Abstract
Thrombosis and thrombocytopaenia secondary to ChAdOx-1 nCov-19 vaccine is a new phenomenon that usually occurs after the first dose of vaccine. Most of these patients are healthy without any prior history of thromboembolic events or heparin use. Hall marks of this condition include detectable antibodies to platelet factor 4 and thrombosis at atypical sites particularly cerebral veins and sinuses mimicking atypical heparin induced thrombocytopaenia. We describe a case of a patient who was diagnosed with this rare condition and treated successfully.
Keywords: COVID-19, vaccination/immunisation
Background
COVID-19 pandemic has taken the world by storm since it first began in January 2020. As of 17 October 2021, 241 million cases of SARS-CoV-2 infections were reported worldwide along with 4.9 million deaths by COVID-19.1 Being a significant global health risk, it led to a relatively quick development of vaccines. As of 14 October, more than 6.4 billion vaccine doses have been administered worldwide,2 with more than 41 million people in England alone receiving their first dose from December 2020 to 6 October 2021.3 An estimated 24.9 million first doses and 24 million second doses of ChAdOx-1 nCov-19 (Oxford/AstraZeneca) vaccine have been administered in the UK by 6 October 2021. Vaccines against SARS-COV 2 are generally safe. With use, reports of thrombosis with thrombocytopaenia in relation to the use of ChAdOx-1 nCov-19 vaccine began to emerge. Several case series have been published in literature describing these patients, the way they were treated and their outcomes.4 5 These patients were generally young (aged 22–54 years), who presented 5–16 days after vaccination with thrombosis at unusual sites, thrombocytopaenia, elevated D-dimers, low/normal fibrinogen, normal PT and aPTT along with elevated titres of antibodies against platelet factor 4 (PF-4) heparin complex without prior exposure to heparin. In the guidelines produced by expert haematology panel (EHP) of British society of Haematology and National Institute for Health and Care Excellence (NICE) in UK, this syndrome has been labelled as vaccine-induced immune thrombosis and thrombocytopaenia (VITT).6 7 The spectrum of clinical features and lab findings strongly resemble autoimmune heparin-induced thrombocytopaenia.8
Up till 6 October 2021, 424 cases have been reported with a fatality rate of 17% and an overall incidence after the first or unknown dose being 15.2 per million doses. Forty-six out of 424 cases have been reported after a second dose with an overall incidence after second dose being 1.9 cases per million doses.3 Such low incidence rates make it a very rare side effect of the vaccine. One hundred and fifty-one out of 424 reported patients had cerebral venous sinus thrombosis (CVST), making this the most common site for thrombosis. CVST carries high mortality in patients with VITT, with odds of death being increased by a factor of 2.7.9 EHP has identified five criteria which can be used to classify a VITT case into one of four categories to prioritise and guide further management. These are definite, probable, possible and unlikely. These criteria are (1) onset of symptoms 5–30 days post COVID-19 vaccine, (2) presence of thrombosis, (3) thrombocytopaenia, (4) D-Dimers>4000 μg/mL (Fibrinogen Equivalent Units) and (5) positive anti-PF-4 antibodies ELISA assay. We describe a case of a patient who presented with CVST in association with ChAdOx-1 nCov-19 vaccine who received prompt treatment and remained well without any complications.
Case presentation
A 48-year-old woman was admitted to accident and emergency unit for severe headache. Three weeks prior, she received the first dose of ChAdOx1 nCoV-19 vaccine. During the first week after vaccination, she noticed influenza-like symptoms including generalised aches and pain, lethargy and mild headache. The headache gradually worsened over the course of next 2 weeks. It was predominantly right sided, radiating from occipital region to the forehead and neck, constant and worsened when turning head to the right or when lying down. Pain was not increased with straining or leaning forward. It was not associated with disturbances in gait, speech, balance, diplopia, decreased vision, abnormal movements, limb weakness or paraesthesia. She did notice flashing lights in her field of vision, photophobia and phonophobia. Hours before presentation, pain was so severe that it woke her up from sleep and she vomited a few times. She did not have any fever, rashes, mouth ulcers, ear pain or discharge. She was a previously fit and physically active person who had recently started a new job. She did not smoke or drink alcohol. There was no past medical history or family history of blood clots or migraine. Three months prior to presentation, she was started on hormone replacement therapy for her perimenopausal symptoms. She did not recall using recent heparin injections. She was not using any other medications or illicit drugs.
On examination, her observations were as follows: heart rate 79, blood pressure 123/73, temperature 36.7°C, respiratory rate 16, oxygen saturation 100% on room air, weight 82 kg and body mass index 25.5 kg/m2. Neurological examination including assessment of cranial nerves, cerebellum and peripheral nervous system was unremarkable. Tenderness was noted on palpation of occiput and neck with pain exacerbated on neck movements. Rest of the general physical and systemic examination was unremarkable.
Investigations
A nasal swab sample taken for SARS-COV-2 RNA was negative by PCR. Initial investigations are shown in table 1. A peripheral blood film confirmed thrombocytopaenia. A CT venogram demonstrated an acute thrombus in right transverse and sigmoid sinus projecting into right internal jugular vein with no evidence of oedema or haemorrhage (figures 1 and 2). A sample for antibodies to PF-4 by ELISA was sent to a different hospital.
Table 1.
Laboratory investigations from admission up to discharge and then on follow-up
| Investigations | On admission to this hospital | On admission to another hospital | Second day | Third day | Fifth day | Seventh day | Follow-up 1 month after discharge | Reference range |
| Haemoglobin, g/L | 143 | 136 | 140 | 134 | 140 | 154 | 139 | 115–164 |
| White cell count, 109/L | 9.2 | 5.8 | 9.3 | 14.2 | 13.8 | 9.2 | 5.9 | 4–11 |
| Neutrophils, 109 /L | 7.7 | 3.9 | 1.9–7.5 | |||||
| Lymphocytes, 109 /L | 0.8 | 1.4 | 1–4 | |||||
| Platelets, 109 /L | 66 | 87 | 110 | 164 | 188 | 200 | 194 | 150–400 |
| Alanine aminotransferase, iU/L |
83 | 87 | 78 | 144 | 0–40 | |||
| Alkaline phosphatase, iU/L | 317 | 296 | 290 | 266 | 30–130 | |||
| Gamma glutamyl transferase, iU/L | 194 | 240 | 6–42 | |||||
| Total bilirubin, μmol/L | 11 | 12 | 10 | 8 | 0–21 | |||
| Albumin, g/L | 42 | 35 | 36 | 47 | 35–50 | |||
| Total protein, g/L | 73 | 74 | 60–80 | |||||
| Prothrombin time, seconds | 13 | 12 | 9–14 | |||||
| International normalised ratio | 1.0 | 1.0 | 1.0 | 0.8–1.2 | ||||
| activated partial thromboplastin time, seconds | 30 | 31 | 24–35 | |||||
| D-dimer assay, ng/mL | 6735 | 235 | Less than 500 | |||||
| Fibrinogen assay, g/L | 4.12 | 3.64 | 2–6 | |||||
| C reactive protein, mg/L | 19 | 25 | 2 | 0–10 | ||||
| Urea, mmol/L | 2.5 | 3.5 | 3.8 | 2.9 | 2.5–7.8 | |||
| Creatinine, μmol/L | 57 | 53 | 57 | 61 | 44–80 | |||
| estimated glomerular filtration rate, mL/min | >90 | >90 | ||||||
| Sodium, mmol/L | 137 | 135 | 139 | 141 | 133–146 | |||
| Potassium, mmol/L | 4.1 | 4.0 | 4.0 | 4.1 | 3.5–5.3 | |||
| Chloride, mmol/L | 104 | 95–108 | ||||||
| Bicarbonate, mmol/L | 27.2 | 22–29 | ||||||
| Glucose, mmol/L | 7.0 | 4.0–7.8 | ||||||
| Lactate, mmol/L | 1.4 | 1–1.5 | ||||||
| pH | 7.48 | 7.35–7.45 | ||||||
| Antiplatelet factor 4 antibodies | >3.0 OD | 2.87 OD | <0.4 negative 0.4–1.0 weakly positive >1.0 positive |
OD, optical density.
Figure 1.
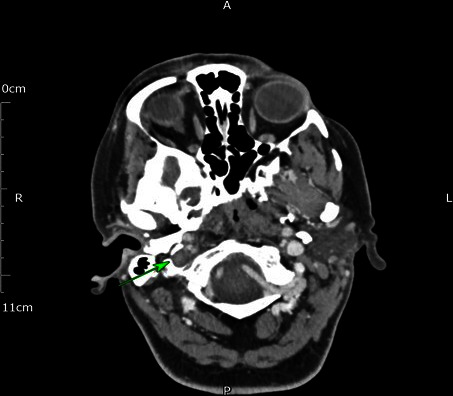
CT venogram on admission demonstrating a thrombus in the right internal jugular vein (green arrow).
Figure 2.
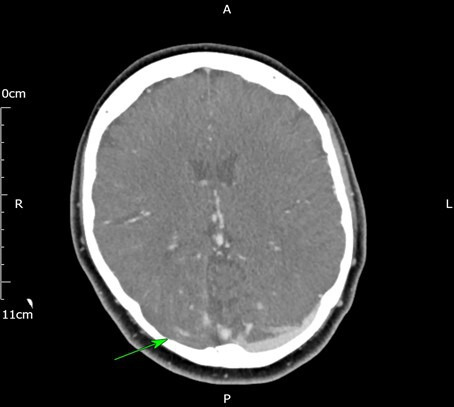
CT venogram on admission showing a filling defect in the right transverse sinus (green arrow) as compared to the left.
Treatment
After discussion with haematology, a diagnosis of probable vaccine induced thrombotic thrombocytopaenia was made. She received fondaparinux 7.5 mg, intravenous immunoglobulin at 1 g/kg and dexamethasone 20 mg/day. Since the clot was extensive, after discussion with neurology and interventional radiology, she was transferred to another hospital for possible clot extraction. Later, antibodies to PF-4 were positive, with the optical density (OD) of more than 3.0, confirming the initial presumptive diagnosis. The test was performed using Immucor PF-4 IgG ELISA kit. A follow-up CT venogram the next day showed stable clot appearances with minor recanalisation along medial and anterior margins. No new areas of thrombosis were demonstrated. Another CT venogram 2 days later showed similar appearances with no propagation. Her headache continued to improve. Dexamethasone was increased to 40 mg per day and given for 3 days while fondaparinux was continued at 7.5 mg per day for further 4 days. Her platelets and D-dimers continued to improve as shown in table 1. An ultrasound abdomen to look for portal and hepatic vein thrombosis was done as initial liver function tests were abnormal but no clot was demonstrated. Dabigatran at 150 mg two times per day was initiated and she was discharged home.
Outcome and follow-up
On follow-up 1 month later, her headaches had improved and she was tolerating dabigatran well. Her platelet counts and D-dimers have remained normal while anti-PF-4 antibodies were persistently positive with OD of 2.87, 1 month after discharge. Her liver function tests were persistently abnormal but stable, for which no cause was identified. Other tests are shown in table 1.
Discussion
VITT is a relatively new phenomenon, recognised after the global roll out of ChAdOx-1 nCov-19 vaccine. The underlying pathogenic mechanism seems to be platelet activation through FcγIIa receptors by IgG antibodies against PF-4.10 11
VITT seems to affect a relatively younger population. In the study by Pavord et al, 56% of the patients were younger than 50 years while 85% were younger than 60 years.9 In a case series of 11 patients by Greinacher et al, all the patients were less than 50 years.5 Patients in earlier published case series presented within 16 days after vaccination whereas the study by Pavord et al showed that overwhelming majority of patients presented between 5 and 30 days after vaccination with 3 % patients presenting between 30 and 48 days.4 5 9 Our patient also presented 3 weeks (21 days) after the first dose of vaccine. Affected patients did not receive any heparin in 3 months prior to presentation and no underlying condition or medication usage has been clearly identified as a risk factor. Thrombosis most commonly involves cerebral venous sinuses. In the study by Pavord et al, CVST was found in 50% patients followed by thrombosis in deep veins and pulmonary arteries in 37% patients. Other less common sites included splanchnic circulation and arterial thrombi in aorta, coronary and cerebral arteries.9 More than one vascular bed may be involved.
In people with a strong clinical suspicion of VITT, NICE recommends a full blood count to look for thrombocytopaenia.7 Thrombocytopaenia is a characteristic feature but up to 5% patients may present without thrombocytopaenia on admission. In such cases, platelet counts should be repeated if strong suspicion remains. Once thrombocytopaenia is confirmed or if strong clinical suspicion remains, D-dimers, PT, aPTT and Clauss fibrinogen should be sent. D-dimers>4000 μg/L and low levels of Fibrinogen increase the likelihood of VITT, although fibrinogen can be normal. On admission, our patient had low platelets, D-dimers>6000 ng/mL, normal fibrinogen, PT and aPTT. In cases of possible VITT with strong suspicion or probable VITT, samples for anti-PF-4 antibodies by HIT ELISA method should be sent.6 In the study by Pavord et al, anti-PF-4 antibodies were detected in overwhelming majority of patients with definite or probable VITT although they were negative in 6 (2.7%) patients. For such cases of probable VITT where anti-PF-4 antibody is negative, NICE recommends discussion in a multidisciplinary team meeting whether treatment should be continued.7 Based on case definition criteria, our patient had definite VITT as all the criteria were met. Appropriate imaging should be pursued based on the site of suspected thrombosis.
The evidence base for the management of VITT is still evolving with many recommendations being expert opinions. EHP and NICE have provided guidance on key aspects of treatment.6 7 Treatment in VITT has two key components: anticoagulation and immunosuppression. Treatment of a probable case should be initiated without waiting for results of anti-PF antibody as VITT can evolve rapidly and is potentially life threatening. For thrombosis, anticoagulation with non-heparin based agents such as direct oral anticoagulants, fondaparinaux, danaparoid and argatroban should be used while heparin-based agents and warfarin should be avoided. In cases where there is high risk of bleeding such as platelets <30×109 /L or when surgery is being considered, Argatroban should be used. Use of fibrinogen concentrate or cryoprecipitate should be considered to keep fibrinogen >1.5 g/L. In a person requiring urgent surgical intervention for thrombosis and low platelets increasing bleeding risk, platelet transfusion is recommended. Our patient was treated with Fondaparinaux initially and later switched to dabigatran on discharge.
Intravenous immunoglobulins at 1 g/kg (divided over 2 days) should be given urgently in definite or probable cases along with consideration for steroids. In a small case series of three patients by Bourguignon et al, intravenous immunoglobulin use was associated with improvement in platelet counts, reduction in D-dimers and increase in serial fibrinogen.10 Since patients with CVST have higher mortality, such patients should be considered for early plasma exchange, high dose steroids and transferred to specialist centre with consideration for neuroradiological intervention, thrombolysis or thrombectomy. Our patient was treated with intravenous immunoglobulin and dexamethasone without waiting for anti-PF-4 antibody result. She was transferred to a specialist centre with expertise in neurosciences. Plasma exchange is recommended in very severe or resistant cases, in those with extensive thrombosis, CVST or platelets <30×109 /L. This was not used in our patient presumably because of stable clot appearances, absence of new thrombi and improvement in platelets. Cases refractory to intravenous immunoglobulin and plasma exchange should be considered for rituximab.
The natural history of VITT is not well understood. EHP and NICE recommends weekly anti-PF-4 antibodies for the first 4 weeks after discharge, followed by monthly tests for the next 6 months.6 7 Anticoagulation should be continued for at least 3 months or till anti-PF-4 antibodies become negative. Patients should be closely followed to detect relapse.
Evidence for best practice in VITT will continue to evolve as more research is conducted. Any suspected case should be discussed with local haematology team and local policies and procedures followed along with consulting the most up to date literature.
Learning points.
Vaccine-induced immune thrombosis and thrombocytopaenia is a potentially life-threatening condition that can occur 5–30 days after ChAdOx-1 nCov-19 vaccine.
Important parameters include presence of thrombosis, thrombocytopaenia, very high D-dimers and positive anti-platelet factor 4 (PF-4) antibodies by ELISA while fibrinogen can be low or normal.
A probable case should be treated immediately with non-heparin anticoagulants, intravenous immunoglobulin and steroids without waiting for anti-PF-4 antibody results and urgent haematology opinion should be sought.
Footnotes
Twitter: @Wahpes
Contributors: SNA: submitting and corresponding author, guarantorDesign and research, writing draft, proof reading. NO: design and research, writing draft, proof reading. MB: design and research, writing draft, proof reading.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Worldometer . Covid-19 coronavirus pandemic, 2021. Available: https://www.worldometers.info/coronavirus/
- 2.World Health Organization . COVID-19 Dashboard, 2021. Available: https://covid19.who.int/
- 3.Coronavirus vaccine-weekly summary of yellow card reporting. Available: www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting
- 4.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2124–30. 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092–101. 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guidance produced by the expert haematology panel focussed on vaccine induced thrombosis and thrombocytopenia (VITT), 2021. Available: https://b-s-h.org.uk/about-us/news/guidance-produced-by-the-expert-haematology-panel-ehp-focussed-on-vaccine-induced-thrombosis-and-thrombocytopenia-vitt/
- 7.Nice guidelines (NG200) on management of VITT. Available: https://www.nice.org.uk/guidance/ng200/resources/fully-accessible-version-of-the-guideline-pdf-pdf-51036811744
- 8.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost 2017;;15:2099–114. Nov. 10.1111/jth.13813 [DOI] [PubMed] [Google Scholar]
- 9.Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med 2021;385:1680–9. 10.1056/NEJMoa2109908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourguignon A, Arnold DM, Warkentin TE, et al. Adjunct immune globulin for vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med 2021;385:720–8. 10.1056/NEJMoa2107051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2202–11. 10.1056/NEJMoa2105385 [DOI] [PMC free article] [PubMed] [Google Scholar]


