Abstract
This study investigates the regulatory effect of plasmacytoid dendritic cells (pDC)/myeloid dendritic cells (mDC) imbalance on balance of Th1/Th2 and Th17/Treg in primary immune thrombocytopenia (ITP). A total of 30 untreated ITP patients and 20 healthy controls were recruited. Compared with healthy control, the pDC proportion of ITP patients was significantly reduced (P = 0.004), while the mDC proportion was not significantly changed (P = 0.681), resulting in a decrease in the pDC/mDC ratio (P = 0.001). Additionally, compared with controls, serum levels of interleukin (IL)-6, IL-12, and IL-23 were increased in ITP patients (P < 0.001), and mRNA levels of IL-12p40, IL-12p35, and IL-23p19 were also increased (P =0.014, P = 0.043, P < 0.001). Compared with the healthy control, the proportion of Th1 and Th17 cells in ITP patients increased (P = 0.001, P = 0.031). Serum levels of interferon gamma (IFN-γ) and IL-17 in ITP patients also increased (P = 0.025, P = 0.005). Furthermore, T-bet and RORγt mRNA levels were increased in peripheral blood of ITP patients (P = 0.018, P < 0.001). Correspondingly, the proportion of Th2 and Treg cells decreased (P = 0.007, P < 0.001), along with a decrease in serum IL-4 and transforming growth factor beta (TGF-β) (P = 0.028, P = 0.042), and an increase in GATA-3 mRNA (P < 0.001). However, there was no significant difference in Foxp3 mRNA levels (P = 0.587). Pearson correlation analysis showed that the proportion of total dendritic cells (DCs) was positively correlated with IL-12 (r = 0.526, P = 0.003) and IL-23 (r = 0.501, P = 0.005) in ITP patients. Th1/Th2 ratio, IFN-γ, and IL-12 levels were negatively correlated with platelet counts (r = −0.494, P = 0.009; r = –0.415, P = 0.028; r = –0.492, P = 0.032). However, IL-23 was positively correlated with IL-17 (r = 0.489, P = 0.006) and negatively correlated with platelet count (r = –0.564, P = 0.001). The ratio of IL-6 and Th17 cells was negatively correlated with platelet count (r = –0.443, P = 0.014; r = –0.471, P = 0.011). The imbalance of pDC/mDC and the increase of IL-6, IL-12, and IL-23 lead to the increased differentiation of CD4+ T cells into Th1 and Th17 cells, which might be the important mechanisms underlying the imbalance of Th1/Th2 and Th17/Treg in ITP patients.
Keywords: Primary immune thrombocytopenia, dendritic cells, CD4 + T cells, cytokines, transcription factors
Impact statement
Dendritic cells (DCs)-mediated T cell overactivation plays an important role in the pathogenesis of immune thrombocytopenia (ITP). However, there are few related studies. In this study, we detected the levels of Th1/Th2, Th17/Treg, and their related cytokines and specific transcription factors in newly diagnosed ITP patients. Meanwhile, we analyzed the imbalance of the ratio of plasmacytoid dendritic cells and myeloid dendritic cells and the cytokine levels of interleukin (IL)-6, IL-12, and IL-23 in newly diagnosed ITP patients. The regulation of DC on the differentiation of CD4+ T cells in newly diagnosed ITP patients was explored, as well as their role in the pathogenesis of ITP.
Introduction
Primary immune thrombocytopenia (ITP) is a common acquired autoimmune hematological disease. It is reported that T cell subgroup dysfunction and ratio imbalance were important factors leading to ITP. 1 Studies have shown that the imbalance of CD4+ T cell subsets in ITP patients is manifested as the imbalance of Th1/Th2, which is shifted toward Th1, 2 , 3 and the imbalance of Th17/Treg, which is shifted toward Th17, 4 , 5 leading to increased secretion of Th1 and Th17 cytokines. Our previous studies4,6 showed consistent results. Thus, T cell subsets and T cell-mediated cellular immunity play an important role in the pathogenesis of ITP.
Dendritic cells (DCs) are a type of antigen-presenting cells, which have the strongest antigen-presenting ability, can activate T cells and B cells, and act as the bridge linking innate and adaptive immunity. 7 Although the number of DCs is less than 1% in peripheral blood, they play an important role in inducing autoimmune diseases and immune tolerance and are closely related to the pathogenesis of ITP.8,9 Activated DCs participate in the body’s immune regulation through secreting cytokines, mainly including interleukin (IL)-12, IL-23, IL-6, IL-2, TNF, and IL-18. 10 Among them, IL-12 and TNF-α induce Th0 to differentiate into Th1 cells, 11 and IL-4 promotes Th0 to differentiate into Th2 cells. 12 IL-6 can mediate Th17/Treg balance and can induce Th17 differentiation from naive T cells together with transforming growth factor beta (TGF-β). On the other hand, IL-6 can inhibit Treg differentiation induced by TGF-β. 13 Human myeloid dendritic cells (mDC), also known as classic DCs, express CD11C, CD11b, CD141, and CD1C. They can induce specific immune response against antigen and maintains self-tolerance. Meanwhile, human plasmacytoid dendritic cell (pDC) expresses CD123 (IL-3R), CD303, and CD304, and its main function is to produce type I interferon (IFN) and activate corresponding T cells when the host is infected. 14
Studies have shown that the imbalance of Th1 and Th2 cells plays a crucial role in the pathogenesis of ITP.15,16 In addition, Treg maintains autoimmune tolerance by secreting cytokines or direct cell contact. 17 It is shown that the number of Tregs is reduced or that Tregs are functionally deficient in ITP patients. 18 In addition, IL-23 and IL-6 also play a key role in inducing human Th0 to Th17 differentiation. 19 Moreover, Th17 cells have been confirmed to be involved in the pathogenesis of ITP.20,21
In this study, we aim to investigate the possible regulatory effect of DCs on CD4+ T cells in ITP patients. The proportions of pDC, mDC, Th1, Th2, Th17, and Treg cells in ITP patients were detected and analyzed. The ratios of pDC/mDC, Th1/Th2, and Th17/Treg cells were also analyzed. Additionally, the levels of serum cytokines (including IL-6, IL-12, IL-23, IFN-γ, IL-4, IL-17, and TGF-β) and the mRNA levels of IL-12p40, IL-12p35, IL-23p19, T-bet, GATA-3, RORγt, and Foxp3 were measured. The correlations of DCs and cytokines were further evaluated. Our finding may shed light on the role of DCs in the immune pathogenesis of ITP patients.
Materials and methods
Subjects
Thirty patients with ITP who were admitted to the Hematologic Disease Center of the First Affiliated Hospital of Xinjiang Medical University from July 2015 to June 2018 were recruited. All cases met the diagnostic criteria proposed by the Blood Branch of the Chinese Medical Association in 2016. 22 In addition, 20 healthy people who received physical examination at the same time period were also recruited as the control group. The clinical data of subjects are shown in Table 1. Peripheral blood was collected from all subjects. This study was approved by Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University, and all subjects signed informed consent.
Table 1.
Clinical data of study subjects.
| Items | Healthy control | Patients | P value |
|---|---|---|---|
| Cases | 20 | 30 | |
| Age (average, years)* | 39.80 ± 1.67 | 40.23 ± 2.28 | >0.05 |
| Gender (Male/Female) | 8/12 | 10/20 | >0.05 |
| Platelet count (average, × 109/L)* | 123.50 ± 5.92 | 12.40 ± 5.08 | <0.001 |
| Platelet count ≤10 × 109/L | 0 | 13 | |
| Platelet count (10–20) × 109/L | 0 | 14 | |
| Platelet count (20–30) × 109/L | 0 | 3 | |
| Platelet count >30 × 109/L | 20 | 0 |
*Data are expressed as mean ± SD.
Calculation of platelet count
The blood samples (20 μL) were mixed with 0.38 mL platelet diluent. After taking the supernatant and washing for three times, the samples were mixed thoroughly immediately and mixed again for 1 min after complete hemolysis. One drop of the above-mentioned platelet suspension was added into the counting pool and incubated for 10–15 min. The number of platelets were counted under high-power lens and were expressed as ×109/L.
Flow cytometry analysis
To detect the proportion of mDC and pDC cells, 50 μL of fresh peripheral blood was incubated with 10 μL of mouse antihuman monoclonal antibodies against lin-FITC, HLA-DR-PE-cy7, CD11c-PerCP, or lin-FITC, HLA-DR-PE-cy7, CD123-PerCP, respectively, at 4°C in the dark for 15 min. To detect the proportion of Th1, Th2, Th17, and Treg cells, 100 μL of peripheral blood was incubated with mouse antihuman monoclonal antibodies against CD4-PECY5, CD127-PE, CD25, BB515, CD4-FITC, CD183-PECY5, and CD196-PE, respectively, at 4°C in the dark for 30 min. All antibodies were obtained from BD Biosciences (San Jose, CA, USA). Then, 1 mL of hemolysin was added. After 8 min of incubation at room temperature, the samples were centrifuged at 1500 rpm for 5 min. After washing once with phosphate-buffered saline, the proportion of DC cell subpopulations was detected with AriaII (BD Biosciences, Franklin Lakes, NY, USA).
Enzyme-linked immunosorbent assay
Serum was isolated from peripheral blood after centrifugation. Enzyme-linked immunosorbent assay (ELISA) kits for IL-6, IL-12, IFN-γ, IL-4, IL-23, IL-17, and TGF-β were purchased from eBioscience (San Diego, CA, USA). Briefly, 50 μL of the diluted standard sample, serum sample, and biotin-labeled antibody were sequentially added to each well and incubated at 37°C for 1 h. After washing, 80 μL of streptavidin–horseradish peroxidase was added to each well and incubated at 37°C for another 30 min. After washing, substrates A and B were added to each well and incubated at 37°C for 10 min. After that, 50 μL of stop solution was added, and the optical density at 450 nm (OD450) was read using xMark™ microplate reader (Bio-Rad, CA, USA). The concentrations of IL-6, IL-12, IFN-γ, IL-4, IL-23, IL-17, and TGF-β were calculated according to the standard curve.
Reverse transcription polymerase chain reaction
RNA was extracted from mononuclear cells in peripheral blood with Trizol (Invitrogen, Carlsbad, CA, USA) and then reverse transcribed into cDNA using cDNA reverse transcription kit (Thermo Scientific, San Jose, CA, USA). SYBR Select Master Mix was purchased from ABI (Foster City, CA, USA). The primers were designed by OEBiotech (Shanghai, China) and synthesized by Invitrogen. The primer sequences are shown in Table 2. The polymerase chain reaction (PCR) reaction system included: 1 μL cDNA template, 0.4 μL upstream primer, 0.4 μL downstream primer, 5 μL TB Green Premix Ex Taq II (2x), 0.2 μL ROX Reference Dye or Dye II (50x), and 3 μL sterilized water. ABI 7500 (ABI) was used for PCR amplification under the conditions: 95°C, 30 s; 40 cycles of 95°C, 5 s and 60°C, 34 s; 95°C, 15 s and 60°C, 1 min; 95°C, 15 s and 60°C, 15 s. The relative level of each gene after standardization with β-actin was calculated using the 2–△△ CT method.
Table 2.
RT-PCR primers.
| Gene | Primer sequences (5′-3′) |
|---|---|
| T-bet | F:5′-AACAAGGGGGCGTCCAA-3′ |
| R:5′-GCTGAGTAATCTCGGCATTCTG-3′ | |
| GATA-3 | F:5′-CAATGCCTGTGGGCTCTACTAC-3′ |
| R:5′-GGCTGAAGGGCGAGATGTG-3′ | |
| RORγt | F:5′-GTGGCCCGGATGTGAGAAG-3′ |
| R:5′-GGAGCCCTTGTCGGATGATG-3′ | |
| Foxp-3 | F:5′-GTGGCCCGGATGTGAGAAG-3′ |
| R:5′-GGAGCCCTTGTCGGATGATG-3′ | |
| IL-12p40 | F:5′-ACGGACAAGACCTCAGCCAC-3′ |
| R:5′-GGGCCCGCACGCTA-3′ | |
| IL-12p35 | F:5′-CCACTCCAGACCCAGGAATG-3′ |
| R:5′-GACGGCCCTCAGCAGGT-3′ | |
| IL-23p19 | F:5′-GAGCCTTCTCTGCTCCCTGAT-3′ |
| R: 5′-AGTTGGCTGAGGCCCAGTAG-3′ | |
| β-actin | F:5′-AGTTGCGTTACACCCTTTCTTG-3′ |
| R:5′-TCACCTTCACCGTTCCAGTTT-3′ |
Statistical analysis
All data were analyzed statistically using SPSS 23.0 and GraphPad Prism 7.0. If the normal distribution and homogeneity of variance are met, the measurement data are expressed as mean ± standard deviation. Data comparison between groups was performed using two independent sample t-tests of measurement data. If the distribution is skewed, the median (interquartile range) is used to represent the data, and Wilcoxon signed rank test of two independent samples was used for data comparison between the two groups. Correlation was analyzed with Pearson linear correlation. A P value <0.05 was considered statistically significant.
Results
Peripheral blood platelet count in ITP group and healthy control group
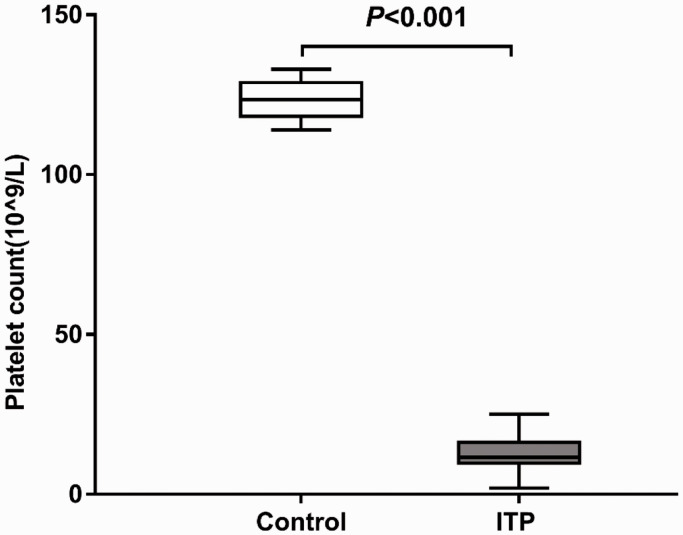
As shown in Figure 1, the platelet count of ITP patients ((12.40 ± 5.08) × 109/L) was significantly lower than that of healthy controls ((123.50 ± 5.92) × 109/L (P < 0.001). Among the 30 patients with ITP, 13 had platelet count ≤10 × 109/L, 14 had platelet count of (10–20) ×109/L, 3 had platelet count of (20–30) ×109/L, and no patient had platelet count >30 × 109/L (Table 1).
Figure 1.
Platelet counts in peripheral blood of healthy controls and ITP patients. Platelet counts in peripheral blood samples from 30 ITP patients and 20 normal controls were detected with a blood cell counter.
ITP: primary immune thrombocytopenia.
Proportion of pDC cells and mDC cells in peripheral blood
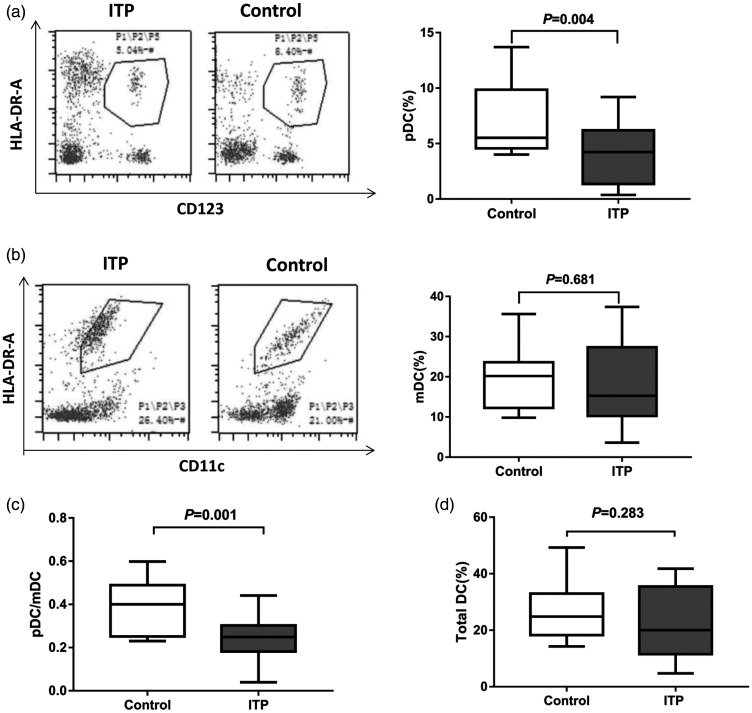
Flow cytometry was used to detect pDC and mDC proportion. The proportion of Lin-CD123 + pDC (4.80 ± 2.44)% in peripheral blood of ITP patients was significantly lower than that of healthy controls (7.29 ± 3.37)% (P < 0.05) (Figure 2a). However, the proportion of Lin-CD11C + mDC (20.56 ± 9.56)% was higher than that of the healthy control group (19.25 ± 7.44)%, but there was no statistical difference (P > 0.05) (Figure 2b). Thus, there was a lower pDC/mDC ratio (0.25 ± 0.08) in ITP patients than in healthy controls (0.39 ± 0.12) (Figure 2c) (P < 0.05). In addition, compared with the healthy control group (10.21 ± 2.83), the total DC ratio of the ITP patient group (12.19 ± 2.49) was slightly increased, but the difference was not statistically significant (P > 0.05) (Figure 2d).
Figure 2.
Proportions of peripheral blood DC subsets in healthy controls and ITP patients. Fresh whole blood of ITP patients (n = 30) and healthy subjects (n = 20) was collected. DC subsets were detected by flow cytometry. (a) Representative flow cytometry of pDC (left) and proportions of peripheral blood pDC (right). (b) Representative flow cytometry of mDC (left) and proportions of peripheral blood mDC (right). (c) Ratio of pDC/mDC in peripheral blood. (d) Proportions of total DC.
ITP: primary immune thrombocytopenia; DC: dendritic cells; pDC: plasmacytoid dendritic cells; mDC: myeloid dendritic cells.
Proportion of CD4+ T cell subsets in peripheral blood
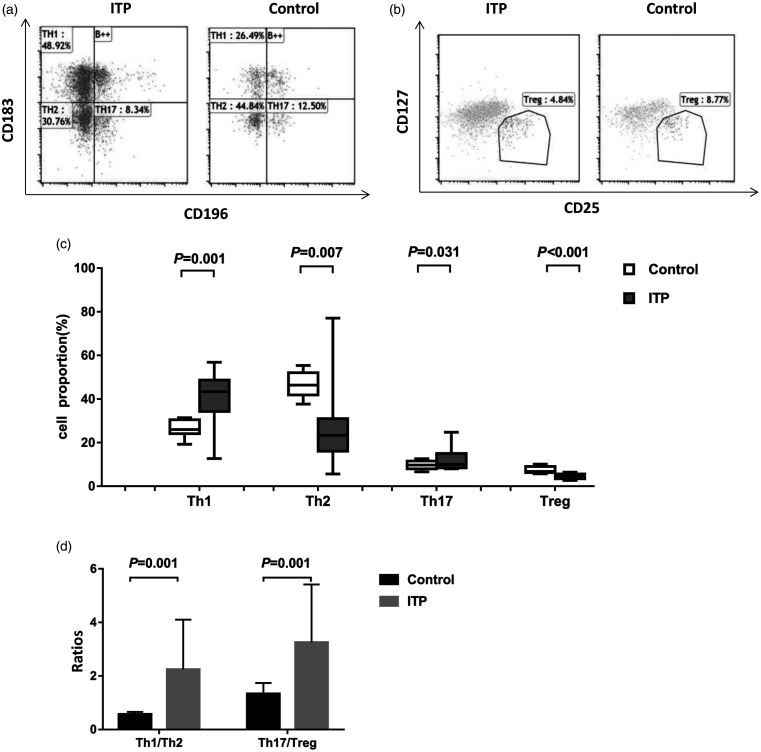
Flow cytometry analysis showed that the proportions of Th1 (38.81 ± 13.15)% and Th17 cells (12.96 ± 5.71)% in peripheral blood of ITP patients were significantly higher than those of healthy controls (Th1: (26.25 ± 3.78)%; Th17: 9.75 ± 2.15)% (Figure 3a and c) (P < 0.05). However, the proportion of Th2 (30.59 ± 22.54)% and Treg cells (4.37 ± 1.11)% in ITP patients was significantly lower than that of healthy controls (Th2: (46.63 ± 5.70)%; Treg: (7.49 ± 1.47)% (P < 0.05) (Figure 3a to c). In addition, Th1/Th2 ratio and Th17/Treg ratio were higher in the ITP patients than in the healthy controls (all P = 0.001) (Figure 3d).
Figure 3.
Proportions of peripheral blood CD4+ T subsets in healthy controls and ITP patients. Fresh whole blood was collected from ITP patients (n = 30) and healthy subjects (n = 20). CD4+ T subsets were analyzed by flow cytometry. (a) Representative flow cytometry results of Th1, Th2, and Th17. (b) Representative flow cytometry results of Treg. (c) Proportions of peripheral blood Th1, Th2, Th17, and Treg. (d) Ratios of Th1/Th2 and Th17/Treg.
ITP: primary immune thrombocytopenia.
IL-6, IL-12, and IL-23 levels in peripheral blood
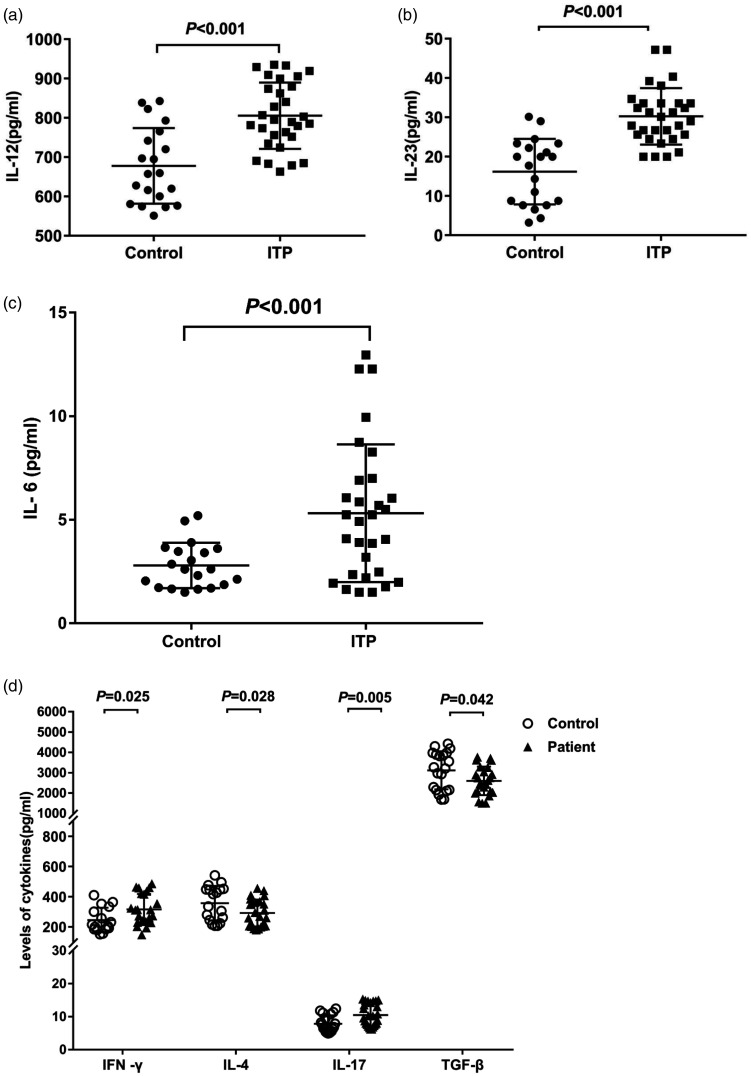
ELISA was used to detect cytokine levels. The results showed that the levels of IL-12, IL-23, and IL-6 in peripheral blood of ITP patients were significantly higher than those in healthy controls (IL-12: 805.43 ± 84.51 pg/mL vs. 677.62 ± 96.15 pg/mL; IL-23: 30.27 ± 7.19 pg/mL vs. 16.19 ± 8.34 pg/mL; IL-6: 5.32 ± 1.32 pg/mL vs. 2.80 ± 1.09 pg/mL) (P < 0.05) (Figure 4a to c).
Figure 4.
Analysis of cytokine levels. Peripheral blood was collected from all patients (n = 30) and healthy subjects (n = 20). Cytokine levels were detected ELISA. (a) Levels of IL-12, (b) IL-23 and (c) IL-6 in ITP patients and controls. (d) Levels of IFN-γ, IL-4, IL -17, and TGF-β in ITP patients and controls.
IL: interleukin; ITP: primary immune thrombocytopenia; IFN-γ: interferon gamma; TGF-β: transforming growth factor beta.
IFN-γ, IL-4, TGF-β, IL-17 levels in peripheral blood
ELISA results also showed that the levels of IFN-γ (316.48 ± 101.42 pg/mL) and IL-17 (10.52 ± 3.30 pg/mL) in the serum of ITP patients were significantly higher than those in the control group (249.69 ± 81.13 pg/mL, 7.88 ± 2.53 pg/mL) (Figure 4d) (P < 0.05). However, the levels of IL-4 (292.22 ± 89.03 pg/mL) and TGF-β (2601.70 ± 703.78 pg/mL) in ITP patients were significantly lower than those in the control group (361.48 ± 110.20 pg/mL, 3121.56 ± 938.84 pg/mL) (Figure 4d) (P < 0.05).
The mRNA levels of IL-12 and IL-23 in peripheral blood
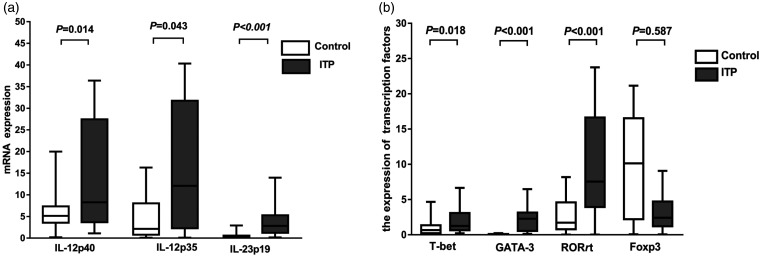
The mRNA levels were measured with reverse transcription PCR (RT-PCR). The results showed that the mRNA levels of IL-12p40, IL-12p35, and IL-23p19 in ITP patients were significantly higher than those in healthy controls (P < 0.05) (Figure 5a).
Figure 5.
Analysis of mRNA expression. (a) Detection of IL-12p40, IL-12p35, and IL-23p19 mRNA expression in peripheral blood of ITP patients (n = 30) and healthy subjects (n = 20) by RT-PCR. (b) Detection of T-bet, GATA-3, RORγt, and Foxp-3 expression in peripheral blood of ITP patients (n = 30) and healthy subjects (n = 20) by RT-PCR.
IL: interleukin; ITP: primary immune thrombocytopenia.
Transcription factor levels in peripheral blood
RT-PCR also showed that the expression levels of T-bet, GATA-3, and RORγt in ITP patients were significantly higher than those in the control group (Figure 5b) (P < 0.05). However, the expression levels of Foxp-3 were not significantly different (P > 0.05).
Pearson correlation analysis
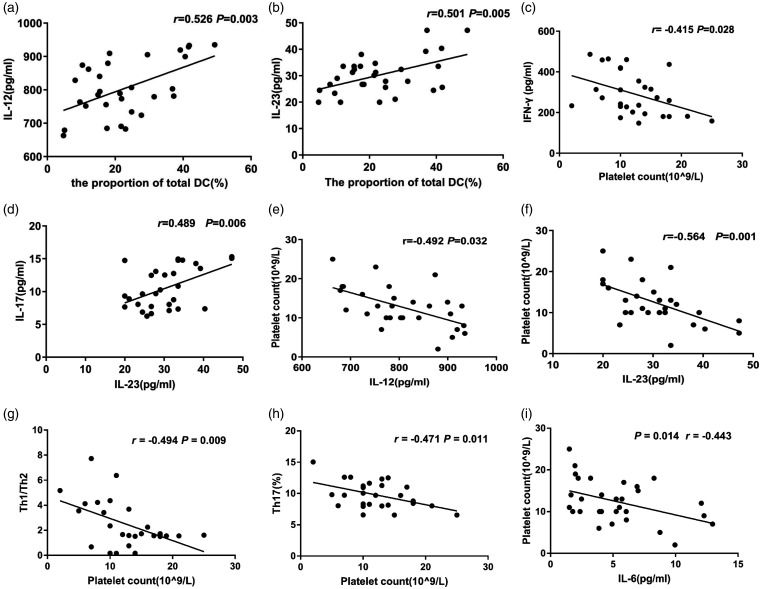
We used Pearson’s correlation to analyze the correlation of total peripheral blood DC proportion, CD4+ T subtypes, IL-6, IL-12, IL-23 and IFN-γ, IL-4, IL-17, TGF-β and platelet count in patients with ITP. The results showed that the total DC proportion of patients was positively correlated with IL-12 (r = 0.526, P = 0.003) (Figure 6a) and IL-23 (r = 0.501, P = 0.005) (Figure 6b). IFN-γ was negatively correlated with platelet count (r = −0.415, P = 0.028) (Figure 6c). IL-23 was positively correlated with IL-17 (r = 0.489, P = 0.006) (Figure 6d). IL-12 was negatively correlated with platelet count (r = −0.492, P = 0.032) (Figure 6e). Similarly, IL-23 was also negatively correlated with platelet count (r = −0.564, P = 0.001) (Figure 6f). Th1/Th2 ratio, Th17 proportion, and IL-6 were all negatively correlated with platelet count (Th1/Th2 ratio: r = −0.494, P = 0.009, Figure 6g; Th17 proportion: r = −0.471, P = 0.011, Figure 6h; IL-6: r = −0.443, P = 0.014, Figure 6i).
Figure 6.
Correlation analysis. Pearson correlation was performed. (a) The correlation between the proportion of total DC (%) and IL-12. (b) The correlation between the proportion of total DC (%) and IL-23. (c) The correlation between platelet count and IFN-γ. (d) The correlation between IL-23 and IL-17. (e) The correlation between IL-12 and platelet count. (f) The correlation between IL-23 and platelet count. (g) The correlation between Th1/Th2 ratio and platelet count. (h) The correlation between Th17 proportion and platelet count. (i) The correlation between IL-6 and platelet count.
IL: interleukin; DC: dendritic cells; IFN-γ: interferon gamma.
Discussion
Cellular immune dysfunction is an important factor of ITP pathogenesis. Studies have shown that CD4+ T cell subgroup imbalance and abnormal secretion of related cytokines are present in ITP patients, which are related to disease occurrence and development.4,16,21 However, the mechanism that causes the imbalance of CD4+ T cell subsets is still unclear.
DC is an antigen-presenting cell that directly activates T cells, which enables the naive T cells to differentiate into effector T cells. Studies have found that DC affects Th0 differentiation and the ratio of Th1/Th2 and Th17/Treg through its maturity, quantitative changes, imbalance of subpopulation ratios, and cytokine secretion.23,24 Therefore, the imbalance of DC subpopulation ratio may be an important mechanism leading to the imbalance of CD4+ T subpopulation in ITP patients. Boggio et al. 25 found that mDC and pDC in peripheral blood of ITP patients were reduced compared with healthy control group. Saito et al. 26 showed that compared with age- and sex-matched healthy controls, the absolute number of pDCs in peripheral blood decreased in patients with primary ITP and Helicobacter pylori-related ITP, while the absolute number of mDC remained unchanged. Hsu et al. 27 reported that after prednisone treatment, the number of mDC and pDC in the peripheral blood of ITP patients decreased, suggesting that glucocorticoids may suppress the immune response and reduce the production of antiplatelet autoantibodies by suppressing the number of circulating DCs. In this study, we also found that compared with healthy controls, pDC was reduced in the serum of ITP patients, but there was no significant change in mDC. This led to a reduction in the patient’s pDC/mDC ratio, confirming the presence of DC subgroup imbalances in ITP patients. However, the subdivision of mDC in this study does not make much sense because pDC seems to be problematic, and it is not critical to understanding the pathophysiology of ITP.
The mDC induces Th0 differentiation into Th1 cells through the induction of IL-12 and TNF-α. 28 Th1 secretes IFN-γ and TNF to exert cellular immune effects and inhibit Th2 proliferation. However, pDC promotes the differentiation of Th0 into Th2 cells, 29 which mainly secrete Th2 type cytokines, including IL-4, IL-5, IL-10, and IL-13. Yang et al. 30 found that IFN-γ and Th1 in serum of ITP patients increased. Lin et al. 3 showed that the Th1/Th2 and T-bet/GATA-3 ratios in patients with ITP were imbalanced. After rhIL-11 treatment, Th1 and T-bet levels were significantly reduced and Th1/Th2 and T-bet/GATA-3 ratio returned to normal. Compared with the healthy control, the proportion of Th1 cells in ITP patients increased, while the proportion of Th2 cells decreased, resulting in an increase in the ratio of Th1/Th2. Consistently, we also confirmed from the protein and gene levels that there was an imbalance in the ratio of Th1/Th2 cells in ITP patients, and ITP patients had Th1 cell immune shift. In addition, the results of this study also showed a decrease in peripheral blood pDC in ITP patients. Therefore, we speculate that the decreased pDC may reduce the differentiation of Th0 into Th2 cells. This further leads to weakened inhibitory effect of Th2 on Th1. Thus, the differentiation Th0 into Th1 is enhanced, forming a Th1 cell bias, which ultimately leads to Th1/Th2 imbalance.
Mature DCs secrete cytokines such as IL-6, IL-12, IL-23, and IL-27, which play completely different roles in regulating Th0 differentiation. 31 IL-12 promotes Th0 differentiation to Th1 cells and induces T cells and natural killer cells to produce IFN-γ. IL-23 induces Th0 to differentiate into Th17 cells and promotes the secretion of IL-17. The synergistic effect of IL-6 and TGF-β promotes the upregulation of STAT3-mediated RORγt, promotes the differentiation of CD4+ initial T cells into Th17, and inhibits Treg. 13 Li et al. 32 found that IL-12 was elevated in peripheral blood and bone marrow in the ITP group. Correlation analysis suggested that the level of IL-12 in peripheral blood of patients with ITP was negatively correlated with platelet count, indicating that the upregulation of IL-12 may be related to the immune response of ITP. In this study, we tested the level of IL-12 in the serum and found that the protein of IL-12 in peripheral blood of ITP patients and mRNA levels increased significantly. Further correlation analysis showed that the proportion of total DC was positively correlated with IL-12; IL-12 was negatively correlated with platelet count; and Th1/Th2 ratio, IFN-γ, and IL-6 were all negatively correlated with platelet count. These results suggest that the increase of Th1/Th2 ratio, IL-6, IL-12, and IFN-γ may be related to the degree of platelet destruction and bleeding of patients.
Th17 mainly secretes IL-17A, IL-17F, IL-21, and IL-22, exerts inflammatory effects, and mediates autoimmune diseases. 33 Treg cells mainly secrete TGF-β and play a role in suppressing inflammatory response and maintaining autoimmune tolerance. If there is imbalance of Th17/Treg, autoimmune diseases may occur. Studies have shown that the proportion of Treg cells in ITP patients is significantly lower than that in healthy controls, the proportion of Th17 cells increases significantly at the time of onset, and that the Treg/Th17 ratio is related to the activity of ITP disease.21,34 Here, in this study, we found that compared with the healthy control group, the proportion of Th17 cells in the ITP group increased, while the proportion of Treg cells decreased, resulting in an increase in the ratio of Th17/Treg. At the same time, we tested the expression of Th17 and Treg cell-related cytokines and transcription factors and found that IL-17 and RORγt were upregulated and TGF-β and Foxp3 were decreased. Thus, ITP patients exhibited Th17 cell bias.
IL-6 and IL-23 is mainly expressed in antigen-presenting cells such as DCs and macrophages and induces Th0 to differentiate into Th17 cells. The study by Zhou et al. 35 showed that compared with the normal control group, Th17-related proinflammatory cytokines (such as IL-17A/F, IL-6, and IL-23) levels were higher, while anti-inflammatory factors (including IL-10 and TGF-β) levels were lower in patients with ITP. The Th17/Treg ratio of the severe ITP patient group was significantly higher than that of the mild ITP patient group and the normal control group, and it was negatively correlated with platelet count. The results of Ye et al. 36 showed that the levels of IL-17 and IL-23 in Th17 cells and serum of patients with ITP increased and that the levels of IL-23 were positively correlated with IL-17 and Th17 cells and negatively correlated with platelet counts. Consistently, in this study, we found that the levels of IL-6 and IL-23 in peripheral blood of ITP patients were higher than those of healthy controls. Further correlation analysis showed that the proportion of total DC was positively correlated with IL-23, and IL-23 was positively correlated with IL-17. However, Th17 proportion, IL-6, and IL-23 were all negatively correlated with platelet count. These results indicate that DC may secrete IL-6 and IL-23 to induce Th0 to differentiate into Th17, which in turn causes a Th17/Treg imbalance. However, we did not find correlations between IL-23 and Th17 cells or between IL-17 and platelet counts, which may be related to the relatively small sample size of this study. The specific mechanism still needs to be further explored.
Conclusions
In summary, we have verified the existence of Th1/Th2 and Th17/Treg imbalance in ITP patients from multiple aspects such as cells, cytokines, and transcription factors. In ITP patients, the immune system exhibited Th1 and Th17 cell immune bias. Furthermore, there was an imbalance of pDC/mDC subpopulations and upregulation of related cytokines (IL-6, IL-12, and IL-23) in ITP patients. Correlation analysis showed that IL-12 and IL-23 were negatively correlated with platelet count, while Th1/Th2 ratio, Th17 proportion, IL-6, and IFN-γ were negatively correlated with platelet count. These can be used to assess the severity of the patient’s condition. However, total DC was positively correlated with IL-12 and IL-23, and IL-23 was positively correlated with IL-17. We speculate that DCs may upregulate the expression of T-bet and RORγt by secreting IL-12, IL-6, and IL-23, promote Th0 differentiation to Th1 and Th17 cell subsets, and cause imbalance of CD4+ T lymphocyte subsets in ITP.
Footnotes
AUTHORS’ CONTRIBUTIONS: QZL, YaL, XJW, MLS, LW, XYW, YiL, and WXF performed the study and collected the data. XJW and XS searched the literature. QZL wrote the manuscript. XHG designed the study, collected the funds, and reviewed the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ETHICAL APPROVAL: This study was approved by Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University, and all subjects signed informed consent.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (No. 81360086).
ORCID iD: Xinhong Guo https://orcid.org/0000-0001-7920-9541
References
- 1.Wei Y, Hou M. T cells in the pathogenesis of immune thrombocytopenia. Semin Hematol 2016; 53: S13–5 [DOI] [PubMed] [Google Scholar]
- 2.Kostic M, Zivkovic N, Cvetanovic A, Marjanović G. CD4+ T cell phenotypes in the pathogenesis of immune thrombocytopenia. Cell Immunol 2020; 351:104096. [DOI] [PubMed] [Google Scholar]
- 3.Lin Y, Zhou X, Guo W, Li Q, Pan X, Bao Y, He M, Zhu B, Lin X, Jin L, Yao R. RhIL-11 treatment normalized Th1/Th2 and T-bet/GATA-3 imbalance in in human immune thrombocytopenic purpura (ITP). Int Immunopharmacol 2016; 38:40–4 [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Pang N, Wang X, Liu Y, Wang X, Wang L, Sun M, Yasen H, Zhao F, Fan W, Guo X, Ding J. Percentages of PD-1(+)CD4(+)T cells and PD-L1(+)DCs are increased and sPD-1 level is elevated in patients with immune thrombocytopenia. Hum Vaccin Immunother 2018; 14:832–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu S, Liu C, Li L, Tian T, Wang M, Hu Y, Yuan C, Zhang L, Ji C, Ma D. Inactivation of notch signaling reverses the Th17/treg imbalance in cells from patients with immune thrombocytopenia. Lab Invest 2015; 95:157–67 [DOI] [PubMed] [Google Scholar]
- 6.Wu D, Liu Y, Pang N, Sun M, Wang X, Haridia Y, Zhao F, Qin Y, Fan W, Guo X, Ding J. PD-1/PD-L1 pathway activation restores the imbalance of Th1/Th2 and treg/Th17 cells subtypes in immune thrombocytopenic purpura patients. Medicine (Baltimore) 2019; 98:e17608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science 2010; 327:656–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang XL, Ma J, Xu M, Meng F, Qu M, Sun J, Qin P, Wang L, Hou Y, Song Q, Peng J, Hou M. Imbalance between CD205 and CD80/CD86 in dendritic cells in patients with immune thrombocytopenia. Thromb Res 2015; 135:352–61 [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Wang Y, Zhang D, Li H, Zhou Z, Yang R. CD70-silenced dendritic cells induce immune tolerance in immune thrombocytopenia patients. Br J Haematol 2020; 191:466–75 [DOI] [PubMed] [Google Scholar]
- 10.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011; 474:298–306 [DOI] [PubMed] [Google Scholar]
- 11.Bousso P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat Rev Immunol 2008; 8:675–84 [DOI] [PubMed] [Google Scholar]
- 12.Yoshimoto T. The hunt for the source of primary interleukin-4: how we discovered that natural killer T cells and basophils determine T helper type 2 cell differentiation in vivo. Front Immunol 2018; 9:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura A, Kishimoto T. IL-6: regulator of treg/Th17 balance. Eur J Immunol 2010; 40:1830–5 [DOI] [PubMed] [Google Scholar]
- 14.Murphy TL, Grajales-Reyes GE, Wu X, Tussiwand R, Briseño CG, Iwata A, Kretzer NM, Durai V, Murphy KM. Transcriptional control of dendritic cell development. Annu Rev Immunol 2016; 34:93–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Wang M, Zhou S, Ma J, Shi Y, Peng J, Hou M, Guo C. Pulsed high-dose dexamethasone modulates Th1-/Th2-chemokine imbalance in immune thrombocytopenia. J Transl Med 2016; 14:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi N, Saitoh T, Gotoh N, Nitta Y, Alkebsi L, Kasamatsu T, Minato Y, Yokohama A, Tsukamoto N, Handa H, Murakami H. The cytokine polymorphisms affecting Th1/Th2 increase the susceptibility to, and severity of, chronic ITP. BMC Immunol 2017; 18:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shevach EM. Foxp3 (+) T regulatory cells: still many unanswered questions-A perspective after 20 years of study. Front Immunol 2018; 9:1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang M, Cheng L, Li F, Wu B, Chen P, Zhan Y, Hua F, Min Z, Ke Y, Liu C, Yuan L, Sun L, Chen H, Ji L, Cheng Y. Transcription factor IRF4 dysfunction affects the immunosuppressive function of treg cells in patients with primary immune thrombocytopenia. Biomed Res Int 2019; 2019:1050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Revu S, Wu J, Henkel M, Rittenhouse N, Menk A, Delgoffe GM, Poholek AC, McGeachy MJ. IL-23 and IL-1β drive human Th17 cell differentiation and metabolic reprogramming in absence of CD28 costimulation. Cell Rep 2018; 22:2642–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Ma T, Zhou X, Chen J, Li J. Circulating level of Th17 cells is associated with sensitivity to glucocorticoids in patients with immune thrombocytopenia. Int J Hematol 2018; 107:442–50 [DOI] [PubMed] [Google Scholar]
- 21.Guo NH, Fu X, Zi FM, Song Y, Wang S, Cheng J. The potential therapeutic benefit of resveratrol on Th17/treg imbalance in immune thrombocytopenic purpura. Int Immunopharmacol 2019; 73:181–92 [DOI] [PubMed] [Google Scholar]
- 22.Thrombosis and Hemostasis Group, Hematology Society, Chinese Medical Association. Consensus of Chinese experts on diagnosis and treatment of adult primary immune thrombocytopenia (version 2016). Zhonghua Xue Ye Xue Za Zhi 2016; 37:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lastic AL, Rodi M, Mouzaki A. Effect of dendritic cell state and antigen-presentation conditions on resulting T-cell phenotypes and Th cytokine profiles. Immunobiology 2016; 221:862–70 [DOI] [PubMed] [Google Scholar]
- 24.Qian C, Cao X. Dendritic cells in the regulation of immunity and inflammation. Semin Immunol 2018; 35:3–11 [DOI] [PubMed] [Google Scholar]
- 25.Boggio E, Gigliotti CL, Rossi D, Toffoletti E, Cappellano G, Clemente N, Puglisi S, Lunghi M, Cerri M, Vianelli N, Cantoni S, Tieghi A, Beggiato E, Gaidano G, Comi C, Chiocchetti A, Fanin R, Dianzani U, Zaja F. Decreased function of Fas and variations of the perforin gene in adult patients with primary immune thrombocytopenia. Br J Haematol 2017; 176:258–67 [DOI] [PubMed] [Google Scholar]
- 26.Saito A, Yokohama A, Osaki Y, Ogawa Y, Nakahashi H, Toyama K, Mitsui T, Hashimoto Y, Koiso H, Uchiumi H, Saitoh T, Handa H, Sawamura M, Karasawa M, Murakami H, Tsukamoto N, Nojima Y. Circulating plasmacytoid dendritic cells in patients with primary and Helicobacter pylori-associated immune thrombocytopenia. Eur J Haematol 2012; 88:340–9 [DOI] [PubMed] [Google Scholar]
- 27.Hsu NC, Chung CY, Horng HC, Chang CS. Corticosteroid administration depresses circulating dendritic cells in ITP patients. Platelets 2004; 15:451–4 [DOI] [PubMed] [Google Scholar]
- 28.Tokumasa N, Suto A, Kagami S, Furuta S, Hirose K, Watanabe N, Saito Y, Shimoda K, Iwamoto I, Nakajima H. Expression of Tyk2 in dendritic cells is required for IL-12, IL-23, and IFN-gamma production and the induction of Th1 cell differentiation. Blood 2007; 110:553–60 [DOI] [PubMed] [Google Scholar]
- 29.Saei A, Hadjati J. Tolerogenic dendritic cells: key regulators of peripheral tolerance in health and disease. Int Arch Allergy Immunol 2013; 161:293–303 [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Weng W, Sun H, Du C, Yin C, Hou H, Fu X, Zhuang W, Sun Z, Liu L. Study on peripheral blood lymphocyte subsets and related cytokines in patients with chronic immune thrombocytopenia. Thromb Hemost 2017; 23:6–9 [Google Scholar]
- 31.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol 2012; 13:722–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q, Yang M, Xia R, Xia L, Zhang L. Elevated expression of IL-12 and IL-23 in patients with primary immune thrombocytopenia. Platelet 2015;26:453--8 [DOI] [PubMed] [Google Scholar]
- 33.Mitsdoerffer M, Lee Y, Jäger A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A 2010; 107:14292–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji L, Zhan Y, Hua F, Li F, Zou S, Wang W, Song D, Min Z, Chen H, Cheng Y. The ratio of treg/Th17 cells correlates with the disease activity of primary immune thrombocytopenia. PLoS One 2012; 7:e50909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou L, Xu F, Chang C, Tao Y, Song L, Li X. Interleukin-17-producing CD4+ T lymphocytes are increased in patients with primary immune thrombocytopenia. Blood Coagul Fibrinolysis 2016; 27:301–7 [DOI] [PubMed] [Google Scholar]
- 36.Ye X, Zhang L, Wang H, Chen Y, Zhang W, Zhu R, Fang C, Deng A, Qian B. The role of IL-23/Th17 pathway in patients with primary immune thrombocytopenia. PLoS One 2015; 10:e0117704. [DOI] [PMC free article] [PubMed] [Google Scholar]