Abstract
Acoustic cavitation has been widely explored for both diagnostic and therapeutic purposes. Ultrasound-induced cavitation, including inertial cavitation and non-inertial cavitation, can cause microstreaming, microjet, and free radical formation. The acoustic cavitation effects on endothelial cells have been studied for drug delivery, gene therapy, and cancer therapy. Studies have demonstrated that the ultrasound-induced cavitation effect can treat cancer, ischaemia, diabetes, and cardiovascular diseases. In this minireview, we will review the impact of ultrasound-induced cavitation on the endothelial cells such as cell permeability, cell proliferation, gene expression regulation, cell viability, hemostasis interaction, oxygenation, and variation in the level of calcium ions, ceramide, nitric oxide (NO) and nitric oxide synthase (NOS) activity. The applications of these effects and the cavitation mechanism involved will be summarized, demonstrating the important role of acoustic cavitation in non-invasive ultrasound treatment of various physiological conditions.
Keywords: Ultrasound cavitation, cellular effects, inertial cavitation, free radical formation, microstreaming
Impact statement
Ultrasound is a well-established technology for the diagnosis and therapy of various diseases. This technology is capable of inducing bioeffects on the endothelial cells through the cavitation mechanism. These bioeffects are stimulated non-invasively and are radiation-free. Also, ultrasound technology can be easily employed in a clinical setting. This article outlines these bioeffects’ results in applications like drug delivery, gene therapy, tumor therapy, anti-angiogenic therapy, and macromolecular delivery. Therefore, this minireview will foster the upcoming research and practical applications of ultrasound cavitation for various treatments and therapies.
Introduction
Ultrasound-induced cavitation mechanisms include stable cavitation,1,2 inertial cavitation,3–13 and their resulting effects such as microstreaming,2,7,10,11,14–16 micromassage, 14 micjets, 15 and free radical formation.15,17,18 These mechanisms induce a list of bioeffects on the endothelial cells such as changes in cell permeabilization,1,3,4,19 cell proliferation,2,5,6,14 gene expression regulation,1,14,20 cell viability,4,16 and levels of calcium ions, 21 ceramide,10,15 oxygenation,13,18 nitric oxide (NO),7–9 and blood coagulation parameters.11,12,17 These bioeffects have a variety of applications including drug delivery,1,3,4,21 gene therapy,2,5,19 wound healing, 6 deoxyribonucleic acid (DNA) and protein synthesis, 14 muscle perfusion, 7 ischaemic bed perfusion, 8 limb perfusion, 9 radiosensitization,10,15 therapeutic angiogenesis, 20 tumor dissection, 17 hepatic sinusoid treatment, 11 vascular occlusion therapy, 12 macromolecular delivery, 13 chemotherapy, 18 and radiation therapy. 18 These applications of the bioeffects induced by cavitation have been found benefitting the treatment of various diseases including cardiovascular disease, 4 cancer,2,5,10,13,15,17,18 coronary artery disease, 19 sickle cell disease, 7 chronic hindlimb ischaemia, 9 and hepatic sinusoid 11 in studies performed in vivo,1,7–12,18–20 in vitro,2,3,5,6,13–17,21 and ex vivo.4,7 The openings of the blood–brain barrier (BBB)1,3,21 and renal glomerular filter barrier 14 are also studied to understand the cavitation bioeffects for applications in drug delivery and DNA and protein synthesis.
Endothelial cell lining is present throughout the vascular system, from the smallest capillary present in the body to the pumping organ of the body. 22 The endothelial cells regulate the transport of materials and white blood cells across the bloodstream. The endothelial cells serve as a barrier between blood and tissues. 23 The cerebral endothelium forms the most inelastic barrier in the brain, known as BBB. 24 The BBB is highly selective, allowing the passage of certain receptors into the brain. On the other hand, the liver sinusoidal endothelial cells are highly permeable with maximum endocytosis capacity in the body. 25 The liver sinusoid endothelial cells maintain the vascular tone during digestion. The endothelial cells in kidneys aid in glomerular filtration to avoid deficiency of proteins. 26 The pulmonary endothelial barrier helps in maintaining the alveolar surface dry for an ideal gas exchange. 27 The disruption in the continuity of the endothelial layer leads to coagulation and thrombosis due to aggregation and activation. In normal conditions, the endothelial cells resist the platelet aggregation by releasing nitric oxide and prostacyclin.28,29 The endothelial cells express factors on their surface that converts thrombin into an anticoagulant enzyme.
This minireview focuses on summarizing the bioeffects of cavitation on the endothelial cells and the related mechanisms and applications, as shown in Table 1. Further, this minireview also summarizes basic information about inertial cavitation, stable cavitation, and their interactions, as well as the existence of bubbles in a multibubble system.
Table 1.
Summary of examples of ultrasound cavitation effect on endothelial cells.
| Cellular effect | Application | Focus | Endothelial cell type | Experiment category | Ultrasound parameters | Cavitation type/results | Ref |
|---|---|---|---|---|---|---|---|
| Cell permeabilization | Drug delivery | Blood–brain barrier | Brain microvascular endothelial cells | In vivo | Power: 2.2 W Exposure time: 30 s Frequency: 1.1 MHz |
Stable cavitation | 1 |
| Porcine brain endothelial cells | In vitro | Frequency: 1 MHz Condition 1: Mode: Continuous Mechanical index: 0.11 Exposure time: 60 s Peak negative pressure: 110 kPa Condition 2: Mechanical index: 0.8 Exposure time: 5 s Duty cycle: 5% Pulse duration: 50 µs Pulse repetition frequency: 1 kHz Peak negative pressure: 800 kPa |
Inertial cavitation | 3 | |||
| Cardiovascular disease | Vascular endothelial cells | Ex vivo | Frequency: 1.1 MHz Energies: 5.0, 66, 630 J/cm2 Duty cycle: 1% |
Inertial cavitation | 4 | ||
| Gene delivery | Coronary artery disease | Vascular endothelial cells | In vivo | Transmitted frequency: 1.8 MHz Received frequency: 3.6 MHz Focal depth: 3 to 4 cm |
Reported as cavitation. | 19 | |
| Cell proliferation | Gene therapy | Tumor angiogenesis, embryonic angiogenesis. | Bovine aortic endothelial cells | In vitro | Frequency: 1 MHz Intensity: 2.2 W/cm2 Duty cycle: 20% Pulse repetition frequency: 40 Hz Pulse length: 5 ms |
Microstreaming | 2 |
| Tumor angiogenesis | Human umbilical vein endothelial cells | In vitro | Transmitted frequency: 1.7 MHz Received frequency: 3.4 MHz Mechanical index: 1.0 Exposure time: 30 s |
Inertial cavitation | 5 | ||
| Wound healing | Injured tissue | Bovine aortic endothelial cells | In vitro | Frequencies: 1 and 3.5 MHz Intensity: 1.2 W/cm2 Exposure time: 15 and 30 min |
Inertial cavitation and thermal effects | 6 | |
| DNA and protein synthesis | Renal glomerulus filter barrier | Human renal glomerular endothelial cells | In vitro | Frequecny: 1 MHz Intensity: 0.3 W/cm2 Duty cycle: 20% Exposure time: 5 min |
Reported as cavitation. | 14 | |
| Calcium ions release | Drug delivery | Blood–brain barrier | Brain microvascular endothelial cells | In vitro | Frequency: 1.25 MHz Cycles: 10 Peak negative pressure: 0.24 MPa |
Reported as cavitation. |
21 |
| Nitric oxide and nitric oxide synthase release | Muscle perfusion | Sickle cell disease | Murine endothelial cells |
In vivo
Ex vivo |
Frequency: 1.3 MHz Mechanical index: 1.3 Exposure time: 10 min |
Inertial cavitation and microstreaming | 7 |
| Ischaemic bed perfusion | Microvascular obstruction | Vascular endothelial cells | In vivo | Frequency: 1 MHz Cycles: 5000 Pulse interval: 3 s Peak negative acoustic pressure: 1.5 MPa |
Inertial cavitation | 8 | |
| Limb perfusion | Chronic hindlimb ischaemia | Murine endothelial cells | In vivo | Frequency: 1.3 MHz Pulse repetition frequency: 9.3 kHz Mechanical index: 0.6, 1.3 |
Inertial cavitation | 9 | |
| Ceramide release | Radiosensitization | Cancer | Human umbilical vein endothelial cells | In vitro | Frequency: 500 kHz Peak negative pressure: 570 kPa Mechanical index: 0.8 Duty cycle: 10% Exposure time: 30 s |
Microstreaming, jets, free radical formation | 15 |
| Vascular endothelial cells | In vivo | Pulse duration: 32 µs Cycles: 16 Pulse repetition frequency: 3 kHz Duty cycle: 0.25% Peak negative pressures: 250, 570, and 750 kPa Center frequency: 500 kHz |
Inertial cavitation, dynamic microstreaming | 10 | |||
| Gene expression regulation | Therapeutic angiogenesis | Ischaemic muscle in diabetes | Vascular endothelial cells | In vivo | Frequency: 1 MHz Power: 2 W Duty cycle: 20% Exposure time: 2 min |
Reported as cavitation. |
20 |
| DNA and protein synthesis | Renal glomerulus filter barrier | Human renal glomerular endothelial cells | In vitro | Frequecny: 1 MHz Intensity: 0.3 W/cm2 Duty cycle: 20% Exposure time: 5 min |
Reported as cavitation. | 14 | |
| Drug delivery | Blood–brain barrier | Brain microvascular endothelial cells | In vivo | Power: 2.2 W Exposure time: 30 s Frequency: 1.1 MHz |
Stable cavitation | 1 | |
| Hemostasis | Tumor dissection | Tumor | Human umbilical vein endothelial cells | In vitro | Frequency: 23.5 kHz Intensity: 10, 50, 100 W/cm2 Exposure time: 2 min |
Free radical formation | 17 |
| Hepatic sinusoid treatment | Hepatic sinusoid | Sinusoid wall endothelial cells | In vivo | Linear transducer: Mechanical index: 0.7 Pulse repetition cycles: 2750 Hz Pulse durations: 0.47 µs Central pulse frequency: 12 MHz Convex transducer: mechanical index: 1.8 Pulse repetition cycles: 1440 Hz Pulse durations: 0.48 µs Central pulse frequency: 8 MHz |
Microstreaming | 11 | |
| Vascular occlusion therapy | Vascular occlusions | Vascular endothelial cells | In vivo | Frequency: 1.17 MHz Peak rarefaction pressure amplitude: 1, 3, 6.5 or 9 MPa Duty cycle: 0.04% or 0.4% Pulse lengths: 500 or 5000 cycles |
Inertial cavitation | 12 | |
| Oxygenation | Chemotherapy and radiation therapy | Hypoxic tumor | Vascular endothelial cells | In vivo | Frequency:4.2 MHz Pulse duration: 1.6 µs Peak negative pressure: 2.5 MPa Pulse repetition frequency: 38 Hz. |
Microstreaming | 18 |
| Cell viability | Drug delivery | Cardiovascular disease | Vascular endothelial cells | Ex vivo | Frequency: 1.1 MHz Energies: 5.0, 66, or 630 J/cm2 |
Inertial cavitation | 4 |
| Drug and gene delivery | Microscale bioeffects | Human umbilical vein endothelial cells | In vitro | Frequency: 1 MHz Peak negative pressure: 0.1 MPa Duty cycle: 0.2% Pulse repetition frequency: 20 Hz Exposure time: 10 s For every minute for three times. |
Microstreaming | 16 |
Overview of acoustic cavitation
Acoustic cavitation
The acoustic cavitation is the formation of a bubble, and its growth and implosive collapse in a liquid, when exposed to an acoustic wave.30,31 An ultrasonic wave propagation with high rarefaction pressure results in the rapid formation of small cavities filled with gas and vapor in the surrounding tissue.32–34 The formation of cavities in an acoustic field is called cavitation nucleation. The ultrasound pressure required to induce cavitation depends on the medium properties and the size of pre-existing cavitation nuclei in the medium. The volumetric pulsation of the bubbles when they are further subjected to ultrasound is known as acoustic cavitation.35–40 Under an acoustic field, the resonance frequency of a gas bubble is given by equation (1)
| (1) |
where is the resonance frequency, is the equilibrium radius of the bubble, is the ambient pressure, is the liquid density, and is the polytropic index. 41 ranges between 1 and ratio of specific heat of the gas at constant temperature and volume.
When a tissue is exposed to ultrasound, it is either subjected to thermal or mechanical mechanisms resulting in biological changes. 42 The safety of the ultrasound is dependent on these mechanisms. Mechanical index is a measure of cavitation likelihood related to non-thermal bioeffects. It is defined as the ratio between peak negative pressure in MPa and square root of frequency of ultrasound in MHz, 43 and is unitless. The FDA approved maximum limit for mechanical index for diagnostic imaging is 1.9 in order to prevent any cavitation-related bioeffect. 42 The significance of mechanical index is that acoustic pressure needs to attain a specific threshold in order to trigger cavitation and cause damage.
Non-inertial cavitation
The two common types of cavitation are inertial and non-inertial cavitation.30,44,45 The type of cavitation to be evoked can be controlled by ultrasound pressure for a specific ultrasound frequency, and the threshold values are also affected by the surrounding medium. Non-inertial cavitation, also sometimes referred as stable cavitation, is sustained linear or non-linear oscillation about the equilibrium radius of an acoustically driven bubble. 30 The compressibility of the gas dominates the cavitation motion dynamics. In a low ultrasound amplitude, a bubble will undergo small symmetric radial oscillations about its equilibrium radius.46,47 However, in a high-amplitude sound field, the oscillations become larger and asymmetric. The bubbles can either gradually dissolve or grow by rectified diffusion.48–50 The stable cavitation results in microstreaming characterized by the creation of fluid flows.51–54 The effect of microstreaming is augmented when the bubbles stimulate the surface waves. The scale of fluid flows is analogous to the bubble dimensions. The microstreaming causes cell lysis and also helps in drug delivery.55–57 The stable cavitation also produces heat due to viscous losses on the surface boundary of the bubble. The scale of both the heat energy and fluid flow is related to the bubble size.
Inertial cavitation
Inertial cavitation is also sometimes called as unstable or transient cavitation. 30 Inertial cavitation is characterized by the unstable expansion and rapid collapse of the bubbles. 30 In inertial cavitation, the bubble expands unstably and collapses in a rapid and violent manner. Inertial cavitation is governed by the inertia of the surrounding medium. Energy will be released into the nearby tissues as broadband acoustic emissions when the bubbles collapse.58,59 When a stable gas-filled cavity is subjected to a high-pressure ultrasound, the rarefaction portion of the pressure causes the bubble to attain a size at which its internal pressure is equal to the vapor pressure. The progression to a vapor-filled cavity will be rapid when the equilibrium radii of the bubble are small at the onset, or at extremely high peak negative pressures. The bubbles then stop growing and collapse unstably due to the inertia of the inrushing surrounding fluid. Due to the collapse of the bubbles during inertial cavitation, a huge local pressure perturbation is produced inside the bubble, and its surrounding fluid since the bubble wall velocity approaches supersonic speeds. 60 This pressure perturbation is known as a shock wave. When the inertial collapse takes place, the gas inside the bubble is highly compressed, giving rise to high heat and pressure. Chemical reactions can also be initiated, and light can be produced.61–63 For a bubble produced near the boundary, the irregular motion of the particles present in the liquid during bubble collapse causes distortion in the cavity. 64 The potential energy of the collapsed bubble is transformed into kinetic energy of liquid jet, which can extend through the inside of the bubble and enters the wall of the opposite bubble. Particularly for large bubbles, the jet often influences the nearby boundary and piles up energy at the impact site. During sonoluminescence, light is emitted due to the high pressure and temperature created during collapse. 65 Light is due to the electric discharge at the site of the implosion of charged bubbles. 61 Single-bubble sonoluminescence (SBSL) and multi-bubble sonoluminescence (MBSL) can be described as the emission of light from a single and a lot of acoustically driven bubbles, respectively. 66 The single bubble in SBSL is produced in a partially degassed liquid.67–69 The SBSL is then produced by irradiating it with a ∼1.5 atm ultrasound wave. MBSL is generated by exposing a gassy liquid to an ultrasound of about 10 to a few 100 kHz with the power of few watts per square centimeter. 66 Inertial cavitation causes several effects in biological tissues 70 like molecular degradation and cell lysis or erosion. 71
Interactions during cavitation
Two main interactions that occur during the process of acoustic cavitation are the interaction between the acoustic field and a bubble, and the interaction between bubbles.72,73 The former is called the primary Bjerknes force and the latter is secondary Bjerknes force. A bubble in an acoustic pressure field will oscillate. When a non-zero pressure gradient is present, it will combine with the bubble oscillation to generate a translational force on the bubble. This translational force produced is known as the primary Bjerknes force and is given by equation (2)
| (2) |
where is particle volume and is acoustic pressure gradient. As a result of the primary Bjerknes force, bubbles with resonance frequency above the acoustic driving frequency will move toward points of largest pressure amplitude, while those with a lower resonance frequency will travel in the other direction.
The secondary Bjerknes force can be calculated by using equation (3)
| (3) |
where is the bubble 2 volume, is the acoustic pressure on bubble 2 from bubble 1, is the rate of change of bubble 1 volume, is the rate of change of bubble 2 volume, r is distance between bubbles, and is the liquid density. The attraction and repulsion between these two bubbles are dependent on the values of their radii with respect to the resonance radius. 74 If both the bubbles are either larger or smaller than the resonance radius, then the bubbles will be attracting each other. On the other hand, if one is larger and the other is smaller than the resonance radius or vice versa, the bubbles will be repelling each other.
Existence of bubbles in a multi-bubble system
In a multi-bubble system, bubble growth involves rectified diffusion, and bubble coalescence processes.30,74–78 In rectified diffusion, the bubble nuclei get attached to the dust present in the liquid or wall of the container. When an ultrasound wave is applied, the rectified diffusion of gas dissolved in the liquid takes place. This process results in the bubble nuclei growing into small bubbles. Bubble coalescence represents the process by which two or more bubbles combine to form a single bubble. These growing bubbles are sometimes undergoing dissolution due to higher surface tension at small radii. On the other hand, the strongly oscillating microbubbles may collapse. When the bubbles collapse, they do not disappear completely. These collapsing bubbles undergo coalescence, fragmentation, or cluster formation. The collapsing bubbles having non-spherical oscillation can fragment into smaller bubbles due to strong nonlinearity. While some of the collapsing bubbles combine to form clusters or clouds due to secondary Bjerknes forces, leading to coalescence. The extreme conditions during collapse result in the generation of highly reactive radicals.79–82 These radicals can be used in the chemical synthesis of nanoparticles or decomposition of organic pollutants. In the case of water, this will result in the formation of H+ and OH− ions. These OH− radicals when interacting with the luminol result in sonochemiluminescence.
Ultrasound cavitation induced bioeffects in endothelial cells
Change in endothelial cell permeability
Cell permeability is found to be affected by acoustic cavitation. Though the cavitation type was non-inertial or inertial in many studies, some studies have not reported any specific cavitation mechanism associated with the change in permeability. One of the main applications for increasing the endothelial cell permeability is drug delivery through BBB and for cardiovascular diseases. The studies were performed in vitro, in vivo, or ex vivo. Lelu et al. studied the important processes that control the ultrasound-induced opening of the BBB. 3 An in vitro experiment performed on primary porcine brain endothelial cells (PBECs) grown on cellZscope showed an increase in cell permeability after treated by ultrasound. The PBEC monolayers were exposed for 5 s at a mechanical index and duty cycle of 0.8 and 5%, respectively, with a pulse length of 50 µs and pulse repetition frequency of 1 kHz for inertial cavitation study. The PBEC monolayers were also exposed to continuous ultrasound wave for 60 s at a mechanical index of 0.11 for stable cavitation study. The peak negative pressures for inertial and non-inertial cavitation studies were 800 and 110 kPa, respectively. In this study, it was found that the non-inertial cavitation had a more pronounced effect on the cells than the inertial cavitation in vitro. The dominance of non-inertial cavitation was expected due to low-pressure waves pushing the bubbles towards the PBEC monolayer, thereby conveying mechanical stimuli directly on this layer for over 60 s. Stable cavitation was also predominant because of the static cellular model used in this study as it provided greater interaction time for a cell with the same bubble. The high-pressure wave of inertial cavitation was expected to cause the destruction of bubbles that were not in contact with the PBEC monolayer. An in vivo study in rats by Deng et al. aimed at assessing whether interrupting the BBB by directing focused ultrasound (FUS) power of 2.2 W and pulse duration of 30 s at 1.1 MHz frequency along with microbubbles could introduce variations in the expression of caveolin-1. 1 The membrane permeability of the brain microvascular endothelial cells was found to increase due to an increase in caveolin-1 after 1 h of ultrasound treatment. With the upregulation of caveolin-1 and eventually caveolae, the permeability of BBB was observed to increase because of the caveolae-mediated transcellular approach. The increase in caveolae was stated to be significantly due to shear stress induced by stable cavitation as a result of the FUS used in this study. Hallow et al. performed targeted delivery using ultrasound in ex vivo arteries. 4 The ex vivo arteries were treated with an ultrasound frequency of 1.1 MHz and a duty cycle of 1% at three different energies, including 5.0, 66, and 630 J/cm2. Reversible permeabilization of vascular endothelial cells was observed, and the inertial cavitation effect of ultrasound was stated as the cause of this effect. The cell viability was preserved with low uptake capacity at the lowest ultrasound energy 5.0 J/cm2. Also, the medial layers showed no intracellular uptake or loss in cell viability when exposed to the low energy pulse. But, the highest intensity has bioeffects in medial layers.
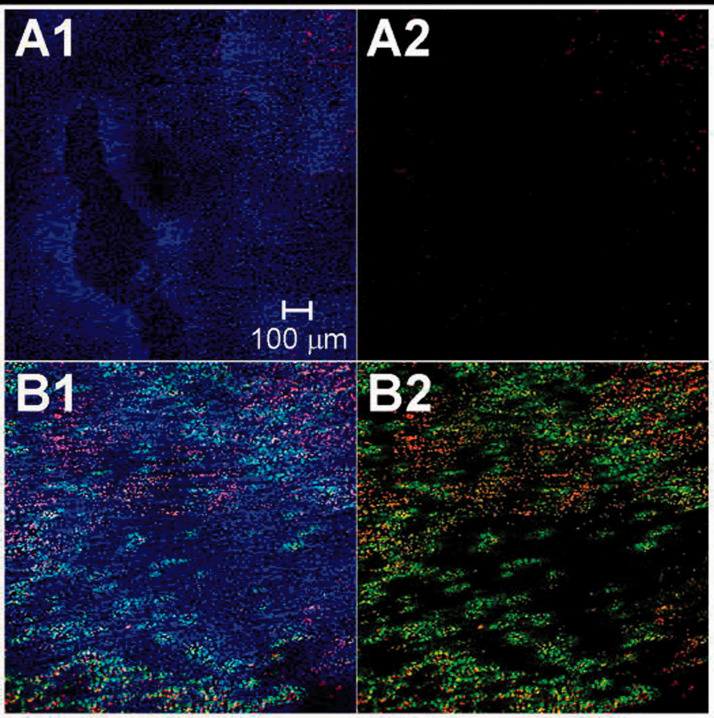
The change in cell permeability is also induced in gene delivery using acoustic cavitation. Figure 1 shows the cellular uptake due to reversible permeabilization and cell viability for intermediate ultrasound energy. The likelihood of gene delivery to the myocardium in rats utilizing ultrasound-mediated microbubble destruction (UMMD) was investigated. 19 A second harmonic ultrasound wave of transmitted and received frequencies of 1.8 and 3.6 MHz with a focal depth of 3 to 4 cm was used. It was found that on the combined treatment of UMMD and vascular endothelial growth factor (VEGF) injection, the increase in membrane permeability and intercellular spaces of vascular endothelial cells were maximum. The change in permeability and intercellular spaces were due to ultrasound cavitation resulting in stimulated angiogenesis. It was concluded that UMMD is a non-invasive technique that could potentially be used for targeting genes to heart.
Figure 1.
Reversible cell permeability for gene delivery. Confocal microscopy images at 10× magnification showing the localization of ultrasound enhanced uptake. (a) is control and (b) is sample treated with intermediate ultrasound energy. (a2) and (b2) were displayed without any blue fluorescence. Nuclei was stained with Hoechst 33342 in blue color, dead cells with propidium iodide in red color and intracellular uptake with TO-PRO®-1 in green color. Reprinted from Hallow et al., 4 Copyright (2007), with permission from Elsevier. (A color version of this figure is available in the online journal.)
Modification in cell proliferation
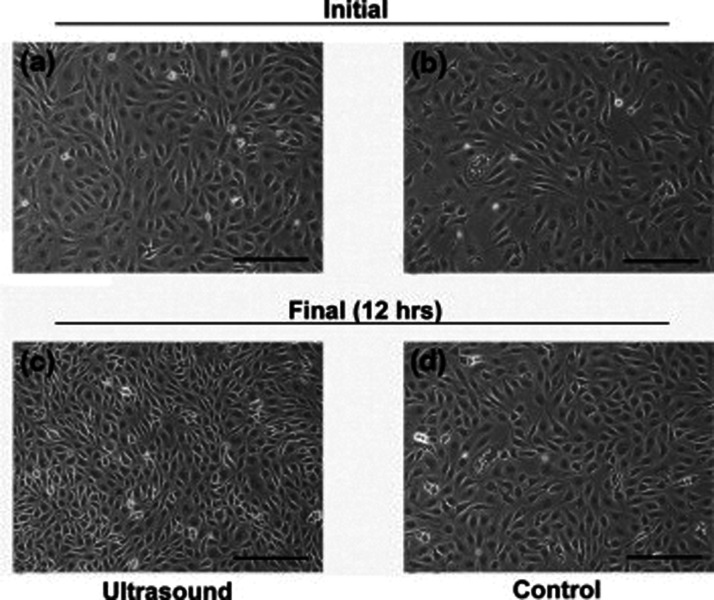
The change in cell proliferation due to the ultrasound cavitation effect is exploited in different applications like gene therapy, wound healing, and controlling the synthesis of DNA and protein. The ultrasound cavitation mechanisms involved in altering the cell proliferation are cavitation, both inertial or non-inertial, induced thermal effects, microstreaming, and micromassage. The angiogenic role of endothelial cells when irradiated with ultrasound was investigated using an in vitro 3D-spheroid bovine aortic endothelial cell (BAEC) culture models. 2 Upon 30-min ultrasound irradiation at 1 MHz with 2.2 W/cm2 energy, 20% duty cycle, 40 Hz pulse repetition frequency, and 5 ms pulse length, down-regulation and redistribution of Flk-1 occurred, and BAEC proliferation rates increased as shown in Figure 2, and migration and sprouting got enhanced in the 3D-spheroid BAEC cultures. The mechanism involved was cavitation-induced micro-streaming stimulating BAECs through localized, high fluid shear stress on the surface of BAECs. Another study reported a reduction in cell proliferation of cultured human renal glomerular endothelial cells (HRGEC) after the combination treatment with low-intensity pulsed ultrasound (LIPUS) and SonoVue. 14 The LIPUS was applied at 1 MHz with 0.3 W/cm2 intensity and 0.2% duty cycle for 5 min. An increase in cell death and repression of the extracellular signal-regulated protein kinases 1 and 2 signaling pathways was also observed only on combinational treatment of LIPUS and SonoVue. Also, the effect of either LIPUS alone or SonoVue alone on cell proliferation was not significant. This combined treatment effect as a result of the enhancement of ultrasound cavitation was observed due to the SonoVue acting as a supply for cavitation nuclei.
Figure 2.
Time-lapse microscopy of ultrasound-mediated variation in cell proliferation of endothelial cell culture. Cells treated with ultrasound for 15 min (a) immediately and (c) 12 h after treatment. Control cells (b) initial and (d) after 12 h. Cell proliferation rate increase was evident in (c). Scale bar: 50 µm. Reprinted from Mizrahi et al., 2 Copyright (2007), with permission from Elsevier.
The potential of therapeutic ultrasound in wound healing applications was studied on cultured BAECs grown on a Petri dish. 6 The therapeutic ultrasound was applied to petri dish at 1 and 3.5 MHz with an intensity of 1.2 W/cm2 for 15 and 30 min in continuous wave mode and pulsed wave mode with a 50% duty cycle. The continuous mode was found to have a stronger effect on cell proliferation for both frequencies when applied for 15 min than the pulsed mode. Also, the change in proliferation rate was independent of the intensity and duration. In both modes, the number of dead cells was less than 3% for all the conditions which was found to be due to cell culture conditions and not due to ultrasound. It was concluded that therapeutic ultrasound sonication could increase BAEC proliferation and migration through inertial cavitation and thermal effects, thereby inducing tissue remodeling. The cellular alterations induced by ultrasound sonication were stated to be due to the transfer of acoustic pressure waves.
The role of ultrasound exposure with microbubbles in increasing liposomal transfection was investigated in vitro on the human umbilical vein endothelial cells (HUVECs) grown on a six-well plate. 5 The cells were exposed to a second harmonic ultrasonic wave of transmitted frequency 1.7 MHz and received frequency of 3.4 MHz with a mechanical index of 1.0 for 30 s along with microbubbles. The UMMD along with liposome augmented the gene transfection by inhibiting the proliferation and migration of HUVECs through inertial cavitation. The inertial cavitation resulted in pores on the cell membrane, increasing the cell permeability resulting in increased delivery of huge molecules into the cells. Also, no apparent cell damage was seen.
Change in calcium ion levels
Acoustic cavitation can elevate the inter- and intra-cellular calcium levels with potential for application in drug delivery. 21 Though the possibility of the change in calcium levels is demonstrated, 21 the area remains largely unexplored as the cavitation mechanisms are unknown. The effects of ultrasound-stimulated microbubbles on murine brain microvascular endothelial cells grown on µ-Slide I0.8 Luer for the localized opening of the BBB was investigated. 21 The ultrasound was applied at 1.25 MHz for 10 cycles with a peak negative pressure of 0.24 MPa. Sonoporation and changes in intracellular calcium concentration ([Ca2+]) were monitored. The membrane poration without any membrane disruption and generation of transient calcium ions were observed. Though the cause of this effect was reported to be cavitation, the imaging frame rate limitation was stated to be a hindrance in the determination of the type of cavitation mechanism behind the bubble dynamics. Hence, no specific cavitation mechanism was mentioned.
Raise in NO levels and NOS activity
Acoustic cavitation is capable of increasing the levels of NO levels and nitric oxide synthase (NOS) activity in endothelial cells.7–9 Hence, the cavitation effect of ultrasound is used in perfusion related studies for diseases like sickle cell anemia, cardiovascular diseases, and chronic hindlimb ischaemia. The ultrasound cavitation mechanism mainly reported to be involved in perfusion is inertial cavitation. Belcik et al. tested whether the increase in shear-dependent muscle perfusion was due to purinergic signaling during cavitation. 7 In vivo evaluation of microvascular perfusion and adenosine triphosphate (ATP) release was performed on proximal hindlimb of mice. The ultrasound was applied to the proximal hindlimb at 1.3 MHz, 1.3 mechanical index for 10 min. In addition, NO release was assessed in samples obtained from muscle samples of mice, and ATP release was evaluated in vitro in both the erythrocytes and murine endothelial cells. The extracellular ATP release from erythrocytes and endothelial cells was found to be related to microstreaming and inertial cavitation. The phosphorylated endothelial NOS (eNOS) was observed to be increased after ultrasound treatment due to the release of ATP stimulating pathways involved in activating eNOS. Gary et al. reported an increase in microperfusion and endothelium-derived NO bioavailability in a rat hind limb model when applying synergistic ultrasound-targeted microbubble cavitation (UTMC) with the NO donor, sodium nitrite. 8 The ultrasound parameters used were 1 MHz frequency, 5000 cycles, 3 s pulse interval, and 1.5 MPa peak negative pressure. Inertial cavitation was found to be the cause of the increase in the NO production by vascular endothelial cells. Also, the NO production was significantly higher in synergistic sodium nitrite with UTMC than sodium nitroprusside, a direct NO donor, with UTMC. Another study reported an ultrasound-mediated increase in perfusion was proved to be increased by microbubble contrast agents that undergo ultrasound-mediated cavitation. 9 The ultrasound was applied to abductor muscles at two different mechanical indices 0.6 and 1.3 at 1.3 MHz, 9.3 kHz pulse repetition frequency, and 5 s pulse interval with and without microbubbles. Passive cavitation detection was used to evaluate the degree of inertial cavitation, which was found to be higher when the mechanical index was 1.3. The study was conducted in vivo to understand cavitation in peripheral artery disease and critical limb ischaemia. An increase in NO level and flow augmentation was observed following treatment with 1.3 mechanical index ultrasound power for 10 min both with and without the microbubble. It was found that activation of eNOS in murine endothelial cells was through inertial cavitation, which caused limb perfusion.
Modulation in ceramide level and its signaling pathway
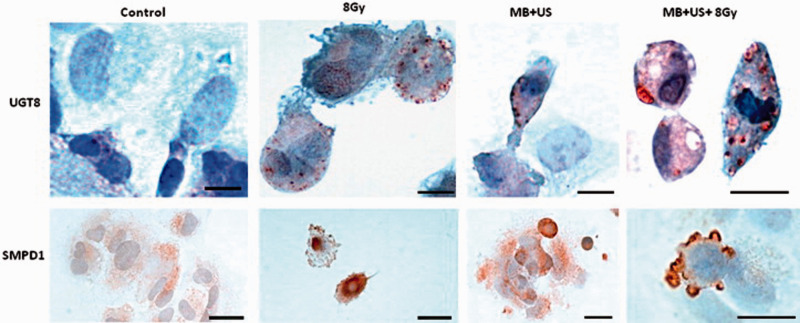
Ultrasound-stimulated microbubbles are shown to increase the radiation sensitivity of tumor tissues and induce cellular apoptosis during radiation therapy. This increase in radiation sensitivity was studied in vivo and in vitro. The variation in gene expression resulting in radiation enhancement caused by ultrasound-stimulated microbubble exposure was studied in vitro on HUVECs. 15 The HUVECs were sonicated at 500 kHz, 570 kPa peak negative pressure, 0.8 mechanical index, and 10% duty cycle for 30 s. The regulation of genes involved in the ceramide signaling pathway on ultrasound treatment was observed to cause the ceramide regulated cell apoptosis in HUVECs grown on a chip. The upregulation of UGT8 gene is shown in Figure 3. The ceramides were expected to be produced due to physical cell membrane disruption by microstreaming or jets due to active or passive cavitation, respectively, or chemically damaged by free radical formation as a result of bubble collapse. Another study investigated the potential of radiosensitization of ultrasound-stimulated microbubbles in mice with prostate xenograft tumors. 10 The animals were treated using ultrasound at 500 kHz, 32 µs pulse duration, 16 cycles, 3 kHz pulse repetition frequency, and 0.25% duty cycle. Three different peak negative pressures were used, including 250, 570, and 750 kPa. For each peak negative pressures, 8, 80, and 1000 µL/kg microbubble concentrations were considered. The increase in ultrasound pressure produced higher apoptosis compared with the increase in radiation dose and microbubble concentration. At low microbubbles concentration, only high power ultrasound had more elevated amounts of ceramide. At 80 and 1000 µL/kg microbubble concentrations, both 570 and 750 kPa had more significant amounts of ceramide. An increase in ceramide level was reported after ultrasound treatment due to vascular endothelial cell damage. This vascular endothelial injury was interpreted to be due to bubble bursting and vasodilation caused by shear stress induced by inertial cavitation and microstreaming, respectively.
Figure 3.
Morphological observation of apoptosis due to the signaling of ceramide pathway using immunohistochemistry. The detection of UGT8 and SMDP1 was shown in top and bottom row. Control: untreated cells, 8 Gy: treated with 8 Gy only, MB + US: treated with ultrasound-activated microbubbles and US + MB + 8Gy: combination of 8 Gy and ultrasound-activated microbubbles. Specific reactivity was observed in damaged cells in US + MB and 8 Gy only group (top). Apoptosis and UGT8 staining were observed in certain compartment of the cells (top). Scale bars (top): 15 µm. Scale bars (bottom): control, 8 Gy, MB + US: 25 µm, and MB + US + 8 Gy: 12 µm. Reprinted from Al-Mahrouki et al., 15 Copyright (2020), with permission from Elsevier. (A color version of this figure is available in the online journal.)
Gene expression regulation in endothelial cells
Ultrasound cavitation has demonstrated great potential in regulating gene expression, which has applications in drug delivery and gene therapy. The ultrasound-induced cavitation was shown to induce angiogenesis in the ischaemic skeletal muscle of diabetic mice. 20 During this study, 2 W of ultrasound energy was applied at 1 MHz and 20% duty cycle to the skeletal muscle for 2 min. An upregulation of proinflammatory cytokines: P-selectin and ICAM-1, which are messenger ribonucleic acid (mRNA) and protein expressions of VEGF, was observed after applying ultrasound on the lower limb of the diabetic mice. The upregulation of these parameters then induced the secretion of endogenous VEGF secretion from vascular endothelial cells promoting angiogenesis and was stated to be due to ultrasonic drilling as a result of the combined cavitation and sonoporation effects. Liu et al. studied the effects of LIPUS and sonovue on the microvascular system and underlying molecular mechanisms in vitro on HRGECs. 14 The LIPUS was applied at 1 MHz with 0.3 W/cm2 intensity and 0.2% duty cycle for 5 min. A significant reduction in phosphorylated ERK1/2 was observed on the combined treatment of ultrasound and sonovue. Reduced cell proliferation increased cell death and inhibited the activation of ERK1/2, thereby, inducing cytotoxicity in these cells. The biological effects including degrading DNA and inhibiting the proliferation of HRGECs were found to be due to the cavitation effects of LIPUS. The possibility of induced changes in the density of caveolae and the expression of the structural protein caveolin-1 by disrupting the BBB in rats by applying FUS combined with microbubbles was studied in vivo. 1 During this study, the 1.1 MHz FUS was applied at 2.2 W power for 30 s to perpendicular to the dorsal surface of the skull. Both the FUS and the combination of FUS and microbubble resulted in a significant increase of caveolin-1. The increase was predominant in combined treatment. Caveolin-1 expression upregulation and increase in permeability of the brain microvascular endothelial cells were observed in the BBB after sonication because of stable cavitation.
Hemostatis and oxygenation
Ultrasound cavitation is proven to induce hemostasis interaction and increase the levels of oxygen and reactive oxygen species in tumors. An in vitro model system with HUVECs was developed to stimulate and measure the hemostatic effects of high power ultrasound applied to the outer surface of blood vessels during tumor dissection. 17 The HUVECs grown on plates were sonicated at 23.5 kHz for 2 min at 10, 50, and 100 W/cm2 intensities. The prostacyclin effect dominated as prostaglandin F1α levels was higher at 100 W/cm2 compared with thromboxane B2. Plasminogen activator inhibitor type 1 secretion was significant at low sound intensity. But, thrombomodulin and thrombospondin did not exhibit any significant response to the ultrasound. Also, enormous morphological damage to the endothelium was observed. The mechanism behind the release of these coagulation parameters was discovered to be cavitation-induced free radical formation. In the other study, the ability of contrast ultrasonography to affect sinusoidal wall endothelial cells and platelets in the liver was examined in in vivo models. 11 Wistar rats were treated with contrast ultrasonography. The air-filled microbubbles were used. The ultrasound was applied initially using a linear transducer for 1 min, followed by 4 min pause, then treated with both the linear and convex probes for 10 min. The linear transducer parameters were 0.7 mechanical index, 2750 Hz pulse repetition rate, 0.47 µs pulse duration, and 12 MHz central pulse frequency. The convex transducer parameters were 1.8 mechanical index, 1440 Hz pulse repetition rate, 0.48 µs pulse duration, and 8 MHz central pulse frequency. The abdomen of the rats was shaved to focus the ultrasound on the liver. Then, light and electron microscopies were used to study the results. It was concluded that contrast ultrasonography causes aggregation of platelets and damage in endothelial cells of the sinusoid wall in rat hepatic sinusoid possibly because of microstreaming. Another study found that the principal mechanism behind vascular endothelium damage was inertial cavitation. 12 In the study, rabbit auricular vessels with the presence of a gas-based microbubble ultrasound contrast agent were used in the experiments. The vessels were sonicated at 1.17 MHz with 1 Hz pulse repetition frequency. The inertial cavitation was detected using passive cavitation detection. The correlation between peak rarefaction pressure, inertial cavitation dose, and cell damage was studied by varying peak rarefaction pressures. This correlation study parameters were 500 cycles pulse length and 0, 1, 3, 6.5, and 9 MPa peak rarefaction pressure for an exposure duration of 120 s. The damage to the vascular endothelium increased with the increase in peak pressures. In the presence of an ultrasound contrast agent, the platelet adhesion increased with peak pressure. The correlation between pulse length, inertial cavitation dose, and cell damage was studied by varying pulse length and duration of the treatment. This correlation study parameters were 500, 5000 cycles pulse length, and 9 MPa peak rarefaction pressure for an exposure duration of 1, 10, and 30 s. Scanning electron microscopic imaging was employed to verify the results of vascular endothelial damage. From both the correlation experiments, it was concluded that the dose of inertial cavitation correlated with the level of vascular endothelium damage in the presence of an ultrasound contrast agent. Also, this could have a potential application in vascular occlusion therapy.
A confined oxygen microbubble delivery was performed to overcome hypoxia before chemo and radiation therapy for cancer. 18 Female nude mice bearing breast tumor was used in this study. Surfactant-shelled microbubbles with oxygen were injected into the tumor site. Ultrasound with a mechanical index of about 0.09 was first used to perfuse the microbubbles into the tumor. Then, destructive ultrasound pulses were applied at 4.2 MHz, 1.6 µs pulse duration, 2.5 MPa peak negative pressure, and 38 Hz pulse repetition frequency to elevate the oxygen level in the tumor tissue by disrupting all the bubbles. An increase in oxygen level was expected to be due to microstreaming as a result of acoustic cavitation, and acoustic radiation forces. Acoustic cavitation increased the membrane permeability of vascular endothelial cells, thereby easing the perfusion of bubbles into tumor site. Hence, the radiosensitivity of the tumor tissue was improved for therapy.
Conclusion and future directions
The cavitation effect of ultrasound possesses many beneficial bioeffects with application in various treatment and therapies.
Two reasons hinder its progress in real-world applications. First, majority of the studies are limited to in vitro experiments on cell lines with only two studies that are performed in vivo experiments. This might be due to two reasons. Firstly, the ultrasound parameters required to produce a desire biological effect have to be optimized and the optimization would require large number of animals. Secondly, the time and cost involved in creating animal models in vivo studies are usually extensive. Hence, the in vivo study can be performed once the parameters are optimized and the hypothesis is confirmed with in vitro experiments. This would save both cost and time involved in in vivo studies. This possesses a greater challenge as the capability of same ultrasound dose to induce similar effect in vivo is still not investigated. Second, the exact cavitation effect behind certain bioeffects is still unexplored. Finally, though bioeffects are promising for some specific applications, there is no established test method to prove the exact cavitation mechanism that is involved.
The ultrasound cavitation is demonstrated to be inducing several biological effects on the endothelial cells including change in cell permeability, proliferation, nitric oxide, ceramide, calcium ions, and cellular pathways. The majority of studies reported the intertial cavitation as the cause. Recently, the ultrasound cavitation capability to alter the expression of genes in the endothelial cells of brain through sonoselective transfection of genes to blood vessels. 83 This study helps to understand the effects of the release of endothelial cell secretions in stimulating nerve growth and shoes the capability of ultrasound cavitation to which is a niche area to be explored. Another study selectively delivered recombinant BRICHOS to the hippocampus and cortex of the brain for treating Alzheimer’s disease. 84 The potential applications include Alzheimer’s and Parkinson’s diseases. The potential for cavitation related bioeffects is always explored for novel application and its current applications are continuously developing. Hence, the cavitation effect on endothelial cells could soon be used for various clinical applications in a healthcare environment.
Footnotes
AUTHORS’ CONTRIBUTIONS: All the authors have contributed equally in writing and editing this article.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part through a National Institutes of Health (NIH) grant R01EY029489.
ORCID iD: Madhumithra Subramanian Karthikesh https://orcid.org/0000-0001-6397-9357
References
- 1.Deng J, Huang Q, Wang F, Liu Y, Wang Z, Wang Z, Zhang Q, Lei B, Cheng Y. The role of caveolin-1 in blood–brain barrier disruption induced by focused ultrasound combined with microbubbles. J Mol Neurosci 2012; 46:677–87 [DOI] [PubMed] [Google Scholar]
- 2.Mizrahi N, Seliktar D, Kimmel E. Ultrasound-induced angiogenic response in endothelial cells. Ultrasound Med Biol 2007; 33:1818–29 [DOI] [PubMed] [Google Scholar]
- 3.Lelu S, Afadzi M, Berg S, Åslund A, Torp SH, Sattler W, Davies CDL. Primary porcine brain endothelial cells as in vitro model to study effects of ultrasound and microbubbles on blood–brain barrier function. IEEE Trans Ultrason Ferroelectr Freq Control 2016; 64:281–90 [DOI] [PubMed] [Google Scholar]
- 4.Hallow DM, Mahajan AD, Prausnitz MR. Ultrasonically targeted delivery into endothelial and smooth muscle cells in ex vivo arteries. J Control Release 2007; 118:285–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C, Zhang X, Liu C, Wang J, Liu X, Li H, Wang J, Wu C. Expression of endostatin mediated by a novel non-viral delivery system inhibits human umbilical vein endothelial cells in vitro. Mol Biol Rep 2010; 37:1755–62 [DOI] [PubMed] [Google Scholar]
- 6.Raz D, Zaretsky U, Einav S, Elad D. Cellular alterations in cultured endothelial cells exposed to therapeutic ultrasound irradiation. Endothelium 2005; 12:201–13 [DOI] [PubMed] [Google Scholar]
- 7.Belcik JT, Davidson BP, Xie A, Wu MD, Yadava M, Qi Y, Liang S, Chon CR, Ammi AY, Field J. Augmentation of muscle blood flow by ultrasound cavitation is mediated by ATP and purinergic signaling. Circulation 2017; 135:1240–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gary Z, Istvanic F, Chen X, Nouraie M, Shiva S, Straub AC, Pacella JJ. Ultrasound-targeted microbubble cavitation with sodium nitrite synergistically enhances nitric oxide production and microvascular perfusion. Ultrasound Med Biol 2020; 46:667–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belcik JT, Mott BH, Xie A, Zhao Y, Kim S, Lindner NJ, Ammi A, Linden JM, Lindner JR. Augmentation of limb perfusion and reversal of tissue ischemia produced by ultrasound-mediated microbubble cavitation. Circ Cardiovasc Imaging 2015; 8:e002979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HC, Al-Mahrouki A, Gorjizadeh A, Karshafian R, Czarnota GJ. Effects of biophysical parameters in enhancing radiation responses of prostate tumors with ultrasound-stimulated microbubbles. Ultrasound Med Biol 2013; 39:1376–87 [DOI] [PubMed] [Google Scholar]
- 11.Shigeta K, Itoh K, Ookawara S, Taniguchi N, Omoto K. Endothelial cell injury and platelet aggregation induced by contrast ultrasonography in the rat hepatic sinusoid. J Ultrasound Med 2004; 23:29–36 [DOI] [PubMed] [Google Scholar]
- 12.Hwang JH, Tu J, Brayman AA, Matula TJ, Crum LA. Correlation between inertial cavitation dose and endothelial cell damage in vivo. Ultrasound Med Biol 2006; 32:1611–9 [DOI] [PubMed] [Google Scholar]
- 13.Jia C, Xu L, Han T, Cai P, Alfred C, Qin P. Generation of reactive oxygen species in heterogeneously sonoporated cells by microbubbles with single-pulse ultrasound. Ultrasound Med Biol 2018; 44:1074–85 [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Wang B, Ding H, Shi H, Liu J, Sun H. Low-intensity pulsed ultrasound in combination with SonoVue induces cytotoxicity of human renal glomerular endothelial cells via repression of the ERK1/2 signaling pathway. Ren Fail 2018; 40:458–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Mahrouki AA, Karshafian R, Giles A, Czarnota GJ. Bioeffects of ultrasound-stimulated microbubbles on endothelial cells: gene expression changes associated with radiation enhancement in vitro. Ultrasound Med Biol 2012; 38:1958–69 [DOI] [PubMed] [Google Scholar]
- 16.Juffermans LJ, van Dijk A, Jongenelen CA, Drukarch B, Reijerkerk A, de Vries HE, Kamp O, Musters RJ. Ultrasound and microbubble-induced intra-and intercellular bioeffects in primary endothelial cells. Ultrasound Med Biol 2009; 35:1917–27 [DOI] [PubMed] [Google Scholar]
- 17.Karsch U, Henn W, Seyfert UT, Steudel WI, Reif J. Effect of high power ultrasound on endothelial cells–an in vitro study of the endothelium–hemostasis interaction. Clin Hemorheol Microcirc 2002; 27:123–35 [PubMed] [Google Scholar]
- 18.Eisenbrey JR, Shraim R, Liu J-B, Li J, Stanczak M, Oeffinger B, Leeper DB, Keith SW, Jablonowski LJ, Forsberg F. Sensitization of hypoxic tumors to radiation therapy using ultrasound-sensitive oxygen microbubbles. Int J Radiat Oncol Biol Phys 2018; 101:88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhigang W, Zhiyu L, Haitao R, Hong R, Qunxia Z, Ailong H, Qi L, Chunjing Z, Hailin T, Lin G. Ultrasound-mediated microbubble destruction enhances VEGF gene delivery to the infarcted myocardium in rats. Clin Imaging 2004; 28:395–8 [DOI] [PubMed] [Google Scholar]
- 20.Song Y, Xie X, Gao Y, Jin L, Wang P. Ultrasound-induced microbubble cavitation promotes angiogenesis in ischemic skeletal muscle of diabetic mice. Int J Clin Exp Med 2016; 9:23345–50 [Google Scholar]
- 21.Park J, Fan Z, Kumon RE, El-Sayed ME, Deng CX. Modulation of intracellular Ca2+ concentration in brain microvascular endothelial cells in vitro by acoustic cavitation. Ultrasound Med Biol 2010; 36:1176–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Blood vessels and endothelial cells. In: Mol biol cell. 4th ed. New York, NY: Garland Science, 2002
- 23.Aman J, Weijers EM, van Nieuw Amerongen GP, Malik AB, van Hinsbergh VW. Using cultured endothelial cells to study endothelial barrier dysfunction: challenges and opportunities. Am J Physiol Lung C 2016; 311:L453–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stamatovic SM, Keep RF, Andjelkovic AV. Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Curr Neuropharmacol 2008; 6:179–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poisson J, Lemoinne S, Boulanger C, Durand F, Moreau R, Valla D, Rautou P-E. Liver sinusoidal endothelial cells: physiology and role in liver diseases. J Hepatol 2017; 66:212–27 [DOI] [PubMed] [Google Scholar]
- 26.Satchell SC, Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am J Physiol-Renal 2009; 296:F947–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rounds S, Lu Q, Harrington EO, Newton J, Casserly B. Pulmonary endothelial cell signaling and function. Trans Am Clin Climatol Assoc 2008; 119:155–67 [PMC free article] [PubMed] [Google Scholar]
- 28.Michiels C. Endothelial cell functions. J Cell Physiol 2003; 196:430–43 [DOI] [PubMed] [Google Scholar]
- 29.Verhamme P, Hoylaerts M. The pivotal role of the endothelium in haemostasis and thrombosis. Acta Clin Belg 2006; 61:213–9 [DOI] [PubMed] [Google Scholar]
- 30.Leighton T. The acoustic bubble. Cambridge, MA: Academic Press, 2012. [Google Scholar]
- 31.Coussios C, Farny C, Ter Haar G, Roy R. Role of acoustic cavitation in the delivery and monitoring of cancer treatment by high-intensity focused ultrasound (HIFU). Int J Hyperthermia 2007; 23:105–20 [DOI] [PubMed] [Google Scholar]
- 32.Church CC. Spontaneous homogeneous nucleation, inertial cavitation and the safety of diagnostic ultrasound. Ultrasound Med Biol 2002; 28:1349–64 [DOI] [PubMed] [Google Scholar]
- 33.Miller MW, Everbach EC, Miller WM, Battaglia LF. Biological and environmental factors affecting ultrasound-induced hemolysis in vitro: 2. Medium dissolved gas (pO2) content. Ultrasound Med Biol 2003; 29:93–102 [DOI] [PubMed] [Google Scholar]
- 34.Apfel R. Acoustic cavitation inception. Ultrasonics 1984; 22:167–73 [Google Scholar]
- 35.Apfel R7. Acoustic cavitation. In: Edmonds PD. (ed.) Methods in experimental physics. London: Academic Press, 1981, pp.355–411 [Google Scholar]
- 36.Neppiras EA. Acoustic cavitation. Phys Rep 1980; 61:159–251 [Google Scholar]
- 37.Apfel R. Acoustic cavitation prediction. J Acoust Soc Am 1981; 69:1624–33 [Google Scholar]
- 38.Crum L, Fowlkes J. Acoustic cavitation generated by microsecond pulses of ultrasound. Nature 1986; 319:52–4 [Google Scholar]
- 39.Holland CK, Apfel RE. Thresholds for transient cavitation produced by pulsed ultrasound in a controlled nuclei environment. J Acoust Soc Am 1990; 88:2059–69 [DOI] [PubMed] [Google Scholar]
- 40.Miller DL. Gas body activation. Ultrasonics 1984; 22:259–60 [DOI] [PubMed] [Google Scholar]
- 41.Leighton T. The principles of cavitation. Ultrasound Food Process 1998; 12:151–82 [Google Scholar]
- 42.Kollmann C, Ter Haar G, Dolezal L, Hennerici M, Salvesen K, Valentin L. Ultrasound output: thermal (TI) and mechanical (MI) indices. Ultraschall Med 2013; 34:422–34 [DOI] [PubMed] [Google Scholar]
- 43.Şen T, Tüfekçioğlu O, Koza Y. Mechanical index. Anatol J Cardiol 2015; 15:334–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flynn H. Physics of acoustic cavitation in liquids. Phys Acoustics 1964; 1B:57–172 [Google Scholar]
- 45.Young F. Cavitation. London: McGraw-Hill, 1989 [Google Scholar]
- 46.Prosperetti A. Thermal effects and damping mechanisms in the forced radial oscillations of gas bubbles in liquids. J Acoust Soc Am 1977; 61:17–27 [Google Scholar]
- 47.Prosperetti A. Bubble phenomena in sound fields: part two. Ultrasonics 1984; 22:115–24 [Google Scholar]
- 48.Holt RG, Roy RA. Bubble dynamics in therapeutic ultrasound. In: Dionikov A (ed.) Bubble and particle dynamics in acoustic fields: Modern trends and applications. Transworld Research Network, Kerala. 2005, pp.108–229.
- 49.Crum LA, Hansen GM. Generalized equations for rectified diffusion. J Acoust Soc Am 1982; 72:1586–92 [Google Scholar]
- 50.Church CC. Prediction of rectified diffusion during nonlinear bubble pulsations at biomedical frequencies. J Acoust Soc Am 1988; 83:2210–7 [DOI] [PubMed] [Google Scholar]
- 51.Elder SA. Cavitation microstreaming. J Acoust Soc Am 1959; 31:54–64 [Google Scholar]
- 52.Nyborg W. Physical acoustics. Vol. 2B. New York, NY: Academic Press, 1965. [Google Scholar]
- 53.Miller DL. Particle gathering and microstreaming near ultrasonically activated gas-filled micropores. J Acoust Soc Am 1988; 84:1378–87 [DOI] [PubMed] [Google Scholar]
- 54.Wu J, Du G. Streaming generated by a bubble in an ultrasound field. J Acoust Soc Am 1997; 101:1899–907 [Google Scholar]
- 55.Datta S, Coussios C-C, McAdory LE, Tan J, Porter T, De Courten-Myers G, Holland CK. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound Med Biol 2006; 32:1257–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rooney JA. Hemolysis near an ultrasonically pulsating gas bubble. Science 1970; 169:869–71 [DOI] [PubMed] [Google Scholar]
- 57.Williams AR. Disorganization and disruption of mammalian and amoeboid cells by acoustic microstreaming. J Acoust Soc Am 1972; 52:688–93 [Google Scholar]
- 58.Mellen RH. Ultrasonic spectrum of cavitation noise in water. J Acoust Soc Am 1954; 26:356–60 [Google Scholar]
- 59.Holt RG, Crum LA. Acoustically forced oscillations of air bubbles in water: experimental results. J Acoust Soc Am 1992; 91:1924–32 [Google Scholar]
- 60.Ohl S-W, Klaseboer E, Khoo BC. Bubbles with shock waves and ultrasound: a review. Interface Focus 2015; 5:20150019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harvey EN. Sonoluminescence and sonic chemiluminescence. J Am Chem Soc 1939; 61:2392–8 [Google Scholar]
- 62.Suslick KS. Sonochemistry. Science 1990; 247:1439–45 [DOI] [PubMed] [Google Scholar]
- 63.Roy R. Physical aspects of sonoluminescence from acoustic cavitation. Ultrason Sonochem 1994; 1:S5–S8 [Google Scholar]
- 64.Brujan EA, Ikeda T, Matsumoto Y. Jet formation and shock wave emission during collapse of ultrasound-induced cavitation bubbles and their role in the therapeutic applications of high-intensity focused ultrasound. Phys Med Biol 2005; 50:4797–809 [DOI] [PubMed] [Google Scholar]
- 65.Young FR. Sonoluminescence. Boca Raton, FL: CRC press, 2004. [Google Scholar]
- 66.Suslick KS, Flannigan DJ. Inside a collapsing bubble: sonoluminescence and the conditions during cavitation. Annu Rev Phys Chem 2008; 59:659–83 [DOI] [PubMed] [Google Scholar]
- 67.Gaitan DF, Crum LA, Church CC, Roy RA. Sonoluminescence and bubble dynamics for a single, stable, cavitation bubble. J Acoust Soc Am 1992; 91:3166–83 [Google Scholar]
- 68.Brenner MP, Hilgenfeldt S, Lohse D. Single-bubble sonoluminescence. Rev Mod Phys 2002; 74:425–84 [DOI] [PubMed] [Google Scholar]
- 69.Putterman SJ, Weninger KR. Sonoluminescence: how bubbles turn sound into light. Annu Rev Fluid Mech 2000; 32:445–76 [Google Scholar]
- 70.Dalecki D. Mechanical bioeffects of ultrasound. Annu Rev Biomed Eng 2004; 6:229–48 [DOI] [PubMed] [Google Scholar]
- 71.Tomita Y, Shima A. Mechanisms of impulsive pressure generation and damage pit formation by bubble collapse. J Fluid Mech 1986; 169:535–64 [Google Scholar]
- 72.Leighton T, Walton A, Pickworth M. Primary bjerknes forces. Eur J Phys 1990; 11:47–50 [Google Scholar]
- 73.Bjerknes V. Fields of force: a course of lectures in mathematical physics delivered December 1 to 23, 1905. Columbia University Press, 1906.
- 74.Choi P-K. Sonoluminescence and acoustic cavitation. Jpn J Appl Phys 2017; 56:07JA01 [Google Scholar]
- 75.Ashokkumar M, Lee J, Kentish S, Grieser F. Bubbles in an acoustic field: an overview. Ultrason Sonochem 2007; 14:470–5 [DOI] [PubMed] [Google Scholar]
- 76.Leighton TG. An introduction to acoustic cavitation. In: Ultrasound in medicine. Philadelphia, PA: Institute of Physics Publishing, 1998, pp.199–223
- 77.Ashokkumar M. The characterization of acoustic cavitation bubbles – an overview. Ultrason Sonochem 2011; 18:864–72 [DOI] [PubMed] [Google Scholar]
- 78.Brennen CE. Cavitation and bubble dynamics. Cambridge: Cambridge University Press, 2014 [Google Scholar]
- 79.Suslick KS. Ultrasound: its chemical, physical, and biological effects. New York, NY: VCH Publishers, 1988 [DOI] [PubMed] [Google Scholar]
- 80.Price GJ. Current trends in sonochemistry. Cambridge: Royal Society of chemistry, 1992 [Google Scholar]
- 81.Ashokkumar M. Sonochemical synthesis of inorganic nanoparticles. In: Advanced wet-chemical synthetic approaches to inorganic nanostructures. Transworld Research Network, 2008, pp.107–31.
- 82.Muthukumaran S, Kentish SE, Stevens GW, Ashokkumar M. Application of ultrasound in membrane separation processes: a review. Rev Chem Eng 2006; 22:155–94 [Google Scholar]
- 83.Gorick C, Mathew A, Garrison W, Thim E, Fisher D, Copeland C, Song J, Klibanov A, Miller G, Price R. Sonoselective transfection of cerebral vasculature without blood–brain barrier disruption. Proc Natl Acad Sci USA 2020; 117:5644–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Galan-Acosta L, Sierra C, Leppert A, Pouliopoulos AN, Kwon N, Noel RL, Tambaro S, Presto J, Nilsson P, Konofagou EE, Johansson J. Recombinant BRICHOS chaperone domains delivered to mouse brain parenchyma by focused ultrasound and microbubbles are internalized by hippocampal and cortical neurons. Mol Cell Neurosci 2020; 105:103498. [DOI] [PubMed] [Google Scholar]