis of the PARROT trial. Pregnancy hypertension. 2021;23:41–47. doi: 10.1016/j.preghy.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duhig KE, Seed PT, Myers JE, et al. Placental growth factor testing for suspected pre-eclampsia: a cost-effectiveness analysis. BJOG: an international journal of obstetrics and gynaecology. 2019;126(11):1390–1398. doi: 10.1111/1471-0528.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarthy FP, Gill C, Seed PT, Bramham K, Chappell LC, Shennan AH. Comparison of three commercially available placental growth factor-based tests in women with suspected preterm pre-eclampsia: the COMPARE study. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2019;53(1):62–67. doi: 10.1002/uog.19051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong S, Kaitu'u-Lino TJ, Hastie R, Brownfoot F, Cluver C, Hannan N. Pravastatin, proton-pump inhibitors, metformin, micronutrients, and biologics: new horizons for the prevention or treatment of preeclampsia. American journal of obstetrics and gynecology. 2020 doi: 10.1016/j.ajog.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Benny PA, Alakwaa FM, Schlueter RJ, Lassiter CB, Garmire LX. A review of omics approaches to study preeclampsia. Placenta. 2020;92:17–27. doi: 10.1016/j.placenta.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McBride N, Yousefi P, Sovio U, et al. Do Mass Spectrometry-Derived Metabolomics Improve the Prediction of Pregnancy-Related Disorders? Findings from a UK Birth Cohort with Independent Validation. Metabolites. 2021;11(8) doi: 10.3390/metabo11080530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sovio U, McBride N, Wood AM, et al. 4-Hydroxyglutamate is a novel predictor of pre-eclampsia. Int J Epidemiol. 2020;49(1):301–311. doi: 10.1093/ije/dyz098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruickshank T, MacDonald TM, Walker SP, et al. Circulating Growth Differentiation Factor 15 Is Increased Preceding Preeclampsia Diagnosis: Implications as a Disease Biomarker. J Am Heart Assoc. 2021;10(16) doi: 10.1161/JAHA.120.020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180(2):499–506. doi: 10.1016/s0002-9378(99)70239-5. Pt 1. [DOI] [PubMed] [Google Scholar]
- 35.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 36.Paiva P, Whitehead C, Saglam B, Palmer K, Tong S. Measurement of mRNA transcripts of very high placental expression in maternal blood as biomarkers of preeclampsia. J Clin Endocrinol Metab. 2011;96(11):E1807–E1815. doi: 10.1210/jc.2011-1233. [DOI] [PubMed] [Google Scholar]
- 37.Whitehead CL, Teh WT, Walker SP, Leung C, Larmour L, Tong S. Circulating MicroRNAs in maternal blood as potential biomarkers for fetal hypoxia in-utero. PLoS One. 2013;8(11):e78487. doi: 10.1371/journal.pone.0078487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whigham CA, MacDonald TM, Walker SP, et al. Circulating GATA2 mRNA is decreased among women destined to develop preeclampsia and may be of endothelial origin. Sci Rep. 2019;9(1):235. doi: 10.1038/s41598-018-36645-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whigham CA, MacDonald TM, Walker SP, et al. Circulating adrenomedullin mRNA is decreased in women destined to develop term preeclampsia. Pregnancy Hypertens. 2019;16:16–25. doi: 10.1016/j.preghy.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Whigham CA, MacDonald TM, Walker SP, et al. MicroRNAs 363 and 149 are differentially expressed in the maternal circulation preceding a diagnosis of preeclampsia. Sci Rep. 2020;10(1):18077. doi: 10.1038/s41598-020-73783-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacDonald TM, Tong S, Myers J, et al. Circulating Tissue Factor Pathway Inhibitor (TFPI) is increased preceding preeclampsia diagnosis and in established preeclampsia. Placenta. 2021;105:32–40. doi: 10.1016/j.placenta.2021.01.018. [DOI] [PubMed] [Google Scholar]
- 42.Yung C, MacDonald TM, Walker SP, et al. Death associated protein kinase 1 (DAPK-1) is increased in preeclampsia. Placenta. 2019;88:1–7. doi: 10.1016/j.placenta.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 43.Lo YM, Tsui NB, Chiu RW, et al. Plasma placental RNA allelic ratio permits noninvasive prenatal chromosomal aneuploidy detection. Nat Med. 2007;13(2):218–223. doi: 10.1038/nm1530. [DOI] [PubMed] [Google Scholar]
- 44.Tsui NB, Lo YM. Placental RNA in maternal plasma: toward noninvasive fetal gene expression profiling. Ann N Y Acad Sci. 2006;1075:96–102. doi: 10.1196/annals.1368.012. [DOI] [PubMed] [Google Scholar]
- 45.Tsui NB, Ng EK, Lo YM. Molecular analysis of circulating RNA in plasma. Methods Mol Biol. 2006;336:123–134. doi: 10.1385/1-59745-074-X:123. [DOI] [PubMed] [Google Scholar]
- 46.Gong S, Gaccioli F, Dopierala J, et al. The RNA landscape of the human placenta in health and disease. Nat Commun. 2021;12(1):2639. doi: 10.1038/s41467-021-22695-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He J, Liu K, Hou X, Lu J. Identification and validation of key non-coding RNAs and mRNAs using co-expression network analysis in pre-eclampsia. Medicine (Baltimore) 2021;100(14):e25294. doi: 10.1097/MD.0000000000025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Wang P, Zhang L, et al. Identification of Key Genes and Long Noncoding RNA-Associated Competing Endogenous RNA (ceRNA) Networks in Early-Onset Preeclampsia. Biomed Res Int. 2020;2020 doi: 10.1155/2020/1673486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tarca AL, Romero R, Erez O, et al. Maternal whole blood mRNA signatures identify women at risk of early preeclampsia: a longitudinal study. J Matern Fetal Neonatal Med. 2020:1–12. doi: 10.1080/14767058.2019.1685964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whigham CA, MacDonald TM, Walker SP, Hannan NJ, Tong S, Kaitu'u-Lino TJ. The untapped potential of placenta-enriched molecules for diagnostic and therapeutic development. Placenta. 2019;84:28–31. doi: 10.1016/j.placenta.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Zhou J, Guo X, Sun Y, Ma L, Zhe R. Levels of serum Hoxb3 and sFlt-1 in pre-eclamptic patients and their effects on pregnancy outcomes. J Obstet Gynaecol Res. 2020;46(10):2010–2018. doi: 10.1111/jog.14397. [DOI] [PubMed] [Google Scholar]
- 52.Hannan NJ, Stock O, Spencer R, et al. Circulating mRNAs are differentially expressed in pregnancies with severe placental insufficiency and at high risk of stillbirth. BMC Med. 2020;18(1):145. doi: 10.1186/s12916-020-01605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noguer-Dance M, Abu-Amero S, Al-Khtib M, et al. The primate-specific microRNA gene cluster (C19MC) is imprinted in the placenta. Hum Mol Genet. 2010;19(18):3566–3582. doi: 10.1093/hmg/ddq272. [DOI] [PubMed] [Google Scholar]
- 54.Hromadnikova I, Dvorakova L, Kotlabova K, Krofta L. The Prediction of Gestational Hypertension, Preeclampsia and Fetal Growth Restriction via the First Trimester Screening of Plasma Exosomal C19MC microRNAs. Int J Mol Sci. 2019;20(12) doi: 10.3390/ijms20122972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carbone IF, Conforti A, Picarelli S, Morano D, Alviggi C, Farina A. Circulating Nucleic Acids in Maternal Plasma and Serum in Pregnancy Complications: Are They Really Useful in Clinical Practice? A Systematic Review. Mol Diagn Ther. 2020;24(4):409–431. doi: 10.1007/s40291-020-00468-5. [DOI] [PubMed] [Google Scholar]
- 56.Townsend R, Khalil A, Premakumar Y, et al. Prediction of pre-eclampsia: review of reviews. Ultrasound Obstet Gynecol. 2019;54(1):16–27. doi: 10.1002/uog.20117. [DOI] [PubMed] [Google Scholar]
- 57.Hughes AE, Sovio U, Gaccioli F, Cook E, Charnock-Jones DS, Smith GCS. The association between first trimester AFP to PAPP-A ratio and placentally-related adverse pregnancy outcome. Placenta. 2019;81:25–31. doi: 10.1016/j.placenta.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Long W, Zhou Q, Wang H, et al. Second-trimester Maternal Serum Screening Biomarkers in the Risk Assessment for Preeclampsia. Ann Clin Lab Sci. 2018;48(3):308–313. [PubMed] [Google Scholar]
- 59.Hu J, Zhang J, He G, et al. First-trimester maternal serum alpha-fetoprotein is not a good predictor for adverse pregnancy outcomes: a retrospective study of 3325 cases. BMC Pregnancy Childbirth. 2020;20(1):104. doi: 10.1186/s12884-020-2789-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bootcov MR, Bauskin AR, Valenzuela SM, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A. 1997;94(21):11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yokoyama-Kobayashi M, Saeki M, Sekine S, Kato S. Human cDNA encoding a novel TGF-beta superfamily protein highly expressed in placenta. J Biochem. 1997;122(3):622–626. doi: 10.1093/oxfordjournals.jbchem.a021798. [DOI] [PubMed] [Google Scholar]
- 62.Bauskin AR, Brown DA, Kuffner T, et al. Role of macrophage inhibitory cytokine-1 in tumorigenesis and diagnosis of cancer. Cancer Res. 2006;66(10):4983–4986. doi: 10.1158/0008-5472.CAN-05-4067. [DOI] [PubMed] [Google Scholar]
- 63.Wollert KC, Kempf T, Wallentin L. Growth Differentiation Factor 15 as a Biomarker in Cardiovascular Disease. Clinical chemistry. 2017;63(1):140–151. doi: 10.1373/clinchem.2016.255174. [DOI] [PubMed] [Google Scholar]
- 64.Wertaschnigg D, Rolnik DL, Nie G, et al. Second- and third-trimester serum levels of growth-differentiation factor-15 in prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2020;56(6):879–884. doi: 10.1002/uog.22070. [DOI] [PubMed] [Google Scholar]
- 65.Lip SV, Boekschoten MV, Hooiveld GJ, et al. Early-onset preeclampsia, plasma microRNAs, and endothelial cell function. Am J Obstet Gynecol. 2020;222(5):497. doi: 10.1016/j.ajog.2019.11.1286. e1- e12. [DOI] [PubMed] [Google Scholar]
- 66.Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 67.Liu VW, Huang PL. Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc Res. 2008;77(1):19–29. doi: 10.1016/j.cardiores.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nemeth B, Muranyi E, Hegyi P, et al. Asymmetric dimethylarginine levels in preeclampsia - Systematic review and meta-analysis. Placenta. 2018;69:57–63. doi: 10.1016/j.placenta.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 69.Yuan J, Wang X, Xie Y, et al. Circulating asymmetric dimethylarginine and the risk of preeclampsia: a meta-analysis based on 1338 participants. Oncotarget. 2017;8(27):43944–43952. doi: 10.18632/oncotarget.16543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saleh L, Verdonk K, Visser W, van den Meiracker AH, Danser AH. The emerging role of endothelin-1 in the pathogenesis of pre-eclampsia. Ther Adv Cardiovasc Dis. 2016;10(5):282–293. doi: 10.1177/1753944715624853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lind Malte A, Uldbjerg N, Wright D, Torring N. Prediction of severe pre-eclampsia/HELLP syndrome by combination of sFlt-1, CT-pro-ET-1 and blood pressure: exploratory study. Ultrasound Obstet Gynecol. 2018;51(6):768–774. doi: 10.1002/uog.17561. [DOI] [PubMed] [Google Scholar]
- 72.Valencia-Ortega J, Zarate A, Saucedo R, Hernandez-Valencia M, Cruz JG, Puello E. Placental Proinflammatory State and Maternal Endothelial Dysfunction in Preeclampsia. Gynecol Obstet Invest. 2019;84(1):12–19. doi: 10.1159/000491087. [DOI] [PubMed] [Google Scholar]
- 73.Wiles K, Bramham K, Seed PT, et al. Diagnostic indicators of superimposed preeclampsia in women with CKD. Kidney Int Rep. 2019;4(6):842–853. doi: 10.1016/j.ekir.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wiles K, Bramham K, Seed PT, et al. Placental and endothelial biomarkers for the prediction of superimposed pre-eclampsia in chronic kidney disease. Pregnancy Hypertens. 2021;24:58–64. doi: 10.1016/j.preghy.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 75.Lan X, Liu Z. Circulating endocan and preeclampsia: a meta-analysis. Biosci Rep. 2020;40(1) doi: 10.1042/BSR20193219. [DOI] [PMC free article] [PubMed] [Google Scholar]