Abstract
The association between the presence of anti-interferon-γ autoantibodies and the onset of immunodeficiency with intracellular infections has been clearly established. No standard regimen to control the production of these pathogenic autoantibodies, apart from antimicrobial therapy to eliminate infections, contributes to the medical burden of this syndrome, which sometimes has a fatal outcome. In this review, we summarize the findings on anti-interferon-γ autoantibodies to facilitate further research and to provide guidance for treatment strategies.
Keywords: Immune deficiency, anti-interferon-γ antibodies, intracellular organism infection, adult-onset immunodeficiency
Impact statement
The frequency of various opportunistic infections associated with anti-interferon (IFN)-γ autoantibodies (AAbs) has increased in the past decade. Numerous studies including clinical manifestations, diagnostic methods, mechanisms of pathogenesis and treatment have been revealed. This review provides comprehensive information on anti-IFN-γ AAbs associated with opportunistic infections.
Introduction
The term “adult-onset immunodeficiency” was first coined by Browne et al. to describe an immunodeficiency syndrome related to the presence of anti-interferon (IFN)-γ autoantibodies (AAbs) in adults with multiple opportunistic infections similar to that observed in patients with advanced human immunodeficiency virus (HIV) infection. 1 Cumulative reports have clearly confirmed that anti-IFN-γ AAbs contribute to immunodeficiency that is accompanied by intracellular infections, mostly in adults. Several studies, including ours, have shown relatively high rates of fatality in these patients.2–6 Most reports of adult-onset immunodeficiency were from academic or university hospitals regarding patients who had been referred from smaller medical care institutions.1,7,8 Thus, we believe that the actual number of cases of adult-onset immunodeficiency is grossly underestimated as this emerging syndrome may not be commonly recognized. Thus far, most treatments for adult-onset immunodeficiency focus on antimicrobial therapy to eliminate infections. There is no standard treatment guideline for controlling the production of anti-IFN-γ AAbs. This review discusses our current understanding of anti-IFN-γ AAbs in terms of etiology, pathology, its effect on the immune system, detection, characteristics, and treatment.
Etiology of anti-IFN-γ AAbs
It is believed that anti-IFN-γ AAbs is the cause of immunodeficiency rather than the result of opportunistic infections. A time series analysis in a patient with disseminated Mycobacterium avium complex infection showed that neutralizing anti-IFN-γ AAbs gradually develop for some time before the onset of opportunistic infections. 9 However, the factor that triggers anti-IFN-γ AAbs production is not known. It has been shown that natural antibodies against IFN-γ can be found in infection-free healthy individuals ranging from newborns to adults. 10 Nevertheless, titers of anti-IFN-γ AAbs were significantly higher in patients suffering from various viral infections. These titers declined, approaching normal levels as the infections gradually resolved. The use of Anaferon, an IFN-γ-specific therapeutic antibody for children, in patients with varicella infection resulted in a significant decrease of anti-IFN-γ AAbs compared to patients receiving a placebo. 11 These data suggest that the production of anti-IFN-γ AAbs could be the result of natural immune regulation following infections, but how these antibodies become pathogenic remains unclear.
Molecular mimicry, when the immune system responds to foreign antigens sharing a similar sequence or structure to self-antigens, is proposed as another possibility in the production of anti-IFN-γ AAbs. 8 The sequence of amino acids of the IFN-γ molecule that the autoantibodies bind to shares 100% homology to amino acids of the ribosome assembly protein Noc2 of Aspergillus terreus, which is highly conserved across all of the Aspergillus spp. 8 A synthetic Aspergillus Noc2 peptide was able to bind to anti-IFN-γ AAbs in patients and induce antibodies that cross-reacted with human IFN-γ and inhibit the IFN-γ-stimulated Phosphorylated−Signal Transducer and Activator of Transcription (pSTAT)−1 upregulation. The difference in the neutralizing capacity of anti-IFN-γ AAbs found in patients and non-neutralizing antibodies in healthy populations may explain pathogenicity. This assumption is supported by evidence showing that patients with anti-IFN-γ AAbs without neutralizing capacity did not have opportunistic infections,1,12 suggesting that not only the amount of the antibody but also IFN-γ–blocking activity is required for pathogenicity. A longitudinal study is required to identify risk factors for the onset of the anti-IFN-γ AAbs and immunodeficiency.
Effect of anti-IFN-γ AAbs on its downstream elements
IFN-γ, a central regulator of the immune system critical for controlling intracellular infections, is secreted by activated T cells, natural killer (NK) cells, and macrophages. The binding of homodimer IFN-γ to its receptor activates the Janus-activated kinase (JAK)-STAT pathway. This JAK-STAT signal harmonizes the transcriptional activation of several genes and mediates various biological responses. 13 High titers of anti-IFN-γ AAbs are able to block the binding of IFN-γ to its receptor which inhibit the early aspects of IFN-γ signal transduction, either STAT-1 phosphorylation or STAT-1 protein expression. These autoantibodies also inhibit the downstream biological consequences of IFN-γ binding which are the up-regulation of tumor necrosis factor (TNF)-α and interleukin (IL)-12 production.14,15 Purified anti-IFN-γ AAbs is able to block the induction of IFN-γ-inducible genes and the upregulation of HLA class II expression on peripheral blood mononuclear cells. 16 Moreover, patients that were anti-IFN-γ AAbs positive could block IFN-γ-mediated antimicrobial immunity in monocytes and macrophages. These include IFN-γ-driven polarization and M1 macrophages activation, the production of cytokines, chemokines and inducible nitric oxide (iNO)/nitric oxide (NO), biosynthesis and reactive oxygen species (ROS) generation, and phagocytosis and degradation efficacy.14,15 These studies clearly demonstrate that anti-IFN-γ AAbs with neutralizing activity can impede the binding of IFN-γ to its receptor, resulting in the absence of downstream signal transduction, directly affecting immune responses against intracellular pathogens.
Characteristics of anti-IFN-γ AAbs
Most anti-IFN-γ AAbs belong to the IgG isotypes, most frequently IgG4 and IgG1 subtypes.1,7,17–21 Several reports showed heterogeneous patterns of isotypes and subtypes among patients. A predominance of IgG3 was also reported in some patients. 18 Our study demonstrated that anti-IFN-γ AAbs mainly exhibited the IgG1 and IgG4 subtypes. However, multiple isotypes and subtypes can be found in some cases. 21 These data suggest that B cells that produce anti-IFN-γ AAbs have undergone antibody class switching.
Several reports found that these autoantibodies recognized an epitope in the C-terminal region of IFN-γ.8,17,21 The data suggest that the major epitope contains the SPAAKTGKRK amino acid sequence, determined by computer modeling. We also found that autoantibodies against IFN-γ in Northern Thai patients recognized the C-terminal linear epitope containing the KRKR motif. 21 This region is critical for IFN-γ receptor (IFNGR) activation and the binding of these autoantibodies impede IFN-γ-mediated activities. 8
Commercially available mouse anti-IFN-γ monoclonal antibodies can bind to distinct epitopes of the IFN-γ molecule with different neutralizing activity. 22 Competitive-binding ELISA using commercial neutralizing mouse anti-IFN-γ monoclonal antibodies showed that anti-IFN-γ AAbs in patients bind to discontinuous epitope of homodimeric IFN-γ. This study also demonstrated the heterogeneity of the auto Abs against IFN-γ in AOID patients and the diverse patterns among individuals. In-depth analysis of binding epitopes in individuals may provide information for designing therapeutic approaches.
Anti-IFN-γ AAbs and pathology
Anti-IFN-γ AAbs has been associated with various opportunistic infections. Disseminated nontuberculous mycobacterial (NTM) infection is the most common infectious disease,1,6 but Talaromyces marneffei, Cryptococcus neoformans, Histoplasma capsulatum, Burkholderia pseudomallei, Salmonella species, and Varicella–zoster virus are also frequently identified as opportunistic pathogens in this patient group.1,2,6,23 Concomitant infections with at least 2 opportunistic pathogens are more accurate parameters to confirm the immunocompromised state in these patients.1,2 Several organ systems are involved, in which lymph nodes, skin, bone and soft tissue appear to be frequently affected.1,2,6 Bone marrow or blood, lung, bladder, liver, and biliary tree are likewise the sites of infection.6,24–26 The clinical manifestations, locations of the infected sites, and causative agents are likely to differ across ethnicities. Rapidly growing mycobacteria (RGM), such as M. abscessus, were the most common NTM species isolated from Thai, Chinese and Filipino patients, whereas M. avium complex (MAC) was predominant among Japanese and non-Asian patients.6,27 Lymph nodes were the most common organ involved in patients with RGM, whereas bone and lung infections were more common in patients with MAC.
Many studies showed that disseminated NTM in patients with anti-IFN-γ autoantibodies can mimic malignancy, cancer, and synovitis-acne-pustulosis-hyperostosis-osteitis (SAPHO) syndrome.26,28,29 Therefore, differential diagnosis should be handled delicately. Most reported cases of patients with anti-IFN-γ AAbs are older adults. Nevertheless, adolescents and young adults with disseminated opportunistic pathogenic infections with anti-IFN-γ AAbs have been reported recently.30,31 The majority of patients reported to have anti-IFN-γ AAbs have been narrowed to Asia-born Asians, particularly those from Southeast Asian populations with human leukocyte antigen (HLA)−DRB1 and DQB1 alleles, especially HLA−DRB1*15:01/16:02 and DQB1*05:01/05:02.32–34 However, opportunistic pathogenic infections have been reported in Caucasian patients with anti-IFN-γ AAbs living in the UK, USA, Germany as well as in patients of African ancestry.16,19,20,35 Although correlations between anti-IFN-γ AAbs, types of opportunistic infections, clinical manifestations, HLA alleles, and ethnicity have been demonstrated, other issues, such as environmental factors and genetic diversity must be elucidated for in-depth understanding of this disease. Interestingly, by fitting a linear mixed model adjusted for age, sex, as well as the presence of infection, antibiotic use and cyclophosphamide or rituximab use, anti-IFN-γ AAbs levels decreased overtime regardless of ethnicity, even in the absence of immunomodulatory therapy. 6 Neither age nor sex was a discriminating factor in the antibody levels over time. Therefore, the factors predicting specific outcomes remain to be revealed.
Detection of anti-IFN-γ AAbs
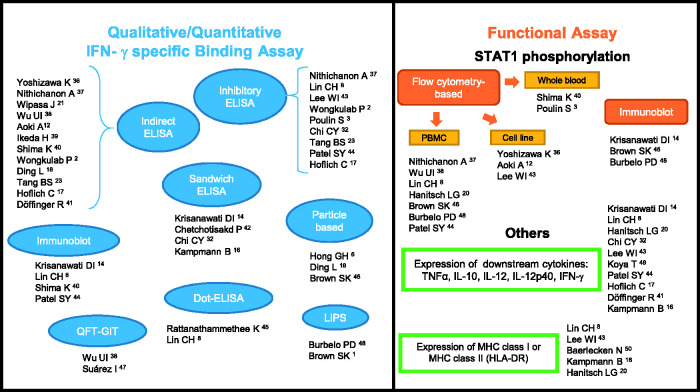
Considering the significance of anti-IFN-γ AAbs-associated pathology, it is important to evaluate antibody detection even though most patients clinically present with disseminated infections. Currently, the approaches used to evaluate anti-IFN-γ AAbs in suspected cases with certain infections vary greatly from simple assays to long tedious bioassays. We have summarized the different wide-ranging methods from qualitative or quantitative IFN-γ-specific binding and biological activity assays based on previous reports (Figure 1). The most common assay used to detect anti-IFN-γ AAbs is the enzyme-linked immunosorbent assay (ELISA), in indirect,2,12,17,18,21,23,36–41 sandwich,14,16,32,42 or inhibitory2,3,8,17,23,32,37,43,44 assay format. For the ELISA-based method, the results could be reported qualitatively (positive or negative) or quantitatively as optical density (OD), 39 titer, 43 or through calculations of arbitrary units 21 or ELISA units (EU).36,39 The easy-to-use, low-cost Dot ELISA strip, which can be read directly on the strip, was developed as a point-of-care screening tool in remote settings.8,45 A particle-based assay with claims as a fast, easy, and relatively inexpensive technique has also been used to detect multiple anti-cytokine autoantibodies simultaneously in human plasma.6,18,46 QuantiFERON-TB Gold In-tube (QFT-GIT), a commercialized IFN-γ release assay commonly used for detection of latent tuberculosis in many hospitals has also been modified for the screening of neutralizing anti-IFN-γ AAbs.38,47 Luciferase immunoprecipitation system (LIPS) is a high throughput quantitative method useful for detecting multiple anti-cytokine autoantibodies at the same time.1,48 Immunoblot of IFN-γ have also been used to verify the exact nature of the binding activity.8,14,40,44
Figure 1.
Summarized methods of detection, the qualitative/quantitative IFN-γ specific binding, and functional assays of anti-IFN-γ AAbs. IFN-γ: interferon gamma; AAbs: autoantibodies; ELISA: enzyme-linked immunosorbent assay; QFT-GIT: QuantiFERON-TB gold in-tube; LIPS: luciferase immunoprecipitation system; STAT1: signal transducer and activator of transcription 1; PBMC: peripheral blood mononuclear cell; TNF: tumor necrosis factor; IL: interleukin; MHC: major histocompatibility complex. (A color version of this figure is available in the online journal.)
Assessment of pSTAT1 is an important approach often used to confirm the anti-IFN-γ AAbs inhibitory function, commonly performed by flow cytometry3,8,12,20,36–38,40,43,44,46,48 or immunoblot.14,46,48 Shima et al. demonstrated that all disseminated NTM participants with inhibited STAT1 phosphorylation have high titers of anti-IFN-γ AAbs. 40 However, non-concomitant results in three participants with high-titer anti-IFN-γ AAbs but without opportunistic infections showed no IFN-γ–blocking activity. 1 A report by Lin et al. illustrated that among healthy individuals who possessed autoantibodies against IFN-γ, no neutralizing activity nor effect to IL-12 production was found. 8 These findings suggest that detection of the existence and the level of anti-IFN-γ AAbs may not be enough for evaluation of clinical management. Other biological activity assays include evaluation of IFN-γ downstream effector molecules.8,14,16,17,20,32,41,43,44,49,50
To date, a standard method has not yet been endorsed. Therefore, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of assays should be taken into consideration when selecting a screening method. Nithichanon et al. demonstrated that inhibitory ELISA is more specific and has greater PPV and NPV than indirect ELISA with comparable sensitivity. 37 Some methodological limitations are of concern. For example, pSTAT1 is diminished in a day dependent manner when kept at 4°C, therefore it is important to take into account the timing of the assay following phlebotomy when using whole blood for pSTAT1 detection. 40 Several methods may be required to confirm the presence and functional activities of anti-IFN-γ AAbs.
Clinical management of anti-IFN-γ AAbs
Sensitive antimicrobial therapy is the most important strategy for eliminating infections, including in patients carrying anti-IFN-γ AAbs. The most common drugs used for the treatment of NTM are a regimen combining rifampin, ethambutol, clarithromycin, linezolid, and amikacin.4,19,26,39,51–53 Anti-fungal medicines that include amphotericin B, itraconazole, and fluconazole have been used for the treatment of Talaromyces marneffei and Cryptococcus spp.54,55 Nevertheless, the disease can be refractory and fatal, despite appropriate and aggressive antimicrobial treatment.3,46,56
In addition to antimicrobial therapy for clinical disease management, there is no standard treatment guideline for anti-IFN-γ AAbs. Treatment with various immunomodulatory agents has been evaluated in order to modulate humoral immunity. Rituximab, a chimeric monoclonal antibody specific to CD20 expressed by B cells, is the most studied immunomodulatory agent in patients carrying anti-IFN-γ AAbs. Treatment with rituximab results in depletion of circulating B cells, reduction of anti-IFN-γ AAbs titers, restoration of IFN-γ signaling, and improvement of clinical conditions and inflammatory markers.46,57,58 A combination of methylprednisolone and rituximab treatment produced favorable outcomes.58,59 Nevertheless, clinical relapse has been observed in some patients and the time to clinical improvement, reduction of anti-IFN-γ AAbs titers, and improved IFN-γ signaling differed among patients. 46 Therefore, optimization of the immunomodulatory agents is needed to constitute appropriate duration and dosage of the therapies. One patient did not respond to multiple doses of anti-CD20 monoclonal antibody, but an anti-CD38 monoclonal antibody targeting plasma cells (daratumumab) successfully suppressed the antibody levels with clinical improvement. 60 Although immunomodulatory agents could successfully offer effective treatment for autoantibodies, these regimens may not be required for patients who respond to antimicrobial therapy alone. 46 Therapies directed at autoantibodies may be important adjunct treatment for patients with high-titer anti-IFN-γ AAbs who continue to have persistent and progressive infection despite long-term antimicrobial therapy.6,46
In settings where rituximab is less accessible, cyclophosphamide, an alkylating agent capable of inhibiting protein synthesis through DNA and RNA crosslinking, may be an alternative option. Treatment of patients carrying anti-IFN-γ AAbs with cyclophosphamide results in the decrease of the antibody titer with clinical improvement, faster complete remission, a longer duration of remission, and a lower incidence of relapsed infection compared with patients receiving rituximab.42,61 This may be due to broader immune suppression on various immune cells.
In addition to immunomodulatory agents, it has been demonstrated that a variant of IFN-γ, in which the anti-IFN-γ AAbs binding epitope was replaced with a corresponding sequence from mouse, is capable of restoring IFN-γ signaling in vitro despite the presence of patient sera. 8 Restoration of IFN-γ responses by using a variant of IFN-γ or an epitope-deprived peptide offers a potential treatment regardless of depletion of the anti-IFN-γ AAbs. Nevertheless, more investigations in vivo are needed to apply this approach for clinical purposes.
Conclusions
Cumulative reports on anti-IFN-γ AAbs reveal increasing impact of anti-IFN-γ AAbs-associated opportunistic infections. This not only affects health, but also impedes the welfare and economic status of the patients and caregivers, as well as nations. This review provides insight into the context of the etiology, pathology, function, diagnostic methodologies, characteristics, and clinical management of anti-IFN-γ AAbs. This information may promote better understanding of adult onset immunodeficiency associated with anti-IFN-γ AAbs.
Footnotes
Authors’ contributions: All authors wrote and approved the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: This report was partly funded by Chiang Mai University, Chiang Mai, Thailand and the Thailand Research Fund (RSA6280065 to JW).
ORCID iD: Jiraprapa Wipasa https://orcid.org/0000-0002-2120-0779
References
- 1.Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, Kirk JL, Jutivorakool K, Zaman R, Ding L, Hsu AP, Patel SY, Olivier KN, Lulitanond V, Mootsikapun P, Anunnatsiri S, Angkasekwinai N, Sathapatayavongs B, Hsueh PR, Shieh CC, Brown MR, Thongnoppakhun W, Claypool R, Sampaio EP, Thepthai C, Waywa D, Dacombe C, Reizes Y, Zelazny AM, Saleeb P, Rosen LB, Mo A, Iadarola M, Holland SM. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med 2012; 367:725–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wongkulab P, Wipasa J, Chaiwarith R, Supparatpinyo K. Autoantibody to interferon-gamma associated with adult-onset immunodeficiency in non-HIV individuals in Northern Thailand. PLoS One 2013; 8:e76371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poulin S, Corbeil C, Nguyen M, St-Denis A, Cote L, Le Deist F, Carignan A. Fatal Mycobacterium colombiense/cytomegalovirus coinfection associated with acquired immunodeficiency due to autoantibodies against interferon gamma: a case report. BMC Infect Dis 2013; 13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weng TP, Syue LS, Lee NY. Disseminated Mycobacterium szulgai infection in a patient with anti-interferon-gamma autoantibodies. IDCases 2020; 21:e00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chetchotisakd P, Anunnatsiri S, Nithichanon A, Lertmemongkolchai G. Cryptococcosis in anti-interferon-gamma autoantibody-positive patients: a different clinical manifestation from HIV-infected patients. Jpn J Infect Dis 2017; 70:69–74 [DOI] [PubMed] [Google Scholar]
- 6.Hong GH, Ortega-Villa AM, Hunsberger S, Chetchotisakd P, Anunnatsiri S, Mootsikapun P, Rosen LB, Zerbe CS, Holland SM. Natural history and evolution of anti-interferon-gamma autoantibody-associated immunodeficiency syndrome in Thailand and the United States. Clin Infect Dis 2020; 71:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kampitak T, Suwanpimolkul G, Browne S, Suankratay C. Anti-interferon-γ autoantibody and opportunistic infections: case series and review of the literature. Infection 2011; 39:65–71 [DOI] [PubMed] [Google Scholar]
- 8.Lin CH, Chi CY, Shih HP, Ding JY, Lo CC, Wang SY, Kuo CY, Yeh CF, Tu KH, Liu SH, Chen HK, Ho CH, Ho MW, Lee CH, Lai HC, Ku CL. Identification of a major epitope by anti-interferon-γ autoantibodies in patients with mycobacterial disease. Nat Med 2016; 22:994–1001 [DOI] [PubMed] [Google Scholar]
- 9.Wu UI, Hung CC, Chang SY, Jhong YT, Sun HY, Wang JT, Hsieh SM, Sheng WH, Liu WC, Chang SC, Chen YC. Neutralizing antiinterferon-γ autoantibodies causing disseminated Mycobacterium avium complex infection in an HIV-infected patient on successful combination antiretroviral therapy. AIDS 2017; 31:2557–9 [DOI] [PubMed] [Google Scholar]
- 10.Caruso A, Bonfanti C, Colombrita D, De Francesco M, De Rango C, Foresti I, Gargiulo F, Gonzales R, Gribaudo G, Landolfo S. Natural antibodies to IFN-gamma in man and their increase during viral infection. J Immunol 1990; 144:685–90 [PubMed] [Google Scholar]
- 11.Don E, van der Meide N, Egorov V, Putilovskiy M, Tarasov S. The level of natural autoantibodies to IFN-gamma in varicella infection treated with antiviral drug anaferon for children: a pilot study. Immunol Lett 2020; 222:90–4 [DOI] [PubMed] [Google Scholar]
- 12.Aoki A, Sakagami T, Yoshizawa K, Shima K, Toyama M, Tanabe Y, Moro H, Aoki N, Watanabe S, Koya T, Hasegawa T, Morimoto K, Kurashima A, Hoshino Y, Trapnell BC, Kikuchi T. Clinical significance of interferon-γ neutralizing autoantibodies against disseminated nontuberculous mycobacterial disease. Clin Infect Dis 2018; 66:1239–45 [DOI] [PubMed] [Google Scholar]
- 13.Saha B, Jyothi Prasanna S, Chandrasekar B, Nandi D. Gene modulation and immunoregulatory roles of interferon gamma. Cytokine 2010; 50:1–14 [DOI] [PubMed] [Google Scholar]
- 14.Krisnawati DI, Liu YC, Lee YJ, Wang YT, Chen CL, Tseng PC, Lin CF. Functional neutralization of anti-IFN-gamma autoantibody in patients with nontuberculous mycobacteria infection. Sci Rep 2019; 9:5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krisnawati DI, Liu YC, Lee YJ, Wang YT, Chen CL, Tseng PC, Shen TJ, Lin CF. Blockade effects of anti-interferon- (IFN-) γ autoantibodies on IFN-γ-regulated antimicrobial immunity. J Immunol Res 2019; 2019:1629258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kampmann B, Hemingway C, Stephens A, Davidson R, Goodsall A, Anderson S, Nicol M, Schölvinck E, Relman D, Waddell S, Langford P, Sheehan B, Semple L, Wilkinson KA, Wilkinson RJ, Ress S, Hibberd M, Levin M. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J Clin Invest 2005; 115:2480–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoflich C, Sabat R, Rosseau S, Temmesfeld B, Slevogt H, Docke WD, Grutz G, Meisel C, Halle E, Gobel UB, Volk HD, Suttorp N. Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium chelonae and burkholderia cocovenenans. Blood 2004; 103:673–5 [DOI] [PubMed] [Google Scholar]
- 18.Ding L, Mo A, Jutivorakool K, Pancholi M, Holland SM, Browne SK. Determination of human anticytokine autoantibody profiles using a particle-based approach. J Clin Immunol 2012; 32:238–45 [DOI] [PubMed] [Google Scholar]
- 19.O'Connell E, Rosen LB, LaRue RW, Fabre V, Melia MT, Auwaerter PG, Holland SM, Browne SK. The first US domestic report of disseminated Mycobacterium avium complex and anti-interferon-gamma autoantibodies. J Clin Immunol 2014; 34:928–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanitsch LG, Lobel M, Muller-Redetzky H, Schurmann M, Suttorp N, Unterwalder N, Monnich U, Meisel C, Wittke K, Volk HD, Scheibenbogen C, Kolsch U. Late-onset disseminated Mycobacterium avium intracellulare complex infection (MAC), cerebral toxoplasmosis and salmonella sepsis in a German caucasian patient with unusual anti-interferon-gamma IgG1 autoantibodies. J Clin Immunol 2015; 35:361–5 [DOI] [PubMed] [Google Scholar]
- 21.Wipasa J, Chaiwarith R, Chawansuntati K, Praparattanapan J, Rattanathammethee K, Supparatpinyo K. Characterization of anti-interferon-gamma antibodies in HIV-negative immunodeficient patients infected with unusual intracellular microorganisms. Exp Biol Med 2018; 243:621–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasamut U, Thongkum W, Moonmuang S, Sakkhachornphop S, Chaiwarith R, Praparattanapan J, Wipasa J, Chawansuntati K, Supparatpinyo K, Lai E, Tayapiwatana C. Neutralizing activity of anti-interferon-γ autoantibodies in adult-onset immunodeficiency is associated with their binding domains. Front Immunol 2019; 10:1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang BS, Chan JF, Chen M, Tsang OT, Mok MY, Lai RW, Lee R, Que TL, Tse H, Li IW, To KK, Cheng VC, Chan EY, Zheng B, Yuen KY. Disseminated penicilliosis, recurrent bacteremic nontyphoidal salmonellosis, and burkholderiosis associated with acquired immunodeficiency due to autoantibody against gamma interferon. Clin Vaccine Immunol 2010; 17:1132–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phoompoung P, Ankasekwinai N, Pithukpakorn M, Foongladda S, Umrod P, Suktitipat B, Mahasirimongkol S, Kiertiburanakul S, Suputtamongkol Y. Factors associated with acquired anti IFN- gamma autoantibody in patients with nontuberculous mycobacterial infection. PLoS One 2017; 12:e0176342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Namkoong H, Asakura T, Ishii M, Yoda S, Masaki K, Sakagami T, Iwasaki E, Yamagishi Y, Kanai T, Betsuyaku T, Hasegawa N. First report of hepatobiliary Mycobacterium avium infection developing obstructive jaundice in a patient with neutralizing anti-interferon-gamma autoantibodies. New Microbes New Infect 2019; 27:4–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh YK, Ding JY, Ku CL, Chen WC. Disseminated Mycobacterium avium complex infection mimicking malignancy in a patient with anti-IFN-gamma autoantibodies: a case report. BMC Infect Dis 2019; 19:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hase I, Morimoto K, Sakagami T, Ishii Y, van Ingen J. Patient ethnicity and causative species determine the manifestations of anti-interferon-gamma autoantibody-associated nontuberculous mycobacterial disease: a review. Diagn Microbiol Infect Dis 2017; 88:308–15 [DOI] [PubMed] [Google Scholar]
- 28.Chang PH, Chuang YC. Anti-interferon-gamma autoantibody-associated disseminated Mycobacterium abscessus infection mimicking parotid cancer with multiple metastases: a case report. Medicine 2017; 96:e8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furuya H, Ikeda K, Miyachi K, Nakamura K, Suzuki K, Furuta S, Tamachi T, Hirose K, Sakagami T, Nakajima H. SAPHO syndrome-like presentation of disseminated nontuberculous mycobacterial infection in a case with neutralizing anti-IFNgamma autoantibody. Rheumatology 2017; 56:1241–3 [DOI] [PubMed] [Google Scholar]
- 30.Liew WK, Thoon KC, Chong CY, Tan NWH, Cheng DT, Chan BSW, Ng MSY, Das L, Arkachaisri T, Huang CH, Kuan JL, Chai LYA, Koh MJA. Juvenile-onset immunodeficiency secondary to anti-interferon-gamma autoantibodies. J Clin Immunol 2019; 39:512–8 [DOI] [PubMed] [Google Scholar]
- 31.Qiu Y, Zhang J, Li B, Shu H. Bacillus cereus isolated from a positive bone tissue culture in a patient with osteolysis and high-titer anti-interferon-gamma autoantibodies: a case report. Medicine 2019; 98:e17609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chi CY, Chu CC, Liu JP, Lin CH, Ho MW, Lo WJ, Lin PC, Chen HJ, Chou CH, Feng JY, Fung CP, Sher YP, Li CY, Wang JH, Ku CL. Anti-IFN-gamma autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02 and the reactivation of latent varicella-zoster virus infection. Blood 2013; 121:1357–66 [DOI] [PubMed] [Google Scholar]
- 33.Pithukpakorn M, Roothumnong E, Angkasekwinai N, Suktitipat B, Assawamakin A, Luangwedchakarn V, Umrod P, Thongnoppakhun W, Foongladda S, Suputtamongkol Y. HLA-DRB1 and HLA-DQB1 are associated with adult-onset immunodeficiency with acquired anti-interferon-gamma autoantibodies. PLoS One 2015; 10:e0128481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ku CL, Lin CH, Chang SW, Chu CC, Chan JF, Kong XF, Lee CH, Rosen EA, Ding JY, Lee WI, Bustamante J, Witte T, Shih HP, Kuo CY, Chetchotisakd P, Kiertiburanakul S, Suputtamongkol Y, Yuen KY, Casanova JL, Holland SM, Doffinger R, Browne SK, Chi CY. Anti-IFN-gamma autoantibodies are strongly associated with HLA-DR*15:02/16:02 and HLA-DQ*05:01/05:02 across southeast Asia. J Allergy Clin Immunol 2016; 137:945–8.e8 [DOI] [PubMed] [Google Scholar]
- 35.van de Vosse E, van Wengen A, van der Meide WF, Visser LG, van Dissel JT. A 38-year-old woman with necrotising cervical lymphadenitis due to histoplasma capsulatum. Infection 2017; 45:917–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshizawa K, Aoki A, Shima K, Tanabe Y, Koya T, Hasegawa T, Kikuchi T, Sakagami T. Serum anti-interferon-gamma autoantibody titer as a potential biomarker of disseminated non-tuberculous mycobacterial infection. J Clin Immunol 2020; 40:399–405 [DOI] [PubMed] [Google Scholar]
- 37.Nithichanon A, Chetchotisakd P, Matsumura T, Takahashi Y, Ato M, Sakagami T, Lertmemongkolchai G. Diagnosis of NTM active infection in lymphadenopathy patients with anti-interferon-gamma auto-antibody using inhibitory ELISA vs. indirect ELISA. Sci Rep 2020; 10:8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu UI, Chuang YC, Sheng WH, Sun HY, Jhong YT, Wang JY, Chang SC, Wang JT, Chen YC. Use of QuantiFERON-TB gold in-tube assay in screening for neutralizing anti-interferon-gamma autoantibodies in patients with disseminated nontuberculous mycobacterial infection. Clin Microbiol Infect 2018; 24:159–65 [DOI] [PubMed] [Google Scholar]
- 39.Ikeda H, Nakamura K, Ikenori M, Saito T, Nagamine K, Inoue M, Sakagami T, Suzuki H, Usui M, Kanemitsu K, Matsumoto A, Shinbo T. Severe disseminated Mycobacterium avium infection in a patient with a positive serum autoantibody to interferon-γ. Intern Med 2016; 55:3053–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shima K, Sakagami T, Tanabe Y, Aoki N, Moro H, Koya T, Kagamu H, Hasegawa T, Suzuki E, Narita I. Novel assay to detect increased level of neutralizing anti-interferon gamma autoantibodies in non-tuberculous mycobacterial patients. J Infect Chemother 2014; 20:52–6 [DOI] [PubMed] [Google Scholar]
- 41.Döffinger R, Helbert MR, Barcenas-Morales G, Yang K, Dupuis S, Ceron-Gutierrez L, Espitia-Pinzon C, Barnes N, Bothamley G, Casanova JL, Longhurst HJ, Kumararatne DS. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis 2004; 38:e10–4 [DOI] [PubMed] [Google Scholar]
- 42.Chetchotisakd P, Anunnatsiri S, Nanagara R, Nithichanon A, Lertmemongkolchai G. Intravenous cyclophosphamide therapy for anti-IFN-gamma autoantibody-associated Mycobacterium abscessus infection. J Immunol Res 2018; 2018:6473629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee WI, Huang JL, Wu TS, Lee MH, Chen IJ, Yu KH, Liu CY, Yang CH, Hsieh MY, Lin YL, Shih YF, Jaing TH, Huang SC, Kuo TT, Ku CL. Patients with inhibitory and neutralizing auto-antibodies to interferon-γ resemble the sporadic adult-onset phenotype of Mendelian susceptibility to mycobacterial disease (MSMD) lacking bacille Calmette-Guerin (BCG)-induced diseases. Immunobiology 2013; 218:762–71 [DOI] [PubMed] [Google Scholar]
- 44.Patel SY, Ding L, Brown MR, Lantz L, Gay T, Cohen S, Martyak LA, Kubak B, Holland SM. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol 2005; 175:4769–76 [DOI] [PubMed] [Google Scholar]
- 45.Rattanathammethee K, Chawansuntati K, Chaiwarith R, Praparattanapan J, Supparatpinyo K, Wipasa J. Dot enzyme-linked immunosorbent assay strip as a screening tool for detection of autoantibody to interferon gamma in sera of suspected cases of adult-onset immunodeficiency. J Clin Lab Anal 2018; 32:e22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Browne SK, Zaman R, Sampaio EP, Jutivorakool K, Rosen LB, Ding L, Pancholi MJ, Yang LM, Priel DL, Uzel G, Freeman AF, Hayes CE, Baxter R, Cohen SH, Holland SM. Anti-CD20 (rituximab) therapy for anti-IFN-γ autoantibody-associated nontuberculous mycobacterial infection. Blood 2012; 119:3933–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suárez I, Lehmann C, Gruell H, Graeb J, Kochanek M, Fätkenheuer G, Plum G, van Wengen A, van de Vosse E, Hartmann P, Hanitsch LG, Rybniker J. Repurposing QuantiFERON for detection of neutralizing interferon-γ autoantibodies in patients with nontuberculous mycobacterial infections. Clin Infect Dis 2017; 65:518–21 [DOI] [PubMed] [Google Scholar]
- 48.Burbelo PD, Browne SK, Sampaio EP, Giaccone G, Zaman R, Kristosturyan E, Rajan A, Ding L, Ching KH, Berman A, Oliveira JB, Hsu AP, Klimavicz CM, Iadarola MJ, Holland SM. Anti-cytokine autoantibodies are associated with opportunistic infection in patients with thymic neoplasia. Blood 2010; 116:4848–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koya T, Tsubata C, Kagamu H, Koyama K, Hayashi M, Kuwabara K, Itoh T, Tanabe Y, Takada T, Gejyo F. Anti-interferon-gamma autoantibody in a patient with disseminated Mycobacterium avium complex. J Infect Chemother 2009; 15:118–22 [DOI] [PubMed] [Google Scholar]
- 50.Baerlecken N, Jacobs R, Stoll M, Schmidt RE, Witte T. Recurrent, multifocal Mycobacterium avium-intercellulare infection in a patient with interferon-gamma autoantibody. Clin Infect Dis 2009; 49:e76-8 [DOI] [PubMed] [Google Scholar]
- 51.Chaononghin S, Visuttichaikit S, Apisarnthanarak A, Khawcharoenporn T. Disseminated Mycobacterium scrofulaceum infection in a patient with anti-interferon-gamma autoantibodies: a case report and review of the literature. Int J Mycobacteriol 2020; 9:91–4 [DOI] [PubMed] [Google Scholar]
- 52.Keragala B, Gunasekera CN, Yesudian PD, Guruge C, Dissanayaka BS, Liyanagama DP, Jinadasa GIM, Constantine SR, Herath H. Disseminated Mycobacterium simiae infection in a patient with adult-onset immunodeficiency due to anti-interferon-gamma antibodies - a case report. BMC Infect Dis 2020; 20:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hase I, Morimoto K, Sakagami T, Kazumi Y, Ishii Y, van Ingen J. Disseminated Mycobacterium gordonae and Mycobacterium mantenii infection with elevated anti-IFN-gamma neutralizing autoantibodies. J Infect Chemother 2015; 21:468–72 [DOI] [PubMed] [Google Scholar]
- 54.Liang X, Si L, Li Y, Zhang J, Deng J, Bai J, Li M, He Z. Talaromyces marneffei infection relapse presenting as osteolytic destruction followed by suspected nontuberculous mycobacterium infection during 6 years of follow-up: a case update. Int J Infect Dis 2020; 93:208–10 [DOI] [PubMed] [Google Scholar]
- 55.Wongkamhla T, Chongtrakool P, Jitmuang A. A case report of talaromyces marneffei oro-pharyngo-laryngitis: a rare manifestation of talaromycosis. BMC Infect Dis 2019; 19:1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nei T, Okabe M, Mikami I, Koizumi Y, Mase H, Matsuda K, Yamamoto T, Takeda S, Tanaka K, Dan K. A non-HIV case with disseminated Mycobacterium kansasii disease associated with strong neutralizing autoantibody to interferon-gamma. Respir Med Case Rep 2013; 8:10–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koizumi Y, Sakagami T, Nishiyama N, Hirai J, Hayashi Y, Asai N, Yamagishi Y, Kato H, Hagihara M, Sakanashi D, Suematsu H, Ogawa K, Mikamo H. Rituximab restores IFN-γ-STAT1 function and ameliorates disseminated Mycobacterium avium infection in a patient with anti-Interferon-γ autoantibody. J Clin Immunol 2017; 37:644–9 [DOI] [PubMed] [Google Scholar]
- 58.Czaja CA, Merkel PA, Chan ED, Lenz LL, Wolf ML, Alam R, Frankel SK, Fischer A, Gogate S, Perez-Velez CM, Knight V. Rituximab as successful adjunct treatment in a patient with disseminated nontuberculous mycobacterial infection due to acquired anti-interferon-γ autoantibody. Clin Infect Dis 2014; 58:e115–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pruetpongpun N, Khawcharoenporn T, Damronglerd P, Suthiwartnarueput W, Apisarnthanarak A, Rujanavej S, Suwantarat N. Disseminated talaromyces marneffei and Mycobacterium abscessus in a patient with anti-Interferon-γ autoantibodies. Open Forum Infect Dis 2016; 3:ofw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ochoa S, Ding L, Kreuzburg S, Treat J, Holland SM, Zerbe CS. Daratumumab (anti-CD38) for treatment of disseminated nontuberculous mycobacteria in a patient with anti-IFN-γ autoantibodies. Clin Infect Dis 2020; DOI: 10.1093/cid/ciaa1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laisuan W, Pisitkun P, Ngamjanyaporn P, Suangtamai T, Rotjanapan P. Prospective pilot study of cyclophosphamide as an adjunct treatment in patients with adult-onset immunodeficiency associated with anti-interferon-γ autoantibodies. Open Forum Infect Dis 2020; 7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]