Abstract
Eosinophilic fasciitis (EF) is a rare subacute fibrosing disorder of unknown aetiology, characterised by thickening of the muscular fascia and subcutaneous tissue, leading to swelling of limbs and trunk and sparing fingers and toes. Eosinophilic infiltration and degranulation may prompt tissue damage and consequent fibrosis due to the accumulation of collagen and extracellular matrix proteins. MRI is the best imaging modality for diagnosis, depicting fascial thickening and enhancement. MRI may also have a significant role in excluding alternative diagnosis and guiding the skin–muscle biopsy.
We report a case of EF with clinical and pathological correlation, highlighting the diagnostic value of MRI for early diagnosis and further treatment.
Keywords: musculoskeletal and joint disorders, skin, rheumatology, radiology
Background
Eosinophilic fasciitis (EF) or Shulman syndrome, was first described in 1974, is a rare variant scleroderma-like disorder, with less than 300 cases reported. Since it may mimic systemic sclerosis (SS), recognising this entity is crucial to avoid misdiagnosis.
This case report highlights the role of MRI to allow early diagnosis and prompt treatment, since this may have a positive effect on patient’s morbidity and disease remission.
Case presentation
A 66-year-old woman without relevant medical history was referred to our rheumatology department with a 1 year history of progressive fatigue, swelling, thickening of lower legs, thighs, forearms and arms, in an additive and symmetrical way, with progressive worsening, sparing hands and toes.
She denied additional symptoms such as Raynaud’s phenomenon, digital ulcers, telangiectasias, inflammatory arthralgia, photosensitivity, dry cough, dyspnoea on exertion and reflux.
Physical examination confirmed the skin thickening of forearms, arms, thighs and legs, with a peau d’orange sign of right thigh (figure 1) and a linear subcutaneous depression in her forearms (figure 2).
Figure 1.
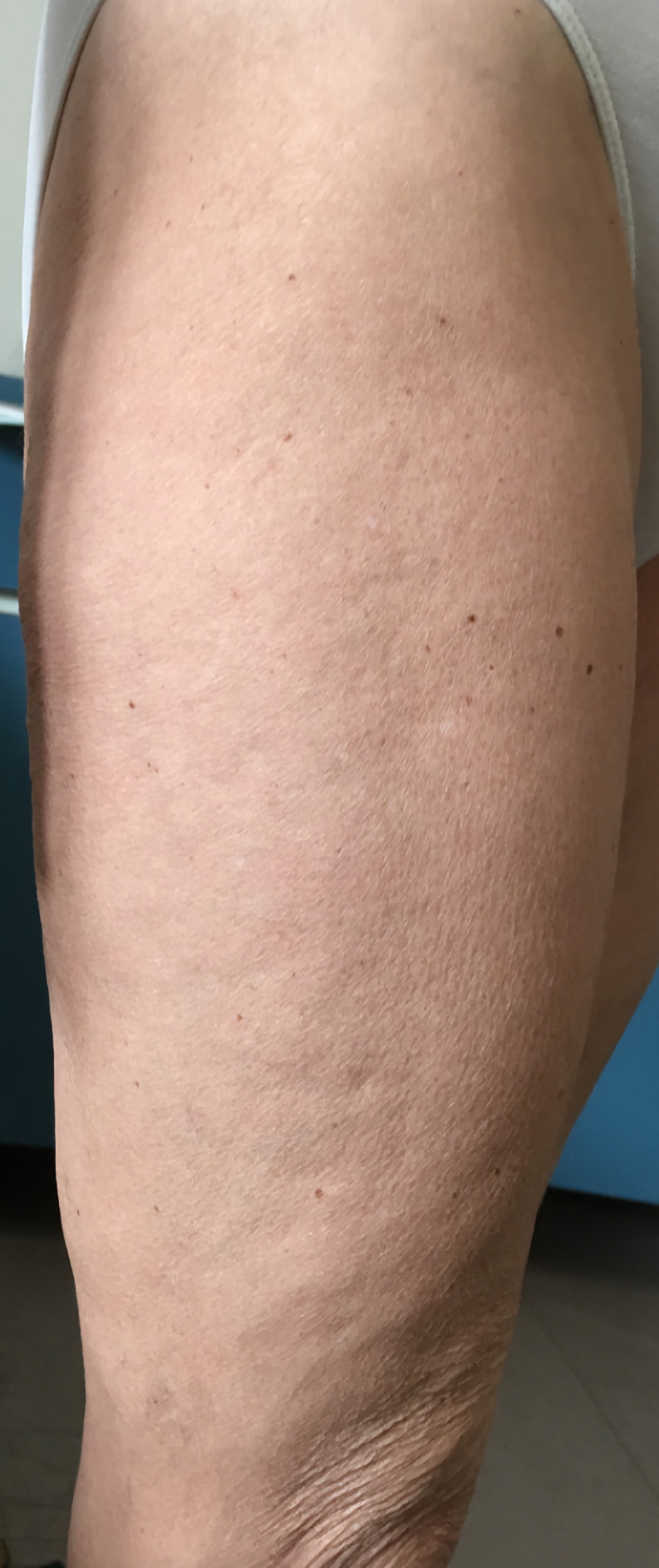
Clinical finding of the orange peel-like appearance in the thigh.
Figure 2.
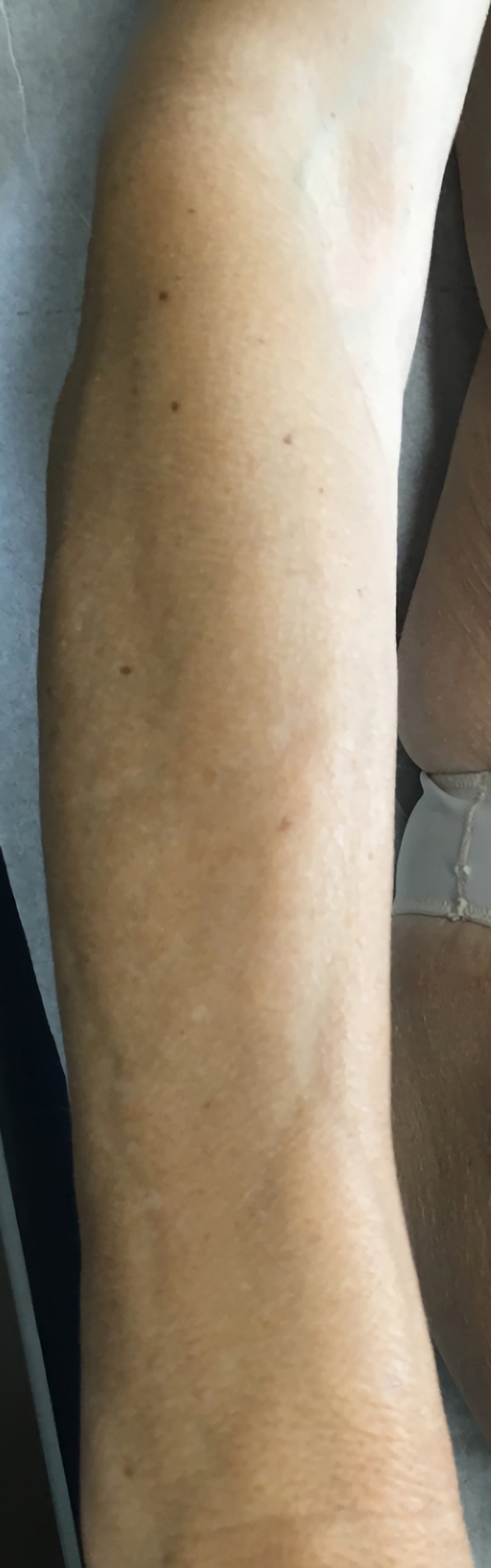
Typical groove sign of the forearm.
Investigations
There was absolute and relative hypereosinophilia 19.1×109 (reference value (RV) <0.5×109 eosinophils/L), the percentage of peripheral eosinophils was 1.39% (RV <0.5%), the erythrocyte sedimentation rate (ESR) was 22 (RV <30 mm/hour), without abnormal polyclonal paraproteinaemia. Blood electrolytes and liver function were normal.
Immunological tests were negative (antinuclear antibodies, anti-Scl 70, anticentromere, anti-JO1, anti-RNP and anticytoplasmic).
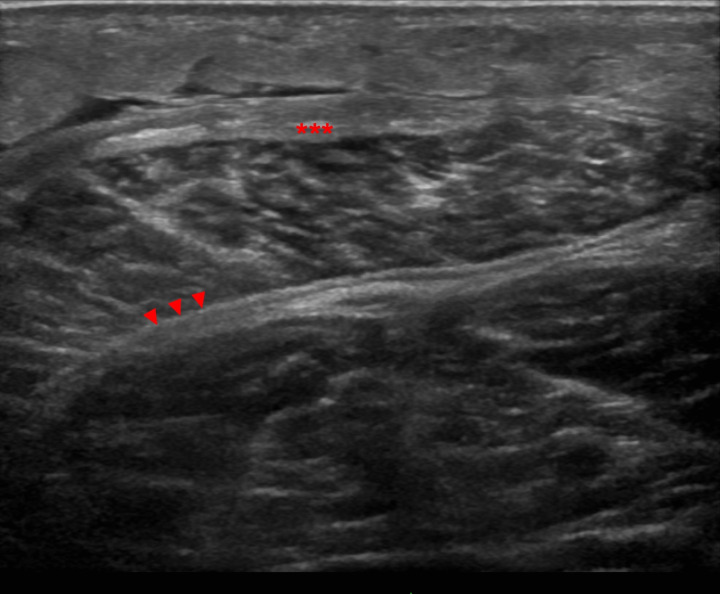
Musculoskeletal ultrasound revealed oedema of subcutaneous tissue, marked thickening of the deep peripheral and deep intermuscular fasciae (figure 3).
Figure 3.
Ultrasound image (linear array transducer 6–15 MHz) of the posterior leg compartment depicts subcutaneous oedema, marked thickening of the deep peripheral fascia (asterisks) and deep intermuscular fascia (arrowheads).
Chest and abdominal-pelvic CT revealed no lesions suggestive of malignancy.
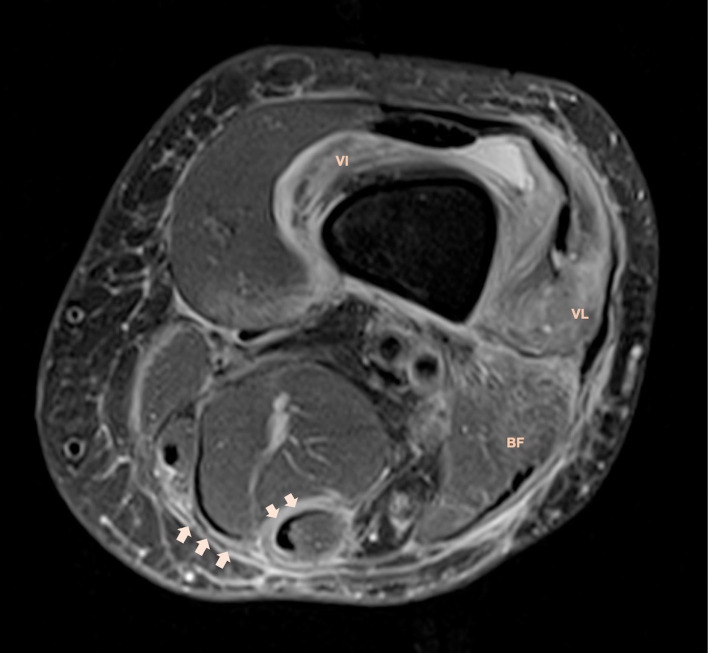
MRI depicted symmetric, diffuse, bilateral fascial thickening (figures 4 and 5), comprising the deep peripheral fascia which surrounds whole muscle groups, and deep intermuscular fascia, mainly in the posterior muscle compartment of the lower limbs.
Figure 4.
Axial fat-suppressed proton density MRI demonstrates thickening and hyperintense signal of deep and intermuscular fasciae (arrows). There is associated muscular oedema of vastus lateralis, vastus intermedius and biceps femoris muscles. Stranding of subcutaneous tissue represents panniculitis.
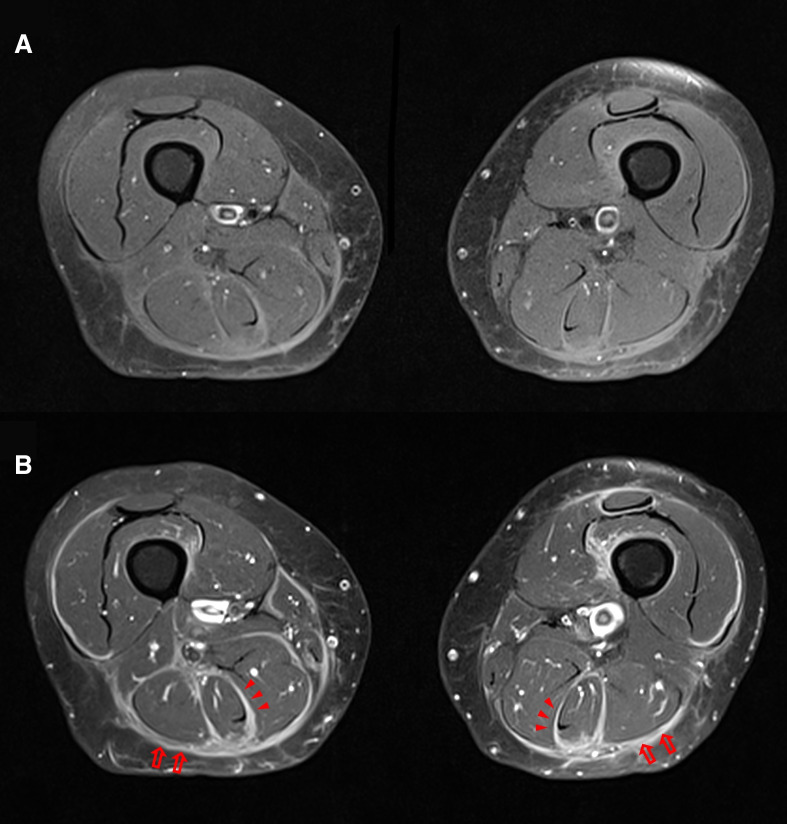
Figure 5.
Axial fat-suppressed T1-weighted MRI before (A) and after gadolinium (B) shows thickening and intense symmetrical fascial enhancement after contrast, mainly involving the deep (arrows) and intermuscular deep fasciae (arrowheads).
Fascial thickening demonstrated moderate hyperintense signal in fluid sensitive sequences with associated intramuscular oedema, specifically in the vastus lateralis, vastus intermedius and biceps femoris muscles (figure 4). After contrast injection, there was strong and symmetrical fascial enhancement (figure 5).
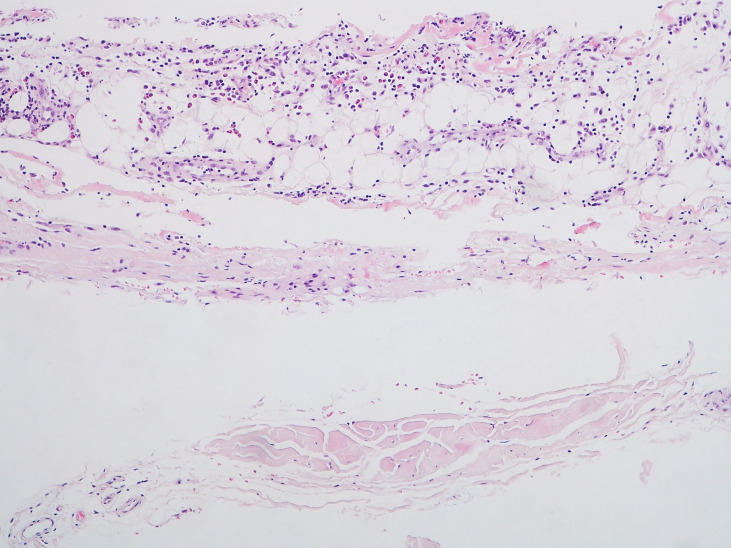
Full-thickness wedge biopsy of left thigh described the presence of fascia tissue and adjacent fibroadipose tissue with slight inflammatory infiltrate consisting of lymphocytes, plasmocytes and eosinophils, that confirmed the suspicion of EF (figure 6).
Figure 6.
Inflammatory infiltrate by lymphocytes, plasma cells and eosinophils in the subcutaneous tissue above the fascia (HE ×100).
Differential diagnosis
The differential diagnosis of EF includes SS, cardiac failure and lymphoma. MRI is useful in showing signal abnormalities centred around the fascia. Perimyositis is also frequent and may increase the potential differential diagnosis.1
SS may be responsible for an extensive cutaneous fibrosis. However, SS is not associated with peripheral eosinophilia, nor satisfying response to corticosteroids and more frequently leads to a visceral involvement (pulmonary or oesophageal). As opposed to SS, in EF the capillaroscopy is usually normal, there is not digital and facial skin sclerosis, and fascial thickening is more diffuse and pronounced.
Stasis oedema is typical of cardiac failure. It is characterised by an infiltration of hypodermic tissue that appears as a hyperintense signal on T2-weighted image (WI), with generally diffuse and symmetric distribution, without enhancement after contrast (‘cold oedema’).2
Peripheral T-cell lymphomas may have a cutaneous and sometimes a fascial involvement, but are easily excluded by the muscle biopsy pathological examination.3
Treatment
The patient was treated with oral prednisolone at a starting dose of 0.5 mg/kg/day anticipated by methylprednisolone pulses (500 mg) on 3 consecutive days and methotrexate at a starting dose of 10 mg/week. Dual-energy X-ray absorptiometry prior to the treatment revealed osteopenia (femoral neck bone mineral density: 766 mg/cm2 with T-score −1.5). Calcium, vitamin D and alendronic acid were added for osteoporosis prevention. Physiotherapy as adjunctive treatment was started.
Outcome and follow-up
Resolution of constitutional symptoms and eosinophilia was verified right after the start of corticoids.
Until now (9 months after onset), with a prednisolone’s dose of 10 mg/daily and methotrexate’s dose of 20 mg/weekly, the effect of treatment was incomplete on the other clinical aspects: skin thickening stabilised and gain in joint amplitudes was partial.
Early start of therapy correlates with better clinical outcomes.4 5 Therefore, our patient partial response is presumably justified due to the long period between onset of symptoms and the final diagnosis.
Discussion
EF is a rare scleroderma-like syndrome.6 It presents classically as a combination of skin induration and diffuse inflammation of the deep fascia, often preceded by oedema and/or erythema which may be painful. As the disease progresses, oedema is gradually replaced by a peau d’orange as deep sclerosis starts to develop. Generally, lower and/or upper limbs are symmetrically involved.7 A depressed vein aspect, named the groove sign, can be present in up to half of patients and seems to be highly suggestive of a deep fibrosis or fascial involvement.
In most cases, involvement of the extremities is symmetrical, whereas unilateral involvement is unusual.
The mean age at onset is between 40 and 50 years. The exact pathophysiology is unknown. Haematological, infectious, autoimmune diseases, intense physical exertion, drugs and chemical compounds have been proposed as possible triggers.8 In 10% of cases it may be paraneoplastic, and the presence of cytopenia may signal an underlying haematological disorder such as hemolytic anaemia, myelodysplasia, lymphoma or multiple myeloma.9
A full-thickness (skin-to-muscle) wedge biopsy of the affected skin is the gold standard for the diagnosis of EF. At histology, it presents with infiltration of lymphocytes, mainly CD8 +with CD4/CD8 ratio <1, plasma cells, histiocytes and variable numbers of eosinophils in the deep reticular dermis and superficial fascia. Unlike SS, the epidermis and superficial dermis are normal, with most pathology located in the subcutaneous tissue, fascia and muscle.10
Eosinophilic infiltrates are present in the majority of patients, however variable and transitory, and can become absent in chronic phase of the disease or after corticosteroid treatment. Interstitial myositis has been observed in 68% of patients with EF, but clinical myositis and muscle degeneration are rarely reported.5 11
The most characteristic laboratory finding in EF is peripheral eosinophilia, which it is present in 63%–93% of patients. Nevertheless, it is not required for the EF diagnosis, and it does not correlate with disease severity. It is also not useful in evaluation of treatment response or further follow-up.12
Inflammatory markers such as C reactive protein, elevated ESR and hypergammaglobulinaemia can be found in more than half of patients.13
Radiographs are usually unremarkable. Ultrasound may depict unspecific fascial thickening although it fails to exclude a possible diagnosis of myositis and other differential diagnosis. MRI is considered the best imaging modality for the diagnosis of EF and it is useful to suggest the optimal location for muscle biopsy.14
MRI findings in active EF are characteristics and radiologists should be familiar with these, which are fascial thickening, hyperintense signal within the fascia on fluid-sensitive sequences and fascial enhancement after contrast administration. Reactive oedema of the adjacent muscles and subcutaneous oedema may also be present, i0.15n a much lesser extent, and as response to the fascial changes (‘contact oedema’).14 15 MRI findings may reflect clinical disease activity, with the degree of T2 hyperintensity within the fascia, fascial thickening on T1-WI and fascial enhancement, all paralleling disease activity, which is useful to evaluate response after treatment.14
The diagnosis of EF is based on clinical, laboratory, imaging and pathohistological findings.
Universally accepted diagnostic criteria in patients with EF are lacking; however, recent criteria have been proposed.8 16 Most physicians consider that the diagnosis can be suggested when characteristic skin lesions with typical hyperintense fascia on MR T2-WI are present, after excluding the various subsets of scleroderma-like lesions.8
Prior studies increasingly favour the combination of systemic corticosteroids and methotrexate for the initial treatment. However, the optimal dose and duration of treatment have not yet been clarified. Several immunomodulators have been recently used including azathioprine, sulfasalazine, cyclosporine, rituximab, infliximab, tocilizumab, intravenous immunoglobulins, tofacitinib and D-penicillamine. Many of these have been combined with corticosteroids and are offered as options for adjuvant therapy in refractory cases, in the setting of corticosteroids dependence or prolonged treatment. However, it is essential to carefully consider the use of these treatments, as current evidence is based on small case series and non-randomised clinical trials. Further studies are needed to determine the role of these biological treatments in EF, such as its effectiveness and duration of treatment.17–20
Learning points.
Eosinophilic fasciitis is a rare subacute fibrosing disorder of unknown aetiology.
MRI is now considered the best imaging modality for diagnosis.
The characteristic MRI findings are abnormal fascial signal intensity and enhancement after contrast. The degree of enhancement is associated with the severity of microscopic inflammation fasciitis.
Definitive diagnosis requires histopathological examination from a full-thickness (epidermis to muscle) biopsy.
Prognosis is favourable with conventional steroids at an early stage, but treatment appears less effective in chronic disease with irreversible fibrotic changes.
Acknowledgments
We thank Dr Vera Las for her useful comments and important clinical contributions.
Footnotes
Contributors: AP and NM reviewed the literature and wrote the first draft. DLP provided the histological diagnosis and the image used in this section. DA helped in the imaging assessment. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Long H, Zhang G, Wang L, et al. Eosinophilic skin diseases: a comprehensive review. Clin Rev Allergy Immunol 2016;50:189–213. 10.1007/s12016-015-8485-8 [DOI] [PubMed] [Google Scholar]
- 2.Kirchgesner T, Dallaudière B, Omoumi P, et al. Eosinophilic fasciitis: typical abnormalities, variants and differential diagnosis of fasciae abnormalities using MR imaging. Diagn Interv Imaging 2015;96:341–8. 10.1016/j.diii.2014.06.018 [DOI] [PubMed] [Google Scholar]
- 3.Lebeaux D, Sène D. Eosinophilic fasciitis (Shulman disease). Best Pract Res Clin Rheumatol 2012;26:449–58. 10.1016/j.berh.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 4.Long H, Zhang G, Wang L, et al. Eosinophilic skin diseases: a comprehensive review. Clin Rev Allergy Immunol 2016;50:189–213. 10.1007/s12016-015-8485-8 [DOI] [PubMed] [Google Scholar]
- 5.Lebeaux D, Francès C, Barete S, et al. Eosinophilic fasciitis (Shulman disease): new insights into the therapeutic management from a series of 34 patients. Rheumatology 2012;51:557–61. 10.1093/rheumatology/ker366 [DOI] [PubMed] [Google Scholar]
- 6.Shulman LE. Diffuse fasciitis with hypergammaglobulinemia and eosinophilia: a new syndrome? J Rheumatol 1984;11:569–70. [PubMed] [Google Scholar]
- 7.Marzano AV, Genovese G. Eosinophilic dermatoses: recognition and management. Am J Clin Dermatol 2020;21:525–39. 10.1007/s40257-020-00520-4 [DOI] [PubMed] [Google Scholar]
- 8.Pinal-Fernandez I, Selva-O' Callaghan A, Grau JM. Diagnosis and classification of eosinophilic fasciitis. Autoimmun Rev 2014;13:379–82. 10.1016/j.autrev.2014.01.019 [DOI] [PubMed] [Google Scholar]
- 9.Urzal J, Cimbron M, Mendonça T, et al. Eosinophilic fasciitis (Shulman's disease): review and comparative evaluation of seven patients. Reumatologia 2019;57:85–90. 10.5114/reum.2019.84813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mejia R, Nutman TB. Evaluation and differential diagnosis of marked, persistent eosinophilia. Semin Hematol 2012;49:149–59. 10.1053/j.seminhematol.2012.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adachi Y, Mizutani Y, Shu E, et al. Eosinophilic fasciitis associated with myositis. Case Rep Dermatol 2015;7:79–83. 10.1159/000381845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samona J. Orthopedic considerations with eosinophilic fasciitis: a case report and literature review. Case Rep Orthop 2012;2012:1–5. 10.1155/2012/865360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fett N, Arthur M. Eosinophilic fasciitis: current concepts. Clin Dermatol 2018;36:487–97. 10.1016/j.clindermatol.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 14.Desvignes-Engelbert A, Saulière N, Loeuille D, et al. From diagnosis to remission: place of MRI in eosinophilic fasciitis. Clin Rheumatol 2010;29:1461–4. 10.1007/s10067-010-1508-1 [DOI] [PubMed] [Google Scholar]
- 15.Moulton SJ, Kransdorf MJ, Ginsburg WW, et al. Eosinophilic fasciitis: spectrum of MRI findings. AJR Am J Roentgenol 2005;184:975–8. 10.2214/ajr.184.3.01840975 [DOI] [PubMed] [Google Scholar]
- 16.Jinnin M, Yamamoto T, Asano Y, et al. Diagnostic criteria, severity classification and guidelines of eosinophilic fasciitis. J Dermatol 2018;45:881–90. 10.1111/1346-8138.14160 [DOI] [PubMed] [Google Scholar]
- 17.Espinoza F, Jorgensen C, Pers Y-M. Efficacy of tocilizumab in the treatment of eosinophilic fasciitis: report of one case. Joint Bone Spine 2015;82:460–1. 10.1016/j.jbspin.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 18.Mazori DR, Femia AN, Vleugels RA. Eosinophilic fasciitis: an updated review on diagnosis and treatment. Curr Rheumatol Rep 2017;19:74. 10.1007/s11926-017-0700-6 [DOI] [PubMed] [Google Scholar]
- 19.Manzini CU, Sebastiani M, Giuggioli D, et al. D-Penicillamine in the treatment of eosinophilic fasciitis: case reports and review of the literature. Clin Rheumatol 2012;31:183–7. 10.1007/s10067-011-1866-3 [DOI] [PubMed] [Google Scholar]
- 20.Vílchez-Oya F, Sánchez-Schmidt JM, Agustí A, et al. The use of tocilizumab in the treatment of refractory eosinophilic fasciitis: a case-based review. Clin Rheumatol 2020;39:1693–8. 10.1007/s10067-020-04952-5 [DOI] [PubMed] [Google Scholar]