Abstract
Romosozumab is a humanized monoclonal anti-sclerostin antibody (Scl-Ab) that binds and inhibits sclerostin, thereby increasing bone formation and decreasing bone resorption. In 2019, the Japanese Ministry of Health, Labor and Welfare, and the FDA approved romosozumab for treating osteoporosis in men and in postmenopausal women at high risk of fracture. In the past decade, pharmacological systemic treatments using molecules in use for the treatment of the osteoporosis have been reported. Herein we reported the case of a 67-year-old woman with nonunion of humerus shaft fracture, in whom bone union could not be achieved after 11 months of conservative treatment; however, successful bone healing was achieved after once-a-month administration of romosozumab for 6 months. To our knowledge, this is the first case reporting the successful use of romosozumab for treating established nonunion. Romosozumab can aid in promoting bone healing of nonunion in patients not willing to undergo surgical intervention.
Keywords: Romosozumab, Nonunion, Fracture, Humerus, Humeral shaft
Introduction
Humeral shaft fractures account for 1%–3% of all fractures [1]. The nonunion rate for these ranges from 0.5% to 13% [2]. Nonunion poses challenges for surgeons and patients. In this report, we present the case of a patient with nonunion of a humeral shaft fracture who was successfully treated with monthly administration of romosozumab, an anti-sclerostin bone-forming antibody [3].
Case presentation
A 67-year-old woman experienced a fracture on the right humerus shaft (AO/OTA classification: 12A1) from a fall in May 2018. The patient originally underwent conservative treatment with immobilization in a hanging cast for 3 weeks and subsequent functional bracing. Radiography at 3 months after injury showed delayed union of the fracture; therefore, treatment with low-intensity pulsed ultrasound (LIPUS) to the fracture site was initiated using Sonic Accelerated Fracture Healing System (Smith & Nephew Inc., Piscataway, NJ, USA) [4]. A functional brace was removed at the end of 2018. In March 2019, after 10 months of the injury, the patient was referred to our hospital because of nonunion of the fracture (Fig. 1).
Fig. 1.
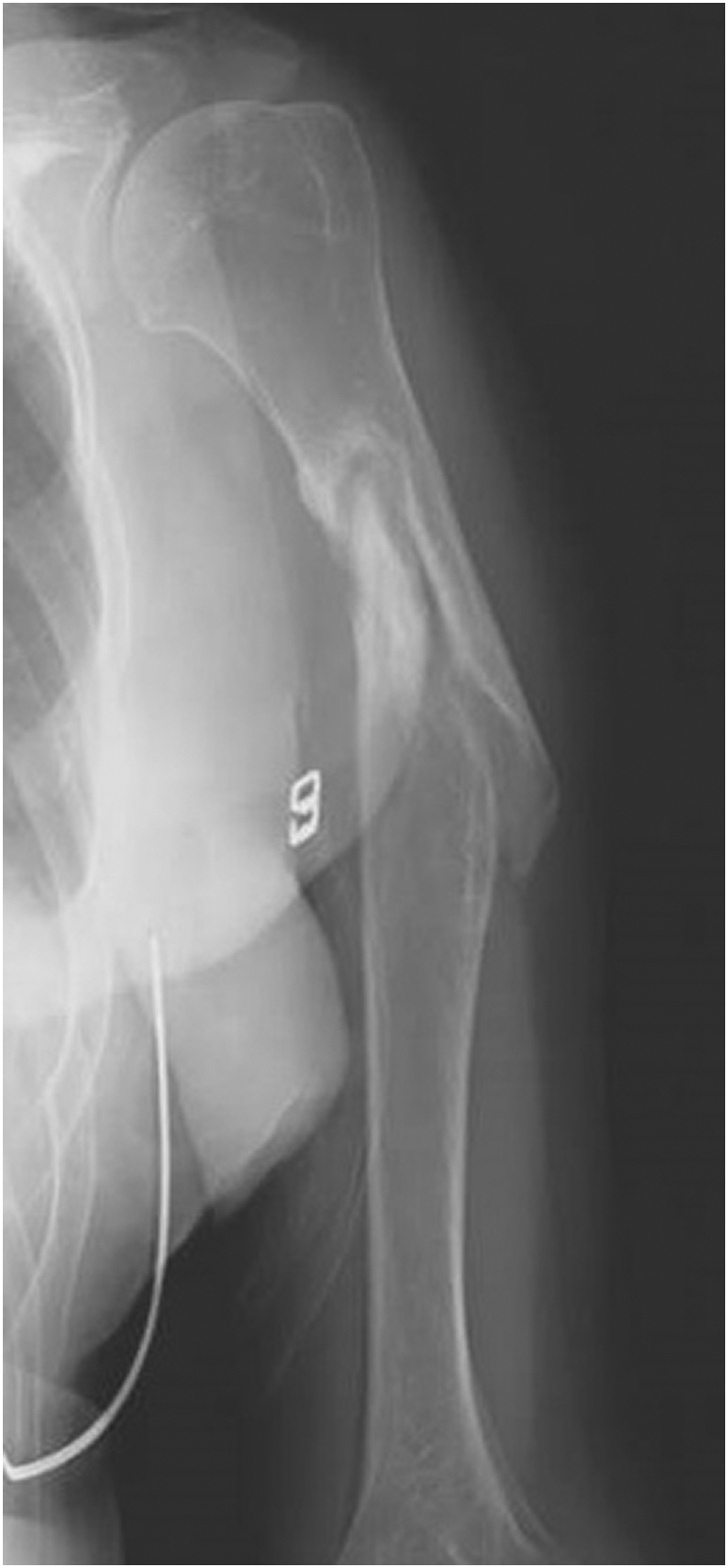
An anteroposterior radiograph of the left humerus taken on the patient's first visit showed the nonunion of a humeral shaft fracture.
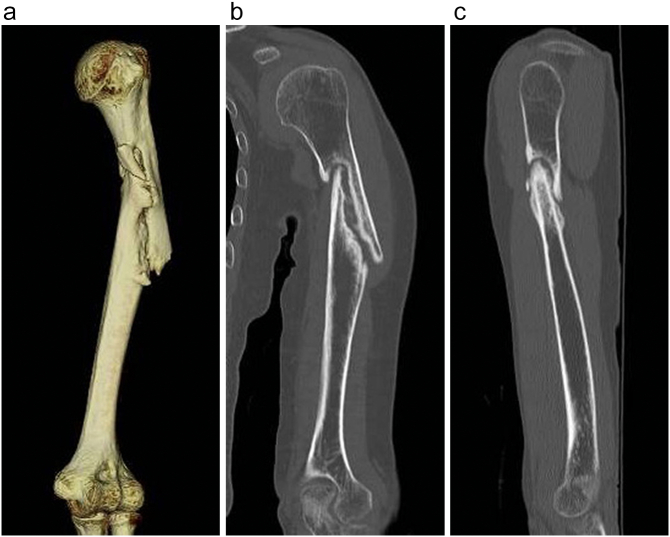
The patient indicated moderate pain at the nonunion site worsened by stress. Computed tomography (CT) revealed nonunion of the proximal third of the humerus shaft with a definite bony gap and sclerotic edges and varus angle deformity (Fig. 2a–c). On physical examination, the patient's active range of motion of the shoulder was not limited. For the past 7 months, the patient had received monthly minodronate as treatment for osteoporosis and had no other known metabolic diseases. She was a non-smoker and did not consume alcohol. Serum 25-hydroxyvitamin D (25(OH)D) was 26.2 ng/ml. A dual energy X-ray absorptiometry (DXA) scan showed severe osteoporosis with low bone mineral density (BMD) in the lumbar spine (T-score of −3.5) and low BMD in the femoral neck (T-score of −2.5).
Fig. 2.
A three-dimensional (3D) computed tomography (CT) image (a), and coronal (b) and sagittal (c) CT images of the left humerus taken on the patient's first visit showed the nonunion of a humeral shaft fracture.
The patient declined an osteosynthesis operation, and consented to therapy with romosozumab (Evenity®; Amgen Inc., Thousand Oaks, CA) at the approved dose for osteoporosis (210 mg/month), in an attempt to treat her osteoporosis as well as the oligotrophic nonunion. We discontinued the minodronate and subcutaneously administrated the romosozumab once per month beginning in April 2019. We continued LIPUS treatment, which was performed for 20 min once daily.
Three months after the initiation of the romosozumab treatment, radiography showed a reduction of the bone gap. After 6 months of treatment, coinciding with the disappearance of pain, radiography (Fig. 3) and CT scan (Fig. 4a–c) showed bridging of the callus, demonstrating that the nonunion was united. After 12 months of treatment, we discontinued the romosoumab and restarted the minodronate. A DXA scan showed that T-scores in the lumbar spine and in the femoral neck were −2.6 and −3.2, respectively. At the patient's final visit (18 months after the initiation of romosozumab), radiography showed complete healing and consolidation. Throughout the follow-up period, the patient reported no side effects.
Fig. 3.
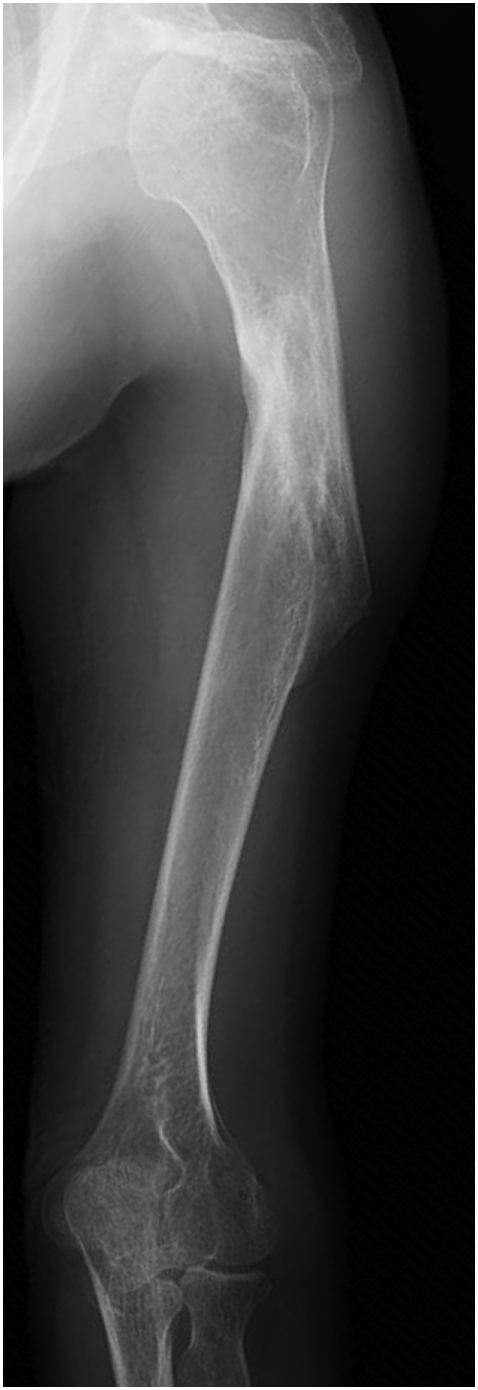
An anteroposterior radiograph of the left humerus taken 6 months after the initiation of romosozumab treatment showed bone union.
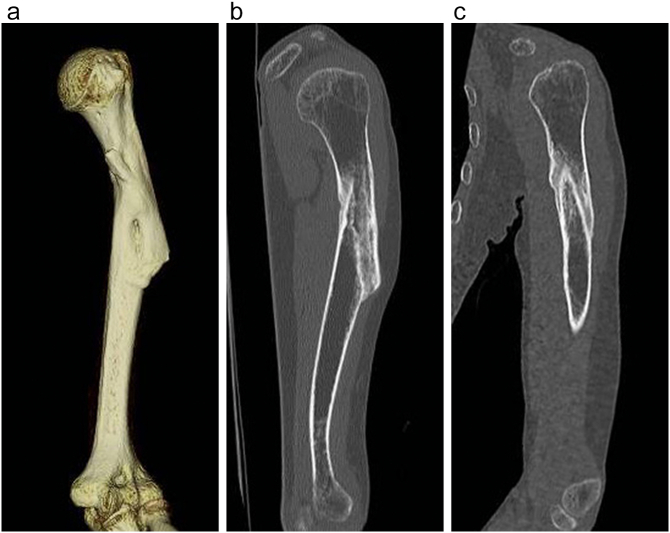
Fig. 4.
A 3D CT image (a), and coronal (b) and sagittal (c) CT images of the left humerus taken 6 months after the initiation of romosozumab treatment showed bone union.
Discussion
When nonunion occurs, treatment often requires a surgical intervention to provide stability and to stimulate bone biology. Nevertheless, not all nonunion cases can be treated surgically because of either patient or technical factor. Researchers have proposed several strategies including LIPUS to improve bone healing in cases of nonunion [4]. In the past decade, pharmacological systemic treatments using molecules in use for the treatment of the osteoporosis have been reported. Teriparatide (recombinant human parathyroid hormone (1–34)) was the first U. S. Food and Drug Administration (FDA) approved anabolic drug for treating osteoporosis in 2002. In addition to its application to osteoporosis, a growing body of evidence indicates the potential for teriparatide to promote the healing of fracture and nonunion [5].
In 2019, the Japanese Ministry of Health, Labor and Welfare, and the FDA approved another anabolic agent, romosozumab for treating osteoporosis in men and in postmenopausal women at high risk of fracture. Romosozumab is a humanized monoclonal anti-sclerostin antibody (Scl-Ab) that binds and inhibits sclerostin, thereby increasing bone formation and decreasing bone resorption [3]. Sclerostin, a glycoprotein encoded by the SOST gene, is a negative regulator of bone formation that is secreted by osteocytes. It inhibits canonical Wnt signaling, which down-regulates osteoblast development and function. A randomized controlled clinical trial (RCT) has demonstrated that romosozumab markedly increased bone formation and therefore BMD, and reduced the prevalence of vertebral and clinical fractures compared with placebo in postmenopausal women with osteoporosis [3].
Recently, two RCTs evaluated the effects of romosozumab on the radiographic and clinical outcomes of patients with hip fractures and tibial diaphyseal fractures [6], [7]. Patients received subcutaneous injections of romosozumab on day 1 and weeks 2, 6, and 12. However, in both studies, the treatment of romosozumab did not accelerate fracture healing. These authors concluded that more studies with patients at high risk of fracture complications such as delayed healing or nonunion are needed to explore the potential of romosozumab to accelerate fracture healing. To date, a few animal studies have investigated the effect of Scl-Ab on bone repair in nonunion [8], [9]. Hamann et al. [8] made a rat bone defect model by creating a 3-mm defect at femur. Rats were then treated twice weekly with subcutaneous injections of Scl-Ab for 12 weeks. At week 12, rats treated with Scl-Ab filled 94% of the femoral defect, whereas rats with a sham treatment filled only 57%. In contrast, Kruck et al. [9] did not find a beneficial effect from Scl-Ab treatment in a mouse refractory fracture model. They created a 0.5-mm open osteotomy in the femur. Mice were then treated with Scl-Ab, twice per week for 21 days. Although the Scl-Ab treatment caused enhanced bone formation, it did not overcome the delays in healing. These discrepancies among previous animal studies may be due to multiple factors, including differences in species, surgical techniques, and duration of treatment. Potential mechanisms by which Scl-Ab may enhance bone healing in fracture and nonunion remains unknown.
Ours is the first case reporting the successful use of romosozumab for treating humeral shaft fracture nonunion that had been treated conservatively. In our patient, after 11 months with no clinical and radiographic evidence of union, bony union was achieved with monthly administration of romosozumab for 6 months. No adverse events occurred during or after treatment. Our patient had established oligotrophic nonunion with a definite bony gap and sclerotic edges, and the nonunion site showed no signs of healing, even after 8 months of treatment with LIPUS. Therefore, it is highly unlikely that our patient's nonunion would have healed spontaneously with continued observation. In this case, daily LIPUS treatment was continued after the initiation of romosozumab, because the patient wanted to continue the treatment. A possibility exists that romosozumab and LIPUS may have additive effects during bone healing. Cessation of bisphosphonate therapy may have affected bone healing in our case. Although a few clinical studies suggest bisphosphonate treatment as potential risk factors for impaired fracture healing, the evidence is still not convincing [10].
Conclusion
We reported the case of a patient with an established nonunion of a humeral shaft fracture who had complete bone healing with cessation of bisphosphonate therapy followed by monthly administration of romosozumab and continuation of LIPUS. Our report suggests that romosozumab may aid in promoting bone healing of nonunion in patients not willing to undergo surgical intervention.
Informed consent
The patient has provided written informed consent to publish the case report.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that have influenced the work reported in this paper.
References
- 1.Papasoulis E., Drosos G.I., Ververidis A.N., Verettas D.A. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury. 2010;41:e21–e27. doi: 10.1016/j.injury.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Peters R.M., Claessen F.M., Doornberg J.N., Kolovich G.P., Diercks R.L., van den Bekerom M.P. Union rate after operative treatment of humeral shaft nonunion–a systematic review. Injury. 2015;46:2314–2324. doi: 10.1016/j.injury.2015.09.041. [DOI] [PubMed] [Google Scholar]
- 3.Cosman F., Crittenden D.B., Adachi J.D., Binkley N., Czerwinski E., Ferrari S., Hofbauer L.C., Lau E., Lewiecki E.M., Miyauchi A., Zerbini C.A., Milmont C.E., Chen L., Maddox J., Meisner P.D., Libanati C., Grauer A. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 2016;375:1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe Y., Matsushita T., Bhandari M., Zdero R., Schemitsch E.H. Ultrasound for fracture healing: current evidence. J. Orthop. Trauma. 2014;24:S56–S561. doi: 10.1097/BOT.0b013e3181d2efaf. [DOI] [PubMed] [Google Scholar]
- 5.Pietrogrande L., Raimondo E. Teriparatide in the treatment of non-unions: scientific and clinical evidences. Injury. 2013;44:S54–S57. doi: 10.1016/S0020-1383(13)70013-5. [DOI] [PubMed] [Google Scholar]
- 6.Bhandari M., Schemitsch E.H., Karachalios T., Sancheti P., Poolman R.W., Caminis J., Daizadeh N., Dent-Acosta R.E., Egbuna O., Chines A., Miclau T. Romosozumab in skeletally mature adults with a fresh unilateral tibial diaphyseal fracture: a randomized phase-2 study. J. Bone Joint Surg. Am. 2020;102:1416–1426. doi: 10.2106/JBJS.19.01008. [DOI] [PubMed] [Google Scholar]
- 7.Schemitsch E.H., Miclau T., Karachalios T., Nowak L.L., Sancheti P., Poolman R.W., Caminis J., Daizadeh N., Dent-Acosta R.E., Egbuna O., Chines A., Maddox J., Grauer A., Bhandari M. A randomized, placebo-controlled study of romosozumab for the treatment of hip fractures. J. Bone Joint Surg. Am. 2020;102(8):693–702. doi: 10.2106/JBJS.19.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamann C., Rauner M., Höhna Y., Bernhardt R., Mettelsiefen J., Goettsch C., Günther K.P., Stolina M., Han C.Y., Asuncion F.J., Ominsky M.S., Hofbauer L.C. Sclerostin antibody treatment improves bone mass, bone strength, and bone defect regeneration in rats with type 2 diabetes mellitus. J. Bone Miner. Res. 2013;28:627–638. doi: 10.1002/jbmr.1803. [DOI] [PubMed] [Google Scholar]
- 9.Kruck B., Zimmermann E.A., Damerow S., Figge C., Julien C., Wulsten D., Thiele T., Martin M., Hamdy R., Reumann M.K., Duda G.N., Checa S., Willie B.M. Sclerostin neutralizing antibody treatment enhances bone formation but does not rescue mechanically induced delayed healing. J. Bone Miner. Res. 2018;33:1686–1697. doi: 10.1002/jbmr.3454. [DOI] [PubMed] [Google Scholar]
- 10.Gorter E.A., Reinders C.R., Krijnen P., Appelman-Dijkstra N.M., Schipper I.B. The effect of osteoporosis and its treatment on fracture healing a systematic review of animal and clinical studies. Bone Rep. 2021;15 doi: 10.1016/j.bonr.2021.101117. [DOI] [PMC free article] [PubMed] [Google Scholar]