Abstract
Antimicrobial resistance (AMR) is widely perceived as a threat to human and animal health and a significant One Health issue with extensive and complex factors contributing to its occurrence and spread. Previous studies have surveyed human and animal health professionals to determine their perceptions regarding AMR and antimicrobial use (AMU). There are limited studies exploring the understanding of veterinary students despite their critical role as future antimicrobial prescribers. A cross-sectional survey was administered to an entire cohort of Doctor of Veterinary Medicine Year 2 (DVM2) students (n = 136) to investigate their knowledge and perceptions regarding AMR and AMU prior to formal education on this issue. Ninety students (66.2% of the cohort) completed the survey. There was overwhelming agreement regarding the immediacy of the problem, with 84.4% of students indicating that ‘We must take action on AMR’. Despite more than 94.4% of students correctly defining AMR, specific knowledge regarding AMR impact, contributory causes to AMR and strategies to solve the challenge of AMR was variable. Most students perceived livestock producers to have a significant role in the perpetuation of AMR due to AMU for prophylaxis (71.1% substantial/moderate contribution) and treatment (56.7% substantial/moderate contribution). Over a third of respondents (37.8%) were unsure if AMR could spread from animals to humans. Respondents perceived that various groups (dentists, doctors, veterinarians, professional organisations) are all important in ameliorating the issue of AMR. The implementation of restrictive measures to reduce veterinary prescription of antimicrobials was viewed as less important than strategies involving education, hygiene, surveillance, and guideline development/availability. To encourage the development of good antimicrobial stewardship (AMS) practices, professional veterinary education needs to foster an understanding of the scientific, behavioural and social issues that contribute to AMR and inappropriate AMU, as well as prescribers' personal contribution to AMR perpetuation and amelioration.
Keywords: Veterinary students, Antimicrobial resistance, Antibiotics, Antibacterials, Antibiotic use, Antimicrobials, Veterinary education, Antimicrobial stewardship
Highlights
-
•
Reports on perceptions of postgraduate veterinary students regarding factors responsible for AMR prior to formal instruction in a DVM program
-
•
Students were unsure about routes of transfer of AMR between humans and animals, and antimicrobial use for routine veterinary procedures
-
•
Students had high agreement that antimicrobial use in livestock contributed to AMR
-
•
Students, like qualified veterinarians, do not like the concept of government restricting their ability to prescribe antimicrobials
1. Introduction
Antimicrobial resistance (AMR) is a significant and expanding global health issue with considerable ramifications for human and animal health [1]. As antimicrobial prescribers, veterinarians are key partners in a One Health approach to AMR as suggested by the World Health Organisation [2]. For veterinarians, AMR is a multifaceted problem given its direct effects on animal treatment outcomes and the potential for zoonotic transfer of resistant microorganisms [3]. Previous research has investigated the practices and perceptions of Australian veterinarians concerning AMR and antimicrobial use (AMU) [[4], [5], [6], [7], [8], [9]].
Understanding the knowledge and perceptions of students who will be future antimicrobial prescribers is equally important for guiding the development of curricula that fosters best practice in antimicrobial stewardship (AMS). There are a limited number of studies exploring the perceptions of veterinary students regarding AMR globally. A study involving Serbian and Croatian veterinary students at various stages of training revealed a lack of cognisance regarding the potential contribution of veterinary medicine to AMR with 42.8% perceiving it had minimal or no contribution [10]. Less than 25% of Nigerian veterinary students at various stages of their training were aware of global efforts to reduce AMR, but greater than 87% exhibited a strong willingness to understand the issue of AMR further [11]. A multicentred investigation of Australian veterinary students' attitudes in the final two years of their degrees, identified a disconnect between preclinical and clinical teaching around AMU [12]. Other studies have surveyed the perceptions of South African veterinary students in pre-final and final years of their veterinary program [13] and veterinary students in their first and final years of their veterinary degree in Bangladesh [14]. These studies concluded that knowledge regarding AMR and AMU increased as students progressed throughout their degree, however, strengthening the alignment between preclinical and clinical teaching was required. These findings highlight the impact of developing evidence-based foundations for prescribing during training and the essential nature of curriculum alignment [[10], [11], [12]].
Similarly, investigations into the knowledge and perceptions of final year Australian medical students regarding infectious diseases versus cardiovascular disease found they lacked confidence and knowledge in the diagnosis and management of infectious diseases compared with cardiovascular disease [15]. Given the range of non-clinical influences on antimicrobial prescribing such as reputation, professional hierarchies, and the pressures to align with clinical practice methods of peers rather than guidelines [16], embedding strong evidence-based foundations for prescribing during professional training is critical.
Australian tertiary programs to train registrable veterinarians have considerable demands on the curriculum to deliver day 1 competencies. Accreditation of these veterinary programs require that students are trained in the importance of antimicrobial stewardship (AMS) and the implications of AMR to animal and human health. However, for teachers to engage and expedite this learning, knowledge of veterinary students' perceptions of AMR issues, prior to formal veterinary training is important in addressing preconceptions. Therefore, the aims of this cross-sectional study were to assess the perceptions of second year veterinary students in the Doctor of Veterinary Medicine (DVM2) at The University of Sydney, prior to their formal training on the impact of AMR and their understanding of the groups/factors responsible for perpetuating and solving the problem. The results of this study will be used to inform the Australian national curriculum on AMR and AMS in veterinary training.
2. Methods
2.1. Study design
A cross-sectional survey was designed to collect information regarding students' perceptions about the impact of AMR and their understanding of the factors and stakeholders responsible for perpetuating and solving the problem. Questions were modified from previous surveys administered to doctors, dentists, and veterinarians [9,17]. The survey for the students was tested prior to administration, using experienced academics and third and fourth year DVM students with their feedback used to refine the final version. The survey used 76 Likert-type responses on a 4- or 5-point scale, with ‘not sure’ options provided. The survey is provided in Supplementary material. The survey was divided into five sections focusing on: ‘antimicrobial resistance and you’; ‘factors influencing the development of antimicrobial resistance’; ‘the impact of antimicrobial resistance’; ‘management of antimicrobial resistance’; and ‘demographic information’. The survey was administered via an online format using REDCap electronic data capture tools https://projectredcap.org/software. The survey was estimated to take 10 to 15 min to complete. This study was approved by the Human Research Ethics Committee of The University of Sydney [Protocol number 2019–1002].
2.2. Recruitment
The survey was administered on February 24th, 2020. A survey link was disseminated to the enrolled students in year 2 of the Doctor of Veterinary Medicine (DVM2) through their university email addresses using a cohort alias. The link was resent on March 2nd, 2020 to remind students to complete the survey. Participation was voluntary and respondents could abandon completing the survey at any stage without penalty.
2.3. Statistical analyses
Statistical analyses were performed using GraphPad Prism® version 8.4.3 (San Diego CA, US). If there was missing data on a single item, the respondent was excluded from the respective analysis. For Likert-type questions, responses were clustered into the following groups: ‘agree / strongly agree’ or ‘disagree / strongly disagree’; ‘substantial / moderate contribution’ or ‘small / no contribution’; ‘moderately / very / extremely important’ or ‘slightly / not important’; and there was also an ‘unsure’ category for all items, as detailed in the result tables. These groups and the ‘unsure’ category for each statement were compared using the Mann-Whitney U test. The Kruskal-Wallis H test was used to compare the mean rank between questions, with Dunn's test used for post-hoc analysis of pairwise comparisons. For all tests, statistical significance was p < 0.05. Uncertainty in the students' response was nominally flagged as ‘high’ when >20% indicated they were ‘unsure’ of their response to the item. The open-ended question was analysed by determining the proportion of respondents providing a response broadly aligning with the WHO statement “antimicrobial resistance happens when microorganisms (such as bacteria, fungi, viruses, and parasites) change when they are exposed to antimicrobial drugs” [2].
3. Results
3.1. Sample population
Ninety out of 136 (66.2%) DVM2 students completed the survey. Most students identified as female (74.4%) consistent with the sample population demographics. Students' median age was 24 years (range 20 to 37 years). All respondents had completed an undergraduate degree before entering the DVM program. Three respondents held a Masters' degree, and none had undertaken a Doctor of Philosophy (PhD) or equivalent. Students intended to work across a variety of interest areas. These included small (53.3%), large (10%) and mixed (48.9%) animal practice, other private practice such as specialisation or exotic species (52.2%) and more broadly in industry (8.9%), diagnostic laboratories (7.8%), government (13.3%) and research/academia (13.3%). Students were able to select multiple checkboxes for this question.
Results have been categorised into the following sections: ‘understanding AMR and its impact’, ‘perceived contributions to AMR’, ‘addressing the problem of AMR’ and ‘strategies for addressing the challenge of AMR’.
3.2. Understanding AMR and its impact
The majority of respondents (78.8%) recognised that the use of antibiotics could select for resistant microbes, which was broadly consistent with the WHO definition of AMR [2]. Conversely, five respondents (5.5%) were incorrect in their assessment that AMR referred to a failure of the patient's own physiological response to antimicrobials. Formal education related to AMR had been undertaken by one-third of respondents in their previous degree and two-thirds of respondents indicated that they wanted further education on the topic, with 37.8% indicating that their understanding was inadequate. Many respondents (67.4%) did not report any personal experience with AMR, while the remaining respondents (32.6%) reported experience with AMR themselves and/or via the medical experience of a family member, friend, or pet.
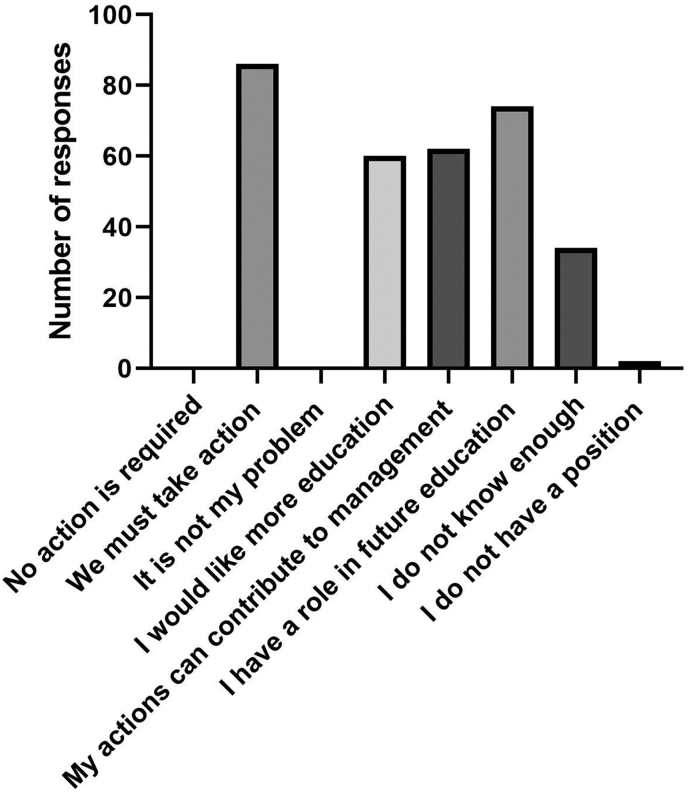
Respondents had various positions on AMR with most agreeing ‘we must take action’ (84.4%) and that their actions could contribute to how AMR is managed (68.9%). None of the respondents believed that ‘no action is required’ (Fig. 1). A small proportion of respondents agreed / strongly agreed [broadly agreed = A], that AMR is overdramatised as a public health problem (A = 4.5%) (Table 1). Respondents overwhelmingly agreed that ‘unnecessary use of antimicrobials leads to reduced future treatment choice’ (A = 91.0%) and that “antimicrobials can affect ‘good’ bacteria that normally live on the skin or in the gut” (A = 92.3%), with a few respondents agreeing that ‘taking antimicrobials has no effect on the bacteria that naturally live in the gut’ (A = 5.6%). While most respondents agreed that the ‘effectiveness of antimicrobials has decreased’ (A = 72.2%), 14.5% disagreed with this statement.
Fig. 1.
Positions of participants regarding antimicrobial resistance and antimicrobial use (respondents could select multiple options).
Table 1.
Students' perceptions on the impact of antimicrobial resistance & antimicrobial use.
| Level of agreement |
Statistical analyses between levels of agreement |
|||||
|---|---|---|---|---|---|---|
| Statement | A (%) | D (%) | U (%) | p (A vs D) | p (A vs U) | p (D vs U) |
| The problem of AMR is overdramatised | 4.5 | 84.3 | 11.2 | <0.0001 | ns | <0.0001 |
| Unnecessary use of antimicrobials leads to reduced future treatment choice | 91.0 | 2.3 | 6.7 | <0.0001 | <0.0001 | ns |
| Antimicrobials can affect ‘good’ bacteria that normally live on the skin and in the gut | 92.3 | 2.1 | 5.6 | <0.0001 | <0.0001 | ns |
| Taking antimicrobials has no effect on the bacteria that naturally live in the gut | 5.6 | 90.0 | 4.4 | <0.0001 | ns | <0.0001 |
| The effectiveness of antimicrobials has decreased | 72.2 | 14.5 | 13.3 | <0.0001 | <0.0001 | ns |
| New antimicrobials are constantly being discovered and developed to keep up with the problem of antimicrobial resistance | 51.2 | 25.5 | 23.3 | 0.0007 | 0.0002 | ns |
| The world is running out of effective antimicrobials | 61.1 | 18.9 | 20 | <0.0001 | <0.0001 | ns |
| Antimicrobial use in one patient may weaken its effectiveness in the same individual in the future | 66.7 | 10.0 | 23.3 | <0.0001 | <0.0001 | 0.03 |
| Antimicrobial use in one patient may weaken its effectiveness for other patients in the future. | 33.3 | 37.8 | 28.9 | ns | ns | ns |
| Antimicrobial resistant bacteria may last a year in a patient after a single use of an antimicrobial | 20 | 7.8 | 72.2 | 0.03 | <0.0001 | <0.0001 |
| Resistance to antimicrobials has spread from human to human | 54.4 | 18.9 | 26.7 | <0.0001 | 0.0002 | ns |
| Resistance to antimicrobials has spread from animals to humans | 44.4 | 17.8 | 37.8 | 0.0002 | ns | 0.0044 |
| Resistance to antimicrobials has spread from humans to animals | 32.2 | 27.7 | 40.0 | ns | ns | ns |
| Emergence of antimicrobial resistance in animals will have a negative effect on human health (1) | 80.1 | 4.3 | 15.6 | <0.0001 | <0.0001 | 0.02 |
| Emergence of antimicrobial resistance in humans will have a negative effect on animal health (2) | 64.1 | 14.6 | 21.3 | <0.0001 | <0.0001 | ns |
A = agree / strongly agree; D = disagree / strongly disagree; U = unsure; ns = not significant. Significance of (1) vs (2) p = 0.04; Bolded signifies that over 20% of respondents were unsure.
There was greater disparity between respondents and high levels of uncertainty (>20%) in response to statements that ‘new antimicrobials are constantly being discovered and developed to keep up with the problem of AMR’ (A = 51.2%; disagree / strongly disagree [D] = 25.6%; unsure [U] = 23.3%); ‘the world is running out of effective antimicrobials’ (A = 61.1%; D = 18.9%; U = 20.0%); or that ‘antimicrobial use in one patient may weaken its effectiveness for other patients in the future’ (A = 33.3%; D = 37.8%; U = 28.9%). Respondents were more likely to agree that ‘antimicrobial use in one patient may weaken its effectiveness in the same individual in the future’ (A = 66.7%; D = 10.0%; U = 23.3%). There was a high level of uncertainty as to whether ‘antimicrobial resistant bacteria could last a year in a patient after a single administration’ (U = 72.2%). Although some respondents agreed that AMR ‘has spread from human to human’ (A = 54.4%), ‘from animals to humans’ (A = 44.4%), and ‘from humans to animals’ (A = 32.2%) there were high levels of uncertainty (U = 26.7%, 37.8% and 40%, respectively) and disagreement (18.9%, 17.8% and 27.7%, respectively) by students regarding these. Students were more certain that the ‘emergence of AMR in animals will have a negative effect on human health’ (A = 80.1%) than the ‘emergence of AMR in humans will have a negative effect on animal health’ (A = 64.1%) (p = 0.004), although 21.3% were unsure of the latter statement.
3.3. Perceived contributors to AMR
3.3.1. Stakeholder groups implicated in contributing to AMR
Students indicated that professional stakeholder groups that make a substantial / moderate contribution (C) to the issue of AMR include ‘doctors prescribing antimicrobials’ (C = 73.3%), ‘veterinarians prescribing antimicrobials’ (C = 60.0%), and ‘livestock producers’, the latter through use in treatment (C = 71.1%) and metaphylaxis (C = 56.7%) (Table 2). Students attributed a significant contribution to ‘transmission of antimicrobial resistance in human hospitals’ (C = 53.9%) and to a lesser extent, animal hospitals (C = 48.9%). However, there was no statistically significant difference between the perceived contribution of human versus animal hospitals to AMR.
Table 2.
Students' perceptions of stakeholder groups or factors contributing to antimicrobial resistance.
| Level of perceived contribution |
Statistical analyses between levels of perceived contribution |
|||||
|---|---|---|---|---|---|---|
| Groups / factors | C (%) | N (%) | U (%) | p (C vs N) | p (C vs U) | p (N vs U) |
| Doctors prescribing antimicrobials | 73.3 | 26.7 | 0 | <0.0001 | <0.0001 | <0.0001 |
| Dentists prescribing antimicrobials | 42.2 | 50.0 | 7.8 | ns | <0.0001 | <0.0001 |
| Veterinarians prescribing antimicrobials | 60.0 | 40.0 | 0 | 0.011 | <0.0001 | <0.0001 |
| Pharmaceutical representatives marketing antimicrobials | 38.9 | 53.3 | 7.8 | 0.011 | <0.0001 | <0.0001 |
| Use of antimicrobials by livestock producers to prevent disease | 71.1 | 22.2 | 6.7 | <0.0001 | <0.0001 | 0.0051 |
| Use of antimicrobials by livestock producers to treat disease | 56.7 | 32.2 | 11.1 | 0.0016 | <0.0001 | 0.001 |
| Transmission of AMR in human hospitals (1) | 53.9 | 34.9 | 11.2 | 0.016 | <0.0001 | 0.0003 |
| Transmission of AMR in animal hospitals (2) | 48.9 | 38.9 | 12.2 | ns | <0.0001 | <0.0001 |
| Using an antimicrobial when benefit to the patient is uncertain | 84.1 | 13.6 | 2.3 | <0.0001 | <0.0001 | 0.0097 |
| Unnecessary use of broad-spectrum antimicrobials | 83.2 | 13.4 | 3.4 | <0.0001 | <0.0001 | 0.028 |
| Patients (human and animal) not finishing their prescribed course of antimicrobials | 82.1 | 16.9 | 1.1 | <0.0001 | <0.0001 | 0.0003 |
| Patients (human and animal) using antimicrobials from previously unfinished prescriptions | 77.8 | 20.0 | 2.2 | <0.0001 | <0.0001 | 0.0002 |
| Human patients requesting antimicrobials | 71.1 | 25.6 | 3.3 | <0.0001 | <0.0001 | <0.0001 |
| Owners of animals requesting antimicrobials | 67.4 | 29.2 | 3.4 | <0.0001 | <0.0001 | <0.0001 |
| Use of over-the-counter antimicrobials in humans | 64.5 | 24.4 | 11.1 | <0.0001 | <0.0001 | 0.031 |
| Use of over-the-counter antimicrobials in animals | 58.6 | 31.3 | 10.1 | 0.0003 | <0.0001 | 0.0007 |
| Long durations of antimicrobial treatment | 57.7 | 31.2 | 11.1 | 0.0005 | <0.0001 | 0.0017 |
| Too low a dose of antimicrobials used in treatment | 51.1 | 37.8 | 11.1 | ns | <0.0001 | <0.0001 |
| Slow development of new antimicrobials | 27.8 | 60.0 | 12.2 | <0.0001 | 0.015 | <0.0001 |
| Antimicrobials are required for routine desexing of companion animals | 48.9 | 26.7 | 24.4 | 0.0033 | 0.0011 | ns |
| Antimicrobials are required for routine dental procedures in companion animals | 48.9 | 30.0 | 21.1 | 0.014 | 0.0002 | ns |
| Antimicrobials are required for surgeries to fix a broken bone in companion animals | 56.7 | 11.1 | 32.2 | <0.0001 | 0.0016 | 0.001 |
C = substantial / moderate contribution; N = small / no contribution; U = unsure; ns = not significant. There was no significant difference between (1) versus (2). Bolded signifies that over 20% of respondents are unsure.
3.3.2. Factors contributing to AMR
Table 2 documents that students believed that the following make a substantial / moderate contribution to AMR: ‘using an antimicrobial when the benefit to the patient is uncertain’ (C = 84.1%), ‘unnecessary use of broad-spectrum antimicrobials’ (C = 83.2%), and ‘patients (human and animal) using antimicrobials from previously unfinished prescriptions’ (C = 77.8%). Students believed that ‘human patients’ and ‘owners of animals requesting antimicrobials’ from the respective health-care provider contributed to AMR (C = 71.1% and 67.4%, respectively).
Students perceived that the following also resulted in a substantial / moderate contribution (C) to AMR: ‘use of over-the-counter antimicrobials in humans’ (C = 64.5%) and ‘in animals’ (C = 58.6%); ‘long durations of antimicrobial treatment’ (C = 57.7%); and ‘too low a dose of antimicrobials used in treatment’ (C = 51.1%). A high proportion of respondents perceived that patients ‘not finishing their prescribed course of antimicrobials’ (C = 82.1%) made a considerable contribution to AMR. Students believed that the ‘slow development of new antimicrobials’ has a small / no contribution (N) to AMR (N = 60%).
There was moderate agreement by students regarding the need for antimicrobials for ‘routine desexing of companion animals’ (C = 48.9%) or ‘routine dental procedures in companion animals’ (C = 48.9%) and ‘surgeries to fix a broken bone in companion animals’ (C = 56.7%). High levels of uncertainty were recorded for each question (24.4%, 21.1%, 32.2% respectively).
3.4. Addressing the problem of AMR
3.4.1. Stakeholders responsible for addressing the problem of AMR
Table 3 demonstrates that students perceived a moderately/very/extremely important role [I] in addressing AMR for most stakeholders including prescribers (such as doctors, veterinarians, and dentists), influential organisations and other groups such as farmers/producers and scientists/microbiologists. Two groups were perceived as having a moderately/very/extremely important role by greater than 80% of respondents: family and friends (I = 80%) and the media (I = 88.9%). Table 3 also documents that students believed human hospitals (I = 100%) and veterinary hospitals (I = 100%) have an important role in addressing the issue of AMR.
Table 3.
Students' perceptions on importance of different stakeholders in addressing antimicrobial resistance.
| Level of perceived importance |
Statistical analyses between levels of perceived contribution |
|||||
|---|---|---|---|---|---|---|
| Stakeholders | I (%) | S (%) | U (%) | p (I vs S) | p (I vs U) | p (S vs U) |
| Doctors | 100 | 0 | 0 | <0.0001 | <0.0001 | ns |
| Veterinarians | 100 | 0 | 0 | <0.0001 | <0.0001 | ns |
| Dentists | 97.8 | 2.2 | 0 | <0.0001 | <0.0001 | ns |
| Professional associations (e.g., Australian Veterinary Association) |
100 | 0 | 0 | <0.0001 | <0.0001 | ns |
| Animal industry organisations (e.g., Meat and Livestock Australia) | 100 | 0 | 0 | <0.0001 | <0.0001 | ns |
| Global organisations (e.g., World Health Organisation, World Organisation for Animal Health) | 100 | 0 | 0 | <0.0001 | <0.0001 | ns |
| Human hospitals | 100 | 0 | 0 | <0.0001 | <0.0001 | ns |
| Veterinary hospitals | 100 | 0 | 0 | <0.0001 | <0.0001 | ns |
| Farmers and producers | 98.9 | 1.1 | 0 | <0.0001 | <0.0001 | ns |
| Government and policy makers | 98.9 | 1.1 | 0 | <0.0001 | <0.0001 | ns |
| Scientists and microbiologists | 98.9 | 1.1 | 0 | <0.0001 | <0.0001 | ns |
| Other veterinary students | 98.9 | 1.1 | 0 | <0.0001 | <0.0001 | ns |
| Pharmaceutical companies | 97.8 | 2.2 | 0 | <0.0001 | <0.0001 | ns |
| Pharmacists | 96.7 | 3.3 | 0 | <0.0001 | <0.0001 | ns |
| Myself | 95.6 | 4.4 | 0 | <0.0001 | <0.0001 | ns |
| Nurses | 94.5 | 5.5 | 0 | <0.0001 | <0.0001 | ns |
| The community and general public | 91.1 | 8.9 | 0 | <0.0001 | <0.0001 | 0.0066 |
| Pet owners | 90.0 | 10.0 | 0 | <0.0001 | <0.0001 | 0.0032 |
| The media | 88.9 | 11.1 | 0 | <0.0001 | <0.0001 | 0.0015 |
| Family and friends | 80.0 | 20.0 | 0 | <0.0001 | <0.0001 | <0.0001 |
I = moderately / very / extremely important; S = slightly / not important; U = unsure; ns = not significant.
Students perceived their family, friends and pet owners as playing a less important role in addressing AMR relative to veterinarians and doctors (all with p ≤ 0.0001). Students perceived their own responsibility in addressing AMR as very/extremely important (I = 95.6%) and 80% indicated that they have a role in the future education of patients and clients (Fig. 1). More than 90% perceived that the community/general public also had a very/extremely important role (I = 91.1%).
3.5. Strategies for addressing the challenge of AMR
Thirteen strategies regarding education, infection control, diagnostic methods, AMU and guideline development had greater than 90% agreement that they were moderately/very/extremely important (I) in addressing the challenge of AMR (Table 4), with low levels of uncertainty (<5%) or disagreement (<6%).
Table 4.
Students' perceptions on the importance of potential strategies to address antimicrobial resistance.
| Level of perceived importance of potential strategies |
Statistical analyses between levels of perceived importance of potential strategies |
|||||
|---|---|---|---|---|---|---|
| I (%) | S (%) | U (%) | P (I vs S) | P (I v U) | p (S vs U) | |
| Local and national AMR surveillance data | 98.9 | 0 | 1.1 | <0.0001 | <0.0001 | ns |
| AMU data in humans, livestock and companion animals | 96.7 | 2.2 | 1.1 | <0.0001 | <0.0001 | ns |
| Research to examine strategies to combat AMR | 97.8 | 1.1 | 1.1 | <0.0001 | <0.0001 | ns |
| Improving existing guidelines on antimicrobial prescribing with research and evidence | 97.8 | 1.1 | 1.1 | <0.0001 | <0.0001 | ns |
| Education sessions on appropriate antimicrobial prescribing for practitioners | 96.7 | 2.2 | 1.1 | <0.0001 | <0.0001 | ns |
| Education programs to raise awareness in the community and public | 96.7 | 2.2 | 1.1 | <0.0001 | <0.0001 | ns |
| Changing client expectations about antimicrobials | 95.5 | 3.4 | 1.1 | <0.0001 | <0.0001 | ns |
| Better hand hygiene in veterinary and human hospitals | 93.4 | 3.3 | 3.3 | <0.0001 | <0.0001 | ns |
| More effective cleaning in human and veterinary hospitals | 92.1 | 4.5 | 3.4 | <0.0001 | <0.0001 | ns |
| Improving diagnostic methods | 91.2 | 4.4 | 4.4 | <0.0001 | <0.0001 | ns |
| Better availability of local and national guidelines and protocols | 93.3 | 5.6 | 1.1 | <0.0001 | <0.0001 | ns |
| Development of new antimicrobials | 93.4 | 4.4 | 2.2 | <0.0001 | <0.0001 | ns |
| Fewer antimicrobial prescriptions | 92.3 | 4.4 | 3.3 | <0.0001 | <0.0001 | ns |
| Reducing or restricting use of antimicrobials in livestock feed | 87.8 | 3.3 | 8.9 | <0.0001 | <0.0001 | ns |
| Using alternative treatments to antimicrobials (e.g. probiotics) | 87.7 | 6.7 | 5.6 | <0.0001 | <0.0001 | ns |
| Prescribing narrowest spectrum antimicrobials | 82.2 | 5.6 | 12.2 | <0.0001 | <0.0001 | ns |
| Restricting veterinary use of antimicrobials considered to be of critical importance in human health | 58.9 | 22.2 | 18.9 | <0.0001 | <0.0001 | ns |
I = moderately / very / extremely important; S = slightly / not important; U = unsure; ns = not significant.
‘Restricting veterinary use of antimicrobials of critical importance in human health’ was considered moderately/very/extremely important (I) in addressing the challenge of AMR by only 58.9% of respondents, with 22.2% disagreeing and 18.9% uncertain.
4. Discussion
Students overwhelmingly agreed that AMR is a major problem and that they have a current (myself I = 95.6, Table 3) and future professional role (veterinarians I = 100%; Table 3) in helping to ameliorate the problem. Such responses aligned with students strongly agreeing that action must be taken to control AMR (A = 84.4%) and no student checked no action should be taken or it is not my problem (Fig. 1). It is encouraging that our students demonstrated significant engagement with the issue prior to educational strategies that seek to foster better AMS [12]. The feasibility of integrating AMS into the behaviour of these surveyed future practitioners is favourable given that 37.8% indicated that their understanding was inadequate, and 66.7% were seeking further education on the topic. Consequently, their responses provide opportunities to identify gaps in their knowledge and address such issues during the DVM program to ultimately graduate competent veterinarians that will contribute to One Health success.
4.1. Students' experience with AMR
Approximately one third of students (32.6%) reported experience with AMR themselves and/or via the medical experience of a family member, friend, or pet. Compared with European countries, where the incidence of AMR infections in people range from 677 to 1188 per million, for <100, or > 200 persons/km2, respectively [18], the specific incidence of AMR cases in people or animals in Australia are not easily accessed. However, rates of resistance in key gram-positive pathogens are classified as moderate to high in people in Australia, Escherichia coli and Klebsiella pneumoniae AMR incidences are relatively low compared to European countries, and resistance rates to fluoroquinolones are increasing [19]. Consequently, it is problematic to make associations between students' AMR experiences with the incidence of AMR infection rates in Australia, or any locality in which they have resided.
4.2. Items with > 20% uncertainty
It was encouraging that most students had a broad, but reasonably accurate understanding of the WHO definition of AMR. Internationally, the general public has been shown to have a similar level of awareness around the definition despite a lack of understanding of key concepts, such as the ineffectiveness of antimicrobials for treating viral infections [20]. However, five students defined AMR as a failure of the patient's own physiological response to antimicrobials. Additionally, the issues for which the students were most unsure involved perceptions on the impact of AMR and AMU, and perceptions on stakeholder groups or factors contributing to AMR. For example, there was uncertainty whether antimicrobial use in one patient may weaken its effectiveness in the same individual in the future (U = 23.3%), as well as strong agreement with this statement (A = 66.7%), (Table 1). However, not only were students uncertain, but there were no significant differences in responses between all three categories [A 33.3%; D 37.8%; U 28.9%] for antimicrobial use in one patient may weaken its effectiveness in the same individual in the future. In contrast, qualified veterinarians had a median ‘agree’ score for both scenarios in a previous survey [17]. It is likely that students did not understand the implications of AMR transmission given their lack of formal education around AMR or experience with these issues in a clinical setting. As future prescribers, veterinary students must be made aware of the potentially extensive impact of antimicrobial administration on other animals, humans and the environment [21]. Additionally, 72.2% were unsure that antimicrobial resistant bacteria may last a year in a patient after a single use of an antimicrobial (Table 1). This level of uncertainty identifies a substantial knowledge gap in predisposing factors and the mechanisms by which antimicrobial resistance occurs in pathogens, both in-vitro and in-vivo.
Over 20% were unsure about the following statements that new antibacterials are constantly being discovered and developed to keep up with the problem of antimicrobial resistance (U = 23.3%) (Table 1). More concerningly, agreement with this item was high at 51.2%. Furthermore, students believed that slow development of new antimicrobials has a small / no contribution to AMR (N = 60.0%, Table 2). Development of new antibacterial drugs has not kept pace with the speed at which some bacteria have developed resistance [22]. Therefore, implementation of AMS for maintaining current antimicrobial therapeutic efficacy and avoiding selection pressures, is vital.
There was uncertainty around the risk of AMR transfer between humans and animals (Table 1). There is clear evidence for the presence of resistant bacteria within companion animals [23,24] and sharing between humans and animal populations [[24], [25], [26], [27], [28]]. Veterinary education programs should highlight the potential for interspecies spread and highlight the need for AMS in veterinary practice to mitigate AMR consequences not just in animal patients but also in the veterinary health professionals, animal owners and in wildlife [24,26].
There was uncertainty or misconceptions regarding the need for antimicrobials in a range of routine veterinary clinical procedures. Although half of the students agreed (46.9%) that antimicrobials were required for routine desexing of companion animals, 24.4% were uncertain (Table 2). Most veterinarians do not use antimicrobials in this circumstance due to current recommendations indicating that prophylaxis is not required [8,29]. There was also uncertainty, or misconception regarding antimicrobials required for routine dental procedures (U = 32.2%; A = 48.9%) and for surgical fixation of a broken bone in companion animals (U = 32.2%; Table 2). Current recommendations are that antimicrobials are required for prophylaxis fracture repair surgeries [30] but rarely recommended for routine dental procedures [31]. Students were not expected to know the specific indications for antimicrobials given their current stage of education, although these perceptions highlight the importance of preclinical education to address such misconceptions.
A sizable proportion of students (37.8%) responded that that too low a dose of antimicrobials used in treatment had a small or no contribution to AMR, and 11.1% were unsure (Table 2). Low dosage of antibiotics resulting in sub-therapeutic concentrations at the site of infection, especially when antibiotic concentration is within the mutant selection window range for the antibiotic vs pathogen, is one of the greatest selection forces for AMR [32]. Students were also divided as to whether transmission of antimicrobial resistance in animal hospitals is a substantial / moderate contribution or had a small / no contribution to AMR (C = 48.9%; N = 38.9%, respectively; Table 2). It is well recognised that transmission of AMR occurs in human hospitals [33,34], but there are fewer studies on AMR in veterinary hospitals. Recent studies document that people working in veterinary hospitals are a high-risk carriage of multidrug resistant (MDR) and extended spectrum beta-lactamase (ESBL) producing bacteria [24,35,36]. It is encouraging that all students perceived that AMS in both human and veterinary hospitals were important in addressing AMR (Table 3).
4.3. Additional misconceptions
The use of over-the-counter antimicrobials in humans (I = 64.5%) and animals (I = 58.6%) (Table 2) were incorrectly identified as a driver of AMR since Australian prescribing legislation in human and veterinary medicine significantly limits the purchasing of antimicrobials, particularly conventional antibacterials, without a prescription [37]. This perception may be attributable to concerns about the contribution of over the counter antimicrobials to AMR in other countries [38]. It is important for veterinary students to engage with prescribing legislation within their future area of practice. Awareness of the Australian legislative framework around antimicrobial use in veterinary education is a critical element in preventing sub-optimal use and the development of AMR.
4.4. Other issues
Students perceived both doctors prescribing antimicrobials (C = 73.3%) and veterinarians prescribing antimicrobials (C = 42.2%) as having a similar contribution towards exacerbating AMR (Table 2). Amongst prescriber groups in Australia, a lack of agreement exists regarding the relative contributions of each profession to the problem [17]. Veterinarians generally perceive the role of animals as minimal while emphasising the importance of human medicine in perpetuating AMR, perhaps because most Australian veterinarians infrequently encounter multi-drug resistant pathogens currently [39]. AMR requires a One Health approach for effective amelioration. The importance of One Health is emphasised throughout the DVM, from the first week of the degree's first year [40]. Acceptance of the concept is evident amongst veterinary students and should be encouraged to promote communication between prescriber groups and prevent transferring responsibility for AMS to another prescribing group, other than their own prescribing group, as noted by Zhuo et al., (2018) [17].
In line with the understanding of other Australian veterinary students, veterinarians, dentists and doctors [9,12,17], our students had high agreement that use of antimicrobials by livestock producers to prevent disease (C = 71.1%) and to treat disease (C = 56.7%) (Table 2) contributed to AMR (p < 0.0001 and p = 0.0016, respectively). They perceived farmers and producers are of high importance in addressing AMR (I = 98.9%, Table 3) and they perceived reducing or restricting use of antimicrobials in livestock feed was also important (I = 87.8%, Table 4). This was the same perception as South African final year veterinary students when surveyed on this issue [13]. Other studies report that many veterinarians (especially livestock practitioners) do not share these perceptions [9,41,42]. The contribution of AMU in agriculture to AMR broadly is poorly substantiated and differs geographically [43]. This is particularly true within the Australian context, where livestock veterinarians are restricted to using a limited range of antimicrobials of generally low importance to human health [7,9] and therefore it is important to dispel exaggerated attribution of blame towards livestock producers.
Most students recognised that the community and general public were important (I = 91.1%) in helping to minimise/address AMR (Table 3). Pet owners and family and friends were also thought to have an important involvement (90.0 and 80.0%, respectively). An effective approach to AMR recognises the role of the community and public, given a greater societal understanding around AMR leads to more appropriate attitudes towards AMU. Members of the public that exhibit a great understanding of antimicrobials also report behaviours that may help to prevent the development of AMR [44]. It is hoped that this perception will encourage respondents to engage with client education around AMR, allowing a collaborative approach to AMS.
It was also encouraging that our students generally were well informed about the following items contributing to AMR and listed in Table 2: using an antimicrobial when the benefit to the patient is uncertain’ (C = 84.1%), unnecessary use of broad-spectrum antimicrobials (C = 83.2%) and patients (human and animal) using antimicrobials from previously unfinished prescriptions (C = 77.8%). These results suggest that these future veterinarians will readily employ AMS principles of antibacterial prescribing. The students were fundamentally correct in that long durations of antimicrobial treatment contribute to AMR (C = 57.7%, Table 2). However, for severe or deep tissue infections such as prostatitis, osteomyelitis, deep pyodermas in dogs, and Rhodococcus equi pneumonia in foals, a long duration of antibiotic treatment is warranted [32] along with patient monitoring to detect the clinical response when antibacterial administration can be terminated.
Client expectations for antimicrobials, such as requesting antimicrobials in the human and veterinary health spheres were perceived by students as an important factor contributing to AMR C = 71.1% and 67.4%, respectively (Table 2). Students also indicated that education programs to raise awareness in the community and public (I = 96.7%, Table 4), would help to ameliorate this factor. Zhou et al. 2018 [17] reported veterinarians and other prescribers attributed little significance to client expectations while another study reported that client expectation and pressure can be a factor influencing prescribing within veterinary practice [39] or that misalignments between client and veterinary expectations exist. This may act as a barrier to appropriate administration if the prescriber cannot recognise these as expectations only and eliminate them from the decision-making process.
The value of interventionist strategies of restricting veterinary use of antimicrobials received the lowest level of agreement as a potential strategy to address AMR (I = 58.9, Table 4). Qualified veterinarians, doctors and dentists have also been shown to perceive strategies to restrict their ability to prescribe as not helpful [9,17]. This represents a challenge to the acceptance of new measures around AMS given veterinary students also appear to disagree with the potential loss of autonomy created by restricted prescribing privileges. Intervention into prescribing practices has been adopted worldwide given evidence that this strategy significantly improves AMS [45]. It may be necessary to incorporate educational strategies to foster acceptance of these potential changes to prescribing policies. However, significant importance was placed on availability of Local and national AMR surveillance data (I = 98.9%, Table 4), improving existing guidelines on antimicrobial prescribing with research and evidence, (I = 97.8%), availability of local and national guidelines and protocols (I = 93.3%), Education sessions on appropriate antimicrobial prescribing for practitioners (I = 96.7%); Education programs to raise awareness in the community and public (I = 96.7%) and better hand hygiene in veterinary and human hospitals (I = 92.1%), as important strategies to address AMR (Table 4).
A limitation of the study was that it did not investigate the specific nature of prior AMR education reported by one third of respondents. If collected, this may have been used to account for differences in perceptions and understanding around AMR. Future studies should investigate the link between education prior to the DVM and the development of perceptions around AMR that could influence AMS and AMU.
5. Conclusions
Veterinary students demonstrated significant understanding of the importance and immediacy of AMR prior to formal veterinary education on AMR issues. The participants had adequate preliminary knowledge of AMU/AMR principles aligning with public perceptions, but a deeper understanding of the problem must be developed through education. The role of livestock in the perpetuation and amelioration of AMR was overstated amongst veterinary students. The idea of control through a multidisciplinary approach was highlighted, with veterinarians perceived as having an equal role to dentists, doctors and professional organisations. Veterinary students were resistant to the implementation of measures restricting the prescribing abilities of veterinarians. It is suggested that collecting baseline information of the AMR knowledge base of incoming students is important for delivering learning tasks that will extend student knowledge and correct their misconceptions. It is the authors' intention to quantify the impact of our educational strategies concerning AMR by surveying students before and after relevant DVM learning activities.
Author contributions
JMN, DD-H and MG conceptualized the study. JM, JMN and MG collected data. JM and MG analysed the data. JM drafted the manuscript. The manuscript was contributed to, and edited by JM, JMN, DD-H and MG.
Funding
This research was funded by the Sydney School of Veterinary Science Research & Enquiry Unit of Study 2020 fund.
Author statement
Jacqueline Norris (JMN), Dale Dominey-Howes (DD-H) and Merran Govendir (MG) conceptualized the study. Josh McClelland (JM), JMN and MG collected data. JM and MG analysed the data. JM drafted the manuscript. The manuscript was contributed to, and edited by JM, JMN, DD-H and MG.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgements
This work was completed in partial fulfillment for the requirements of the Doctor of Veterinary Medicine degree, The University of Sydney (student JM). The authors thank The University of Sydney Year 2 DVM students 2020 (graduating class of 2022) for their involvement in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2021.100366.
Contributor Information
Josh W. McClelland, Email: jmcc6036@uni.sydney.edu.au.
Jacqueline M. Norris, Email: jacqui.norris@sydney.edu.au.
Dale Dominey-Howes, Email: dale.dominey-howes@sydney.edu.au.
Merran Govendir, Email: merran.govendir@sydney.edu.au.
Appendix A. Supplementary data
Survey administered to the Year 2 DVM students
References
- 1.Australian Government Responding to the Threat of Antimicrobial Resistance - Australia's First National Antimicrobial Resistance Strategy 2015–2019. 2015. http://www.health.gov.au/internet/main/publishing.nsf/Content/1803C433C71415CACA257C8400121B1F/%24File/amr-strategy-2015-2019.pdf
- 2.WHO World Health Organisation, Global Action Plan on Antimicrobial Resistance. 2015. https://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/ Accessed 20 November, 2020.
- 3.Gibbons J.F., Boland F., Buckley J.F., Butler F., Egan J., Fanning S., Markey B.K., Leonard F.C. Influences on antimicrobial prescribing behaviour of veterinary practitioners in cattle practice in Ireland. Vet. Rec. 2013;172:14–18. doi: 10.1136/vr.100782. [DOI] [PubMed] [Google Scholar]
- 4.Crabb H.K., Hardefeldt L.Y., Bailey K.E., Billman-Jacobe H., Gilkerson J.R., Browning G.F. Survey of veterinary prescribing for poultry disease. Aus. Vet. J. 2019;97:288. doi: 10.1111/avj.12812. [DOI] [PubMed] [Google Scholar]
- 5.Hardefeldt L.Y., Browning G.F., Thursky K., Gilkerson J.R., Billman-Jacobe H., Stevenson M.A., Bailey K.E. Antimicrobials used for surgical prophylaxis by companion animal veterinarians in Australia. Vet. Microbiol. 2017;203:301–307. doi: 10.1016/j.vetmic.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Hardefeldt L.Y., Browning G.F., Thursky K., Gilkerson J.R., Billman-Jacobe H., Stevenson M.A., Bailey K.E. Antimicrobials used for surgical prophylaxis by equine veterinary practitioners in Australia. Equine Vet. J. 2018 doi: 10.1111/evj.12709. [DOI] [PubMed] [Google Scholar]
- 7.Hardefeldt L.Y., Browning G.F., Thursky K.A., Gilkerson J.R., Billman-Jacobe H., Stevenson M.A., Bailey K.E. Cross-sectional study of antimicrobials used for surgical prophylaxis by bovine veterinary practitioners in Australia. Vet. Rec. 2017;181:6. doi: 10.1136/vr.104375. [DOI] [PubMed] [Google Scholar]
- 8.Hardefeldt L.Y., Holloway S., Trott D.J., Shipstone M., Barrs V.R., Malik R., Burrows M., Armstrong S., Browning G.F., Stevenson M. Antimicrobial prescribing in dogs and cats in Australia: results of the Australasian infectious disease advisory panel survey. J. Vet. Intern. Med. 2017;31:1100–1107. doi: 10.1111/jvim.14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norris J.M., Zhuo A.N., Govendir M., Rowbotham S.J., Labbate M., Degeling C., Gilbert G.L., Dominey-Howes D., Ward M.P. Factors influencing the behaviour and perceptions of Australian veterinarians towards antibiotic use and antimicrobial resistance (vol 14, e0223534, 2019) PLoS One. 2019;14 doi: 10.1371/journal.pone.0224844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovacevic Z., Blagojevic B., Suran J., Horvat O. Mapping knowledge and comprehension of antimicrobial stewardship and biosecurity among veterinary students. PLoS One. 2020;15:0235866. doi: 10.1371/journal.pone.0235866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odetokun I.A., Akpabio U., Alhaji N.B., Biobaku K.T., Oloso N.O., Ghali-Mohammed I., Biobaku A.J., Adetunji V.O., Fasina F.O. Knowledge of antimicrobial resistance among veterinary students and their personal antibiotic use practices: a national cross-sectional survey. Antibiotics. 2019;8 doi: 10.3390/antibiotics8040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardefeldt L., Nielsen T., Crabb H., Gilkerson J., Squires R., Heller J., Sharp C., Cobbold R., Norris J., Browning G. Veterinary students' knowledge and perceptions about antimicrobial stewardship and biosecurity-a national survey. Antibiotics. 2018;7 doi: 10.3390/antibiotics7020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith P.W., Agbaje M., LeRoux-Pullen L., Van Dyk D., Debusho L.K., Shittu A., Sirdar M.M., Fasanmi O.G., Adebowale O., Fasina F.O. Implication of the knowledge and perceptions of veterinary students of antimicrobial resistance for future prescription of antimicrobials in animal health, South Africa. J. S. Afr. Vet. Assoc. 2019;90:1–8. doi: 10.4102/jsava.v90i0.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapot L., Sarker M.S., Begum R., Hossain D., Akter R., Hasan M.M., Bupasha Z.B., Bayzid M., Salauddin M., Parvej M.S. Knowledge, attitudes and practices regarding antibiotic use and resistance among veterinary students in Bangladesh. Antibiotics. 2021;10:332. doi: 10.3390/antibiotics10030332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weier N., Thursky K., Z. S.T.R Antimicrobial knowledge and confidence amongst final year medical students in Australia. PLoS One. 2017;12:0182460. doi: 10.1371/journal.pone.0182460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broom A., Broom J., Kirby E. Cultures of resistance? A Bourdieusian analysis of doctors' antibiotic prescribing. Soc. Sci. Med. 2014;110:81–88. doi: 10.1016/j.socscimed.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 17.Zhuo A., Labbate M., Norris J.M., Gilbert G., Ward M., Bajorek B., Degeling C., Rowbotham S., Dawson A., Nguyen K., Hill-Cawthorne G., Sorrell T., Govendir M., Kesson A., Iredell J., Dominey-Howes D. Comparing prescriber groups’ knowledge, perceptions, and behaviours highlights opportunities and challenges to taking a one health approach to antimicrobial resistance. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collignon P., Beggs J.J. CON: COVID-19 will not result in increased antimicrobial resistance prevalence. JAC-AMR. 2020;2 doi: 10.1093/jacamr/dlaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AURA Third Australian Report on Antimicrobial Use and Resistance in Human Health. 2019. 2019. www.safetyandquality.gov.au/antimicrobial-use-and-resistance-in-australia/aura-2019/ (accessed 24 Jun 2021)
- 20.Gu J., Zhao J., Huang Y., Yang W., Ren Z., Li W., Fan Y., Zhang Q., Zhang F., Fu Y. Use of antibiotics by urban and rural residents in Heilongjiang Province, China: cross-sectional study. Tropical Med. Int. Health. 2015;20:7. doi: 10.1111/tmi.12602. [DOI] [PubMed] [Google Scholar]
- 21.Palma E., Tilocca B., Roncada P. Antimicrobial resistance in veterinary medicine: a overview. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21061914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giguère S. In: Antimicrobial Therapy in Veterinary Medicine. Giguère S., Prescott J., Dowling P., editors. John Wiley & Sons; Ames, Iowa: 2013. Antimicrobial drug action and interaction: An introduction; pp. 3–10. [Google Scholar]
- 23.Saputra S., Jordan D., Mitchell T., Wong H.S., Abraham R.J., Kidsley A., Turnidge J., Trott D.J., Abraham S. Antimicrobial resistance in clinical Escherichia coli isolated from companion animals in Australia. Vet. Microbiol. 2017;211:43–50. doi: 10.1016/j.vetmic.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Worthing K.A., Abraham S., Pang S., Coombs G.W., Saputra S., Jordan D., Wong H.S., Abraham R.J., Trott D.J., Norris J.M. Molecular characterization of methicillin-resistant Staphylococcus aureus isolated from Australian animals and veterinarians. Microb. Drug Resist. 2018;24:203–212. doi: 10.1089/mdr.2017.0032. [DOI] [PubMed] [Google Scholar]
- 25.Weese J.S., Giguere S., Guardabassi L., Morley P.S., Papich M., Ricciuto D.R., Sykes J.E. ACVIM consensus statement on therapeutic antimicrobial use in animals and antimicrobial resistance. J. Vet. Intern. Med. 2015;29:487–498. doi: 10.1111/jvim.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukerji S., Stegger M., Truswell A.V., Laird T., Jordan D., Abraham R.J., Harb A., Barton M., O’Dea M., Abraham S. Resistance to critically important antimicrobials in Australian silver gulls (Chroicocephalus novaehollandiae) and evidence of anthropogenic origins. J. Antimicrob. Chemother. 2019;74:2566–2574. doi: 10.1093/jac/dkz242. [DOI] [PubMed] [Google Scholar]
- 27.Sahibzada S., Pang S., Hernández-Jover M., Jordan D., Abraham S., O'Dea M., Heller J. Prevalence and antimicrobial resistance of MRSA across different pig age groups in an intensive pig production system in Australia. Zoonoses Public Hlth. 2020;67:576–586. doi: 10.1111/zph.12721. [DOI] [PubMed] [Google Scholar]
- 28.Abraham S., Trott D.J., Jordan D., Gordon D.M., Groves M.D., Fairbrother J.M., Smith M.G., Zhang R., Chapman T.A. Phylogenetic and molecular insights into the evolution of multidrug-resistant porcine enterotoxigenic Escherichia coli in Australia. Int. J. Antimicrob. Agents. 2014;44:105–111. doi: 10.1016/j.ijantimicag.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Holloway S., Trott D.J., Shipstone M., Barrs V.R., Malik R., Burrows M. Australasian Infectious Diseases Advisory Panel, Antibiotic prescribing detailed guidelines. 2013. https://animalmedicinesaustralia.org.au/wp-content/uploads/2019/11/AIDAP-Australian-Infectious-Diseases-Advisory-Panel-and-Antibiotic-Prescribing-Detailed-Guidelines-.pdf (accessed 12 August 2020) [DOI] [PMC free article] [PubMed]
- 30.Gans I., Jain A., Sirisreetreerux N., Haut E.R., Hasenboehler E.A. Current practice of antibiotic prophylaxis for surgical fixation of closed long bone fractures: a survey of 297 members of the Orthopaedic trauma association. Patient Saf. Surg. 2017;11:1–6. doi: 10.1186/s13037-016-0118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellows J., Dennis S., Snyder C.J. AAHA dental care guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 2019;55:49–56. doi: 10.5326/JAAHA-MS-6933. [DOI] [PubMed] [Google Scholar]
- 32.Baggot J., Giguère S. In: Antimicrobial Therapy in Veterinary Medicine John Wiley & Sons. Giguère S., Prescott J., Dowling P., editors. Ames; Iowa: 2013. Principles of antimicrobial drug bioavailability and disposition; pp. 41–77. [Google Scholar]
- 33.Amann S., Neef K., Kohl S. Antimicrobial resistance (AMR) Eur. J. Hosp. Pharm. 2019;26:175–177. doi: 10.1136/ejhpharm-2018-001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Accolti M., Soffritti I., Mazzacane S., Caselli E. Fighting AMR in the healthcare environment: microbiome-based sanitation approaches and monitoring tools. Int. J. Mol. Sci. 2019;20:1535–1548. doi: 10.3390/ijms20071535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Royden A., Ormandy E., Pinchbeck G., Pascoe B., Hitchings M.D., Sheppard S.K., Williams N.J. Prevalence of faecal carriage of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli in veterinary hospital staff and students. Vet. Rec. Open. 2019;6 doi: 10.1136/vetreco-2018-000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jordan D., Simon J., Fury S., Moss S., Giffard P., Maiwald M., Southwell P., Barton M.D., Axon J.E., Morris S.G., Trott D.J. Carriage of methicillin-resistant Staphylococcus aureus by veterinarians in Australia. Aus. Vet. J. 2011;89:152–159. doi: 10.1111/j.1751-0813.2011.00710.x. [DOI] [PubMed] [Google Scholar]
- 37.Sakeena M.H.F., Bennett A.A., Carter S.J., McLachlan A.J. A comparative study regarding antibiotic consumption and knowledge of antimicrobial resistance among pharmacy students in Australia and Sri Lanka. PLoS One. 2019;14:0213520. doi: 10.1371/journal.pone.0213520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llor C., Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014;5:12. doi: 10.1177/2042098614554919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardefeldt L.Y., Gilkerson J.R., Billman-Jacobe H., Stevenson M.A., Thursky K., Bailey K.E., Browning G.F. Barriers to and enablers of implementing antimicrobial stewardship programs in veterinary practices. J. Vet. Intern. Med. 2018;32:1092–1099. doi: 10.1111/jvim.15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mor S.M., Norris J.M., Bosward K.L., Toribio J., Ward M.P., Gongora J., Vost M., Higgins P.C., McGreevy P.D., White P.J., Zaki S. One health in our backyard: design and evaluation of an experiential learning experience for veterinary medical students. One Health. 2018;5:57–64. doi: 10.1016/j.onehlt.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hockenhull J., Turner A.E., Reyher K.K., Barrett D.C., Jones L., Hinchliffe S., Buller H.J. Antimicrobial use in food-producing animals: a rapid evidence assessment of stakeholder practices and beliefs. Vet. Rec. 2017;181 doi: 10.1136/vr.104304. [DOI] [PubMed] [Google Scholar]
- 42.Postma M., Speksnijder D.C., Jaarsma A.D., Verheij T.J., Wagenaar J.A., Dewulf J. Opinions of veterinarians on antimicrobial use in farm animals in Flanders and the Netherlands. Vet. Rec. 2016;179:68–77. doi: 10.1136/vr.103618. [DOI] [PubMed] [Google Scholar]
- 43.Chang Q., Wang W., Regev-Yochay G., Lipsitch M., Hanage W.P. Antibiotics in agriculture and the risk to human health: how worried should we be? Evol. Appl. 2015;8:240–247. doi: 10.1111/eva.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vallin M., Polyzoi M., Marrone G., Rosales-Klintz S., Tegmark Wisell K., C. Stalsby Lundborg, knowledge and attitudes towards antibiotic use and resistance - a latent class analysis of a Swedish population-based sample. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davey P., Marwick C.A., Scott C.L., Charani E., McNeil K., Brown E., Gould I.M., Ramsay C.R., Michie S. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017;2 doi: 10.1002/14651858.CD003543.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survey administered to the Year 2 DVM students