Abstract
Study design
Meta-analysis.
Objectives
We aim to identify the clinically significant ideal Mesenchymal Stem Cell (MSC) count in the management of osteoarthritis of knee from Randomized Controlled Trials (RCTs) available in the literature.
Materials and methods
We conducted independent and duplicate electronic database searches including PubMed, Embase, Web of Science, and Cochrane Library till August 2021 for RCTs conducted in the management of knee osteoarthritis using MSC therapy specifying the quantity of MSCs delivered. We categorized the studies based on the MSC count utilized in them into four groups namely <1 × 107 MSCs (Group I), 1-5x107 MSCs (Group II), 5-10 × 107 MSCs (Group III), and >10 × 107 MSCs (Group IV). Visual Analog Score (VAS) for Pain, Western Ontario McMaster Universities Osteoarthritis Index (WOMAC), Lysholm score, Knee Osteoarthritis Outcome Score (KOOS), and adverse events were the outcomes analyzed. Analysis was performed in R-platform using OpenMeta [Analyst] software.
Results
14 studies involving 564 patients were included for analysis. We noted incremental decrease in the VAS with increasing dosage of MSCs at 12 months [Group I,WMD = 2.641(p = 0.854); Group II, WMD = −4.853(p = 0.379); Group III, WMD = −12.154 (p = 0.316); Group IV, WMD = −15.935(p = 0.116)], and 24 months [Group I,WMD = −6(p = 0.001); Group II, WMD = −15(p = 0.001); Group IV, WMD = −20(p = 0.001)]. We also noted incremental improvement in the WOMAC, KOOS with increasing dosage of MSCs at 12 months [Group I, WMD = 7(p = 0.001); Group II, WMD = 28(p = 0.001); Group IV, WMD = 30(p = 0.001)] and [Group II, WMD = −2.562(p = 0.676); Group III, WMD = 7.670(p = 0.099); Group IV, WMD = 13.475(p = 0.261)] respectively. However, we noted significant reduction in the Lysholm score in Group IV, compared to the others at 12 months (WMD = −12.5, 95%CI[-25.883,0.883]) and 24 months (WMD = −6.6, 95%CI[-23.596,10.396]). We did not find any significant increase in the adverse events with incremental dosage of MSCs in any of the groups compared.
Conclusion
Compared to the four dosage groups of MSCs analyzed, Group III showed consistent significant improvement in pain and functional outcomes analyzed compared to the other groups. Hence, we recommend a cell volume of 5-10 × 107 cells to be delivered to the target site to obtain superior benefits out of the procedure. However, we urge future trials of sufficient quality to validate our findings to arrive at a consensus on the ideal count of MSCs to be delivered in the cellular therapy for knee osteoarthritis.
Keywords: Mesenchymal stem cell, Bone-marrow derived mesenchymal stem cell, Cell count, Cartilage regeneration, Knee osteoarthritis, Meta-analysis
1. Introduction
Due to its versatile nature, global researchers considered the Mesenchymal Stromal Cells (MSCs) as a potential regenerative tool for cartilage repair in osteoarthritis (OA) knee. Lopa et al. stated MSCs as ‘medicinal signaling cells’ since they could sense the nature of their biological milieu and secrete appropriate cytokines, pro-angiogenic, anti-apoptotic, and anti-inflammatory molecules to maintain the native homeostasis.1 Hence, the application of MSCs into the knee joint plays a major role in cartilage regeneration and rejuvenation.
Although bone marrow and adipose tissue were considered to be the common source of these MSCs, the quantity of MSCs present in either bone marrow aspirate concentrate (BMAC) or stromal vascular fraction (SVF) is usually lower when compared to their culture-expanded clinical-grade MSCs or MSCs expanded from any sources.2,3 However, the lower number of MSCs in the concentrate does not imply an inferior nature of the treatment modalities that employ them. A successful outcome of MSC-based cellular therapy in OA knee relies not only on the quantity but also on the nature of the source, and quality of MSCs.4 The transplanted MSCs should be of sufficient potential to regenerate the repaired cartilaginous tissue.
Various researchers have used various doses of MSC count (ranged from 5 × 106 to 150 × 106 MSCs) for regenerating the cartilage.5, 6, 7 Vangsness et al. demonstrated that 50 × 106 and 150 × 106 intra-articular allogenic bone marrow MSCs (BM-MSCs) had regenerated the cartilage and meniscus along with a significant pain reduction in the patients with OA knee following partial medial meniscectomy.7 Koh et al. observed a significant improvement in the functional knee score after 26 months of follow-up with 1.18 × 106 adipose tissue-derived MSCs (AD-MSCs) along with platelet-rich plasma.8 Pers et al. reported the functional improvement in OA knee with low (2 × 106) dose than medium (10 × 106) and high (50 × 106) doses of autologous AD-MSCs.9 Jo et al. demonstrated significant functional improvement in OA knee pain after 2 years of follow-up with MSC dose of 100 × 106 cells compared to 10 × 106 cells and 50 × 106 cells.10 Jo et al. reported that hyaline-like articular regeneration with 100 × 106 AD-MSCs than 10 × 106 and 50 × 106 AD-MSCs.11
Due to varying doses of MSCs used by various researchers, it is a need for an hour to perform dose-escalation clinical trials to identify the optimal therapeutic dose for cartilage regeneration. To standardize the treatment of knee osteoarthritis with MSC-based cellular therapy, we aim to identify the clinically significant ideal cell count needed to be injected in the management of osteoarthritis of the knee.
2. Materials & methods
This meta-analysis was conducted following the guidelines of the Back Review Group of Cochrane Collaboration12 and reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.13
2.1. Search strategy
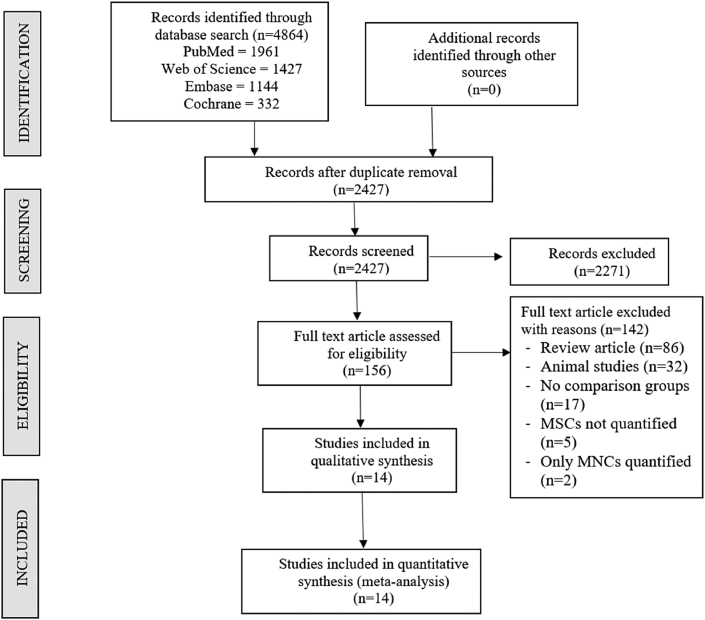
Two reviewers performed an independent electronic literature search for studies evaluating the safety and efficacy of stem cell therapy for spinal cord injury. We searched the following databases: PubMed, Embase, Web of Science, and the Cochrane Library up to August 2021. No language or date restrictions were applied. Keywords used for the search were as follows: “Knee Osteoarthritis”, “Knee Degeneration”, “Stem Cell Therapy” and “Mesenchymal Stem Cells”, “Bone marrow”, “Adipose”. A sample search strategy used in one of the included databases was presented in Supplementary File 1. The reference list of the selected articles was also searched to identify studies not identified in the primary search. As per the inclusion and exclusion criteria, eligible studies were included for meta-analysis. The discrepancy between the authors was resolved through discussion until a consensus was obtained. A detailed study selection flow diagram is given in Fig. 1.
Fig. 1.
PRISMA flow diagram of the included studies.
2.2. Inclusion criteria
Studies were included for quantitative review if they met the following PICOS criteria:
Population: Patients with knee osteoarthritis.
Intervention: MSC-based cellular therapy with clear reporting on the amount of MSCs delivered to the target site.
Comparator: Control.
Outcomes: Visual Analog Score (VAS) for Pain, Western Ontario McMaster Universities Osteoarthritis Index (WOMAC), Lysholm Knee Scale (Lysholm), Knee Osteoarthritis Outcome Score (KOOS), and adverse events.
Study Design: Randomized Controlled Trials.
2.3. Exclusion criteria
Trials were excluded if they had the following characteristics:
-
1.
RCTs without a clear mention about the quantity of the MSCs delivered to the intervention group.
-
2.
RCTs mentioning only the quantity of mononuclear cells without a clear quantification of the MSCs within them.
-
3.
Observational studies and interventional studies without a comparator group.
-
4.
Animal studies involving stem cell therapy for knee osteoarthritis models.
-
5.
Review articles and in-vitro studies involving stem cell therapy.
2.4. Data extraction
Two reviewers retrieved independently relevant data from articles included for analysis. Following data were extracted:
-
1.
Study characteristics: year of publication, authors, country, level of evidence, number of patients enrolled.
-
2.
Baseline characteristics: mean age, gender proportions, Kellgren Lawrence grade of osteoarthritis, Source of MSC utilized, intervention for both the groups, amount of MSCs delivered, follow-up duration, and assessment parameters utilized.
-
3.
Efficacy Outcomes: VAS for pain, Functional outcomes like WOMAC score, Lysholm score, and KOOS.
-
4.
Safety Outcomes: Adverse events in the included studies.
We categorized the studies included into four groups based on the quantity of the MSCs utilized in them. We categorized the studies using a low volume of MSCs (<1 × 107 cells) as Group I, a moderate volume of MSCs (1-5x107 cells) as Group II, high volume of MSCs (5-10 × 107 cells) as Group III, and a very high volume of MSCs (>10 × 107 cells) as Group IV for analytical purposes. Any disagreement in data collection was resolved until a consensus was attained by discussion.
2.5. Risk of bias and quality assessment
The methodological quality of the included studies was assessed independently by two reviewers using The Cochrane Collaboration's ROB2 tool for randomized studies which has five domains of bias assessment including randomization process, deviation from intended intervention, missing outcome data, measurement of the outcome, and selection of the reported results.14
2.6. Statistical analysis
Meta-analysis was conducted in the R platform with OpenMeta[Analyst].15 For dichotomous variable outcomes, risk ratio (RR) with 95% Confidence Interval (CI) was used and for continuous variable outcomes, weighted mean difference (WMD) with 95% CI was used. Heterogeneity was assessed using the I2 test.16 If I2 < 50% and p > 0.1, we used a fixed-effects model to evaluate, otherwise, a random-effects was used. A p-value <0.05 was considered significant. Sensitivity analyses were performed to explore the source of heterogeneity when it existed. Publication bias was analyzed with a funnel plot and normal quantile plot for the outcomes in the included studies and Egger's regression test.
3. Results
3.1. Search results
Electronic database search resulted in 4864 articles which after initial screening for duplicate removal gave a total of 2427 articles. Title and abstract screening were done in those 2427 articles and 2271 of them were excluded. 156 articles qualified for full-text review of which 142 were excluded. We included 14 studies5, 6, 7,17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 with 564 patients into the analysis. PRISMA flow diagram of study selection is given in Fig. 1. 6/14 studies19,20,24, 25, 26, 27 utilized MSC from adipose tissue, of which 1 used allogenic source and rest 5 studies utilized autogenous source of AD-MSCs. 8/14 studies5, 6, 7,17,18,21, 22, 23 utilized MSC from bone marrow, of which 3 used allogenic sources, and the rest 5 studies utilized autogenous sources of BM-MSCs. There was no uniformity among the included studies for the outcome measures utilized. The general characteristics of the studies included were given in Table 1. The protocol of intervention used in the case and control groups along with the measures of outcome assessment were given in Table 2.
Table 1.
Characteristics of included studies.
| Sl. No |
Authors | Year | Country | Nature of Study | Kellgren Lawrence Grade | Sample size | Treatment/Control | Mean Age (SD) |
Male/Female |
MSC Type | MSC Source | Follow-up (months) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Group | Control Group | Treatment Group | Control Group | |||||||||||
| 1 | A Vega | 2014 | Spain | RCT | II, III, IV | 30 | 15/15 | 56.6 ± 9.24 | 57.3 ± 9.09 | 06/09 | 05/10 | BM | Allo | 12 |
| 2 | CT Vangness Jr | 2014 | USA | RCT | NR | 55 | 36/19 | 44.6 ± 9.82 | 47.8 ± 8 | 25/11 | 13/06 | BM | Allo | 24 |
| 3 | D Garay Mendoza | 2017 | Mexico | RCT | NR | 61 | 30/31 | 55.57 ± 12.02 | 59.32 ± 10.85 | 07/23 | 09/22 | BM | Auto | 6 |
| 4 | D Kuah | 2018 | Australia | RCT | I, II, III | 20 | 16/4 | 50.8 ± 7.29 | 55.0 ± 10.42 | 11/05 | 01/03 | AD | Allo | 12 |
| 5 | J Freitag | 2019 | Australia | RCT | II, III | 30 | 20/10 | 54.6 ± 6.3 | 51.5 ± 6.1 | 11/09 | 01/09 | AD | Auto | 12 |
| 6 | JM Lamo-Espinosa | 2016 | Spain | RCT | II, III, IV | 30 | 20/10 | 65.9 | 60.3 | 12/08 | 07/03 | BM | Auto | 12 |
| 7 | KL Wong | 2013 | Singapore | RCT | NR | 56 | 28/28 | 53 | 49 | 15/13 | 14/14 | BM | Auto | 24 |
| 8 | M Emadedin | 2018 | Iran | RCT | II, III, IV | 43 | 19/24 | 51.7 ± 9.2 | 54.7 ± 5.3 | 12/07 | 15/09 | BM | Auto | 6 |
| 9 | PK Gupta | 2016 | India | RCT | II, III | 60 | 40/20 | 58.10 ± 8.23 | 54.90 ± 8.27 | 12∖28 | 4∖16 | BM | Allo | 12 |
| 10 | S Wakitani | 2002 | Japan | I, II | 24 | 12/12 | NR | NR | NR | NR | BM | Auto | 16 | |
| 11 | TDX Tran | 2019 | Taiwan | RCT | II, III | 33 | 15/18 | 58.2 ± 5.70 | 59.0 ± 6.04 | 03/12 | 05/13 | AD | Auto | 24 |
| 12 | WS Lee | 2019 | South Korea | RCT | II, III, IV | 24 | 12/12 | 62.2 ± 6.5 | 63.2 ± 4.2 | 03/09 | 03/09 | AD | Auto | 6 |
| 13 | YG Koh | 2012 | South Korea | RCT | IV | 50 | 25/25 | 54.2 ± 9.3 | 54.4 ± 11.3 | 08/17 | 08/17 | AD | Auto | 16 |
| 14 | YG Koh | 2014 | South Korea | RCT | I, II, III | 44 | 23/21 | 52.3 ± 4.9 | 54.2 ± 2.9 | 06/17 | 05/16 | AD | Auto | 24 |
AD – Adipose derived; Allo – Allogenic; Auto – Autologous; BM – Bone Marrow derived; MSC – Mesenchymal Stem Cell; NR – Not Reported; RCT – Randomized Controlled Trial; SD – Standard Deviation; USA – United States of America.
Table 2.
Stem cell transplantation protocol of the included studies.
|
Study |
MSC Type | MSC Source | MSC Preparation |
MSC count (107 cells) | Treatment group Intervention | Control group Intervention | Outcome measures |
|---|---|---|---|---|---|---|---|
| A Vega | BM | Allo | CE-BMMSC | 4 | sIA Injection of MSC | sIA Injection of 60 mg HA | VAS, WOMAC |
| CT Vangness Jr | BM | Allo | CE-BMMSC | 5/15 | sIA Injection of MSC + 20 mg HA | sIA Injection of 20 mg HA | VAS, Lysholm Score |
| D Garay Mendoza | BM | Auto | BMC | 2 | 600 μg/day G-CSF for 3 consecutive days before the procedure + sIA Injection of MSC | Oral acetaminophen 500 mg every 8 h for 6 months |
VAS, WOMAC |
| D Kuah | AD | Allo | CE-ADMSC | 0.39–0.67 | sIA Injection of MSC | Placebo sIA Injection of cell culture media and cryopreservative | VAS, WOMAC, MRI assessment |
| J Freitag | AD | Auto | CE-ADMSC | 10/20 | sIA Injection of MSC ± 2nd injection at 6 months | Conservative management | VAS, WOMAC, KOOS, MRI assessment |
| JM Lamo-Espinosa | BM | Auto | CE-BMMSC | 1/10 | sIA Injection of MSC + 60 mg HA | sIA Injection of 60 mg HA | VAS, WOMAC, MRI assessment |
| KL Wong | BM | Auto | CE-BMMSC | 1.46 | HTO + Microfracture + sIA Injection of MSC + 20 mg HA | HTO + Microfracture + sIA Injection of 20 mg HA |
Tegner Score, Lysholm Score |
| M Emadedin | BM | Auto | CE-BMMSC | 4.1 | sIA Injection of MSC | Placebo sIA Injection of Normal Saline | VAS, WOMAC |
| PK Gupta | BM | Allo | CE-BMMSC | 2.5/5/7.5/15 | sIA Injection of MSC + 20 mg HA | Placebo sIA Injection of 20 mg HA | VAS, WOMAC, MRI assessment |
| S Wakitani | BM | Auto | CE-BMMSC | 1 | HTO + Microfracture + sIA Injection of MSC | HTO + Microfracture + Placebo injection | MRI assessment, HSS Knee rating scale |
| TDX Tran | AD | Auto | SVF | 10 | Arthroscopic micro fracture + sIA Injection of MSC | Arthroscopic micro fracture | WOMAC, MRI assessment |
| WS Lee | AD | Auto | CE-ADMSC | 10 | sIA Injection of MSC | Placebo injection with Normal Saline | WOMAC, MRI assessment |
| YG Koh | AD | Auto | SVF | 0.189 | Arthroscopic debridement + sIA Injection of MSC + PRP | Arthroscopic debridement + PRP | VAS, Tegner Score, Lysholm Score |
| YG Koh | AD | Auto | CE-ADMSC | 0.411 | HTO + sIA Injection of MSC + PRP | HTO + PRP | VAS, Lysholm Score |
AD – Adipose derived; Allo – Allogenic; Auto – Autologous; BM – Bone Marrow derived; BMC – Bone Marrow Concentrate; CE-ADMSC – Culture Expanded Adipose Derived MSC; CE-BMMSC – Culture Expanded Bone Marrow MSC; HA – Hyaluronic Acid;; HSS – Hospital for Special Surgeries; HTO – High Tibial Osteotomy; IA – Intra-articular; IKDC – International Knee Documentation Committee, KOOS – Knee Osteoarthritis Outcome Score; PRP – Platelet Rich Plasma; MRI – Magnetic Resonance Imaging; MSC – Mesenchymal Stem Cells; sIA – Single Intra-articular; SVF – Stromal Vascular Fraction; VAS – Visual Analog Score; WOMAC – Western Ontario Mc-Master Universities Osteoarthritis Index.
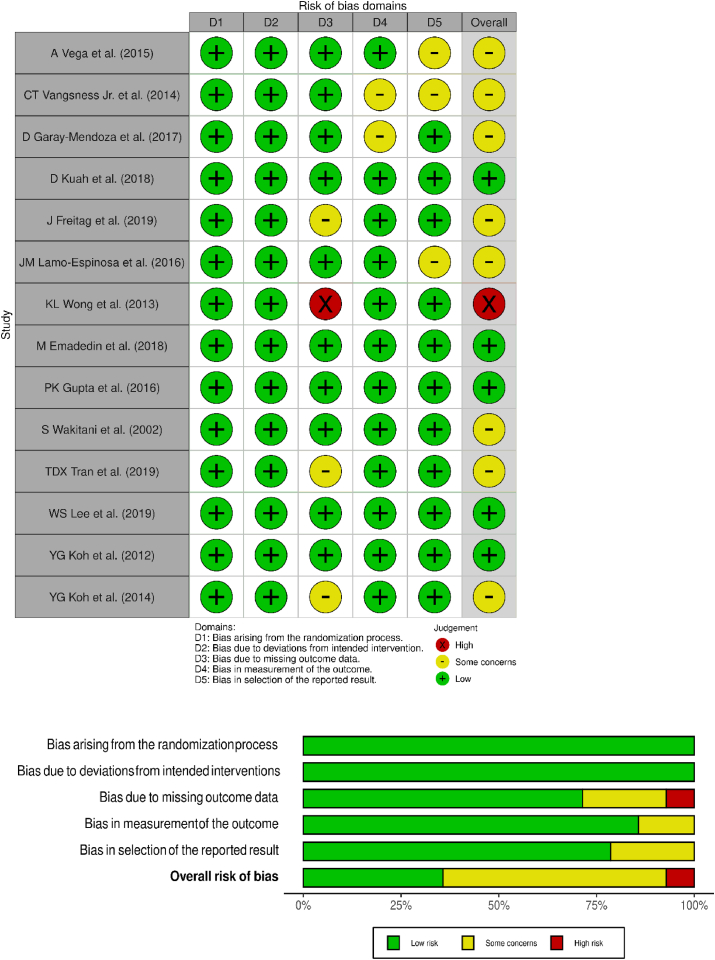
3.2. Quality assessment
The methodological quality of the included studies evaluated as per the RoB2 tool was presented in Fig. 2. None of the included studies had a high risk of bias to be excluded from the analysis.
Fig. 2.
Methodological quality and risk of bias assessment of all the included studies.
3.3. Efficacy Outcomes
3.3.1. Visual analog scale for pain
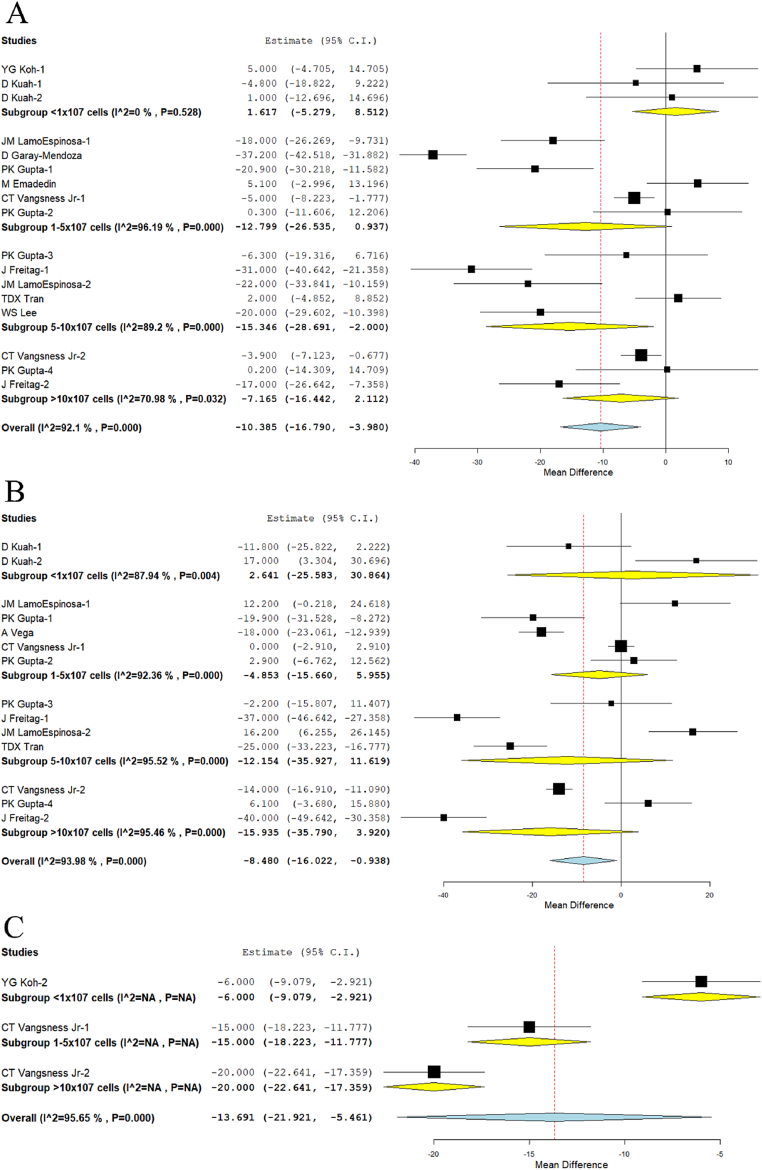
We analyzed 2 studies19,26 in Group I, 5 studies5, 6, 7,18,22 in Group II, 5 studies5,6,20,24,25 in Group III, and 3 studies6,7,20 in Group IV reporting the VAS outcome at 6 months. There was a significant heterogeneity observed between the included studies. (I2>80%, p < 0.001). Hence, the random-effects model was used for analysis across all time points. On analysis, we noted an incremental reduction in the VAS with the increase in the dosage of the MSCs delivered. However, a significant reduction in VAS score was noted compared to their controls at 6 months only in Group III (WMD = −15.346, 95% CI [−28.691, −2.000], p = 0.024); Fig. 3A.
Fig. 3.
Forest plot of the included studies comparing various doses of MSCs compared to their controls. A: VAS at 6 months; B: VAS at 12 months; C: VAS at 24 months.
We analyzed one study19 in Group I, 4 studies5, 6, 7,17 in Group II, 4 studies5,6,20,24 in Group III, and 3 studies6,7,20 in Group IV reporting the VAS outcome at 12 months. There was a significant heterogeneity observed between the included studies. (I2>80%, p < 0.001). Hence, the random-effects model was used for analysis across all time points. On analysis, we noted a similar incremental reduction in the VAS with the increase in the dosage of the MSCs delivered as noted at 6 months. However, a significant reduction in VAS score was not noted compared to their controls at 12 months in any of the groups analyzed (Fig. 3B).
We analyzed one study27 in Group I, one study7 in Group II, and one study7 in Group IV reporting the VAS outcome at 24 months. There was a significant heterogeneity observed between the included studies. (I2>80%, p < 0.001). Hence, the random-effects model was used for analysis across all time points. On analysis, we noted a similar incremental reduction in the VAS with the increase in the dosage of the MSCs delivered as noted at 6, 12 months. Moreover, a significant reduction in VAS score was noted compared to their controls in Group I (WMD = −6, 95% CI [−9.079, −2.921], p < 0.001), Group II (WMD = −15, 95% CI [−18.223, −11.777], p < 0.001), and Group IV (WMD = −20, 95% CI [−22.641, −17.359], p < 0.001) at 24 months (Fig. 3C) respectively.
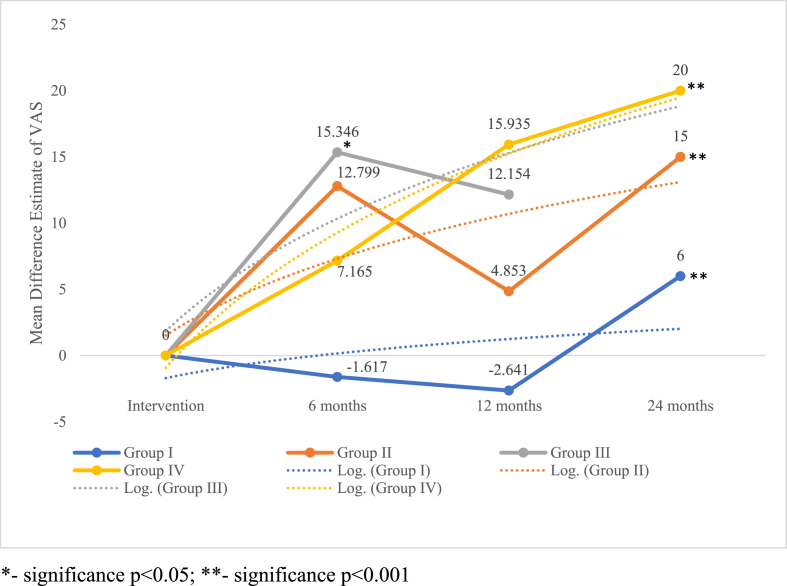
On critical analysis of the pain reduction potential of the dosage groups compared, it is noted as shown in Fig. 4 that despite the lack of significance in the pain reduction at 12 months, we noted a dose-response relationship in the VAS outcome compared to the controls. Although all the groups produced significant results around 24 months, consistent results were delivered by Group III (5-10 × 107 cells). However, the inconsistencies in the results of the other groups could also be accounted to the heterogeneity in the studies included for analysis.
Fig. 4.
Dose-response relationship in pain reduction across incremental dosage groups of MSCs at various timepoints based on VAS score.∗- significance p < 0.05; ∗∗- significance p < 0.001.
3.3.2. KOOS, WOMAC & lysholm score
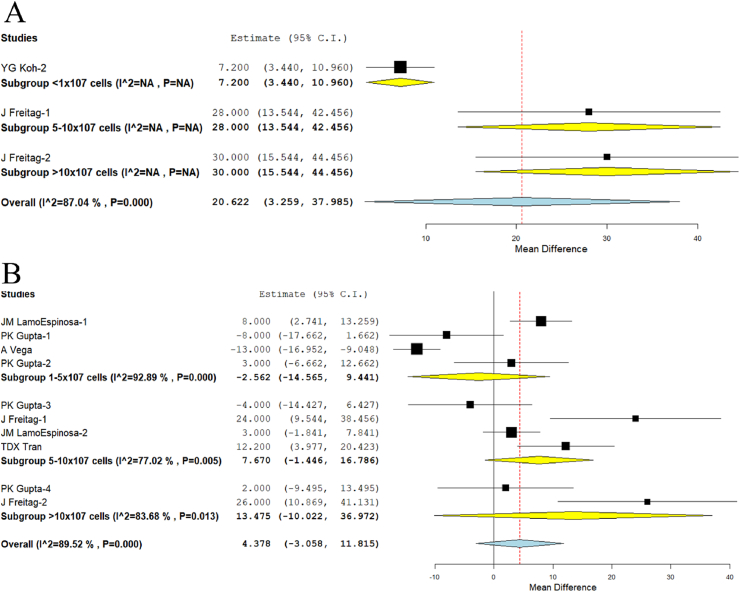
We analyzed the quality of life outcomes such as KOOS reported in one study27 of Group I, one study20 of Group III, and one study20 of Group IV. There was a significant heterogeneity observed between the included studies. (I2>80%, p < 0.001). Hence, the random-effects model was used for analysis across all time points. On analysis, we noted significant improvement in the outcomes scores across all the groups (p = 0.020) in an incremental fashion with WMD = 7.2, 95% CI [3.440,10.960] in Group I, WMD = 28, 95% CI [13.544,42.456] in Group III, and WMD = 30, 95% CI [15.544,44.456] in Group IV respectively as shown in Fig. 5A.
Fig. 5.
Forest plot of the included studies comparing various doses of MSCs compared to their controls. A: KOOS at 12 months, B: WOMAC at 12 months.
We analyzed the functional outcome parameter such as WOMAC reported in three studies5,6,17 of Group II, four studies5,6,20,24 of Group III, and two studies6,20 of Group IV. There was a significant heterogeneity observed between the included studies. (I2>80%, p < 0.001). Hence, the random-effects model was used for analysis across all time points. On analysis, although we did not note a significant improvement in the outcomes scores across the groups analyzed (p = 0.249), we noted an incremental dose-response relationship in the improvement of WOMAC score with the increasing dose of the MSCs delivered, WMD = −2.562, 95% CI [−14.565,9.441] in Group II, WMD = 7.670, 95% CI [−1.446,16.786] in Group III, and WMD = 13.475, 95% CI [−10.022,36.972] in Group IV, as shown in Fig. 5B.
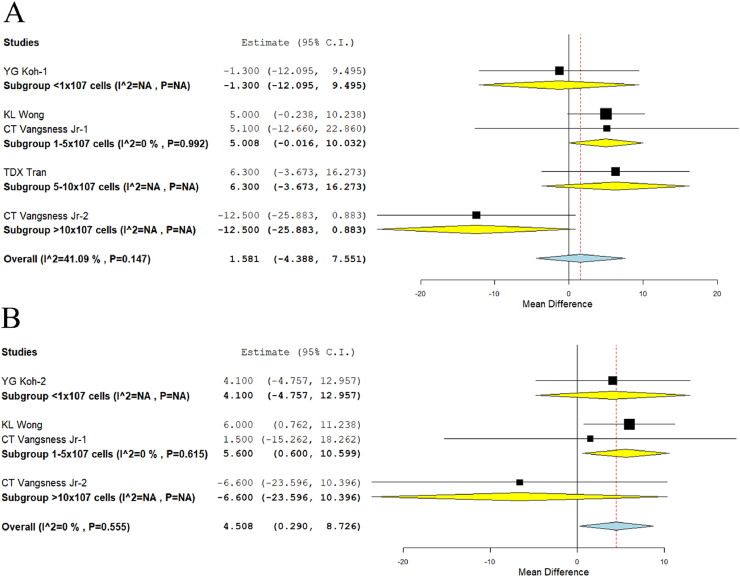
With regards to the lysholm score, we analyzed one study26 in Group I, two studies7,21 in Group II, one study23 in Group III, and one study7 in Group IV reporting the outcome at 12 months. There was a significant heterogeneity observed between the included studies. (I2>80%, p < 0.001). Hence, the random-effects model was used for analysis across all time points. Although we did not note significant improvement across any of the groups analyzed, we noted an incremental response in Group I (WMD = −1.300, 95%CI [−12.095, 9.495]), Group II (WMD = 5.008, 95%CI [−0.016, 10.032]), and Group III (WMD = 6.300, 95%CI [−3.673, 16.273]). We noted a significant decrease (p < 0.001) in the outcome of Group IV (WMD = −12.500, 95%CI [−25.883, 0.883]) compared to the other groups analyzed as shown in Fig. 6A. Moreover, on analysis of the lysholm score reported at 24 months, we also noted similar significant deterioration (p < 0.001) in the outcome of Group IV (WMD = −6.600, 95% CI [−23.596, 10.396]) compared to the other groups analyzed. We also noted significant improvement in the outcome compared to the controls in Group II (WMD = 5.600, 95% CI [0.600, 10.599]; p = 0.028) while data was not available for Group III at the specified time point analyzed for the given outcome score as shown in Fig. 6B.
Fig. 6.
Forest plot of the included studies comparing various doses of MSCs compared to their controls. A: Lysholm score at 12 months, B: Lysholm score at 24 months.
On overall analysis of the KOOS, WOMAC, and Lysholm score, it is noted that incremental dose-response relationship in the outcome score was noted from Group I-III while Group IV demonstrated an inverse relationship compared to the other groups analyzed.
3.3.3. Safety
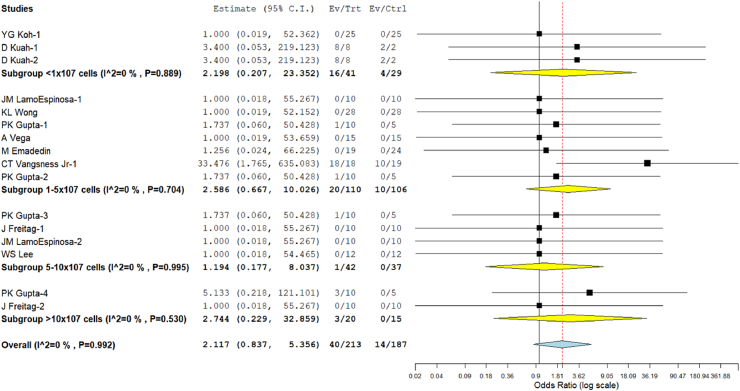
Ten studies5, 6, 7,17,19, 20, 21, 22,25,26 involving 400 patients reported adverse effects with low heterogeneity among the included studies across all the doses utilized. (I2 = 0.0%, p = 0.992). Hence, a fixed-effects model was used for analysis. Across all the groups analyzed, we did not note any significant increase in the adverse events compared to the controls (RR = 2.117, 95% CI [0.837, 5.356], p = 0.113; Fig. 6). No major serious adverse events with permanent effects such as death, tumor, or immune reaction to the intervention were noted during follow-up in either of the doses of MSCs utilized. We also noted that the least estimate of the risk of adverse events in Group III (RR = 1.194, 95% CI [0.177, 8.037], p = 0.856; Fig. 7).
Fig. 7.
Forest plot of the included studies comparing adverse events across incremental dosage groups of MSCs compared to their controls.
3.3.4. Sensitivity analysis
A sensitivity analysis was performed in each analysis. All the results (VAS for Pain, KOOS, Lysholm, and adverse events) were not significantly altered by sequentially omitting each study in the meta-analysis. On the other hand, the consistency of the results was maintained after reanalysis by changing to the random-effects model.
3.3.5. Publications bias
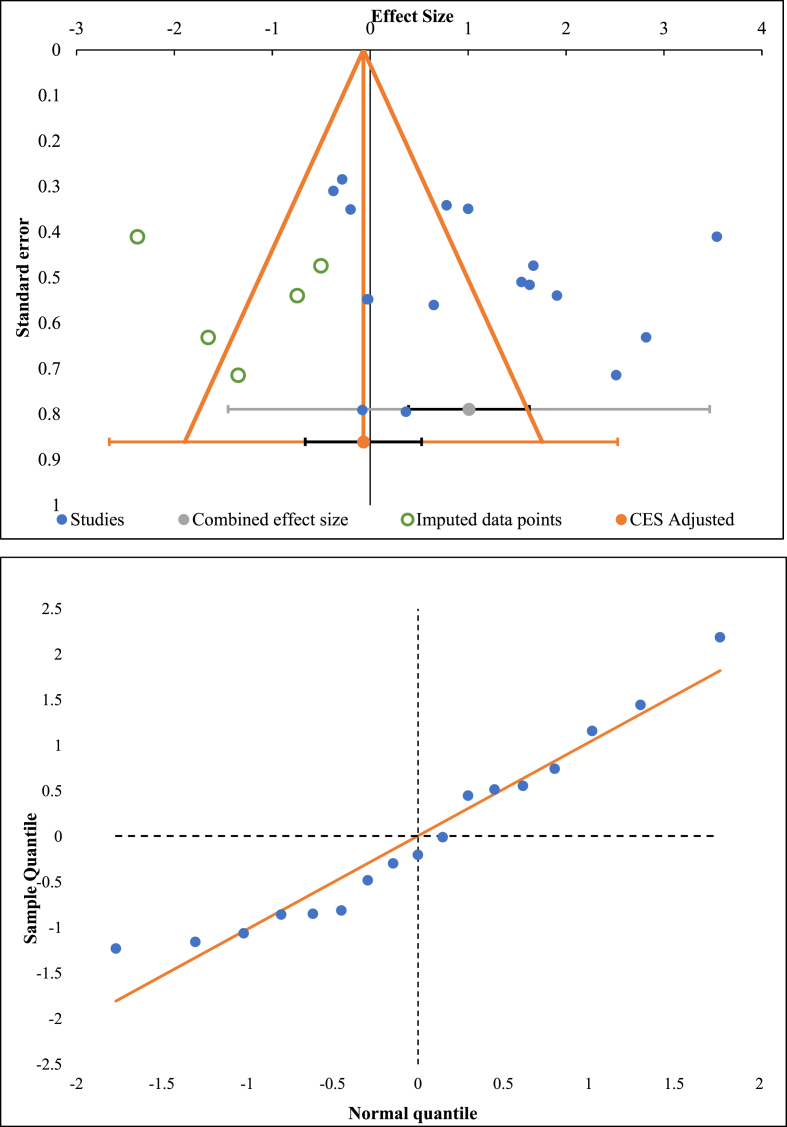
Publication bias was analyzed utilizing the funnel plot, normal quantile plot, and Egger's regression test for the meta-analysis performed. There was no evidence of publication bias by funnel plot and normal quantile plot as shown in Fig. 8 or by Egger's regression test (p = 0.564). All the studies lied close to the 95% CI and no significant heterogeneity was noted in the distribution of the studies about the axes, implying minimal publication bias.
Fig. 8.
Publication bias assessment with funnel plot and quantile plot for Visual Analog Score at 6 months in the included studies.
4. Discussion
When MSCs are injected locally, they modulate the joint environment and halt the progression of the disease process in OA knee. With a short in-vivo half-life, MSCs release the biomicromolecules inside the joint and alters the disease environment effectively.28 The quantification of the survival time of MSCs inside the joint which is hypoxic, with low pH, and rich inflammatory mediators, to produce a therapeutic effect depends on the number of MSCs delivered into them. The dose-response relationship in MSC therapy depends not only on the cellular count but also on a vast grade of complex factors which makes it difficult for global researchers to standardize the therapy for optimal functional outcome. However, the clarity in the dose of MSCs was poorly understood as it represents the total number of MSCs for each shot of injection or the number of injected MSCs per kg body weight. To add to the ambiguity, some studies estimate the entire mononuclear cell count in dosage calculation which includes a heterogeneous population of cells apart from MSCs. Variability is also noted in the dosage frequency utilized for cartilage regeneration irrespective of the source of MSCs, cultured or uncultured, and autologous or allogeneic in origin. Upon utilization of the culture-expanded cells, there is no consensus in the available literature regarding the ideal and effective number of passages needed to obtain the optimal cell population with the required characteristics.29 Identification of the minimum effective count of MSCs required for the healing cascade and definitive outcomes in terms of regeneration was suggested since the larger the doses of MSCs transplanted into the joint, the greater the competition for nutrients for their survival within the grafted area occurs.30
MSCs act by direct cell-to-cell contact (membrane-bounded proteins and receptors) and by paracrine signaling (cytokines, chemokines, and trophic factors) in the transplanted environment.31 Under physiological conditions, MSCs reside in the perivascular niche whereas they get activated upon signals from injured cartilage to re-establish the joint homeostasis. As the result, MSCs limit inflammation and apoptosis thereby increasing the reparative capacity of the recipient site.1 The priming of MSCs in OA synovial fluid which contains INF-γ and TNF-α increases the concentration of indoleamine 2,3-dioxygenase and interleukin-6 (IL-6) expression. These MSCs derived mediators inhibit the proliferation of T cells, downregulate IL-1β and ADAMTS5, and upregulate interleukin 1 receptor antagonist (IL-1Ra) and suppressor of cytokine signaling (SOCS-1).1.
Allogenic products namely CARTISTEM (allogenic cord blood-derived MSCs – 2.5 × 106 cells/500 μL/cm2 area of knee cartilage),32 Stempeucell (allogenic ex-vivo cultured pooled human BM-MSCs – 2 × 108 cells cryopreserved and stored in 15 mL cryo-bags),6 and JointStem (autologous AD-MSCs – 10 × 107 cells)33 have launched the MSC derived products with a definite dosage for cartilage injuries.
Similar to the work of Herningou et al.34 on the estimation of the native load of MSCs in the femoral head which would be necessary to restore in cellular therapy for its osteonecrosis, no such study is available to date on the OA knee. Hence estimation of the ideal cell count by stratification of the studies based on the cell counts utilized and reported outcomes became the necessary analysis.
4.1. Main finding
We made a stratified analysis of all the available RCTs using MSC therapy based on the MSC count delivered at the target site for knee osteoarthritis and found that.
-
1.
An incremental decrease in the VAS with increasing dosage of MSCs at 12 months [Group I, WMD = 2.641 (p = 0.854); Group II, WMD = −4.853 (p = 0.379); Group III, WMD = −12.154 (p = 0.316); Group IV, WMD = −15.935 (p = 0.116)], and 24 months [Group I, WMD = −6 (p = 0.001); Group II, WMD = −15 (p = 0.001); Group IV, WMD = −20 (p = 0.001)]. We also noted significant incremental increase in the KOOS with increasing dosage of MSCs at 12 months [Group I, WMD = 7 (p = 0.001); Group II, WMD = 28 (p = 0.001); Group IV, WMD = 30 (p = 0.001)]. However, we noted significant reduction in the lysholm score in Group IV, compared to the others at 12 months (WMD = −12.5, 95%CI[-25.883,0.883]) and 24 months (WMD = −6.6, 95%CI[-23.596,10.396]).
-
2.
On comparing the relative improvement between the groups with various parameters such as VAS, KOOS, and Lysholm score, Group III (5-10 × 107 cells) gave a consistent improvement in the outcome parameters analyzed without significant deterioration compared to the other groups at various time points analyzed.
-
3.
We did not find any significant increase in the adverse events with an incremental dosage of MSCs in any of the groups compared.
MSCs exhibit a varied spectrum of immunoregulating capabilities on both innate and adaptive immune systems. Due to the prevailing inflammatory environment, specific toll-like receptor (TLR) ligands affect the MSC-based cellular therapy in the management of OA knee.35, 36, 37 In the early stages of OA knee, TLR2/4 on MSC surface may result in polarization into MSC1, which promotes further immunogenic response to protect joint tissues in OA pathogenesis.36 In later stages of OA knee, TLR3 accumulation leads to polarization into MSC2 which further prevents articular surface damage in the OA knee.36 Failure of inflammatory attenuation leads to the continuation of the vicious cycle in OA pathogenesis.
In such a pro-inflammatory milieu, the quiescent resident MSCs are attenuated and may not be induced in the lines of regeneration of the articular cartilage and hence infusion of MSCs into the knee joint may not only repair and regenerate the joint cartilage but also helps in stimulating the resident MSCs to attain joint homeostasis in favor of regeneration. MSCs suppress host immune responses by inhibiting immune cells proliferation and maturation in a non-MHC restricted manner.38 MSCs are considered to be hypoimmunogenic, which express low levels of HLA 1 and are deprived of HLA class II and co-stimulatory molecule expression.39,40 Numerous studies have also demonstrated MSCs to suppress the activity of a broad range of immune cells, including T cells, natural killer cells, dendritic cells, B cells, neutrophils, monocytes, macrophages.41
There is no consensus on the viability of MSCs to exert immunomodulatory effects in the local or systemic environment. Once administered, MSCs undergo radical biological and morphological changes and disappear from the site of injection within few days of intra-articular injection.11 Despite the disappearance of administered MSCs from the site, they elaborate the cytokines and chemokines which work with paracrine signaling and induces the quiescent and resident MSCs to enhance the significant therapeutic effects.42 Hence the dead MSCs create an immunosuppressive or immune-privileged environment by secreting IL-10 and TGF-β and inhibition of lipopolysaccharide (LPS) stimulated macrophages by the secretion of IL-1β and TNF-α.43 Indirectly, phagocytes eliminate MSCs and result in hyporesponsiveness to LPS and switching off the immunological cellular environment to an anti-inflammatory environment.44 Upon cell-to-cell contact, T cells release perforin and granzyme B and induce apoptosis of infused MSCs.45 IDO, a soluble factor, secreted by phagocytes is responsible for immunomodulation.42 Mancuso et al. emphasized that apoptotic MSCs retrieved from OA knee exhibited higher immunosuppressive effects than viable MSCs.46
Complement cascade induces phagocytosis and recruit progenitor cells to the site of injury. Complement systems exhibit an interplay between MSCs to influence the biological features of engrafted cells. When a complement molecule binds to MSC, it induces phagocytosis by macrophages and in turn, M2 polarization occurs to establish a tolerogenic environment.47 With the rapid clearance of MSCs from the systemic circulation, a therapeutic response being observed was due to the interplay between complement and monocyte-phagocyte system.48 MSCs attenuates autophagy and promote cellular survival, proliferation, differentiation, and restore the functional tissues in the degenerative environment.49 Senescent MSCs exhibit minimal proliferation, differentiation, immunoregulatory, and secretory ability in OA knee.50
4.2. Future directives
Our study gives a baseline range of MSCs (5-10 × 107 cells) needed to demonstrate an effective and favorable outcome from MSC-based therapy for OA knee. However, appropriate dose-escalation studies of sufficient power and duration must be carried out to validate the findings of our study and arrive at a definitive consensus on the prevailing ambiguity in the volume and count of MSCs needed in the management of knee osteoarthritis. Although commercial vendors have their proprietary methods of dosage of MSCs, an objective and conventional method of estimation of the appropriate number of MSCs needed in the management of the disease needs to be estimated. Despite identifying the appropriate number of MSCs needed, objective measures to identify their regenerative potential with colony-forming unit (CFU) assays are also needed which is not provided in any of the included studies. Hence, we recommend reporting future trials of MSCs in OA knee to report not only the outcome of the MSCs used but also the efficacy of the source utilized based on the measures such as CFU assays.
4.3. Limitations
Our analysis has some limitations. Blinding was not established in most of the studies which might invite room for treatment bias from patients or observers. Heterogeneity was observed in most of the outcomes reported across the studies which might be due to the variability in the treatment protocols followed in the individual studies as shown in Table 2. Moreover, patients in various stages of the disease process were included in the studies which might also contribute to the heterogeneity of their results. Hence a large multicentric trial with standardized dosage and frequency protocol with established outcome assessment measures, without any adjuvant procedures is needed to further confirm the results of our analysis.
5. Conclusion
Compared to the four dosage groups of MSCs analyzed, Group III showed consistent significant improvement in pain and functional outcomes analyzed compared to the other groups. Hence, we recommend a cell volume of 5-10 × 107 cells to be delivered to the target site to obtain superior benefits out of the procedure. However, we urge future trials of sufficient quality to validate our findings to arrive at a consensus on the ideal count of MSCs to be delivered in the cellular therapy for knee osteoarthritis.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcot.2021.101744.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lopa S., Colombini A., Moretti M., de Girolamo L. Injective mesenchymal stem cell-based treatments for knee osteoarthritis: from mechanisms of action to current clinical evidences. Knee Surg Sports Traumatol Arthrosc. 2019;27(6):2003–2020. doi: 10.1007/s00167-018-5118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Matteo B., Vandenbulcke F., Vitale N.D., et al. Minimally manipulated mesenchymal stem cells for the treatment of knee osteoarthritis: a systematic review of clinical evidence. Stem Cell Int. 2019;2019:1735242. doi: 10.1155/2019/1735242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolia I.K., Bougioukli S., Hill W.J., et al. Clinical efficacy of bone marrow aspirate concentrate versus stromal vascular fraction injection in patients with knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. Published online June. 2021;8 doi: 10.1177/03635465211014500. [DOI] [PubMed] [Google Scholar]
- 4.Iijima H., Isho T., Kuroki H., Takahashi M., Aoyama T. Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: a meta-analysis toward the establishment of effective regenerative rehabilitation. NPJ Regen Med. 2018;3:15. doi: 10.1038/s41536-018-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamo-Espinosa J.M., Mora G., Blanco J.F., et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II) J Transl Med. 2016;14(1):246. doi: 10.1186/s12967-016-0998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta P.K., Chullikana A., Rengasamy M., et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther. 2016;18(1):301. doi: 10.1186/s13075-016-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vangsness C.T., Farr J., Boyd J., Dellaero D.T., Mills C.R., LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96(2):90–98. doi: 10.2106/JBJS.M.00058. [DOI] [PubMed] [Google Scholar]
- 8.Koh Y.-G., Jo S.-B., Kwon O.-R., et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 2013;29(4):748–755. doi: 10.1016/j.arthro.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Pers Y.-M., Rackwitz L., Ferreira R., et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med. 2016;5(7):847–856. doi: 10.5966/sctm.2015-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jo C.H., Chai J.W., Jeong E.C., et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am J Sports Med. 2017;45(12):2774–2783. doi: 10.1177/0363546517716641. [DOI] [PubMed] [Google Scholar]
- 11.Jo C.H., Lee Y.G., Shin W.H., et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells Dayt Ohio. 2014;32(5):1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 12.Furlan A.D., Malmivaara A., Chou R., et al. Updated method guideline for systematic reviews in the Cochrane Back and neck group. Spine. 2015;2015;40(21):1660–1673. doi: 10.1097/BRS.0000000000001061. [DOI] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group T.P. Preferred reporting Items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 15.Wallace B.C., Dahabreh I.J., Trikalinos T.A., Lau J., Trow P., Schmid C.H. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Software. 2012;49(5):1–15. doi: 10.18637/jss.v049.i05. [DOI] [Google Scholar]
- 16.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vega A., Martín-Ferrero M.A., Del Canto F., et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–1690. doi: 10.1097/TP.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 18.Garay-Mendoza D., Villarreal-Martínez L., Garza-Bedolla A., et al. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int J Rheum Dis. 2018;21(1):140–147. doi: 10.1111/1756-185X.13139. [DOI] [PubMed] [Google Scholar]
- 19.Kuah D., Sivell S., Longworth T., et al. Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: a randomized double-blind placebo-controlled single ascending dose study. J Transl Med. 2018;16(1):49. doi: 10.1186/s12967-018-1420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freitag J., Bates D., Wickham J., et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019;14(3):213–230. doi: 10.2217/rme-2018-0161. [DOI] [PubMed] [Google Scholar]
- 21.Wong K.L., Lee K.B.L., Tai B.C., Law P., Lee E.H., Hui J.H.P. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years' follow-up. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 2013;29(12):2020–2028. doi: 10.1016/j.arthro.2013.09.074. [DOI] [PubMed] [Google Scholar]
- 22.Emadedin M., Labibzadeh N., Liastani M.G., et al. Intra-articular implantation of autologous bone marrow-derived mesenchymal stromal cells to treat knee osteoarthritis: a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy. 2018;20(10):1238–1246. doi: 10.1016/j.jcyt.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Wakitani S., Imoto K., Yamamoto T., Saito M., Murata N., Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10(3):199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 24.Tran T.D.X., Wu C.-M., Dubey N.K., et al. Time- and Kellgren−Lawrence grade-dependent changes in intra-articularly transplanted stromal vascular fraction in osteoarthritic patients. Cells. 2019;8(4):E308. doi: 10.3390/cells8040308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee W.-S., Kim H.J., Kim K.-I., Kim G.B., Jin W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8(6):504–511. doi: 10.1002/sctm.18-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh Y.-G., Choi Y.-J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902–907. doi: 10.1016/j.knee.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Koh Y.-G., Kwon O.-R., Kim Y.-S., Choi Y.-J. Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 2014;30(11):1453–1460. doi: 10.1016/j.arthro.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 28.Parekkadan B., Milwid J.M. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pittenger M.F., Discher D.E., Péault B.M., Phinney D.G., Hare J.M., Caplan A.I. Mesenchymal stem cell perspective: cell biology to clinical progress. Npj Regen Med. 2019;4(1):1–15. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liebergall M., Schroeder J., Mosheiff R., et al. Stem cell-based therapy for prevention of delayed fracture union: a randomized and prospective preliminary study. Mol Ther J Am Soc Gene Ther. 2013;21(8):1631–1638. doi: 10.1038/mt.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang M., Yuan Q., Xie L. Mesenchymal stem cell-based immunomodulation: properties and clinical application. Stem Cell Int. 2018;2018 doi: 10.1155/2018/3057624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park Y., Ha C., Lee C., Yoon Y.C., Park Y. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 Years of extended follow-up. Stem Cells Transl Med. 2017;6(2):613–621. doi: 10.5966/sctm.2016-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss J.N. In: Weiss J.N., editor. Springer International Publishing; 2021. A Phase 3 Study to Evaluate the Efficacy and Safety of JointStem in the Treatment of Osteoarthritis; pp. 199–203. (Orthopedic Stem Cell Surgery). [DOI] [Google Scholar]
- 34.Hernigou P., Beaujean F., Lambotte J.C. Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg Br. 1999;81(2):349–355. doi: 10.1302/0301-620x.81b2.8818. [DOI] [PubMed] [Google Scholar]
- 35.Ham O., Lee C.Y., Kim R., et al. Therapeutic potential of differentiated mesenchymal stem cells for treatment of osteoarthritis. Int J Mol Sci. 2015;16(7):14961–14978. doi: 10.3390/ijms160714961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao X., Zhao Y., Sun X., Xing Y., Wang X., Yang Q. Immunomodulation of MSCs and MSC-derived extracellular vesicles in osteoarthritis. Front Bioeng Biotechnol. 2020 doi: 10.3389/fbioe.2020.575057. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kangari P., Talaei-Khozani T., Razeghian-Jahromi I., Razmkhah M. Mesenchymal stem cells: amazing remedies for bone and cartilage defects. Stem Cell Res Ther. 2020;11(1):492. doi: 10.1186/s13287-020-02001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang W., Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2019;53(1) doi: 10.1111/cpr.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ankrum J.A., Ong J.F., Karp J.M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y., Huang J., Gong L., et al. The plasticity of mesenchymal stem cells in regulating surface HLA-I. iScience. 2019;15:66–78. doi: 10.1016/j.isci.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghannam S., Bouffi C., Djouad F., Jorgensen C., Noël D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther. 2010;1(1):2. doi: 10.1186/scrt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang X., Ding Y., Zhang Y., Tse H.-F., Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23(9):1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 43.Ma S., Xie N., Li W., Yuan B., Shi Y., Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21(2):216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss A.R.R., Dahlke M.H. Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front Immunol. 2019;10:1191. doi: 10.3389/fimmu.2019.01191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osińska I., Popko K., Demkow U. Perforin: an important player in immune response. Cent-Eur J Immunol. 2014;39(1):109–115. doi: 10.5114/ceji.2014.42135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mancuso P., Murphy M.J., Barry F. Immunomodulatory effect of mesenchymal stem cells following intra-articular injection in a model of osteoarthritis: a potential role for apoptosis. Osteoarthritis Cartilage. 2017;25:S386–S387. doi: 10.1016/j.joca.2017.02.663. [DOI] [Google Scholar]
- 47.Ye J., Xie C., Wang C., et al. Promoting musculoskeletal system soft tissue regeneration by biomaterial-mediated modulation of macrophage polarization. Bioact Mater. 2021;6(11):4096–4109. doi: 10.1016/j.bioactmat.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gavin C., Meinke S., Heldring N., et al. The complement system is essential for the phagocytosis of mesenchymal stromal cells by monocytes. Front Immunol. 2019;10:2249. doi: 10.3389/fimmu.2019.02249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu C., Zhao L., Wu D., Li L. Modulating autophagy in mesenchymal stem cells effectively protects against hypoxia- or ischemia-induced injury. Stem Cell Res Ther. 2019;10(1):120. doi: 10.1186/s13287-019-1225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J., Ding Y., Liu Z., Liang X. Senescence in mesenchymal stem cells: functional alterations, molecular mechanisms, and rejuvenation strategies. Front Cell Dev Biol. 2020;8:258. doi: 10.3389/fcell.2020.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.