Summary
Autoimmune thyroid disease (AITD) is caused by aberrant activation of the immune system allowing autoreactive B and T cells to target the thyroid gland leading to disease. Although AITD is more frequently diagnosed in adults, children are also affected but rarely studied. Here, we performed phenotypic and functional characterization of peripheral blood immune cells from pediatric and adult-onset AITD patients and age-matched controls using mass cytometry. Major findings indicate that unlike adult-onset AITD patients, pediatric AITD patients exhibit a decrease in anergic B cells (BND) and DN2 B cells and an increase in immature B cells compared to age-matched controls. These results indicate alterations in peripheral blood immune cells seen in pediatric-onset AITD could lead to rapid progression of disease. Hence, this study demonstrates diversity of AITD by showing differences in immune cell phenotypes and function based on age of onset, and may inform future therapies.
Subject areas: Immunology, Immunological methods, Cell biology
Graphical abstract

Highlights
-
•
Penetrance of high-risk HLA-DR3 haplotype is higher in pediatric AITD patients
-
•
Pediatric AITD patients display altered frequency of autoreactive B cell subsets
-
•
Immune cell subset frequency and function is similar in adult AITD and controls
Immunology; Immunological methods; Cell biology
Introduction
Autoimmune thyroid disease (AITD), including Hashimoto's thyroiditis (hypothyroidism) and Graves' disease (hyperthyroidism), is one of the most commonly diagnosed autoimmune disorders in the United States (Ragusa et al., 2019; Antonelli et al., 2020a, 2020b). Although AITD is rarely life threatening, it is associated with many serious comorbidities, such as abnormal growth and development in children, cardiovascular disease, mental health issues, polycystic ovary syndrome (PCOS) in women, and increased risk for thyroid cancer (Noureldine and Tufano, 2015; Radhakrishnan et al., 2013; Ho et al., 2020; Fugger et al., 2018; Hanley et al., 2016). In addition, about 20% of AITD patients develop other organ specific or systemic autoimmune disorders, such as celiac disease, type 1 diabetes (T1D), systemic lupus erythematosus (SLE), and rheumatoid arthritis (RA) (Pan et al., 2015a, 2015b; Lu et al., 2016; Sun et al., 2016). Secondary AITD is an immune-related Adverse Event (irAE) commonly seen following treatment with immune-checkpoint antibodies, such as anti-PD-1/PD-L1 and anti-CTLA4 (Villadolid and Amin, 2015; Abdel-Wahab et al., 2016; Bertrand et al., 2015). Hence, given its prevalence and comorbidities, more studies aimed at understanding the underlying pathogenesis of AITD are needed.
Currently it is thought that a combination of environmental triggers, genetic predisposition, and alterations in the immune system contribute to development of AITD. Although AITD is more commonly diagnosed in adulthood with a female-to-male ratio of 9:1, when AITD is diagnosed in childhood the gender distribution tends to be equal among females and males (Manji et al., 2006). This finding suggests a genetic risk may play a bigger role in males, whereas environmental triggers (e.g., dietary iodine, stress, and smoking), which can take time to accumulate, may be more important in females. In line with this, studies have shown the susceptibility genes for AITD are similar in children and adults but can vary in the frequency in which they are found in young versus adult-onset AITD (Manji et al., 2006). For example, studies have shown that the high risk conferring HLA-DR3 haplotype is more common in young onset Graves' disease patients than older onset (Farid et al., 1980b; Jurecka-Lubieniecka et al., 2013; Lavard et al., 1997). In line with this, clinical studies have demonstrated different patterns of autoimmune comorbidities associated with pediatric versus adult AITD (Ruggeri et al., 2018). It was shown that AITD co-occurring with celiac disease or T1D is more common in pediatric patients, whereas AITD and connective tissue diseases occur more commonly in adult patients (Ruggeri et al., 2017). These findings likely reflect the difference in high risk HLA haplotypes (HLA-DR3 and HLA-DR4) found in pediatric versus adult AITD. Although differences in genetic risk alleles and environmental exposures have been well-studied in pediatric and adult-onset AITD patients, very few studies have sought to analyze differences in immune cell populations and age of onset.
AITD is characterized by the breakdown of immune tolerance against thyroid antigens, such as thyroglobulin (Tg) and thyroid peroxidase (TPO), leading to infiltration of autoreactive B and T cells into the thyroid gland and production of autoantibodies directed against the thyroid. Studies have demonstrated that the most predominant cell types infiltrating the thyroid gland are CD4+ T cells, CD8+ T cells, and B cells (Antonelli et al., 2015). It is worth noting that of the genes known to confer risk for AITD, roughly 70% are involved in T cell function (e.g., CTLA4, PTPN22, and IL2RA), implicating T cells heavily in the pathogenesis of AITD (Simmonds, 2013). Hence, previous studies have analyzed the phenotype of peripheral and intrathyroidal T cells in AITD patients, though for most of these studies the variables of age of onset and time since diagnosis were not considered. Many studies have implicated Th1 cells in the initial phase of the pathogenesis of AITD through production of pro-inflammatory cytokines, such as IFN-g, TNF-a, and IL-6, that can stimulate other T cells and NK cells and impair the functional activity of thyrocytes, further perpetuating the inflammatory process (Weetman et al., 1997; Antonelli et al., 2020a; Ragusa et al., 2019; Rapoport and McLachlan, 2014). In addition, IL-17 producing Th17 cells have been shown to be necessary for the induction of AITD in an animal model (Horie et al., 2009) and are increased in the peripheral blood and thyroid tissue from AITD patients (Li et al., 2013; Peng et al., 2013; Qin et al., 2012). Furthermore, some studies have shown that activated HLA-DR + T cells are increased in the peripheral blood of AITD patients (Gessl and Waldhausl, 1998; Fountoulakis et al., 2008), whereas another study found they are decreased (Gessl and Waldhausl, 1998; Nada and Hammouda, 2014). Finally, expression of the exhaustion marker, PD-1, has been shown to be higher on T cells from the peripheral blood of AITD patients compared to controls (Alvarez-Sierra et al., 2019).
Mouse models of AITD have demonstrated an important role for B cells in the pathogenesis of disease (Yu et al., 2001, 2006; Hong and Braley-Mullen, 2014; Braley-Mullen and Yu, 2000). In humans and mice, B cells produce anti-thyroid antibodies that are both hallmarks of disease but also aggravate cell-mediated thyroid damage by mediating complement fixation and antibody-dependent cellular cytotoxicity (ADCC) in HT and through stimulation of the TSH receptor (TSH-R) in GD (Chiovato et al., 1993). B cells also act as potent antigen-presenting cells (APCs) to cognate T cells, particularly when antigen concentration is low. B cells can produce pro-inflammatory cytokines, such as IL-6, TNF-a, and IFN-g, that contribute to the inflammatory microenvironment. Analysis of the thyroid glands of AITD patients has revealed the presence of B cells in and around the thyroid, forming lymphoid follicles, which are not present in healthy controls (Segundo et al., 2004; Armengol et al., 2001). Previous studies in our lab have demonstrated a decrease in anergic (unresponsive) Tg-reactive and TPO-reactive B cells in the peripheral blood of recent onset AITD subjects, which we hypothesize reflects the activation and departure of these cells from the peripheral blood, and entry into the thyroid gland (Smith et al., 2017). Although studies suggest that B cells are essential in the pathogenesis of AITD, few studies have analyzed changes in peripheral B cell subsets, their activation status, and cytokine production during onset of disease.
In addition to B and T lymphocytes, myeloid and NK cells are also thought to contribute to disease. Monocytes, macrophages, and dendritic cells act as APCs to naive T cells and are potent cytokine producers that influence the differentiation of T helper cells into their various subtypes, such as Th1, Th2, and Th17, based on the cytokines they release in the vicinity. Recent evidence suggests that NK cells can have either a cytotoxic (CD56lo) or regulatory (CD56hi) phenotype and studies suggest that in AITD patients, the protective activity of NK cells is impaired (Gallo et al., 2020). Given the putative roles of T and B lymphocytes, myeloid cells, and NK cells in the pathogenesis of AITD, we measured phenotypic and functional (intracellular cytokines) perturbations in pediatric and adult AITD blood cells using high-dimensional mass cytometry to explore how cellular and molecular changes could drive AITD development differently based on age of onset. We show that pediatric AITD subjects exhibit a significant decrease in peripheral anergic (BND) B cells and DN2 B cells, and a tendency of decreased NK cells, which may reflect departure of these cells from blood and entry into the thyroid. Adult onset AITD subjects exhibit a decrease in IL-23p19+ and IL-6+ monocytes compared to age-matched controls. This study reveals correlates of disease that may inform our understanding of disease development.
Results
The high risk HLA-DR3 haplotype is more common in pediatric-onset AITD subjects
Given that the underlying immunopathogenesis of AITD likely involves perturbations in multiple immune cells, such as T, B, and myeloid cells, as well as an imbalance in pro-inflammatory cytokine production, that conspire to cause immune-mediated destruction of the thyroid gland, we utilized high dimensional single-cell mass cytometry (CyTOF) to analyze phenotypic (cell surface) and functional (cytokine) markers on immune cells from the peripheral blood of pediatric and adult onset AITD patients (Figure S1). For this study we recruited 10 pediatric onset AITD patients, 22 pediatric age/sex matched controls, 13 adult onset AITD patients, and 22 adult age/sex matched controls. HLA genotyping revealed that 60% (6/10) of the pediatric AITD subjects and 30% (4/13) of the adult AITD subjects carried the high risk HLA-DR3 haplotype (DRB1∗03-DQA1∗05-DQB1∗02) (Table 1). These findings are consistent with previous reports that the HLA-DR3 haplotype is more common in young onset AITD patients compared to older onset AITD (Farid et al., 1980a; Jurecka-Lubieniecka et al., 2013). Interestingly, despite previous findings of gender disparity in adult AITD (i.e., increased female:male ratio), such disparity is less apparent in pediatric AITD. Our pediatric AITD cohort was 80% female, similar to our adult AITD cohort, which may reflect our relatively small pediatric sample size (n = 10).
Table 1.
Study participant demographics, related to STAR Methods
| Subjects (n) | Age (mean ± SD) | Sex (% F) | Diagnosis | % HLA-DR3 + |
|---|---|---|---|---|
| Pediatric AITD (10) | 12.00 ± 2.67 | 80% | HT (n = 5) | 60% |
| GD (n = 5) | ||||
| Pediatric controls (22) | 13.71 ± 4.39 | 83% | ||
| Adult AITD (13) | 38.15 ± 15.26 | 77% | HT (n = 4) | 30.7% |
| GD (n = 9) | ||||
| Adult controls (22) | 35.92 ± 12.87 | 77% |
AITD, autoimmune thyroid disease; SD, standard deviation; F, female; HT, Hashimoto's thyroiditis; GD, Graves' disease.
Mass cytometry analysis revealed alterations in frequency of B cell subpopulations in the pediatric new-onset AITD subjects
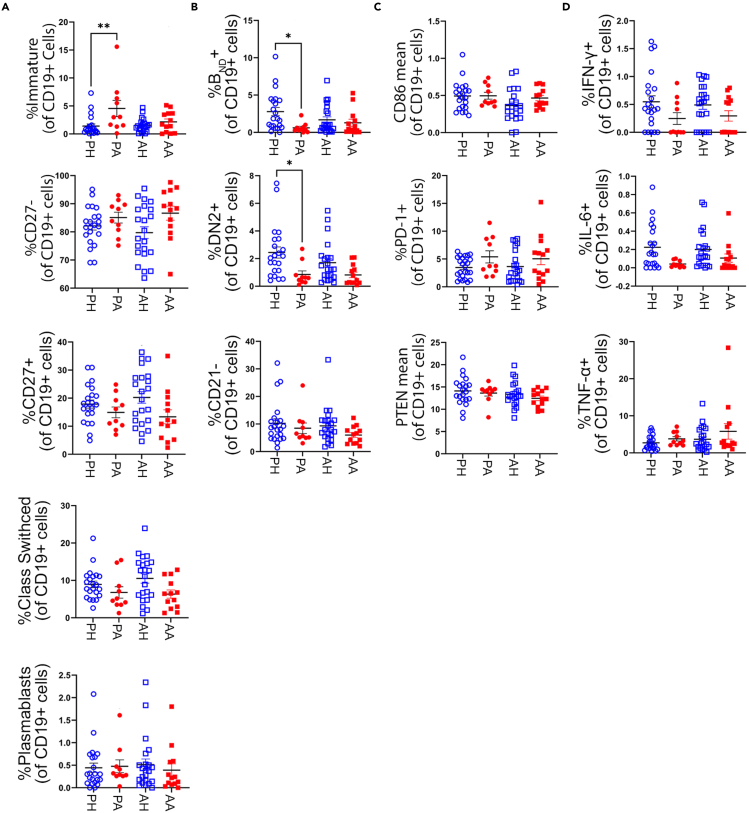
The role of B cells in the pathogenesis of AITD is thought to be three-fold: 1. their terminally differentiated daughters produce destructive (HT) and/or stimulatory (GD) anti-thyroid antibodies, 2. they produce pro-inflammatory cytokines, such as TNFa-, IFN-g, and IL-6, and 3. they act as potent antigen-presenting cells to cognate T cells. Hence, we sought to analyze differences in B cell subpopulations, their phenotype, and functional capabilities in pediatric and adult-onset AITD patients. Traditional manual gating strategies (Figure S2) for mass cytometry data were used to delineate major B cell subpopulations, including naive, memory, immature, and plasmablast populations. In addition, B cell population frequencies previously implicated in development of AITD and other autoimmune disorders were also analyzed. This included the anergic BND (CD27−IgMloIgD+) B cell population, which was previously shown to be a reservoir of autoreactive yet unresponsive B cells in healthy individuals (Duty et al., 2009). Previous studies have found anergic B cells are absent from peripheral blood of newly diagnosed AITD patients, as well as T1D, RA, and SLE patients (Smith et al., 2015, 2017; Liubchenko et al., 2013; Malkiel et al., 2016). We also analyzed the frequency of DN2 (CD27−IgD-CD21−CXCR5-) cells, which are thought to be a source of extrafollicular plasmablasts and have been implicated in the pathogenesis of SLE (Jenks et al., 2018; Wang et al., 2018), and the CD21- B cell population, which is thought to be activated and/or exhausted B cells and have been shown to be increased in RA and SLE patients (Isnardi et al., 2010; Wehr et al., 2004; Freudenhammer et al., 2020). Analysis of major B cell subpopulations revealed that pediatric AITD subjects have a statistically significant increase in frequency of immature (CD27−IgMhiCD38hi) B cells compared to age matched controls, a decrease, though not to be significant, in class-switched memory (CD27+IgD−) B cells, and no difference in frequencies of naive (CD27-), memory (CD27+), or plasmablasts (CD38hiCD27hi) (Figure 1A). In addition, pediatric AITD subjects had a decreased frequency of anergic BND and DN2 cells compared to controls (Figure 1B), suggesting these two potentially pathogenic populations may have trafficked from the peripheral blood to the thyroid where they are participating in disease. B cell frequencies of adult-onset AITD subjects were similar to age matched controls (Figures 1A and 1B).
Figure 1.
Pediatric AITD patients have altered frequencies of potentially pathogenic B cell subsets (immature, Bnd, and DN2)
(A) Frequencies of immature (CD27−IgMhiCD38hi), naive (CD27-), memory (CD27+), class-switched memory (CD27+IgD−), and plasmablast (CD27hiCD38hi) B cells in adult-onset AITD (AA), adult healthy controls (AH), pediatric-onset AITD (PA), and pediatric healthy controls (PH) reveals PA patients have increased frequency of immature B cells compared to age matched controls.
(B) Frequencies of anergic Bnd (CD27−IgMloIgD+), DN2 (CD27−IgD-CD21−CXCR5-), and CD21- (CD19+CD21-) B cells in AITD patients and controls reveals PA patients have reduced frequencies of Bnd and DN2 B cells compared to PH.
(C) Mean metal intensity of CD86 and PTEN in CD19 + B cells and frequency of PD-1+ B cells is similar between AITD patients and controls, suggesting total B cells do not show increased activation in AITD patients.
(D) B cells from AITD patients do not secrete increased levels of the pro-inflammatory cytokines, IFN-y, IL-6, or TNF-a, at baseline compared to controls. Data are represented as mean ± SEM. Samples were processed as depicted in Figure S1. Frequency of B cell subsets, activation markers, and cytokine production were determined by manual gating, as depicted in Figures S2 and S3. See also Figure S7 for comparison of manually gated BND cells to algorithm determined BND cells. Statistical significance determined by One-way ANOVA with Sidak multiple comparisons post-test. ∗p < 0.05
We next investigated the activation status of B cells by analyzing expression levels of the co-stimulation molecule, CD86, the inhibitory molecule PD-1, and PTEN, a negative regulator of the PI3-kinase pathway previously shown to be decreased in B cells from SLE, T1D, and AITD patients (Smith et al., 2019; Wu et al., 2014). Total B cells from both pediatric and adult AITD patients showed normal expression of CD86 and PD1, and despite previous findings of decreased PTEN expression in AITD patients, our findings showed slightly decreased but not statistically significant expression of PTEN in pediatric AITD patients and normal expression of PTEN in adult AITD patients (Figure 1C). Differences in PTEN expression compared to previous reports could be because of the method used (e.g., flow cytometry vs. CyTOF), how expression levels of fluorescence vs metal intensities is calculated, and/or differences in sample sizes. Given the decrease in frequency of BND and DN2 cells in the peripheral blood of pediatric AITD patients, which may suggest they have become activated and migrated to the thyroid gland, we also examined expression of CD86, PD1, and PTEN in these specific B cell subsets for evidence of activation. However, there was no significant difference in expression of these markers in pediatric or adult AITD subjects compared to age-matched controls (data not shown).
Next, we wanted to investigate whether peripheral B cells from pediatric and adult AITD patients produce more pro-inflammatory cytokines, including IFN-g, IL-6, and TNF-a, immediately ex vivo. Not unexpectedly, the percent positive cytokine producing B cells, as determined by positive control PMA/ionomycin stimulation of an anchor control sample (Figure S3), was low among all cohorts and demonstrated no increase in IFN-g, IL-6, or TNF-a producing B cells in AITD subjects, irrespective of age of onset (Figure 1D). Although it is likely in vitro stimulation of B cells with a protein transport inhibitor would show increased cytokine production, in this study we aimed to study cytokine production immediately ex vivo without prior stimulation. Future studies may be aimed at analyzing differences in cytokine production in the presence of a protein transport inhibitor with or without stimulation. Taken together, manual gating of B cells from peripheral blood of pediatric and adult-onset AITD patients revealed a decrease in frequency of BND and DN2 cells, but did not reveal differences in B cell activation or cytokine production.
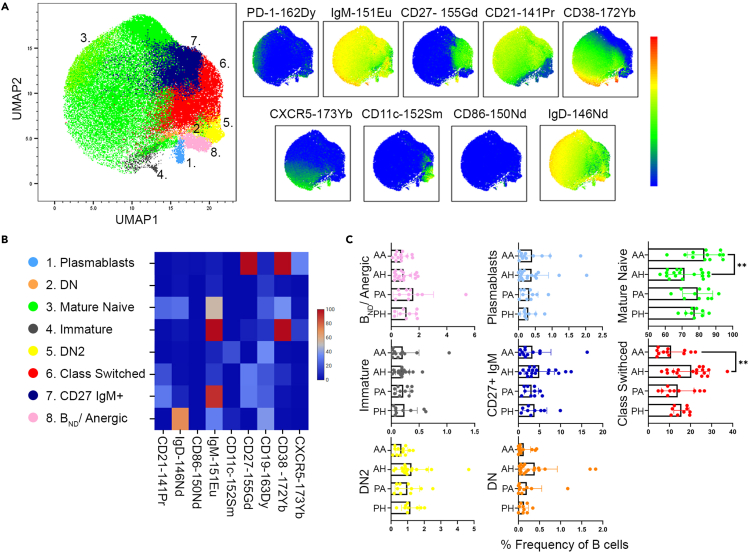
Finally, to comprehensively explore the immunophenotype of pediatric and adult-onset AITD patients and controls, we analyzed major immune cell subsets using the unsupervised clustering algorithms, PhenoGraph and X-Shift. These dimension reduction programs generate cell clusters based on selected surface markers. Unbiased analysis of patient and control B cells (gated as CD45+CD19+CD14-) revealed seven major B cell subpopulations based on varying surface marker expression (Figure 2A). All of the B cell populations were also identified using manual gating strategies, and therefore, unsupervised clustering did not reveal any novel B cell subpopulations, likely a result of the relatively few surface markers used to cluster on (Figure 2B). Results demonstrated that similar to the manual gated population, the frequency of DN2 cells (CD27−IgD-CD21−CD11c+) were decreased, though non-significantly, in the pediatric and adult AITD patients compared to their age-matched controls (Figure 2C). In addition, adult AITD patients demonstrated a significant increase in mature naive (CD27−IgM+IgD+) B cells with a corresponding significant decrease in class-switched memory B cells (CD27+IgD−). Pediatric AITD patients showed a similar trend compared to their age-matched controls (Figure 2C). In conflict with manual gated subpopulations, frequencies of immature (CD27−CD38hiIgMhi) and BND cells were similar among AITD patients and controls, irrespective of their age of onset (Figure 2C). Differences in frequencies of manually gated B cell populations and algorithm defined populations are likely because of inherent differences in the two methods used, such as the varying number of subjects analyzed in each method, the number of markers used to define subpopulations, and how algorithm based analysis identifies statistically significant clusters from their nearest neighbors. As seen in manual gating, markers of activation, such as CD86, PTEN, and PD-1, as well as cytokine levels, were similar across subjects (data not shown).
Figure 2.
Unsupervised clustering and dimension reduction analysis of B cells reveals similar frequencies of B cell subpopulations in AITD patients and controls
(A) B cells were manually gated as CD45 + CD19 + CD3-CD14-and then analyzed using the unsupervised clustering and dimension reduction programs PhenoGraph and X-shift to generate distinct clusters based on B cell surface marker expression (PD-1, IgM, CD27, CD21, CD38, CXCR5, CD11c, CD86, and IgD). The UMAP plot was created using total B cells from concatenated adult AITD, adult healthy control, pediatric AITD, and pediatric healthy control samples with a minimum of 5,000 B cell events. Eight unique B cell subpopulations were identified. UMAP plots of each surface marker are displayed to demonstrate varying expression levels among the B cell subpopulations.
(B) Heatmap of B cell surface marker expression for identification of the 8 B cell subpopulations identified using PhenoGraph/X-Shift. DN: double-negative (CD27-IgD-CD21-), DN2: double-negative CD11c+ (CD27-IgD-CD21-CD11c+).
(C) Frequencies of B cell subpopulations in adult AITD (AA), adult healthy control (AH), pediatric AITD (PA), and pediatric healthy control (PH) subjects demonstrate AA patients have increased mature naive B cells and decreased class-switched B cells compared to AH. Data are represented as mean ± SEM. Samples were processed as depicted in Figure S1. See also Figure S7 for comparison of manually gated BND cells to algorithm determined BND cells. Statistical significance determined by Mann-Whitney non-parametric unpaired Student's t tests.
Immunophenotyping of T cells from AITD subjects reveals similar frequencies, activation, and cytokine production compared to age matched controls
Given the role of T cells in the pathogenesis of AITD, we next analyzed frequencies of major T cell subpopulations, their activation status, and cytokine production. Manual gating of major CD3+ T cell subpopulations (Figure S4) revealed pediatric and adult-onset AITD patients have similar frequencies of CD4+ and CD8+ T cells to age-matched controls (Figure S5). We also analyzed the frequency of CD4-CD8-double-negative (DN) T cells, which have previously been implicated in the pathogenesis of animal models of autoimmune disease, as well as in SLE and Sjogren's syndrome patients, through production of IL-17 and promotion of autoantibody production (Masuda et al., 1991; Alunno et al., 2013; Crispin et al., 2008; Sieling et al., 2000). We found a similar frequency of DN T cells in AITD patients and age-matched controls (Figure S5). Next, we analyzed the frequency of naive, central memory (Tcm), effector memory (Tem), and CD45RA + effector memory (TEMRA) CD4+ and CD8+ T cells. Not unexpected, there was a decrease in CD4+ and CD8+ Tcm cells with a correlated increase in naive CD4+ and CD8+ T cells in all pediatric subjects, irrespective of disease state (Figures 3A and 3B). Given the role of B cells in AITD, we also analyzed frequencies of circulating T follicular helper (Tfh) and T peripheral helper (Tph) cells, which are T cells that interact directly with B cells either inside or outside the germinal center, respectively, to promote B cell differentiation and plasmablast formation. Although Tfh and Tph cells have previously been shown to be expanded in many other autoimmune disorders, including SLE, RA, T1D, and Sjogren's syndrome (Bocharnikov et al., 2019; Ekman et al., 2019; Choi et al., 2015; Verstappen et al., 2017; Fortea-Gordo et al., 2019), we found an increase, though not significant, in Tph cells in pediatric AITD subjects compared to controls and no difference in adult AITD subjects (Figure 3C).
Figure 3.
Frequencies of T cell subsets, their activations status, and cytokine production are similar between AITD subjects and controls
(A) Frequencies of CD4+ naive (CD27 + CD45RA+), central memory (Tcm; CD27 + CD45RA-), effector memory (Tem; CD27-CD45RA-), and effector memory CD45RA+ (TEMRA; CD27-CD45RA+) T cells reveals an increase in naive and a correlated decrease in Tcm cells in pediatric subjects (PA, PH) compared to adults (AA, AH), irrespective of disease status.
(B) Frequencies of CD8+ naive, Tcm, Tem, and TEMRA cells are not statistically different in AITD patients compared to controls.
(C) Frequencies of Tfh (CD4+PD-1+CXCR5+ICOS+) and Tph (CD4+PD-1+CXCR5−ICOS+) cells in AITD patients and controls. The frequency of Tph cells is increased, but not significantly, in AITD subjects compared to controls.
(D and E) T cells from AITD patients do not appear more activated than controls based on PD-1 or HLA-DR positivity.
(F) CD4+ T cells from AITD patients do not produce more Th1 cytokines (IFN-y, IL-6, and TNF-a) or the Th17 cytokine, IL-17, compared to controls. PA: pediatric AITD, PH: pediatric healthy control, AA: adult AITD, AH: adult healthy control. Data are represented as mean ± SEM. Samples were processed as depicted in Figure S1. Frequency of T cell subsets, activation markers, and cytokine production were determined by manual gating, as depicted in Figures S3 and S4. See also Figure S5 for frequencies of CD4, CD8, and DN (CD4-CD8-) T cells. Statistical significance determined by One-way ANOVA with Sidak multiple comparisons post-test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
As mentioned above, previous studies have identified an increase in activated PD-1+ T cells, as well as HLA-DR + T cells (with conflicting reports) in peripheral blood of AITD subjects compared to controls (Fountoulakis et al., 2008; Alvarez-Sierra et al., 2019). However, in this study we found similar frequencies of PD-1+ CD4+/CD8+ and HLA-DR + CD4+/CD8+ T cells in AITD subjects compared to controls, irrespective of their age of onset (Figures 3D and 3E). We believe these contradictions with previous studies may reflect our relatively small sample sizes, how gates were drawn to determine PD-1 and HLA-DR positivity, and/or difference in methods used (flow versus mass cytometry). Next, given studies have implicated both Th1 and Th17 cells in the pathogenesis of AITD, we analyzed production of the pro-inflammatory Th1 cytokines, IFN-g, IL-6, and TNF-a, and the Th17 cytokine, IL-17, in CD4+ T cells immediately ex vivo. We found no significant difference in cytokine production between AITD subjects and controls (Figure 3F), suggesting frequencies of Th1 and Th17 cells, as measured at baseline immediately ex vivo, are not increased in AITD subjects. The frequency of IL-17 + DN T cells was also not increased in AITD subjects (data not shown).
Finally, to complete our analysis of T cells in AITD subjects using the unbiased clustering algorithms, PhenoGraph and X-Shift, we analyzed clusters generated from CD45+CD19−CD3+ cells and the T cell surface markers in our antibody panel (Figures 4A and 4B). Although we were able to find the majority of the cell types identified with manual gating, there were no identifiable clusters specific for Tfh, Tph, or CD8+ PD-1hi T cells (Figures 4A and 4B). However, similar to manual gating, we were able to identify a CD4-CD8- DN T cell population that could further be separated into CD27+CD45RA−, CD27+CD45RA+, or CD27−CD45RA+. As shown in Figure 4C, there were no significant differences in frequencies of T cell subpopulations among our cohort. However, similar to results from manual gating, the altered ratio of naive to memory T cells in the pediatric subjects compared to adult subjects, irrespective of their disease status, was observed (Figure 4C). As seen in manual gating, markers of activation, such as PD-1 and HLA-DR, as well as cytokine levels, were similar across subjects (data not shown).
Figure 4.
Unsupervised clustering and dimension reduction analysis of T cells reveals similar frequencies of T cell subpopulations in AITD patients and controls
(A) T cells were manually gated as CD45 + CD3+CD19-and then analyzed using the unsupervised clustering and dimension reduction programs PhenoGraph and X-shift to generate distinct clusters based on T cell surface marker expression (PD-1, ICOS, CD7, CD27, CD45RA, CD3, CXCR5, CD123, CD8, and CD4). A UMAP plot was created using total T cells from concatenated adult AITD, adult healthy control, pediatric AITD, and pediatric healthy control samples with a minimum of 9,000 T cell events. Twelve unique T cell subpopulations were identified. UMAP plots of each surface marker are displayed to demonstrate varying expression levels among the T cell subpopulations.
(B) Heatmap of T cell surface marker expression for identification of the 12 T cell subpopulations identified using PhenoGraph/X-Shift. DN: double-negative (CD3+CD4-CD8-), TEMRA: T effector memory CD45RA+, TEM: T effector memory, Tcm: T central memory.
(C) Frequencies of T cell subpopulations in adult AITD (AA), adult healthy control (AH), pediatric AITD (PA), and pediatric healthy control (PH) subjects demonstrate normal frequencies in AITD patients compared to controls. Data are represented as mean ± SEM. Samples were processed as depicted in Figure S1. Statistical significance determined by Mann-Whitney non-parametric unpaired Student's t tests.
Immunophenotyping of myeloid and NK subpopulations
In addition to B and T cells, myeloid cells and NK cells can also participate in AITD pathogenesis. Myeloid cells can act as APCs to T cells and are potent producers of pro-inflammatory cytokines that help aggravate the immune response to self-antigens. Hence, we first analyzed the frequency and cytokine production of major myeloid cell subsets from peripheral blood of AITD subjects and controls using manual gating strategies (Figure S6). We found no difference in the frequency of major dendritic cell (DC) subsets, including cDC1, cDC2, and pDC cells, in AITD subjects compared to age matched controls (Figure 5A). Next, we analyzed frequencies of major monocyte subsets, including classical, non-classical, and intermediate monocytes. We found pediatric AITD subjects have an increase in frequency of classical monocytes compared to age-matched controls (Figure 5B). To determine the possibility that these monocytes are producing more pro-inflammatory cytokines, we analyzed their baseline cytokine production of 12 known monocyte derived cytokines, including IL-1a, IL-1b, TNF-a, MCP-1, and MIP-1B. We found that adult-onset AITD subjects had a decrease in IL-6+ and IL-23p19 + monocytes compared to age-matched controls. However, we found no difference in cytokine production in pediatric-onset AITD subjects compared to age-matched controls, suggesting the increased frequency of classical monocytes found in the blood is not associated with an increase in production of pro-inflammatory cytokines (Figure 5C). Hence, the biological significance of a moderate increase in peripheral blood monocytes seen in pediatric AITD subjects remains to be determined.
Figure 5.
Manual gating analysis of myeloid and NK cell subsets reveals pediatric AITD patients show a tendency of reduced frequencies of total NK and the cytotoxic CD56lo NK subset compared to pediatric controls
(A) Frequencies of dendritic cell (DC) subsets are similar among AITD and control subjects. Conventional type 1 DCs (cDC1) were gated as CD3-CD19-HLA-DR + CD14-CD16-CD11c + CD1c-, conventional type 2 DCs (cDC2) were gated as CD3-CD19-HLA-DR + CD14-CD16-CD11c + CD1c+, and plasmacytoid DC (pDC) were gated as CD3-CD19-HLA-DR + CD45RA + CD123 + CD11c-.
(B) The frequency of classical monocytes (CD3-CD19-HLA-DR + CD14+) is increased in pediatric AITD subjects compared to pediatric controls, whereas the frequency of intermediate monocytes (CD3-CD19-HLA-DR + CD14 + CD16+) and non-classical monocytes (CD3-CD19-HLA-DR + CD14-CD16+) are similar among AITD and control subjects.
(C) Frequencies of cytokine producing monocytes at baseline demonstrates adult AITD patients have reduced frequencies of IL-23p19+ and IL-6+ monocytes compared to adult controls.
(D) The frequency of natural killer (NK; CD3-CD19-HLA-DR-CD16+) cells is reduced, but not significantly, in pediatric AITD and adult AITD patients compared to controls. The reduced frequency of NK cells appears relegated to the cytotoxic CD56lo NK cell subset, as opposed to the regulatory CD56hi NK subset. PA: pediatric AITD, PH: pediatric healthy control, AA: adult AITD, AH: adult healthy control. Data are represented as mean ± SEM. Samples were processed as depicted in Figure S1. Frequency of myeloid and NK cell subsets, and cytokine production were determined by manual gating, as depicted in Figures S3 and S6. Statistical significance determined by One-way ANOVA with Sidak multiple comparisons post-test. ∗p < 0.05, ∗∗p < 0.01.
To complete our analysis of immune cells by manual gating, we analyzed the frequency of NK cells in blood from our cohort of subjects. We found that both pediatric and adult-onset AITD subjects exhibited a decrease, though not significant, in NK cells compared to their age-matched controls. Given previous reports that NK cells can have either a regulatory (CD56hi) or pathogenic (CD56lo) phenotype, and that AITD subjects have shown an impaired regulatory NK cell phenotype (Gallo et al., 2020), we analyzed the relevant NK subsets. We found that the tendency of a decrease in total NK cells seen in AITD subjects appears relegated to the cytotoxic CD56lo NK subset, whereas the frequency of regulatory CD56hi NK cells is similar in AITD subjects and controls (Figure 5D). Hence, we hypothesize the pathogenic CD56lo NK subset may have trafficked from the peripheral blood to the thyroid gland, where it participates in disease through cytotoxic activity.
Finally, we analyzed myeloid and NK cells, gated as CD45+CD19−CD3-, using dimension reduction and unsupervised clustering (Figure 6). We identified a few clusters that could not be identified as a particular cell type, e.g., myeloid or NK, using Cluster Explorer and comparison to manual gating, and therefore, these populations were excluded from the analysis (data not shown). In addition, although we were able to identify known myeloid and NK cell subsets that were identified by manual gating, we were unable to identify a pDC cluster (Figures 6A and 6B). Of the seven identifiable clusters, we found that classical monocytes were not increased in the pediatric AITD subjects, which differed from our manual gating analysis (Figure 6C). In addition, in parallel with manual gating, pediatric and adult AITD patients demonstrated a decreased, but not significantly, frequency of CD56lo NK cells compared to their age/sex-matched controls (Figure 6C). These data support the hypothesis that cytotoxic NK cells may traffic from the periphery to the thyroid gland to participate in cytotoxic activity. Lastly, we found no significant difference in cytokine production in our monocyte populations (data not shown).
Figure 6.
Unsupervised clustering and dimension reduction analysis of myeloid and NK cells reveals similar frequencies of subpopulations in AITD patients and controls
(A) Non-T/non-B cells which encompass myeloid and natural killer (NK) cells were manually gated as CD45 + CD3-CD19-and then analyzed using the unsupervised clustering and dimension reduction programs PhenoGraph and X-shift to generate distinct clusters based on cell surface marker expression (CD15, CD1c, CD11b, HLA-DR, CD56, CD11c, CD14, and CD16). A UMAP plot was created using total non-B/non-T cells from concatenated adult AITD, adult healthy control, pediatric AITD, and pediatric healthy control samples with a minimum of 17,000 cell events. Seven unique cell subpopulations were identified. UMAP plots of each surface marker are displayed to demonstrate varying expression levels among the myeloid and NK cell subpopulations.
(B) Heatmap of cell surface marker expression for identification of the 5 myeloid and 2 NK cell subsets identified using PhenoGraph/X-Shift. cDC1: conventional type 1 dendritic cell; cDC2: conventional type 2 dendritic cell.
(C) Frequencies of myeloid and NK cell subsets in adult AITD (AA), adult healthy control (AH), pediatric AITD (PA), and pediatric healthy control (PH) subjects demonstrate normal frequencies in AITD patients compared to controls. Data are represented as mean ± SEM. Samples were processed as depicted in Figure S1. Statistical significance determined by Mann-Whitney non-parametric unpaired Student's t tests.
Discussion
Here we demonstrate that immunophenotyping and functional analysis of newly diagnosed pediatric and adult-onset AITD subjects reveals alterations in immune cell subsets unique to age of onset. Specifically, we identified the frequency of immature B cells is increased in, and the frequency of BND, DN2, and cytotoxic NK cells are reduced in the peripheral blood of pediatric-onset AITD subjects but not adult-onset AITD subjects using manual gating strategies. These cellular subsets are of particular interest because they have been implicated in development of AITD and/or other autoimmune disorders. Immature B cells have been shown to be higher in T1D, RA, SLE, and Sjogren's syndrome patients because of defective central and peripheral tolerance mechanisms, and are shown to be enriched in autoreactivity (Glauzy et al., 2017; Kinnunen et al., 2013; Menard et al., 2011). Hence, we suspect that a similar phenomenon is occurring in AITD patients, thus, providing a pool of autoreactive B cells that can participate in disease if they receive the necessary signals.
We have previously identified a loss of peripheral thyroid-reactive and total BND cells in adult-onset AITD subjects (Smith et al., 2017). Although in the current study we were only able to identify a loss of total anergic B cells in pediatric but not adult AITD subjects, we believe this could be because of a difference in genetic risk alleles between the two cohorts. Dogma suggests autoreactive B cells become anergic in the periphery because of chronic antigen binding in the absence of second signals provided by “helper” T cells (signal 1 without signal 2). Hence, properly timed provision of T cell help may activate anergic cells. The gene conferring the highest susceptibility for AITD is in the HLA locus. It is has been shown that the HLA class II haplotype HLA-DR3, followed by HLA-DR4, confer the highest risk (Tomer, 2014) and in our study 80% (8/10) of the pediatric AITD patients vs 54% (7/13) of adult AITD patients were carriers of either the HLA-DR3 or HLA-DR4 haplotype. It is hypothesized that these class II molecules are structurally permissive for binding of Tg and TSH-R peptides that lead to cognate interaction with T cells, giving rise to loss of anergy and development of AITD (Jacobson et al., 2008). Hence, it is possible that loss of anergic B cells was seen only in our pediatric AITD but not the adult AITD patients because of their increased frequency of HLA-DR3 and HLA-DR4 alleles, which enhance the ability of pediatric AITD B cells to preferentially present thyroid antigens and thus receive T cell help. In line with this, in our previous study 92% (11/12) of our newly diagnosed adult AITD patients carried one or both of these haplotypes (Smith et al., 2017). We suspect other factors are in play, such as the environment and other genetic risk alleles that allow escape from tolerance of B cells, setting the stage for cognate interactions with T cells.
Although DN2 B cells have been extensively reported to be enriched in SLE patients using manual gating strategies (Jenks et al., 2018; Wang et al., 2018), in this study we found a decrease in peripheral DN2 cells in our pediatric AITD patients. One possible explanation for this is SLE is considered a systemic autoimmune disorder, in that autoimmunity is directed against antigens expressed throughout the body (e.g., dsDNA), whereas AITD is a very organ-specific autoimmune disorder in which B and T cells target antigens expressed predominantly, and in some cases, exclusively in the thyroid. Hence, we hypothesize loss of DN2 cells from the peripheral blood in our pediatric AITD patients reflects their migration to the thyroid where they participate in disease through extrafollicular antibody production. Given the reduction, though not significantly, of cytotoxic NK cells from the blood of pediatric AITD subjects, we believe a similar phenomenon is occurring with this subset. Future studies in our lab are focused on analyzing thyroid tissue to determine whether BND, DN2, and CD56lo NK cells have migrated to the site of autoimmune attack.
In this study we used a combination of manual gating and unsupervised clustering and dimension reduction to deeply evaluate the immunophenotype and function of immune cells in AITD subjects and controls. Importantly, each analytical strategy rendered varying results, with a difference in immature and BND cells only seen by manual gating. This finding necessitates a brief discussion of the advantages and disadvantages of the two methods and how to interpret the varying results. Manual gating is simple and intuitive, but the user can be biased toward expected populations and drawing gates can be subjective. On the other hand, unsupervised analysis is unbiased, more automated, and can identify novel cell populations. Although comparative studies have shown that manual gating and unbiased analysis can achieve the same results (Conrad et al., 2019), this is not always the case. For example, when we overlay our manually gated BND population with the BND-like cluster identified by PhenoGraph and X-shift (Figure S7), the two populations do not overlap significantly with only about 18% of the manually gated-assigned BND cells overlapping with the algorithm-assigned BND-like cells. Many of the BND-like cells express normal levels of IgM compared to the manually gated BND cells, suggesting the former are not anergic. In addition, using heatmap analysis, the algorithm based BND-like cells are also CD21-and CD38-with slightly higher levels of CD11c compared to manually gated BND cells and mature naive cells (Figure S7). Their spatial relation and phenotype suggest they are more similar to DN2 cells, except they express much higher levels of IgD. Hence, although unbiased analysis of high-dimensional datasets has many advantages, comparison of subpopulations between the two methods is not always straight-forward and requires proper interpretation.
Limitations of the study
Although this is the first study to interrogate the immunophenotype and function of immune cells from pediatric and adult-onset AITD patients concurrently using high-dimensional mass cytometry, this study does have its limitations. Although the pathogenesis of AITD involves both autoreactive B and T cells, we did not find a disease-relevant immune signature in the T cell compartment in either of our AITD cohorts. We hypothesize that because AITD is an organ-directed autoimmune disorder and only antigen-reactive T (and B) cells should, in theory, participate in disease development, that analysis of changes that occur (only) at the level of antigen-reactive cells, e.g., thyroglobulin-reactive T and B cells, would reveal a disease-relevant immune signature. Furthermore, it is possible we were only able to observe a difference in frequency of immature, BND, and DN2 B cell populations since these subsets have previously been shown to be enriched in autoreactive B cell clones (Duty et al., 2009; Jenks et al., 2018). Hence, future studies analyzing thyroid-reactive T (and B) cells in AITD, using methods compatible with mass cytometry (Stensland and Smith, 2021; Wiedeman et al., 2020), are needed. In addition, future studies analyzing other relevant immune cell subpopulations during development of autoimmunity, such as regulatory B and T cells, and potentially cytokine production in the presence of a protein transport inhibitor, are warranted. Lastly, we acknowledge that validation of these results with a second cohort is needed, and these studies are currently underway. In conclusion, immunophenotyping of peripheral blood of AITD patients reveals alterations in pathogenic immune cells frequencies in pediatric AITD subjects that are normalized in adult AITD. These findings may reflect differences in frequencies of genetic risk alleles that lead to rapid progression of disease in young-onset AITD.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-human CD45 (clone HI30) | Fluidigm | Cat#3089003B RRID:AB_2661851 |
| Mouse monoclonal anti-human CD66 (clone B1.1) | BD | Cat#551354 RRID:AB_394166 |
| Mouse monoclonal anti-human CD15 (clone HI98) | BD | Cat#555400 RRID:AB_395800 |
| Mouse monoclonal anti-human CD21 (clone Bu32) | BioLegend | Cat#354902 RRID:AB_11219188 |
| Mouse monoclonal anti-human CD8 (clone SK1) | BioLegend | Cat#344727 RRID:AB_2563762 |
| Mouse monoclonal anti-human CD123 (clone 9F5) | BD | Cat#555642 RRID:AB_395999 |
| Mouse monoclonal anti-human CD3 (clone UCHT1) | BD | Cat#555330 RRID:AB_395737 |
| Mouse monoclonal anti-human IgD (clone IA6-2) | BioLegend | Cat#348235 RRID:AB_2563775 |
| Mouse monoclonal anti-human CD7 (clone M-T701) | BD | Cat#555359 RRID:AB_395762 |
| Mouse monoclonal anti-human CD86 (clone IT2.2) | Fluidigm | Cat#3150020B RRID:AB_2687852 |
| Mouse monoclonal anti-human IgM (clone MHM-88) | BioLegend | Cat#314527 RRID:AB_314527 |
| Mouse monoclonal anti-human CD11c (clone 3.9) | BioLegend | Cat#301639 RRID:AB_2562812 |
| Mouse monoclonal anti-human CD45RA (clone HI100) | BioLegend | Cat#304143 RRID:AB_2562822 |
| Mouse monoclonal anti-human CD14 (clone M5E2) | BioLegend | Cat#301843 RRID:AB_2562813 |
| Mouse monoclonal anti-human CD27 (clone L128) | Fluidigm | Cat#3155001B RRID:AB_2687645 |
| Mouse monoclonal anti-human ICOS (clone C398.4A) | BioLegend | Cat#313502 RRID:AB_416326 |
| Mouse monoclonal anti-human CD1c (clone L161) | BioLegend | Cat#331502 RRID:AB_1088995 |
| Mouse monoclonal anti-human PD-1 (clone EH12.2H7) | BioLegend | Cat#329902 RRID:AB_940488 |
| Mouse monoclonal anti-human CD19 (clone SJ25C1) | BioLegend | Cat#363001 RRID:AB_2563988 |
| Mouse monoclonal anti-human CD16 (clone B73.1) | eBioscience | Cat#16-0167-025 RRID:AB_2865639 |
| Mouse monoclonal anti-human HLA-DR (clone L243) | BioLegend | Cat#307602 RRID:AB_314680 |
| Mouse monoclonal anti-human CD56 (clone REA196) | Miltenyi Biotech | Cat#130-108-016 RRID:AB_2658728 |
| Mouse monoclonal anti-human CD38 (clone HIT2) | BioLegend | Cat#303502 RRID:AB_314354 |
| Mouse monoclonal anti-human CXCR5 (clone RF8B2) | BD | Cat#552032 RRID:AB_394324 |
| Mouse monoclonal anti-human CD4 (clone SK3) | Fluidigm | Cat#3174004B RRID:AB_2687862 |
| Mouse monoclonal anti-human CD11b (clone ICRF44) | Fluidigm | Cat#3209003B RRID:AB_2687654 |
| Mouse monoclonal anti-human IFN-g (clone 4S.B3) | BioLegend | Cat#502501 RRID:AB_315226 |
| Mouse monoclonal anti-human IL-1a (clone 364-3B3-14) | BioLegend | Cat#500104 RRID:AB_315083 |
| Mouse monoclonal anti-human IL-17a (clone BL168) | BioLegend | Cat#512331 RRID:AB_2563779 |
| Mouse monoclonal anti-human IL-1RA (clone AS17) | Santa Cruz | Cat#sc-57275 RRID:AB_784001 |
| Mouse monoclonal anti-human MIP1b (clone D21-1351) | BD | Cat#562900 RRID:AB_2737877 |
| Mouse monoclonal anti-human PTEN (clone A2B1) | BD | Cat#559600 RRID:AB_397290 |
| Mouse monoclonal anti-human IL-8 (clone E8N1) | BioLegend | Cat#511402 RRID:AB_893460 |
| Mouse monoclonal anti-human IL-6 (clone MQ2-13A5) | BioLegend | Cat#501115 RRID:AB_2562841 |
| Mouse monoclonal anti-human TNF-a (clone Mab11) | BioLegend | Cat#502901 RRID:AB_315253 |
| Mouse monoclonal anti-human IL1b (clone H1b-98) | BioLegend | Cat#511601 RRID:AB_1236517 |
| Mouse monoclonal anti-human MCP1 (clone 5D3-F7) | eBioscience | Cat#14-7099-81 RRID:AB_468432 |
| Mouse monoclonal anti-human IL-12p40 (clone C8.6) | BioLegend | Cat#501702 RRID:AB_2255186 |
| Mouse monoclonal anti-human IFN-a (clone LT27.295) | Miltenyi Biotech | Cat#130-108-050 RRID:AB_2659989 |
| Mouse monoclonal anti-human IL-23p19 (clone 23dcdp) | eBioscience | Cat#12-7823-42 RRID:AB_10668835 |
| Biological samples | ||
| Blood from Donors | University of Colorado Anschutz Medical Center, Colorado Children’s Hospital, and Barbara Davis Center for Childhood Diabetes | University of Colorado Institutional Review Board (COMIRB 01-384) |
| Chemicals, peptides, and recombinant proteins | ||
| R848 (Resiquimod) | Invivogen | Cat#tlrl-r848 |
| LPS (LPS-EK) | Invivogen | Cat#tlrl-eklps |
| Protein transport inhibitory cocktail | eBioscience | Cat#00-4980-93 |
| Critical commercial assays | ||
| Cell-ID 20-Plex Pd Barcoding Kit | Fluidigm | Cat#PRD023 |
| Cell-ID Intercalator-Ir | Fluidigm | Cat#201192A |
| Maxpar Antibody Labeling Kit | Fluidigm | Cat#201160B |
| Perm/Wash buffer 1 | BD | Cat#558050 |
| Phosflow lyse/fix buffer | BD | Cat#558049 |
| DNA midi kit | Qiagen | Cat#51185 |
| HLA typing | Barbara Davis Center for Diabetes | Autoantibody/HLA Service Center at the Barbara Davis Center for Diabetes |
| Deposited data | ||
| All mass cytometry data | Smith Lab | Stensland, Z. (2021), “AITD CyTOF study normalized data set”, Mendeley Data, V1: https://doi.org/10.17632/s85cgbb4r5.1 |
| Software and algorithms | ||
| FlowJo | BD | v10.8.0 |
| Cell Engine | Primity Bio, Inc. | |
| Matlab Normalizer and Debarcoder Tool | Nolan Lab | Matlab Normalizer, Matlab Single Cell Debarcoder |
| Phenograph – FlowJo plugin | BD | FlowJo Exchange Phenograph |
| X-shift – FlowJo plugin | BD | FlowJo Exchange XShift |
| UMAP – FlowJo plugin | BD | FlowJo Exchange UMAP |
| Downsample – FlowJo plugin | BD | FlowJo Exchange DownSample |
| Cluster explorer – FlowJo plugin | BD | FlowJo Exchange ClusterExplorer |
| GraphPad Prism9 | GraphPad Software, Inc | v9.0.0 |
| CytofBatchAdjust | Schuyler et al., 2019 | https://www.frontiersin.org/articles/10.3389/fimmu.2019.02367/full |
Resource availability
Lead contact
-
•
For further information and requests for resources and reagenets should be direct to and will be fulfilled by the lead contact, Mia Smith (mia.smith@cuanschutz.edu).
Materials availability
-
•
This study did not generate new unique reagents.
Experimental model and subject details
All participates were enrolled under study protocols approved by the Colorado Multipe Institutional Review Board (COMIRB #01-384). Written informed consent was obtained from each adult participant, or from parents or guardians of participants less than 18 years old. Assent was obtained from participants over the age of 7 years who were cognitively able to consent. All procedures were performed in accordance with COMIRB guidelines and regulations. Demographics and other data about research participants can be found in Tables 1 and S1.
Method details
Study participants
Samples were obtained with informed consent at the University of Colorado Anschutz Medical Center, Colorado Children’s Hospital, and the Barbara Davis Center for Childhood Diabetes. Eligible subjects were male or female, who had been diagnosed with either Graves’ disease (GD) or Hashimoto’s thyroiditis (HT) within 2 months of blood collection. Only subjects that had not begun treatment or had minimal treatment with thyroid replacement or antithyroid drugs were enrolled, since treatment could alter the phenotype of peripheral blood cells. Presence of antibodies against Tg, TPO, and TSH-R, as well as TSH, Free T4, and Total T3 tests were used to confirm a diagnosis of GD or HT. A one-time blood collection was obtained from 13 adult (>18 years old) onset AITD, 22 age/sex matched adult controls, 10 pediatric (<18 years old) onset AITD, and 22 pediatric age/sex matched controls. Initially we sought to recruit an equal number of HT and GD patients in our adult AITD cohort. However, this was made difficult by the fact the treatment of HT patients is typically begun prior to entering specialist care. Therefore, the majority of HT patients coming to our center do not meet eligibility requirements. Nonetheless, from our experience both disorders likely follow a similar early pathogenic progression (Smith et al., 2017), and therefore, we believe findings are relevant to both HT and GD. Demographics and clinical data of research participants can be found in Tables 1 and S1, respectively.
Sample processing
Whole blood was collected in heparinized vacutainers. Blood was divided for CyTOF analysis and DNA isolation for HLA analysis. DNA for HLA typing was extracted from whole blood using the DNA midi kit (Qiagen). HLA typing was performed at the Autoantibody/HLA Service Center at the Barbara Davis Center for Diabetes. Blood for CyTOF analysis was processed directly ex vivo, using a protocol adapted from previous studies (O'Gorman et al., 2017; Baxter et al., 2018). Patient and control samples were analyzed at baseline with no exogenous stimulation. Red blood cells from approximately 0.75 mL of whole blood were lysed and cells were fixed with Phosflow lyse/fix buffer (BD, 558049) for 15 minutes at 37°C. Cells were washed once with ice-cold PBS and then once with complete staining media (CSM) (1% BSA + PBS + 0.01% sodium azide). Cell pellets were stored at -80°C until ready for mass antibody staining and barcoding. For normalization between barcoding sets, blood from an “anchor” control was collected and divided in half. One half of the sample was processed in the same way as above at baseline in replicates to be included in each barcode set. In order to serve as a positive control for cytokine antibody staining, the other half of the “anchor” blood sample was stimulated with 0.1 ug/mL each of the TLR7/8 and TLR4 agonists, R848 (Invivogen, tlrl-r848-5) and LPS (Invivogen, tlrl-eklps), respectively, and protein transport inhibitory cocktail (eBioscience, 00-4980-93), as previously described (Baxter et al., 2018), at 37°C for 6 hours, and then stored at -80°C until processing. On the day of barcoding and staining, cell pellets were thawed, barcoded with a combination of palladium isotope mass tags (Fluidigm, PRD023) in sets of 20 to minimize technically variability, pooled together, surface stained in a single tube with our metal-labeled surface antibody panel (Table S2), then permeabilized with Perm/Wash buffer I (BD, 558050) and stained with intracellular metal-labeled antibodies (Table S2). All antibodies were either purchased pre-conjugated to metal isotopes or were conjugated using the Maxpar Antibody Labeling Kit (Fluidigm, 201160B). Stained cells were labeled with Cell-ID Intercalator-Ir (Fluidigm, 201192A), then analyzed on a Helios mass cytometer (Fluidigm) (Figure S1). Data was acquired using internal normalization beads.
Mass cytometry analysis
Files were instrument/bead normalized and debarcoded using Matlab Normalizer and Debarcoder Tool. Individual FCS files were normalized using CytofBatchAdjust (Schuyler et al., 2019). Manual gating was performed using CellEngine (Primity Bio, Inc.). For unsupervised clustering analysis, the following programs were utilized: PhenoGraph, Downsample, X-shift, UMAP, and Cluster explorer, which are available as plugins with FlowJo (TreeStar). Both X-Shift and PhenoGraph were used to identify immune cell frequency distributions while UMAP was used for non-linear dimension reduction and visualization using cell specific surface markers.
Pre-processing for analysis
For each subject, non-granulocyte (CD66-) cells were gated to identify major immune cell subsets. B cells were gated as CD45+CD19+CD14-, T cells were gated as CD45+CD19-CD3+, and myeloid/NK cells were gated as CD45+CD19-CD3-. Subsets were then normalized by down-sampling leaving 5,000 B cells from each sample, 9,000 T cells from each sample, and 17,000 myeloid/NK cells from each sample for individual analysis. Samples that had less than the threshold for each subset were excluded leaving 10 pediatric AITD, 9 pediatric controls, 12 adult AITD, and 12 adult controls. All samples were annotated to reflect their sample ID, disease state, and age before subsets were concatenated into individual workspaces for further analysis.
Topographical analysis
Concatenated subsets were first visualized using UMAP for dimension reduction using Eucledian distance with nearest neighbors set to 15 and minimum spanning distance set to 0.5 for each analysis. To generate the UMAP for B cells, the following surface markers were utilized: PD-1, IgM, CD27, CD21, IgD, CXCR5, CD11c, CD38, and CD86. For the T cell UMAP projection, the following markers were used: PD-1, ICOS, CD7, CD27, CD45RA, CD3, CXCR5, CD123, CD8, and CD4. For the myeloid/NK UMAP the following markers were utilized: CD15, CD1c, CD11b, HLADR, CD16, CD56, CD11c, and CD14.
Clustering analysis
For each analysis X-shift was utilized to partition single events into subpopulations within each subset using the same phenotypic markers as their topographical analyses. For each analysis angular distance was utilized with B cells using K: 132 and a subsampling limit of 250,000 (5,000 from each sample), T cells using K: 102 and a subsampling limit of 450,000 (9,000 from each sample), and myeloid/NK cells using K: 112 and a subsampling of 850,000 (17,000 from each sample). PhenoGraph was additionally used to confirm the existence of major and minuet X-shift clusters. Clusters identified by both algorithms were kept and clusters that were only found in one program were further interrogated to confirm their identity and existence. Clusters were identified using heatmaps created in both FlowJo and CellEngine of the cell surface markers utilized to generate the UMAP. In addition, comparison to manual gated populations and Cluster Explorer were used to help confirm the identify of individual clusters. Clusters that were indistinguishable from one another based on surface marker expression were merged. A minimum of three repeat runs of each analysis with identical parameters confirmed the results were reproducible (data not shown).
Quantification and statistical analysis
Data were analyzed using Prism software (GraphPad Software, Inc.). Differences in frequencies of manually gated populations between cohorts were analyzed using One-way ANOVA with Sidak multiple comparisons post-test. Mann-Whitney non-parametric unpaired Student’s t tests were used to determine differences among populations found using unbiased analysis. A p-value of ≤ 0.05 was considered statistically significant and is denoted by an asterisk, in which ∗≤ 0.05, ∗∗≤ 0.01, and ∗∗∗≤ 0.001.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R21AI124488 (Cambier), K23AR070897 (Hsieh), P30DK116073 (Sussel), F30OD021477 (Smith), and K01OD028759 (Smith)).
Author contributions
E.W.H., P.A.G., K.A.S., V.D.S., J.C.C., and M.J.S. designed the research and reviewed and edited the manuscript; E.W.H., K.A.S., and V.D.S. recruited subjects; E.W.H., J.C.C., and M.J.S. provided funding. M.J.S., R.M.B, and B.M.C. performed experiments. M.J.S. and Z.C.S. analyzed the data and prepared figures; M.R. was the study coordinator; Z.C.S., J.C.C., and M.J.S. wrote the manuscript.
Declaration of interest
The authors declare no competing interests.
Published: January 21, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.103626.
Supplemental information
Table S1. AITD patient demographics, HLA haplotype, and available clinical information, related to STAR Methods
Data and code availability
Data has been deposited at Mendeley: https://doi.org/10.17632/s85cgbb4r5. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Abdel-Wahab N., Shah M., Suarez-Almazor M.E. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One. 2016;11:e0160221. doi: 10.1371/journal.pone.0160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alunno A., Bistoni O., Bartoloni E., Caterbi S., Bigerna B., Tabarrini A., Mannucci R., Falini B., Gerli R. IL-17-producing CD4-CD8- T cells are expanded in the peripheral blood, infiltrate salivary glands and are resistant to corticosteroids in patients with primary Sjogren's syndrome. Ann. Rheum. Dis. 2013;72:286–292. doi: 10.1136/annrheumdis-2012-201511. [DOI] [PubMed] [Google Scholar]
- Alvarez-Sierra D., Marin-Sanchez A., Ruiz-Blazquez P., De Jesus Gil C., Iglesias-Felip C., Gonzalez O., Casteras A., Costa R.F., Nuciforo P., Colobran R., Pujol-Borrell R. Analysis of the PD-1/PD-L1 axis in human autoimmune thyroid disease: insights into pathogenesis and clues to immunotherapy associated thyroid autoimmunity. J. Autoimmun. 2019;103:102285. doi: 10.1016/j.jaut.2019.05.013. [DOI] [PubMed] [Google Scholar]
- Antonelli A., Ferrari S.M., Corrado A., Di Domenicantonio A., Fallahi P. Autoimmune thyroid disorders. Autoimmun. Rev. 2015;14:174–180. doi: 10.1016/j.autrev.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Antonelli A., Fallahi P., Elia G., Ragusa F., Paparo S.R., Ruffilli I., Patrizio A., Gonnella D., Giusti C., Virili C., et al. Graves' disease: clinical manifestations, immune pathogenesis (cytokines and chemokines) and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2020;34:101388. doi: 10.1016/j.beem.2020.101388. [DOI] [PubMed] [Google Scholar]
- Antonelli A., Ferrari S.M., Ragusa F., Elia G., Paparo S.R., Ruffilli I., Patrizio A., Giusti C., Gonnella D., Cristaudo A., et al. Graves' disease: epidemiology, genetic and environmental risk factors and viruses. Best Pract. Res. Clin. Endocrinol. Metab. 2020;34:101387. doi: 10.1016/j.beem.2020.101387. [DOI] [PubMed] [Google Scholar]
- Armengol M.P., Juan M., Lucas-Martin A., Fernandez-Figueras M.T., Jaraquemada D., Gallart T., Pujol-Borrell R. Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active intrathyroidal germinal centers. Am. J. Pathol. 2001;159:861–873. doi: 10.1016/S0002-9440(10)61762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter R.M., Kong D.S., Garcia-Perez J.E., O'gorman W.E., Hsieh E.W.Y. Single-cell analysis of immunophenotype and cytokine production in peripheral whole blood via mass cytometry. J. Vis. Exp. 2018;136:57780. doi: 10.3791/57780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand A., Kostine M., Barnetche T., Truchetet M.E., Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocharnikov A.V., Keegan J., Wacleche V.S., Cao Y., Fonseka C.Y., Wang G., Muise E.S., Zhang K.X., Arazi A., Keras G., et al. PD-1hiCXCR5- T peripheral helper cells promote B cell responses in lupus via MAF and IL-21. JCI Insight. 2019;4:e130062. doi: 10.1172/jci.insight.130062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braley-Mullen H., Yu S. Early requirement for B cells for development of spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J. Immunol. 2000;165:7262–7269. doi: 10.4049/jimmunol.165.12.7262. [DOI] [PubMed] [Google Scholar]
- Chiovato L., Bassi P., Santini F., Mammoli C., Lapi P., Carayon P., Pinchera A. Antibodies producing complement-mediated thyroid cytotoxicity in patients with atrophic or goitrous autoimmune thyroiditis. J. Clin. Endocrinol. Metab. 1993;77:1700–1705. doi: 10.1210/jcem.77.6.7903315. [DOI] [PubMed] [Google Scholar]
- Choi J.Y., Ho J.H., Pasoto S.G., Bunin V., Kim S.T., Carrasco S., Borba E.F., Goncalves C.R., Costa P.R., Kallas E.G., et al. Circulating follicular helper-like T cells in systemic lupus erythematosus: association with disease activity. Arthritis Rheumatol. 2015;67:988–999. doi: 10.1002/art.39020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad V.K., Dubay C.J., Malek M., Brinkman R.R., Koguchi Y., Redmond W.L. Implementation and validation of an automated flow cytometry analysis pipeline for human immune profiling. Cytometry A. 2019;95:183–191. doi: 10.1002/cyto.a.23664. [DOI] [PubMed] [Google Scholar]
- Crispin J.C., Oukka M., Bayliss G., Cohen R.A., Van Beek C.A., Stillman I.E., Kyttaris V.C., Juang Y.T., Tsokos G.C. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J. Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty J.A., Szodoray P., Zheng N.Y., Koelsch K.A., Zhang Q., Swiatkowski M., Mathias M., Garman L., Helms C., Nakken B., et al. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J. Exp. Med. 2009;206:139–151. doi: 10.1084/jem.20080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman I., Ihantola E.L., Viisanen T., Rao D.A., Nanto-Salonen K., Knip M., Veijola R., Toppari J., Ilonen J., Kinnunen T. Circulating CXCR5(-)PD-1(hi) peripheral T helper cells are associated with progression to type 1 diabetes. Diabetologia. 2019;62:1681–1688. doi: 10.1007/s00125-019-4936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farid N.R., Moens H., Larsen B., Payne R., Saltman K., Fifield F., Ingram D.W. HLA haplotypes in familial Graves' disease. Tissue Antigens. 1980;15:492–500. [PubMed] [Google Scholar]
- Farid N.R., Stone E., Johnson G. Graves' disease and HLA: clinical and epidemiologic associations. Clin. Endocrinol. (Oxf.) 1980;13:535–544. doi: 10.1111/j.1365-2265.1980.tb03421.x. [DOI] [PubMed] [Google Scholar]
- Fortea-Gordo P., Nuno L., Villalba A., Peiteado D., Monjo I., Sanchez-Mateos P., Puig-Kroger A., Balsa A., Miranda-Carus M.E. Two populations of circulating PD-1hiCD4 T cells with distinct B cell helping capacity are elevated in early rheumatoid arthritis. Rheumatology (Oxford) 2019;58:1662–1673. doi: 10.1093/rheumatology/kez169. [DOI] [PubMed] [Google Scholar]
- Fountoulakis S., Vartholomatos G., Kolaitis N., Frillingos S., Philippou G., Tsatsoulis A. HLA-DR expressing peripheral T regulatory cells in newly diagnosed patients with different forms of autoimmune thyroid disease. Thyroid. 2008;18:1195–1200. doi: 10.1089/thy.2008.0089. [DOI] [PubMed] [Google Scholar]
- Freudenhammer M., Voll R.E., Binder S.C., Keller B., Warnatz K. Naive- and memory-like CD21(low) B cell subsets share core phenotypic and signaling characteristics in systemic autoimmune disorders. J. Immunol. 2020;205:2016–2025. doi: 10.4049/jimmunol.2000343. [DOI] [PubMed] [Google Scholar]
- Fugger G., Dold M., Bartova L., Kautzky A., Souery D., Mendlewicz J., Serretti A., Zohar J., Montgomery S., Frey R., Kasper S. Comorbid thyroid disease in patients with major depressive disorder - results from the European Group for the Study of Resistant Depression (GSRD) Eur. Neuropsychopharmacol. 2018;28:752–760. doi: 10.1016/j.euroneuro.2018.03.011. [DOI] [PubMed] [Google Scholar]
- Gallo D., Piantanida E., Gallazzi M., Bartalena L., Tanda M.L., Bruno A., Mortara L. Immunological drivers in graves' disease: NK cells as a master switcher. Front. Endocrinol. (Lausanne) 2020;11:406. doi: 10.3389/fendo.2020.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessl A., Waldhausl W. Elevated CD69 expression on naive peripheral blood T-cells in hyperthyroid Graves' disease and autoimmune thyroiditis: discordant effect of methimazole on HLA-DR and CD69. Clin. Immunol. Immunopathol. 1998;87:168–175. doi: 10.1006/clin.1998.4524. [DOI] [PubMed] [Google Scholar]
- Glauzy S., Sng J., Bannock J.M., Gottenberg J.E., Korganow A.S., Cacoub P., Saadoun D., Meffre E. Defective early B cell tolerance checkpoints in sjogren's syndrome patients. Arthritis Rheumatol. 2017;69:2203–2208. doi: 10.1002/art.40215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley P., Lord K., Bauer A.J. Thyroid disorders in children and adolescents: a review. JAMA Pediatr. 2016;170:1008–1019. doi: 10.1001/jamapediatrics.2016.0486. [DOI] [PubMed] [Google Scholar]
- Ho C.W., Chen H.H., Hsieh M.C., Chen C.C., Hsu S.P., Yip H.T., Kao C.H. Increased risk of polycystic ovary syndrome and it's comorbidities in women with autoimmune thyroid disease. Int. J. Environ. Res. Public Health. 2020;17:2422. doi: 10.3390/ijerph17072422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.H., Braley-Mullen H. Follicular B cells in thyroids of mice with spontaneous autoimmune thyroiditis contribute to disease pathogenesis and are targets of anti-CD20 antibody therapy. J. Immunol. 2014;192:897–905. doi: 10.4049/jimmunol.1301628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie I., Abiru N., Nagayama Y., Kuriya G., Saitoh O., Ichikawa T., Iwakura Y., Eguchi K. T helper type 17 immune response plays an indispensable role for development of iodine-induced autoimmune thyroiditis in nonobese diabetic-H2h4 mice. Endocrinology. 2009;150:5135–5142. doi: 10.1210/en.2009-0434. [DOI] [PubMed] [Google Scholar]
- Isnardi I., Ng Y.S., Menard L., Meyers G., Saadoun D., Srdanovic I., Samuels J., Berman J., Buckner J.H., Cunningham-Rundles C., Meffre E. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood. 2010;115:5026–5036. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson E.M., Huber A., Tomer Y. The HLA gene complex in thyroid autoimmunity: from epidemiology to etiology. J. Autoimmun. 2008;30:58–62. doi: 10.1016/j.jaut.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks S.A., Cashman K.S., Zumaquero E., Marigorta U.M., Patel A.V., Wang X., Tomar D., Woodruff M.C., Simon Z., Bugrovsky R., et al. Distinct effector B cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity. 2018;49:725–739 e6. doi: 10.1016/j.immuni.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurecka-Lubieniecka B., Ploski R., Kula D., Krol A., Bednarczuk T., Kolosza Z., Tukiendorf A., Szpak-Ulczok S., Stanjek-Cichoracka A., Polanska J., Jarzab B. Association between age at diagnosis of Graves' disease and variants in genes involved in immune response. PLoS One. 2013;8:e59349. doi: 10.1371/journal.pone.0059349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen T., Chamberlain N., Morbach H., Cantaert T., Lynch M., Preston-Hurlburt P., Herold K.C., Hafler D.A., O'connor K.C., Meffre E. Specific peripheral B cell tolerance defects in patients with multiple sclerosis. J. Clin. Invest. 2013;123:2737–2741. doi: 10.1172/JCI68775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavard L., Madsen H.O., Perrild H., Jacobsen B.B., Svejgaard A. HLA class II associations in juvenile Graves' disease: indication of a strong protective role of the DRB1∗0701,DQA1∗0201 haplotype. Tissue Antigens. 1997;50:639–641. doi: 10.1111/j.1399-0039.1997.tb02922.x. [DOI] [PubMed] [Google Scholar]
- Li D., Cai W., Gu R., Zhang Y., Zhang H., Tang K., Xu P., Katirai F., Shi W., Wang L., et al. Th17 cell plays a role in the pathogenesis of Hashimoto's thyroiditis in patients. Clin. Immunol. 2013;149:411–420. doi: 10.1016/j.clim.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Liubchenko G.A., Appleberry H.C., Striebich C.C., Franklin K.E., Derber L.A., Holers V.M., Lyubchenko T. Rheumatoid arthritis is associated with signaling alterations in naturally occurring autoreactive B-lymphocytes. J. Autoimmun. 2013;40:111–121. doi: 10.1016/j.jaut.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M.C., Chang S.C., Huang K.Y., Koo M., Lai N.S. Higher risk of thyroid disorders in young patients with type 1 diabetes: a 12-year nationwide, population-based, retrospective cohort study. PLoS One. 2016;11:e0152168. doi: 10.1371/journal.pone.0152168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkiel S., Jeganathan V., Wolfson S., Orduno N.M., Marasco E., Aranow C., Mackay M., Gregersen P.K., Diamond B. Checkpoints for autoreactive B cells in peripheral blood of lupus patients assessed by flow cytometry. Arthritis Rheumatol. 2016;68:2210–2220. doi: 10.1002/art.39710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji N., Carr-Smith J.D., Boelaert K., Allahabadia A., Armitage M., Chatterjee V.K., Lazarus J.H., Pearce S.H., Vaidya B., Gough S.C., Franklyn J.A. Influences of age, gender, smoking, and family history on autoimmune thyroid disease phenotype. J. Clin. Endocrinol. Metab. 2006;91:4873–4880. doi: 10.1210/jc.2006-1402. [DOI] [PubMed] [Google Scholar]
- Masuda T., Ohteki T., Abo T., Seki S., Nose S., Nagura H., Kumagai K. Expansion of the population of double negative CD4-8- T alpha beta-cells in the liver is a common feature of autoimmune mice. J. Immunol. 1991;147:2907–2912. [PubMed] [Google Scholar]
- Menard L., Saadoun D., Isnardi I., Ng Y.S., Meyers G., Massad C., Price C., Abraham C., Motaghedi R., Buckner J.H., et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J. Clin. Invest. 2011;121:3635–3644. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada A.M., Hammouda M. Immunoregulatory T cells, LFA-3 and HLA-DR in autoimmune thyroid diseases. Indian J. Endocrinol. Metab. 2014;18:574–581. doi: 10.4103/2230-8210.137524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noureldine S.I., Tufano R.P. Association of Hashimoto's thyroiditis and thyroid cancer. Curr. Opin. Oncol. 2015;27:21–25. doi: 10.1097/CCO.0000000000000150. [DOI] [PubMed] [Google Scholar]
- O'Gorman W.E., Kong D.S., Balboni I.M., Rudra P., Bolen C.R., Ghosh D., Davis M.M., Nolan G.P., Hsieh E.W. Mass cytometry identifies a distinct monocyte cytokine signature shared by clinically heterogeneous pediatric SLE patients. J. Autoimmun. 2017 doi: 10.1016/j.jaut.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X.F., Gu J.Q., Shan Z.Y. Increased risk of thyroid autoimmunity in rheumatoid arthritis: a systematic review and meta-analysis. Endocrine. 2015;50:79–86. doi: 10.1007/s12020-015-0533-x. [DOI] [PubMed] [Google Scholar]
- Pan X.F., Gu J.Q., Shan Z.Y. Patients with systemic lupus erythematosus have higher prevalence of thyroid autoantibodies: a systematic review and meta-analysis. PLoS One. 2015;10:e0123291. doi: 10.1371/journal.pone.0123291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D., Xu B., Wang Y., Guo H., Jiang Y. A high frequency of circulating th22 and th17 cells in patients with new onset graves' disease. PLoS One. 2013;8:e68446. doi: 10.1371/journal.pone.0068446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q., Liu P., Liu L., Wang R., Yan N., Yang J., Wang X., Pandey M., Zhang J.A. The increased but non-predominant expression of Th17- and Th1-specific cytokines in Hashimoto's thyroiditis but not in Graves' disease. Braz. J. Med. Biol. Res. 2012;45:1202–1208. doi: 10.1590/S0100-879X2012007500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan R., Calvin S., Singh J.K., Thomas B., Srinivasan K. Thyroid dysfunction in major psychiatric disorders in a hospital based sample. Indian J. Med. Res. 2013;138:888–893. [PMC free article] [PubMed] [Google Scholar]
- Ragusa F., Fallahi P., Elia G., Gonnella D., Paparo S.R., Giusti C., Churilov L.P., Ferrari S.M., Antonelli A. Hashimotos' thyroiditis: epidemiology, pathogenesis, clinic and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019;33:101367. doi: 10.1016/j.beem.2019.101367. [DOI] [PubMed] [Google Scholar]
- Rapoport B., McLachlan S.M. Graves' hyperthyroidism is antibody-mediated but is predominantly a Th1-type cytokine disease. J. Clin. Endocrinol. Metab. 2014;99:4060–4061. doi: 10.1210/jc.2014-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri R.M., Giuffrida G., Campenni A. Autoimmune endocrine diseases. Minerva Endocrinol. 2018;43:305–322. doi: 10.23736/S0391-1977.17.02757-2. [DOI] [PubMed] [Google Scholar]
- Ruggeri R.M., Trimarchi F., Giuffrida G., Certo R., Cama E., Campenni A., Alibrandi A., De Luca F., Wasniewska M. Autoimmune comorbidities in Hashimoto's thyroiditis: different patterns of association in adulthood and childhood/adolescence. Eur. J. Endocrinol. 2017;176:133–141. doi: 10.1530/EJE-16-0737. [DOI] [PubMed] [Google Scholar]
- Schuyler R.P., Jackson C., Garcia-Perez J.E., Baxter R.M., Ogolla S., Rochford R., Ghosh D., Rudra P., Hsieh E.W.Y. Minimizing batch effects in mass cytometry data. Front. Immunol. 2019;10:2367. doi: 10.3389/fimmu.2019.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segundo C., Rodriguez C., Aguilar M., Garcia-Poley A., Gavilan I., Bellas C., Brieva J.A. Differences in thyroid-infiltrating B lymphocytes in patients with Graves' disease: relationship to autoantibody detection. Thyroid. 2004;14:337–344. doi: 10.1089/105072504774193159. [DOI] [PubMed] [Google Scholar]
- Sieling P.A., Porcelli S.A., Duong B.T., Spada F., Bloom B.R., Diamond B., Hahn B.H. Human double-negative T cells in systemic lupus erythematosus provide help for IgG and are restricted by CD1c. J. Immunol. 2000;165:5338–5344. doi: 10.4049/jimmunol.165.9.5338. [DOI] [PubMed] [Google Scholar]
- Simmonds M.J. GWAS in autoimmune thyroid disease: redefining our understanding of pathogenesis. Nat. Rev. Endocrinol. 2013;9:277–287. doi: 10.1038/nrendo.2013.56. [DOI] [PubMed] [Google Scholar]
- Smith M.J., Packard T.A., O'neill S.K., Henry Dunand C.J., Huang M., Fitzgerald-Miller L., Stowell D., Hinman R.M., Wilson P.C., Gottlieb P.A., Cambier J.C. Loss of anergic B cells in prediabetic and new-onset type 1 diabetic patients. Diabetes. 2015;64:1703–1712. doi: 10.2337/db13-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.J., Rihanek M., Coleman B.M., Gottlieb P.A., Sarapura V.D., Cambier J.C. Activation of thyroid antigen-reactive B cells in recent onset autoimmune thyroid disease patients. J. Autoimmun. 2017;89:82–89. doi: 10.1016/j.jaut.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.J., Ford B.R., Rihanek M., Coleman B.M., Getahun A., Sarapura V.D., Gottlieb P.A., Cambier J.C. Elevated PTEN expression maintains anergy in human B cells and reveals unexpectedly high repertoire autoreactivity. JCI Insight. 2019;4:e123384. doi: 10.1172/jci.insight.123384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensland Z.C., Smith M.J. Enrichment and detection of antigen-binding B cells for mass cytometry. Magnetochemistry. 2021;7:92. doi: 10.3390/magnetochemistry7070092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Lu L., Yang R., Li Y., Shan L., Wang Y. Increased incidence of thyroid disease in patients with celiac disease: a systematic review and meta-analysis. PLoS One. 2016;11:e0168708. doi: 10.1371/journal.pone.0168708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer Y. Mechanisms of autoimmune thyroid diseases: from genetics to epigenetics. Annu. Rev. Pathol. 2014;9:147–156. doi: 10.1146/annurev-pathol-012513-104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstappen G.M., Meiners P.M., Corneth O.B.J., Visser A., Arends S., Abdulahad W.H., Hendriks R.W., Vissink A., Kroese F.G.M., Bootsma H. Attenuation of follicular helper T cell-dependent B cell hyperactivity by abatacept treatment in primary sjogren's syndrome. Arthritis Rheumatol. 2017;69:1850–1861. doi: 10.1002/art.40165. [DOI] [PubMed] [Google Scholar]
- Villadolid J., Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl. Lung Cancer Res. 2015;4:560–575. doi: 10.3978/j.issn.2218-6751.2015.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Wang J., Kumar V., Karnell J.L., Naiman B., Gross P.S., Rahman S., Zerrouki K., Hanna R., Morehouse C., et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet(+) B cells in SLE. Nat. Commun. 2018;9:1758. doi: 10.1038/s41467-018-03750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weetman A.P., Ajjan R.A., Watson P.F. Cytokines and graves' disease. Baillieres Clin. Endocrinol. Metab. 1997;11:481–497. doi: 10.1016/s0950-351x(97)80708-2. [DOI] [PubMed] [Google Scholar]
- Wehr C., Eibel H., Masilamani M., Illges H., Schlesier M., Peter H.H., Warnatz K. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin. Immunol. 2004;113:161–171. doi: 10.1016/j.clim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Wiedeman A.E., Muir V.S., Rosasco M.G., Deberg H.A., Presnell S., Haas B., Dufort M.J., Speake C., Greenbaum C.J., Serti E., et al. Autoreactive CD8+ T cell exhaustion distinguishes subjects with slow type 1 diabetes progression. J. Clin. Invest. 2020;130:480–490. doi: 10.1172/JCI126595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.N., Ye Y.X., Niu J.W., Li Y., Li X., You X., Chen H., Zhao L.D., Zeng X.F., Zhang F.C., et al. Defective PTEN regulation contributes to B cell hyperresponsiveness in systemic lupus erythematosus. Sci. Transl. Med. 2014;6:246ra99. doi: 10.1126/scitranslmed.3009131. [DOI] [PubMed] [Google Scholar]
- Yu S., Maiti P.K., Dyson M., Jain R., Braley-Mullen H. B cell-deficient NOD.H-2h4 mice have CD4+CD25+ T regulatory cells that inhibit the development of spontaneous autoimmune thyroiditis. J. Exp. Med. 2006;203:349–358. doi: 10.1084/jem.20051438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Medling B., Yagita H., Braley-Mullen H. Characteristics of inflammatory cells in spontaneous autoimmune thyroiditis of NOD.H-2h4 mice. J. Autoimmun. 2001;16:37–46. doi: 10.1006/jaut.2000.0458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. AITD patient demographics, HLA haplotype, and available clinical information, related to STAR Methods
Data Availability Statement
Data has been deposited at Mendeley: https://doi.org/10.17632/s85cgbb4r5. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.