Summary
Background
Although the BNT162b2 COVID-19 vaccine is known to induce IgG neutralizing antibodies in serum protecting against COVID-19, it has not been studied in detail whether it could generate specific immunity at mucosal sites, which represent the primary route of entry of SARS-CoV-2.
Methods
Samples of serum and saliva of 60 BNT162b2-vaccinated healthcare workers were collected at baseline, two weeks after the first dose and two weeks after the second dose. Anti-S1-protein IgG and IgA total antibodies titres and the presence of neutralizing antibodies against the Receptor Binding Domain in both serum and saliva were measured by quantitative and by competitive ELISA, respectively.
Findings
Complete vaccination cycle generates a high serum IgG antibody titre as a single dose in previously infected seropositive individuals. Serum IgA concentration reaches a plateau after a single dose in seropositive individuals and two vaccine doses in seronegative subjects. After the second dose IgA level was higher in seronegative than in seropositive subjects. In saliva, IgG level is almost two orders of magnitude lower than in serum, reaching the highest values after the second dose. IgA concentration remains low and increases significantly only in seropositive individuals after the second dose. Neutralizing antibody titres were much higher in serum than in saliva.
Interpretation
The mRNA BNT162b2 vaccination elicits a strong systemic immune response by drastically boosting neutralizing antibodies development in serum, but not in saliva, indicating that at least oral mucosal immunity is poorly activated by this vaccination protocol, thus failing in limiting virus acquisition upon its entry through this route.
Funding
This work was funded by the Department of Medicine and Surgery, University of Insubria, and partially supported by Fondazione Umberto Veronesi (COVID-19 Insieme per la ricerca di tutti, 2020).
Keywords: BNT162b2 mRNA vaccine, COVID-19, SARS-CoV-2, Saliva, IgA
Abbreviations: S, Spike glycoprotein; NAb, neutralizing antibodies; sIgA, secretory IgA; RBD, Receptor-Binding Domain; Ab, antibodies; HCW, healthcare workers; AUC, Area Under the ROC Curve; CLIA, chemiluminescent immunoassay; NAAT, Nucleic Acid Amplification Test; ELISA, enzyme-linked immunosorbent assay; INH, inhibition activity; GMC, geometric mean concentration; Se, sensitivity; Sp, specificity; SP, seropositive subjects; SN, seronegative subjects; R, IgA responders; NR, IgA non-responders; CI, Confidence Interval; ÷, mathematical symbol for range of values
Research in context.
Evidence before this study
On December 6th, 2021, we searched PubMed website for published peer-reviewed research articles written in English using the search terms “BNT162b2 vaccine”, “salivary antibodies” and “oral mucosal immunity”. Several studies have shown that BNT162b2 COVID-19 mRNA vaccine induces neutralizing antibody responses in healthy adults and one single dose promotes a similar or even higher immune response in individuals with prior SARS-CoV-2 infection than in infection naïve individuals receiving two-dose immunization. However, we identified only three studies investigating the activation of a specific oral mucosal immunity after mRNA vaccination. They reported that within 1-2 weeks after the second dose, vaccinated subjects had S-protein IgG antibodies in their saliva, while IgA were detected in a substantial proportion, but results were inconsistent. Assessing the mucosal immune response might provide further information about the vaccine efficacy in protecting the host from the infection, since the mucosal sites represent the first route of entry of the virus.
Added value of this study
This is the first report assessing the development of both total IgG and IgA antibodies in the saliva and the neutralizing activity against both the wild-type and Delta variant of the Receptor Binding Domain in individuals who underwent the BNT162b2 vaccination protocol. Our findings demonstrate that although mRNA vaccination elicits a strong systemic immune response associated with high serum IgG antibody titres, it is not able to promote an effective activation of the mucosal immune response. The neutralizing activity in saliva was lower than that measured in the serum and was mainly provided by salivary IgG that are exuded from the serum. Only in previously exposed individuals the increase of salivary IgA was more pronounced.
Implications of all the available evidence
Our findings indicate that intramuscular administration of the mRNA BNT162b2 vaccine promotes a strong systemic immune response but only weakly induces the production of salivary IgG and in less extent salivary IgA. The low IgA antibodies titre in saliva suggests a lack of stimulation of secretory IgA and therefore an ineffective activation of mucosal immunity by systemic vaccination. Distinct routes of immunization, such as the nasal or oral, might represent a new challenge in the booster doses of vaccine to increase oral or respiratory mucosal immunity against SARS-CoV-2, as at these sites the first contact with the virus takes place. This should be desirable to block primary virus infection more efficiently and to obtain sterilizing immunity.
Alt-text: Unlabelled box
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of coronavirus disease 2019 (COVID-19), an acute respiratory syndrome with a complex and highly variable disease pathology. The appearance of SARS-CoV-2 has led to a rapidly spreading pandemic with more than 260 million cases and over 5,200,000 deaths reported worldwide.1 Several features of SARS-CoV-2, including its ability to efficiently replicate in the mucosa of upper respiratory tract, the presence of many asymptomatic and paucisymptomatic infected individuals producing infectious virus, and its variable incubation time of about 3 to 14 days, have likely contributed to its rapid spread.2
The severe and worldwide effect of the pandemic on human society calls for the rapid development of safe and effective preventive and therapeutic drugs to control SARS-CoV-2 spreading.3 The BNT162b2 and mRNA-1273 COVID-19 vaccines constitute an unprecedented innovative tool for mass immunization, since they represent the first two vaccines based on the messenger RNA (mRNA) technology distributed at a large-scale in the general population.4 The BNT162b2, a mRNA vaccine that expresses the prefusion stabilized full Spike glycoprotein (S) of SARS-CoV-2 isolate Wuhan-Hu-1 (GenBank accession number, MN908947.3), is 95% effective against the onset of severe COVID-19 in a phase III trial after 7 days from the second dose injection.5
Nevertheless, although several reports have assessed the generation of vaccine-induced IgG neutralizing antibodies (NAb) in the serum of immunized individuals, few data are available regarding the activation of a specific mucosal immunity.6 The respiratory tract,7 the oral mucosa,8 and conjunctival surfaces9 represent the primary route of entry of SARS-CoV-2, thus anti-SARS-CoV-2 vaccines inducing specific immune response in these districts might become a crucial tool to counteract the pandemic.
Mucosal humoral immunity is mainly constituted by secretory IgA (sIgA), which play an important role in host defense against respiratory pathogens, as SARS-CoV-2.10 sIgA may prevent SARS-CoV-2 adhesion to target epithelial cells via neutralization of the coronavirus Spike protein or binding to the SARS-CoV-2 Nucleocapsid protein.11 Furthermore, salivary sIgA might represent a non-invasive tool to stratify the population into different risk categories and inform individual and collective decisions relating to appropriate vaccine prioritization.12 Here we assessed the mucosal immune response in healthy individuals who underwent the mRNA BNT162b2 COVID-19 vaccination by measuring total IgG and IgA antibodies against the S protein of the virus both in serum and saliva, as well as the specific NAb against SARS-CoV-2 Receptor-Binding Domain (RBD) in both the compartments. We report the unprecedented finding that the IgG titer threshold of 1.54 ng/ml in saliva is predictive of seropositivity, suggesting that salivary IgG antibodies (Ab) might represent a suitable indication of seroconversion.
Methods
Study design and participants
We performed an observational cohort study recruiting 60 healthcare workers (HCW) of our hospital (ASST dei Sette Laghi) who underwent a complete BNT162b2 vaccination protocol (two doses, three weeks apart) between December 30th, 2020, and January 20th, 2021, to assess both the systemic and mucosal antibody response elicited by the vaccination. The clinical protocol for sample and data collection and the informed consent were approved by the Institutional Ethics Committee (Comitato Etico dell'Insubria, n° 165/2020).
At study design, we considered the Area Under the ROC Curve (AUC) as a measure of accuracy for salivary IgG vs. serum chemiluminescent assay (CLIA) as gold standard. We hypothesized an AUC of 0.75 to identify positive (i.e., CLIA ≥ 15 AU/ml) subjects, and we assumed a positive:negative ratio ranging from 5 to 6. Under these assumptions, the study with a planned sample size of 60 workers has a probability of 80% to detect a true AUC larger than 0.5 at a type-I error of 5%.
Eligible participants were 18 years old or older, and negative for SARS-CoV-2 infection as assessed by Nucleic Acid Amplification Test (NAAT). Glucocorticosteroid and/or immunosuppressant drugs, autoimmune disorders, and pregnancy were indicated as exclusion criteria.
The samples of serum and saliva were collected at three different time points: day of the first dose (T0), two weeks after the first dose (T1) and two weeks after the second dose (T2). Blood samples were collected into a sterile 5 ml container with gel separator, and then centrifuged at 3,000 g for 5 minutes to obtain the serum fraction. Saliva was self-collected by spitting under the supervision of a trained provider13 (Supplementary Appendix).
The following clinical data were recorded: age, sex, and the presence of side effects after each vaccine injection. For those subjects who reported a previous exposure to SARS-CoV-2, confirmed either by serological testing or NAAT, additional information about the date of the diagnosis, the presence of symptoms and the severity of the disease were recorded.
Antibody measurement
A commercial enzyme-linked immunosorbent assay (ELISA) specific for S1 protein of SARS-CoV-2 was used to measure both IgG and IgA Ab titres in serum and saliva samples according to the manufacturer's instructions (Anti-nCoV19 S1 IgG/IgA HS Immunospark, Pomezia, Italy). Serum samples were analyzed at 1:100, 1,1000 and 1:10,000 dilution, while the saliva samples were diluted at 1:3, 1:10, 1:100, and 1:1000. The results were expressed as ng/ml.
Serum IgG against SARS-Cov-2 S1/S2 Spike protein subunits were measured by CLIA (LIAISON® DiaSorin).14 The results were expressed as arbitrary units (AU/ml), with a negative and positive cut-off of 15 AU/ml and 400 AU/ml, respectively. Values greater than 400 AU/ml were expressed as 400.
The presence of anti-Receptor Binding Domain (RBD) NAb in serum and saliva was assessed by competitive ELISA, following the manufacturer's instructions (cPASSTM SARS-CoV-2 Neutralization Antibody Detection Kit, GenScript). Briefly, 10 µl of serum were diluted in 90 µl of sample dilution buffer, while the saliva was used undiluted. Positive and negative serum controls provided within the kit were used as reference for the serum NAb, while known positive and negative salivary samples were used as reference for the salivary NAb. The optical density (OD) average of the negative controls was used to calculate the percentage of inhibition according to the formula: (1 – OD value of the sample / OD value of negative control) x 100%. A cut-off value of 30% was used to discriminate between the presence or absence of NAb, according to the manufacturer's instructions. In case of positivity, the percentage of inhibitory activity (INH) was also assessed. To verify the efficacy of the mucosal immune response against the variants of concern, the serum and the salivary samples of those individuals who showed inhibitory activity in their saliva against the wild-type antigen (i.e., Wuhan-Hu-1 RBD) were also tested against the RBD of the Delta variant (lineage B.1.617.2; E484Q and L452R).
Statistical analysis
Participant characteristics and side effects after the first and second vaccine doses were summarized using standard descriptive statistics, in individuals with and without previous SARS-CoV-2 infection taken separately.
To account for their skewed distributions, at each visit time, we calculated sample medians and interquartile range for serum and salivary IgG and IgA, in the overall sample and according to previous SARS-CoV-2 infection. To estimate time trends in serum and salivary IgG and IgA, we used repeated-measure regression models, with baseline value, time visit, previous SARS-CoV-2 infection status, and the interaction between time and infection status as independent variables. Again, we modelled log-transformed IgG and IgA values, and reported the geometric mean concentrations (GMC). We used an unstructured variance-covariance matrix, to allow a flexible intra-individual correlation between IgG and IgA at different times and robust standard error estimates (empirical GEE). At T1 and T2, differences in logarithmic IgG and IgA from baseline (with 95% confidence intervals) were estimated using SAS macro NLEstimate.15
In addition, we estimated the AUC for both serum and salivary IgG at CLIA threshold for positivity (i.e., ≥ 15 AU/ml) and at a value indicative of a good immune response after the vaccination (i.e., ≥90 AU/ml)16 from logistic regression models. We also reported sensitivity (Se) and specificity (Sp) (with 95% confidence intervals) at the best serum and salivary IgG thresholds as defined according to the Youden method17 and estimated through the ‘OptimalCutpoints’ package in R (3.6.3 release).18 The software for the remaining analyses was SAS Software (9.4 release).
Role of funders
This work was funded by the Department of Medicine and Surgery, University of Insubria, and partially supported by Fondazione Umberto Veronesi (COVID-19 Insieme per la ricerca di tutti, 2020). The funders had no role in the experimental design, collection of data or writing the paper.
Results
Participants
Sixty healthy HCW were enrolled in the study and vaccinated with the BNT162b2 COVID-19 vaccine. The mean age of the study participants was 41.2 ± 10.4 years (range, 26-62 years) and 40 subjects (i.e., 66.7%) were female. Clinical data and systemic side effects to each injection were reported in Table S1.
BNT162b2-vaccine induced antibody response
Concentrations of S1-binding IgG and IgA Ab were assessed both in serum and saliva samples at baseline (T0), two weeks after the BNT162b2 priming dose (T1) and two weeks after the boosting dose (T2).
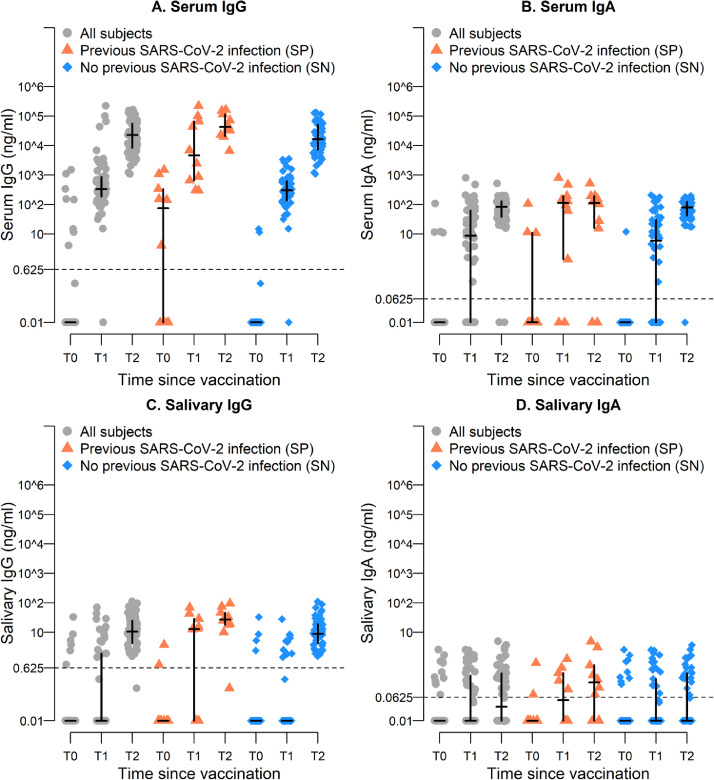
Firstly, all the serum samples were analyzed by the CLIA assay, and we could measure the presence of an IgG antibody titre in 10 (from now named seropositive subjects, SP) out of the 60 enrolled subjects, but not in the other 50 subjects (from now named seronegative subjects, SN) (Figure S1, Table S2). Of note, this value (T0GMC: 64.2 AU/ml, SP) was similar to that measured in SN after the first dose (T1GMC: 43.2 AU/ml, SN), confirming the need for a single dose in subjects with previous natural SARS-CoV-2 infection,19 and that a boosting dose is necessary to increase antibody concentration in unexposed individuals.20
In the meantime, we could measure specific anti-S1 IgG Ab titres in SP individuals by the ELISA assay at T0, with GMC of 3.7 ng/ml (Figure 1A, Table 1). Substantially higher serum IgG titres were achieved after the booster dose, with significant differences between SN and SP groups (T1GMC: 255.6 ng/ml SN, 5,962.2 ng/ml SP; T2GMC: 17,341.4 ng/ml SN, 45,603.0 ng/ml SP) (Figure 1A, Table 1). These data were consistent with those obtained by CLIA (Figure S1, Table S2).
Figure 1.
Distribution of serum (panels A and B) and salivary (panels C and D) IgG and IgA, at different times, for all the recruited individuals and according to previous SARS-CoV-2 status at T0. In each group, the horizontal line represents the sample median, while the vertical line the interquartile range.
Table 1.
Time trends for IgG and IgA antibodies in both serum and saliva, overall sample and by previous SARS-CoV-2 exposure.
| Variable, time | All subjects (n=60) |
No previous SARS-CoV-2 (SN; n=50) |
Previous SARS-CoV-2 (SP; n=10) |
p-value2 | p-value3 | |||
|---|---|---|---|---|---|---|---|---|
| Mean1 | Δ (IC 95%) | Mean1 | Δ (IC 95%) | Mean1 | Δ (IC 95%) | |||
| Serum | ||||||||
| Serum IgG (ng/ml)a | ||||||||
| T0 | 0.04 | - | 0.01 | - | 3.7 | - | 0.005 | 0.45 |
| T1 | 432.1 | 432.1 (219.3; 644.8) | 255.6 | 255.6 (112.0; 399.2) | 5962.2 | 5958.5 (-956.4; 12873.4) | 0.11 | |
| T2 | 20373.65 | 20373.6 (11486.0; 29261.2) | 17341.4 | 17341.4 (10262.7; 24420.1) | 45603.0 | 45599.3 (14787.7; 76410.8) | 0.08 | |
| Serum IgA (ng/ml)a | ||||||||
| T0 | 0.02 | - | 0.01 | - | 0.1 | - | <0.0001 | 0.24 |
| T1 | 1.71 | 1.7 (0.01; 3.4) | 1.10 | 1.1 (-0.1; 2.3) | 16.1 | 16 (-22.2; 54.1) | 0.44 | |
| T2 | 49.59 | 49.6 (21.1; 78.0) | 61.6 | 61.6 (29.5; 93.7) | 16.7 | 16.6 (-21.6; 54.9) | 0.08 | |
| Saliva | ||||||||
| Salivary IgG (ng/ml)a | ||||||||
| T0 | 0.02 | - | 0.02 | - | 0.03 | - | 0.04 | <0.0001 |
| T1 | 0.07 | 0.1 (0; 0.1) | 0.04 | 0.02 (-0.01; 0.06) | 1.08 | 1.1 (-1.2; 3.3) | 0.36 | |
| T2 | 10.8 | 10.8 (6.8; 14.8) | 9.8 | 9.7 (4.1; 15.4) | 18.1 | 18.1 (-7.2; 43.3) | 0.53 | |
| Salivary IgA, (ng/ml)a | ||||||||
| T0 | 0.02 | - | 0.02 | - | 0.02 | - | 0.22 | 0.06 |
| T1 | 0.05 | 0.02 (0.01; 0.05) | 0.04 | 0.02 (-0.01; 0.05) | 0.06 | 0.04 (-0.02; 0.11) | 0.59 | |
| T2 | 0.07 | 0.05 (0.02; 0.08) | 0.06 | 0.04 (0.01; 0.07) | 0.16 | 0.14 (-0.05; 0.32) | 0.29 | |
a: Change in geometric mean concentration, modelled through a log-linear regression model for repeated measures, with unstructured variance-covariance matrix and adjusting for baseline.
1: Geometric mean concentration
2: p-value testing homogeneity of trends between subjects with and without previous SARS-CoV-2 infection.
3: p-value testing homogeneity of geometric mean values in IgG and IgA between subjects with and without previous SARS-CoV-2 infection, at each time.
On average, positive S1-specific serum IgA titres increased after the second dose, with a trend similar to that observed for serum IgG (T1GMC: 1.71 ng/ml; T2GMC: 49.59 ng/ml) (Figure 1B, Table 1). However, differently from what found for serum IgG, after the second dose, despite the higher IgA titre in SP at T1 compared to SN (T1GMC: 1.10 ng/ml SN, 16.1 ng/ml SP), the concentration of IgA remained stable in SP while significantly increased in SN (T2GMC: 61.6 ng/ml SN, 16.7 ng/ml SP) (Figure 1B, Table 1).
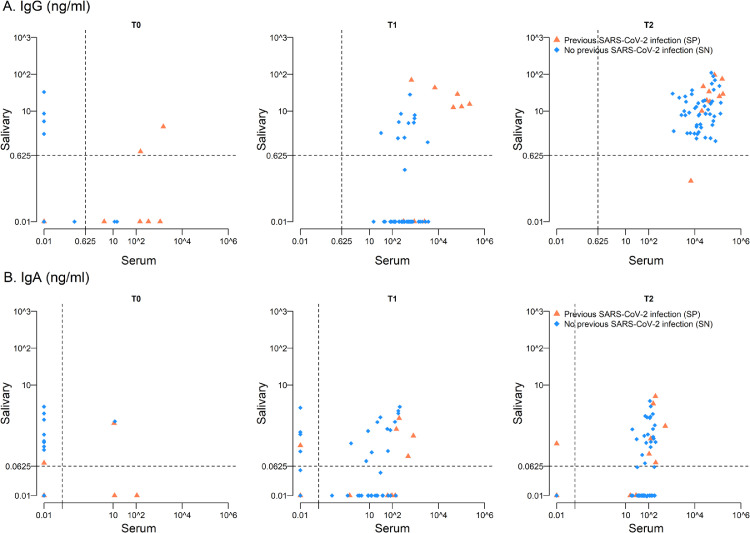
To further characterize the mucosal immune response elicited by the vaccination, we investigated both S1-specific IgG and IgA titres in saliva. Differently from serum, salivary IgG were not detected at T0 in SP, but slightly increased at T1 and boosted after the second dose (T1GMC: 1.08 ng/ml; T2GMC: 18.1 ng/ml, SP) (Figure 1C, Table 1), while in SN salivary IgG were measurable only at T2 (T1GMC: 0.04 ng/ml; T2GMC: 9.8 ng/ml, SN) (Figure 1C, Table 1). However, at T2 the IgG serum/saliva ratio was comparable between the two groups (around 2,500 for SP and 1,800 for SN) (Table 1). Moreover, looking for a correlation between serum and salivary IgG, we observed the presence of IgG in saliva when serum IgG titre was higher than 103 (Pearson correlation coefficient at T2 0.392, p=0.002) (Figure 2A).
Figure 2.
Scatter-plot for salivary and serum IgG (panel A) and IgA (panel B) at different times according to previous SARS-CoV-2 status at T0.
As far as the IgA, we observed a broad range of salivary IgA concentration without a clear increased titre after the boosting dose (T1GMC: 0.05 ng/ml; T2GMC: 0.07 ng/ml) (Figure 1D, Table 1). However, the IgA levels slightly increased at T2 in SP (T1GMC: 0.06 ng/ml; T2GMC: 0.16 ng/ml, SP) but not in SN (T1GMC: 0.04 ng/ml; T2GMC: 0.06 ng/ml, SN), indicating that specific mucosal IgA could be elicited by the vaccination in subjects with a previous infection. Consistent with this hypothesis, we observed that the IgA serum/saliva ratio at T2 was higher in SN than in SP (around 1,000 and 100, respectively).
Differently from what observed for IgG, about 50% of enrolled subjects developed salivary IgA when the level of IgA in serum was higher than 102 (Pearson correlation coefficient at T2 0.291, p=0.02) (Figure 2B). To further clarify this point, we looked for correlation between serum IgA and salivary IgA levels in subjects having measurable IgA in saliva at T2 (IgA responders, R) or subjects with undetectable salivary IgA (IgA non-responders, NR). As indicated in Table S3, serum IgA titres were higher in R than in NR (T2GMC: 117.0 ng/ml, R; 51.7 ng/ml, NR), suggesting that most of salivary IgA are exuded from serum.
When Ab concentrations were compared with clinical symptoms, reported side-effects to vaccination did not significantly affect IgG and IgA levels both in serum and saliva (Table S4). In addition, we did not observe any significant correlation between Ab levels and elapsed time between onset of symptoms and vaccination or the severity of COVID-19 disease in SP (Table S5).
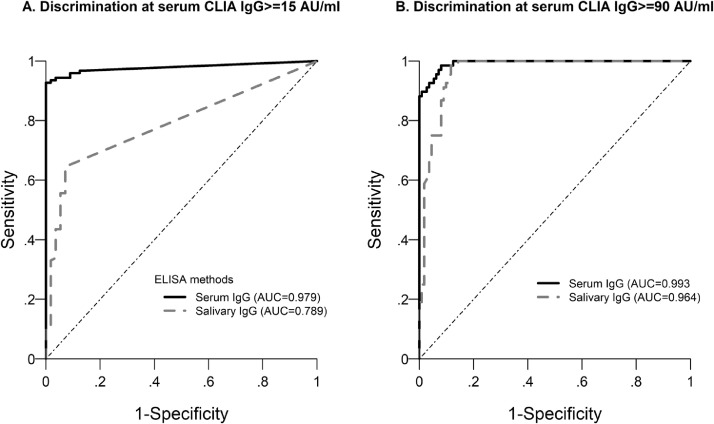
Finally, ROC analysis assessed that the IgG titre thresholds of 125.8 ng/ml in serum and 0.127 ng/ml in saliva were predictive of CLIA seropositivity (i.e., ≥ 15 AU/ml) with higher accuracy in serum (Se 0.93, 95% CI 0.87 – 0.97; SP 1.0, 95% CI 0.94 – 1.0), compared to saliva (Se 0.65, 95% CI 0.55 – 0.73; Sp 0.93, 95% CI 0.83 – 0.98). However, considering 90 AU/ml in CLIA analysis16 as a cut-off value for an effective immune response, the thresholds of 904.5 ng/ml IgG in serum and 1.54 ng/ml IgG in saliva were strongly predictive for both samples (Serum Se 0.99, 95% CI 0.92 – 1.0; Sp 0.92, 95% CI 0.85 – 0.96; Saliva Se 0.99, 95% CI 0.92 – 1.0; Sp 0.88, 95% CI 0.81 – 0.94) (Figure 3, Table S6), indicating that salivary IgG Ab might represent a suitable indication of seroconversion.
Figure 3.
ROC curve for serum and salivary IgG to identify individuals with CLIA above 15 (A) and 90 (B) AU/ml
BNT162b2 vaccine-elicited neutralizing antibodies in saliva
To investigate the presence of IgG and IgA NAb both in serum and saliva we tested all samples against SARS-CoV-2 RBD, by using a competitive ELISA assay. At T0 serum NAb were detected in 70% of SP (INH: 32.6 ÷ 91.9%, T0m 60.9 ± 23.5%). Of note, the SP subjects without NAb reported a previous asymptomatic infection, and two of them presented low IgG NAb detected by CLIA. At T0, all enrolled subjects scored negative for NAb in saliva (Table S7).
At T1, serum NAb were found in more than 50% of participants (INH: 30.3 ÷ 92.6%, T1m 60.1 ± 21.3%) including all the SP (INH: 46.3 ÷ 92.6%, T1m 80.6 ± 17.1%, SP), and only 50% of SN with lower values (INH: 30.3 ÷ 91.1%, T1m 51.9 ± 16.7%, SN). In saliva, only 8 subjects resulted positive (INH: 31.2 ÷ 78.9%, T1m 49.4 ± 16.8%), and 6 of them were SP (INH: 31.2 ÷ 78.9%, T1m 53.2 ± 18.1%, SP) and 2 SN (36.9 and 39.6% respectively) (Table S7).
At T2, we found NAb in all serum samples (INH: 87.3 ÷ 93.0%, T2m 91.5 ± 1.1%), with no significant differences between the SP (INH: 90.6 ÷ 93.0%, T2m 92.2 ± 0.7%) and SN (INH: 87.3 ÷ 92.7%, T2m 91.4 ± 1.1%). In contrast, only 15 saliva samples were positive (INH: 30.3 ÷ 76.4%, T2m 56.4 ± 14.6%), with a significant difference between the two subgroups, indeed 60% of SP (INH: 37.1 ÷ 72.7%, T2m 62.8 ± 13.2%, SP) and only 18% of SN (INH: 30.3 ÷ 76.4%, T2m 52.2 ± 14.5%, SN) showed NAb (Table S7).
The serum and salivary samples of those 15 subjects who showed inhibitory activity in their saliva were tested also against the antigen of the Delta variant, and they showed levels of INH like those recorded against the wild-type antigen (Serum INH: 84.3 ÷ 90.89%, T2m 89.5 ± 1.8%; Salivary INH: 30.2 ÷ 74.2, T2m 53.5 ± 15.7) (Table S8).
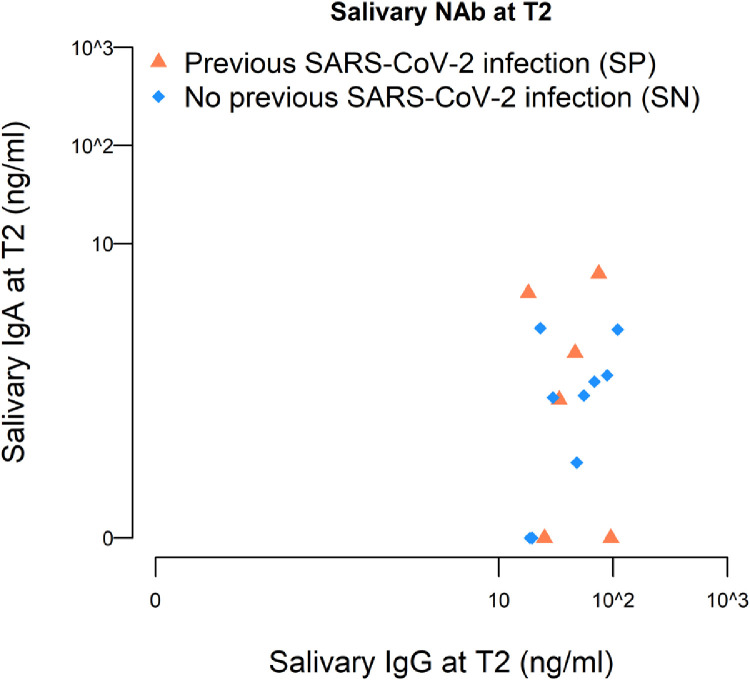
Interestingly, the presence of salivary NAb was correlated with the amount of total IgG detected in the serum (p=0.01), as well as with the quantity of salivary IgG (p <0.0001) and IgA (p=0.04), but not with serum IgA (p=0.77) (Table S9). As reported in Figure 4, the major contribution to salivary NAb come from salivary IgG rather than IgA, at least for SN. Of note, we observed that SP with salivary NAb present salivary IgA titers higher than those found in SN (median value 0.51 ng/ml SP, 0.28 ng/ml SN).
Figure 4.
Scatter-plot for salivary IgG and IgA among individuals with positive salivary NAb (n=15) at T2, according to previous SARS-CoV-2 status at T0.
Discussion
Approved mRNA COVID-19 vaccines have been shown to be effective in blocking the disease and significantly reducing the mortality rate associated with the infection21; their capacity to protect from infection and spreading, at least for BNT162b2 and mRNA-1273 vaccines, is however still matter of debate.22,23 Indeed, cases of vaccinated subjects who become infected are commonly observed, especially several months after the injection.24 This is due to the fact that these vaccines induce a specific and potent systemic immunity against the virus but are not very efficient in promoting a specific immunity at the level of the mucosae which represent the virus route of entry.25,26 In order to better investigate this aspect, we undertook a comparative analysis of serum and mucosal anti-SARS-CoV-2 antibodies by focusing also on the more prevalent antibody class present in the mucosa, the IgA antibody isotype.
Here we showed that a complete BNT162b2 mRNA vaccination induces strong systemic humoral responses that are quantified in serum samples, but low IgG and even lower IgA antibodies to the S-protein and the RBD in saliva. Thus, the mucosal immune response was substantially weaker than the systemic-evoked immunity, as salivary IgG Ab titers detectably increased only after the boosting dose among individuals that did not experience previous contact with the virus before undergoing vaccination (here designed as seronegative or SN). As far as the IgA, it is known that in saliva they are mostly represented by dimeric secretory IgA (sIgA).27 The finding that the BNT162b2 vaccination poorly induces salivary IgA suggests that these Ab were not mucosal-evoked sIgA but originated most likely from serum, by transudation from the blood circulation. Thus, in the examined population, the low mucosal immunity detected is mostly represented by salivary IgG rather than IgA. Consistently, after the boosting dose of vaccine only 18% of SN showed neutralizing activity in saliva.
On the contrary, 60% of virus-exposed subjects before vaccination (here designed as seropositive or SP) developed NAb in their saliva after vaccine administration. Indeed, in this group the increase of salivary IgA was more pronounced, and the serum/saliva IgA ratio was ten-fold higher than in unexposed individuals. This observation suggests that in subjects with previous SARS-CoV-2 natural infection the presence of some “mucosal” immunity mirrors the activation of B cells that can switch toward the production of IgA after vaccination, as also suggested by recent reports.26,28
In conclusion, the results reported in this study significantly contribute, in our opinion, to clarify several points of interest for future focusing on adopting optimal strategies of anti-SARS-CoV-2 vaccination. To briefly summarize, first we substantiated previous findings showing that two doses of vaccine are required to generate a high IgG antibody titre in the serum of SN individuals, while a single dose is sufficient in previously infected SP individuals. In the latter individuals a second vaccine dose results in higher antibody concentration as compared to SN individuals.
Second, serum IgA concentration seems to reach a plateau after a single dose of vaccine in SP individuals and does not further increase after a second dose. In SN subjects IgA were boosted after the second dose, reaching higher concentration compared to the SP group. This may be explained by the fact that previous virus exposure might first elicit mucosal IgA response similarly to other viral infections, rather than systemic immunity. Consistently with this hypothesis, it was reported that in infected individuals mucosal IgA response inversely correlated with symptom severity, being particularly abundant in asymptomatic COVID 19 patients, reinforcing their role in controlling virus penetration in the body.29
Third, in saliva, IgG concentration is almost two orders of magnitude lower than in serum and reaches maximum levels after two vaccine doses. IgA concentration remains low and increases significantly only in SP individuals after the second vaccine dose (20 to 30 times lower than in serum, anyway). Generally speaking, NAb titres for both IgG and IgA isotypes follow the same rules as total anti-S1 antibody concentration both in serum and saliva, being thus much higher in serum than in saliva. This indicates that vaccination with the BNT162b2 vaccine equally favors the production of total anti-S1 and neutralizing antibodies.
Thus, intramuscular vaccination drastically increases both total and NAb concentration in the serum but not in the salivary compartment, indicating that at least oral mucosal immunity is poorly activated by this vaccination protocol.
The vaccination campaign has significantly reduced the cases of severe COVID-19, hospitalizations, and deaths.30
However, although the vaccination strongly contributed to limit the number of infected people among the vaccinated individuals, some vaccinated person can be infected, often asymptomatically.31 Furthermore, the role of emerging new viral variants, as the Delta and the Omicron variants, continuously represents a challenge for the scientific community to block the circulation of the virus, which represents a considerable risk for global health.32 Indeed, even though the efficacy of COVID-19 vaccines has been demonstrated to be maintained against at least for the Delta variant,33 as also confirmed by our findings, it is very likely that an incomplete cycle of vaccination or the persistent circulation of the virus can lead to a decrease in efficacy or to the onset of new variants able to escape the immune response elicited by the vaccines.34
Within this frame, reconsidering the strategy of vaccination to prevent not only the severe disease but the viral infection (i.e., the so-called ‘sterilizing immunity’), should represent the goal for the generation of second line COVID-19 vaccines, aimed to reinforce the mucosal immune response.35
Thus, distinct routes of immunizations, such as nasal or oral, could constitute the way to increase oral or respiratory mucosal immunity against SARS-CoV-2, as at these sites the first contact with the virus takes place.36 Regarding the vaccination via nasal route, that represents the main entrance for SARS-CoV-2 in the body, results that have been achieved in vivo studies look promising,37 and phase 1 clinical trials should be published within the next months.38 Studies on oral mucosal vaccination, which represents the main route of SARS-CoV-2 transmission are still preliminary.39
On the other hand, governments have recently approved the administration of the booster dose for the mRNA vaccines (i.e., the third dose), and data about the development of specific mucosal immune response could clarify the role of these vaccines in protecting against the infection, since the systemic evoked response seems stronger than after the primary vaccination cycle.40
Contributors
Conceptualization: L. Azzi, G. Forlani.
Data curation: L. Azzi, D. Dalla Gasperina, M. Shallak, A. Baj, V. Maurino, G. Forlani.
Investigation: L. Azzi, D. Dalla Gasperina, M. Shallak, G. Ietto, D. Iovino, V. Maurino, G. Forlani.
Formal analysis: G. Veronesi, M.M. Ferrario.
Visualization: G. Veronesi.
Methodology: D. Dalla Gasperina, F. Gianfagna, F. Dentali.
Validation: F. Maggi, G. Forlani.
Supervision: D. Dalla Gasperina, F. Maggi, M.M. Ferrario, R.S. Accolla.
Project administration: L. Azzi, F. Dentali, G. Carcano, L.S. Maffioli, G. Forlani.
Resources: A. Tagliabue, L.S. Maffioli.
Funding acquisition: A. Tagliabue.
Writing – original draft: L. Azzi, G. Veronesi, G. Forlani.
Writing – review and editing: D. Dalla Gasperina, D. Focosi, R.S. Accolla.
Declaration of interests
None to declare.
Acknowledgements
We thank all the colleagues who participated to this study, and we are grateful to Giulia Cappellari, Virna Bolognesi and Elisabetta Forlini for their precious support in collecting serum and salivary samples. This work was funded by the Department of Medicine and Surgery, University of Insubria, and partially supported by Fondazione Umberto Veronesi (COVID-19 Insieme per la ricerca di tutti, 2020).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103788.
Supplementary materials
References
- 1.World Health Organization . 2021. Coronavirus disease (COVID-19) dashboard.https://covid19.who.int accessed on December 5th, 2021. [PubMed] [Google Scholar]
- 2.Lavezzo E, Franchin E, Ciavarella C, et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature. 2020;584:425–429. doi: 10.1038/s41586-020-2488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodgson SH, Mansatta K, Mallett G, et al. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021;21:e26–e35. doi: 10.1016/S1473-3099(20)30773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ketas TJ, Chaturbhuj D, Cruz-Portillo VM, et al. Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva. Pathog Immun. 2021;6:116–134. doi: 10.20411/pai.v6i1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fröberg J, Diavatopoulos DA. Mucosal immunity to severe acute respiratory syndrome coronavirus 2 infection. Curr Opin Infect Dis. 2021;34:181–186. doi: 10.1097/QCO.0000000000000724. [DOI] [PubMed] [Google Scholar]
- 8.Huang N, Pérez P, Kato T, et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021;27:892–903. doi: 10.1038/s41591-021-01296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azzolini C, Donati S, Premi E, et al. SARS-CoV-2 on ocular surfaces in a cohort of patients with COVID-19 from the Lombardy region, Italy. JAMA Ophtalmol. 2021 doi: 10.1001/jamaophtalmol.2020.5464. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Jin L, Chen T. The effects of secretory IgA in the mucosal immune system. Biomed Res Int. 2020;2020 doi: 10.1155/2020/2032057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterlin D, Mathian A, Miyara M, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13:eabd2223. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varadhachary A, Chatterjee D, Garza J, et al. Salivary anti-SARS-CoV-2 IgA as an accessible biomarker of mucosal immunity against COVID-19. medRxiv. 2020 [Google Scholar]
- 13.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2021. Considerations for the use of saliva as sample material for COVID-19 testing. 3 May 2021. [Google Scholar]
- 14.Bonelli F, Sarasini A, Zierold C, et al. Clinical and analytical performance of an automated serological test that identifies S1/S2-neutralizing IgG in COVID-19 patients semiquantitatively. J Clin Microbiol. 2020;58:e01224. doi: 10.1128/JCM.01224-20. -20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SAS Notes: Estimating nonlinear combinations of model parameters. Available at: https://support.sas.com/kb/58/775.html
- 16.Moscato G, Mazzetti P, Lucenteforte E, et al. Assessment of automated high-throughput serological assays for prediction of high-titer SARS-CoV-2 neutralizing antibody. J Clin Virol Plus. 2021;1 doi: 10.1016/j.jcvp.2021.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.López-Ratón M, Rodríguez-Álvarez MX, Cadarso-Suárez C, et al. OptimalCutpoints: an R package for selecting optimal cutpoints in diagnostic tests. J Stat Softw. 2014;61(8):1–36. doi: 10.18637/jss.v061.i08. [DOI] [Google Scholar]
- 19.Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favresse J, Bayart J-L, Mullier F, et al. Early antibody response in health-care professionals after two doses of SARS-CoV-2 mRNA vaccine (BNT162b2) Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.05.004. S1198-743X(21)00224-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keehner J, Horton LE, Pfeffer MA, et al. SARS-CoV-2 infection after vaccination in health care workers in California. N Engl J Med. 2021;384:1774–1775. doi: 10.1056/NEJMc2101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. CDC real-world study confirms protective benefits of mRNA COVID-19 vaccines ( https://www.cdc.gov/media/releases/2021/p0329-COVID-19-Vaccines.html ), accessed on June 10th, 2021
- 24.Israel A, Merzon E, Schäffer AA, et al. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: test negative design study. BMJ. 2021 doi: 10.1136/bmj-2021-067873. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park JH, Lee HK. Delivery routes for COVID-19 vaccines. Vaccines (Basel) 2021;9:524. doi: 10.3390/vaccines9050524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mortari EP, Russo C, Vinci MR, et al. Highly specific memory B cells generation after the 2nd dose of BNT162b2 vaccine compensate for the decline of serum antibodies and absence of mucosal IgA. Cells. 2021;10:2541. doi: 10.3390/cells10102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell MW, Moldoveanu Z, Ogra PL, et al. Mucosal immunity in COVID-19: a neglected but critical aspect of SARS-CoV-2 infection. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.611337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapić I, Šegulja D, Rogić D. Assessment of salivary antibody response to BNT162b2 mRNA COVID-19 vaccination. J Med Virol. 2021;93:5257–5259. doi: 10.1002/jmv.27096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soffritti I, D'Accolti M, Fabbri C, et al. Oral microbiome dysbiosis is associated with symptoms severity and local immune/inflammatory response in COVID-19 patients: a cross-sectional study. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.687513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chodick G, Tene L, Rotem RS, et al. The effectiveness of the two-dose BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab438. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayawi K, Shahriar S, Serhani MA, et al. Vaccine versus Variants (3Vs): are the COVID-19 vaccines effective against the variants? A systematic review. Vaccines (Basel) 2021;9:1305. doi: 10.3390/vaccines9111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuzmina A, Wattad S, Khalaila Y, et al. SARS-CoV-2 Delta variant exhibits enhanced infectivity and a minor decrease in neutralization sensitivity to convalescent or post-vaccination sera. iScience. 2021 doi: 10.1016/j.isci.2021.103467. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mudgal R, Nehul S, Tomar S. Prospects for mucosal vaccine: shutting the door on SARS-CoV-2. Hum Vaccin Immunother. 2020;16:2921–2931. doi: 10.1080/21645515.2020.1805992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan RWY, Liu S, Cheung JY, et al. The mucosal and serological immune responses to the novel coronavirus (SARS-CoV-2) vaccines. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.744887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chavda VP, Vora LK, Pandya AK, et al. Intranasal vaccines for SARS-CoV-2: from challenges to potential in COVID-19 management. Drug Discov Today. 2021;26:2619–2636. doi: 10.1016/j.drudis.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lapuente D, Fuchs J, Willar J, et al. Protective mucosal immunity against SARS-CoV-2 after heterologous systemic prime-mucosal boost immunization. Nat Commun. 2021;12:6871. doi: 10.1038/s41467-021-27063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin R. Trying to block SARS-CoV-2 transmission with intranasal vaccines. JAMA. 2021;326:1661–1663. doi: 10.1001/jama.2021.18143. [DOI] [PubMed] [Google Scholar]
- 39.Daniell H, Nair SK, Esmaeili N, et al. Debulking SARS-CoV-2 in saliva using angiotensin converting enzyme 2 in chewing gum to decrease oral virus transmission and infection. Mol Ther. 2021 doi: 10.1016/j.ymthe.2021.11.008. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.